ABSTRACT
Curcumin (diferuloylmethane) is a natural polyphenolic compound that targets multiple signaling molecules and helps in supporting health benefits as it aids in the management of oxidative and inflammatory conditions. However, curcumin by itself has poor absorption, rapid metabolism, and fast elimination, which makes its bioavailability very low. Therefore, curcumin is more active when combined with piperine to provide major health benefits. Several studies indicated that curcumin has powerful effects on post-surgical outcomes such as in ischemia-related surgeries, it improves postoperative pain and fatigue following laparoscopic cholecystectomy. Also, it is effective against traumatic brain injury outcomes through several molecular signaling pathways that include oxidative stress, inflammation, apoptosis, and autophagy. Finally, it has topical applications that improve granulation tissue formation, deposition of collagen, wound contraction, and remodeling of the tissue. In conclusion, curcumin has been shown to have strong antioxidant and anti-inflammatory effects that could result in improved post-surgical outcomes.
Introduction
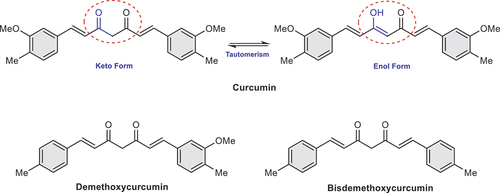
Turmeric is a spice that has been traditionally used in Asian countries for culinary purposes as well as for its beneficial health effect. [Citation1] Turmeric, also called Curcuma longa, is a rhizomatous herbaceous perennial plant of the ginger family and is a major source of curcumin.[Citation2] The chemical structure of curcumin contains two feruloyl groups connected by a methylene carbon and with two keto-enol tautomeric forms as shown in .[Citation3] It is a natural polyphenol derivative that showed to target multiple signaling molecules at the cellular level and help in supporting health benefits by acting as a potent anti-inflammatory and antioxidant compound.[Citation4] It has been shown to be beneficial in the treatment of inflammatory conditions,[Citation5] metabolic syndrome,[Citation6] wounds,[Citation7] post-surgical inflammation and outcomes.[Citation8,Citation9] Turmeric contains three curcuminoids: curcumin, bisdemethoxycurcumin, and demethoxycurcumin (), which are evaluated by the US Food and Drug administration (FDA) and got approved to be “generally recognized as safe” (GRAS).[Citation4] Clinical trials evaluated their safety and tolerability even at doses ranging from 400 to 800 mg/day, and showed to have good tolerability and safety profiles.[Citation10]
In a review about curcumin and its effects on human health, it has been mentioned that curcumin is a polyphenol that is integrated into multiple activities at both molecular and cellular levels, which in turn supports many health benefits.[Citation1] However, a major problem with the use of curcumin is its bioavailability, as it has been proved that ingesting curcumin by itself has very low bioavailability due to ineffective absorption, and fast metabolism and excretion.[Citation1] This issue can be solved by combining curcumin with piperine (a key active ingredient in black pepper) to create a curcumin complex that is readily absorbed and metabolized in the body.[Citation1] Curcumin is widely known and has multiple worldwide applications. In India, it has been used in curries; in China, it is used as a colorant; in Japan, people serve it in tea, while others in Thailand, use it in cosmetics; in the United States, curcumin is used in cheese, mustard sauce, butter, food preservation, and as a coloring agent.[Citation1] Interestingly, in Pakistan, curcumin has been highly recognized there for its beneficial effects and is used for its anti-inflammatory properties.[Citation4]
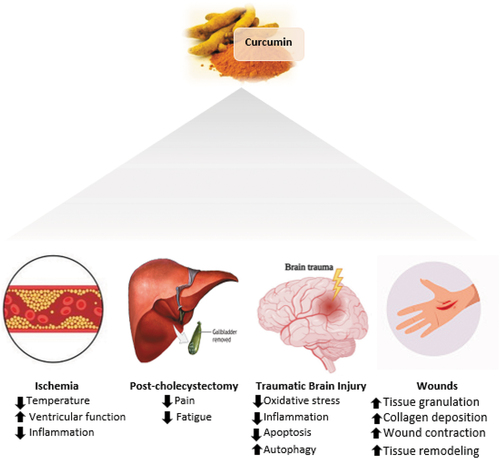
Several studies mentioned the potential antioxidant and anti-inflammatory power of curcumin, which can justify its therapeutic effects in different post-surgical procedures.[Citation9,Citation11–13] The aim of this review is to study the effect of curcumin as indicated in the literature on surgical-related outcomes. This review studies the effect of curcumin on ischemia, traumatic brain injury, cholecystostomy, and wound healing.
Curcumin and ischemia
Curcumin has powerful anti-inflammatory and antioxidant properties, yet only limited studies have aimed to assess the effect of curcumin supplementation on ischemia[Citation14,Citation22,Citation23] as shown in . In a study done on rats in favor of identification of the effect of curcumin treatment on protein expression in focal cerebral ischemia, they suggest that curcumin exerts a neuroprotective effect, as it has been shown in focal cerebral ischemia, curcumin regulates the expression of several proteins that are associated with cellular processes and differentiation.[Citation14] These proteins include ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), isocitrate dehydrogenase (ICDH), adenosyl-homocysteinase, and eukaryotic initiation factor 4A (eIF4A).[Citation14] In which all these proteins were reduced in the vehicle-treated rats, increasing the severity of the injury.[Citation14] However, curcumin treated rats diminished the injury induced by the decrease of these proteins, as curcumin diminishes the decreased expression of UCH-L1, mediates the levels of ICDH, adenosyl-homocysteinase as well as maintain the levels of eIF4A.[Citation14] These proteins are important for the maintenance of physiological cellular function. UCH-L1 is considered a novel biomarker for severe traumatic brain injury,[Citation25] it is required for the regular synaptic and cognitive function [Citation26] as it exerts protection against oxidative stress on neurons. UCH-L1 downregulation can lead to neuronal degeneration [Citation25] and increase the susceptibility of apoptosis.[Citation27]
Table 1. Summary of impact of curcumin on endo/exogenous trauma.
Moreover, in an RCT that was done on children with tetralogy of Fallot to assess the effectiveness of curcumin as an antioxidant on decreasing myocardial ischemia reperfusion injury during the cardiopulmonary bypass through the evaluation of serum malondialdehyde and glutathione concentrations, activity of nuclear factor-kappa B, c-Jun N terminal kinase, caspase-3, and post-operative clinical outcome.[Citation22] In this research, it was found that the intervention group that received curcumin and placebo group had almost similar malondialdehyde and glutathione concentrations.[Citation22] Also, there was no significant difference between both groups in NF-kB.[Citation22] However, c-Jun N terminal kinase significantly decreased in the intervention group from the pre-ischemia to the re-perfusion phase.[Citation22] Moreover, the intervention group has a lower temperature and better ventricular function, but no difference was observed in mechanical ventilation days or length of hospital stay in the two groups.[Citation22] This study has limitations such as malondialdehyde and glutathione levels were only measured after both groups received curcumin or placebo, no measurements were made at the baseline. Also, they tested a single curcumin dose (45 mg/day) for all patients of different body weights and size.[Citation22]
It has been shown that curcuminoids have remarkable antioxidant and anti-inflammatory properties because they inhibit inflammatory mediators such as NF-kB, cyclooxygenase-2, lipoxygenase, and inducible nitric oxide synthase.[Citation28] Curcuminoids as a preventative agent against myocardial infarction (MI) after coronary artery bypass grafting (CABG) were evaluated in a randomized controlled trial.[Citation23] In this study, the incidence of in-hospital MI as a primary end point decreased from 30% to 13.1% in the placebo and curcuminoid group respectively.[Citation23] Moreover, C-reactive protein, plasma malondialdehyde, and N-terminal pro-B type natriuretic peptide levels all of which represented the secondary end point in the study, were all significantly lower in curcuminoid when compared to the placebo group.[Citation23] This concludes the antioxidant and anti-inflammatory effect of curcuminoids reduced MI associated with CABG.[Citation23] Other in-vitro studies have also demonstrated additional evidence supporting the cardioprotective effect of curcuminoids is that they inhibit human platelet activation which eventually lead to decreased occurrence of myocardial ischemia.[Citation11] Curcumin’s antioxidant properties were found to reduce adriamycin-induced cardiotoxicity and may help prevent diabetic cardiovascular complications.[Citation29] Curcumin also has an anti-thrombotic, anti-proliferative, and anti-inflammatory properties, and can lower serum cholesterol levels, all of which may protect against atherosclerosis.[Citation29] In animal models, curcumin have been shown to reduce the development of cardiac hypertrophy and heart failure.[Citation30]
Curcumin post-cholecystectomy
The effect of curcumin on patient reported outcomes post laparoscopic cholecystectomy was studied in a double-blinded randomized controlled trial. Patient outcomes were reported inform of pain or fatigue.[Citation24] Follow-up was made on regular basis with the patients. They found that curcumin improves postoperative pain and fatigue following a laparoscopic surgery.[Citation24] This is the only trial we found in the literature on curcumin and cholecystectomy. More studies are required to be done to reveal this association.
Curcumin and traumatic brian injury
It has also been indicated that curcumin may be effective against traumatic brain injury (TBI) outcomes through several molecular signaling pathways that include oxidative stress, inflammation, apoptosis, and autophagy.[Citation31]
Curcumin is a dietary antioxidant that can ameliorate TBI-induced damage in several experimental studies.[Citation13,Citation16,Citation18,Citation31,Citation32] An important mechanism that leads to the pathogenesis of TBI is oxidative stress, which eventually results in neuronal function impairment.[Citation31] Moreover, oxidative stress induces cognition impairment which might be related to the levels of brain-derived neurotrophic factor (BDNF),[Citation32] which has a neuroprotective impact and can inhibit oxidative damage.[Citation32] In an experimental study done on rats, curcumin was effective as it significantly reduced oxidative stress and up-regulated the expression of BDNF and cAMP response element binding protein (CREB), indicating that curcumin improves the impact of TBI on synaptic plasticity and cognitive function effect by regulating the oxidative stress.[Citation15]
Furthermore, curcumin has an ameliorative effect on the inflammatory cytokines and aid in the suppression of edema following TBI.[Citation16,Citation18] Curcumin decreases the expression of NF-kB which increase following a neurotrauma, thereby decreasing the levels of IL-1β-induced aquaporin-4 expression in astrocytes.[Citation16] In another study, curcumin when administered in 100 mg/kg for 2 weeks in a weight drop model of cortical contusion trauma in rats, there was a significant decrease in the size of lesions in the brain.[Citation17] In line with that, 25-50 mg of Tetrahydrocurcumin when administered 30 min post TBI inhibits the apoptotic cell death incused by modulating autophagy mediated by the AKT pathway.[Citation18] Pretreatment with curcumin, have also shown to improve acute inflammatory response post TBI by modulating toll-like receptor 4 (TLR4) expression in mice.[Citation19] This has resulted in improving brain edema and dysfunction through lowering inflammation, TLR4-positive macrophages as well as cell apoptosis.[Citation19] In this trial, the anti-inflammatory effect of curcumin post TBI was evaluated through the neuroinflammatory cascade initiated by TBI which has contributed to neuronal damage and behavioral impairment, in which TLR4 is an important mediator of this cascade.[Citation19] The administration of curcumin post-injury has shown to improve outcomes by reducing acute macroglia/macrophages activation and neuronal apoptosis through downregulation of the expression of TLR4 and its known downstream effectors (MyD88/Nf-kB) signaling pathway.[Citation19]
Curcumin has a protective role in neurological disorders.[Citation13,Citation18,Citation32] In a study identifying the role of curcumin, and its underlying molecular mechanism in TBI through the Nrf2-ARE pathway in mice, it was found that 100 mg/kg curcumin was significant in improving secondary brain injury (brain water content, oxidative stress, neurological severity score, and neuronal apoptosis).[Citation13] At the molecular level, administration of curcumin elevated Bcl-2 content and reduced cleaved caspase-3 which showed an anti-apoptotic effect of curcumin.[Citation13] In addition, curcumin markedly improved Nrf2 translocation which increased the expression of heme oxygenase 1 (HO1) and NAD(P)H, quinone oxidoreductase 1 (NQO1) and prevented the decline of antioxidant enzyme activities.[Citation13] All of which resulted in increased antioxidant activity, activated Nrf2-ARE pathway, and eventually attenuated brain injury in mice.[Citation13]
Curcumin in wounds
Curcumin has been shown to have significant wound healing properties. Curcumin hastens wound healing through the activation of various stages involved in natural wound healing. It guards against bacterial infection of the wound tissue,[Citation33] decreases the body’s response to inflammation and oxidation,[Citation34] and stimulates cell proliferation to aid in the repair of damaged tissue[Citation35] that arise from cutaneous wounds. Curcumin properties in wound healing have been discussed recently in the literature, which provides evidence about its ability in improving granulation tissue formation, deposition of collagen, wound contraction, and remodeling of tissues.[Citation35] To maximize curcumin therapeutic effects on skin wounds, the topical application of curcumin must be optimized.
Granulation tissue formation
Granulation tissue is characterized by the infiltration of fibroblasts and the small capillaries formation at the site of the wound, which eventually produces the extracellular matrix.[Citation36] It serves as the foundation for the epithelial cells to move and close the wound gap, thereby improving tissue re-epithelialization.[Citation37] In animal studies, excised wounds on rats treated with curcumin-loaded chitosan-alginate (CA-CD-Cur) showed accelerated closure rates, enhanced histopathological results, and decreased SOD, lipid peroxidation, PI3K, and p-AKT levels compared to the gauze-treated control group.[Citation20] Hydroxyproline, which is a protein marker predominantly expressed during collagen synthesis was found to increase in curcumin incorporated collagen matrix treated rats compared to their control counterparts. Hence, this hydroxyproline reflects the abundance of myofibroblasts in the wound.[Citation38] Since myofibroblasts are differentiated fibroblasts during granulation tissue formation, myofibroblasts form an adequate marker of this mechanism.[Citation38] In line with that, curcumin-treated wounds in rats showed better and more advanced organization of granulation tissues and myofibroblasts were more abundant.[Citation39]
Collagen deposition
Extracellular matrix formation requires various proteins and polysaccharides such as granulation tissue and collagen to provide support to the cells.[Citation40] Collagen is an important component of the extracellular matrix of the skin, and it comprises 70%–80% of the skin.[Citation40] Formation of the scar tissue, which is the result of wound repair process, is mostly made from collagenous fibers.[Citation41] Thereby, adequate formation and deposition of collagen in the wound will result in an optimal wound repair process.[Citation41] In a rat model, curcumin treated rats were shown to have higher collagen content in wounds compared to the gauze-treated control group.[Citation7] The collagen was thicker, more dense, and well aligned in the curcumin treated group.[Citation7] In another trial, the role of curcumin was revealed in a rat model that showed an increase in the content of collagen post curcumin treatment with a higher aldehyde content than collagen formed in their control counterparts.[Citation39] The high aldehyde content led to the development of highly cross-linked collagen bed in the curcumin treated wounds.[Citation39] Another study supported these findings by showing not only an increase in collagen content in the wound but also that collagen fibers matured earlier when wounds were treated topically with curcumin.[Citation42] This was due to the substantial increase in tensile strength and temperature shrinkage of the wounds treated with curcumin.[Citation42]
Wound contraction
In the final stage of wound healing, wound contraction involves complex interactions between cells, extracellular proteins, and cytokines.[Citation7] Wound contraction begins post differentiation of fibroblasts into myofibroblasts. Where wound contraction increases by myofibroblasts that induce expression of α-smooth muscle actin in the granulation tissue.[Citation43] It is stimulated by the transformation of growth factor β and platelet-derived growth factors during the cross-linkage that occurs in collagen bundles.[Citation38] Several studies provided evidence about the ability of curcumin in increasing the rate of wound contraction, thus accelerating wound healing.[Citation7] A study measured wound area planimetrically in rats, traced the size of wounds and found that topical application of curcumin significantly increase wound contraction by 20% compared to the control group.[Citation7] Another study showed that wounds in curcumin treated rats contracted by 90% 12 days post injury compared to gauze treated group, which means that there was a 74% contraction in curcumin treated wounds.[Citation44] Also, it was reported in one of the studies that maximum wound contraction. An important cytokine is TGF- β which is involved in the repair, chemotaxis, and deposition of collagen in the wound tissue, is released by different cells including fibroblasts.[Citation45] Studies showed that curcumin-treated wounds have a greater number of fibroblasts, which were positive for TGF- β compared to the control group.[Citation7] Similarly, topically treated diabetic mice using curcumin had a significant increase in TGF- β expression in their granulation tissues of wounds.[Citation7]
Re-epithelialization and remodeling
Epidermis forms an important barrier that protect the host from environmental interactions that could be physical, chemical, or microbial.[Citation7] The process of keratinocyte migration from the lower layers of skin for cell division is known as Epithelialization.[Citation37] Robust re-epithelialization is required as a final stage of wound healing to restore the epidermis’s adequate barrier function.[Citation7] Topical treatment of curcumin on wounds results in a complete cellular epithelialization in a rat model.[Citation38] When compared to the control group, the total epithelialization period was significantly reduced from 23 to 11 days.[Citation38] There was a significant increase in wound re-epithelialization in rats, with an optimum re-epithelialization in 12 days of continuous curcumin treatment.[Citation38] Similarly, curcumin-treated diabetic wounds in rats had accelerated re-epithelialization in addition to enhanced epithelial cell migration and a reduced wound gap and width when compared to the control group.[Citation38] Wound organization was also shown to be advanced in curcumin-treated rats, concluding that they had an enhanced remodeling of wounds.[Citation38]
Plausible mechanisms of action
The anti-inflammatory and antioxidant properties explain the major mechanisms of Curcumin beneficial effects on health.[Citation46] It has been shown that Curcumin increases serum superoxide dismutase (SOD), glutathione peroxidase (GSH), catalase, and lipid peroxidases.[Citation47] These are enzymes active in the neutralization of free radicals such as reactive oxygen species (ROS) and Reactive Nitrogen Species (RNS). Also, Curcumin can suppress ROS-generating enzymes such as lipoxygenase/cyclooxygenase and xanthine hydrogenase/oxidase. Moreover, curcumin is considered a chain-breaking antioxidant due to its lipophilic property, which makes it effective in scavenging peroxyl radicals.[Citation1]
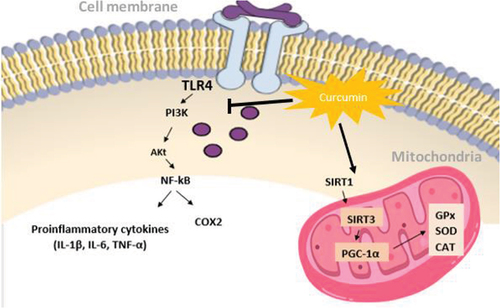
Pathological processes of oxidative stress are closely related to those of inflammation. This is as inflammatory cells release many reactive species at the site of inflammation which eventually lead to oxidative stress.[Citation1] Intracellular signaling cascades are initiated by ROS and RNS to enhance pro-inflammatory gene expression, which in turn aid in the development of chronic diseases and conditions such as Alzheimer’s disease, cerebral injury, metabolic syndrome, depression, and multiple sclerosis. Tumor necrosis factorα (TNFα) is an inflammatory biomarker that mediates inflammation in major diseases. TNFα is regulated by the activation of nuclear factor (NF-kB), a transcription factor. Besides TNFα, inflammatory cytokines, gram-negative bacteria, viruses, and physiological stress are also activators of NF-kB pathway. It has been shown that curcumin can block NF-kB activation that has been raised by many inflammatory stimuli.[Citation1] In another RCT, curcumin was found to increase cellular total antioxidant capacity, decrease high sensitivity C-reactive protein (hs-CRP) and interlukin-6 (IL-6).[Citation48]
Sirtuins (SIRT) are intracellular regulatory proteins that participate in various cellular mechanisms such as aging, stress, metabolic regulation, and transcription. Several studies suggest that SIRT1 and SIRT3 are involved in the inhibition of cellular oxidative stress,[Citation49] while SIRT2 triggers the formation of ROS.[Citation50] The activation of SIRT proteins by curcumin has been shown to increase levels of antioxidants by activating SIRT1 and SIRT3 and inhibiting SIRT2.[Citation51] Curcumin increases SIRT1 expression[Citation49] which is a protein that increases the binding of Forkhead box O (FOXO) to DNA and activates FOXO transcription factors. This binding regulates antioxidant genes such as SOD and CAT, that reduce levels of ROS.[Citation52,Citation53] Peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α) regulate genes expression that contributes to mitochondrial metabolism and oxidative stress.[Citation54] PGC-1α is activated by SIRT1 protein to improve expression of antioxidant genes in the mitochondria, which include GPx, CAT, and SOD[Citation55] thereby reducing oxidative stress (). Moreover, overexpression of SIRT3 boost the expression of PGC-1α and decrease ROS production.[Citation51] A study showed that curcumin upregulate the expression of SIRT3 and PGC-1α and decrease oxidative stress by lowering malondialdehyde (MDA) and upregulating SOD, GPx, and CAT levels. The authors of this study concluded that curcumin augment PGC-1α/SIRT3 signaling pathway and may possibly improve oxidative stress.[Citation56] SIRT2 levels increase in the cell upon oxidative stress.[Citation50] In an animal-based model, curcumin treatment showed significant reduction in SIRT2 expression in hippocampus of rats. Curcumin’s protective effect against oxidative stress might be due to its ability to increase SIRT1, SIRT3 and decrease SIRT2.[Citation57]
Figure 2. Antioxidant and anti-inflammatory effects of curcumin.
Cutaneous wound healing is divided into three consequent phases, the inflammatory, proliferation, and remodeling phase.[Citation20] Various radicals are generated as a result of the injury during the inflammation phase, which is where majority of the biological change occurs.[Citation34,Citation58] Radicals are highly linked to oxidative stress, which induces lipid peroxidation and impairs wound healing.[Citation34,Citation58] Because SOD and catalase are enzymes that aid in the scavenging of free radicals, an increase in their levels should reflect the increase in oxidative stress.[Citation20] Reduced oxidative stress enhances the inflammatory response by the downregulation of NFKB, resulting in decreased SOD gene expression.[Citation59] In a study, the administration of curcumin-containing sponge in rats resulted in lower SOD and lipid peroxidation than non-curcumin-containing and gauze groups, indicating a microenvironment with lower oxidative stress. The administration of Curcumin can increase the levels of catalase enzymes.[Citation60] This could be due to the conversion of the superoxide radicals into hydrogen peroxide, which further stimulates catalase expression.[Citation60]
Curcumin was studied in preclinical cancer research, and it has been shown that it inhibits carcinogenesis in several cancer types such as pancreatic, gastric, hepatic, and leukemia. Curcumin induced anti-inflammatory effects through inhibition of NF-kB and COX-2, inhibition of lipoxygenase and scavenging of free radicals generated in arachidonic acid metabolism, down regulate expression of inflammatory cytokines IL1b, IL-6, and TNF-a (), resulting in growth inhibition of cancer cell lines, and down-regulation of enzymes that mediate inflammation and tumor-cell proliferation (such as protein kinase C).[Citation61] In another post hoc analysis of randomized clinical trials, they concluded that serum TNF-a, IL-6, TGF-b, and MCP-1 significantly decreased in subjects with metabolic syndrome following curcumin supplementation.[Citation6]
Curcumin, in addition to its antioxidant and anti-inflammatory properties, has been shown in studies to target other powerful molecules. As previously mentioned, curcumin has been postulated to induce positive effects on ischemia-related injuries.[Citation14,Citation22,Citation23] Protein ubiquitination and de-ubiquitination are critical mechanisms for cellular function maintenance.[Citation62] UCH-L1 protects neurons from oxidative stress, and decreased levels results in neuronal degeneration.[Citation14,Citation25] Curcumin administration, on the other hand, has shown to reduce the decreased expression of UCH-L1 in middle cerebral artery occlusion injury (MCAO), preventing neuronal cell death caused by MCAO.[Citation14] Moreover, curcumin has been shown to actively mediate energy metabolism and cell survival in ischemic brain injury by maintaining ICDH levels, which is an enzyme that regulates energy metabolism, cell survival, and cell destiny.[Citation14] As a result, curcumin was proposed to have a neuroprotective effect by maintaining the levels of ICDH.[Citation14] Adenosylhomocysteinase is a neuromodulatory enzyme in the central nervous system that protects against brain ischemia.[Citation14] Curcumin has been shown to preserve adenosine levels by preserving adenosylhomocysteinase levels, resulting in a neuroprotective effect during MCAO.[Citation14] eIF4A is a polypeptide involved in protein translation initiation,[Citation63] and it has been shown that curcumin maintains the levels of eIF4A2 during MCAO injury. Thus, mediating the protection of neuronal cells during MCAO injury.[Citation14]
Conclusion
In conclusion, this review paper shows that curcumin is highly beneficial and has strong antioxidant and anti-inflammatory effects that could result in improved post-surgical outcomes. These benefits are maximized when curcumin is coupled with agents such as piperine, that significantly increase its bioavailability. Studies have shown that curcumin has a powerful impact post surgeries especially in ischemia, cholecystectomy, traumatic brain injury and wound healing. Furthermore, sufficient trials on the effect of curcumin supplementation and post-surgical outcomes are conducted on animal models. However, very scarce human clinical trials are in the literature as only four studies were found. Indicating the need for further studies to make a robust conclusion on the effect of curcumin supplementation on other surgical conditions.
Abbreviations
| UCH-L1 | = | ubiquitin carboxy-terminal hydrolase L1 |
| MI | = | myocardial infarction |
| CABG | = | coronary artery bypass grafting |
| TBI | = | traumatic brain injury |
| BDNF | = | brain-derived neurotrophic factor |
| CREB | = | cAMP response element binding protein |
| HO1 | = | heme oxygenase 1 |
| NQO1 | = | NAD(P)H,quinone oxidoreductase 1 |
| GSH | = | glutathione peroxidase |
| ROS | = | reactive oxygen species |
| RNS | = | Reactive Nitrogen Species |
| hs-CRP | = | high sensitivity C-reactive protein |
| FOXO | = | Forkhead box O |
| MDA | = | malondialdehyde |
| TLR4 | = | Toll like receptor 4 |
| Pl3k | = | Phosphoinositide 3-kinases |
| Akt | = | protein kinase B |
| NF-kB | = | nuclear factor kappa light chain enhancer of activated B cells |
| COX2 | = | Cyclooxygenase-2 |
| IL-1β | = | Interleukin-1β |
| IL-6 | = | Interleukin-6 |
| TNF-α | = | Tumor necrosis factor-α |
| SIRT 1 | = | Sirtuin 1 |
| SIRT3 | = | Sirtuin 3 |
| PGC-1α | = | Peroxisome proliferator-activated receptor gamma coactivator 1-α |
| GPx | = | Glutathione peroxidase |
| SOD | = | superoxide dismutase |
| CAT | = | Catalase |
Acknowledgment
Qatar national library for supporting APC chargesOpen Access funding provided by the Qatar National Library.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hewlings, S. J.; Kalman, D. S. Curcumin: A Review of Its Effects on Human Health. Foods. 2017, 6(10), 92. DOI: 10.3390/foods6100092.
- Priyadarsini, K. I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules. 2014, 19(12), 20091–20112. DOI: 10.3390/molecules191220091.
- Yusuf, M.; Sadiya, A.; Ahmed, B.; Gulfishan, M. Modern Perspectives of Curcumin and Its Derivatives as Promising Bioactive and Pharmaceutical Agents. Biointerface Res. Apl. Chem. 2022, 12(6), 28.
- Gupta, S. C.; Patchva, S.; Aggarwal, B. B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15(1), 195–218. DOI: 10.1208/s12248-012-9432-8.
- Tabrizi, R.; Vakili, S.; Akbari, M.; Mirhosseini, N.; Lankarani, K. B.; Rahimi, M.; Mobini, M.; Jafarnejad, S.; Vahedpoor, Z.; Asemi, Z. The Effects of Curcumin-containing Supplements on Biomarkers of Inflammation and Oxidative Stress: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Phytotherapy Res. 2019, 33(2), 253–262. DOI: 10.1002/ptr.6226.
- Panahi, Y.; Hosseini, M. S.; Khalili, N.; Naimi, E.; Simental-Mendía, L. E.; Majeed, M.; Sahebkar, A. Effects of Curcumin on Serum Cytokine Concentrations in Subjects with Metabolic Syndrome: A Post-Hoc Analysis of a Randomized Controlled Trial. Biomed. Pharmacother. 2016, 82, 578–582. DOI: 10.1016/j.biopha.2016.05.037.
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a Wound Healing Agent. Life Sci. 2014, 116(1), 1–7. DOI: 10.1016/j.lfs.2014.08.016.
- Shah, F. A.; Park, D. J.; Gim, S. A.; Koh, P. O. Curcumin Treatment Recovery the Decrease of Protein Phosphatase 2A Subunit B Induced by Focal Cerebral Ischemia in Sprague-Dawley Rats. Lab. Anim. Res. 2015, 31(3), 134–138. DOI: 10.5625/lar.2015.31.3.134.
- Zhang, H. J.; Xing, Y. Q.; Jin, W.; Li, D.; Wu, K.; Lu, Y. Effects of Curcumin on Interleukin-23 and Interleukin-17 Expression in Rat Retina After Retinal Ischemia-Reperfusion Injury. Int. J. Clin. Exp. Pathol. 2015, 8(8), 9223–9231.
- Basnet, P.; Skalko-Basnet, N. Curcumin: An Anti-Inflammatory Molecule from a Curry Spice on the Path to Cancer Treatment. Molecules. 2011, 16(6), 4567–4598. DOI: 10.3390/molecules16064567.
- Keihanian, F.; Saeidinia, A.; Bagheri, R. K.; Johnston, T. P.; Sahebkar, A. Curcumin, Hemostasis, Thrombosis, and Coagulation. J. Cell. Physiol. 2018, 233(6), 4497–4511. DOI: 10.1002/jcp.26249.
- Dai, L. Y.; Cheng, B. H.; Li, J. Effect of Curcumin on Cerebral Ischemia-Reperfusion Injury in Rats. Zhong yao cai. 2015, 38(2), 344–349.
- Dai, W.; Wang, H.; Fang, J.; Zhu, Y.; Zhou, J.; Wang, X.; Zhou, Y.; Zhou, M. Curcumin Provides Neuroprotection in Model of Traumatic Brain Injury via the Nrf2-ARE Signaling Pathway. Brain Res. Bull. 2018, 140, 65–71. DOI: 10.1016/j.brainresbull.2018.03.020.
- Shah, F. A.; Gim, S. A.; Sung, J. H.; Jeon, S. J.; Kim, M. O.; Koh, P. O. Identification of Proteins Regulated by Curcumin in Cerebral Ischemia. J. Surg. Res. 2016, 201(1), 141–148. DOI: 10.1016/j.jss.2015.10.025.
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Dietary Curcumin Counteracts the Outcome of Traumatic Brain Injury on Oxidative Stress, Synaptic Plasticity, and Cognition. Exp. neurol. 2006, 197(2), 309–317. DOI: 10.1016/j.expneurol.2005.09.004.
- Laird, M. D.; Sukumari-ramesh, S.; Swift, A. E.; Meiler, S. E.; Vender, J. R.; Dhandapani, K. M. Curcumin Attenuates Cerebral Edema Following Traumatic Brain Injury in Mice: A Possible Role for Aquaporin-4? J. Neurochem. 2010, 113(3), 637–648. DOI: 10.1111/j.1471-4159.2010.06630.x.
- Samini, F.; Samarghandian, S.; Borji, A.; Mohammadi, G. Curcumin Pretreatment Attenuates Brain Lesion Size and Improves Neurological Function Following Traumatic Brain Injury in the Rat. Pharmacol. Biochem. Behav. 2013, 110, 238–244. DOI: 10.1016/j.pbb.2013.07.019.
- Gao, Y.; Li, J.; Wu, L.; Zhou, C.; Wang, Q.; Li, X.; Zhou, M.; Wang, H. Tetrahydrocurcumin Provides Neuroprotection in Rats After Traumatic Brain Injury: Autophagy and the PI3K/AKT Pathways as a Potential Mechanism. J. Surg. Res. 2016, 206(1), 67–76. DOI: 10.1016/j.jss.2016.07.014.
- Zhu, H. -T.; Bian, C.; Yuan, J. -C.; Chu, W. -H.; Xiang, X.; Chen, F.; Wang, C. -S.; Feng, H.; Lin, J. -K. Curcumin Attenuates Acute Inflammatory Injury by Inhibiting the Tlr4/myd88/nf-κB Signaling Pathway in Experimental Traumatic Brain Injury. J. Neuroinflammation. 2014, 11(1), 1–17. DOI: 10.1186/1742-2094-11-59.
- Zhao, Y.; Dai, C.; Wang, Z.; Chen, W.; Liu, J.; Zhuo, R.; Yu, A.; Huang, S. A Novel Curcumin-Loaded Composite Dressing Facilitates Wound Healing Due to Its Natural Antioxidant Effect. Drug Des. Devel. Ther. 2019, 13, 3269. DOI: 10.2147/DDDT.S219224.
- Li, X.; Nan, K.; Li, L.; Zhang, Z.; Chen, H. In vivo Evaluation of Curcumin Nanoformulation Loaded Methoxy Poly (Ethylene Glycol)-Graft-Chitosan Composite Film for Wound Healing Application. Carbohydr. Polym. 2012, 88(1), 84–90. DOI: 10.1016/j.carbpol.2011.11.068.
- Sukardi, R.; Sastroasmoro, S.; Siregar, N. C.; Djer, M. M.; Suyatna, F. D.; Sadikin, M.; Ibrahim, N.; Rahayuningsih, S. E.; Witarto, A. B. The Role of Curcumin as an Inhibitor of Oxidative Stress Caused by Ischaemia Re-Perfusion Injury in Tetralogy of Fallot Patients Undergoing Corrective Surgery. Cardiol. Young. 2016, 26(3), 431–438. DOI: 10.1017/S1047951115000360.
- Wongcharoen, W.; Jai-Aue, S.; Phrommintikul, A.; Nawarawong, W.; Woragidpoonpol, S.; Tepsuwan, T.; Sukonthasarn, A.; Apaijai, N.; Chattipakorn, N. Effects of Curcuminoids on Frequency of Acute Myocardial Infarction After Coronary Artery Bypass Grafting. Am. J. card. 2012, 110(1), 40–44. DOI: 10.1016/j.amjcard.2012.02.043.
- Agarwal, K. A.; Tripathi, C.; Agarwal, B. B.; Saluja, S. Efficacy of Turmeric (Curcumin) in Pain and Postoperative Fatigue After Laparoscopic Cholecystectomy: A Double-Blind, Randomized Placebo-Controlled Study. Surg. endosc. 2011, 25(12), 3805–3810. DOI: 10.1007/s00464-011-1793-z.
- Papa, L.; Akinyi, L.; Liu, M. C.; Pineda, J. A.; Tepas, J. J., III; Oli, M. W.; Zheng, W.; Robinson, G.; Robicsek, S. A.; Gabrielli, A. Ubiquitin C-Terminal Hydrolase is a Novel Biomarker in Humans for Severe Traumatic Brain Injury. Crit. Care Med. 2010, 38(1), 138. DOI: 10.1097/CCM.0b013e3181b788ab.
- Gong, B.; Cao, Z.; Zheng, P.; Vitolo, O. V.; Liu, S.; Staniszewski, A.; Moolman, D.; Zhang, H.; Shelanski, M.; Arancio, O. Ubiquitin Hydrolase Uch-L1 Rescues β-Amyloid-Induced Decreases in Synaptic Function and Contextual Memory. Cell. 2006, 126(4), 775–788. DOI: 10.1016/j.cell.2006.06.046.
- Chu, K.; Li, H.; Wada, K.; Johnson, J. Ubiquitin C-Terminal Hydrolase L1 is Required for Pancreatic Beta Cell Survival and Function in Lipotoxic Conditions. Diabetologia. 2012, 55(1), 128–140. DOI: 10.1007/s00125-011-2323-1.
- Bengmark, S. Curcumin, an Atoxic Antioxidant and Natural NfκB, Cyclooxygenase-2, Lipooxygenase, and Inducible Nitric Oxide Synthase Inhibitor: A Shield Against Acute and Chronic Diseases. J. Parenteral Enteral Nutr. 2006, 30(1), 45–51. DOI: 10.1177/014860710603000145.
- Wongcharoen, W.; Phrommintikul, A. The Protective Role of Curcumin in Cardiovascular Diseases. Int. J. Cardiol. 2009, 133(2), 145–151. DOI: 10.1016/j.ijcard.2009.01.073.
- Marcu, M. G.; Jung, Y. -J.; Lee, S.; Chung, E. -J.; Lee, M. -J.; Trepel, J.; Neckers, L. Curcumin is an Inhibitor of P300 Histone Acetylatransferase. Med. Chem. 2006, 2(2), 169–174. DOI: 10.2174/157340606776056133.
- Farkhondeh, T.; Samarghandian, S.; Roshanravan, B.; Peivasteh-Roudsari, L. Impact of Curcumin on Traumatic Brain Injury and Involved Molecular Signaling Pathways. Recent pat. food, nutr. agric. 2020, 11(2), 137–144. DOI: 10.2174/2212798410666190617161523.
- Sahin, N.; Kilic, E.; Ates, N.; Balcikanli, Z.; Orhan, C.; Tuzcu, M.; Sahin, K.; Juturu, V. Curcumin Plays Neuroprotection Activity by Modulation of Neurotrophic Factor BDNF, GAP-43 and GFAP in Mice with Traumatic Brain Injury (P06-043-19). Curr. Dev. Nutr. 2019, 3(Supplement_1), nzz031. P06-043–19. DOI: 10.1093/cdn/nzz031.P06-043-19.
- Huang, Y.; Dan, N.; Dan, W.; Zhao, W. Reinforcement of Polycaprolactone/Chitosan with Nanoclay and Controlled Release of Curcumin for Wound Dressing. ACS Omega. 2019, 4(27), 22292–22301. DOI: 10.1021/acsomega.9b02217.
- Mohanty, C.; Sahoo, S. K. Curcumin and Its Topical Formulations for Wound Healing Applications. Drug Discovery Today. 2017, 22(10), 1582–1592. DOI: 10.1016/j.drudis.2017.07.001.
- Fereydouni, N.; Darroudi, M.; Movaffagh, J.; Shahroodi, A.; Butler, A. E.; Ganjali, S.; Sahebkar, A. Curcumin Nanofibers for the Purpose of Wound Healing. J. Cell. Physiol. 2019, 234(5), 5537–5554. DOI: 10.1002/jcp.27362.
- Alhajj, M.; Goyal, A. Physiology, Granulation Tissue. In StatPearls [Internet] 2020. https://www.ncbi.nlm.nih.gov/books/NBK554402/
- Rousselle, P.; Braye, F.; Dayan, G. Re-Epithelialization of Adult Skin Wounds: Cellular Mechanisms and Therapeutic Strategies. Adv. Drug Delivery Rev. 2019, 146, 344–365. DOI: 10.1016/j.addr.2018.06.019.
- Choudhary, V.; Shivakumar, H. A Review on Curcumin: Wound Healing Properties and Biomarkers of Wound Healing. Int. Res. J. Pharm. 2018, 9(9), 1–5. DOI: 10.7897/2230-8407.099179.
- Mohanty, C.; Das, M.; Sahoo, S. K. Sustained Wound Healing Activity of Curcumin Loaded Oleic Acid Based Polymeric Bandage in a Rat Model. Mol. Pharmaceutics. 2012, 9(10), 2801–2811. DOI: 10.1021/mp300075u.
- Haydont, V.; Bernard, B. A.; Fortunel, N. O. Age-Related Evolutions of the Dermis: Clinical Signs, Fibroblast and Extracellular Matrix Dynamics. Mech. Ageing Dev. 2019, 177, 150–156. DOI: 10.1016/j.mad.2018.03.006.
- Petrucci, T.; Gallicchio, V. The Use of Human Stem Cells to Promote Tissue Healing in Skin Burn Wound Injuries. J. Stem. Cell. Res. 2021, 2(1), 1–14. DOI: 10.52793/JSCR.2021.2(1)-15.
- Kant, V.; Gopal, A.; Pathak, N. N.; Kumar, P.; Tandan, S. K.; Kumar, D. Antioxidant and Anti-Inflammatory Potential of Curcumin Accelerated the Cutaneous Wound Healing in Streptozotocin-Induced Diabetic Rats. Int. Immunopharmacol. 2014, 20(2), 322–330. DOI: 10.1016/j.intimp.2014.03.009.
- Gabbiani, G. 50 Years of Myofibroblasts: How the Myofibroblast Concept Evolved. In Myofibroblasts; Hinz, B. and Lagares, D. Eds., Springer, 2021; pp. 1–5.
- Dai, M.; Zheng, X.; Xu, X.; Kong, X.; Li, X.; Guo, G.; Luo, F.; Zhao, X.; Wei, Y. Q.; Qian, Z. Chitosan-Alginate Sponge: Preparation and Application in Curcumin Delivery for Dermal Wound Healing in Rat. J. Biomed. Biotechnol. 2009, 2009, 1–8. DOI: 10.1155/2009/595126.
- Maxson, S.; Lopez, E. A.; Yoo, D.; Danilkovitch-Miagkova, A.; LeRoux, M. A. Concise Review: Role of Mesenchymal Stem Cells in Wound Repair. Stem cells transl. med. 2012, 1(2), 142–149. DOI: 10.5966/sctm.2011-0018.
- Lutgendorf, S. K.; Aggarwal, B. B.; Sood, A. K., Curcumin InhibitsTumor Growth and Angiogenesis in Ovarian Carcinoma byTargeting the Nuclear Factor-KB Pathway.
- Sahebkar, A.; Serban, M. -C.; Ursoniu, S.; Banach, M. Effect of Curcuminoids on Oxidative Stress: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Funct. Foods. 2015, 18, 898–909. DOI: 10.1016/j.jff.2015.01.005.
- Saraf-bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of Curcumin Supplementation on Markers of Inflammation and Oxidative Stress Among Healthy Overweight and Obese Girl Adolescents: A Randomized Placebo-controlled Clinical Trial. Phytotherapy Res. 2019, 33(8), 2015–2022. DOI: 10.1002/ptr.6370.
- Miao, Y.; Zhao, S.; Gao, Y.; Wang, R.; Wu, Q.; Wu, H.; Luo, T. Curcumin Pretreatment Attenuates Inflammation and Mitochondrial Dysfunction in Experimental Stroke: The Possible Role of Sirt1 Signaling. Brain Res. Bull. 2016, 121, 9–15. DOI: 10.1016/j.brainresbull.2015.11.019.
- Nie, H.; Hong, Y.; Lu, X.; Zhang, J.; Chen, H.; Li, Y.; Ma, Y.; Ying, W. SIRT2 Mediates Oxidative Stress-Induced Apoptosis of Differentiated PC12 Cells. Neuroreport. 2014, 25(11), 838–842. DOI: 10.1097/WNR.0000000000000192.
- Alizadeh, M.; Kheirouri, S. Curcumin Reduces Malondialdehyde and Improves Antioxidants in Humans with Diseased Conditions: A Comprehensive Meta-Analysis of Randomized Controlled Trials. BioMedicine. 2019, 9(4), 4. DOI: 10.1051/bmdcn/2019090423.
- Lai, L.; Yan, L.; Gao, S.; Hu, C. -L.; Ge, H.; Davidow, A.; Park, M.; Bravo, C.; Iwatsubo, K.; Ishikawa, Y. Type 5 Adenylyl Cyclase Increases Oxidative Stress by Transcriptional Regulation of Manganese Superoxide Dismutase via the SIRT1/FoxO3a Pathway. Circulation. 2013, 127(16), 1692–1701. DOI: 10.1161/CIRCULATIONAHA.112.001212.
- Hejazi, J.; Rastmanesh, R.; Taleban, F. -A.; Molana, S. -H.; Hejazi, E.; Ehtejab, G.; Hara, N. Effect of Curcumin Supplementation During Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study. Nutr. Cancer. 2016, 68(1), 77–85. DOI: 10.1080/01635581.2016.1115527.
- Houten, S. M.; Auwerx, J. PGC-1α: Turbocharging Mitochondria. Cell. 2004, 119(1), 5–7. DOI: 10.1016/j.cell.2004.09.016.
- Merksamer, P. I.; Liu, Y.; He, W.; Hirschey, M. D.; Chen, D.; Verdin, E. The Sirtuins, Oxidative Stress and Aging: An Emerging Link. Aging (Albany NY). 2013, 5(3), 144. DOI: 10.18632/aging.100544.
- Zhang, M.; Tang, J.; Li, Y.; Xie, Y.; Shan, H.; Chen, M.; Zhang, J.; Yang, X.; Zhang, Q.; Yang, X. Curcumin Attenuates Skeletal Muscle Mitochondrial Impairment in COPD Rats: PGC-1α/SIRT3 Pathway Involved. Chem.-Biol. Interact. 2017, 277, 168–175. DOI: 10.1016/j.cbi.2017.09.018.
- Wang, F.; Nguyen, M.; Qin, F. X. F.; Tong, Q. SIRT2 Deacetylates Foxo3a in Response to Oxidative Stress and Caloric Restriction. Aging Cell. 2007, 6(4), 505–514. DOI: 10.1111/j.1474-9726.2007.00304.x.
- Thangapazham, R. L.; Sharad, S.; Maheshwari, R. K. Skin Regenerative Potentials of Curcumin. BioFactors. 2013, 39(1), 141–149. DOI: 10.1002/biof.1078.
- Singer, A. J.; Clark, R. A. Cutaneous Wound Healing. New Engl. J. Med. 1999, 341(10), 738–746. DOI: 10.1056/NEJM199909023411006.
- Gong, C.; Wu, Q.; Wang, Y.; Zhang, D.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A Biodegradable Hydrogel System Containing Curcumin Encapsulated in Micelles for Cutaneous Wound Healing. Biomaterials. 2013, 34(27), 6377–6387. DOI: 10.1016/j.biomaterials.2013.05.005.
- Jurenka, J. S. Anti-Inflammatory Properties of Curcumin, a Major Constituent of Curcuma Longa: A Review of Preclinical and Clinical Research. Altern. med. rev. 2009, 14(2), 143–144.
- Alves-Rodrigues, A.; Gregori, L.; Figueiredo-Pereira, M. E. Ubiquitin, Cellular Inclusions and Their Role in Neurodegeneration. Trends Neurosci. 1998, 21(12), 516–520. DOI: 10.1016/S0166-2236(98)01276-4.
- Imataka, H.; Sonenberg, N. Human Eukaryotic Translation Initiation Factor 4G (eIF4G) Possesses Two Separate and Independent Binding Sites for eIF4A. Mol. Cell. Biol. 1997, 17(12), 6940–6947. DOI: 10.1128/MCB.17.12.6940.