Abstract
Introduction: Chronic kidney disease (CKD) promotes gut dysbiosis, and enteric glial reactivity, a feature of intestinal inflammation. Brazil nut modulated enteric glial profile in healthy animals and could modulate these cells in 5/6 nephrectomized rats.
Methods: A 5/6 nephrectomy-induced CKD and Sham-operated rats were divided as follows: CKD and Sham received a standard diet and CKD-BN and Sham-BN received a 5% Brazil nut enriched-diet. The protein content of glial fibrillary acid protein (GFAP), enteric glial marker, and GPx protein content and activity were assessed in the colon. The major phyla of gut microbiota were assessed.
Results: CKD-BN group presented a decrease in GFAP content (p = 0.0001). The CKD-BN group modulated the abundance of Firmicutes, increasing its proportion compared to the CKD group. The CKD-BN group showed increased GPx activity in the colon (p = 0.0192), despite no significant difference in protein content.
Conclusion: Brazil nut-enriched diet consumption decreased enteric glial reactivity and modulated gut microbiota in the CKD experimental model.
Introduction
Chronic kidney disease (CKD) is an important trigger for other metabolic repercussions that are clinically important for patients. There are alterations in antioxidant enzyme activity, predisposing oxidative stress, and disorders in the gastrointestinal tract (GIT), including modifications in GIT transit, gut microbiota profile, and enteric glial cells molecular pattern (Citation1–3). Enteric glial cells are the most numerous component of the enteric nervous system (ENS) and is a key cell in neurogastroenterology (Citation4). Data from the experimental model shows that CKD induces signs of enteric glial reactivity, through overexpression of glial fibrillary acid protein (GFAP) (Citation1). GFAP is the most studied enteric glial marker for intestinal disorders with ENS involvement in chronic non-communicable diseases (Citation5–8). The observed response is commonly associated with intestinal inflammation and oxidative stress, making enteric glia an important target in CKD management.
Non-pharmacological strategies in CKD, especially those with few contraindications and easy adhesion by patients, have gained attention from researchers. In this sense, nutritional interventions, with food sources of nutrients and bioactive compounds, exhibit a vast array of evidence suggesting the potential to modulate intestinal responses in chronic diseases like CKD, including the enteric glial profile. Among them, Brazil nut (Bertholletia excelsa H.B.K.), an Amazonian native seed, is considered one of the main dietary sources of selenium, besides also being a source of unsaturated fatty acids, fibers, and bioactive compounds (Citation9–11). Previous experimental data shows that Brazil nut-enriched diet modulates GFAP content in the colonic neuromuscular layer and retards gastric emptying of healthy animals (Citation12). In addition, despite the potential of Brazil nut to modulate gut microbiota being poorly explored, some compounds found in the nut, such as phenolic acids and selenium, can alter the proliferation of commensal bacteria in vitro and in vivo (Citation13–16). Moreover, regarding CKD, Brazil nut supplementation is known mainly for increasing glutathione peroxidase (GPx) activity in the bloodstream of hemodialysis patients (Citation17–18).
Nevertheless, despite the advances reported about health benefits associated with the consumption of this nut, until now, no data is showing if Brazil nut supplementation could modulate enteric glial cells and gut microbiota or promote tissue-specific responses regarding GPx activity. Thus, this study investigated the impact of a Brazil nut-enriched diet on the abundance of gut microbiota’s major phyla, enteric glia cell state through GFAP, and antioxidant activity in the liver and colonic neuromuscular layer after renal fibrosis induced by 5/6 nephrectomy, a CKD animal model.
Material and methods
Animals
Male Wistar rats (n = 36, 400–450 g), 12-week-old, were obtained from Nucleo de Animais de Laboratorio from Federal Fluminense University (NAL-UFF). The study was conducted following the Brazilian Society of Science and Laboratory Animals and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, revised 1996). All the animals were housed in an adequate environment, with temperature- and humidity-controlled (22 ± 2 °C and 25% humidity), an artificial 12h alternating cycle of the dark/light (light from 7 am to 7 pm), and free access to water and a standard diet (Nuvilab-CR1, Parana, Brazil). The following animal protocols were approved by the Fluminense Federal University Animal Ethics Committee (number 956/2017).
Experimental protocol
Animals were randomly divided into two groups: Sham (n = 18) and CKD (n = 18). The 5/6 nephrectomy was performed as previously described (Citation1). Only male rats were choosen for this experimental design due to higher CKD-related adverse events in men and due to the possible protective role of estrogen in CKD (Citation19–21). Five weeks after the procedure, the animals were subdivided into four groups, with equivalent body mass, according to the diet to be received: Sham (n = 9), which received the control diet, Sham-BN (n = 9), which received Brazil nut-enriched diet, CKD (n = 9) which received the control diet, and CKD-BN (n = 9), which received Brazil nut-enriched diet. The sample size was calculated as previously described by Cruz-Orive and Weibel (1990) (Citation22), in which, considering the probability p = 1/2 of altering the variables, n = 5 was the minimum significant number of animals to be used in the statistical analysis. After the beginning of Brazil nut’s offer, the follow-up lasted eight weeks. Animals were weighed every week, and water and food intake were measured three times a week. This experiment considers every single animal as an experimental unit. Due to the huge metabolic repercussion of the 5/6 nephrectomy and two different types of diet, the authors involved in the animal care were aware of the group allocation during the conduct of experiment e in vivo assessments and procedures.
Diet
The four groups received chows formulated into pellets and stored at 4 °C as the American Institute of Nutrition (AIN-93M) recommended for rodent diets (Citation23). Sham e CKD received the control diet, and Sham-BN and CKD-BN received a diet containing ground Brazil nut, added at a 5% (w/w) concentration. The Brazil nut-enriched diet was balanced to obtain chows equivalent in macronutrients and calorie supply, as described in . Brazil nuts used in this study were obtained from the Amazonian region (Itacoatiara city, Agropecuaria Aruana, Amazonas, Brazil). According to previous data, a single nut weighed 5 g and contained 0.75 g protein, 0.45 g carbohydrate, 3.53 g lipid, and 290.5 µg selenium (Citation24).
Table 1. Experimental diets composition.
Urine analysis
Animals were allocated to cleaned metabolic cages containing free access to water and food in the fifth and last week of follow-up to collect 24h-urine. Total protein excretion was determined using Bioclin® kits and an automatic biochemical analyzer (Bioclin, Santa Branca, Brazil). To minimize confounders, the samples were analyzed blinded.
Fasting glycemia
Animals were previously kept for 12h fasting to assess glycemia with an automatic glucometer in the last week of follow-up (ACCU CHECK-Active, Roche, Basel, Switzerland). To minimize confounders, the animals were intercalated among the groups and reassure equal conditions.
Fecal water content
In the last week of the experiment, animals from the four groups were kept individually in an open field for 1 h to estimate colonic motility indirectly, as previously described (Citation25). Feces were immediately collected to assess the water content. The samples were subjected to humidity waste at 65 °C for 12 h. The wet weight was compared with the dry weight, to obtain the content of fecal water (%), calculated by the following formula: [(wet weight – dry weight)/wet weight] × 100. To minimize confounders, the animals were intercalated among the groups to reassure equal conditions.
Biological material collection
At the end of the experiment, animals were anesthetized with intraperitoneal ketamine (40 mg/kg) and xylazine (8 mg/kg) (Laboratório Virbac, São Paulo, Brazil) and euthanized by cardiac puncture. Blood samples were collected in tubes and centrifuged (15 min, 4500 × g, 4 °C) to obtain plasma and serum. Kidney, colon, and liver tissues were carefully excised for further analysis. Fecal pellets were removed, counted to evaluate gastrointestinal transit (Citation26), and reserved for further analysis. To minimize confounders, the animals were intercalated among the groups to reassure equal conditions.
Serum biochemical analysis
Urea, creatinine, uric acid, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were determined in all groups using Bioclin® kits and a BS-120 automatic biochemical analyzer (Bioclin, Santa Branca, Brazil). To minimize confounders, the samples were analyzed blinded.
LPS concentration
The plasma concentration of LPS was evaluated following the manufacturer’s instructions (LPS ELISA Kit, No. EU3126, Wuhan Fine Biotech, Wuhan, China). The color change is measured spectrophotometrically at a wavelength of 450 nm. The concentration of LPS in the samples was determined by comparing the samples to the standard curve. To minimize confounders, the samples were analyzed blinded.
Kidney histopathology
Formalin‐fixed kidney tissue was embedded in paraffin and 5‐μm‐thick sections were stained with hematoxylin and eosin to evaluate general histopathology and Picrosirius red to evaluate the fibrosis. The photomicrographs were obtained from Leica® microscope DFC310 FX, Wetzlar, Germany. For fibrosis evaluation, ten random fields were selected and estimated using the Image-Pro Plus (Media Cybernetics, Silver Spring, MD, USA), using the density threshold selection tool. The results are expressed as a percentage in relation to the Sham group (n = 5/group). To minimize confounders, the photomicrographs were analyzed blinded.
Colon morphometry
Paraformaldehyde-fixed (4%) colonic tissue, embedded in paraffin, was cut into 5‐μm‐thick slices. Cuts were stained with hematoxylin-eosin for general histopathology. The photomicrographs were obtained from Leica® microscope DFC310 FX, Wetzlar, Germany. Representative fields from different colon segments of each animal were selected to determine colon neuromuscular layer thickness and estimated using ImageJ 1.43 software (National Institutes of Health, Bethesda, MD, USA). The results are expressed as μm. To minimize confounders, the photomicrographs were analyzed blinded.
Western blotting
The neuromuscular layer of the colonic tissue was delaminated and instantaneously homogenized in RIPA buffer containing Protease Inhibitor Cocktail (Sigma-Aldrich, Missouri, USA). Protein concentration, electrophoresis, and immunoblot were performed as previously described (Citation1). The specification of the primary antibodies was GFAP: mouse polyclonal antibody, 1:1000 (Abcam, Cambridge®, United Kingdom) and GPx: mouse monoclonal antibody, 1:1000 (Santa Cruz Biotechnology®, California, USA). The revelation was made through a chemiluminescence reaction using ECL (Bio-Rad Laboratories®, California, USA) in an adequate imaging system (ChemiDoc MP, Bio-Rad Laboratories®, California, USA). Quantity One 4.6.8 software analyzed Western blot bands densitometry (Bio-Rad Laboratories®, California, USA). Results are expressed in corrected densitometry compared to β-Actin (mouse monoclonal antibody 1:1000, Santa Cruz Biotechnology®, California, USA).
Analysis of gut microbiota profile
The feces collected from the colon during euthanasia were used for DNA extraction. Real-time polymerase chain reaction (RT-PCR) assays were used for the relative quantification of specific microorganism phyla in the gut microbiota through the detection of 16S rRNA genes. Samples of 180–220 mg per animal from four experimental groups were used in this process using the QIAamp® Fast DNA Stool Mini Kit (Qiagen, Düsseldorf, Germany), according to the manufacturer’s instructions. The relative quantification and abundance of the phyla were normalized by ΔCt of the total quantification of bacteria present in the sample (Citation27). The amplification reactions were performed in the StepOnePlus™ Real-Time PCR System equipment (Life Technologies, California, EUA) programmed for the following cycle: 95 °C/10 min; 95 °C/15s, 60 °C/1 min (45 cycles). To a reaction mixture containing 6 µL of SYBR green RT-PCR mix (Life Technologies, California, EUA), 0.2 µM of each of the primers and 4 µL of DNA from the samples were added. The specific primers for the 16 rDNA genes’ conserved regions are described in (Citation28, Citation29). Each reaction was adjusted to the total volume of 12 µL with water. The analysis occurred on MicroAmpOptical 96-well plates sealed with MicroAmp® 96-Well OpticalAdhesiveFilm (Life Technologies, California, EUA). Samples were analyzed in duplicate for each primer pair. All runs were performed using negative controls with no DNA added to detect possible contamination of the reaction. The results were visualized using StepOne™ Software v2.3 (Life Technologies, California, EUA) and expressed as relative abundance (%) (n = 6/group). To minimize confounders, the samples were analyzed blinded.
Table 2. Specific primers for the 16 rDNA genes’ conserved region.
Antioxidant enzymes activity in the liver and the neuromuscular layer
ELISA kits assessed glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity in the liver and neuromuscular layer homogenates (GPx assay kit No. 703102 – absorbance measured at 340 nm; SOD assay kit No. 706002 - absorbance measured at 450 nm; Cayman Chemical®, Michigan, USA). Absorbances were measured on the PowerWave XS microplate reader (Biotek®, Vermont, EUA). To minimize confounders, the samples were analyzed blinded.
Statistical analyses
Statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software, California, USA). The distribution of variables was analyzed using the Shapiro-Wilk test. The results are expressed as the mean and standard deviation. Differences among groups were analyzed using a one-way analysis of variance (ANOVA) followed by Sidak’s posthoc test (parametric data) or Kruskal–Wallis test followed by Dunn’s posthoc test (non-parametric data). Statistical significance was set at p < 0.05.
Results
In the fifth week after the 5/6 nephrectomy, the modeling was already successful in reducing kidney function. CKD group presented increased urinary excretion of creatinine and total proteins in 24h urine. No significant differences were observed for urinary excretion of urea. The CKD group also showed a lower body mass than the Sham group (). Concerning the effects of the Brazil nut-enriched diet on body mass, the CKD-BN group presented higher values than the CKD group.
Table 3. Body mass and urinary excretion of renal markers 5 wk after 5/6 nephrectomy procedure.
No significant differences between the CKD and CKD-BN groups were found regarding water intake. CKD and CKD-BN groups presented increase in markers of renal function such as serum creatinine, urea, and total proteins urinary when comparing with Sham group. However, these parameters were not decreased in CKD-BN group when comparing to CKD group. The experimental groups displayed no significant differences in serum uric acid, AST, ALT, LPS levels, and fasting glycemia ().
Table 4. Effects of Brazil nut-enriched diet in the general parameters of experimental groups.
The CKD and CKD-BN animals presented dilatation of the renal tubules () in relation to their counterparts (). Regarding fibrosis, the Sham and Sham-BN presented a preserved morphology of their renal parenchyma (). CKD and CKD-BN group showed a significant rise in tubulointerstitial fibrosis compared to the Sham group (p = 0.0095); however, the consumption of a Brazil nut-enriched diet did not impact the progression of the CKD ().
Figure 1. Representative photomicrographs [a–h] and fibrosis quantification [I] of renal tissue of experimental groups. Sham [a, b] and Sham-BN [a, d] groups show preserved parenchyma and no fibrosis. In contrast, CKD [e, f] and CKD-BN [g, h] groups presented dilated tubules (indicated by arrows), and several regions with fibrosis (indicated by arrowhead). Hematoxylin and eosin [a, c, e, g] and Picrosirius [b, d, f, h], 40x magnification. (*) means statistical difference compared to the Sham group (p = 0.0095). Statistical significance was considered when p < 0.05. One-way ANOVA with Sidak post-test. [i] Sham, n = 5; Sham-BN, n = 5; CKD: n = 5; CKD-BN: n = 5.
![Figure 1. Representative photomicrographs [a–h] and fibrosis quantification [I] of renal tissue of experimental groups. Sham [a, b] and Sham-BN [a, d] groups show preserved parenchyma and no fibrosis. In contrast, CKD [e, f] and CKD-BN [g, h] groups presented dilated tubules (indicated by arrows), and several regions with fibrosis (indicated by arrowhead). Hematoxylin and eosin [a, c, e, g] and Picrosirius [b, d, f, h], 40x magnification. (*) means statistical difference compared to the Sham group (p = 0.0095). Statistical significance was considered when p < 0.05. One-way ANOVA with Sidak post-test. [i] Sham, n = 5; Sham-BN, n = 5; CKD: n = 5; CKD-BN: n = 5.](/cms/asset/c3c7ce1c-642b-4dfa-8e98-3fbac54d0068/uacn_a_2247057_f0001_c.jpg)
Regarding the fecal water content, no significant differences were found between the experimental groups (p = 0.6852). When observing the number of fecal pellets in the colon, the CKD group showed a significantly higher amount than the Sham group (p = 0.0027). However, no significant differences were observed between CKD and CKD-BN groups (). CKD and CKD-BN animals presented inflammatory infiltrates in the colonic tissue (). The neuromuscular layer thickness evidenced that the CKD group showed minor values compared to the Sham group (p = 0.0375). This parameter was not impacted by consuming a Brazil nut-enriched diet ().
Figure 2. Indirect assessments of gut motility evaluating fecal water content (%) [a] and fecal pellets unit (unit) [b] of experimental groups. (*) means statistical difference compared to the Sham group (p = 0.0027). Statistical significance was considered when p < 0.05. One-way ANOVA with Sidak post-test. [a] Sham, n = 7; Sham-BN, n = 6; CKD: n = 5; CKD-BN: n = 6. [b] Sham, n = 6; Sham-BN, n = 6; CKD: n = 6; CKD-BN: n = 6.
![Figure 2. Indirect assessments of gut motility evaluating fecal water content (%) [a] and fecal pellets unit (unit) [b] of experimental groups. (*) means statistical difference compared to the Sham group (p = 0.0027). Statistical significance was considered when p < 0.05. One-way ANOVA with Sidak post-test. [a] Sham, n = 7; Sham-BN, n = 6; CKD: n = 5; CKD-BN: n = 6. [b] Sham, n = 6; Sham-BN, n = 6; CKD: n = 6; CKD-BN: n = 6.](/cms/asset/1e3413f6-6288-44b8-83a1-b920171c7845/uacn_a_2247057_f0002_c.jpg)
Figure 3. Representative photomicrographs of the colonic tissue [a, b, c, d] and colon neuromuscular layer thickness [e] of experimental groups. The Sham and Sham-BN groups presented preserved parenchyma [a, b]. In contrast, the CKD and CKD-BN group exhibited inflammatory foci between the mucosal and submucosal layers (indicated by the arrow) [c, d]. The arrowhead indicates the neuromuscular layer. Hematoxylin and eosin, 10× magnification. (*) means statistical difference compared to the Sham group (p = 0.0375). Statistical significance was considered when p < 0.05. One-way ANOVA with Sidak post-test. [e] Sham, n = 5; Sham-BN, n = 5; CKD: n = 5; CKD-BN: n = 4.
![Figure 3. Representative photomicrographs of the colonic tissue [a, b, c, d] and colon neuromuscular layer thickness [e] of experimental groups. The Sham and Sham-BN groups presented preserved parenchyma [a, b]. In contrast, the CKD and CKD-BN group exhibited inflammatory foci between the mucosal and submucosal layers (indicated by the arrow) [c, d]. The arrowhead indicates the neuromuscular layer. Hematoxylin and eosin, 10× magnification. (*) means statistical difference compared to the Sham group (p = 0.0375). Statistical significance was considered when p < 0.05. One-way ANOVA with Sidak post-test. [e] Sham, n = 5; Sham-BN, n = 5; CKD: n = 5; CKD-BN: n = 4.](/cms/asset/179e7e81-80de-4ad3-87cd-ab86417829a4/uacn_a_2247057_f0003_c.jpg)
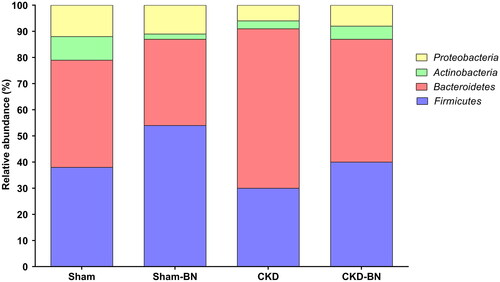
shows that the CKD group increases GFAP content (p = 0.0106). Animals that received Brazil-nut enriched diet presented modulation of enteric glial cells since the CKD-BN group displayed a decrease in GFAP protein content compared to CKD (p = 0.0084). GPx protein content did not show a statistical difference among experimental groups. However, considering the higher values observed in the CKD-BN group, there is a numerical increase in GPx protein content compared to the CKD group, despite no statistical significance (p = 0.1892). Observing gut microbiota composition, the CKD group presents different proportions of Firmicutes and Bacteroidetes than the Sham group, with superiority in the relative abundance of the phylum Bacteroidetes. When observed the effects of the Brazil nut-enriched diet, the CKD-BN group demonstrated a change in this proportion; the two main phyla are more proportionally balanced and more remarkably similar to the relative abundance found in the Sham group ().
Figure 4. Densitometric analysis [a, b] and representative blots [c] of GFAP and GPx protein content in the colonic neuromuscular layer of experimental groups. The values were normalized to β-actin; statistical significance was considered when p < 0.05. One-way ANOVA with Sidak post-test. (*) means statistical difference compared to Sham group (p = 0.0106); (†) means statistical difference compared to CKD group (p = 0.0084). [a] Sham, n = 5; Sham-BN, n = 3; CKD: n = 5; CKD-BN: n = 5. [b] Sham, n = 3; Sham-BN, n = 5; CKD: n = 4; CKD-BN: n = 5.
![Figure 4. Densitometric analysis [a, b] and representative blots [c] of GFAP and GPx protein content in the colonic neuromuscular layer of experimental groups. The values were normalized to β-actin; statistical significance was considered when p < 0.05. One-way ANOVA with Sidak post-test. (*) means statistical difference compared to Sham group (p = 0.0106); (†) means statistical difference compared to CKD group (p = 0.0084). [a] Sham, n = 5; Sham-BN, n = 3; CKD: n = 5; CKD-BN: n = 5. [b] Sham, n = 3; Sham-BN, n = 5; CKD: n = 4; CKD-BN: n = 5.](/cms/asset/809bf77a-8373-4a65-9545-9b0c349d4db6/uacn_a_2247057_f0004_c.jpg)
Figure 5. Relative abundance of gut microbiota’s major phyla from colonic fecal samples of experimental groups. Sham, n = 6; Sham-BN, n = 6; CKD, n = 6; CKD-BN, n = 6.
When observing the enzyme’s activity in the neuromuscular layer, GPx activity was decreased in the CKD group (p = 0.0488). CKD-BN group modulated this parameter, showing increased GPx activity compared to the CKD group (p = 0.0192) (). No significant differences were found in SOD activity in the neuromuscular layer of experimental groups (). Moreover, no significant differences were found in the hepatic antioxidant enzyme activity ().
Figure 6. GPx [a, c] and SOD [b, d] activity in the colonic neuromuscular layer and the liver of experimental groups. One-way ANOVA with Sidak post-test. (*) means statistical difference compared to Sham group (p = 0.0192); (†) means statistical difference compared to CKD group (p = 0.0488). [a] Sham, n = 5; Sham-BN, n = 5; CKD: n = 4; CKD-BN: n = 5. [b] Sham, n = 5; Sham-BN, n = 5; CKD: n = 5; CKD-BN: n = 5. [c] Sham, n = 4; Sham-BN, n = 4; CKD: n = 4; CKD-BN: n = 4. [d] Sham, n = 4; Sham-BN, n = 4; CKD: n = 4; CKD-BN: n = 4. GPx: Glutathione peroxidase; SOD: superoxide dismutase.
![Figure 6. GPx [a, c] and SOD [b, d] activity in the colonic neuromuscular layer and the liver of experimental groups. One-way ANOVA with Sidak post-test. (*) means statistical difference compared to Sham group (p = 0.0192); (†) means statistical difference compared to CKD group (p = 0.0488). [a] Sham, n = 5; Sham-BN, n = 5; CKD: n = 4; CKD-BN: n = 5. [b] Sham, n = 5; Sham-BN, n = 5; CKD: n = 5; CKD-BN: n = 5. [c] Sham, n = 4; Sham-BN, n = 4; CKD: n = 4; CKD-BN: n = 4. [d] Sham, n = 4; Sham-BN, n = 4; CKD: n = 4; CKD-BN: n = 4. GPx: Glutathione peroxidase; SOD: superoxide dismutase.](/cms/asset/960c205c-85cb-4b06-885a-113ef12dd5b9/uacn_a_2247057_f0006_c.jpg)
Discussion
The results from this study demonstrated that Brazil nut-enriched diet could modulate gut microbiota and attenuate the increase in GFAP content in the colonic neuromuscular layer, which might represent an attenuation of enteric glial reactivity in a 5/6 nephrectomy model. To the best of our knowledge, this is the first study that used a nutritional strategy to modulate enteric glial cells in CKD and the first to show Brazil nut modulating gut microbiota profile. Nonetheless, Brazil nut-enriched diet promoted tissue-specific responses regarding GPx activity, with increased activity of such enzyme in the colonic neuromuscular layer and no changes in hepatic tissue.
Previous experimental studies have also explored the impact of Brazil nuts on the renal injury. Using a renal occlusion model, the offer of Brazil nut before the procedure attenuated inflammation, apoptosis, and macrophage migration in renal tissue and reduced plasma levels of phosphorus and urea (Citation30, Citation31). Unlike the studies above, CKD and CKD-BN groups did not display differences in creatinine, urea, and uric acid levels. This response can be explained by the beginning of the intervention after the establishment of renal dysfunction, strengthening our aim of verifying the impact of a Brazil nut-enriched diet as a non-pharmacological strategy in a chronic condition.
Another parameter modulated by Brazil nut-enriched diet was body mass, attenuating its decrease when comparing to CKD group. Lower body mass is a risk factor for mortality in patients with CKD (Citation32). Usually, it is resulting from the protein-energy wasting (PEW), chronic condition characterized by nutritional and metabolic disorders that progressively decreases the body compartments of adipose tissue and muscle (Citation33, Citation34). It is estimated that 11 to 50% of patients diagnosed with CKD stages 3 to 5 are affected by PEW (Citation35, Citation36). Nutritional strategies are important to prevent PEW in CKD (Citation37). In the present study, Brazil nut was shown to suppress weight loss. Previous data demonstrate that a Brazil-nut enriched diet increased final body mass and improves body composition without changes in food consumption in healthy animals (Citation38). This effect can be due to the nutrient-dense characteristic of the Brazil nut, with high apport of lipids, and also the bioactive compounds content in this nut (Citation10). Recently, Wang K et al. (2022) (Citation39) described that nut consumption 1–6 times a week is inversely associated with mortality in CKD patients, which could be due improvements in body mass promoted by this augment in diet of lipids.
The involvement of enteric glial cells in a reactive status, including overexpression of GFAP, is an essential feature in gut pathophysiology, including in non-communicable chronic diseases (Citation5, Citation6, Citation40). In a CKD experimental model, GFAP protein content and immunoreactivity are increased in the neuromuscular layer, switching to a reactive phenotype associated with inflammation represented by inflammatory foci and upregulation of the nuclear factor-kappa B (Citation1). In this sense, nutritional strategies focused on modulating the enteric glial responses are a growing interest in the neurogastroenterology field. Brazil nut was previously reported as a modulator of enteric glia. Almeida et al. (2022) (Citation1) demonstrated that healthy male Wistar rats receiving a Brazil-nut enriched diet in a 5% concentration increased GFAP immunoreactivity. In this study, an opposite response was obtained. Nevertheless, the modulation of such cells is very context-dependent (Citation4). Therefore, the CKD animal model has processes affecting the enteric glial profile. However, a nutritional intervention’s ability to attenuate glial reactivity in the gut represents a step forward in the patient management and development of future therapies.
Among the possible pathways that allowed such glial response, it is possible to highlight the influence of Brazil nut composition, which has some nutrients with data in the literature that support the modulation of neural cells. For example, ellagic acid has neuroprotective potential concerning the central nervous system (Citation41, Citation42). In addition, Brazil nut is a source of unsaturated fatty acids, such as omega-3 (Citation10). Although it is not the main source of this nutrient, the literature reports that it has the potential to promote differentiation and induce attenuation of the inflammatory response of astrocytes, which may be contributing to the decrease in GFAP expression observed in CKD-BN group (Citation43, Citation44). In addition, the Brazil nut is the most abundant food source of selenium, a mineral whose consumption is described in the literature as a promoter of increased GPx activity in the colon and with neuroprotective activity on astrocytes in the CNS, including attenuating its reactivity (Citation45–47). CKD-BN group also displayed an increase in GPx activity in the colonic neuromuscular layer, which was primarily due to the consumption of a Brazil nut-enriched diet, and probably resonated in the enteric glial modulation given the susceptibility of such cells in response to oxidative stress (Citation5, Citation48).
Despite the modulation of GPx, SOD activity remained unchanged in the colonic neuromuscular layer. SOD is considered the first defense against oxidative stress, neutralizing the superoxide anion to form hydrogen peroxide and oxygen (Citation49). Glial reactivity is reported to upregulate in mRNA transcripts of SOD in human enteric glial cells in culture and appears to have neuroprotective effect in astrocytes from CNS (Citation50–52). In our study we did not find any differences between Sham and CKD group, which could be due to the temporal changes in SOD activity reported by some authors (Citation53–55).
Another highlight data is the prebiotic effect of the Brazil nut-enriched diet. CKD-BN group presented an increased proportion of Firmicutes and Actinobacteria. Both phyla have essential species for butyrate production in the intestinal lumen. Roseburia spp., Faecalibacterium spp, Dorea spp., Lactobacillus spp., belonging to the phylum Firmicutes, and Bifidobacterium spp., belonging to the phylum Actinobacteria, are examples of bacteria that generate butyrate and may be increased in the intestinal microbiota of CKD-BN animals, favoring the proliferation and differentiation of enterocytes and intestinal immune response (Citation56). It is important to highlight that Sham-BN group presented this same characteristics of gut microbiota profile described above, with increased proportion of the Firmicutes, demonstrating that Brazil nut can also present prebiotic effect in healthy conditions.
Gut microbiota modulation promoted by Brazil nut-enriched diet may be another pathway that promotes GFAP modulation in the colonic neuromuscular layer. Glial reactivity is commonly occurring with dysbiosis given their dynamic crosstalk (Citation57, Citation58). Gut microbiota can signalize enteric glial renovation and proliferation, and, despite the lack of evidence linking enteric glial modulation by commensal bacteria through nutritional strategies, it is evident that as long as Brazil nut can restore gut microbiota profile to a symbiotic pattern, it impacts other compounds of GIT physiology, including enteric glial cells (Citation59). Additionally, improvement in gut microbiota profile is strongly related to alleviation of PEW through different mechanisms. In a recent review, Martin-Del-Campo et al. (2023) (Citation60) discussed that dysbiosis in CKD leads some metabolic consequences (e.g., inflammation, uremic toxins retention and impaired signaling of hormones related to appetite) that promotes repercussion in nutrition parameters such as anorexia, muscle catabolism, increased energy expenditure and fat wasting. Moreover, it is described that CKD progression can affect the abundance of butyrate producing bacteria. Their abundance is positively correlated with better anthropometric parameters (handgrip strength, mid-upper arm circumference; mid-upper arm muscle circumference and body mass index) (Citation61). As Brazil nut-enriched diet increased the filum containing several butyrate producing bacteria, this can be a pathway that Brazil nut improves weight gain in CKD animals.
The gut-liver axis also has metabolic repercussions in chronic diseases, which CKD needs further exploration. Nutritional strategies focused on modulation of gut microbiota were found to be associated with alleviating hepatic oxidative stress (Citation62, Citation63). However, in this study, despite Brazil nut-enriched diet effect in modulating the Firmicutes, no differences were found in SOD and GPx activity in CKD and CKD-BN groups. The hepatic activity of selenoproteins like GPx decreases under selenium deficiency (Citation64). Recently, Kume et al. (2021) (Citation65) offered diets containing Brazil nut flour (5 and 15%) for 15 days to healthy animals and, despite observing an increase in selenium serum levels, no changes in oxidative stress markers, such as lipid peroxidation and protein carbonylation were observed compared to the control group. Furthermore, previous studies showed that selenium supplementation over a 0.1 µg/g diet does not increase GPx1 activity in rodents’ liver (Citation66). Also, Cardoso et al. (2021) (Citation67) demonstrated that high selenium intake (≥0.08 µg Se g−1) does not necessarily promote increased incorporation of selenium into selenoproteins in the liver.
Taking into consideration the results, we consider that the Brazil nut-enriched diet modulated three principal aspects in CKD animals, nutritional, metabolic and intestinal, which should be considered the main mechanisms of action of Brazil nut. The nutritional includes the intrinsic characteristics of Brazil nut, with increase apport of lipids, suppressing the weight loss that occurs in CKD (Citation10, Citation36). The intestinal includes the prebiotic effect of Brazil, increasing the proportion of Firmicutes and Actinobacteria, crucial for butyrate production, and the neuroprotective effect upon enteric glial cells. It is especially important when considering the dysbiosis and glial reactivity that occurs in CKD (Citation1, Citation68). The metabolic effects of Brazil nut intervention in CKD are well-established by researchers in the field, describing how this nut can attenuate inflammation and oxidative stress in CKD patients (Citation17, Citation18, Citation24, Citation69). Despite characterizing these tree features, it is impossible to isolate them, given the complex interaction that occurs in the organism. Improvements in metabolic condition, with decrease of inflammation and oxidative stress, ameliorate the nutritional status as well as improving dysbiosis and the intestinal environment can mitigate inflammatory and oxidative responses (Citation33, Citation37, Citation70, Citation71). This highlights Brazil nut as complete intervention able to improve CKD outcomes that should be expanded to clinical trials.
We believe that our data are relevant in demonstrating that Brazil nut can modulate gut microbiota and glial reactivity in CKD. The novelty of this finding gives new perspectives to understand how nutritional strategies can modulate gut pathophysiological outcomes reported in this disease. However, this study has some limitations. We did not analyze sex-dependent responses or performed temporal-related evaluation to understand how the modulation develop over time, which could represent interesting approaches to future studies.
Conclusion
In conclusion, Brazil nut-enriched diet intake for eight weeks enhanced GPx activity and decreased GFAP content in the colonic neuromuscular layer, indicating an effect in attenuating enteric glial reactivity. This study’s pioneering shows that a nutritional strategy modulating enteric glial cells in CKD points the way for robust studies with Brazil nut supplementation potentially impacting gut-related outcomes in CKD patients. In addition, the consumption of this nut promoted a prebiotic effect by modulating the main phyla of gut microbiota. With the advances in the nephrology field showing gut microbiota as a crucial therapeutical target in CKD, the data reported in this study will optimistically encourage more studies to be carried out with Brazil nut interventions to understand better its effect on the quality of life of CKD patients.
Ethics approval
This protocol was approved by the Fluminense Federal University’s Ethical Committee on Animal Research, Niterói-RJ, Brazil (protocol 956/2017).
Supplemental Material
Download MS Excel (37.5 KB)Acknowledgments
We are thankful to the Agropecuaria Aruana for donating Brazil nuts. We are also grateful to the following laboratories for their technical assistance: Experimental Nutrition Laboratory (LabNe) – Fluminense Federal University, including the use of animal houses, Unidade de Pesquisa Clínica (UPC), and Multi-user Physiology and Pharmacology Laboratory (LAMFFA), including the use of ChemiDoc MP. The authors would also like to thank all workers involved in the routine activities of the laboratories.
Disclosure statement
The authors declare that there are no conflicts of interest.
Data availability statement
The dataset used in this study is available as an Excel format file as Supplementary Material.
Additional information
Funding
References
- Almeida PP, de Moraes Thomasi BB, Menezes AC, Da Cruz BO, da Silva Costa N, Brito ML, D’Avila Pereira A, Castanon CR, Degani VAN, Magliano DC, et al. 5/6 Nephrectomy affects enteric glial cells and promotes impaired antioxidant defense in the colonic neuromuscular layer. Life Sci. 2022;298:120494. doi:10.1016/j.lfs.2022.120494.
- Daenen K, Andries A, Mekahli D, Van Schepdael A, Jouret F, Bammens B. Oxidative stress in chronic kidney disease. Pediatr Nephrol. 2019;34(6):975–91. doi:10.1007/s00467-018-4005-4.
- Kim JE, Kim H-E, Park JI, Cho H, Kwak M-J, Kim B-Y, Yang SH, Lee JP, Kim DK, Joo KW, et al. The association between gut microbiota and uremia of chronic kidney disease. Microorganisms. 2020;8(6):907. doi:10.3390/microorganisms8060907.
- Seguella L, Gulbransen BD. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat Rev Gastroenterol Hepatol. 2021;18(8):571–87. doi:10.1038/s41575-021-00423-7.
- Antonioli L, D’Antongiovanni V, Pellegrini C, Fornai M, Benvenuti L, di Carlo A, van den Wijngaard R, Caputi V, Cerantola S, Giron MC, et al. Colonic dysmotility associated with high-fat diet-induced obesity: role of enteric glia. FASEB J. 2020;34(4):5512–24. doi:10.1096/fj.201901844R.
- Hosseinifard ES, Morshedi M, Bavafa-Valenlia K, Saghafi-Asl M. The novel insight into anti-inflammatory and anxiolytic effects of psychobiotics in diabetic rats: possible link between gut microbiota and brain regions. Eur J Nutr. 2019;58(8):3361–75. doi:10.1007/s00394-019-01924-7.
- Luo P, Liu D, Li C, He WX, Zhang CL, Chang MJ. Enteric glial cell activation protects enteric neurons from damage due to diabetes in part via the promotion of neurotrophic factor release. Neurogastroenterol Motil. 2018;30(10):e13368. doi:10.1111/nmo.13368.
- Stenkamp-Strahm C, Patterson S, Boren J, Gericke M, Balemba O. High-fat diet and age-dependent effects on enteric glial cell populations of mouse small intestine. Auton Neurosci. 2013;177(2):199–210. doi:10.1016/j.autneu.2013.04.014.
- Bodnar M, Szczyglowska M, Konieczka P, Namiesnik J. Methods of selenium supplementation: bioavailability and determination of selenium compounds. Crit Rev Food Sci Nutr. 2016;56(1):36–55. doi:10.1080/10408398.2012.709550.
- Cardoso BR, Duarte GBS, Reis BZ, Cozzolino SMF. Brazil nuts: nutritional composition, health benefits and safety aspects. Food Res Int. 2017;100(Pt 2):9–18. doi:10.1016/j.foodres.2017.08.036.
- Kieliszek M. Selenium(-)fascinating microelement, properties and sources in food. Molecules. 2019;24(7):1298. doi:10.3390/molecules24071298.
- Almeida PP, Thomasi BBM, Costa NDS, Valdetaro L, Pereira AD, Gomes ALT, Stockler-Pinto MB. Brazil Nut (Bertholletia excelsa H.B.K) retards gastric emptying and modulates enteric glial cells in a dose-dependent manner. J Am Nutr Assoc. 2022;41(2):157–65. doi:10.1080/07315724.2020.1852981.
- Callejon-Leblic B, Selma-Royo M, Collado MC, Gomez-Ariza JL, Abril N, Garcia-Barrera T. Untargeted gut metabolomics to delve the interplay between selenium supplementation and gut microbiota. J Proteome Res. 2022;21(3):758–67. doi:10.1021/acs.jproteome.1c00411.
- Gangadoo S, Dinev I, Chapman J, Hughes RJ, Van TTH, Moore RJ, Stanley D. Selenium nanoparticles in poultry feed modify gut microbiota and increase abundance of Faecalibacterium prausnitzii. Appl Microbiol Biotechnol. 2018;102(3):1455–66. doi:10.1007/s00253-017-8688-4.
- John JA, Shahidi F. Phenolic compounds and antioxidant activity of Brazil nut (Bertholletia excelsa). J Funct Foods. 2010;2(3):196–209. doi:10.1016/j.jff.2010.04.008.
- Pacheco-Ordaz R, Wall-Medrano A, Goni MG, Ramos-Clamont-Montfort G, Ayala-Zavala JF, Gonzalez-Aguilar GA. Effect of phenolic compounds on the growth of selected probiotic and pathogenic bacteria. Lett Appl Microbiol. 2018;66(1):25–31. doi:10.1111/lam.12814.
- Stockler-Pinto MB, Mafra D, Moraes C, Lobo J, Boaventura GT, Farage NE, Silva WS, Cozzolino SF, Malm O. Brazil nut (Bertholletia excelsa, H.B.K.) improves oxidative stress and inflammation biomarkers in hemodialysis patients. Biol Trace Elem Res. 2014;158(1):105–12. doi:10.1007/s12011-014-9904-z.
- Stockler-Pinto MB, Malm O, Moraes C, Farage NE, Silva WS, Cozzolino SM, Mafra D. A follow-up study of the chronic kidney disease patients treated with Brazil nut: focus on inflammation and oxidative stress. Biol Trace Elem Res. 2015;163(1–2):67–72. doi:10.1007/s12011-014-0167-5.
- Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14(3):151–64. doi:10.1038/nrneph.2017.181.
- Diwan V, Small D, Kauter K, Gobe GC, Brown L. Gender differences in adenine-induced chronic kidney disease and cardiovascular complications in rats. Am J Physiol Renal Physiol. 2014;307(11):F1169–78. doi:10.1152/ajprenal.00676.2013.
- Swartling O, Rydell H, Stendahl M, Segelmark M, Trolle Lagerros Y, Evans M. CKD progression and mortality among men and women: a nationwide study in Sweden. Am J Kidney Dis. 2021;78(2):190–1. doi:10.1053/j.ajkd.2020.11.026.
- Cruz-Orive LM, Weibel ER. Recent stereological methods for cell biology: a brief survey. Am J Physiol. 1990;258(4 Pt 1):L148–56. doi:10.1152/ajplung.1990.258.4.L148.
- Reeves PG, Nielsen FH, Fahey GC. Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123(11):1939–51. doi:10.1093/jn/123.11.1939.
- Stockler-Pinto MB, Mafra D, Farage NE, Boaventura GT, Cozzolino SM. Effect of Brazil nut supplementation on the blood levels of selenium and glutathione peroxidase in hemodialysis patients. Nutrition. 2010;26(11–12):1065–9. doi:10.1016/j.nut.2009.08.006.
- Zhu HC, Zhao J, Luo CY, Li QQ. Gastrointestinal dysfunction in a Parkinson’s disease rat model and the changes of dopaminergic, nitric oxidergic, and cholinergic neurotransmitters in myenteric plexus. J Mol Neurosci. 2012;47(1):15–25. doi:10.1007/s12031-011-9560-0.
- Swaminathan M, Fung C, Finkelstein DI, Bornstein JC, Foong JPP. Alpha-synuclein regulates development and function of cholinergic enteric neurons in the mouse colon. Neurosci. 2019;423:76–85. doi:10.1016/j.neuroscience.2019.10.029.
- Hermann-Bank ML, Skovgaard K, Stockmarr A, Larsen N, Molbak L. The gut microbiotassay: a high-throughput qPCR approach combinable with next generation sequencing to study gut microbial diversity. BMC Genomics. 2013;14:788. doi:10.1186/1471-2164-14-788.
- Bacchetti De Gregoris T, Aldred N, Clare AS, Burgess JG. Improvement of phylum- and class-specific primers for real-time PCR quantification of bacterial taxa. J Microbiol Methods. 2011;86(3):351–6. doi:10.1016/j.mimet.2011.06.010.
- Ferreira RB, Gill N, Willing BP, Antunes LC, Russell SL, Croxen MA, Finlay BB. The intestinal microbiota plays a role in Salmonella-induced colitis independent of pathogen colonization. PLOS One. 2011;6(5):e20338. doi:10.1371/journal.pone.0020338.
- Anselmo NA, Paskakulis LC, Garcias RC, Botelho FFR, Toledo GQ, Cury MFR, Carvalho NZ, Mendes GEF, Iembo T, Bizotto TSG, et al. Prior intake of Brazil nuts attenuates renal injury induced by ischemia and reperfusion. J Bras Nefrol. 2018;40(1):10–7. doi:10.1590/1678-46a85-jbn-3819.
- Cury MFR, Olivares EQ, Garcias RC, Toledo GQ, Anselmo NA, Paskakulis LC, Botelho FFR, Carvalho NZ, Silva AAD, Agren C, et al. Inflammation and kidney injury attenuated by prior intake of Brazil nuts in the process of ischemia and reperfusion. J Bras Nefrol. 2018;40(4):312–8. doi:10.1590/2175-8239-JBN-2018-0016.
- Rahimlu M, Shab-Bidar S, Djafarian K. Body mass index and all-cause mortality in chronic kidney disease: a dose-response meta-analysis of observational studies. J Ren Nutr. 2017;27(4):225–32. doi:10.1053/j.jrn.2017.01.016.
- Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, Mitch WE, Price SR, Wanner C, Wang AY, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr. 2013;23(2):77–90. doi:10.1053/j.jrn.2013.01.001.
- Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–8. doi:10.1038/sj.ki.5002585.
- Carrero JJ, Thomas F, Nagy K, Arogundade F, Avesani CM, Chan M, Chmielewski M, Cordeiro AC, Espinosa-Cuevas A, Fiaccadori E, et al. Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the international society of renal nutrition and metabolism. J Ren Nutr. 2018;28(6):380–92. doi:10.1053/j.jrn.2018.08.006.
- Koppe L, Fouque D, Kalantar-Zadeh K. Kidney cachexia or protein-energy wasting in chronic kidney disease: facts and numbers. J Cachexia Sarcopenia Muscle. 2019;10(3):479–84. doi:10.1002/jcsm.12421.
- Hanna RM, Ghobry L, Wassef O, Rhee CM, Kalantar-Zadeh K. A practical approach to nutrition, protein-energy wasting, sarcopenia, and cachexia in patients with chronic kidney disease. Blood Purif. 2020;49(1–2):202–11. doi:10.1159/000504240.
- da Silva Costa N, Almeida PP, Da Cruz BO, Brito ML, Maldonado-Campos J, Menezes AC, Figueiredo MS, Magliano AC, Pereira AD, Stockler-Pinto MB. Supplementation of diet with Brazil nut modulates body composition, bone parameters, and lipid peroxidation in Wistar rats. J Food Biochem. 2022;46(10):e14294.doi:10.1111/jfbc.14294.
- Wang K, Qian D, Hu Y, Cheng Y, Ge S, Yao Y. Nut consumption and effects on chronic kidney disease and mortality in the United States. Am J Nephrol. 2022;53(6):503–12. doi:10.1159/000524382.
- Grubisic V, Verkhratsky A, Zorec R, Parpura V. Enteric glia regulate gut motility in health and disease. Brain Res Bull. 2018;136:109–17. doi:10.1016/j.brainresbull.2017.03.011.
- de Oliveira MR. The effects of ellagic acid upon brain cells: a mechanistic view and future directions. Neurochem Res. 2016;41(6):1219–28. doi:10.1007/s11064-016-1853-9.
- Wang GQ, He XM, Zhu GF, Li DD, Shi JS, Zhang F. Ellagic acid supports neuron by regulating astroglia Nrf2. Biotechnol Appl Biochem. 2019;66(5):738–43. doi:10.1002/bab.1791.
- Yu JZ, Wang J, Sheridan SD, Perlis RH, Rasenick MM. N-3 polyunsaturated fatty acids promote astrocyte differentiation and neurotrophin production independent of cAMP in patient-derived neural stem cells. Mol Psychiatry. 2021;26(9):4605–15. doi:10.1038/s41380-020-0786-5.
- Zgorzynska E, Dziedzic B, Markiewicz M, Walczewska A. Omega-3 PUFAs suppress IL-1beta-induced hyperactivity of immunoproteasomes in astrocytes. IJMS. 2021;22(11):5410. doi:10.3390/ijms22115410.
- Abdelfattah MS, Badr SEA, Lotfy SA, Attia GH, Aref AM, Abdel Moneim AE, Kassab RB. Rutin and selenium co-administration reverse 3-nitropropionic acid-induced neurochemical and molecular impairments in a mouse model of Huntington’s disease. Neurotox Res. 2020;37(1):77–92. doi:10.1007/s12640-019-00086-y.
- Rahn J, Lennicke C, Kipp AP, Muller AS, Wessjohann LA, Lichtenfels R, Seliger B. Altered protein expression pattern in colon tissue of mice upon supplementation with distinct selenium compounds. Proteomics. 2017;17(11):1600486. doi:10.1002/pmic.201600486.
- Turovsky EA, Mal’tseva VN, Sarimov RM, Simakin AV, Gudkov SV, Plotnikov EY. Features of the cytoprotective effect of selenium nanoparticles on primary cortical neurons and astrocytes during oxygen-glucose deprivation and reoxygenation. Sci Rep. 2022;12(1):1710. doi:10.1038/s41598-022-05674-1.
- Shi C, Yue F, Shi F, Qin Q, Wang L, Wang G, Mu L, Liu D, Li Y, Yu T, et al. Selenium-containing amino acids protect dextran sulfate sodium-induced colitis via ameliorating oxidative stress and intestinal inflammation. J Inflamm Res. 2021;14:85–95. doi:10.2147/JIR.S288412.
- Wang Y, Branicky R, Noe A, Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018;217(6):1915–28. doi:10.1083/jcb.201708007.
- Cheng Y, Takeuchi H, Sonobe Y, Jin S, Wang Y, Horiuchi H, Parajuli B, Kawanokuchi J, Mizuno T, Suzumura A. Sirtuin 1 attenuates oxidative stress via upregulation of superoxide dismutase 2 and catalase in astrocytes. J Neuroimmunol. 2014;269(1–2):38–43. doi:10.1016/j.jneuroim.2014.02.001.
- Linan-Rico A, Turco F, Ochoa-Cortes F, Harzman A, Needleman BJ, Arsenescu R, Abdel-Rasoul M, Fadda P, Grants I, Whitaker E, et al. Molecular signaling and dysfunction of the human reactive enteric glial cell phenotype: implications for GI infection, IBD, POI, neurological, motility, and GI disorders. Inflamm Bowel Dis. 2016;22(8):1812–34. doi:10.1097/MIB.0000000000000854.
- Xiang J, Zhang J, Cai X, Yang F, Zhu W, Zhang W, Cai M, Yu Z, Li X, Wu T, et al. Bilobalide protects astrocytes from oxygen and glucose deprivation-induced oxidative injury by upregulating manganese superoxide dismutase. Phytother Res. 2019;33(9):2329–36. doi:10.1002/ptr.6414.
- DeKosky ST, Taffe KM, Abrahamson EE, Dixon CE, Kochanek PM, Ikonomovic MD. Time course analysis of hippocampal nerve growth factor and antioxidant enzyme activity following lateral controlled cortical impact brain injury in the rat. J Neurotrauma. 2004;21(5):491–500. doi:10.1089/089771504774129838.
- Saidi SA, Abdelkafi S, Jbahi S, van Pelt J, El-Feki A. Temporal changes in hepatic antioxidant enzyme activities after ischemia and reperfusion in a rat liver ischemia model: effect of dietary fish oil. Hum Exp Toxicol. 2015;34(3):249–59. doi:10.1177/0960327114531991.
- Yi SS, Hwang IK, Kim DW, Shin JH, Nam SM, Choi JH, Lee CH, Won MH, Seong JK, Yoon YS. The chronological characteristics of SOD1 activity and inflammatory response in the hippocampi of STZ-induced type 1 diabetic rats. Neurochem Res. 2011;36(1):117–28. doi:10.1007/s11064-010-0280-6.
- Fu X, Liu Z, Zhu C, Mou H, Kong Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit Rev Food Sci Nutr. 2019;59(sup1):S130–S52. doi:10.1080/10408398.2018.1542587.
- Seguella L, Palenca I, Franzin SB, Zilli A, Esposito G. Mini-review: interaction between intestinal microbes and enteric glia in health and disease. Neurosci Lett. 2023;806:137221. doi:10.1016/j.neulet.2023.137221.
- Seguella L, Pesce M, Capuano R, Casano F, Pesce M, Corpetti C, Vincenzi M, Maftei D, Lattanzi R, Del Re A, et al. High-fat diet impairs duodenal barrier function and elicits glia-dependent changes along the gut–brain axis that are required for anxiogenic and depressive-like behaviors. J Neuroinflammation. 2021;18(1):115. doi:10.1186/s12974-021-02164-5.
- Vicentini FA, Keenan CM, Wallace LE, Woods C, Cavin JB, Flockton AR, Macklin WB, Belkind-Gerson J, Hirota SA, Sharkey KA. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome. 2021;9(1):210. doi:10.1186/s40168-021-01165-z.
- Martin-Del-Campo F, Avesani CM, Stenvinkel P, Lindholm B, Cueto-Manzano AM, Cortes-Sanabria L. Gut microbiota disturbances and protein-energy wasting in chronic kidney disease: a narrative review. J Nephrol. 2023;36(3):873–83. doi:10.1007/s40620-022-01560-1.
- Hu J, Zhong X, Liu Y, Yan J, Zhou D, Qin D, Xiao X, Zheng Y, Wen L, Tan R, et al. Correlation between intestinal flora disruption and protein-energy wasting in patients with end-stage renal disease. BMC Nephrol. 2022;23(1):130. doi:10.1186/s12882-022-02762-2.
- Ding X, Jian T, Li J, Lv H, Tong B, Li J, Meng X, Ren B, Chen J. Chicoric acid ameliorates nonalcoholic fatty liver disease via the AMPK/Nrf2/NFkappaB signaling pathway and restores gut microbiota in high-fat-diet-fed mice. Oxid Med Cell Longev. 2020;2020:9734560. doi:10.1155/2020/9734560.
- Gao Y, Liu Y, Ma F, Sun M, Song Y, Xu D, Mu G, Tuo Y. Lactobacillus plantarum Y44 alleviates oxidative stress by regulating gut microbiota and colonic barrier function in Balb/C mice with subcutaneous d-galactose injection. Food Funct. 2021;12(1):373–86. doi:10.1039/d0fo02794d.
- Zoidis E, Seremelis I, Kontopoulos N, Danezis GP. Selenium-dependent antioxidant enzymes: actions and properties of selenoproteins. Antioxidants. 2018;7(5):66. doi:10.3390/antiox7050066.
- Kume WT, de Jesus Porto EP, de Lara Spada EC, Lisboa DR, Stachack FFF, Terezo AJ, Hernandes T, Takeuchi KP, Dos Santos Elias MP, Gai BM, et al. Acute supplementation of growing rats with Brazil nut flour increases hepatic lipid content but prevents oxidative damage in the liver. J Food Biochem. 2021;45(8):e13834. doi:10.1111/jfbc.13834.
- Barnes KM, Evenson JK, Raines AM, Sunde RA. Transcript analysis of the selenoproteome indicates that dietary selenium requirements of rats based on selenium-regulated selenoprotein mRNA levels are uniformly less than those based on glutathione peroxidase activity. J Nutr. 2009;139(2):199–206. doi:10.3945/jn.108.098624.
- Cardoso BR, Lago L, Dordevic AL, Kapp EA, Raines AM, Sunde RA, Roberts BR. Differential protein expression due to Se deficiency and Se toxicity in rat liver. J Nutr Biochem. 2021;98:108831. doi:10.1016/j.jnutbio.2021.108831.
- Chung S, Barnes JL, Astroth KS. Gastrointestinal microbiota in patients with chronic kidney disease: a systematic review. Adv Nutr. 2019;10(5):888–901. doi:10.1093/advances/nmz028.
- Stockler-Pinto MB, Lobo J, Moraes C, Leal VO, Farage NE, Rocha AV, Boaventura GT, Cozzolino SM, Malm O, Mafra D. Effect of Brazil nut supplementation on plasma levels of selenium in hemodialysis patients: 12 months of follow-up. J Ren Nutr. 2012;22(4):434–9. doi:10.1053/j.jrn.2011.08.011.
- Abot A, Fried S, Cani PD, Knauf C. Reactive oxygen species/reactive nitrogen species as messengers in the gut: impact on physiology and metabolic disorders. Antioxid Redox Signal. 2022;37(4–6):394–415. doi:10.1089/ars.2021.0100.
- Lau WL, Kalantar-Zadeh K, Vaziri ND. The gut as a source of inflammation in chronic kidney disease. Nephron. 2015;130(2):92–8. doi:10.1159/000381990.
