Abstract
The mechanism of pyroptosis in trigeminal neuralgia (TN) progression remains unclear. The discovery of tRNA-derived small RNAs (tsRNAs) in the mechanism of pyroptosis is a breakthrough. We aim to study whether tsRNA is involved in TN progression through pyroptosis. A TN rat model was constructed by chronic constriction injury of infraorbital nerve and then mechanical hyperalgesia was quantitated using a withdrawal threshold test. Mechanical hyperalgesia was significantly elevated by constriction injury. Immunofluorescence revealed that expression of GSDMD and NLRP3 were significantly enhanced in TN rats. The expression of caspase-1, IL-1β, and IL-18 was significantly boosted after TN induction. CoPP + H2O2 exposure induced cell injury and pyroptosis of rat Schwann RSC96 cells, but reversed by VX-765 (a caspase-1 inhibitor). A total of 87 differentially expressed tsRNAs (DEtsRNAs) were identified in TN rats. KEGG pathway enrichment showed that DEtsRNAs were mainly involved in pyroptosis-related pathways, such as MAPK, ErbB, and mTOR signaling pathways. RT-qPCR showed that pyroptosis-related DEtsRNAs of tsRNA003489, tsRNA001840, and tsRNA003424 were downregulated in TN. Network analysis indicated tsRNA003489 and tsRNA001840 interact with NLRP3, a key regulator of pyroptosis. tsRNA has potential to participate in TN progression by targeting pyroptosis-related genes, which provides a new perspective for understanding pathology of TN.
Introduction
Trigeminal neuralgia (TN) is a severe unilateral neuropathic pain which has a substantial negative influence on the quality of life. TN patients are often associated with cognitive dysfunction and a higher risk of developing central nervous system degeneration (Ren et al. Citation2021). TN is one of the most difficult neuropathic pains to treat, characterized by spontaneous pain, hyperalgesia, and allodynia in the orofacial area (Tao et al. Citation2021). Neurogenic inflammation is implicated in neuropathic pains which is attributed to inflammatory mediators, including pro-inflammatory cytokines and chemokines (Chen et al. Citation2013; Martini et al. Citation2016). For example, inflammatory cytokines tumor necrosis factor-α, interleukin (IL)-1β, and IL-6 play an important role in the pathogenesis of TN by modulating the immune response in the peripheral periphery of trigeminal ganglion neurons (Liu et al. Citation2019; Xu et al. Citation2020). However, the cause of neurogenic inflammation in TN remains a matter of debate and is still left to be understood.
The NOD-like receptor family pyrin domain containing three (NLRP3) inflammasome triggers pyroptosis. NLRP3 inflammasome activated-pyroptosis releases large amounts of the inflammatory mediators IL-1β and IL-18 thereby recruiting more inflammatory cells to the site of the incident resulting in expanded inflammation (Yu et al. Citation2021). NLRP3 inflammasome has been reported to be linked to neuropathic pain. For example, the NLRP3 inflammasome level was elevated in microglia after nerve damage and NLRP3 knockdown significantly activated mechanical allodynia and attenuated cell death in microglia in TN rats (Sun et al. Citation2021a). In complete Freund's adjuvant-treated prosopalgia mice, the expression of NLRP3, IL-1β, and IL-18 in trigeminal ganglions was significantly increased (Chen et al. Citation2018). MCC950, a specific NLRP3 inflammasome blocker, not only significantly inhibited NLRP3 expression in TN rats but also alleviated neurogenic inflammation and neurodegeneration (Ren et al. Citation2021). These findings indicate that NLRP3 inflammasome appear as candidates for controlling neuropathic pain in TN. However, many gaps in its mechanism still exist.
tRNA-derived small RNAs (tsRNAs) is a class of 18–40 nt non-coding RNAs. tsRNAs are divided into three major types based on their cleavage position within the tRNA transcript: tRNA halves (tiRNAs), tRNA-derived small fragments (tRFs), and 3′U tRNA fragments (Xiao et al. Citation2022). Decades ago, tsRNAs were considered as 'junk' until researchers discovered that they had a specific biological origin and was sensitive to external stress such as oxidative stress, virus infection, or heat shock (Wang et al. Citation2013) and metabolic disorders (Zhang et al. Citation2021). In recent years, the role of tsRNAs in neurological diseases has been gradually revealed. For example, abnormal expression of tsRNAs in sperm in aged males is associated with anxiety-like behavior in offspring (Guo et al. Citation2021). Altered tsRNAs may contribute to experimental intracerebral hemorrhage (Li et al. Citation2020) and regulate gene expression in a sequence-specific manner in the primate hippocampus (Jehn et al. Citation2020). However, the function of tsRNAs in TN has not been reported. Unlike tsRNAs, the regulatory role of other types of non-coding RNAs in TN has been reported, including lncRNAs (Xu et al. Citation2020), miRNAs (Xiong et al. Citation2019), and so on. Considering that tsRNAs differ from other non-coding RNAs in their structure and mode of action, the researchers believe it is important to probe the function of tsRNAs (Zhao et al. Citation2023; Zong et al. Citation2021). Besides, to our knowledge, until now, there is only one report on the regulation of NLRP3 inflammation by tsRNAs, showing that tRF3-Thr-AGT suppressed the ZBP1/NLRP3 pathway to ameliorate acute pancreatitis (Sun et al. Citation2021b). Therefore, exploring whether tsRNAs can regulate NLRP3 to participate in TN progression is quite meaningful.
In this study, we aimed to confirm the significance of NLRP3-mediated pyroptosis in TN development and to resolve the tsRNAs expression profile in trigeminal ganglions in TN rats to explore the key tsRNAs-associated with NLRP3. We will provide new therapeutic targets for targeted therapy of TN.
Materials and methods
Animal study and ethics statement
A total of 12 healthy SPF-grade Sprague–Dawley male rats (180–220 g) were purchased from Spelford (Beijing) Biotechnology Co. Rats were housed in a standard room at 24–26°C with 55–60% humidity and light cycles controlled in 12 h/12 h alternating light and dark for a week. All rats received an ad libitum diet and water. Potential confounding factors for this study include body mass index, breed, batch effect, and environmental factors. All rat experimental operations were approved by the Ethics Committee of The First Affiliated Hospital of Nanchang University [SYXK (gan) 2021-0003], according to the ARRIVE 2.0 Guidelines (Percie du Sert et al. Citation2020), and the NIH guidelines. A minimum sample size of 12 was determined based on empirical experience. All experimental animals were included in the analysis and no exclusions were made. A computer-generated random numbering method in excel was used for grouping. In animal experiments, the researchers who took samples and tested the indicators did not know the grouping situation.
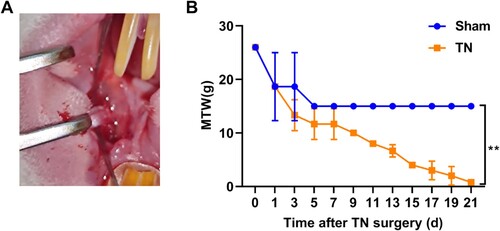
Twelve rats were randomly divided into two groups. Using 4% pentobarbital sodium (50 mg/kg) (4390-16-3, Merck), rats were fixed in a supine position. In the experimental TN group (n = 6), on the left intraoral soft palate site (next to the first molar), a longitudinal 1 cm incision was opened to expose the infraorbital nerve (Figure (A)). Next, the infraorbital nerve was loosely ligated with two 4-0 chromic guts spaced 2 mm apart. The ligation tightness required that the diameter of the nerve be reduced but was not to interrupt the epineural circulation. The wound was sutured and the contralateral side of all rats was intact. The sham group (n = 6) was performed as above but without ligation. The left trigeminal ganglion on the surgical side was collected at 21-day post-surgery under 4% pentobarbital sodium anesthesia following transcardially perfused with 0.1 M PBS and 4% formaldehyde/12.5% picric acid solution in 0.1 M PBS. Three of the six animals in each group were fixed for immunofluorescence and the other three animals tissue collected fresh for other analyses.
Orofacial mechanical withdrawal threshold (MWT) test
To minimize the bias of MTT test, each experiment was performed by the same experimentalist over the same period of time. The MWT was used to evaluate mechanical hyperalgesia of rats at different time-points after TN surgery (0, 1, 3, 5, 7, 9, 11, 13, 15, 17, 19, 21 d) between 10:00 and 14:00 in the afternoon. Rats were placed in an individual transparent box (22 × 12 × 22 cm) with the stainless-steel mesh at the bottom to acclimate the laboratory environment for at least 1 h. A series of normalized von Frey filaments was utilized to stimuli the skin within the infraorbital territory. The location of stimulation was around the central vibrissal pad of the nasal region and the surrounding hairy skin (the sensory innervation area of the infraorbital nerve in the face). Stimulus intensity was constantly increased until a withdrawal response appeared or the threshold force of 2.0 g was reached. The test was repeated five times for each filament at 5-s intervals. The positive response was defined as rapid retreat, dodging, turning, attacking, and scratch action. The MWT was defined as the lowest force in grams that produced at least three positive reactions in five consecutive applications.
RNA isolation
Total RNA was extracted from the left trigeminal ganglion in the sham group (n = 3) and TN group (n = 3) using Trizol (Invitrogen, USA). The concentration and purity of total RNA were measured by micro-spectrophotometer (TGem, TIANGEN) and integrity was accessed by agarose gel electrophoresis. The qualified RNA was stored at −80°C for RT-qPCR analyses and small RNA sequencing.
RT-qPCR analyses
The qualified RNA was reverse-transcribed into cDNA using specific primers and then amplified by PCR using 2 × Master Mix (Roche). Next, the PCR amplification procedure was 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 60 s on the ABI Q6 system (Applied Biosystems Inc., USA). The gene expression was determined by the 2−ΔΔCT method, mRNA was relative to GAPDH and tsRNA was relative to U6. All the primer sequences used in this study are shown in Supplemental Table 1.
Immunofluorescence (IF)
The fresh tissue of trigeminal ganglion was fixed in 4% paraformaldehyde for 30 min and embedded in paraffin and then placed in the EDTA antigen repair buffer (pH 8.0) for antigen repair. Next, drew a circle using an immunohistochemical pen to enclose the tissue following blocked it with bovine serum albumin for 30 min. Sections were incubated with antibodies of GSDMD Rabbit mAb (1:1000, A20728, ABclonal) or NLRP3 Rabbit (1:100, FNab10120, FineTest) at 4°C overnight and conjugated with fluorescence secondary antibody of IgG (H + L) (FITC conjugated Goat Anti-Rabbit IgG (H + L)) (1:100, GB22303, Servicebio) for 50 min at 25°C. After that, the DAPI dye solution (G1012, Servicebio) was dripped into the circle and incubated for 10 min at 25°C followed by being treated with a spontaneous fluorescence quencher (G1221, Servicebio). Finally, sections were sealed with anti-fluorescence quencher (G1401, Servicebio) and observed under the fluorescence microscope (NIKON DS-U3). The FITC-labeled secondary antibody with an excitation wavelength of 492 nm was detected at emission wavelengths from 515 to 555 nm. The image Pro plus 6.0 software was used to calculate the fluorescence intensity/area. Three random visual fields for each replicate and three replicates for each group were performed.
Western blot
Protein samples were prepared by RIPA buffer (Thermo, USA) and protein concentration was determined by the bicinchoninic acid (BCA) method (Thermo, USA). An equal amount (20 µg) of protein was separated by 10% SDS/PAGE and then transferred onto polyvinylidene fluoride (PVDF) membranes. After sealed by Tris Buffered Saline with Tween-20 (TBST) solution with 5% skim milk, membranes were incubated with primary antibodies, including GSDMD (1:5000, 20770-1-AP, Proteintech), IL-18 (1:10000, 10663-1-AP, Proteintech), IL-1β (1:2000, 26048-1-AP, Proteintech), caspase-1 (1:200, Sc-56036, SentaCruz), and GAPDH (1:15000, 60004-1-lg, Proteintech) at 4°C overnight followed by incubated with secondary antibody Goat Anti-Mouse IgG H&L(HRP) (1:1000, ab205719, Abcam) at 25°C for 2 h. Proteins were detected with ECL luminescence kits (Thermo, USA) and observed on a chemiluminescent imaging system (Shanghai Qinxiang Scientific Instrument Co., Ltd.).
Cell culture and treatment
Rat Schwann RSC96 cells (iCell-r030) were purchased from Saibaikang Biotechnology and cultured in DMEM medium (10-013-CVRC, Corning) supplemented with 10% FBS and 1% P/S in an incubator at 37°C with 5% CO2.
The TN cell model construction was referred to the previous study (Liu et al. Citation2023). Briefly, RSC96 cells were first pretreated with 40 mM CoPP (HY-W250116, MCE) for 24 h, followed by incubation with 300 µM H2O2 for 24 h to induce cellular damage. To inhibit pyroptosis, RSC96 cells were pretreated with 50 M VX-765 (a caspase-1 inhibitor) (HY-13205, MCE) for 24 h prior to modeling.
CCK8 assays
Cell viability of Schwann cells was measured by CCK8 kit (C0038, Beyotime). Briefly, Schwann cells were digested by Trypsin-EDTA (25200072, GIBCO) to make a single-cell suspension. After counting, cell concentration was readjusted to 1 × 104 cells/mL. Next, 100 µL cells were added to each well and six wells for each sample. After TN model induction, 10 µL CCK8 working solution was added into well, after 1 h, OD values at 450 nm were detected by a microplate reader.
Flow cytometry
Cell apoptosis of Schwann cells was measured by flow cytometry. Following treatment, the cell pellets were harvested from centrifugation and re-suspended in 195 μL of binding buffer to make cell concentration at 2 × 105 cells/mL, and then 5 μL of Annexin V-FITC solution was applied prior to incubation at room temperature for 15 min. After washing, cells were re-suspended in 195 μL of binding buffer and added 10 μL Propidium iodide (PI). A Beckman instrument was used to measure Annexin V and PI staining by flow cytometry.
ELISA
The concentration of IL-18 and IL-1β in RSC96 cells was measured by using Rat IL-1β ELISA Kit (ml028510, Shanghai Enzyme Linked Biology Co., Ltd.) and Rat IL-1β ELISA Kit (ml028514, Shanghai Enzyme Linked Biology Co., Ltd.), respectively, according to the ELISA kit instruction steps. A microplate reader was used to detect absorption at 450 nm.
Small RNA sequencing
Small RNA sequencing was entrusted to Yingbio Technology (Shanghai, China). In brief, RNA underwent 3’ adaptor ligation and hybridized with reverse primers following 5’ adaptor ligation. Next, RNA products of bidirectional adaptor sequences were used for the first-strand cDNA synthesis and amplifying into cDNA libraries. The cDNA fragments were separated by 8% gel electrophoresis and the length of 135–170 bp fragments were excised and recovered and purified for sequencing. The quality of libraries was accessed by Agilent 2100 BioAnalyzer. Small RNA sequencing was performed on the Illumina HiSeq2500 platform in a 50 bp single-end run.
Raw reads were filtered by Fast-QC software to obtain clean reads. Next, to exclude the interference of miRNA and piRNA, clean reads need to be aligned onto miRbase (https://www.mirbase.org/) and piRNAclusterDB 2.0 (https://www.smallrnagroup.uni-mainz.de/piRNAclusterDB). After that, the remaining clean reads aligned onto tRFdb (http://genome.bioch.virginia.edu/trfdb/) and MINTbase (https://cm.jefferson.edu/MINTbase/) to find out tsRNA sequences and the counts of tsRNA were considered as expression level. Differentially expressed tsRNAs (DEtsRNAs) were defined as |log2 fold change| > 1 and p-value < 0.05. All the DEtsRNAs were annotated to GO and KEGG analysis based on their target gene.
Network construction
The network of DEtsRNAs-mRNA-pathway was constructed using Cytoscape v.3.6.1 software. This work included 5 DEtsRNAs, 110 mRNAs, and 9 pathways. The mRNA that was predicted target gene of DEtsRNAs by RNAhybrid and Miranda has an edge with DEtsRNA.
Statistical analyses
Data statistics were conducted utilizing GraphPad Prism 9.0.0. Data are expressed as mean ± standard deviation (SD). Comparisons between two groups were performed with t test and among multiple groups were performed with one-way analysis of variance following Tukey’s test. p < 0.05 was considered statistically significant.
Results
Impaired MWT in TN rat
As shown in Figure (A), chronic constriction injury (CCI) of the infraorbital nerve (ION) (CCI-ION) was used to construct TN rat models. The nociception of rats was evaluated by MWT test on the time-point of 0, 1, 3, 5, 7, 9, 11, 13, 15, 17, 19, 21 d after CCI-ION surgery. The results of MWT showed that the MWT of rats in the sham group decreased sharply on the first postoperative day and stabilized without further decrease five days after surgery. However, the MWT of rats in the TN group continued to decrease without a plateau after CCI-ION surgery and was significantly lower than that of the sham rats (Figure (B)). The results obtained are consistent with the previous studies (Luo et al. Citation2019; Tao et al. Citation2021).
TN accompanied by pyroptosis activation
To investigate whether NLRP3 inflammasome-mediated pyroptosis is associated with TN progression, we used IF to detect the activation of GSDMD and NLRP3 of pyroptosis response cascade. Compared with the sham group, the green fluorescent signals of GSDMD and NLRP3 were significantly enhanced in the TN group (Figure (A)). Moreover, inflammatory mediators such as IL-1β and IL-18, that act downstream of the NLRP3 inflammasome were also significantly boosted after TN induction as measured by RT-qPCR (Figure (B)) and western blot (Figure (C)). The protein expression of caspase-1 (cleaves pro-IL-1β and pro-IL-18 to induce the secretion of mature IL-1β and IL-18) also significantly elevated after TN induction (Figure (D)).
Figure 2. TN accompanied by pyroptosis activation. (A) Representative images of immunofluorescence of NLRP3 (green) and GSDMD (green) staining. Scale bar: 20 μm. (B) The mRNA expression of inflammatory mediators of IL-1β and IL-18 was detected by RT-qPCR. (C) The protein level of inflammatory mediators of IL-1β and IL-18 was detected by western blot. (D) The protein level of caspase-1 was detected by western blot. * p < 0.05, *** p < 0.001.
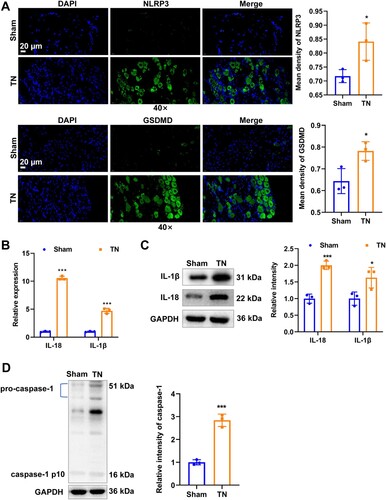
To further clarify the role of pyroptosis in TN, we utilized CoPP + H2O2 exposure to induce rat Schwann RSC96 cell injury to construct a TN cell model and introduced the pyroptosis inhibitor VX-765 (a caspase-1 inhibitor) treatment. The results showed that CoPP + H2O2 exposure resulted in a significant increase in apoptosis and a decrease in viability of RSC96 cells compared to the control group, indicating cellular damage (Figure (A–C)). However, VX-765 treatment significantly restored cell viability and reduced apoptosis of RSC96 cells (Figure (A–C)). Expectedly, CoPP + H2O2 exposure led to a significant increase in the expression of GSDMD and the release of IL-1β and IL-18 in RSC96 cells, whereas VX-765 treatment significantly suppressed this effect, suggesting that pyroptosis inhibition ameliorates TN in the cell model.
Figure 3. Inhibition of pyroptosis ameliorates TN in RSC96 cell model. (A) Cell apoptosis of RSC96 was detected by flow cytometry. (B) Statistical results of flow cytometry. (C) Cell viability of RSC96 was detected by CCK8. (D) The protein level of GSDMD in RSC96 cells was detected by western blot. (E) The concentration of IL-1β and IL-18 was detected by ELISA. ** p < 0.01, *** p < 0.001.
TN led to changes in tsRNA expression profile
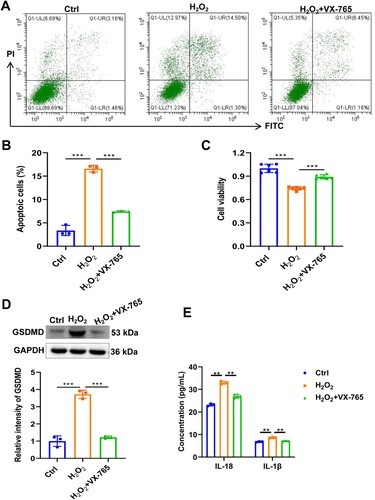
We next explored the intrinsic molecular mechanism of pyroptosis-related TN progression. The tsRNA landscape of trigeminal ganglion in the sham and TN groups was characterized using small RNA sequencing. Compared with the sham group, a total of 87 tsRNAs were significantly changed in the TN group, of which 15 tsRNAs were up-regulated and 72 tsRNAs were down-regulated (Figure , Supplemental Table 2). In short, these indicated that TN led to altered tsRNA expression profile.
Figure 4. TN led to changes in tsRNA expression profile. Volcano plots presenting the DEtsRNAs between the sham and TN groups. Red dots indicated that tsRNAs expression was higher in the TN group than the sham group. Blue dots indicated that tsRNAs expression was lower in the TN group than the sham group.
DEtsRNAs involved in pyroptosis-related pathway
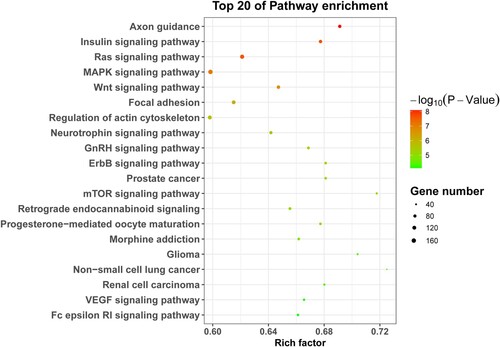
To further depict the pathway of targets of DEtsRNAs involvement in TN, we annotated the target of DEtsRNAs into the KEGG pathway. Top 20 of KEGG pathway enrichment showed that these DEtsRNAs were mainly involved in pyroptosis-related pathways, such as MAPK signaling pathway, ErbB signaling pathway, and mTOR signaling pathway (Figure ). Therefore, we speculated that DEtsRNAs may be involved in TN progression by regulating the pyroptosis pathway.
Network of DEtsRNAs
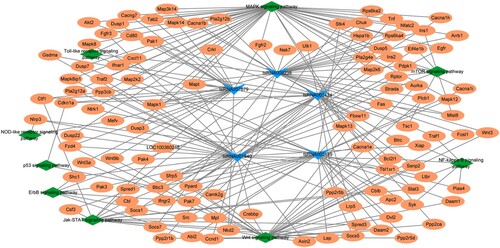
To further understand the interactions among the altered DEtsRNAs and pyroptosis, a tsRNA-target-pathway-pyroptosis network was performed. Five DEtsRNAs targeting pyroptosis-related pathways from Figure were selected for the construction of network, including tsRNA003489, tsRNA001840, tsRNA003424, tsRNA007679, and tsRNA009238. As shown in Figure , both tsRNA003489 and tsRNA001840 target NLRP3 and participate in the NOD-like receptor signaling pathway; tsRNA007679 and tsRNA001840 target GSMDA, tsRNA009238 target Nek7. NLRP3, GSMDA, and Nek7 are all typical pyroptosis-related markers. These results implied that DEtsRNAs may be involved in TN progression through pyroptosis.
RT-qPCR validation
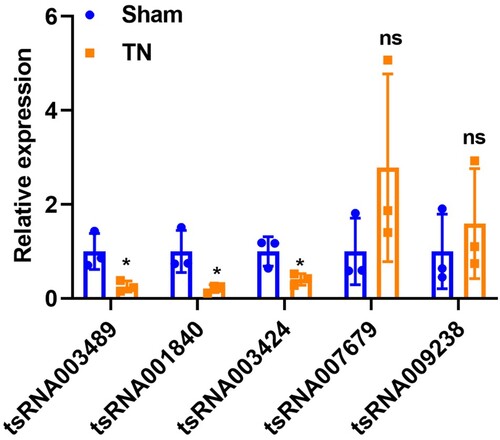
To validate the reliability of RNA sequencing results, five pyroptosis-related DEtsRNAs in the above network were selected for RT-qPCR verification. In the small RNA sequencing results, tsRNA007679 and tsRNA009238 were significantly up-regulated after TN induction, while tsRNA003489, tsRNA001840, and tsRNA003424 were all down-regulated. In the results of RT-qPCR verification, compared with the sham group, the expression of tsRNA003489, tsRNA001840, and tsRNA003424 were decreased in the TN group, which was consistent with RNA sequencing (Figure ). However, the expression of tsRNA007679 and tsRNA009238 showed no difference between the two groups. Therefore, we speculate that the involvement of pyroptosis in TN progression may be related to tsRNA003489 (CGGTCCCCGGCACCTCCACCA), tsRNA001840 (CAAGGCCCTGGGTTCGGTCCCCAGCTC), and tsRNA003424 (CGGGAGGCCCGGGTTCGATTCCCGGCCCATGCACCA), but not to tsRNA007679 and tsRNA009238.
Discussion
TN severely affects the quality of life of patients, yet there is currently no cure for the disease as its pain mechanism remains much debated. This study demonstrates that TN progression is associated with NLRP3 inflammatory-mediated pyroptosis, inhibition of pyroptosis ameliorated TN in RSC96 cell model, and reveals for the first time that the tsRNA landscape of trigeminal ganglion tissue. Our study made a superficial contribution to the pathogenesis of pyroptosis mediated by new small molecules of tsRNA in the progression of TN.
In the canonical pyroptosis pathway, an inflammasome protein complex, composed of pattern recognition receptors (PRRs) (NLRP1, NLRP3, and NLRC4 are the most common PRRs), caspase-recruitment domain (ASCs), and inflammatory caspases, receives stimuli from external factors to activate caspase-1, which cleaves GSDMD resulting in the pore formation and releasing IL-18 and IL-1β (Rao et al. Citation2022). Hence, pyroptosis is also known as a pro-inflammatory mode of cell death due to its expanded inflammatory response. Therefore, pyroptosis is involved in the progression of many inflammatory-related diseases, including neuralgia. For example, NLRP3 expression was reported to be highly expressed in TN rats who have obvious cognitive and memory deficits, and specific blockade of NLRP3 significantly improved neurodegeneration (Ren et al. Citation2021). The high expression of NLRP3 in CCI-treated trigeminal ganglion was revealed by RNA sequencing, the study also found that NLRP3 was regulated by RIPK1 through the MAPK pathway (Liu et al. Citation2022). Liu et al. also proved that expression of GSDMD, IL-1β, IL-18, and caspase-1 were increased in CCI-induced TN rat model, and VX-765 (an inhibitor of caspase-1) inhibited their expression (Liu et al. Citation2023). Our results were consistent with these studies, where NLRP3 and GSDMD-mediated pyroptosis were activated in CCI-induced TN rats. In addition, the inflammatory cytokines IL-1β and IL-18, products of pyroptosis, are released in large quantities into the cellular microenvironment to trigger localized inflammation. Interestingly, many recent studies have shown that inflammation is associated with neuropathic pain. For example, Yao et al. reveal that inflammation plays a close and important role in the progression and etiology of TN (Yao et al. Citation2020). Liu et al. prove that inflammatory cytokines were significantly upregulated in the blood of TN patients and significantly positively correlated with TN severity (r = 0.943, p < 0.05) (Liu et al. Citation2019). Thus, the upregulation of IL-1β and IL-18 may be one of the culprits of the pathological features of TN, including the triggering of neuropathic pain and mechanical hyperalgesia. The above results lead us to speculate whether targeted therapy for pyroptosis could be an effective strategy for the treatment of TN? In fact, our study cannot answer this question. Intriguingly, Mu et al. contribute to answer this question. They confirmed that targeted inhibition of NLRP3-caspase-1-GSDMD-mediated pyroptosis in microglia by hydrogen-producing Si-based agent significantly improved TN through suppressing chronic neuroinflammation and nerve demyelination (Mu et al. Citation2022). Despite all this, the mechanism of pyroptosis in TN still requires more research.
The physiological importance of tsRNA in the nervous system has been proven and accumulating studies have revealed the involvement of tsRNAs in neurological-related disease progression (Fagan et al. Citation2021), especially in neurodegenerative diseases, such as Alzheimer's disease (Wu et al. Citation2021; Zhang et al. Citation2019, Citation2020) and Parkinson's disease (Magee et al. Citation2019). In this study, the expression of 87 tsRNAs was altered in response to the TN, suggesting that they may be related to TN progression. To my knowledge, no such previous study could be identified. In addition, studies on tsRNA regulation of pyroptosis are rare. Sun et al demonstrated that tRF3-Thr-AGT prevents pyroptosis through ZBP1/NLRP3 pathway to attenuate acute pancreatitis (Sun et al. Citation2021b). Zhu et al. found that tectorigenin inactivates pyroptosis and inhibits autophagy by enhancing tRF-47-58ZZJQJYSWRYVMMV5BO expression to protect liver against nonalcoholic steatohepatitis (Zhu et al. Citation2022). These results suggest that tsRNAs have the potential to regulate pyroptosis. The present study initially found that tsRNA003489 and tsRNA001840 could act on pyroptosis regulation by targeting NLRP3. At present, there are many known mechanisms of action of tsRNAs (Du et al. Citation2023), unfortunately, this study did not delve into the action mechanism and the biological significance of tsRNA-pyroptosis in TN. This will also be the subject of our subsequent study.
Although this study is the first report to characterize the tsRNA expression profile in TN rats and to reveal the involvement of tsRNA-regulated pyroptosis in TN pathology, there are still some limitations. First, the specific molecular mechanism of TN mediated by pyroptosis is not clear. Second, we did not conduct in-depth verification of the identified tsRNAs and did not explore how they specifically regulate pyroptosis in TN.
Conclusions
Confirmation of the involvement of NLRP3-GSDMD-IL18/IL1β-mediated pyroptosis in TN development was demonstrated by an in vivo TN rat model. Inhibition of pyroptosis ameliorated cellular damage in the TN cell model. For the first time, we profiled tsRNA expression landscape using small RNA sequencing, and a total of 87 DEtsRNAs were identified in TN rats. The potential of tsRNAs to regulate pyroptosis was initially revealed by the DEtsRNAs-mRNA-pathway-pyroptosis network. The present study provides a new perspective on understanding TN development.
Authors’ contributions
Conceptualization: Mizhen Qiu, Daying Zhang, and Shibiao Chen; Methodology: Mizhen Qiu, Fei Zeng, and Daying Zhang; Formal analysis and investigation: Mizhen Qiu, Fei Zeng, and Yi Yan; Writing – original draft preparation: Mizhen Qiu, Fei Zeng, Yi Yan, Mu Xu, and Xuexue Zhang; Writing – review and editing: Zhijian Wang, Yutong Wang, Daying Zhang, and Shibiao Chen; Resources: Mizhen Qiu, Fei Zeng, Yi Yan, Mu Xu, and Xuexue Zhang; Supervision: Xuexue Zhang, Zhijian Wang, Yutong Wang. All authors read and approved the manuscript vision.
Supplemental Table 2.xlsx
Download MS Excel (16.2 KB)Supplemental Table 1.docx
Download MS Word (16.6 KB)Data availability statement
The data that support the findings of this study are openly available in NCBI, accession number PRJNA911011 at https://www.ncbi.nlm.nih.gov/search/all/?term=PRJNA911011. The raw data for western blot, mechanical withdrawal threshold test, immunofluorescence of GSDMD and NLRP3, CCK8, ELISA, flow cytometry, and RT-qPCR have been deposited to Zenodo ( https://doi.org/10.5281/zenodo.10369206
).Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chen ML, Lin K, Lin SK. 2018. NLRP3 inflammasome signaling as an early molecular response is negatively controlled by miR-186 in CFA-induced prosopalgia mice. Braz J Med Biol Res. 51(9):e7602. doi:10.1590/1414-431X20187602.
- Chen NF, Huang SY, Chen WF, Chen CH, Lu CH, Chen CL, Yang SN, Wang HM, Wen ZH. 2013. TGF-beta1 attenuates spinal neuroinflammation and the excitatory amino acid system in rats with neuropathic pain. J Pain. 14(12):1671–1685. doi:10.1016/j.jpain.2013.08.010.
- Du J, Huang T, Zheng Z, Fang S, Deng H, Liu K. 2023. Biological function and clinical application prospect of tsRNAs in digestive system biology and pathology. Cell Commun Signal. 21(1):302. doi:10.1186/s12964-023-01341-8.
- Fagan SG, Helm M, Prehn JHM. 2021. tRNA-derived fragments: a new class of non-coding RNA with key roles in nervous system function and dysfunction. Prog Neurobiol. 205:102118. doi:10.1016/j.pneurobio.2021.102118.
- Guo Y, Bai D, Liu W, Liu Y, Zhang Y, Kou X, Chen J, Wang H, Teng X, Zuo J, et al. 2021. Altered sperm tsRNAs in aged male contribute to anxiety-like behavior in offspring. Aging Cell. 20(9):e13466. doi:10.1111/acel.13466.
- Jehn J, Treml J, Wulsch S, Ottum B, Erb V, Hewel C, Kooijmans RN, Wester L, Fast I, Rosenkranz D. 2020. 5’ tRNA halves are highly expressed in the primate hippocampus and might sequence-specifically regulate gene expression. RNA. 26(6):694–707. doi:10.1261/rna.073395.119.
- Li PF, Guo SC, Liu T, Cui H, Feng D, Yang A, Cheng Z, Luo J, Tang T, Wang Y. 2020. Integrative analysis of transcriptomes highlights potential functions of transfer-RNA-derived small RNAs in experimental intracerebral hemorrhage. Aging (Albany NY). 12(22):22794–22813. doi:10.18632/aging.103938.
- Liu M, Wang Y, Li S, Hou X, Li T, Xu Z, Chen F, Zhou Y, Xia L, Wang W. 2023. Attenuates reactive oxygen species: induced pyroptosis via activation of the Nrf2/HO-1 signal pathway in models of trigeminal neuralgia. Sci Rep. 13(1):18111. doi:10.1038/s41598-023-44013-w.
- Liu MX, Zhong J, Xia L, Dou NN, Li ST. 2019. A correlative analysis between inflammatory cytokines and trigeminal neuralgia or hemifacial spasm. Neurol Res. 41(4):335–340. doi:10.1080/01616412.2018.1564188.
- Liu Y, Dong Y, Liu Z, Wang Y, Chai Y, Han Z, Wei W, Chen M. 2022. RIPK1-mediated NLRP3 activation via MAPK signaling pathway in the pathogenesis of trigeminal neuralgia. Res Square. PREPRINT (Version 1) available at Research Square. doi:10.21203/rs.3.rs-2158508/v1.
- Luo D, Lin R, Luo L, Li Q, Chen T, Qiu R, Li Y. 2019. Glial plasticity in the trigeminal root entry zone of a rat trigeminal neuralgia animal model. Neurochem Res. 44(8):1893–1902. doi:10.1007/s11064-019-02824-2.
- Magee R, Londin E, Rigoutsos I. 2019. TRNA-derived fragments as sex-dependent circulating candidate biomarkers for Parkinson's disease. Parkinsonism Relat Disord. 65:203–209. doi:10.1016/j.parkreldis.2019.05.035.
- Martini AC, Berta T, Forner S, Chen G, Bento AF, Ji RR, Rae GA. 2016. Lipoxin A4 inhibits microglial activation and reduces neuroinflammation and neuropathic pain after spinal cord hemisection. J Neuroinflammation. 13(1):75. doi:10.1186/s12974-016-0540-8.
- Mu G, Wu D, Jiang Q, Wang L, Li Q, Lu B, Yu X. 2022. Hydrogen-producing silicon-based agent inhibits microglial pyroptosis to improves nerve demyelination in trigeminal neuralgia rats.
- Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, et al. 2020. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. 18(7):e3000410. doi:10.1371/journal.pbio.3000410.
- Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao C, Liu W, Deng H, Li J, Ning P, et al. 2022. Pyroptosis in inflammatory diseases and cancer. Theranostics. 12(9):4310–4329. doi:10.7150/thno.71086.
- Ren C, Chen M, Mu G, Peng S, Liu X, Ou C. 2021. NLRP3 inflammasome mediates neurodegeneration in rats with chronic neuropathic pain. Shock. 56(5):840–849. doi:10.1097/SHK.0000000000001832.
- Sun B, Chen Z, Chi Q, Zhang Y, Gao B. 2021b. Endogenous tRNA-derived small RNA (tRF3-Thr-AGT) inhibits ZBP1/NLRP3 pathway-mediated cell pyroptosis to attenuate acute pancreatitis (AP). J Cell Mol Med. 25(22):10441–10453. doi:10.1111/jcmm.16972.
- Sun X, Cao L, Ge JL, Ge JY, Yang XF, Du BX, Song J. 2021a. The NLRP3-related inflammasome modulates pain behavior in a rat model of trigeminal neuropathic pain. Life Sci. 277:119489. doi:10.1016/j.lfs.2021.119489.
- Tao R, Huang F, Lin K, Lin SW, Wei DE, Luo DS. 2021. Using RNA-Seq to explore the Hub genes in the trigeminal root entry zone of rats by compression injury. Pain Physician. 24(5):E573–e581.
- Wang Q, Lee I, Ren J, Ajay SS, Lee YS, Bao X. 2013. Identification and functional characterization of tRNA-derived RNA fragments (tRFs) in respiratory syncytial virus infection. Mol Ther. 21(2):368–379. doi:10.1038/mt.2012.237.
- Wu W, Lee I, Spratt H, Fang X, Bao X. 2021. tRNA-Derived fragments in Alzheimer's disease: implications for new disease biomarkers and neuropathological mechanisms. J Alzheimers Dis. 79(2):793–806. doi:10.3233/JAD-200917.
- Xiao L, Wang J, Ju S, Cui M, Jing R. 2022. Disorders and roles of tsRNA, snoRNA, snRNA and piRNA in cancer. J Med Genet. 59(7):623–631. doi:10.1136/jmedgenet-2021-108327.
- Xiong W, Tan M, Tong Z, Yin C, He L, Liu L, Shen Y, Guan S, Ge H, Li G, et al. 2019. Effects of long non-coding RNA uc.48 + on pain transmission in trigeminal neuralgia. Brain Res Bull. 147:92–100. doi:10.1016/j.brainresbull.2019.02.009.
- Xu M, Yan Y, Zhu M, Wang Z, Zhang X, Zhang D. 2020. Effects of long non-coding RNA Gm14461 on pain transmission in trigeminal neuralgia. J Inflamm (Lond). 17:1. doi:10.1186/s12950-019-0231-1.
- Yao Y, Chang B, Li S. 2020. Relationship of inflammation with trigeminal neuralgia. J Craniofac Surg. 31(2):e110–e113. doi:10.1097/scs.0000000000005879.
- Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. 2021. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 6(1):128. doi:10.1038/s41392-021-00507-5.
- Zhang S, Li H, Zheng L, Li H, Feng C, Zhang W. 2019. Identification of functional tRNA-derived fragments in senescence-accelerated mouse prone 8 brain. Aging (Albany NY). 11(22):10485–10498. doi:10.18632/aging.102471.
- Zhang X, Trebak F, Souza LAC, Shi J, Zhou T, Kehoe PG, Chen Q, Feng Earley Y. 2020. Small RNA modifications in Alzheimer's disease. Neurobiol Dis. 145:105058. doi:10.1016/j.nbd.2020.105058.
- Zhang Y, Ren L, Sun X, Zhang Z, Liu J, Xin Y, Yu J, Jia Y, Sheng J, Hu GF, et al. 2021. Angiogenin mediates paternal inflammation-induced metabolic disorders in offspring through sperm tsRNAs. Nat Commun. 12(1):6673. doi:10.1038/s41467-021-26909-1.
- Zhao Y, Li X, Ye C, Huang C, Lv X, Li J. 2023. The biogenesis, mechanism and function of the tRNA-derived small RNA (tsRNA): a review compared with microRNA. Am J Cancer Res. 13(5):1656–1666.
- Zhu J, Wen Y, Zhang Q, Nie F, Cheng M, Zhao X. 2022. The monomer TEC of blueberry improves NASH by augmenting tRF-47-mediated autophagy/pyroptosis signaling pathway. J Transl Med. 20(1):128. doi:10.1186/s12967-022-03343-5.
- Zong T, Yang Y, Zhao H, Li L, Liu M, Fu X, Tang G, Zhou H, Aung LHH, Li P, et al. 2021. tsRNAs: novel small molecules from cell function and regulatory mechanism to therapeutic targets. Cell Prolif. 54(3):e12977. doi:10.1111/cpr.12977.