?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Citrus juice is widely consumed due to its high nutritional values, desirable taste, and color. The juice color is the key indicator for quality attributes affecting the consumer’s attention. Conventional thermal treatment is the common method in juice processing which adversely affects its nutritional value and appearance. Measuring the color properties of the juice is an intelligent way to control the juice quality and estimating the alteration in chemicals. In this study, the effects of different treatments on the color aspects of citrus juice including L*, a* & b* values, browning index, total color difference, chroma, and hue index were reviewed. New thermal methods such as microwave, ohmic heating, and infrared radiation are used which result in the shortened process come up time (CUT). In addition, non-thermal treatments such as pulsed electric fields, high-pressure processing, high-pressure carbon dioxide, sonication, and ultraviolet are applied to process the juice, which results in better preservation of the nutritional values. These methods are less effective in the inactivation of the enzymes playing a key role in the juices stability. Therefore, a combination of thermal and non-thermal methods is suggested to increase the effectiveness of the process as well as preserving the juice quality.
Introduction
Fruits and vegetables are low-cost foods with high nutritional values containing vitamins, fiber, and minerals. Based on epidemiological studies, diets containing fruit and vegetables are effective in reducing the risk of several non-communicable diseases like atherosclerosis, stroke, cancer, diabetes, and arthritis.[Citation1]
The genus Citrus L. belonging to the family “Rutaceae” comprises about 40 species. As the most important fruit crop, it is cultivated in tropical and subtropical parts of the world.[Citation2,Citation3] Different products are obtained from citrus fruits such as juice, jam, marmalade, and puree.
According to the Codex Alimentarius Commission definition, the juice is “the unfermented but fermentable liquid which is obtained by mechanical extraction from the edible part of appropriately mature and fresh fruit” (FAO/WHO, 2000). Citrus juice is widely consumed due to its availability, high nutritional values, desirable taste, odor, and color. Citrus juice contains water, vitamins, minerals, sugars (mainly sucrose, fructose & glucose), carbohydrates, acids, phytochemicals and other organic compounds.[Citation3] Citrus fruits are known as a major natural source of citric acid (2-hydroxy-1, 2, 3-propanetricar-boxylic acid) which results in lowering the pH and producing the unique sour taste of the ceitrus juices.[Citation4] Oxalic, tartaric, malic, ascorbic and lactic acids are other acids found in the citrus fruit.[Citation5] During mechanical extraction, the pulp (containing fiber and insoluble particles) partly disperses in the serum phase of the juice which causes desirable cloudy and turbid appearance. The type of juice extraction method determines the fiber content that ends up in the juice. Therefore, a clarified or cloudy juice will be respectively produced by completely or partially separation of the pulp.[Citation6] Citrus juice holds a small amount of protein and contains no fat (free of saturated fat and cholesterol).[Citation3] It is also a good source of calcium, phosphorus, sulfur, folate, zinc, selenium, potassium, magnesium, iron, and calcium.[Citation7] Recognized vitamins in citrus products are thiamin, riboflavin, niacin and other vitamins with antioxidant activity including vitamins C and E and provitamin A carotenoids.[Citation2,Citation8] Furthermore, citrus juices are known as a good source of bioactive compounds such as various phenolic compounds, carotenoids and flavonoids.[Citation9] Mainly the content of anthocyanins, carotenoids, pulps and other some chemicals determine and influence the color and turbidity of the juices.
“Consumers buy with their eyes”,[Citation10] which means that the color is the first aspect of food products affecting the consumer’s attraction; therefore, color is a determining factor in the food industry.[Citation11] The color of the juice is decisive in juice acceptance by the consumers as it reflects the quality, safety, and nutritional values,[Citation12] whereby the consumer instantly identifies the flavor, for example, the orange-colored drinks should taste like orange or carrot.[Citation13,Citation14] On the other hand, the stability of the juice color will encourage consumers to buy it repeatedly. The juice color is affected by the fruit characteristics (a.o. variety, ripening, and growth conditions), juice extraction method, the treatment or processing technology (pasteurization, sterilization, etc.), product formulation, packaging and storage conditions. As some pigments and carotenoids are heat sensitive, color also can reflect the insufficiency or adequacy of a juice treatment.[Citation15]
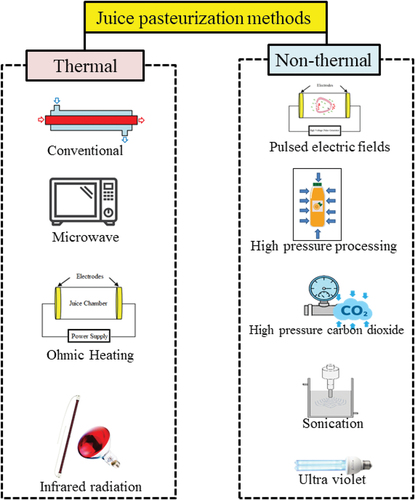
Generally, the target of juice processing should be considered as 1 to 3 weeks shelf life for fresh fruit juices and more than 18 months for storing processed juice.[Citation16] Different thermal treatments (conventional treatment, microwave (MW), ohmic heating (OH) and infrared radiation (IR)) and non-thermal treatments (pulsed electric fields (PEF), high-pressure processing (HPP), high-pressure carbon dioxide (HPCD), sonication and ultraviolet (UV-C)) are used to process the juice to assure its safety, stability and to prolong its shelf life by inactivating microorganisms and enzymes (). Application of these methods can also cause different chemical reactions resulting in loss of nutrients and other chemical compounds such as pigments. The alteration in chemical composition influences the physical properties and juice appearance and color, thereby affecting consumer appreciation. Though it was also found that some juice processing methods had more advantages in preserving the fresh-like juice color than others.[Citation17–19] Therefore, measuring the color properties of the juice is an intelligent way to control the juice quality as well as assessing the changes of chemical compounds.
As sensorial analysis qualitatively defines the juice color, different instruments such as colorimeters, spectrophotometers, spectroradiometers as well as Image processing methods produce quantitative color aspects including L*, a* & b* values, browning index (BI), total color differences (TCD), chroma and hue index.[Citation20] In this paper, the effect of different thermal and non-thermal processing methods on the color aspects of citrus juice is reviewed including various common applied color measurements as shown in .
Table 1. Effect of thermal and non-thermal treatments on color properties of different citrus juices.
What Is Color and Its Measurement Methods?
When a material is exposed to the light source, the light is absorbed, reflected, or passed through it. Depending on the wavelength of the rejected light by the material, the received light by the eyes is interpreted as a color by the human brain.[Citation10] In foods, this is directly influenced by the presence of chemical components and their structure such as water content, pigments, thickness, etc.
Analysis of food color can be operated via qualitatively and/or quantitatively procedures. Qualitative analysis means judging the visual inspections based on comparing various samples. While quantitative analysis refers to extracting data showing the color distribution.[Citation21] In other words, the color of food products can be evaluated based on visual and instrumental methods. Visual evaluation is done via sensorial experiments to compare the samples. This experiment is valuable as it can be expanded to the consumer’s demands and satisfaction. Besides, a visual color analysis which is performed via the panel tests is cheaper than the instrumental methods.[Citation20] While, it is hard and unconfident to evaluate the effect of process conditions only based on the visual experience of persons as uncontrollable factors such as observer genetic, age, eye health, culture, light, etc. can affect the final results.[Citation13,Citation14]
In some cases, the color changes of juice cannot be recognizable by the naked eyes; therefore, the instrumental method should be applied to overcome this limitation of the visual analysis method. In this method, colorimeters, spectrophotometers, and spectroradiometers are used as instruments to express the color coordinates.[Citation20] Colorimeters analyze the color of primary (emitted light) and secondary (reflected or transmitted external light from the object) radiation sources.[Citation20] Spectrophotometers analyze the spectral distribution of transmittance/reflectance as a function of the wavelength which is depending on sample properties, not illumination nor the observer.[Citation22] Spectroradiometers work based on the measure of both the wavelength and amplitude of the light emitted from a light source. Availability, simplicity, high accuracy and easy handling of these instruments lead to the color analysis being as a routine test in assessing the food quality.[Citation23,Citation24] While using these instruments, some factors should be controlled such as position, deepness, dimensions of the sample, the intensity of the light source, blank sample, environmental effects.[Citation25,Citation26] The major limitation of these instruments is related to the need for a homogenous sample to obtain uniform and reliable, and thereby meaningful results.[Citation10] The preparation of samples (grinding, homogenizing, etc.) is time-consuming and will also destroy the food. Therefore, image processing is developed as a quick and efficient tool to evaluate the color of heterogeneous as well as homogenous samples. Image processing techniques using various software such as ImageJ & Photoshop are frequently used as a non-destructive method in analyzing the taken digital image of the food samples. This software extracts data by manipulating the numerical representation of images based on different algorithms.[Citation10,Citation25]
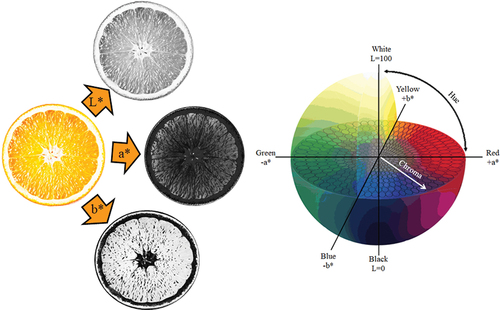
Colorimetry is a vast scientific area beneficial in quantifying, representing and scaling the color in different agricultural fields and industries especially in the food area.[Citation10] There are different color coordinate systems to describe the color of the food products including Munsell, Hunter Lab, Lovibond, CIE & CIELAB. CIE shows the required amount of red, green and blue by a standard observer to give a color match using mathematical coordinates (X, Y & Z).[Citation23] International Commission of Illumination (CIE) recommended the CIELAB as the most appropriate one for the color specification in the food[Citation27] (). This system is mostly preferred to use in the food industry as the results are close to sensorial and chemical evaluations of the products.[Citation28,Citation29] Due to its uniform distribution of colors and its approximation of results by the human eye, CIELAB is frequently used in food studies.[Citation23] In fact, the main difference between the human’s eyes and color analysis instruments is measuring the color; human’s eyes see the color more complex in terms of lightness, hue, and chroma, while these instruments extract the pure coordinates (L*, a* & b* values). The combination of these color space values helps us to have a desirable conclusion near the human’s observations.[Citation15]
CIELAB is defined by three colorimetric coordinates.[Citation30] L* ranging from 0 to 100 reflects a measure of ‘darkness’ to ‘lightness’, respectively. Positive and negative values of a* are in the direction of ‘redness’ and ‘greenness’, respectively. Positive values of b* attributed ‘yellowness’ and the negative values reflect the ‘blueness’.
Humans identify the color of each sample through brightness and chromaticity. Chromaticity combines hue and saturation (chroma) describing the color attributes via the CIELAB system.[Citation31] The hue angle, the qualitative expression of color, illustrates a color with respect to grey color with the same lightness.[Citation26,Citation30] Based on the difference in wavelength absorbance, hue has identified an angle.[Citation10] It is derived from arctan (b* value/ a* value) expressing on a 360° grid where 0°, 90°, 180°, and 270° represents the bluish-red, yellow, green and blue color, respectively.[Citation32] Chroma, calculating as (a*2 + b*[Citation1,Citation2/], is a quantitative expression of colorfulness representing intensity or saturation.[Citation30] This, ranging from 0 to positive values, allows each hue to be distinguished by its difference in comparison to a grey level with the same lightness.[Citation10,Citation26]
Total color difference (TCD) describes the color distance in the 3-dimensional color space and is calculated by . Which the
,
and
refers to the difference between the color parameters of the fresh juice and the treated one. TCD is an effective index in evaluating the color of the treated juice in comparison to the fresh one. To represent the effect of treatment on the food color, TCD limits can be analytically categorized as: 1) TCD > 3 (very dissimilar), 2) 1.5 < TCD < 3 (dissimilar) 3) and TCD < 1.5 (small difference).[Citation33] It is reported that TCD values higher than 3 reflect the undesirable color changes for many food products.[Citation34]
Browning reaction is a common phenomenon during the preparation, processing, and storage of citrus juice which directly influences the quality and acceptance of the product. In general, browning reactions included caramelization, Maillard reaction, and degradation of ascorbic acid (AA). In juiced with low AA content like apple juice, the Maillard’s reaction is more effective in browning development.[Citation35] While as a result of the high acidity of citrus juice, Maillard’s reaction, via sugar-amino acid reaction, is of minor importance.[Citation36] Considering the thermal sensitivity of AA and its relation with the Browning Index (BI), measuring the BI is known as a common method to control the quality of the juice treatment conditions and loss of juice nutritional value.[Citation37] In browning of citrus juices, AA has a key role in color stability. BI defines as the brown color purity and is enough sensitive to reflect the effect of process conditions on the color and quality of the juice.[Citation37,Citation38] The higher thermal sensitivity of BI during thermal processing is related to the higher AA degradation.[Citation35] This index can be measured by reading the juice absorbance using a spectrophotometer or also via EquationEq. 1(1)
(1) :
Chemical compounds and reactions affecting the citrus juice color
Citrus fruits and their derived products are good sources of antioxidants which their consumption reduces the risk of inflammations, mutagenicity, and carcinogenicity.[Citation39,Citation40] Antioxidants, such as carotenoids, anthocyanins, flavonoids and ascorbic acid, preserve the cell structure and its functionality in the human body by affecting free radicals and inhibition of lipid peroxidation.[Citation2] For example, studies showed that the flavonoids can act as anti-lipid oxidation agent in vitro apart from reducing the quality of peroxide formation in vivo.[Citation41] Generally, in vitro assays demonstrated that most antioxidant activity of citrus fruits depends on the content of phenolic compounds and ascorbic acid.[Citation42] Various pre-harvest and post-harvest processing factors influence the content of antioxidants in food products. The post-harvest factors relating to the food industries include preparation methods, processing conditions and storage factors such as temperature, humidity and light.[Citation2]
Carotenoids are responsible for yellow, orange, red, or red-bluish look colors in different citrus fruits. Most carotenoids contain a 40-carbon basal structure including conjugated double bonds. The pattern of these bonds affects the antioxidant activity of the carotenoids. The central chain may include cyclic groups. Based on their structure, carotenoids are categorized as carotenes (containing only carbon and hydrogen atoms) and oxocarotenoids (xanthophylls) that carry at least one oxygen atom.[Citation8] The health effects of carotenoids and other species depend on the ingested amount, their bioavailability, etc. The in vitro methods demonstrated the bioaccessibility of carotenoids and their bioavailability. The carotenoids should be incorporated into micelles to be absorbable for the intestinal enterocytes.[Citation30] Depending on the type of carotenoids, they are mostly stable during thermal processes such as blanching, cooking and canning.[Citation43] Besides, their bioaccessibility could be affected by the thermal treatments.[Citation44] Carotenoids are susceptible to oxidation, isomerization and any alteration during juice processing and storage due to their unsaturated structure.[Citation45]
Anthocyanins are unstable during juice processing and storage which brings some limitation in the thermal treatment of the citrus juice especially the juice of blood orange. Their degradation causes decolorization or developing undesirable brown-colored compounds in the juice.[Citation46] Polyphenoloxidase, peroxidase and glycosidase enzymes can involve in their degradation. These enzymes are naturally found in plants or sourced from mold contamination.[Citation32] The colored polymerized pigments can be produced as a result of the reaction between anthocyanins and other phenolic compounds.[Citation32] Flavonoids in citrus, naringin, hesperidin and nargenin, play an antioxidant role in the body and reduce the formation of peroxide in vivo.[Citation2,Citation41]
The human body is not able to synthesize AA so it should be taken up from the diet, mainly fruits and vegetables.[Citation47] AA is a heat-sensitive vitamin that is considered as a citrus juice quality indicator.[Citation48] Depending on some external factors, such as oxygen, heat, light and process or storage duration, degradation of AA can take place in aerobic and/or anaerobic conditions. In general, oxidation and anaerobic destruction of vitamin C occur during juice processing and storage, respectively.[Citation49] Oxygen induces the rate of the AA degradation but in anaerobic conditions dominates the non-enzymatic browning.[Citation50] Steps of aerobic degradation include hydration of AA to ketogulonic acid and decarboxylation and dehydration to furfural. Anaerobic conditions lead to the formation of furfural with 3-deoxy-L-pentosonic acid as an intermediate compound.[Citation51] The further reaction of the formed carbonyl compounds such as hydroxymethylfurfural (HMF) via aldol condensation or reaction with amino groups and polymerization yields brown pigments.[Citation49,Citation50] HMF will also be formed during the Maillard reaction and reducing sugar degradation.[Citation49] However, these processes have a minimum effect on juice browning in comparison to the impact of AA degradation.[Citation37] To control and prevent the development of visible brown color, it is essential to identify these compounds at the initial steps of the reaction by measuring the color attributes of the juice.[Citation37]
Cloud stability is a critical quality aspect in citrus juice due to the influence on juice appearance, flavor, and mouthfeel. Pectin methylesterase (PME) activity causes de-esterification of the methoxylated pectin resulting in easy access of the calcium cations to the pectin and production of the insoluble pectate. Considering the key role of pectin in the cloud stability of the juice, the PME inactivation is important in juice processing to preserve the cloudiness and color aspects (especially the lightness) of the juice during the shelf life.[Citation52]
Effect of thermal treatments on citrus juices color
Different time-temperature combinations (intensity of the thermal process) are applied to control the stability, safety and shelf life of food products. Food products can be categorized into two groups; low-acids (pH > 4.6) and high-acids (pH < 4.6). Low acid food products should be processed at temperatures higher than 100°C, called sterilization, due to the possible presence of thermal resistant microorganisms (vegetative or spore forms) such as Clostridium botulinum. However, high-acid food products are subjected to be spoiled by the growth of relatively thermal sensitive microorganisms.[Citation53] Thermal treatment with a temperature lower than 100°C (pasteurization) is therefore sufficient to ensure the safety of these kinds of foods.[Citation52,Citation54]
Citrus juices are categorized as high-acid food products. Pasteurization of citrus juice is essential as it may contain various pathogens such as E. coli, Salmonella, Cryptosporidium species which cause diseases like hemolytic uremic syndrome.[Citation6] It was found that at least a 5-log reduction in the population of the target pathogens is required for guaranteeing the juice safety.[Citation55] Besides, some enzymes such as pectin methylesterase (PME) and polyphenol oxidase should be inactivated to guarantee the stability of the appearance and nutritional value of the juice during shelf life. That is the main reason for the limited shelf life (a few days) of unpasteurized juice especially without storing the juice under a defined condition such as a cold chain. However, pasteurization process intensity is not sufficient to inactivate all kinds of vegetative bacteria, spore formers and thermal resistant isoforms of the enzymes in the juice; hence, preserving the pasteurized juice to extend the shelf life could be achieved by storing it in a specific condition such as refrigeration temperature or using some additives (acids or sugars) in the juice formula before the heating process. For example, the packed juice in laminated cartons, immediately after heating over 90°C, will be stable for a long time (about 12 months) under temperate conditions. But, using laminated plastic to pack the juice as a cup drink generally have about 6 months shelf life.[Citation56]
Pasteurization is accomplished using different time-temperature combinations including low-temperature long time (LTLT) and high-temperature short time (HTST). LTLT refers to heating the juice at 63 to 65°C for a long time (about 20 to 30 min). The adverse effects of the LTLT process on nutritional values and physical aspects of the product results in the development of HTST. During HTST, the juice is subjected to a high-temperature treatment for a short duration; for example, this process for orange juice treatment includes heating at 90 to 95°C for only 15 to 30 s.[Citation57] HTST induces better preservation of the pigments and vitamins in the juice in comparison with the LTLT. Ultra-high temperature (UHT) is another pasteurization method that is introduced in the dairy industry. UHT can be used in producing the citrus juice (for example at 135°C for 2 s)[Citation58]; however, considering the thermal sensitivity of the spoilage microorganisms in high acidic juice, the product stability could already be achieved by using HTST and/or LTLT.
Several systems with different heating mechanisms are used in juice processing. Some of these systems are based on indirect heating mechanisms, using a liquid medium (conventional method) and some of them acted as indirect methods (radiations and electrical currency).[Citation52,Citation59–61] In all thermal treatments, there are three sequential steps: 1) come up step, rising in the initial temperature of the products up to process temperature; 2) holding step, holding the juice at a specific temperature for a defined period & 3) cooling step, reducing the juice temperature to prevent from adverse overheating effects. The main difference between the various thermal treatment methods is related to the speed of heating the juice in the first step (come up time (CUT)). In comparison to the conventional treatment, CUT is shorter in novel thermal methods such as IR, MW and OH.[Citation60] The shorter CUT results in lower nutrient loss and preventing undesirable alteration in the physical properties of the juice like color and appearance.
Conventional thermal pasteurization of citrus juices
Conventional thermal pasteurization refers to indirect heating of the juice by transferring the heat from a medium (usually water or steam) or a hot surface (heat exchangers) to the juice.[Citation62] These processes can be operated in batch (packaged juice) or continuous (before packaging) modes. Using various combinations of temperature and time showed different effects on the color aspects of citrus juices.
Aghajanzadeh et al. (2016) studied the effect of thermal treatment (60–90℃ for 15 min) on color aspects of key lime juice.[Citation52] They observed that the L* value decreased during heating at higher temperatures and thermal inactivation of PME which takes place during the process improves the juice turbidity. They also found that higher temperatures induce a decrease in AA content causing a raise in BI, a* and b* values. Moreover, an adverse linear relation (R[Citation2]=0.98) was found between the content of AA and BI. Demirdöven and Baysal (2014) reported that the L*, a* & b* values of fresh orange juice were 55.9, 5.3 & 52.1, respectively.[Citation59] A decrease in L* (5.5%), a* (98.1%) and b* (11.5%) values were observed during heating of the juice at 95℃ for 1 min. In another study, AA degradation (16%) brought a rise in BI from 0.14 (fresh juice) to 0.27 after the thermal treatment of fresh Shamuti orange at 90℃ for the 50 s. Also, a decrease in the lightness of the orange juice was found from 99.3 (fresh juice) to 97.2 by thermal inactivation of the PME (95%). Based on the sensory evaluation, a significant alteration between the color of the fresh and pasteurized samples was recognized.[Citation63] In another study, sweet orange juice was heated in a water bath at 50 to 90℃ for 15 min; it was found that the higher temperature brought lower values of a* × b* due to more thermal degradation of AA.[Citation64] Mandarin juice has been subjected to different thermal pasteurization conditions (65℃ for 15 s, 85℃ for 15 s and 92℃ for 30 s).[Citation65] Higher ascorbic acid degradation, PME inactivation and total carotenoids content decrease were found in the samples treated at the higher temperature. The turbidity (transmittance percentage) of juice (being 16.07% in the fresh state) decreased to 9.50, 9.88 and 8.63 after heating at 65, 85℃ and 92℃, respectively. These changes resulted in the highest increase in lightness (41.18) after processing at 85℃ for 15 in compared to the fresh one (36.08). Up to 22% increase in chroma and 2% decrease in hue index were observed after heating of the juice at different conditions. The heated juice at 85℃ showed the highest TCD (11.04) followed by 92℃ (10.12) and 65℃ (10.10). Lightness, a* and b* values of fresh mandarin juice were 43.88, 9.95 and 28.01, respectively.[Citation66] Conventional thermal pasteurization (90℃ and 30 s) of the mandarin juice affected the L* (47.15), a* (6.91) and b* (30.06) values resulting in a TCD of 2.69. During heating of grapefruit juice at 70℃ for 90 s in a water bath, the lightness (6.65), a* (3.80) and b* (4.95) of the fresh juice reduced to 6.33, 3.51 and 4.67, respectively.[Citation67]
Upon pasteurization of Valencia orange juice at 90℃ for the 30 s, the partial precipitation of unstable suspended particles affected the lightness of the orange juice. As well, losses of carotenoid pigments, especially violaxanthin, cis-violaxanthin and antheraxanthin and the isomerization of the 5,6-epoxid carotenoids to 5,8-epoxids produced a lighter and more saturated juice with increased chroma and hue angle values. The juice became more yellowish and less reddish after the thermal treatment as b* value increased from 17.26 to 20.02 and a* value reduced from −1.75 to −2.64. The estimated total color difference (TCD) was 2.92 reflecting a considerable effect of thermal treatment on the color of the orange juice.[Citation43] Ultrafreezing is an effective way to preserve the nutritional values and organoleptic properties of juices.[Citation44] Mapelli-Brahm et al. (2018) studied the effect of thermal treatment (90℃, 30s) and thawing conditions (room temperature, refrigeration temperature (4℃) and MW treatment (800 W, 20 s)) on the color properties of ultrafrozen Pinalate orange juice (using liquid nitrogen).[Citation30] The light-yellowish appearance of Pinalate juice was attributed to the presence of some carotenoids, mainly ζ-carotene which has a lower b* value and chroma in comparison to the main carotenoids in common orange juices. Besides, the pale color of this juice could also be related to the presence of the high amount of colorless carotenoids (phytoene and phytofluene) and the low content of xanthophylls. While thawing conditions had no effect, it was found that thermal treatment lowered the juice lightness. The chroma and hue index remained almost unchanged during pasteurization and thawing. High thermal degradation of carotenoids (67%) induced the highest TCD in heated juice. The high TCD (more than 2.8) in the thawed samples were observed at refrigeration temperature and MW treatment probably due to the loss of Violaxanthin (about 76.5%) and carotenoids (>42%).
Anthocyanins, especially cyaniding-3-glucosid and cyaniding-3-(6”-malonyl) glucoside, have a key role in the appealing color of the blood orange juice.[Citation68,Citation69] Cao et al. (2011) studied the effect of thermal treatment (70–90℃) on the color of the Tarocco blood oranges juice.[Citation46] It was found that a higher degradation rate of anthocyanins occurs as the juice is processed at higher temperatures, resulting in a significant a* value reduction. However, the decreaes rate of a* value was higher than anthocyanins. A linear relation between the content of anthocyanins and the a* value was found (R[Citation2] > 0.98) which can be helpful in online checking of the juice quality in industries.[Citation46] Lee and Coats (1999) studied the changes in the color aspects of the juices of two different grapefruits cultivars (Ruby red & Star ruby) during thermal treatment (91℃, 63.3 ml/s) in a plate heat exchanger.[Citation45] The same treatment was applied to the juices throughout the seven months (from November to May). They observed that the processed juices showed a more noticeable yellowish color. The TCD of Ruby red & Star ruby grapefruit juices ranged from 2.5 to 4.8 and from 2.1 to 3.1 after treatment, respectively. The contents of lycopene and β-carotene, pigments responsible for the pink-red color of grapefruit, were unchanged after processing, representing their thermal stability. Hence, the change in color could be related to chromoplasts degradation and carotene in cellular lipids, as well as the effect of the thermal process in the structure of the suspended particles and pulps. In addition, carotenoids are mostly adsorbed by the pulp and their amounts in the juice serum are low due to xanthophylls are fatty acid esters.[Citation70] This could be an explanation why some processing methods such as heating and homogenization which produce smaller juice particles (broken pulps) result in a more yellow light color while juices containing larger particles show an orange color.
Microwave heating of citrus juices
Microwave (MW) is a part of the electromagnetic spectrum waves with a frequency ranging between 300 MHz to 300 GHz. The Federal Communications Commission chose two frequencies of 915 and 2450 MHz for use in the food industry.[Citation71] The interaction of electromagnetic radiation with dielectric materials at certain frequencies causes MW heating.[Citation72] Dipole rotation and ionic polarization are the dominant mechanisms in the generation of heat in food products containing water and ionic compounds.[Citation71,Citation73] There is no need for intermediate surface or fluids to heat the food; therefore, MW pasteurization is faster, more effective and economical than the conventional heat treatments.[Citation74] The heat is inherently generated due to the friction between the ions or dipole molecules. Therefore, the direct MW heating causes shorter CUT compared with the conventional heating method.[Citation60,Citation75]
Sweet orange (Citrus sinensis) contain the most complex carotenoids between the citrus, especially esterified cis-violaxanthin, β-cryptoxanthin, lutein, zeaxanthin and cis-antheraxanthin.[Citation16] The temperature of the sweet orange juice linearly increased (33℃/min) during batch MW heating at 60 to 85℃ for 0.25 to 5 min.[Citation74] Raise in temperature caused 39.3% decrease in the PME activity (at 70℃ for 1 min) as well as 3% (60℃-2.5 min) to 52% (85℃-1 min) reduction in total carotenoids contents. β-cryptoxanthin, considered as a pro-vitamin A, was accounted for about 64% of total carotenoids and it reduced about 14% after MW treatment (70℃ for 1 min). While β-carotene and zeaxanthin that are pigments influencing the color of the orange juice reduced 27% and 22%, respectively. When the orange juice was heated at 70℃ for 1 min and 5 min, 3% to 12% fall in AA content was observed, respectively. The changes in the content of the chemical compounds influenced the color of the orange juice. The chroma didn’t change during heating at 70℃, while it was enhanced as the higher temperature was applied for a longer period (9.93 to 13.97). Based on TCD analysis, it was revealed that MW treatment of the juice at 60℃ for 5 min is more effective in the preservation of the juice appearance. During continuous MW process of Navel orange juice, 93.0% (50 ml/min- 900 W- 75℃) to 95.4% (40 ml/min- 900 W- 83℃) reductions in PME activity were found.[Citation72] At these conditions, the content of pectin increased from 2.6 to 17.2%. The MW treatment was effective in AA preservation as only a 9% reduction in its content was reported. When more intense MW was applied (50 ml/min & 900 W) the juice became slightly lighter than the fresh one. But, a* and b* reduced after MW heating. In both MW treatment conditions, the TCD was changed between 1.9 (50 ml/min- 900 W) to 3.1 (40 ml/min- 900 W) reflecting the effect of lower flow rate on rising temperature and the undesirable effects of temperature on the degradation of chemical compounds. Vikram et al. (2005) studied the effect of different MW power (245 to 455 W) and process time (0.5 to 15 min) on the color of orange juice.[Citation64] Due to a lack of control on the raise in the juice temperature, its final temperature increased up to 100 to 125℃. This is why the rate of AA degradation during MW heating (0.050 to 0.194 1/min) was higher than the conventional treatment (0.035 to 0.178 1/min). These results caused the high-speed reduction in the combination of a* & b* values (a*×b*). During microwave processing (800 W, 80℃ and 70 s) of mandarin juice, an increase in lightness and yellowness and a decrease in redness were observed. The theory of cell membrane rupture during MW heating and release of different chemical compounds can explain changes in the juice color.[Citation66]
The effect of thawing conditions on the color of ultrafrozen Valencia orange juice (using liquid nitrogen) was investigated at different conditions including room temperature, refrigeration temperature (4℃) and MW subjection (800 W, 20 s).[Citation44] The MW thawing decreased the contents of provitamin A carotenoids (-cryptoxanthin,
-carotene and
- carotene), lutein, zeaxanthin and antioxidants in comparison to the fresh juice and thawed samples at room and refrigeration temperatures. These brought a high TCD in MW treated sample (3.01) rather than the thawed juices at room (1.91) and refrigeration temperatures (2.21).
Ohmic heating of citrus juices
During ohmic heating (OH) which is also known as electrical resistance heating, the juice should be placed or passed through two electrodes. In OH, heating the juice as well as the occurrence of electro-plasmolysis (electropermeabilization) is resulted from passing the electrical current through the juice.[Citation48,Citation76] The base of this method relies on the presence of abundant dissolved ionic salts in a liquid medium and a reduction in electrical conductivity which enhances the alteration in the juice temperature.[Citation61,Citation75] The rise in the temperature depends on other properties of the juice such as electrical conductivity, particle size, ionic concentration, field strength and also properties of the electrodes.[Citation76] The electro-plasmolysis is a non-thermal phenomenon which causes disruptive pores in the cell wall with different sizes because of changing the natural dielectric strength of this wall.[Citation77] Therefore, release out of the content of the cell enhances the inactivation of microorganisms and enzymes as well as the extraction of some chemical compounds.[Citation48] The main advantage of this thermal treatment, rather than the conventional method, is its short CUT and reduction in adverse effects of overheating the juice.
About 96% reduction in PME activity was found when Navel orange juice was treated using a continuous OH system,[Citation61] and a 2% increase in the content of pectin (at 44 V/cm, 70℃) indicated the electro-plasmolysis effect. While the pectin content of the conventional heated sample (411.2 mg/L) was similar as the fresh juice (410.3 mg/L). The fresh juice contained 48.6 mg/100 mL AA; this amount reduced up to 43.1 mL/100 mL during OH at 44 V/cm. The small reduction in vitamin content can be explained by the application of moderate temperature besides the short CUT. The OH treated juice showed a darker color with lower a* and b* values in comparison to the fresh one. The cloudiness of the juice developed at higher content of the pectin so the lightness of the juice reduced. TCD of the heated samples at 42 V/cm and 44 V/cm were 8.2 and 11.2, respectively. The chroma changed between 7.6 to 10.7 during OH treatment at 42 V/cm to 44 V/cm. Hence, it can be concluded that the larger changes in the appearance of the juice happened when the juice was heated at the more intense condition.
A continuous OH system (50 Hz, 20 cm electrode gap, 8 kV maximum voltage) was used to process the Shamuti orange juice.[Citation18] No significant difference was found between the content of AA and lightness in ohmic treated juice and conventionally processed one (90℃ for 50 s) during storage. OH considerably increased the BI of the juice, even more than the conventional treatment. This higher level was also observed during storage at 4℃ for 100 days. In another study, the researcher evaluated the same conventional treatment with continuous OH up to 90, 120 & 150℃ with 3, 4 & 5 L/min.[Citation63] A 90% to 98% reduction in PME was found as a result of the alteration in the juice temperature while the flow rate showed no significant impact on the inactivation of this enzyme. Similarly, the rise in temperature caused about 7 to 25% decrease in the AA content. Based on the spectroscopy and sensorial evaluation, it was revealed that the BI of the processed juice was slightly higher than the fresh one without any difference in the appearance. Reduction in the vitamin content caused BI increment as well as decrease in lightness.
During ohmic heating (42 V/cm) of the sweet orange juice at 50 to 90℃ for 0.5 to 15 min, it was revealed that the high internal heat generation brought the low-speed reduction in AA content (0.024 to 0.157 1/min) in comparison to the conventional treatment.[Citation64] In OH, the short CUT was effective in retaining the AA content as well as the color aspect (a*×b*). When the batch OH system with the fixed electric field strength (16 V/cm) was used to heating the orange juice to 50 to 60℃ for 1 min, no significant difference was found between the color aspects (Lab-values) of the processed and the fresh juice.[Citation78]
The infrared radiation of citrus juices
Infrared radiation (IR) with wavelength ranging from 0.5 to 100 μm is part of the electromagnetic radiation spectrum.[Citation79] Based on the wavelength, infrared is categorized into near – IR (0.75–1.4 μm), mid – IR (1.4–3.0 μm) and far – IR (3.0–1000 μm).[Citation80] Most food components can absorb the energy of far – IR.[Citation79] However, the food composition and its structure affect the penetration depth of this radiation. Direct heat penetration of IR creates high energy efficiency with fast and uniform heating with a short CUT.[Citation81] IR is applied in different areas of food processing such as heating, dehydration, baking, cooking, roasting, thawing and blanching.[Citation79] Unlike conventional heating in a liquid medium, the temperature of the IR processed juice linearly increases with time which is the reason for the short CUT in IR heating.[Citation52] Researchers used IR heating to study its effect on the properties of key lime juice.[Citation52] The juice was heated with a 1500 W IR modules adjusted in 8.5 cm from the sample up to 60, 70, 80 & 90℃. In comparison to the use of a water bath, IR treatment was effective in preserving AA content with lower PME inactivation because of the shorter CUT. During conventional treatment of the key lime juice, the constant rate of AA degradation (0.018 to 0.319 1/min) was higher than the IR process (0.013 to 0.213 1/min). During IR heating (0.065 to 0.495 1/min), the inactivation rate of the PME heat resistance fraction was lower than the conventional one (0.056 to 0.469 1/min). A 10℃ rise in temperature improved the cloud value of the IR treated juice (1.44 times higher than the fresh juice) which was similar to the conventional processed juice. The color of the juice became darker at higher temperatures. Unlike the temperature, the heating time had a significant effect on rise in BI. Due to AA degradation and developing of browning reactions, a* and b* values increased during thermal treatment.
In another study, where sweet orange juice was exposed to IR treatment (250 W) the rate of temperature increase was higher for the conventional method, while, it was lower when using ohmic heating. The reaction rate of AA degradation during IR heating was changed from 0.044 to 0.228 1/min. In this study, the most effective methods in preserving the juice color were ohmic heating, followed by IR and MW treatment.[Citation64]
Effect of non-thermal treatments on citrus juices color
Conventional thermal treatment is commonly used in the food industry. Even if there are lots of benefits, studies showed important disadvantages in the quality of the thermally treated juice. Heat sensitive chemical compounds such as ascorbic acid are the key indicator determining the final quality of processed juice; control or preventing loss of nutrients is the main reason for the emergence of novel non-thermal treatments such as PEF, HPP, HPCD, ultrasound and UV-C radiation. The basis of these methods is destroying the microorganisms and enzymes by changing their biological and chemical structures during short processing time. These changes take place without a considerable rise in the juice temperature; hence, the undesirable degradation of chemical compounds, changes in physical aspects and organoleptic properties of the juice are significantly reduced.
Pulse Electric Field treatment of citrus juices
Pulse Electric Field (PEF) is a novel non-thermal juice treatment which exerts a high voltage to the juice for a very short time. The final temperature of the juice will slightly increase when the juice is processed using this system. A considerable increase in juice temperature may occur in uncontrolled PEF treatment due to the effect of electrical resistance heating. The PEF treatment influences the juice properties due to alteration in the juice temperature in addition to the occurrence of the electroporation phenomenon.[Citation82]
Cortés et al. (2008) compared the effect of thermal treatment (90°C for 20 s) with continuous PEF (30 kV/cm for 100 μs) on the color properties of Navel orange juice.[Citation11] The HMF content of the PEF treated and the fresh juices were the same (0.089); while, thermal treatment enhanced slightly the HME development (up to 0.115). It was reported that the BI of PEF treated and fresh orange juice was almost similar (0.086) but thermally processed juice had a higher BI (0.093). The lightness of the PEF processed juice (52.23) and the heated one (52.41) and was higher than the fresh orange juice (51.36). While the a* value of the fresh juice (4.56) was higher than the PEF (2.99) and the heated (1.57) juices. The b* value of the fresh, PEF and thermally treated juice were 50.73, 53.62 and 57.61, respectively. Chroma of the fresh juice (50.93) increased up to 53.70 and 57.63 after the application of PEF and heat treatment. In addition, it was revealed that heating had more impact on the TCD of the juice than PEF treatment.
Cortés et al. (2006) applied various PEF intensities (20–40 kV/cm) for 30 to 340 μs to process the Navel orange juice.[Citation83] The juice temperature increased as higher PEF intensity was used for a longer period. It was found that the carotenoids content remained constant or reduced at 25 kV/cm. While, the results were different at 35 and 40 kV/cm, as in some cases the carotenoids content (13-cis-violaxanthin and 7,8,7ʹ,8ʹ-tetrahydrolycopene) increased. In comparison to the fresh juice, the total carotenoids showed a 12.6% reduction in pasteurized juice (90°C for 20 s) using a heat exchanger. The content decreased 9.6%, 6.4% and 7.8% when the juice processed at 25 kV/cm- 340 μs, 30 kV/cm- 240 μs and 40 kV/cm- 130 μs, respectively. 11.1%, 9.9% and 15.6% reduction were observed in vitamin A content as the juice treated at 25 kV/cm- 340 μs, 40 kV/cm- 130 μs and 90°C-20 s, respectively. The L*, a* & b* values of the fresh juice were 72.61, 6.15 and 77.14, respectively. The color of all PEF treated samples shifted toward the lower a* value (4.41) and the more positive b* value (84.24) with the constant lightness (72.61). The higher rise in temperature (72℃) was achieved when the juice was processed at 25 kV/cm for 340 μs which resulted in the 7.32 alteration in TCD. To evaluate the effect of carotenoids content on the juice color, linear regression models were used. It was reported that the 9-cis--Carotene, 13-cis-
-carotene and 13-cis-Violaxanthin had a linear relationship a* (positive) and L* (negative). While Neoxanthin+9-cis-violaxanthin mixture, lutein, antheraxanthin, zeaxanthin, isolutein,
-Cryptoxanthin,
-Carotene, phytoene+phytofluene and 9-cis-
-Carotene had a linear relationship with a* (positive), b* (positive) and L* (negative). The L* values were 48.6, 49.2, and 50.7 and the hue angles were 53.4, 54.0, and 54.5 for thermally processed (90℃ for 90 s), PEF-processed (40 kV/cm for 97 ms), and fresh Rohde Valance orange juice, respectively.[Citation17] The AA content in the fresh and PEF processed juice was around 55 mg/100 ml, but the thermal treatment caused 19% reduction. Therefore, BI of thermally processed juice increased from 0.20 to 0.34 and that of PEF-processed orange juice increased from 0.18 to 0.31 during storage at 4°C for 196 days. The PEF treated orange juice showed a lighter color in comparison to the heated juiced in a heat exchanger.
A mix of orange & carrot juice (4:1) was processed using a continuous PEF system (25 kV/cm for 280 s) and a plate heat exchanger (98℃ for 21 s).[Citation84] It was found that the turbidity of the fresh juice (0.64) increased during the thermal (1.31) and PEF processes (1.06). The more turbid appearance of the heated juice can be related to the higher PME inactivation levels in comparison to the PEF treated one. HMF content was the same in all samples. Lightness and chroma were not affected by the processing conditions. However, the hue index shifted toward the yellowish. Cserhalmi et al. (2006) processed different citrus juices (grapefruit, orange, lemon & tangerine) at 28 kV/cm for 100 μs.[Citation85] About 6.2% reduction in AA content was observed after treatment. It was found that HMF and BI of all samples were not affected by the PEF treatment. After PEF treatment, a* value of all samples, L*& b* values of the grapefruit and tangerine juices were reduced. These changes influenced the TCD of the juice as the juice of grapefruit, orange, lemon & tangerine showed TCD of 0.45, 0.47, 0.59 & 2.44, respectively. Yeom et al. (2000) compared the effect of heat treatment (94.6°C for 30 s) with PEF (35 kV/cm for 59 μs) on the properties of the Valencia orange juice.[Citation86] 98% and 88% decrease in the PME activity was reported when juice was respectively heated and PEF treated. It could be concluded that during the short PEF process time, the enzyme inactivation resulted from the electroporation phenomena rather than the rise in the juice temperature. The equivalent surface area mean diameters of the particle size in the PEF and thermally processed juice were 41.70 and 49.62, respectively. In comparison to the thermally processed sample, the AA content of the PEF treated juice was higher which produced lower BI. During storage at 4°C, the PEF-treated orange juice showed higher lightness and hue angle than the pasteurized orange juice.
Mixed mandarin and Hallabong tangor juice was firstly heated prior to PEF processing (70℃-16 kV/cm-89 s and 55℃-19 kV/cm-102
s)[Citation87] The processing conditions had no significant effect on the juice lightness. There was also no considerable changes between a* value of the fresh (22.97) and treated juice at 70℃-16 kV/cm-89
s (22.35). Whereas, a decrease in a* value (22.15) of the juice was observed after processing at 55℃-19 kV/cm-102
s. The b* value of the fresh juice was 78.30 which increased to 79.19 and 80.19 after processing at 70℃-16 kV/cm-89
s and 55℃-19 kV/cm-102
s, respectively. TCD of the treated juice at 55℃-19 kV/cm-102
s brought higher TCD (2.17) than processing at 70℃-16 kV/cm-89
s (1.33). The processing conditions had the same effect on increasing the BI of the fresh juice. These could be related to the reduction in the content of ascorbic acid and antioxidant activity of the treated juices.
High-pressure processing of citrus juices
High-pressure processing (HPP) is known as one of the most innovative methods for inactivating the microorganisms and enzymes of thermo-sensitive products. In HPP, the juice is uniformly subjected to pressures at around room temperature.[Citation88] Depending on the food ingredients, the temperature can increase by approximately 3℃ per 100 MPa.[Citation89] The minor rise in temperature is insufficient to produce degradable effects on the nutritional values of the juice. On the other hand, the stability of covalent bonds for HPP causes a minimal alternating effect on the low-molecular-weight components such as flavoring agents, pigments and vitamins resulting in better preservation of the organoleptic and color properties of the juice.[Citation90] In industrial applications, the pressure ranging from 400 to 600 MPa is suggested to operate an optimum process; this pressure range shows the reversible or irreversible effects on micro/ macro molecules of the food compounds.[Citation90] Selecting the operating pressure and process time is essential to prevent adverse effects on the juice properties.[Citation91] It was mentioned that the application of the 600 MPa would be inappropriate for commercial use due to a rise in the operational expenses; hence, a combination of the HPP with mild thermal treatment can be a strategic way to overcome these limitations.[Citation92]
Lacroix et al. (2005) used dynamic high pressure (DHP) and pre-heating (50℃ for 10 min) to process the Valencia orange juice.[Citation93] During DPH, the juice was forced for 3 or 5 times through a narrow and variable orifice at high pressure (170 MPa) and high velocity. DHP is different from a static high-pressure process in which rheological phenomena such as cavitation, friction, shear and turbulence can take place. A 20% reduction in PME activity was observed in the DPH processed juice while the combination of DPH and pre-heating decreased the enzyme activity by 50–75%. Homogenization reduced the size of suspended particles in the juice and thereby prevented their aggregation and juice clarification. Heating helped this effect by disrupting the hydrogen bonds and improving the unfolding and swelling of macromolecules to be more sensitive for the DPH destruction. In comparison to the fresh juice, loss of opalescence was delayed for one and three days after DPH with 3 and 5 passes, respectively. Besides, the combination of these two methods resulted in the opalescence stability of the juice for 8 days. The residual activity of PME was 6.2% in the processed orange juice at 800 MPa for 1 min.[Citation92] More than 80% of AA content reduced during storage (3 months at 4℃ & 2 months at 37℃) due to the reaction of this vitamin with dissolved oxygen in the not deaerated juice. These reactions caused the development of the BI especially during storage at a higher temperature (37℃). At this storage condition, the lightness and yellowness decreased but a rise in redness was observed that brought a considerable change in TCD (>9) of the juice. However, there was no alteration in the color aspects of the stored processed juice at 4, 15, or 26℃. Bull et al. (2004) reported that a 45% reduction in PME activity of Navel orange juice was observed when HPP was applied (600 MPa, 1 min).[Citation94] Evaluating the juice clarification (cloud loss) reflected that this factor increased from 14% to 20% immediately after HPP up to 12 weeks of storage at 10℃. BI of the fresh and HPP juice were 0.05 and 0.08, respectively. While no difference between TCD of both samples was observed (TCD = 0). During storage, the BI and TCD increased up to 0.11 and 9.02, respectively. In addition, there was no difference between pH, Brix, viscosity, titratable acid content and alcohol insoluble solids of the treated & fresh juices. The effect of HPP (450 MPa, 5 min, 11.5℃) was investigated on the color aspects of the prebiotic orange juice.[Citation95] While 9.57% decrease in the hue index was observed during HPP, the lightness and chroma of the juice increased by 4.52% and 36.40%, respectively. The TCD increased to 2.10 after the juice treatment. These changes induced a desirable slightly lighter and more vivid color in juice. Torres et al. (2011) reported that HPP (400–600 MPa, 15 min, 20℃) improved the lightness and redness of the blood orange juice color.[Citation96] While the content of cyanidin-3-glucoside was constant, higher pressure resulted in more AA degradation (5.5% to 8.1%). The content of AA and cyanidin-3-glucoside decreased after 10 days of storage at 20℃. However, the HPP was effective in color preservation as the TCD of the stored control sample was 18.2 which was higher than the treated juice at 600 MPa for 15 min (TCD = 10.7).
A 95% reduction in PME activity was reported when Greek Navel orange juice was heated in a tubular heat exchanger at 80℃ for 1 min; similarly, the processed juice using the HPP system (600 MPa for 4 min) showed PME inactivation by 93%.[Citation91] After 1 month storage at 5℃, the AA retention in HPP and heated juices were 84% and 72%, respectively. So, in comparison to the conventional heating, the HPP was more effective in preserving the AA, which resulted in the lower rates of BI alteration in HPP juice (0.00065–0.0062 1/days) than the thermally processed one (0.00160–0.00876 1/days) during storage at 0 to 15℃. While, there was no difference found between the effects of both applied method in the rate (0.21 1/days) of the BI development during storage at 30℃, it reflected the temperature dependency of the browning reaction. The results of the sensorial evaluations represented the color of the HPP processed juice was the same as the fresh one, showing the advantages of using this novel method in comparison to the thermal treatment. Shiikuwasha (Citrus depressa Hayata) juice was subjected to HPP (600 MPa, 2.5 min, 25℃) and HTST (93℃, 3 min).[Citation97] The BI of the juice increased from 0.1 (fresh juice) to 0.6 and 1.0 after HPP and thermal processing, respectively. The highest BI was observed in stored heated juice (28 days at 7℃). TCD of the control, HPP, and HTST treated juices were 5.23, 5.4, and 4.37, respectively. These color changes were attributed to the higher HMF concentration in HTST treated juice as well as changes in total phenol and flavonoids contents.
HPP of mandarin juice at 150 MPa for 15 s (68℃) induced a 60.77%, 3.92% and 59.75% decrease in PME activity, ascorbic acid content and total carotenoids content, respectively.[Citation65] 2.46% increase in turbidity (transmittance percentage) of the juice was observed during HPP, confirming the effect of particle size reduction on cloudiness. Lightness (36.08), chroma (42.23) and hue index (84.41) of the fresh juice increased respectively to 45.30, 56.40 and 85.47 during HPP, with the total color difference of 16.93. Cheng et al. (2020) found that HPP (600 MPa, 4℃ and 1.5 min) of mandarin juice caused an increase in brightness, deepening the yellowish and fading the redness in different degrees with TCD of 2.26.[Citation66] Researchers reported that these alterations in the juice color were due to the inactivation of endogenous enzymes and HPP promoting the dissolution of chromogenic substances in cells.
The grapefruit juice was pressurized at 150, 200 and 250 MPa, at 40, 50 and 60℃ for 3 min.[Citation67] The content of total carotenoids, total anthocyanins and antioxidant capacity increased during the juice treatment. The maximum improvement in the contents of total carotenoids (36.84%), total anthocyanins (36.18%), and antioxidant capacity (34.57%) were observed during processing at 250 MPa at 60°C for 3 min. These results could be related to the development in the extraction of compounds via the distraction of some cells. In this study, a 67.43% decrease in the ascorbic acid content was reported. PME and PPO activity decreased with an increase in pressure and temperature. These chemical changes caused decrease in the L* (2–3.2%), a* (3.9–68%) and b* (3.2–5.3%) values.
High-pressure carbon dioxide treatment of citrus juices
High-pressure carbon dioxide (HPCD) is a cold pasteurization technology that is useful in destroying the microorganisms and enzymes as a result of CO2 utilization under 50 MPa. Application of CO2, a non-toxic, non-flammable and cheap gas, shows no undesirable effects quality of the food products.[Citation98,Citation99] When the pressure is subjected, carbonic acid is produced by dissolving the CO2 in the juice causing a fall in pH, this pH change is temporal until removal of the pressure.[Citation100]
During HPCD treatment of the Valencia orange juice, the highest PME inactivation (56%) was observed at 72 MPa for 10 min. The cloud value of the juice increased by about 846% when the pressure (38 MPa – 1.18 CO2/juice (w/w) ratio) was subjected. It was found that the gas/juice ratio had more impact on cloud improvement in comparison to the applied pressure. After HPCD treatment (107 MPa & 0.99 gas/juice ratio), lightness and the yellowness values of the juice increased while the redness decreased. The treated juice at 72 MPa and 0.64 CO2/juice (w/w) ratio showed the highest TCD (13.83) followed by 9.43 for the 38 MPa and 0.40 CO2/juice (w/w) ratio.[Citation101]
Sonication of citrus juices
Ultrasound is categorized as vibrational energy which produces the sound energy from electrical or mechanical energy. In juice processing, a low frequency-high power ultrasound (20–100 kHz, 10–1000 W/cm[Citation2]) is recently used to inactivate the enzymes and microorganisms via the physical and chemical effects such as mechanical cavitation and free radicals formation.[Citation102] During ultrasonication of the juice, cavitation bubbles are created as a result of alteration in pressure. The bubbles collapse in the consequence of compression cycles of the sonic waves.[Citation33] To increase its efficiency, this method is applied separately or in combination with thermal treatment, HPP and also both of them called thermosonication, manosonication and manothermosonication, respectively. This method is beneficial to save processing time and input energy.
Different levels of ultrasound intensity ranging from 8.61 to 22.79 W/cm[Citation2] were applied to process the Valencia orange juice for 2 to 10 min.[Citation33] It was found that this method had a significant effect on cloud value as during the lowest intensity level (8.61 W/cm2 for 2 min) 222% rise in this value was observed which was related to the breakage of the linear structure of the pectin and reduction in its molecular weight during ultrasonication. The color of the orange juice became lighter after treatment for 2 min at all intensity levels (62.03 to 63.54) in comparison to the fresh sample (59.7). However, a longer processing time reduced the L* value ranging from 60.05 to 61.46, which was related to the partial precipitation of unstable suspended particles and the occurrence of oxidation reactions. The a* and b* values of the fresh juice were about 7.4 and 56.3, respectively. Unlike the b* value, a* value reduced as higher intensities were applied for longer periods. Because of the destruction of carotenoids, BI increased as a more intense process was used, for example, 261% increase in BI was reported during treatment at 8.61 W/cm2 for 2 min. Degradation of these pigments can occur due to enhanced oxidation reactions by the interaction with formed free radicals such as hydroxyl radicals. Besides, during cavitation contact between dissolved gasses like water vapor and O2 may be increased by the substrate. TCD of the processed juice at 17.17 and 22.79 for 10 min were higher than 3 representing the distinct alteration in the juice color. Tiwari et al. (2009) observed an increase in color aspects as well as the cloud value of the orange juice after using different amplitudes of the system (40–100%) under the same condition (2–10 min, 20 kHz, 1500 W).[Citation103] The AA content (45.45 to 34.38 mg/100 ml) and cloud value (0.839 to 0.459) showed a decrease during storage. While AA content of the fresh juice was 45.77 mg/100 ml; higher retention in the vitamin content (74.5%) was achieved after treating at 100% amplitude for 10 min. BI of the juice increased from 0.138 to 0.236 during 30 days of storage. L*, a* and b* values of the fresh juice were 58.70, 11.86 & 60.53, respectively. After juice processing and 30 days storage (10℃) L*, a* and b* values raised respectively up to 60.83, 11.42 & 61.75 and 61.73, 12.88 and 66.01. These caused TCD to change between 2.35 to 6.23 during 0 to 30 days of storage.
Aadil et al. (2013) reported that the cloud value of the fresh grapefruit juice was 0.42.[Citation104] Application of ultrasound (28 kHz, 600 W) for 30, 60 & 90 min enhanced the cloud value of grapefruit juice up to 1.04, 1.09 & 1.11 respectively; which resulted from reducing the size of suspended molecules by cavitation effects and also homogenization of the juice. The AA content of the fresh juice (27.83 mg/100 ml) increased after juice processing for 90 min (35.75 mg/100 ml) due to cavitation impacted on the removal of the entrapped oxygen. Breakage of the structure of the pigments caused a raise in BI from 0.221 to 0.315 in fresh and ultrasound treated juice for 90 min. L*, a* and b* values of the fresh juice were 7.58, 5.50 & −11.84 respectively. Treating the juice for 90 min reduced all of the color values (L* = 6.95, a* = 4.56 and b* = −12.11) which were not recognizable to the naked eyes. During the processing of mandarin juice (50℃, 750 W and 36 min), an increase in lightness (from 43.88 to 45.80), a decrease in a* value (from 9.95 to 7.17) and an increase in b* value (from 28.01 to 31.86) were observed. The TCD of the processed juice was 2.60.[Citation66] The researcher attributed these color alteration to the inactivation of polyphenol oxidase, removal of dissolved oxygen, enzymatic browning inhibition, destruction of cell wall and release of natural pigment compounds to some extent.
Thermosonication processing (35 kHz, 750 W, 50 and 60℃, 5 and 10 min) of tangerine juice was compared with heating treatment at 85℃ for 5 min in a water bath as a control.[Citation105] Regardless of the temperature and time, the thermosonicated samples showed higher total polyphenols content (11 to 13%) when compared to the control (31.24 mg/100 ml). This could be related to the release of polyphenols from intracellular structures and disruption of the binding with the polysaccharides and cell wall proteins during the cavitation phenomenon. Carotenoids are important pigments in the coloring of the tangerine juice. The carotenoids contents of all samples were statistically the same (1.04 to 1.33 mg -carotene/kg), except the processed juice at 50℃ for 5 min (0.92 mg
-carotene/kg). Thermosonication treatment showed effectiveness in ascorbic acid preservation (21.6 to 24.4 mg/100 ml) than the control (19.4 mg/100 ml). The thermosonication increased the cloud value. Thermosonication application at 60℃ for 10 min (35.94) and 50℃ for 5 min (35.38) led to a higher L* value in comparison to other processing conditions. Juice treatment had no effect on chroma. The thermosonicated samples (0.36 to 0.95) showed lower hue index when compared to the control (1.32), reflecting the presence of more reddish pigments in the tangerine juice.
Ultraviolet application on citrus juices
Ultraviolet radiation (UV) classifies in the electromagnetic spectrum ranging from 100 to 400 nm. which can be divided into UV-A (320–400 nm), UV-B (280–320 nm) and UV-C (200 to 280 nm). UV-C with 254 nm is suggested to be applied in water and juice treatment.[Citation106,Citation107] Application of UV-C in food processing shows the potential to destroy different microorganisms a result of the light-absorbing effect by the structure of microbial DNA or RNA. The use of UV-C showed no adverse effects such as toxicity or production of toxic by-products, off taste and odor. While UV treatment does result in low energy consumption in comparison with thermal pasteurization.[Citation106,Citation108]
It was also found that this method was disabled in PME inactivation to preserve the cloud stability of the juice in addition to showing no adverse effect on the juice color.[Citation106] The turbidity of the fresh lime juice (12567 NTU) reduced by 50% after exposure of the UV-C lamp at 44.24 mJ/cm2.[Citation109] Researchers explained this result by inactivation of the yeasts and molds besides the less sedimentation of the fragmented pectin. Higher intensity caused more changes in the color properties of the juice. After processing at 44.24 mJ/cm2, the lightness (61.47), b* value (8.54) and chroma (9.09) of the fresh juice reduced to 55.28, 1.64 and 3.03, respectively. The a* value and hue angle of fresh juice were −3.12 and 110.04, respectively. Applying the more intense treatment (227.6–442.4 J/m2) increased a* value (−2.98 to −2.55), hue angle (113.58 to 147.28) and TCD (1.78 to 9.30). In another study, grapefruit juice was treated using a batch UV-C system (0–3.94 J/cm[Citation2]).[Citation108] After treatment at 1.83, 2.84 and 3.94 J/cm2, AA content reduced respectively up to 17%, 29% and 35% which can be explained by metal-catalyzed oxidation mechanism due to the presence of the oxygen in the headspace of the package. The contents of total phenol and naringin remained constant after UV-C treatment. The TCD of the processed juice at 1.83, 2.84 and 3.94 J/cm2 were 0.7, 1.7 and 1.1, respectively. However, no development in the browning reaction was detected. The obtained result reflects the efficiency of this method in preserving the nutritional values and juice quality.
Gayán et al. (2012) combined the pre-heating (55℃) with UV-C (27.1 J/ml) to process Navel orange juice.[Citation110] It was found that the combined method was more effective in PME inactivation (63.96%) than only heating treatment (47.84%). The AA content of the fresh juice (52.45 mg/100 ml) decreased 16.47% after heating-UV treatment while heating showed no effect on vitamin degradation. This unwanted effect is related to the ability of the UV-C in producing radicals through different phytochemical reactions. In comparison to the TCD of the heated sample (0.23), the combined method was more effective in preserving the color of the juice as the fresh one.
Conclusion
The color of the citrus juice is known as a key quality attribute affecting the consumer’s acceptance and a quality indicator for the process. Therefore, qualitative and quantitative analysis of color is suggested to control the effect of applied pasteurization methods on juice quality. In conventional thermal treatment, holding time and CUT are important in the degradation of heat-sensitive compounds (a.o. pigments, vitamins, enzymes) as well as the inactivation of microorganisms. As alteration in the content of these compounds resulted in color changes, reducing the CUT is an effective way to preserve the juice quality. Novel juice thermal methods such as microwave treatment, ohmic heating and infrared irradiation are introduced to shorten the come up time depending on the applied process conditions. Other innovative non-thermal treatments (PEF, HPP, HPCD & UV-C) are more operative in preserving the nutritional value of the juice. However, these methods showed less effectiveness in the inactivation of the PME which has a key role in the cloud stability of the citrus juices. Based on the hurdle concept, a combination of mild thermal and non-thermal methods can be therefore suggested to increase the effectiveness of the process as well as preserving the juice quality.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Jaiswal, A. K.; Abu-Ghannam, N. Degradation Kinetic Modelling of Color, Texture, Polyphenols and Antioxidant Capacity of York Cabbage after Microwave Processing. F. Re. Int. 2013, 53(1), 125–133.
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant Activity of Citrus Fruits. Food Chem. 2016, 196, 885–896.
- Rampersaud, G. C.; Valim, M. F. 100% Citrus Juice: Nutritional Contribution, Dietary Benefits, and Association with Anthropometric Measures. Crit. Rev. Food Sci. Nutr. 2017, 57(1), 129–140.
- Penniston, K. L.; Nakada, S. Y.; Holmes, R. P.; Assimos, D. G. Quantitative Assessment of Citric Acid in Lemon Juice, Lime Juice, and Commercially-available Fruit Juice Products. J. Endourol. 2008, 22(3), 567–570.
- Violeta, N.; Trandafir, I.; Ionica, M. E. HPLC Organic Acid Analysis in Different Citrus Juices under Reversed Phase Conditions. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2010, 38(1), 44–48.
- Heyman, M. B.; Abrams, S. A. Fruit Juice in Infants, Children, and Adolescents: Current Recommendations. Pediatrics. 2017, 139(6), e20170967.
- Zhou, Z. Citrus Fruits Nutrition; Science Press: Beijing, China, 2012.
- Stahl, W.; Sies, H. Antioxidant Activity of Carotenoids. Mol Aspects Med. 2003, 24(6), 345–351.
- Xu, G.; Liu, D.; Chen, J.; Ye, X.; Ma, Y.; Shi, J. Juice Components and Antioxidant Capacity of Citrus Varieties Cultivated in China. Food Chem. 2008, 106(2), 545–551.
- Sandoval, J. R. M.; Rosas, M. E. M.; Sandoval, E. M.; Velasco, M. M. M.; De Ávila, H. C. . Colorimetry Image Process. Travieso-Gonzalez, C. M. (Rijeka, Croatia: InTech Publishing) 2018 Color Analysis and Image Processing Applied in Agriculture , 61.
- Cortés, E. M.; Frígola, A., J. Color of Orange Juice Treated by High Intensity Pulsed Electric Fields during Refrigerated Storage and Comparison with Pasteurized Juice. Food Control. 2008, 19(2), 151–158.
- Fernandez-Vazquez; Stinco; Hernanz Vila; Heredia,; Chaya; Vicario. Internal Preference Mapping of Milk–fruit Beverages: Influence of Color and Appearance on Its Acceptability. Food Sci. Nutr. 2018, 6(1), 27–35.
- Spence, C.; Piqueras-Fiszman, B. Food Color and Its Impact on Taste/flavor Perception. In Multisensory Flavor Perception. Woodhead Publishing, 2016,107–132.
- Garber, J. L.; Hyatt, L.; Nafees, L., E. M. The Effects of Food Color on Perceived Flavor: A Factorial Investigation in India. J. Food Prod. Marketing. 2016, 22(8), 930–948.
- Fernandez-Vazquez; Stinco; Melendez-Martinez; Heredia; Vicario. Visual and Instrumental Evaluation of Orange Juice Color: A Consumers’ Preference Study. J. Sen. Stu. 2011, 26(6), 436–444.
- Achir, N.; Dhuique-Mayer, C.; Hadjal, T.; Madani, K.; Pain, J.-P.; Dornier, M. Pasteurization of Citrus Juices with Ohmic Heating to Preserve the Carotenoid Profile. Innovative Food Sci. Emerg. Technol. 2016, 33, 397–404.
- Min, S.; Jin, Z.; Min, S.; Yeom, H.; Zhang, Q. Commercial‐Scale Pulsed Electric Field Processing of Orange Juice. J. Food Sci. 2003, 68(4), 1265–1271.
- Leizerson, S.; Shimoni, E. Stability and Sensory Shelf Life of Orange Juice Pasteurized by Continuous Ohmic Heating. J. Agric. Food Chem. 2005, 53(10), 4012–4018.
- Aghajanzadeh, S.; Kashaninejad, M.; Ziaiifar, A. M. Cloud Stability of Sour Orange Juice as Affected by Pectin Methylesterase during Come up Time: Approached through Fractal Dimension. Int. J. Food Prop. 2017, 20(sup3), S2508–S2519.
- Melendez-Martinez; Vicario; Heredia. Instrumental Measurement of Orange Juice Colour: A Review. J. Sci. Food Agric. 2005, 85(6), 894–901.
- Yam, K. L.; Papadakis, S. E. A Simple Digital Imaging Method for Measuring and Analyzing Color of Food Surfaces. J. Food Eng. 2004, 61(1), 137–142.
- Meléndez‐Martínez, A. J.; Vicario, I. M.; Heredia, F. J. Instrumental Measurement of Orange Juice Colour: A Review. J. Sci. Food Agric. 2005, 85(6), 894–901.
- Giusti, M. M.; Wrolstad, R. E.; Smith, D. E. CIE Color Specifications Calculated from Reflectance or Transmittance Spectra. In Food Analysis Laboratory Manual. Springer, Cham, 2017, 219–224.
- Fernández‐Vázquez, R.; Stinco, C. M.; Hernanz Vila, D.; Heredia, F. J.; Chaya, C.; Vicario, I. M. Internal Preference Mapping of Milk–fruit Beverages: Influence of Color and Appearance on Its Acceptability. Food Sci. Nutr. 2018, 6(1), 27–35.
- Stinco Scanarotti, C. M.; Fernández Vázquez, R.; Heredia Mira, F. J.; Meléndez Martínez, A. J.; Vicario Romero, I. Spectroradiometry Vs. Digital Image Analysis in Colour Measurement in Juices from Different Orange and Mandarin Varieties. Óptica pura y aplicada. 2014, 47(2), 139–144.
- Melendez-Martinez, A. J.; Vicario, I. M.; Heredia, F. J. Influence of White Reference Measurement and Background on the Color Specification of Orange Juices by Means of Diffuse Reflectance Spectrophotometry. J.-Aoac Int. 2006, 89(2), 452.
- Mapelli-Brahm, P.; Rodríguez-Pulido, F. J.; Stinco, C. M.; Heredia, F. J.; Meléndez-Martínez, A. J. Applications of Visible Spectroscopy and Color Measurements in the Assessments of Carotenoid Levels in Foods. In Plant and Food Carotenoids. Humana, New York, NY, 2020, 103–116.
- García-Marino, M.; Escudero-Gilete, M. L.; Heredia, F. J.; Escribano-Bailón, M. T.; Rivas-Gonzalo, J. C. Color-copigmentation Study by Tristimulus Colorimetry (CIELAB) in Red Wines Obtained from Tempranillo and Graciano Varieties. F. Re. In.t. 2013, 51(1), 123–131.
- Esparza, I.; Santamaría, C.; Calvo, I.; Fernández, J. M. Significance of CIELAB Parameters in the Routine Analysis of Red Wines Relevancia de Los Parámetros CIELAB En El Análisis de Rutina de Vinos Tintos. CyTA–J. Food. 2009, 7(3), 189–199.
- Mapelli-Brahm, P.; Stinco, C. M.; Rodrigo, M. J.; Zacarías, L.; Meléndez-Martínez, A. J. Impact of Thermal Treatments on the Bioaccessibility of Phytoene and Phytofluene in Relation to Changes in the Microstructure and Size of Orange Juice Particles. J. Funct. Foods. 2018, 46, 38–47.
- Lazaro, A.; Boada, M.; Villarino, R.; Girbau, D. Color Measurement and Analysis of Fruit with a Battery-Less NFC Sensor. Sensors. 2019, 19(7), 1741.
- Wrolstad, R. E.; Durst, R. W.; Lee, J. Tracking Color and Pigment Changes in Anthocyanin Products. Trends Food Sci. Technol. 2005, 16(9), 423–428.
- Tiwari,; Muthukumarappan, K.; O’Donnell, C.; Cullen, P. Effects of Sonication on the Kinetics of Orange Juice Quality Parameters. J. Agric. Food Chem. 2008, 56(7), 2423–2428.
- Francis, F. J.; Clydesdale, F. M. Food Colorimetry: Theory and Applications; Westport: AVI Publishing Co. Inc, 1975.
- Roig, M.; Bello, J.; Rivera, Z.; Kennedy, J. Studies on the Occurrence of Non-enzymatic Browning during Storage of Citrus Juice. F. Re. Int. 1999, 32(9), 609–619.
- Koca, N.; Burdurlu, H. S.; Karadeniz, F. Kinetics of Nonenzymatic Browning Reaction in Citrus Juice Concentrates during Storage. Turk. J. Agric. For. 2004, 27(6), 353–360.
- Cohen, E.; Birk, Y.; Mannheim, C.; Saguy, I. A Rapid Method to Monitor Quality of Apple Juice during Thermal Processing. LWT Food Sci. Technol. 1998, 31(7), 612–616.
- Lunadei, L. Galleguillos, P.; Diezma Iglesias, B.; Lleó García, L. Evaluation of Enzymatic Browning in Fresh-cut Apple Slices Applying a Multispectral Vision System. 2010.
- Zhang, H.; Xi, W.; Yang, Y.; Zhou, X.; Liu, X.; Yin, S.; Zhang, J.; Zhou, Z. An On-line HPLC-FRSD System for Rapid Evaluation of the Total Antioxidant Capacity of Citrus Fruits. Food Chem. 2015, 172, 622–629.
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E. N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and Human Diseases. Clin. Chim. Acta. 2014, 436, 332–347.
- Nakao, K.; Murata, K.; Itoh, K.; Hanamoto, Y.; Masuda, M.; Moriyama, K.; Shintani, T.; Matsuda, H. Anti-hyperuricemia Effects of Extracts of Immature Citrus Unshiu Fruit. J. Traditional Med. 2011, 28(1), 10–15.
- Rekha, C.; Poornima, G.; Manasa, M.; Abhipsa, V.; Devi, J. P.; Kumar, H. T. V.; Kekuda, T. R. P. Ascorbic Acid, Total Phenol Content and Antioxidant Activity of Fresh Juices of Four Ripe and Unripe Citrus Fruits. Chem. Sci. Trans. 2012, 1(2), 303–310.
- Lee; Coates. Effect of Thermal Pasteurization on Valencia Orange Juice Color and Pigments. LWT Food Sci. Technol. 2003, 36(1), 153–156.
- Stinco, C. M.; Fernández-Vázquez, R.; Heredia, F. J.; Meléndez-Martínez, A. J.; Vicario, I. M. Bioaccessibility, Antioxidant Activity and Colour of Carotenoids in Ultrafrozen Orange Juices: Influence of Thawing Conditions. LWT Food Sci. Technol. 2013, 53(2), 458–463.
- Lee; Coates. Thermal Pasteurization Effects on Color of Red Grapefruit Juices. J. Food Sci. 1999, 64(4), 663–666.
- Cao, S.-Q.; Liang, L.; Pan, S.-Y. Thermal Degradation Kinetics of Anthocyanins and Visual Color of Blood Orange Juice. Agric. Sci. China. 2011, 10(12), 1992–1997.
- Lee; Kader. Preharvest and Postharvest Factors Influencing Vitamin C Content of Horticultural Crops. Postharvest. Biol. Technol. 2000, 20(3), 207–220.
- Kaur; Aggarwal. Effect of Chemical Preservation over Thermal Processing on Storage Stability of Tomato Juice. Asian J. Dairy Food Res. 2015, 34(1), 49–53.
- Burdurlu, H. S.; Koca, N.; Karadeniz, F. Degradation of Vitamin C in Citrus Juice Concentrates during Storage. J. Food Eng. 2006, 74(2), 211–216.
- Graumlich, T. R.; Marcy, J. E.; Adams, J. Aseptically Packaged Orange Juice and Concentrate: A Review of the Influence of Processing and Packaging Conditions on Quality. J. Agric. Food Chem. 1986, 34(3), 402–405.
- Kurata, T.; Sakurai, Y. Degradation of L-ascorbic Acid and Mechanism of Nonenzymic Browning Reaction: Part II. Non-oxidative Degradation of L-ascorbic Acid Including the Formation of 3-deoxy-L-pentosone Part III. Oxidative Degradation of L-ascorbic Acid (Degradation of dehydro-L-ascorbic Acid). Agric Biol Chem. 1967, 31(2), 170–184.
- Aghajanzadeh, S.; Kashaninejad, M.; Ziaiifar, A. M. Effect of Infrared Heating on Degradation Kinetics of Key Lime Juice Physicochemical Properties. Innovative Food Sci. Emerg. Technol. 2016, 38, 139–148.
- Turtoi, M. Inactivation of Saccharomyces Cerevisiae Using New Non-thermal Technologies. A Review. Rom. Biotechnol. Lett. 2014, 19(1), 8901.
- McGlynn, W. G. The Importance of Food pH in Commercial Canning Operations. Oklahoma Cooperative Extension Service, Division of Agricultural Sciences and Natural Resources; Stillwater, Oklahoma: Oklahoma State University, 2003.
- FDA. Hazard Analysis and Critical Point (HACCP); Procedures for the Safe and Sanitary Processing and Importing of Juice; Final Rule. Fed. Regist. 2001, 66, 6137–6202.
- Ashurst, P. The Stability and Shelf Life of Fruit Juices and Soft Drinks. In The Stability and Shelf Life of Food; Woodhead Publishing, 2016,347–374.
- Rupasinghe, H. V.; Yu, L. J. . Food additive El-Samragy, Y. (Rijeka, Croatia: InTech Publishing) Emerging preservation methods for fruit juices and beverages , 2012, 65–82.
- Wang, Y.; Li, W.; Ma, Y.; Zhao, X.; Zhang, C. Effect of Thermal Treatments on Quality and Aroma of Watermelon Juice. J. Food Qual. 2018,1, 1–7 doi:10.1155/2018/9242675.
- Aghajanzadeh, S.; Ziaiifar, A. M.; Kashaninejad, M.; Maghsoudlou, Y.; Esmailzadeh, E. Thermal Inactivation Kinetic of Pectin Methylesterase and Cloud Stability in Sour Orange Juice. J. Food Eng. 2016, 185, 72–77.
- Tajchakavit, S.; Ramaswamy, H. Thermalvs. Microwave Inactivation Kinetics of Pectin Methylesterase in Orange Juice under Batch Mode Heating Conditions. LWT Food Sci. Technol. 1997, 30(1), 85–93.
- Demirdöven, A.; Baysal, T. Optimization of Ohmic Heating Applications for Pectin Methylesterase Inactivation in Orange Juice. J. Food Sci. Technol. 2014, 51(9), 1817–1826.
- Ramaswamy, H. S.; Marcotte, M. Food Processing: Principles and Applications; New York: CRC Press, 2005.
- Leizerson, S.; Shimoni, E. Effect of Ultrahigh-temperature Continuous Ohmic Heating Treatment on Fresh Orange Juice. J. Agric. Food Chem. 2005, 53(9), 3519–3524.
- Vikram, V.; Ramesh, M.; Prapulla, S. Thermal Degradation Kinetics of Nutrients in Orange Juice Heated by Electromagnetic and Conventional Methods. J. Food Eng. 2005, 69(1), 31–40.
- Sentandreu, E.; Stinco, C. M.; Vicario, I. M.; Mapelli-Brahm, P.; Navarro, J. L.; Meléndez-Martínez, A. J. High-pressure Homogenization as Compared to Pasteurization as a Sustainable Approach to Obtain Mandarin Juices with Improved Bioaccessibility of Carotenoids and Flavonoids. J. Cleaner Prod. 2020, 262, 121325.
- Cheng, C.-X.; Jia, M.; Gui, Y.; Ma, Y. Comparison of the Effects of Novel Processing Technologies and Conventional Thermal Pasteurisation on the Nutritional Quality and Aroma of Mandarin (Citrus Unshiu) Juice. Innovative Food Sci. Emerg. Technol. 2020, 64, 102425.
- Aadil, R. M.; Zeng, X.-A.; Jabbar, S.; Nazir, A.; Mann, A. A.; Khan, M. K. I.; Ramzan, A. Quality Evaluation of Grapefruit Juice by Thermal and High Pressure Processing Treatment. Pak. J. Agric. Res. 2017, 30, 3.
- Mondello, L.; Cotroneo, A.; Errante, G.; Dugo, G.; Dugo, P. Determination of Anthocyanins in Blood Orange Juices by HPLC Analysis. J. Pharm. Biomed. Anal. 2000, 23(1), 191–195.
- Lee, H. S. Characterization of Major Anthocyanins and the Color of Red-fleshed Budd Blood Orange (Citrus Sinensis). J. Agric. Food Chem. 2002, 50(5), 1243–1246.
- Arena, E.; Fallico, B.; Maccarone, E. Influence of Carotenoids and Pulps on the Color Modification of Blood Orange Juice. J. Food Sci.Chicago. 2000, 65(3), 458–460.
- Benlloch-Tinoco, M.; Igual, M.; Rodrigo, D.; Martínez-Navarrete, N. Comparison of Microwaves and Conventional Thermal Treatment on Enzymes Activity and Antioxidant Capacity of Kiwifruit Puree. Innovative Food Sci. Emerg. Technol. 2013, 19, 166–172.
- Demirdöven, A.; Baysal, T. Inactivation Effect of Microwave Heating on Pectin Methylesterase in Orange Juice. Ukr. Food J. 2016, 5(2), 248–411.
- Ahmed, J.; Ramaswamy, H. S.; Kasapis, S.; Boye, J. I. Novel Food Processing: Effects on Rheological and Functional Properties; New York: CRC Press, 2009.
- Cinquanta, L.; Albanese, D.; Cuccurullo, G.; Di Matteo, M. Effect on Orange Juice of Batch Pasteurization in an Improved Pilot‐Scale Microwave Oven. J. Food Sci. 2010, 75(1), E46–E50.
- Aghajanzadeh, S.; Ziaiifar, A. M. A Review of Pectin Methylesterase Inactivation in Citrus Juice during Pasteurization. Trends Food Sci. Technol. 2018, 71, 1–12.
- Kaur, N.; Singh, A. Ohmic Heating: Concept and Applications-A Review. Crit. Rev. Food Sci. Nutr. 2016, 56(14), 2338–2351.
- Evrendilek, G. A.; Baysal, T.; Icier, F.; Yildiz, H.; Demirdoven, A.; Bozkurt, H. Processing of Fruits and Fruit Juices by Novel Electrotechnologies. Food Eng. Rev. 2012, 4(1), 68–87.
- Lee; Kim; Kang. Effect of pH for Inactivation of Escherichia Coli O157: H7, Salmonella Typhimurium and Listeria Monocytogenes in Orange Juice by Ohmic Heating. LWT Food Sci. Technol. 2015, 62(1), 83–88.
- Cullen, P. J.; Tiwari, B. K.; Valdramidis, V. P. Novel Thermal and Non-thermal Technologies for Fluid Foods; New York: Academic Press is an imprint of Elsevier, 2011.
- Krishnamurthy, K.; Khurana, H. K.; Soojin, J.; Irudayaraj, J.; Demirci, A. Infrared Heating in Food Processing: An Overview. Compr. Rev. Food Sci. Food Saf. 2008, 7(1), 2–13.
- Rastogi, N. K. Recent Trends and Developments in Infrared Heating in Food Processing. Crit. Rev. Food Sci. Nutr. 2012, 52(9), 737–760.
- Buckow, R.; Baumann, P.; Schroeder, S.; Knoerzer, K. Effect of Dimensions and Geometry of Co-field and Co-linear Pulsed Electric Field Treatment Chambers on Electric Field Strength and Energy Utilisation. J. Food Eng. 2011, 105(3), 545–556.
- Cortés; Esteve; Rodrigo; Torregrosa, F.; Frigola. Changes of Colour and Carotenoids Contents during High Intensity Pulsed Electric Field Treatment in Orange Juices. Food Chem. Toxicol. 2006, 44(11), 1932–1939.
- Rivas, A.; Rodrigo, D.; Martínez, A.; Barbosa-Cánovas, G.; Rodrigo, M. Effect of PEF and Heat Pasteurization on the Physical–chemical Characteristics of Blended Orange and Carrot Juice. LWT Food Sci. Technol. 2006, 39(10), 1163–1170.
- Cserhalmi, Z.; Sass-Kiss, A.; Tóth-Markus, M.; Lechner, N. Study of Pulsed Electric Field Treated Citrus Juices. Innovative Food Sci. Emerg. Technol. 2006, 7(1–2), 49–54.
- Yeom, H. W.; Streaker, C. B.; Zhang, Q. H.; Min, D. B. Effects of Pulsed Electric Fields on the Quality of Orange Juice and Comparison with Heat Pasteurization. J. Agric. Food Chem. 2000, 48(10), 4597–4605.
- Lee, S. J.; Bang, I. H.; Choi, H.-J.; Min, S. C. Pasteurization of Mixed Mandarin and Hallabong Tangor Juice Using Pulsed Electric Field Processing Combined with Heat. Food Sci. Biotechnol. 2018, 27(3), 669–675.
- Raso, J.; Calderon, M. L.; Gongora, M.; Barbosa‐Canovas, G. V.; Swanson, B. G. Inactivation of Zygosaccharomyces Bailii in Fruit Juices by Heat, High Hydrostatic Pressure and Pulsed Electric Fields. J. Food Sci. 1998, 63(6), 1042–1044.
- Rastogi, N.; Raghavarao, K.; Balasubramaniam, V.; Niranjan, K.; Knorr, D. Opportunities and Challenges in High Pressure Processing of Foods. Crit. Rev. Food Sci. Nutr. 2007, 47(1), 69–112.
- Chakraborty, S.; Kaushik, N.; Rao, P. S.; Mishra, H. High‐pressure Inactivation of Enzymes: A Review on Its Recent Applications on Fruit Purees and Juices. Compr. Rev. Food Sci. Food Saf. 2014, 13(4), 578–596.
- Polydera, A.; Galanou, E.; Stoforos, N.; Taoukis, P. Inactivation Kinetics of Pectin Methylesterase of Greek Navel Orange Juice as a Function of High Hydrostatic Pressure and Temperature Process Conditions. J. Food Eng. 2004, 62(3), 291–298.
- Nienaber, U.; Shellhammer, T. High‐pressure Processing of Orange Juice: Combination Treatments and a Shelf Life Study. J. Food Sci. 2001, 66(2), 332–336.
- Lacroix, N.; Fliss, I.; Makhlouf, J. Inactivation of Pectin Methylesterase and Stabilization of Opalescence in Orange Juice by Dynamic High Pressure. F. Re. Int. 2005, 38(5), 569–576.
- Bull, M. K.; Zerdin, K.; Howe, E.; Goicoechea, D.; Paramanandhan, P.; Stockman, R.; Sellahewa, J.; Szabo, E. A.; Johnson, R. L.; Stewart, C. M. The Effect of High Pressure Processing on the Microbial, Physical and Chemical Properties of Valencia and Navel Orange Juice. Innovative Food Sci. Emerg. Technol. 2004, 5(2), 135–149.
- Almeida, F. D. L.; Gomes, W. F.; Cavalcante, R. S.; Tiwari, B. K.; Cullen, P. J.; Frias, J. M.; Bourke, P.; Fernandes, F. A.; Rodrigues, S. Fructooligosaccharides Integrity after Atmospheric Cold Plasma and High-pressure Processing of a Functional Orange Juice. F. Re. Int. 2017, 102, 282–290.
- Torres, B.; Tiwari, B.; Patras, A.; Cullen, P.; Brunton, N.; O’Donnell, C. Stability of Anthocyanins and Ascorbic Acid of High Pressure Processed Blood Orange Juice during Storage. Innovative Food Sci. Emerg. Technol. 2011, 12(2), 93–97.
- Lai, -Y.-Y.; Chen, J.-H.; Liu, Y.-C.; Hsiao, Y.-T.; Wang, C.-Y. Evaluation of Microbiological Safety, Physicochemical and Aromatic Qualities of Shiikuwasha (Citrus Depressa Hayata) Juice after High Pressure Processing. J. Food Sci. Technol. 2021,58, 1–11.
- Truong, T.; Boff, J.; Min, D.; Shellhammer, T. Effects of Carbon Dioxide in High‐Pressure Processing on Pectinmethylesterase in Single‐strength Orange Juice. J. Food Sci. 2002, 67(8), 3058–3062.
- Damar, S.; Balaban, M. O. Review of Dense Phase CO2 Technology: Microbial and Enzyme Inactivation, and Effects on Food Quality. J. Food Sci. 2006, 71, 1.
- Bertoloni, G.; Bertucco, A.; De Cian, V.; Parton, T. A Study on the Inactivation of Micro‐organisms and Enzymes by High Pressure CO2. Biotechnol. Bioeng. 2006, 95(1), 155–160.
- Kincal, D.; Hill, W.; Balaban, M.; Portier, K.; Sims, C.; Wei, C.; Marshall, M. A Continuous High‐pressure Carbon Dioxide System for Cloud and Quality Retention in Orange Juice. J. Food Sci. 2006, 71, 6.
- O’donnell, C.; Tiwari, B.; Bourke, P.; Cullen, P. Effect of Ultrasonic Processing on Food Enzymes of Industrial Importance. Trends Food Sci. Technol. 2010, 21(7), 358–367.
- Tiwari, O. C.; Muthukumarappan, P.; Cullen, P. J., K. Effect of Sonication on Orange Juice Quality Parameters during Storage. Int. J. Food Sci. Technol. 2009, 44(3), 586–595.
- Aadil, R. M.; Zeng, X.-A.; Han, Z.; Sun, D.-W. Effects of Ultrasound Treatments on Quality of Grapefruit Juice. Food Chem. 2013, 141(3), 3201–3206.
- Alves, L. D. L.; Dos Santos, R. L.; Bayer, B. L.; Devens, A. L. M.; Cichoski, A. J.; Mendonça, C. R. B. Thermosonication of Tangerine Juice: Effects on Quality Characteristics, Bioactive Compounds, and Antioxidant Activity. J. Food Process. Preserv. 2020, 44(12), e14914.
- Keyser, M.; Műller, I. A.; Cilliers, F. P.; Nel, W.; Gouws, P. A. Ultraviolet Radiation as a Non-thermal Treatment for the Inactivation of Microorganisms in Fruit Juice. Innovative Food Sci. Emerg. Technol. 2008, 9(3), 348–354.
- Kaya, Z.; Yıldız, S.; Ünlütürk, S. Effect of UV-C Irradiation and Heat Treatment on the Shelf Life Stability of a Lemon–melon Juice Blend: Multivariate Statistical Approach. Innovative Food Sci. Emerg. Technol. 2015, 29, 230–239.
- La Cava, E. L.; Sgroppo, S. C. Evolution during Refrigerated Storage of Bioactive Compounds and Quality Characteristics of Grapefruit [Citrus Paradisi (Macf.)] Juice Treated with UV-C Light. LWT Food Sci. Technol. 2015, 63(2), 1325–1333.
- Mohd-Hanif, H.; Shamsudin, R.; Adzahan, N. M. UVC Dosage Effects on the Physico-chemical Properties of Lime (Citrus Aurantifolia) Juice. Food Sci. Biotechnol. 2016, 25(1), 63–67.
- Gayán, E.; Serrano, M.; Monfort, S.; Álvarez, I.; Condón, S. Combining Ultraviolet Light and Mild Temperatures for the Inactivation of Escherichia Coli in Orange Juice. J. Food Eng. 2012, 113(4), 598–605.