ABSTRACT
Mushrooms have been an acclaimed food for their unique flavor and medicinal properties since ancient times. Modern research shows that mushrooms are rich in various nutrients and biologically active substances. In recent years, researchers have become increasingly interested in mushrooms because they contain important secondary metabolites, such as phenolic compounds with significant bioactive properties. This review introduces the nutritional components and secondary metabolites in mushrooms, focusing on the bioactive functions and potential applications of mushroom polyphenols. Finally, the current challenges and future research trends of mushroom polyphenols are briefly discussed. In the aspect of nutritional value, mushrooms are high in protein and insoluble fiber, while low in fat and sodium, making them a low-energy, healthy food. Mushrooms contain a large amount of beneficial bioactive substances for health, including phenolic compounds, as well as tocopherols, terpenoids, and phytosterols. Mushroom polyphenols have antioxidant, anti-inflammatory, anti-cancer, anti-tyrosine, antihyperglycemic, and other biological activities beneficial to human health and medical applications, especially in the various degenerative disease and cancer treatments. However, based on the properties of phenolic compounds, research and development in commercial applications still face many issues that need to be addressed by researchers.
Introduction
Mushrooms are a kind of human food with a long history of consumption and are currently widely cultivated around the world. Although many people would classify them as a plant, mushrooms are actually a species that belongs to the kingdom of fungi. According to current findings, there are more than 40,000 species of mushrooms in the world,[Citation1] of which 2,189 have been identified as edible species.[Citation2] By the beginning of the 21st century, 60 species were commercialized.[Citation3] Because mushrooms growth depends on the influence of the geographical environment, the history of edible mushrooms in different regions has its own characteristics. Historically, China was the first country to cultivate fungi, starting around 600 AD. The main varieties cultivated were Auricularia auricula and Lentinus edodes (shiitake). Later, in 1600, France started growing Agaricus bisporus. Meanwhile, in the United States in 1900, the main cultivated mushroom species was Pleurotus ostreatus, commonly known as oyster mushrooms.[Citation4] Nowadays, various cultivated mushrooms are being promoted worldwide due to the development and progress of the times. Among the commercially cultivated mushroom species, the most common types of edible mushrooms are A. bisporus, L. edodes, P. ostreatus, Flammulina velutipes (commonly known as needle mushrooms or enoki mushrooms), and Volvariella volvacea (straw mushrooms). Wild mushrooms are also popularly consumed as food in several European countries, but it is important to note that some of them are poisonous.[Citation5] shows some common commercially cultivated mushroom species.
Figure 1. Some common commercially cultivated mushroom species: a. Agaricus bisporus; b. Lentinus edodes; c. Auricularia polytricha; d. Pleurotus ostreatus; e. Pleurotus eryngii; f. Flammulina velutipes.

Mushrooms are popular due to their distinct umami flavor, as well as their high nutrient and bioactive compound content.[Citation6] Studies have shown that mushrooms are a low-calorie, low-fat food that is rich in insoluble fiber, protein, vitamins, minerals and other beneficial nutrients.[Citation7,Citation8] Mushrooms also contain a mass of biologically active compounds, such as phenolic compounds, polysaccharides, tocopherols, terpenoids, and phytosterols, which give mushrooms medicinal values, like anti-oxidative, anti-inflammatory, anti-cancer, anti-tyrosine, anti-hyperlipidemic, and other bioactivity characteristics.[Citation9] In recent years, more and more studies have been carried out on the nutritional value and biological activity of mushrooms. Based on the biologically active substances in mushrooms, the research and development of functional foods containing mushrooms has sparked the interest of researchers, especially the research on phenolic compounds in mushroom extracts.[Citation10] This article provides a brief introduction to the nutrients in mushrooms, with a focus on the phenolic compounds present in different types of mushrooms and their biological activities. It also discusses the commercial applications and future development trends of phenolic extracts from mushrooms. Finally, the article briefly addresses the challenges currently faced by researchers in this field.
The nutritional content of mushrooms
Mushrooms have been an important component of the human diet since ancient times, providing unique flavor and textural properties. Their nutritional value has been proven in many studies.[Citation11] In addition, the unique flavor of mushrooms is also widely welcomed by consumers. Hundreds of flavor compounds have been identified in mushrooms, and the Maillard reaction during cooking also contributes to the unique flavor of mushrooms.[Citation12] Rizzo et al.[Citation8] summarizes the nutritional and energy composition of some mushroom species in .
Table 1. Nutrient composition and energy composition of some mushroom species (modified from Rizzo et al.[Citation8]).
Carbohydrates
The primary component of mushrooms is carbohydrates (50–65%), consisting mainly of monosaccharides, their derivatives, and oligosaccharides.[Citation13] Although there are some differences between mushroom species, the main sugars in mushrooms are mannitol and trehalose, with small amounts of sucrose, glucose, raffinose, fructose, and xylose.[Citation5,Citation14] Mushrooms also contain a small amount of glycogen, glucan, mannan, and pectin.[Citation5,Citation10] In addition, mushrooms are a good source of cellulose. Studies have shown that mushrooms contain 20–33% insoluble fiber and a small amount of soluble fiber (4–9%).[Citation10,Citation15] The insoluble fiber in mushrooms is mainly composed of chitin and β-glucan.[Citation6] Chitin is a polymer composed of 1000–3000 acetylglucosamine residues interconnected by 1,4-glycosidic chains and is mainly found in the cell wall of mushrooms (80–90% of dry weight).[Citation5] β-glucan is an emerging research direction of mushroom polysaccharides. Studies have shown that β-glucan can stimulate the production of macrophages, natural killer cells, T cells, and cytokines, making it an effective biological response regulator.[Citation16,Citation17]
Proteins and lipids
Protein is one of the most important nutrients in the human diet, and mushrooms are relatively high in protein and contain all the essential amino acids. The protein content of mushrooms varies depending on the species and growth stage, typically ranging from 18 to 37%.[Citation18] Cheung[Citation15] pointed out that mushrooms have a premium protein quality based on the standards of the Food and Agriculture Organization (FAO). Kato et al.[Citation19] also reported that mushroom protein could be an alternative source of animal protein for vegetarians. Mushrooms also contain all the essential amino acids,[Citation13] ranging from 30–50 g/100 g protein (on dry matter basis).[Citation20] However, Aletor[Citation21] reported that the content of essential amino acids in mushrooms varies from species to species. The mushroom protein contains high concentrations of several amino acids, including glutamic acid, L-aspartic acid, arginine, and threonine.[Citation15] In addition, some mushroom varieties were found to contain two limited amino acids, leucine and lysine, as well as two rare amino acids, γ-aminobutyric acid and ornithine.[Citation13]
Mushrooms have a low fat concentration, typically accounting for 2–6% of their dried mass.[Citation6] Several studies have shown that mushrooms contain high amounts of oleic acid and linoleic acid, which are unsaturated fatty acids, while palmitic acid, a saturated fatty acid, is also present in large quantities. The ratio of unsaturated to saturated fatty acids in mushrooms is approximately 2:1.[Citation22,Citation23] Moreover, linolenic acid serves as the precursor of 1-octen-3-ol, the main aromatic compound in mushrooms that imparts their distinctive flavor.[Citation14]
Vitamins and minerals
Mushrooms are a rich source of vitamin B compounds. Wang et al.[Citation24] reported that mushrooms contain high concentrations of riboflavin (vitamin B2), niacin (vitamin B3), and folic acid (vitamin B9), as well as trace amounts of thiamine (vitamin B1) and cobalamin (vitamin B12). Riboflavin is an important vitamin in mushrooms. Manzi et al.[Citation20] reported that riboflavin levels in some species of A. bisporus are comparable to those in egg and cheese. Another study found that Pleurotus eryngii, P. ostreatus, and A. bisporus have higher levels of niacin compared to other mushroom species, with P. eryngii having the highest levels (5.9 mg/kg).[Citation6] Vitamin D concentrations are relatively high in wild mushrooms but low in cultivated mushrooms.[Citation14] The vitamin D content is usually limited in the cultivated mushrooms as they are grown in centralized darkrooms. However, cultivated mushrooms contain a large amount of ergosterol, a precursor to vitamin D2.[Citation9] Simon et al.[Citation25] reported that under ultraviolet (UV) or sunlight exposure, ergosterol in mushrooms can be converted into vitamin D2. Similarly, Taofiq et al.[Citation26] demonstrated that mushrooms can convert ergosterol to vitamin D2 in a relatively short period of time when exposed to UV light. As a result, mushrooms can also be considered as a good source of vitamin D2.
Mushrooms also contain minerals, primarily potassium, phosphorus, magnesium, and a trace of calcium, iron, and zinc.[Citation13] Some commonly cultivated mushrooms, such as A. bisporus, were reported to be high in potassium, phosphorus, magnesium and zinc.[Citation15] Zhang et al.[Citation27] reported high concentrations of nickel, chromium and cadmium in mushrooms collected in industrial areas. The concentration of toxic metals and metalloids in mushrooms may reflect the ambient level of these contaminants. Among agricultural crops, edible mushrooms contain particularly high concentrations of heavy metals, indicating their remarkable capability to uptake toxic metals from the soil.[Citation28,Citation29] Mushroom fruit bodies may accumulate toxic metals and metalloids in polluted areas, and their consumption may pose a risk to human health.[Citation29] Therefore, it is critical to cultivate mushrooms in areas with low pollution from industrial, urban, or transportation sources.[Citation30,Citation31] In general, commercially cultivated mushroom species do not have this problem. However, in countries where wild edible mushrooms are preferred, caution must be exercised.
Classification of phenolic compounds in mushrooms
Generally, compounds with a combination of aromatic rings (single or multiple) and hydroxyl groups (single or multiple) are called phenolic compounds.[Citation32] Phenolic compounds are secondary metabolites produced by the majority of plant life activities.[Citation33] There are approximately 8,000 phenolic compounds that have been identified[Citation32] and the subcategories include phenolic acids, flavonoids, tannins, and other complex phenols.[Citation34] Mushrooms are rich in phenolic compounds, similar to plants. In mushrooms, the common phenols are mainly phenolic acids, flavonoids, tannins, and other polyphenols. shows the four main classes of phenolic compounds and their chemical structures.
Figure 2. Four major classes of phenolic compounds and their chemical structures: (A) phenolic acid-hydroxybenzoic acid derivatives, (B) phenolic acid-hydroxycinnamic acid derivatives, (C) flavonoid compounds, (D) tannins.
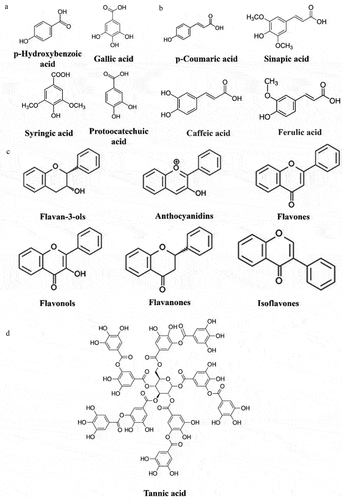
Phenolic acids
Phenolic acids, which are a major class of phenolic compounds found in mushrooms,[Citation35] can be divided into two subclasses: hydroxybenzoic acid and its derivatives, and hydroxycinnamic acid and its derivatives. These subclasses are both formed by the synthesis of L-phenylalanine or L-tyrosine through the shikimic acid pathway.[Citation36] Hazafa et al.[Citation37] noted that hydroxybenzoic acid typically includes gallic acid, vanillic acid, and ellagic acid. In contrast, hydroxycinnamic acid has many subclasses, such as caffeic acid, cinnamic acid, chlorogenic acid, coumaric acid, erucic acid and ferulic acid.[Citation37]
A study of mushrooms from Brazil determined the types and levels of phenolic acids in samples, with gallic acid, categorchol, and p-hydroxybenzoic acid detected in most samples, and the remaining phenolic acids including protocatechuic acid, gentian acid, and p-hydroxybenzoic acid, trans-cinnamic acid, p-coumaric acid, ferulic acid and vanillin were only quantified in one or two kinds of mushrooms.[Citation38] In the study of Bach et al.,[Citation38] gallic acid and p-hydroxybenzoic acid concentrations in Agaricus brasiliensis were significantly higher than those of other mushroom species, 492 μg/g and 333 μg/g dry weight, respectively. Nowacka et al.[Citation39] used LC-ESI-MS/MS method to detect 19 wild edible fungi in Poland and found a total of 14 phenolic acids in the samples, among which synaptic acid and rosmarinic acid were the main phenolic acids. In addition, Lin et al.[Citation40] detected chlorogenic, ferulic, gallic, protocatechuic, and sinapic acids in Agrocybe aegerita species. In another study, gallic acid, 3,4-dihydroxybenzoic acid, and gentisic acid were mostly quantified in mushroom varieties but no ferulic acid, salicylic acid, or chlorogenic acid was detected in 43 mushroom samples.[Citation41] In addition, Çayan et al.[Citation42] identified and quantified phenolic acids in 26 mushrooms, and the results showed a total of 16 kinds of phenolic and organic acid compounds were obtained. Most mushroom samples contained gallic acid, fumaric acid, protocatechuic acid, catechin hydrate and trans-cinnamic acid. The mushroom species with the highest phenolic acid content were Suillus granulatus (71.8 µg/g), followed by Lepista nuda (68.4 µg/g), and the species with the lowest content were Clitocybe odora and Russula aurora, 4.38 µg/g and 4.39 µg/g, respectively. Among these mushroom species, gallic acid was the main phenolic acid in Russula auror species (2.96 µg/g), the main phenolic acid in A. bisporus was fumaric acid (10.10 µg/g), and 6,7-dihydroxy coumarin was the main phenolic acid in Armillaria tabescens (2.07 µg/g) and Leucoagaricus leucothites (9.02 µg/g).[Citation42] In another study, Palacios et al.[Citation34] studied six wild and two cultivated mushrooms and found gallic acid, p-hydroxybenzoic acid, gentisic acid, and protocatechuic acid in all mushroom samples. Gallic acid was found in the highest concentrations in P. ostreatus, p-hydroxybenzoic acid and protocatechuic acid in Boletus edulis, and homogentisic acid was the only free acid quantified significantly in Calocybe gambosa.[Citation34] Selli et al.[Citation43] investigated the impact of two cooking methods on the phenolic and antioxidant properties of two popular edible mushroom types: Champignon (Agaricus bisporus) and Oyster (Pleurotus ostreatus) marketed in Turkey. They found that cinnamic acid, a compound belonging to the hydroxycinnamic acid group, was the dominant phenolic compound in both mushroom types. The amount of cinnamic acid was found to be 14.80–22.60 mg/kg in champignon samples and 3.47–13.10 mg/kg in oyster mushroom samples.
Flavonoids
Flavonoids are another type of phenolic compound found in mushroom species. They are the most abundant class of phenolic compounds, accounting for more than half of all phenolic compounds discovered.[Citation44] The basic skeleton of flavonoids is C6-C3-C6, and their subclasses include flavonols, flavones, flavanones, isoflavones, anthocyanidins, and flavanols (including catechins and proanthocyanidins), classified according to the oxidation state of the central ring.[Citation45] These subclasses are then hydroxylated, methoxylated, pentenylated or glycosylated to form further structures.[Citation46] Flavonoid is derived from two different primary metabolic pathways: phenylpropanoid derivatives and malonyl-CoA. The phenylpropanoid derivative (4-coumaroyl-CoA) condenses with three malonyl-CoA molecules to produce naringenin chalcone, which is then isomerized by chalcone flavanone isomerase and produces flavanone.[Citation47]
Some studies have shown that mushrooms contain various flavonoids,[Citation35,Citation48] but Gil-Ramírez et al.[Citation47] reported that the mushroom itself does not have the ability to synthesize flavonoids, and the flavonoids present in mushrooms may be absorbed from the growth substrate of mushrooms or neighboring plants. Current research shows that the main types of flavonoids in mushrooms are quercetin, catechin, rutin, kaempferol and myricetin.[Citation49] For instance, an ethanolic extract of Ganoderma lucidum contains four flavonoids: quercetin, kaempferol, hesperetin, and naringenin, with the latter two having relatively high concentrations.[Citation50] The flavonoids detected in another study on P. ostreatus were quercetin, catechin and chrysin.[Citation51] Liu et al.[Citation52] studied five wild mushrooms in southwestern China, and the results showed the two flavonoids with the highest content were quercetin and catechin. Among them, the highest catechin content was found in Laccaria amethystea extract with 30 µg/g dry weight, while the highest quercetin content was found in Laccaria ventricosum extract with 70 µg/g dry weight.
In another study, quercetin-hexoside, isoquercetin-hexoside, myricetin, procyanidin, and kaempferol were measured in Lactarius indigo species, among which the myricetin content was the highest at 53 µg/g dry weight.[Citation53] Similarly, Palacios et al.[Citation34] measured seven species of mushrooms, and the main flavonoid compound among them was myricetin, while catechins were only measured in Cantharellus cibarius and A. bisporus.[Citation34] In addition, Xiaokang et al.[Citation54] detected rutin and quercetin in L. edodes, the contents of which were 2.1 and 0.091 mg/g dry weight, respectively. In a study of wild edible mushrooms in Thailand, researchers detected three flavonoids: rutin, quercetin, and apigenin.[Citation55] Kaewnarin et al.[Citation55] also reported that Russula emetica species had the highest quercetin content at 1,762 µg/100 g of dried sample, and rutin was only detected in Rugiboletus extremiorientalis and R. emetica. In addition, rutin and quercetin were also detected in the extract of Pleurotus citrinopileatus, and the study showed that the concentration of flavonoids in ethanol extracts is higher than that in aqueous extracts.[Citation56] summarizes the phenolic acids and flavonoids in some mushroom species.
Table 2. The phenolic acids and flavonoids in some mushroom species.
Tannins
Tannins are also important secondary plant metabolites, divided into hydrolyzable and condensed tannins.[Citation59] Hydrolyzable tannins are composed of gallic acid or ellagic acid esterified glucose, and condensed tannins are composed of polymers of flavan 3-ols or flavan 3,4-diols.[Citation60] Hydrolyzable tannins have various structures due to various possibilities of oxidative bonds formed by intermolecular oxidation reactions and are generally oligomers (molecular weight = 2000–5000 Daltons).[Citation60] Condensed tannins are also known as procyanidins because they can be broken down into anthocyanins by acid-catalyzed reactions.[Citation46] Pizzi[Citation61] reported that 90% of the tannins extracted each year globally are condensed tannins. Akindahunsi and Oyetayo[Citation62] measured the total tannin content of Pleurotus tuber-regium to be 310 µg catechin equivalent/g dry weight. Garrab et al.[Citation63] also reported that total tannin content in Agaricus silvaticus, Hydnum rufescens and Meripilus giganteus was 45.2, 48.4, and 82.4 mg catechin equivalent/g methanolic extract, respectively. One study of Astraeus hygrometricus, a wild mushroom species in southwest India, showed that the tannin content was significantly reduced under the cooking pressure.[Citation64] Nevertheless, compared with another mushroom species, Termitomyces umkowaan and Astraeus hygrometricus had a higher tannin content regardless of whether it is cooked or not.[Citation65] Kant Mishra et al.[Citation66] studied the methanol and water extracts of the caps and stalks of L. edodes, and the results showed that the content of condensed tannins in the extracts of different solvents ranged from 1.23 to 3.26 mg catechin/g extract. However, the amount of qualitative and quantitative research on tannin compounds in mushrooms is still insufficient and further research will be needed to collect more data in the future.
Other phenolic compounds in mushrooms
There are some other phenolic compounds in mushrooms that have been discovered in other studies, such as lignans, stilbene, and pyrogallol. Lignans have a chemical structure similar to sterols, so they are also considered phytoestrogens.[Citation67] Lignans are composed of two propylbenzene units to form dimers, which are divided into eight structural subgroups according to the oxygen binding mode and cyclization mode.[Citation68] Li et al.[Citation69] detected lignans, coumarins and terpenoids in two wild bolete species. Stilbene is another natural phenolic compound, generally divided into resveratrol, piceatannol, and pterostilbene.[Citation37] In a study of 29 different wild mushrooms in northwestern Greece, a methanol extract was harvested from six species of L. edodes, and the results showed that L. edodes extract induced apoptosis and necrosis of A549 cells, and the HPLC identification results showed that piceatannol is one of the effective ingredients.[Citation70] In another study, rosmarinic acid was detected in Morchella esculenta, ellagic acid in A. bisporus, and vanillin in Collybia dryophila.[Citation42] Witkowska et al.[Citation71] found pyrogallol in Lactarius deliciosus, Cantharellus cibarius, and A. bisporus species. In another study, three new grifolin derivatives were detected in the methanol extract of Boletus pseudocalopus.[Citation72]
Other bioactive compounds in mushrooms
Tocopherols
Tocopherol is a form of vitamin E. Tocopherol in nature consists of 4 structures, namely α-tocopherol, β-tocopherol, γ-tocopherol and δ-tocopherol.[Citation73] Tocopherols usually consist of a 6-chromanol ring and a saturated side chain, while if the compound is composed of a geranyl side chain and three double bonds it is called tocotrienol.[Citation74] Tocopherol is known for its powerful antioxidant activity. Numerous studies have confirmed the bioactive properties of tocopherols, including their ability to reduce blood lipid levels, blood pressure, and inflammation, as well as their antioxidant and neuroprotective properties.[Citation75] Tocopherol can also maintain food stability and extend the shelf life of food.[Citation76] One study measured tocopherol levels in five mushroom species, and the results were 1.16 µg/g (Agaricus silvicola), 3.23 µg/g (Agaricus silvaticus), 1.29 µg/g (Agaricus romagnesii), 1.22 µg/g (Agaricus arvensis), and 2.41 µg/g (A. bisporus), respectively.[Citation77] In addition, in a study of 18 mushroom species, Lepista inversa species had the highest α-tocopherol content (0.28 μg/g), Laccaria laccata species had the highest β-tocopherol content (7.06 μg/g), Clitocybe alexandri species had the highest γ-tocopherol content (1.34 μg/g), and Lepista inversa species had the highest content of δ-tocopherol (0.64 μg/g).[Citation78] Quintero-Cabello et al.[Citation79] studied the Neolentinus lepideus species, which is eaten more in Central America and Asia, and α-tocopherol (3370 mg/100 g dry weight) and γ-tocopherol (18.5 mg/100 g dry weight) were detected in these mushroom species. In another study on Laetiporus sulphureus, the researchers detected three different structures of tocopherols, α-, γ-, and δ-tocopherol, and their contents were 109, 62.1, and 18.4 μg/100 g dry weight, respectively.[Citation80] Moreover, a study on Morchella esculenta showed that the content of total tocopherol in the extract was 38 µg/g extract, as well as other phenolic compounds including ellagic acid, catechol, quercetin, cinnamic acid, rutin, and p-coumaric acid.[Citation81]
Terpenes
Terpenes are naturally occurring unsaturated hydrocarbons consisting of compounds with the chemical formula (C5H8)n. Based on the number of carbon atoms in their chemical structure, terpenes can be divided into monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30) and carotenoids (C40).[Citation6] Terpenes in mushrooms have antioxidant, anticancer, anti-inflammatory, and other biological activities.[Citation82] Ganoderma lucidum is a typical example of this, and one study showed that Ganoderic acid A contained in G. lucidum is a triterpenoid with anti-inflammatory, antioxidant and anti-fibrotic effects.[Citation83] Moreover, Podkowa et al.[Citation84] observed that the carotenoids in mushrooms are effective components that prevent atherosclerosis and also play an important role in the prevention of nervous and circulatory system diseases.
Phytosterols and phytostanols
Phytosterols and phytostanols, collectively referred to as phytosteroids, are an important class of phytochemicals that are usually present in plant cell membranes.[Citation85] They have sparked widespread interest owing to their similar structure and biological function to cholesterol, and according to the branched chains and their saturation, they can be divided into β-sitosterol, campesterol, cholesterol, stigmasterol and stigmastanol.[Citation86] Campesterol and brassicasterol have been detected in some mushroom species, while other phytosterols were barely detectable.[Citation87,Citation88] Cholesterol reduction is one of the most important bioactive functions of phytosterols.[Citation85] According to several research, people should consume at least 2 g of phytosterols and phytostanols daily to regulate their blood lipid levels.[Citation89,Citation90] In addition, in a study evaluating plant sterols and plant sterols in controlling blood cholesterol levels, it was discovered that the two substances were not significantly different in lowering cholesterol, especially for lowering low-density lipoprotein cholesterol levels.[Citation91] Ergosterol is also a sterol, but in general, ergosterol is found in fungi and not in plants, so it cannot be classified as phytosterol.
Biological activity of phenolic compounds in mushrooms
Antioxidant activity
For organisms, the oxidation process in the body provides the energy required for physiological activities. However, in some specific cases, oxidative stress will occur in the body, resulting in excessive production of reactive oxygen species (ROS) in cells, disrupting the balance of ROS in the body, which is associated with negative health effects.[Citation92] ROS is essentially a free radical that reacts with cells in the body, causing oxidative damage to RNA and DNA, which in turn leads to cell damage, affects body tissues and organs, and causes body diseases.[Citation93] The antioxidant compounds of mushroom extracts have become an important source of alternative synthetic antioxidant materials in the pharmaceutical and food industries, supplying products with biologically active ingredients.[Citation94] The quantitative and qualitative characteristics of phenolic compounds in mushrooms determine the antioxidant activity of the extract.[Citation95] Several studies have also confirmed that the antioxidant activity of mushroom varieties has a strong positive correlation with the content of phenolic compounds, which may be based on the scavenging ability of phenolic compounds to hydroxyl groups.[Citation95,Citation96]
The antioxidant activity of mushroom extracts, which is generally related to the level of polyphenols, is also affected by the different varieties and extraction solvents, and these findings support the increased use of these extracts in food and medicine to improve human health.[Citation63] Bahadori et al.[Citation97] used DPPH assay to analyze the in vitro antioxidant activity of Melanoleuca stridula and Melanoleuca cognata; the results showed that the antioxidant activity descended in the order of water extract > methanol extract > ethyl acetate extract. Another study conducted in Turkey confirmed the previous relationship between phenol content and in vitro antioxidant activity. Researchers found that under the same conditions of ethanol extracts of mushrooms, Polyporus pinicola and Polyporus volvatus extracts with high phenol content exhibited high antioxidant activity.[Citation98] Similarly, Tahidul et al.[Citation99] studied 43 common mushrooms in China, and their results showed that all mushroom varieties exhibited antioxidant activity. They also adopted different detection methods of antioxidant activity, such as DPPH scavenging ability, FRAP, ABTS free radical scavenging ability and metal chelating ability, and confirmed that the content of phenolic compounds in mushrooms had a corresponding relationship with its antioxidant ability.[Citation99] Therefore, the phenolic compounds extracted from mushrooms have different antioxidant mechanisms. They may act as electron donors, neutralize free radicals, and maintain cellular antioxidant effects. In addition, mushroom polyphenols can also chelate metal elements to generate ROS. Mushroom polyphenols can also inhibit the formation of free radicals by inhibiting enzymes, mainly oxidases.[Citation35]
Anti-tumor activity
Research on mushroom extracts containing anti-tumor substances has recently received much attention. There is evidence that mushroom extracts can control cancer.[Citation100] Studies have shown that mushroom extracts can exert their anti-tumor activity by inhibiting cancer cell proliferation and tumor growth.[Citation101,Citation102] Various studies have demonstrated that phenolic compounds found in mushrooms have anticancer activity. For example, Albatrellus confluence has been proven to have certain inhibitory effects on human gastric and ovarian cancer cell lines.[Citation103,Citation104] Auricularia polytricha species were found to be effective against colon cancer (COLO-205), breast cancer (MCF-7), and kidney cancer (ACHN) cell lines.[Citation105] Inonotus obliquus, Ganoderma lucidum and Coprinus atramentarius have shown anticancer activity against leukemia,[Citation106] human lung cancer[Citation107] and human colon cancer cell lines,[Citation108] respectively.
A significant relationship has been established between flavonoids and anti-cancer proliferation effects.[Citation109,Citation110] In addition, the water extract of A. aegerita may overlap with multiple stages of angiogenesis during the growth of solid tumors.[Citation111] Ukaegbu et al.[Citation112] studied the anti-proliferative effects of methanolic and water extracts of Hypsizygus tessellatus and Flammulina velutipes caps on two breast cancer cell lines (MCF-7 and MDA-MB-231). They found that the two mushroom species exhibited high anti-proliferative activity, and the water extract had potent antioxidant activity against DPPH and high anti-proliferative effect. They also speculated that the underlying mechanism of mushroom extract’s anti-proliferative activity might be that the hydroxyl groups in the phenolic compounds interact with the polar receptor sites of mitochondrial cytochrome P450 enzymes to induce cell apoptosis.[Citation112] Similarly, there are studies in which the phenolic compounds detected in the phenol-rich water extract of A. aegerita mushroom mainly include ferulic, chlorogenic, protocatechuic, gallic acid and erucic acid. Therefore, it is believed that the phenol-rich water extract of A. aegerita mushroom had strong anti-tumor activity, which may have broad application prospects in tumor prevention and treatment.[Citation40]
Clinical studies on cancer have shown that lentinan, a bioactive polysaccharide extracted from Shiitake mushrooms (L. edodes), can complement chemotherapy during cancer treatment, improving the quality of life and extending the survival period of patients.[Citation113–115] However, the majority of these clinical studies are only pilot or phase I studies, which means that these findings are still preliminary. Additional follow-up experiments are required to further investigate the role of lentinan in cancer treatment.
Anti-inflammatory activity
As a defense mechanism of the body, inflammation usually occurs in response to internal or external stimuli. Under normal circumstances, the inflammation usually resolves itself after healing, but there is an overreaction of defenses that leads to damage.[Citation116] Therefore, anti-inflammatory substances are needed to inhibit the excessive development of some inflammations. Inflammation is normally regulated by immune cells, and an excessively developed inflammatory response can lead to immune cells producing inflammatory mediators that trigger various degenerative diseases.[Citation117] Bioactive substances in mushrooms, such as polyphenols, have anti-inflammatory activity by reducing this inflammatory mediator.[Citation118] Moro et al.[Citation119] also observed that pyrotriol isolated from Lactarius deliciosus, Cantharellus cibarius, and A. bisporus extracts showed significant anti-inflammatory effects. In addition, the phenolic-rich extracts of P. eryngii were found anti-inflammatory and anti-colon cancer effects.[Citation120] In addition, Taofiq et al.[Citation121] found that the phenolic extracts of A. bisporus, Boletus impolitus, Macrolepiota procera, and P. ostreatus have strong anti-inflammatory ability by inhibiting the production of nitric oxide. Among these mushrooms species, the extract of P. ostreatus showed the greatest effect in reducing nitric oxide production, possibly due to its cinnamic acid content (619 μg/g).
In a randomized, single-blinded, placebo-controlled trial,[Citation122] patients with ulcerative colitis and Crohn’s disease were given 60 mL of AndoSanTM, an extract from medicinal Basidiomycetes mushrooms, or a placebo for three weeks. The findings confirmed the reduced plasma levels of IL-2 in Crohn’s disease group and IL-5 in ulcerative colitis group. Further investigation revealed an improvement in the clinical symptoms and quality of life of patients in both groups who had received the mushroom extract product.[Citation123,Citation124]
Anti-hyperglycemic activity
Diabetes mellitus is a chronic metabolic disease characterized by chronic hyperglycemia. The body is exposed to a long-term high blood sugar level due to insufficient insulin secretion or damaged biological effects, causing a series of complications and various tissue damage or dysfunction.[Citation125] Research has showed that inhibiting intestinal α-glucosidase or pancreatic α-amylase is an effective strategy for controlling the risk of type 2 diabetes.[Citation126] Studies also showed that the anti-diabetic activity of phenolic compounds in mushrooms is due to their ability to inhibit the activity of these two enzymes.[Citation52,Citation127] Kato et al.[Citation19] also pointed out that the phenolic compounds in mushrooms can exhibit anti-diabetic activity through aldose reductase. Kaewnarin et al.[Citation55] studied some edible mushrooms in Thailand and found that when the mushroom species have a higher content of polyphenols and flavonoids, they exhibit higher inhibitory activity against α-glucosidase, and Rugiboletus extremiorientalis showed the strongest inhibitory activity. In another study, Chinese scholars selected eight mushrooms for research, and Ganoderma lucidum had the highest content of total phenols and total flavonoids and showed the highest inhibition of α-glycosidase and aldose reductase activity simultaneously.[Citation128] Similarly, in another study of Turkish mushrooms, six mushroom samples also showed an enzyme inhibitory activity of phenolic extracts and demonstrated a positive correlation between phenolic compounds content and their enzyme inhibitory activity.[Citation129] The results showed that the inhibitory levels of Lycoperdon utriforme species on α-amylase and α-glucosidase were 0.22 and 2.97 mmol acarbose equivalents/g extract, respectively, which were the highest among all samples.[Citation129] In a clinical trial, Jayasuriya et al.[Citation130] confirmed the hypoglycemic effect of P. ostreatus and Pleurotus cystidiosus at a dose of 50 mg/kg body weight when orally administered to Type 2 diabetic patients and healthy individuals challenged with glucose. The mechanism by which the mushrooms exert their hypoglycemic activity was explained by increasing glucokinase activity and stimulating insulin secretion, which increased glucose utilization by peripheral tissues[Citation130]
Antibacterial activity
The antibacterial activity of mushrooms had been widely confirmed, and research also showed that the antibacterial activity of mushrooms mainly comes from their phenolic compounds.[Citation131] At the same time, research also showed that the antibacterial activity of mushrooms was related to the variety of mushroom, the content of phenolic compounds in mushroom and the kind of pathogenic bacteria.[Citation132] Nowacka et al.[Citation133] reported that phenolic extracts from some mushrooms exhibit significant antibacterial activity. In a different study, Macrolepiota procera and Lactarius deliciosus were used to detect the potential antibacterial activity of 15 microorganisms, and the results show that Lactarius deliciosus has better antibacterial activity than the other species, the inhibitory concentration of which can be as low as 2.5 mg/mL.[Citation134] In addition, five phenolic compounds were extracted from the medicinal mushroom Phellinus linteus, all of which showed potent anti-influenza virus activity.[Citation135] Research on the antibacterial properties of five phenolic mushroom extracts of Shiitake, Flammulina velutipes, A. bisporus, and Brazilian mushrooms against pathogenic bacteria, such as Escherichia coli, Streptococcus aureus, Staphylococcus aureus, and Bacillus cereus, showed that these mushrooms have varying levels of antibacterial activity.[Citation38] This has been explained as the cell membrane of gram-negative bacteria has an additional lipopolysaccharide barrier that limits the excretion of many compounds.[Citation38,Citation136]
Other biological activities
The mushroom phenolic compounds also exhibit other biological activities, such as anti-osteoporosis and anti-tyrosinase. Osteoporosis is a bone disease caused by low bone density and low bone mass, and its incidence increases with age.[Citation137] Tanaka et al.[Citation138] investigated syringic acid in shiitake mushrooms and discovered that it may influence thigh formation and bone resorption, prevent bone loss and microstructure damage, and aid in the prevention of osteoporosis. In addition, Wei et al.[Citation139] extracted 11 bioactive compounds from Hericium erinaceum, four of which exhibited anti-osteoporosis activity. The mechanism was that these compounds inhibit a factor that can induce osteoclast differentiation. Another study extracted and measured phenolic compounds from beds of five species of mushrooms, and the results indicated that the contents of vanillic acid and syringic acid in beds of shiitake mushrooms were the highest, 0.45 and 0.31 mg/g dry weight, respectively. They also used mouse cells to confirm that vanillic acid and clove acid are resistant to obstinacy.[Citation140]
Tyrosinase is a copper-bearing oxidoreductase with a complex structure containing several subunits, more commonly known as polyphenol oxidase. Increased tyrosinase activity can lead to hyperpigmentation and the increased production of melanin in human skin.[Citation49] In addition, the increased activity of tyrosinase can cause the browning of fresh-cut vegetables and fruits. Therefore, tyrosinase inhibitors have received widespread attention in preventing the browning of vegetables and fruits and skin pigmentation.[Citation141] Phenolic compounds have strong anti-tyrosinase activity due to their antioxidant activity.[Citation55] According to a study, the ethyl acetate extract of the Tricholosporum goniospermum species showed the highest tyrosinase inhibitory activity among the three solvent extracts tested.[Citation142] Alkan et al.[Citation143] studied the anti-tyrosine activity of eight mushroom extracts and speculated that the presence of phenolic compounds such as p-hydroxybenzoic acid and p-coumaric acid in the mushroom leads to a significant inhibitory effect. In addition, some studies have pointed out that the anti-tyrosinase activity of a methanol extract of L. edodes was the highest among other solvent extracts of the same species.[Citation144] At the same time, Abd Razak et al.[Citation95] reported that the factors affecting the antioxidant activity and tyrosinase activity of mushroom extract included extraction temperature and time. The best extraction conditions for Schizophyllum commune species were found to be 1 hour at 30°C, resulting in the highest tyrosinase inhibition (96.6%).[Citation95] summarizes the biological activities possessed by some mushroom species.[Citation9]
Table 3. The biological activity of some mushroom species (modified from Anusiya et al.[Citation9]).
Potential applications of mushrooms
Mushroom extracts as functional foods and health products
Since ancient times, mushrooms have been not only a delicious food but also used as medicinal materials in some countries.[Citation188] In recent years, the pharmacological properties of mushrooms based on their biologically active ingredients have resulted in development of functional foods and health products.[Citation82] A study showed that adding mushroom species (A. bisporus) extract and dates to bread can effectively improve the content of protein, iron and other nutrients and help the body regulate anemia and malnutrition.[Citation145] Vital et al. (2019) added the water-alcoholic extract of Agaricus blazei mushrooms to milk, and after simulating the in vitro digestion process, they concluded that the milk extracted with mushrooms showed stronger antioxidant activity and effectively prevented the oxidation of lipids in milk.[Citation189] In addition, some mushroom varieties were added to grains as raw material to increase the phenolic content of these grains. In this way, the contents of active ingredients such as antioxidants and anti-diabetes were increased in functional foods.[Citation190] In a study on puffed snacks, researchers added A. bisporus, L. edodes, and Boletus edulis powder to the product, and the results showed that the antioxidant and anti-hyperglycemic activity of the products were effectively improved with the addition of mushrooms.[Citation191] The other two studies used mushrooms containing phenolic compounds as raw materials in cereal bars and muffins. This increased the phenolic content of the products and improved their nutritional properties.[Citation192,Citation193]
The biologically active substances found in mushrooms have also been utilized as dietary supplements in the development of health products, with promising results. Sabaratnam et al.[Citation194] suggested using Hericium erinaceus extract to produce health products that can prevent neurodegenerative diseases. This is because the proteoglycan component of this species can induce neuron differentiation and promote neuron survival. Another mushroom species that has been widely studied as a health supplement is Ganoderma lucidum. Besides having anti-inflammatory and antioxidant properties, this mushroom species prevented alcohol-induced steatohepatitis damage by reducing lipid levels in the livers of mice[Citation195] and induced the activation and maturation of mouse bone marrow-derived dendritic cells.[Citation196] Simultaneously, the polysaccharides contained in Ganoderma lucidum are also believed to enrich the intestinal microbiota, regulate intestinal bacteria, and increase the immune shielding function of the intestinal tract.[Citation197]
Mushroom extracts as cosmeceutical agents
Due to the demands of commercial competition and advances in product development, the cosmetic industry has been seeking ingredients from natural sources with low toxicity. At present, mushroom extracts and their biologically active compounds are ingredients of interest in cosmetic formulation research. According to research findings, using mushroom extract as a cosmetic ingredient in products has a significant effect on some skin problems such as pigmentation, skin ageing, and wrinkles.[Citation198,Citation199] A study extracted Tremella fuciformis polysaccharide for testing and found that it inhibited the formation of blackness and lightening spots[Citation200] and also had an excellent moisturizing effect.[Citation201] Kim et al.[Citation202] pointed out that Matsutake mycelium extract can significantly reduce elastase activity, reduce the degradation of elastin, and inhibit or repair wrinkle formation during skin ageing. Three kinds of ergosterols were isolated from the mycelium of Grifola frondosa, which were confirmed to have inhibitory effect on the key anti-aging process of Cyclooxygenase 2 (Cox-2).[Citation203]
Inhibition of pigmentation mainly inhibits the formation of melanin, and the formation of various melanin in organisms is mainly regulated by tyrosinase.[Citation204] Therefore, tyrosinase inhibition is the main method to inhibit pigmentation.[Citation205] Some studies have proven that in melanoma cells, Phellinus igniarius fruiting bodies and Flammulina velutipes extract inhibit tyrosinase activity and melanin synthesis.[Citation206,Citation207] Chien et al.[Citation208] reported that among the basidiomycetes tested, Ganoderma lucidum showed the strongest inhibitory effect on tyrosinase activity. Therefore, Ganoderma lucidum extracts are widely used as cosmeceutical products. Although some of the active compounds in mushrooms have been partially identified and are being developed, the available mushroom species only represent a small portion of what exists. Many more mushrooms have yet to be discovered, verified, and commercialized.
Mushroom as pharmaceutical raw material
About 5,000 years ago, some mushroom species appeared in human life generally as medicine, so some mushrooms are also called medicinal mushrooms.[Citation209] Some of the active substances in mushrooms have been shown in numerous studies to reduce the risk of neurodegenerative diseases. Active substances in some mushroom species have been studied by researchers for the treatment of neurodegenerative diseases.[Citation210] In several clinical trials involving mushrooms, research specifically on Hericium erinaceus demonstrated significant improvements in cognitive function. A 16-week Japanese study found that the experimental group, who continued to take a tablet of *H. erinaceus* extract (250 mg, three times a day), showed a significant improvement in cognitive function compared to the control group.[Citation211] The bioactive substances in mushrooms also have great potential in cancer treatment. Panda and Luyten[Citation212] summarized the relevant data of medicinal mushrooms in clinical research, and the results showed that the treatment of cancer accounted for 16% in clinical research, which is the largest proportion of all diseases treated. A double-blind, randomized clinical trial conducted in Taiwan used Antrodia Cinnamon as a cancer drug. The results showed that the medicinal mushroom reduced mortality in cancer patients, but the holistic treatment did not improve the prognosis of cancer patients and was at risk of reducing platelet count.[Citation213
The health-promoting properties of mushroom polysaccharides have recently gained attention in the food and pharmaceutical industries. Clinical trials have confirmed their immunomodulatory and anticancer effects.[Citation214,Citation215] Mushroom polysaccharides exhibit anticarcinogenic activity through the induction of apoptosis, inhibition of tumor cell growth, and stimulation of the immune response.[Citation214] Lentinan is a polysaccharide extracted from L. edodes, the world’s second-most-cultivated edible mushroom. Several clinical trials have supported its efficacy in treating various types of cancer, mainly due to its immunostimulatory and cytotoxic effects.[Citation215–217] Both in vitro and in vivo studies support the role of lentinan in activating natural killer cells, enhancing the proliferation of macrophages and cytotoxic T cells, and inducing nonspecific immunological responses.[Citation218–220]
Ribotoxin-like proteins have been isolated from the fruiting bodies of edible mushrooms, including Boletus edulis,[Citation221] P. ostreatus,[Citation222] Agrocybe aegerita,[Citation223] P. eryngii,[Citation224] and A. bisporus.[Citation225] Several in vitro studies have shown that Ageritin, isolated from Agrocybe aegerita, has cytotoxic activities against some tumor cell lines.[Citation226–228] Eryngitins isolated from P. eryngii showed no cytotoxicity to human HUVEC cells but exhibited cytotoxic activity to insect Sf9 cells. This suggests that they could be used to develop bioinsecticides. Scientists believe that ribotoxin-like proteins provided an important evolutionary advantage in mushrooms by allowing them to defend against viruses, molds, and predators.[Citation223,Citation229] Ribotoxin-like proteins may have potential future applications in biomedicine as therapeutic agents or in the development of bio-pesticide products. However, further research is necessary to fully understand the biological function of ribotoxin-like proteins in mushrooms.
Use of mushrooms in food product development
The unique characteristics of mushrooms, including their rich flavor, abundance of healthful active ingredients and nutrients, and low allergen content, have led to their increased use in meat products. This is particularly evident in the development of meat analogue-based products, which have enormous commercial potential.[Citation230–233] More recently, Yuan et al.[Citation234] successfully developed a mushroom-based meat analogue using edible mushrooms such as Coprinus comatus, L. edodes, and P. ostreatus with soybean protein isolate through a thermos-extrusion process, resulting in a product that had a satisfactory sensory profile and a textural profile similar to beef. Stephan et al.[Citation235] reported that sausages containing Pleurotus sapidus mushrooms were found to have a satisfactory flavor and texture, in comparison to commercial protein and meat sausages.
The high perishability of fresh mushrooms, mainly due to their high moisture content, requires immediate consumption or preservation. Drying is a cost-effective and feasible method for long-term storage of mushrooms.[Citation236] Incorporating powdered mushrooms into bakery products significantly enriches the final product with fiber, protein, vitamins, minerals, and polyphenols. According to Salehi et al.,[Citation237] the optimal sensory properties were achieved by incorporating 4–10% powdered mushrooms into bakery products. Cirlincione et al.[Citation238] created functional breads by adding 10% P. eryngii. Sensory evaluations showed that, despite some undesired changes in color, odor, and aroma intensity, the breads made with Mentana flour and P. eryngii had more favorable sensorial characteristics compared to breads made without mushrooms. Spim et al.[Citation193] reported that using L. edodes mushroom powder to produce sweet cereal bars resulted in a finished product with favorable textural and sensory properties, including flavor, appearance, and aroma. More recently, Amerikanou et al.[Citation239] developed a healthy snack using Pleurotus eryngii mushrooms with high nutritional and biological values. Sensory evaluation, which included flavor, appearance, texture, and taste, indicated an overall acceptable sensory quality of the final product. The recent shift in consumer preferences towards healthier food choices is likely to accelerate research and development for the introduction of innovative mushroom-based products in the food industry. Further research is also necessary to optimize the dosages of mushrooms in foods.
Research trends and current challenges of phenolic compounds in mushrooms
Recently, phenolic compounds have become more and more popular due to their various biological activities and their widespread existence in nature. However, the stability and solubility of polyphenol compounds can be affected to varying degrees during storage and processing, which can limit their bioavailability and restrict their industrial development to a certain extent.[Citation240] Singhal et al.[Citation241] investigated the effect of various heat treatments on the phenolic content of A. bisporus. The study concluded that the total phenolic content in mushroom samples was significantly reduced, regardless of the specific heat treatment conditions used. They pointed out that the loss of total phenols may be due to their decomposition during processing and the leaching of phenols in the solution.[Citation241] Furthermore, prior research has indicated that storage conditions, including high temperature, light exposure, and gastrointestinal conditions (such as pH and enzyme interactions with other body components) can affect phenolic compounds to varying degrees. As a result, this negatively affects their addition to functional foods and their digestion in the body.[Citation242]
The research of phenolic compounds in mushrooms as nano-products has become an emerging direction in the research of mushroom polyphenols. Due to the development of new drug delivery systems, the efficacy of therapeutic compounds has increased, while their toxicity, dosage and side effects have decreased.[Citation243] Therefore, a study pointed out that biologically active compounds found in mushroom species can be produced as nano-particle materials and added to functional foods or health products.[Citation113] At the same time, Abdelshafy et al.[Citation49] also reported that mushroom polyphenols extracted using ultrasonic encapsulation technology could be used to control different types of chronic diseases. Moreover, there is a growing interest in using mushroom polyphenols as functional foods or as an ingredient in fortified foods. These products have exhibited various biological activities, such as anti-tumor, antioxidant, and antibacterial effects.
Conclusion
Many studies have been conducted on the types, contents, and biological activities of phenolic compounds in mushrooms. Phenolic acids and flavonoids have been identified as the major phenolic compounds in mushrooms, although they may differ from species to species. Phenolic acids in mushrooms mainly include p-coumaric acid, caffeic acid, p-hydroxybenzoic acid, ferulic acid and gallic acid, while flavonoids include naringenin, hesperidin, kaempferol, catechin, rutin and quercetin. Phenolic compounds extracted from mushrooms have various biological activities, such as antioxidant, anti-tumor, hypoglycemic, antibacterial, anti-osteoporosis, anti-tyrosinase and anti-inflammatory activities. As a result of these properties, mushroom polyphenol compounds are largely used as functional food and nutriment in commercial development. In addition, mushroom phenolic compounds in skincare products, cosmetics and other applications have also been confirmed by several studies. In recent years, more and more attention has been paid to studying mushroom phenolic compounds. Some researchers have developed mushroom nanophase preparation research directions and shown some possible results. However, based on the instability and bioavailability of phenolic compounds and the possible losses in production and transportation, more research should be focused on improving the efficiency of phenolic compounds.
Disclosure statement
This is to acknowledge that the authors and funding body do not have any financial interest or benefit that has arisen from the direct applications of this research.
Additional information
Funding
References
- He, M. Q.; Zhao, R. L.; Liu, D. M.; Denchev, T. T.; Begerow, D.; Yurkov, A.; Kemler, M.; Millanes, A. M.; Wedin, M.; McTaggart, A. R., et al. Species Diversity of Basidiomycota. Fungal Diversity. 2022, 114(1), 281. DOI: 10.1007/s13225-021-00497-3.
- Li, H.; Tian, Y.; Menolli, N.; Ye, L.; Karunarathna, S. C.; Perez-Moreno, J.; Rahman, M. M.; Rashid, M. H.; Phengsintham, P.; Rizal, L., et al. Reviewing the World’s Edible Mushroom Species: A New Evidence-Based Classification System. Compr. Rev. Food Sci. Food Saf. 2021, 20(2), 1982.
- Chang, S. -T. Overview of Mushroom Cultivation and Utilization as Functional Foods. In Mushrooms as Functional Foods, 1st ed.; Cheung, P.C.K., Ed.; Wiley; New Jersey, 2008; pp. 1–34.
- Zhang, Y.; Venkitasamy, C.; Pan, Z.; Wang, W. Recent Developments on Umami Ingredients of Edible Mushrooms – a Review. Trends Food Sci. Technol. 2013, 33(2), 78. DOI: 10.1016/j.tifs.2013.08.002.
- Kalač, P. A Review of Chemical Composition and Nutritional Value of Wild-Growing and Cultivated Mushrooms. J. Sci. Food Agric. 2013, 93(2), 209. DOI: 10.1002/jsfa.5960.
- Cateni, F.; Gargano, M. L.; Procida, G.; Venturella, G.; Cirlincione, F.; Ferraro, V. Mycochemicals in Wild and Cultivated Mushrooms: Nutrition and Health. Phytochem. Rev. 2021, 21(2), 339–383. DOI: 10.1007/s11101-021-09748-2.
- Djibril, M. B.; Xiang, G.; Laila, A. -S.; Joshua, M.; Vernon, M. C.; Paddy, S.; Xinyuan, Z.; Guodong, L.; Robert, B. B.; John, P. R. Prospective Study of Dietary Mushroom Intake and Risk of Mortality: Results from Continuous National Health and Nutrition Examination Survey (NHANES) 2003-2014 and a Meta-Analysis. Nutr. J. 2021, 20(1), 1. DOI: 10.1186/s12937-021-00738-w.
- Rizzo, G.; Goggi, S.; Giampieri, F.; Baroni, L. A Review of Mushrooms in Human Nutrition and Health. Trends Food Sci. Technol. 2021, 117, 60. DOI: 10.1016/j.tifs.2020.12.025.
- Anusiya, G.; Gowthama Prabu, U.; Yamini, N. V.; Sivarajasekar, N.; Rambabu, K.; Bharath, G.; Banat, F. A Review of the Therapeutic and Biological Effects of Edible and Wild Mushrooms. Bioengineered. 2021, 12(2), 11239. DOI: 10.1080/21655979.2021.2001183.
- Marçal, S.; Sousa, A. S.; Taofiq, O.; Antunes, F.; Morais, A. M. M. B.; Freitas, A. C.; Barros, L.; Ferreira, I. C. F. R.; Pintado, M. Impact of Postharvest Preservation Methods on Nutritional Value and Bioactive Properties of Mushrooms. Trends Food Sci. Technol. 2021, 110, 418. DOI: 10.1016/j.tifs.2021.02.007.
- Jacinto-Azevedo, B.; Valderrama, N.; Henríquez, K.; Aranda, M.; Aqueveque, P. Nutritional Value and Biological Properties of Chilean Wild and Commercial Edible Mushrooms. Food Chem. 2021, 356, 129651. DOI: 10.1016/j.foodchem.2021.129651.
- Rotola-Pukkila, M.; Yang, B.; Hopia, A. The Effect of Cooking on Umami Compounds in Wild and Cultivated Mushrooms. Food Chem. 2019, 278, 56. DOI: 10.1016/j.foodchem.2018.11.044.
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom Nutraceuticals for Improved Nutrition and Better Human Health: A Review. PharmaNutrition. 2017, 5(2), 35. DOI: 10.1016/j.phanu.2017.02.001.
- Guillamón, E.; García-Lafuente, A.; Lozano, M.; D´arrigo, M.; Rostagno, M. A.; Villares, A.; Martínez, J. A. Edible Mushrooms: Role in the Prevention of Cardiovascular Diseases. Fitoterapia. 2010, 81(7), 715. DOI: 10.1016/j.fitote.2010.06.005.
- Cheung, P. C. K. The Nutritional and Health Benefits of Mushrooms. Nutr. Bull. 2010, 35(4), 292. DOI: 10.1111/j.1467-3010.2010.01859.x.
- Maity, P.; Sen, I. K.; Chakraborty, I.; Mondal, S.; Bar, H.; Bhanja, S. K.; Mandal, S.; Maity, G. N. Biologically Active Polysaccharide from Edible Mushrooms: A Review. Int. J. Biol. Macromol. 2021, 172, 408. DOI: 10.1016/j.ijbiomac.2021.01.081.
- Yu, Z.; Qiang, L.; Yamin, S.; Hongjing, W.; Ziming, Z.; Jinglin, W.; Kaiping, W. Induction of Apoptosis in S180 Tumour Bearing Mice by Polysaccharide from Lentinus Edodes via Mitochondria Apoptotic Pathway. J. Funct. Foods. 2015, 15, 151. DOI: 10.1016/j.jff.2015.03.025.
- González, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez, R.; Contreras, J.; Belmares, R. Edible Mushrooms as a Novel Protein Source for Functional Foods. Food & Function. 2020, 11(9), 7400. DOI: 10.1039/D0FO01746A.
- Kato, A.; Yasuko, H.; Goto, H.; Hollinshead, J.; Nash, R. J.; Adachi, I. Inhibitory Effect of Rhetsinine Isolated from Evodia Rutaecarpa on Aldose Reductase Activity. Phytomedicine. 2009, 16(2), 258. DOI: 10.1016/j.phymed.2007.04.008.
- Manzi, P.; Aguzzi, A.; Pizzoferrato, L. Nutritional Value of Mushrooms Widely Consumed in Italy. Food Chem. 2001, 73(3), 321. DOI: 10.1016/S0308-8146(00)00304-6.
- Aletor, V. A. Compositional Studies on Edible Tropical Species of Mushrooms. Food Chem. 1995, 54(3), 265. DOI: 10.1016/0308-8146(95)00044-J.
- Corrêa, R. C. G.; Brugnari, T.; Bracht, A.; Peralta, R. M.; Ferreira, I. C. F. R. Biotechnological, Nutritional and Therapeutic Uses of Pleurotus Spp. (Oyster Mushroom) Related with Its Chemical Composition: A Review on the Past Decade Findings. Trends Food Sci. Technol. 2016, 50, 103. DOI: 10.1016/j.tifs.2016.01.012.
- Sande, D.; de Oliveira, G. P.; E Moura, M. A. F.; de Almeida Martins, B.; Lima, M. T. N. S.; Takahashi, J. A. Edible Mushrooms as a Ubiquitous Source of Essential Fatty Acids. Food Res. Int. 2019, 125, 108524. DOI: 10.1016/j.foodres.2019.108524.
- Wang, X. -M.; Zhang, J.; Wu, L. -H.; Zhao, Y. -L.; Li, T.; Li, J. -Q.; Wang, Y. -Z.; Liu, H. -G. A Mini-Review of Chemical Composition and Nutritional Value of Edible Wild-Grown Mushroom from China. Food Chem. 2014, 151, 279. DOI: 10.1016/j.foodchem.2013.11.062.
- Simon, R. R.; Phillips, K. M.; Horst, R. L.; Munro, I. C. Vitamin D Mushrooms: Comparison of the Composition of Button Mushrooms (Agaricus Bisporus) Treated Postharvest with UVB Light or Sunlight. J. Agric. Food Chem. 2011, 59(16), 8724. DOI: 10.1021/jf201255b.
- Taofiq, O.; Fernandes, Â.; Barros, L.; Barreiro, M. F.; Ferreira, I. C. F. R. UV-Irradiated Mushrooms as a Source of Vitamin D2: A Review. Trends Food Sci. Technol. 2017, 70, 82. DOI: 10.1016/j.tifs.2017.10.008.
- Zhang, Y.; Cao, Y. R.; Xu, H. Evaluation of Heavy Metal Contents in Some Wild Edible Mushrooms from Panzhihua; China: SICHUAN UNIVERSITY, 2012Vol. 49. p. 246
- Wang, X.; Liu, H.; Zhang, J.; Li, T.; Wang, Y. Evaluation of Heavy Metal Concentrations of Edible Wild-Grown Mushrooms from China. J. Environ. Sci. Health Part B. 2017, 52(3), 178. DOI: 10.1080/03601234.2017.1261545.
- Dowlati, M.; Sobhi, H. R.; Esrafili, A.; FarzadKia, M.; Yeganeh, M. Heavy Metals Content in Edible Mushrooms: A Systematic Review, Meta-Analysis and Health Risk Assessment. Trends Food Sci. Technol. 2021, 109, 527. DOI: 10.1016/j.tifs.2021.01.064.
- Carvalho, M.; Pimentel, A.; Fernandes, B. Study of Heavy Metals in Wild Edible Mushrooms Under Different Pollution Conditions by X-Ray Fluorescence Spectrometry. Anal. Sci. 2005, 21(7), 747. DOI: 10.2116/analsci.21.747.
- Campos, J. A.; Tejera, N. A.; Sánchez, C. J. Substrate Role in the Accumulation of Heavy Metals in Sporocarps of Wild Fungi. BioMetals. 2009, 22(5), 835. DOI: 10.1007/s10534-009-9230-7.
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56(11), 317. DOI: 10.1111/j.1753-4887.1998.tb01670.x.
- Alara, O. R.; Abdurahman, N. H.; Ukaegbu, C. I. Extraction of Phenolic Compounds: A Review. Curr Res Food Sci. 2021, 4, 200. DOI: 10.1016/j.crfs.2021.03.011.
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M. A.; Martínez, J. A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant Properties of Phenolic Compounds Occurring in Edible Mushrooms. Food Chem. 2011, 128(3), 674. DOI: 10.1016/j.foodchem.2011.03.085.
- Ferreira, C. F. R. I.; Barros, L.; Abreu, M. V. R. Antioxidants in Wild Mushrooms. Curr. Med. Chem. 2009, 16(12), 1543. DOI: 10.2174/092986709787909587.
- Rice-Evans, C. A.; Miller, N. J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radical Biol. Med. 1996, 20(7), 933. DOI: 10.1016/0891-5849(95)02227-9.
- Hazafa, A.; Iqbal, M. O.; Javaid, U.; Tareen, M. B. K.; Amna, D.; Ramzan, A.; Piracha, S.; Naeem, M. Inhibitory Effect of Polyphenols (Phenolic Acids, Lignans, and Stilbenes) on Cancer by Regulating Signal Transduction Pathways: A Review. Clin. Transl. Oncol. 2021, 24(3), 432–445. DOI: 10.1007/s12094-021-02709-3.
- Bach, F.; Zielinski, A. A. F.; Helm, C. V.; Maciel, G. M.; Pedro, A. C.; Stafussa, A. P.; Ávila, S.; Haminiuk, C. W. I. Bio Compounds of Edible Mushrooms: In vitro Antioxidant and Antimicrobial Activities. LWT. 2019, 107, 214. DOI: 10.1016/j.lwt.2019.03.017.
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Antibacterial, Antiradical Potential and Phenolic Compounds of Thirty-One Polish Mushrooms. PLoS One. 2015, 10(10), 1. DOI: 10.1371/journal.pone.0140355.
- Lin, S.; Ching, L. T.; Lam, K.; Cheung, P. C. K. Anti-Angiogenic Effect of Water Extract from the Fruiting Body of Agrocybe Aegerita. LWT. 2017, 75, 155. DOI: 10.1016/j.lwt.2016.08.044.
- Islam, T.; Yu, X.; Xu, B. Phenolic Profiles, Antioxidant Capacities and Metal Chelating Ability of Edible Mushrooms Commonly Consumed in China. LWT - Food Sci. Technol. 2016, 72, 423. DOI: 10.1016/j.lwt.2016.05.005.
- Çayan, F.; Deveci, E.; Tel-Çayan, G.; Duru, M. E. Identification and Quantification of Phenolic Acid Compounds of Twenty-Six Mushrooms by HPLC–DAD. J. Food Meas. Charact. 2020, 14(3), 1690. DOI: 10.1007/s11694-020-00417-0.
- Selli, S.; Guclu, G.; Sevindik, O.; Kelebek, H. Variations in the Key Aroma and Phenolic Compounds of Champignon (Agaricus Bisporus) and Oyster (Pleurotus Ostreatus) Mushrooms After Two Cooking Treatments as Elucidated by GC–MS-O and LC-DAD-ESI-MS/ms. Food Chem. 2021, 354, 129576. DOI: 10.1016/j.foodchem.2021.129576.
- Harborne, J. B.; Baxter, H.; Moss, G. P. Phytochemical Dictionary : A Handbook of Bioactive Compounds from Plants, 2nd ed. ed.; London: Taylor & Francis, 1999.
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79(5), 727. DOI: 10.1093/ajcn/79.5.727.
- Dai, J.; Mumper, R. J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules. 2010, 15(10), 7313. DOI: 10.3390/molecules15107313.
- Gil-Ramírez, A.; Pavo-Caballero, C.; Baeza, E.; Baenas, N.; Garcia-Viguera, C.; Marín, F. R.; Soler-Rivas, C. Mushrooms Do Not Contain Flavonoids. J. Funct. Foods. 2016, 25, 1. DOI: 10.1016/j.jff.2016.05.005.
- Babak Bahadori, M.; Sarikurkcu, C.; Yalcin, O. U.; Cengiz, M.; Gungor, H. Metal Concentration, Phenolics Profiling, and Antioxidant Activity of Two Wild Edible Melanoleuca Mushrooms (M. Cognata and M. Stridula). Microchem. J. 2019, 150, 104172. DOI: 10.1016/j.microc.2019.104172.
- Abdelshafy, A. M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.; Li, L. A Comprehensive Review on Phenolic Compounds from Edible Mushrooms: Occurrence, Biological Activity, Application and Future Prospective. Crit. Rev. Food Sci. Nutr. 2021, 62(22), 1. DOI: 10.1080/10408398.2021.1898335.
- Veljovic, S.; Veljovic, M.; Nikicevic, N.; Despotovic, S.; Radulovic, S.; Niksic, M.; Filipovic, L. Chemical Composition, Antiproliferative and Antioxidant Activity of Differently Processed Ganoderma Lucidum Ethanol Extracts. J. Food Sci. Technol. 2017, 54(5), 1312. DOI: 10.1007/s13197-017-2559-y.
- Hassan, A. I.; Ghoneim, M. A. M.; Mahmoud, M. G.; Asker, M. S. Assessment Role of Total Phenols and Flavonoids Extracted from Pleurotus Columbinus Mushroom on the Premature Ovarian Failure Induced by Chemotherapy in Rats. J. Genet. Eng. Biotechnol. 2021, 19(1), 182. DOI: 10.1186/s43141-021-00278-0.
- Liu, Y. -T.; Sun, J.; Luo, Z. -Y.; Rao, S. -Q.; Su, Y. -J.; Xu, R. -R.; Yang, Y. -J. Chemical Composition of Five Wild Edible Mushrooms Collected from Southwest China and Their Antihyperglycemic and Antioxidant Activity. Food Chem. Toxicol. 2012, 50(5), 1238. DOI: 10.1016/j.fct.2012.01.023.
- Yahia, E. M.; Gutierrez-Orozco, F.; Moreno-Perez, M. A. Identification of Phenolic Compounds by Liquid Chromatography-Mass Spectrometry in Seventeen Species of Wild Mushrooms in Central Mexico and Determination of Their Antioxidant Activity and Bioactive Compounds. Food Chem. 2017, 226, 14. DOI: 10.1016/j.foodchem.2017.01.044.
- Xiaokang, W.; Brunton, N. P.; Lyng, J. G.; Harrison, S. M.; Carpes, S. T.; Papoutsis, K. Volatile and Non-Volatile Compounds of Shiitake Mushrooms Treated with Pulsed Light After Twenty-Four Hour Storage at Different Conditions. Food Biosci. 2020, 36, 100619. DOI: 10.1016/j.fbio.2020.100619.
- Kaewnarin, K.; Suwannarach, N.; Kumla, J.; Lumyong, S. Phenolic Profile of Various Wild Edible Mushroom Extracts from Thailand and Their Antioxidant Properties, Anti-Tyrosinase and Hyperglycaemic Inhibitory Activities. J. Funct. Foods. 2016, 27, 352. DOI: 10.1016/j.jff.2016.09.008.
- Gogoi, P.; Chutia, P.; Singh, P.; Mahanta, C. L. Effect of Optimized Ultrasound-Assisted Aqueous and Ethanolic Extraction of Pleurotus Citrinopileatus Mushroom on Total Phenol, Flavonoids and Antioxidant Properties. J. Food Process Eng. 2019, 42(6), e13172. DOI: 10.1111/jfpe.13172.
- Fogarasi, M.; Socaci, S. A.; Dulf, F. V.; Diaconeasa, Z. M.; Fărcaș, A. C.; Tofană, M.; Semeniuc, C. A. Bioactive Compounds and Volatile Profiles of Five Transylvanian Wild Edible Mushrooms. Molecules. 2018, 23(12), 3272. DOI: 10.3390/molecules23123272.
- Koutrotsios, G.; Kalogeropoulos, N.; Stathopoulos, P.; Kaliora, A. C.; Zervakis, G. I. Bioactive Compounds and Antioxidant Activity Exhibit High Intraspecific Variability in Pleurotus Ostreatus Mushrooms and Correlate Well with Cultivation Performance Parameters. World J. Microbiol. Biotechnol. 2017, 33(5), 98. DOI: 10.1007/s11274-017-2262-1.
- Das, A. K.; Islam, M. N.; Faruk, M. O.; Ashaduzzaman, M.; Dungani, R. Review on Tannins: Extraction Processes, Applications and Possibilities. South African J. of Bot. 2020, 135, 58. DOI: 10.1016/j.sajb.2020.08.008.
- Khanbabaee, K.; van Ree, T. Tannins: Classification and Definition. Nat. Prod. Rep. 2001, 18(6), 641.
- Pizzi, A. Recent Developments in Eco-Efficient Bio-Based Adhesives for Wood Bonding: Opportunities and Issues. J. Adhes. Sci. Technol. 2006, 20(8), 829. DOI: 10.1163/156856106777638635.
- Akindahunsi, A. A.; Oyetayo, F. L. Nutrient and Antinutrient Distribution of Edible Mushroom, Pleurotus Tuber-Regium (Fries) Singer. LWT - Food Sci. Technol. 2006, 39(5), 548. DOI: 10.1016/j.lwt.2005.04.005.
- Garrab, M.; Edziri, H.; El Mokni, R.; Mastouri, M.; Mabrouk, H.; Douki, W. Phenolic Composition, Antioxidant and Anticholinesterase Properties of the Three Mushrooms Agaricus Silvaticus Schaeff., Hydnum rufescens Pers. and Meripilus Giganteus (Pers.) Karst. in Tunisia. South African J. of Bot. 2019, 124, 359. DOI: 10.1016/j.sajb.2019.05.033.
- Pavithra, M.; Sridhar, K. R.; Greeshma, A. A.; Tomita-Yokotani, K. Bioactive Potential of the Wild Mushroom Astraeus hygrometricus in South-West India. Mycology. 2016, 7(4), 191. DOI: 10.1080/21501203.2016.1260663.
- Karun, N. C.; Sridhar, K. R.; Niveditha, V. R.; Ghate, S. D., Chapter 17 - Bioactive Potential of Two Wild Edible Mushrooms of the Western Ghats of India. In Fruits, Vegetables, and Herbs, Watson, R. R. Chapter 17 - Bioactive Potential of Two Wild Edible Mushrooms of the Western Ghats of India. In Fruits, Vegetables, and Herbs; Preedy, V.R., Ed.; London: Academic Press, 2016; p. 343.
- Kant Mishra, K.; Singh Pal, R.; Chandra Bhatt, J. Comparison of Antioxidant Properties in Cap and Stipe of Lentinula Edodes - a Medicinal Mushroom. Emir. J. Food Agric. 2015, 27(7), 562. DOI: 10.9755/ejfa.184591.
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J. J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules. 2019, 24(5), 917. DOI: 10.3390/molecules24050917.
- Li, Y.; Xie, S.; Ying, J.; Wei, W.; Gao, K. Chemical Structures of Lignans and Neolignans Isolated from Lauraceae. Molecules. 2018, 23(12), 12. DOI: 10.3390/molecules23123164.
- Li, J.; Wu, H.; Wang, L.; Huang, Y.; Wang, L. Key Taste Components in Two Wild Edible Boletus Mushrooms Using Widely Targeted Metabolomics. Biochem. Syst. Ecol. 2021, 96, 104268. DOI: 10.1016/j.bse.2021.104268.
- Vasdekis, E. P.; Karkabounas, A.; Giannakopoulos, I.; Savvas, D.; Lekka, M. E. Screening of Mushrooms Bioactivity: Piceatannol Was Identified as a Bioactive Ingredient in the Order Cantharellales. Eur. Food Res. Technol. 2018, 244(5), 861. DOI: 10.1007/s00217-017-3007-y.
- Witkowska, A. M.; Zujko, M. E.; Mirończuk-Chodakowska, I. Comparative Study of Wild Edible Mushrooms as Sources of Antioxidants. Int. J. Med. Mushrooms. 2011, 13(4), 335. DOI: 10.1615/IntJMedMushr.v13.i4.30.
- Song, J.; Manir, M. M.; Moon, S. -S. Cytotoxic Grifolin Derivatives Isolated from the Wild Mushroom Boletus Pseudocalopus (Basidiomycetes). Chem. Biodivers. 2009, 6(9), 1435. DOI: 10.1002/cbdv.200800217.
- Malekbala, M. R.; Soltani, S. M.; Hosseini, S.; Eghbali Babadi, F.; Malekbala, R. Current Technologies in the Extraction, Enrichment and Analytical Detection of Tocopherols and Tocotrienols: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57(14), 2935. DOI: 10.1080/10408398.2015.1020532.
- Sen, C. K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E Beyond Tocopherols. Life Sci. 2006, 78(18), 2088. DOI: 10.1016/j.lfs.2005.12.001.
- Mocchegiani, E.; Costarelli, L.; Giacconi, R.; Malavolta, M.; Basso, A.; Piacenza, F.; Ostan, R.; Cevenini, E.; Gonos, E. S.; Franceschi, C., et al. Vitamin E-Gene Interactions in Aging and Inflammatory Age-Related Diseases: Implications for Treatment. A Systematic Review. Ageing Res. Rev. 2014, 14(1), 81. DOI: 10.1016/j.arr.2014.01.001.
- Minhajuddin, M.; Beg, Z. H.; Iqbal, J. Hypolipidemic and Antioxidant Properties of Tocotrienol Rich Fraction Isolated from Rice Bran Oil in Experimentally Induced Hyperlipidemic Rats. Food Chem. Toxicol. 2005, 43(5), 747. DOI: 10.1016/j.fct.2005.01.015.
- Barros, L.; Correia, D. M.; Ferreira, I. C. F. R.; Baptista, P.; Santos-Buelga, C. Optimization of the Determination of Tocopherols in Agaricus Sp. Edible Mushrooms by a Normal Phase Liquid Chromatographic Method. Food Chem. 2008, 110(4), 1046. DOI: 10.1016/j.foodchem.2008.03.016.
- Heleno, S. A.; Barros, L.; Sousa, M. J.; Martins, A.; Ferreira, I. C. F. R. Tocopherols Composition of Portuguese Wild Mushrooms with Antioxidant Capacity. Food Chem. 2010, 119(4), 1443. DOI: 10.1016/j.foodchem.2009.09.025.
- Quintero-Cabello, K. P.; Palafox-Rivera, P.; Lugo-Flores, M. A.; Gaitán-Hernández, R.; González-Aguilar, G. A.; Silva-Espinoza, B. A.; Tortoledo-Ortiz, O.; Ayala-Zavala, J. F.; Monribot-Villanueva, J. L.; Guerrero-Analco, J. A. Contribution of Bioactive Compounds to the Antioxidant Capacity of the Edible Mushroom Neolentinus Lepideus. Chem. Biodivers. 2021, 18(7), e2100085. DOI: 10.1002/cbdv.202100085.
- Petrović, J.; Stojković, D.; Reis, F. S.; Barros, L.; Glamočlija, J.; Ćirić, A.; Ferreira, I. C. F. R.; Soković, M. Study on Chemical, Bioactive and Food Preserving Properties of Laetiporus Sulphureus (Bull.: Fr.) Murr. Food Funct. 2014, 5(7), 1441. DOI: 10.1039/C4FO00113C.
- Wagay, J. A.; Nayik, G. A.; Wani, S. A.; Mir, R. A.; Ahmad, M. A.; Rahman, Q. I.; Vyas, D. Phenolic Profiling and Antioxidant Capacity of Morchella Esculenta L. by Chemical and Electrochemical Methods at Multiwall Carbon Nanotube Paste Electrode. J. Food Meas. Charact. 2019, 13(3), 1805. DOI: 10.1007/s11694-019-00099-3.
- Ma, G.; Yang, W.; Zhao, L.; Pei, F.; Fang, D.; Hu, Q. A Critical Review on the Health Promoting Effects of Mushrooms Nutraceuticals. Food Sci. Hum. Wellness. 2018, 7(2), 125. DOI: 10.1016/j.fshw.2018.05.002.
- Ma, J. Q.; Zhang, Y. J.; Tian, Z. K. Anti-Oxidant, Anti-Inflammatory and Anti-Fibrosis Effects of Ganoderic Acid a on Carbon Tetrachloride Induced Nephrotoxicity by Regulating the Trx/TrxR and JAK/ROCK Pathway. Chem.-Biol. Interact. 2021, 344, 109529. DOI: 10.1016/j.cbi.2021.109529.
- Podkowa, A.; Kryczyk-Poprawa, A.; Opoka, W.; Muszyńska, B. Culinary–Medicinal Mushrooms: A Review of Organic Compounds and Bioelements with Antioxidant Activity. Eur. Food Res. Technol. 2021, 247(3), 513. DOI: 10.1007/s00217-020-03646-1.
- Nattagh-Eshtivani, E.; Barghchi, H.; Pahlavani, N.; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; Ziaei, R., et al. Biological and Pharmacological Effects and Nutritional Impact of Phytosterols: A Comprehensive Review. Phytotherapy Res. 2022, 36(1), 299. DOI: 10.1002/ptr.7312.
- Piironen, V.; Lindsay, D. G.; Miettinen, T. A.; Toivo, J.; Lampi, A. -M. Plant Sterols: Biosynthesis, Biological Function and Their Importance to Human Nutrition. J. Sci. Food Agric. 2000, 80(7), 939. DOI: 10.1002/(SICI)1097-0010(20000515)80:7<939:AID-JSFA644>3.0.CO;2-C.
- Sapozhnikova, Y.; Byrdwell, W. C.; Lobato, A.; Romig, B. Effects of UV-B Radiation Levels on Concentrations of Phytosterols, Ergothioneine, and Polyphenolic Compounds in Mushroom Powders Used as Dietary Supplements. J. Agric. Food Chem. 2014, 62(14), 3034. DOI: 10.1021/jf403852k.
- Phillips, K. M.; Ruggio, D. M.; Horst, R. L.; Minor, B.; Simon, R. R.; Feeney, M. J.; Byrdwell, W. C.; Haytowitz, D. B. Vitamin D and Sterol Composition of 10 Types of Mushrooms from Retail Suppliers in the United States. J. Agric. Food Chem. 2011, 59(14), 7841. DOI: 10.1021/jf104246z.
- Association, A. D. Nutrition Recommendations and Interventions for Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2008, 31(Supplement_1), S61.
- Smith, S. C.; Allen, J.; Blair, S. N.; Bonow, R. O.; Brass, L. M.; Fonarow, G. C.; Grundy, S. M.; Hiratzka, L.; Jones, D.; Krumholz, H. M., et al. AHA/ACC Guidelines for Secondary Prevention for Patients with Coronary and Other Atherosclerotic Vascular Disease: 2006 Update: Endorsed by the National Heart, Lung, and Blood Institute. J. Am. Coll. Cardiol. 2006, 47(10), 2130. DOI: 10.1016/j.jacc.2006.04.026.
- Talati, R.; Sobieraj, D. M.; Makanji, S. S.; Phung, O. J.; Coleman, C. I. The Comparative Efficacy of Plant Sterols and Stanols on Serum Lipids: A Systematic Review and Meta-Analysis. J. Am. Diet. Assoc. 2010, 110(5), 719. DOI: 10.1016/j.jada.2010.02.011.
- Lushchak, V. I. Free Radicals, Reactive Oxygen Species, Oxidative Stress and Its Classification. Chem.-Biol. Interact. 2014, 224, 164. DOI: 10.1016/j.cbi.2014.10.016.
- Mwangi, R. W.; Macharia, J. M.; Wagara, I. N.; Bence, R. L. The Antioxidant Potential of Different Edible and Medicinal Mushrooms. Biomed. Pharmacother. 2022, 147, 112621. DOI: 10.1016/j.biopha.2022.112621.
- Zielinski, A. A. F.; Haminiuk, C. W. I.; Beta, T. Multi-Response Optimization of Phenolic Antioxidants from White Tea (Camellia Sinensis L. Kuntze) and Their Identification by LC–DAD–Q-TOF–MS/MS. LWT - Food Sci. Technol. 2016, 65, 897. DOI: 10.1016/j.lwt.2015.09.020.
- Abd Razak, D. L.; Mohd Fadzil, N. H.; Jamaluddin, A.; Abd Rashid, N. Y.; Sani, N. A.; Abdul Manan, M. Effects of Different Extracting Conditions on Anti-Tyrosinase and Antioxidant Activities of Schizophyllum Commune Fruit Bodies. Biocatal. Agric. Biotechnol. 2019, 19, 101116. DOI: 10.1016/j.bcab.2019.101116.
- Contato, A. G.; Inácio, F. D.; de Araújo, C. A. V.; Brugnari, T.; Maciel, G. M.; Haminiuk, C. W. I.; Bracht, A.; Peralta, R. M.; de Souza, C. G. M. Comparison Between the Aqueous Extracts of Mycelium and Basidioma of the Edible Mushroom Pleurotus Pulmonarius: Chemical Composition and Antioxidant Analysis. J. Food Meas. Charact. 2020, 14(2), 830. DOI: 10.1007/s11694-019-00331-0.
- Bahadori, M. B.; Sarikurkcu, C.; Yalcin, O. U.; Cengiz, M.; Gungor, H. Metal Concentration, Phenolics Profiling, and Antioxidant Activity of Two Wild Edible Melanoleuca Mushrooms (M. Cognata and M. Stridula). Microchem. J. 2019, 150, 104172. DOI: 10.1016/j.microc.2019.104172.
- Orhan, I.; Üstün, O. Determination of Total Phenol Content, Antioxidant Activity and Acetylcholinesterase Inhibition in Selected Mushrooms from Turkey. J. Food Compost. Anal. 2011, 24(3), 386. DOI: 10.1016/j.jfca.2010.11.005.
- Tahidul, I.; Xiaoming, Y.; Baojun, X. Phenolic Profiles, Antioxidant Capacities and Metal Chelating Ability of Edible Mushrooms Commonly Consumed in China. LWT – Food Sci. Technol. 2016, 72, 423. DOI: 10.1016/j.lwt.2016.05.005.
- Ding, X.; Hou, Y.; Hou, W. Structure Feature and Antitumor Activity of a Novel Polysaccharide Isolated from Lactarius Deliciosus Gray. Carbohydr. Polym. 2012, 89(2), 397. DOI: 10.1016/j.carbpol.2012.03.020.
- Liu, K.; Xiao, X.; Wang, J.; Chen, C. Y. O.; Hu, H. Polyphenolic Composition and Antioxidant, Antiproliferative, and Antimicrobial Activities of Mushroom Inonotus Sanghuang. LWT - Food Sci. Technol. 2017, 82, 154. DOI: 10.1016/j.lwt.2017.04.041.
- Nowacka-Jechalke, N.; Olech, M.; Nowak, R. Chapter 11 - Mushroom Polyphenols as Chemopreventive Agents. In Polyphenols: Prevention and Treatment of Human Disease, Second Edition) ed.; Watson, R., Preedy, V.R. and Zibadi, S., Eds.; London: Academic Press, 2018; p. 137.
- Wu, Z.; Li, Y. Grifolin Exhibits Anti-Cancer Activity by Inhibiting the Development and Invasion of Gastric Tumor Cells. Oncotarget. 2017, 8(13), 13. DOI: 10.18632/oncotarget.15250.
- Yan, H.; Che, X.; Lv, Q.; Zhang, L.; Dongol, S.; Wang, Y.; Sun, H.; Jiang, J. Grifolin Induces Apoptosis and Promotes Cell Cycle Arrest in the A2780 Human Ovarian Cancer Cell Line via Inactivation of the ERK1/2 and Akt Pathways. Oncol. Lett. 2017, 13(6), 4806. DOI: 10.3892/ol.2017.6092.
- Arora, S.; Goyal, S.; Balani, J.; Tandon, S. Enhanced Antiproliferative Effects of Aqueous Extracts of Some Medicinal Mushrooms on Colon Cancer Cells. Int. J. Med. Mushrooms. 2013, 15(3), 301. DOI: 10.1615/IntJMedMushr.v15.i3.70.
- Nakajima, Y.; Nishida, H.; Matsugo, S.; Konishi, T. Cancer Cell Cytotoxicity of Extracts and Small Phenolic Compounds from Chaga [Inonotus Obliquus (Persoon) Pilat]. J. Med. Food. 2009, 12(3), 501. DOI: 10.1089/jmf.2008.1149.
- Chen, Y.; Lv, J.; Li, K.; Xu, J.; Li, M.; Zhang, W.; Pang, X. Sporoderm-Broken Spores of Ganoderma Lucidum Inhibit the Growth of Lung Cancer: Involvement of the Akt/mTOR Signaling Pathway. Nutr. Cancer. 2016, 68(7), 1151. DOI: 10.1080/01635581.2016.1208832.
- Khan, A. A.; Gani, A.; Ahmad, M.; Masoodi, F. A.; Amin, F.; Kousar, S. Mushroom Varieties Found in the Himalayan Regions of India: Antioxidant, Antimicrobial, and Antiproliferative Activities. Food Sci. Biotechnol. 2016, 25(4), 1095. DOI: 10.1007/s10068-016-0176-6.
- Saltarelli, R.; Palma, F.; Gioacchini, A. M.; Calcabrini, C.; Mancini, U.; De Bellis, R.; Stocchi, V.; Potenza, L. Phytochemical Composition, Antioxidant and Antiproliferative Activities and Effects on Nuclear DNA of Ethanolic Extract from an Italian Mycelial Isolate of Ganoderma Lucidum. J. Ethnopharmacol. 2019, 231, 464. DOI: 10.1016/j.jep.2018.11.041.
- Veljović, S.; Veljović, M.; Nikićević, N.; Despotović, S.; Radulović, S.; Nikšić, M.; Filipović, L. Chemical Composition, Antiproliferative and Antioxidant Activity of Differently Processed Ganoderma Lucidum Ethanol Extracts. J. Food Sci. Technol. 2017, 54(5), 1312. DOI: 10.1007/s13197-017-2559-y.
- Araújo, J. R.; Gonçalves, P.; Martel, F. Chemopreventive Effect of Dietary Polyphenols in Colorectal Cancer Cell Lines. Nutr. Res. 2011, 31(2), 77. DOI: 10.1016/j.nutres.2011.01.006.
- Ukaegbu, C. I.; Shah, S. R.; Hamid, H. A.; Alara, O. R.; Sarker, M. Z. I. Phenolic Compounds of Aqueous and Methanol Extracts of Hypsizygus Tessellatus (Brown and White Var.) and Flammulina Velutipes Caps: Antioxidant and Antiproliferative Activities. Pharm. Chem. J. 2020, 54(2), 170. DOI: 10.1007/s11094-020-02174-2.
- Wang, W.; Wu, Z.; Wang, X.; Li, C.; Zhang, K.; Zhou, J.; Cheng, S.; Lu, F. Enzymatic Hydrolysis Combined with High-Pressure Homogenisation for the Preparation of Polysaccharide-Based Nanoparticles from the By-Product of Flammulina Velutipes. Int. J. Food Sci. Technol. 2018, 53(10), 2422. DOI: 10.1111/ijfs.13836.
- Sakamoto, J.; Morita, S.; Oba, K.; Matsui, T.; Kobayashi, M.; Nakazato, H.; Ohashi, Y. Efficacy of Adjuvant Immunochemotherapy with Polysaccharide K for Patients with Curatively Resected Colorectal Cancer: A Meta-Analysis of Centrally Randomized Controlled Clinical Trials. Cancer Immunol. Immunother. 2006, 55(4), 404. DOI: 10.1007/s00262-005-0054-1.
- Trivedi, S.; Patel, K.; Belgamwar, V.; Wadher, K. Functional Polysaccharide Lentinan: Role in Anti-Cancer Therapies and Management of Carcinomas. Pharmacol. Res. Mod. Chin. Med. 2022, 2, 100045. DOI: 10.1016/j.prmcm.2022.100045.
- Jayasuriya, W. J. A. B. N.; Handunnetti, S. M.; Wanigatunge, C. A.; Fernando, G. H.; Abeytunga, D. T. U.; Suresh, T. S. Anti-Inflammatory Activity Pleurotus Ostreatus , a Culinary Medicinal Mushroom, in Wistar Rats, a Culinary Medicinal Mushroom, in Wistar Rats…18th Congress of the International Society for Mushroom Science, China. Evidence-Based Complementary Altern. Med. (Ecam). 2020, 2020, 1. DOI: 10.1155/2020/6845383.
- Ruksiriwanich, W.; Khantham, C.; Linsaenkart, P.; Chaitep, T.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Režek Jambrak, A.; Nazir, Y.; Yooin, W., et al. Anti-Inflammation of Bioactive Compounds from Ethanolic Extracts of Edible Bamboo Mushroom (Dictyophora Indusiata) as Functional Health Promoting Food Ingredients. Int. J. Food Sci. Technol. 2022, 57(1), 110. DOI: 10.1111/ijfs.15338.
- Elsayed, E. A.; El Enshasy, H.; Wadaan, M. A. M.; Aziz, R. Mushrooms: A Potential Natural Source of Anti-Inflammatory Compounds for Medical Applications. Mediators Inflammation. 2014, 2014, 805841. DOI: 10.1155/2014/805841.
- Moro, C.; Palacios, I.; Lozano, M.; D’Arrigo, M.; Guillamón, E.; Villares, A.; Martínez, J. A.; García-Lafuente, A. Anti-Inflammatory Activity of Methanolic Extracts from Edible Mushrooms in LPS Activated RAW 264.7 Macrophages. Food Chem. 2012, 130(2), 350. DOI: 10.1016/j.foodchem.2011.07.049.
- Hu, Q.; Yuan, B.; Xiao, H.; Zhao, L.; Wu, X.; Rakariyatham, K.; Zhong, L.; Han, Y.; Muinde Kimatu, B.; Yang, W. Polyphenols-Rich Extract from Pleurotus Eryngii with Growth Inhibitory of HCT116 Colon Cancer Cells and Anti-Inflammatory Function in RAW264.7 Cells. Food & Function. 2018, 9(3), 1601. DOI: 10.1039/C7FO01794D.
- Taofiq, O.; Calhelha, R. C.; Heleno, S.; Barros, L.; Martins, A.; Santos-Buelga, C.; Queiroz, M. J. R. P.; Ferreira, I. C. F. R. The Contribution of Phenolic Acids to the Anti-Inflammatory Activity of Mushrooms: Screening in Phenolic Extracts, Individual Parent Molecules and Synthesized Glucuronated and Methylated Derivatives. Food Res. Int. 2015, 76, 821–827. DOI: 10.1016/j.foodres.2015.07.044.
- Therkelsen, S.; Hetland, G.; Lyberg, T.; Lygren, I.; Johnson, E. Cytokine Levels After Consumption of a Medicinal Agaricus Blazei Murill‐based Mushroom Extract, AndoSan™, in Patients with Crohn’s Disease and Ulcerative Colitis in a Randomized Single‐blinded Placebo‐controlled Study. Scand. J. Immunol. 2016, 84(6), 323. DOI: 10.1111/sji.12476.
- Therkelsen, S. P.; Hetland, G.; Lyberg, T.; Lygren, I.; Johnson, E. Effect of the Medicinal Agaricus Blazei Murill-Based Mushroom Extract, Andosantm, on Symptoms, Fatigue and Quality of Life in Patients with Crohn’s Disease in a Randomized Single-Blinded Placebo Controlled Study. PLoS One. 2016, 11(7), e0159288. DOI: 10.1371/journal.pone.0159288.
- Therkelsen, S. P.; Hetland, G.; Lyberg, T.; Lygren, I.; Johnson, E. Effect of a Medicinal Agaricus Blazei Murill-Based Mushroom Extract, AndoSan™, on Symptoms, Fatigue and Quality of Life in Patients with Ulcerative Colitis in a Randomized Single-Blinded Placebo Controlled Study. PLoS One. 2016, 11(3), e0150191. DOI: 10.1371/journal.pone.0150191.
- Shobana, S.; Sreerama, Y. N.; Malleshi, N. G. Composition and Enzyme Inhibitory Properties of Finger Millet (Eleusine Coracana L.) Seed Coat Phenolics: Mode of Inhibition of α-Glucosidase and Pancreatic Amylase. Food Chem. 2009, 115(4), 1268. DOI: 10.1016/j.foodchem.2009.01.042.
- Koike, D.; Yamadera, K.; DiMagno, E. P. Effect of a Wheat Amylase Inhibitor on Canine Carbohydrate Digestion, Gastrointestinal Function, and Pancreatic Growth. Gastroenterol. 1995, 108(4), 1221. DOI: 10.1016/0016-5085(95)90223-6.
- Garduño-Diaz, S. D.; Khokhar, S. Prevalence, Risk Factors and Complications Associated with Type 2 Diabetes in Migrant South Asians. Diabetes/metab. res. rev. 2012, 28(1), 6. DOI: 10.1002/dmrr.1219.
- Wu, T.; Xu, B. Antidiabetic and Antioxidant Activities of Eight Medicinal Mushroom Species from China. Int. J. Med. Mushrooms. 2015, 17(2), 129. DOI: 10.1615/IntJMedMushrooms.v17.i2.40.
- Akata, I.; Zengin, G.; Picot, C. M. N.; Mahomoodally, M. F. Enzyme Inhibitory and Antioxidant Properties of Six Mushroom Species from the Agaricaceae Family. South African J. of Bot. 2019, 120, 95. DOI: 10.1016/j.sajb.2018.01.008.
- Jayasuriya, W. B. N.; Wanigatunge, C. A.; Fernando, G. H.; Abeytunga, D. T. U.; Suresh, T. S. Hypoglycaemic Activity of Culinary Pleurotus Ostreatus and P. Cystidiosus Mushrooms in Healthy Volunteers and Type 2 Diabetic Patients on Diet Control and the Possible Mechanisms of Action. Phytotherapy Res. 2015, 29(2), 303. DOI: 10.1002/ptr.5255.
- Erjavec, J.; Ravnikar, M.; Brzin, J.; Grebenc, T.; Blejec, A.; Gosak, M. Ž.; Sabotič, J.; Kos, J.; Dreo, T. Antibacterial Activity of Wild Mushroom Extracts on Bacterial Wilt Pathogen Ralstonia solanacearum. Plant Dis. 2016, 100(2), 453. DOI: 10.1094/PDIS-08-14-0812-RE.
- Kosanić, M.; Ranković, B.; Dašić, M. Mushrooms as Possible Antioxidant and Antimicrobial Agents. Iran. J. Pharm. Res. 2012, 11(4), 1095.
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Analysis of Phenolic Constituents, Antiradical and Antimicrobial Activity of Edible Mushrooms Growing Wild in Poland. LWT - Food Sci. Technol. 2014, 59(2), 689. DOI: 10.1016/j.lwt.2014.05.041.
- Kosanić, M.; Ranković, B.; Rančić, A.; Stanojković, T. Evaluation of Metal Concentration and Antioxidant, Antimicrobial, and Anticancer Potentials of Two Edible Mushrooms Lactarius Deliciosus and Macrolepiota Procera. J. Food Drug Anal. 2016, 24(3), 477. DOI: 10.1016/j.jfda.2016.01.008.
- Hwang, B. S.; Lee, I. -K.; Choi, H. J.; Yun, B. -S. Anti-Influenza Activities of Polyphenols from the Medicinal Mushroom Phellinus Baumii. Bioorg. Med. Chem. Lett. 2015, 25(16), 3256. DOI: 10.1016/j.bmcl.2015.05.081.
- Oliveira, D. A.; Angonese, M.; Gomes, C.; Ferreira, S. R. S. Valorization of Passion Fruit (Passiflora Edulis Sp.) By-Products: Sustainable Recovery and Biological Activities. J. Supercrit. Fluids. 2016, 111, 55. DOI: 10.1016/j.supflu.2016.01.010.
- Choi, H. -G.; Kwon, B. -C.; Yim, S. -H.; Youk, H.; Lee, J. -W. Weight Change is Associated with Osteoporosis: A Cross Sectional Study Using the Korean Community Health Survey. Int. J. Environ. Res. Public Health. 2021, 18(24), 13368.
- Tanaka, T.; Kawaguchi, N.; Zaima, N.; Moriyama, T.; Fukuta, Y.; Shirasaka, N. Antiosteoporotic Activity of a Syringic Acid Diet in Ovariectomized Mice. J. Nat. Med. 2017, 71(4), 632. DOI: 10.1007/s11418-017-1105-6.
- Wei, L.; Sang Hyun, L.; Hae Dong, J.; Jin Yeul, M.; Young Ho, K. Antioxidant and Anti-Osteoporotic Activities of Aromatic Compounds and Sterols from Hericium Erinaceum. Molecules. 2017, 22(1), 108. DOI: 10.3390/molecules22010108.
- Tanaka, T.; Onuma, H.; Shigihara, T.; Kimura, E.; Fukuta, Y.; Shirasaka, N.; Moriyama, T.; Homma, Y. Anti-Osteoporotic Effects of Syringic Acid and Vanilic Acid in the Extracts of Waste Beds After Mushroom Cultivation. J. Biosci. Bioeng. 2019, 128(5), 622. DOI: 10.1016/j.jbiosc.2019.04.021.
- Carcelli, M.; Rogolino, D.; Bartoli, J.; Pala, N.; Compari, C.; Ronda, N.; Bacciottini, F.; Incerti, M.; Fisicaro, E. Hydroxyphenyl Thiosemicarbazones as Inhibitors of Mushroom Tyrosinase and Antibrowning Agents. Food Chem. 2020, 303, 125310. DOI: 10.1016/j.foodchem.2019.125310.
- Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Tirillini, B.; Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Di Simone, S. C., et al. Evaluation of Antioxidant, Antimicrobial and Tyrosinase Inhibitory Activities of Extracts from Tricholosporum Goniospermum, an Edible Wild Mushroom. Antibiotics. 2020, 9(8), 513. DOI: 10.3390/antibiotics9080513.
- Alkan, S.; Uysal, A.; Kasik, G.; Vlaisavljevic, S.; Berežni, S.; Zengin, G. Chemical Characterization, Antioxidant, Enzyme Inhibition and Antimutagenic Properties of Eight Mushroom Species: A Comparative Study. J. Fungi. 2020, 6(3), 166. DOI: 10.3390/jof6030166.
- Yoon, K. N.; Alam, N.; Lee, K. R.; Shin, P. G.; Cheong, J. C.; Yoo, Y. B.; Lee, T. S. Antioxidant and Antityrosinase Activities of Various Extracts from the Fruiting Bodies of Lentinus Lepideus. Molecules. 2011, 16(3), 2334. DOI: 10.3390/molecules16032334.
- Ahmad, K.; Singh, N. Evaluation of Nutritional Quality of Developed Functional Bread Fortified with Mushroom and Dates. Clarion Int. Multidiscip. J. 2016, 5(1), 23. DOI: 10.5958/2277-937X.2016.00004.6.
- Firenzuoli, F.; Gori, L.; Lombardo, G. The Medicinal Mushroom Agaricus Blazei Murrill: Review of Literature and Pharmaco-Toxicological Problems. Evid. Based Complement. Altern. Med. 2008, 5(1), 659263. DOI: 10.1093/ecam/nem007.
- Liu, Q.; Zhu, M.; Geng, X.; Wang, H.; Ng, T. B. Characterization of Polysaccharides with Antioxidant and Hepatoprotective Activities from the Edible Mushroom Oudemansiella Radicata. Molecules. 2017, 22(2), 234. DOI: 10.3390/molecules22020234.
- Zhang, Y.; Zeng, Y.; Men, Y.; Zhang, J.; Liu, H.; Sun, Y. Structural Characterization and Immunomodulatory Activity of Exopolysaccharides from Submerged Culture of Auricularia Auricula-Judae. Int. J. Biol. Macromol. 2018, 115, 978. DOI: 10.1016/j.ijbiomac.2018.04.145.
- Song, G.; Du, Q. Structure Characterization and Antitumor Activity of an α β-Glucan Polysaccharide from Auricularia Polytricha. Food Res. Int. 2012, 45(1), 381. DOI: 10.1016/j.foodres.2011.10.035.
- Zhao, S.; Rong, C.; Liu, Y.; Xu, F.; Wang, S.; Duan, C.; Chen, J.; Wu, X. Extraction of a Soluble Polysaccharide from Auricularia Polytricha and Evaluation of Its Anti-Hypercholesterolemic Effect in Rats. Carbohydr. Polym. 2015, 122, 39. DOI: 10.1016/j.carbpol.2014.12.041.
- Bovi, M.; Cenci, L.; Perduca, M.; Capaldi, S.; Carrizo, M. E.; Civiero, L.; Chiarelli, L. R.; Galliano, M.; Monaco, H. L. BEL β-Trefoil: A Novel Lectin with Antineoplastic Properties in King Bolete (Boletus Edulis) Mushrooms. Glycobiology. 2012, 23(5), 578. DOI: 10.1093/glycob/cws164.
- Sarikurkcu, C.; Tepe, B.; Yamac, M. Evaluation of the Antioxidant Activity of Four Edible Mushrooms from the Central Anatolia, Eskisehir – Turkey: Lactarius Deterrimus, Suillus Collitinus, Boletus Edulis, Xerocomus Chrysenteron. Bioresour. Technol. 2008, 99(14), 6651. DOI: 10.1016/j.biortech.2007.11.062.
- Badshah, H.; Ullah, F.; Khan, M. U.; Mumtaz, A. S.; Malik, R. N. Pharmacological Activities of Selected Wild Mushrooms in South Waziristan (FATA), Pakistan. South African J. of Bot. 2015, 97, 107. DOI: 10.1016/j.sajb.2014.12.002.
- Kivrak, I.; Kivrak, S.; Harmandar, M. Bioactive Compounds, Chemical Composition, and Medicinal Value of the Giant Puffball, Calvatia Gigantea (Higher Basidiomycetes), from Turkey. Int. J. Med. Mushrooms. 2016, 18(2), 97. DOI: 10.1615/IntJMedMushrooms.v18.i2.10.
- Khalili, M.; Ebrahimzadeh, M. A.; Kosaryan, M.; Abbasi, A.; Azadbakht, M. Iron Chelation and Liver Disease Healing Activity of Edible Mushroom (Cantharellus Cibarius), in vitro and in vivo Assays. Rsc. Adv. 2015, 5(7), 4804. DOI: 10.1039/C4RA11561A.
- Lemieszek, M. K.; Nunes, F. M.; Cardoso, C.; Marques, G.; Rzeski, W. Neuroprotective Properties of Cantharellus Cibarius Polysaccharide Fractions in Different in vitro Models of Neurodegeneration. Carbohydr. Polym. 2018, 197, 598. DOI: 10.1016/j.carbpol.2018.06.038.
- Ridwan, A. Y.; Wu, J.; Choi, J. -H.; Hirai, H.; Kawagishi, H. Bioactive Compounds from the Edible Mushroom Cortinarius Caperatus. Mycoscience. 2018, 59(2), 172. DOI: 10.1016/j.myc.2017.08.015.
- Guo, M. Z.; Meng, M.; Feng, C. C.; Wang, X.; Wang, C. L. A Novel Polysaccharide Obtained from Craterellus Cornucopioides Enhances Immunomodulatory Activity in Immunosuppressive Mice Models via Regulation of the TLR4-NF-Κb Pathway. Food & Function. 2019, 10(8), 4792. DOI: 10.1039/C9FO00201D.
- Pérez-Moreno, J.; Martínez-Reyes, M. Edible Ectomycorrhizal Mushrooms: Biofactories for Sustainable Development. In Biosystems Engineering: Biofactories for Food Production in the Century XXI; Guevara-Gonzalez, R. and Torres-Pacheco, I., Eds.; Springer International Publishing: Cham, 2014; p. 151.
- Rodríguez-Seoane, P.; González-Muñoz, M. J.; Falqué, E.; Domínguez, H. Pressurized Hot Water Extraction of β-Glucans from Cantharellus Tubaeformis. Electrophoresis. 2018, 39(15), 1892. DOI: 10.1002/elps.201700399.
- Sánchez, C. Bioactives from Mushroom and Their Application. In Food Bioactives: Extraction and Biotechnology Applications; Puri, M., Ed.; Springer International Publishing: Cham, 2017; p. 23.
- Pang, X.; Yao, W.; Yang, X.; Xie, C.; Liu, D.; Zhang, J.; Gao, X. Purification, Characterization and Biological Activity on Hepatocytes of a Polysaccharide from Flammulina Velutipes Mycelium. Carbohydr. Polym. 2007, 70(3), 291. DOI: 10.1016/j.carbpol.2007.04.010.
- Wu, M.; Luo, X.; Xu, X.; Wei, W.; Yu, M.; Jiang, N.; Ye, L.; Yang, Z.; Fei, X. Antioxidant and Immunomodulatory Activities of a Polysaccharide from Flammulina Velutipes. J. Traditional Chin. Med. 2014, 34(6), 733. DOI: 10.1016/S0254-6272(15)30089-3.
- Ai-Lati, A.; Liu, S.; Ji, Z.; Zhang, H.; Mao, J. Structure and Bioactivities of a Polysaccharide Isolated from Ganoderma Lucidum in Submerged Fermentation. Bioengineered. 2017, 8(5), 565. DOI: 10.1080/21655979.2017.1283459.
- Wu, S. -J.; Lu, T. -M.; Lai, M. -N.; Ng, L. -T. Immunomodulatory Activities of Medicinal Mushroom Grifola Frondosa Extract and Its Bioactive Constituent. Am. J. Chin. Med. 2013, 41(01), 131. DOI: 10.1142/S0192415X13500109.
- Kim, S. P.; Nam, S. H.; Friedman, M. Hericium Erinaceus (Lion’s Mane) Mushroom Extracts Inhibit Metastasis of Cancer Cells to the Lung in CT-26 Colon Cancer-Tansplanted Mice. J. Agric. Food Chem. 2013, 61(20), 4898. DOI: 10.1021/jf400916c.
- Friedman, M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium Erinaceus (Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J. Agric. Food Chem. 2015, 63(32), 7108. DOI: 10.1021/acs.jafc.5b02914.
- Chien, R. -C.; Yang, Y. -C.; Lai, E. I.; Mau, J. -L. Anti-Inflammatory Effects of Extracts from the Medicinal Mushrooms Hypsizygus Marmoreus and Pleurotus Eryngii (Agaricomycetes). Int. J. Med. Mushrooms. 2016, 18(6), 477. DOI: 10.1615/IntJMedMushrooms.v18.i6.20.
- Shah, S. R.; Ukaegbu, C. I.; Hamid, H. A.; Alara, O. R. Evaluation of Antioxidant and Antibacterial Activities of the Stems of Flammulina Velutipes and Hypsizygus Tessellatus (White and Brown Var.) Extracted with Different Solvents. J. Food Meas. Charact. 2018, 12(3), 1947. DOI: 10.1007/s11694-018-9810-8.
- Finimundy, T. C.; Gambato, G.; Fontana, R.; Camassola, M.; Salvador, M.; Moura, S.; Hess, J.; Henriques, J. A. P.; Dillon, A. J. P.; Roesch-Ely, M. Aqueous Extracts of Lentinula Edodes and Pleurotus Sajor-Caju Exhibit High Antioxidant Capability and Promising in vitro Antitumor Activity. Nutr. Res. 2013, 33(1), 76. DOI: 10.1016/j.nutres.2012.11.005.
- Hearst, R.; Nelson, D.; McCollum, G.; Millar, B. C.; Maeda, Y.; Goldsmith, C. E.; Rooney, P. J.; Loughrey, A.; Rao, J. R.; Moore, J. E. An Examination of Antibacterial and Antifungal Properties of Constituents of Shiitake (Lentinula Edodes) and Oyster (Pleurotus Ostreatus) Mushrooms. Complementary Ther. Clin. Pract. 2009, 15(1), 5. DOI: 10.1016/j.ctcp.2008.10.002.
- Cheng, X. -D.; Wu, Q. -X.; Zhao, J.; Su, T.; Lu, Y. -M.; Zhang, W. -N.; Wang, Y.; Chen, Y. Immunomodulatory Effect of a Polysaccharide Fraction on RAW 264.7 Macrophages Extracted from the Wild Lactarius Deliciosus. Int. J. Biol. Macromol. 2019, 128, 732. DOI: 10.1016/j.ijbiomac.2019.01.201.
- Liu, C.; Sun, Y.; Mao, Q.; Guo, X.; Li, P.; Liu, Y.; Xu, N. Characteristics and Antitumor Activity of Morchella Esculenta Polysaccharide Extracted by Pulsed Electric Field. Int. J. Mol. Sci. 2016, 17(6), 986. DOI: 10.3390/ijms17060986.
- Nitha, B.; Fijesh, P. V.; Janardhanan, K. K. Hepatoprotective Activity of Cultured Mycelium of Morel Mushroom, Morchella Esculenta. Exp. Toxicol. Pathol. 2013, 65(1), 105. DOI: 10.1016/j.etp.2011.06.007.
- Hu, T.; Lin, Q.; Guo, T.; Yang, T.; Zhou, W.; Deng, X.; Yan, J. -K.; Luo, Y.; Ju, M.; Luo, F. Polysaccharide Isolated from Phellinus Linteus Mycelia Exerts Anti-Inflammatory Effects via MAPK and PPAR Signaling Pathways. Carbohydr. Polym. 2018, 200, 487. DOI: 10.1016/j.carbpol.2018.08.021.
- Ajith, T. A.; Janardhanan, K. K. Antioxidant and Antihepatotoxic Activities of Phellinus Rimosus (Berk) Pilat. J. Ethnopharmacol. 2002, 81(3), 387. DOI: 10.1016/S0378-8741(02)00042-9.
- Teplyakova, T. V.; Psurtseva, N. V.; Kosogova, T. A.; Mazurkova, N. A.; Khanin, V. A.; Vlasenko, V. A. Antiviral Activity of Polyporoid Mushrooms (Higher Basidiomycetes) from Altai Mountains (Russia). Int. J. Med. Mushrooms. 2012, 14(1), 37. DOI: 10.1615/IntJMedMushr.v14.i1.40.
- Harms, M.; Lindequist, U.; Al-Resly, Z.; Wende, K. Influence of the Mushroom Piptoporus Betulinus on Human Keratinocytes. Planta. med. 2013, 79(13), C4. DOI: 10.1055/s-0033-1351998.
- Zhang, Y.; Hu, T.; Zhou, H.; Zhang, Y.; Jin, G.; Yang, Y. Antidiabetic Effect of Polysaccharides from Pleurotus Ostreatus in Streptozotocin-Induced Diabetic Rats. Int. J. Biol. Macromol. 2016, 83, 126. DOI: 10.1016/j.ijbiomac.2015.11.045.
- Zhao, S.; Zhao, Y.; Li, S.; Zhao, J.; Zhang, G.; Wang, H.; Ng, T. B. A Novel Lectin with Highly Potent Antiproliferative and HIV-1 Reverse Transcriptase Inhibitory Activities from the Edible Wild Mushroom Russula Delica. Glycoconjugate J. 2010, 27(2), 259. DOI: 10.1007/s10719-009-9274-5.
- Teoh, Y. P.; Don, M. M.; Ujang, S. Nutrient Improvement Using Statistical Optimization for Growth of Schizophyllum Commune, and Its Antifungal Activity Against Wood Degrading Fungi of Rubberwood. Biotechnol. Prog. 2012, 28(1), 232. DOI: 10.1002/btpr.714.
- Hobbs, C. The Chemistry, Nutritional Value, Immunopharmacology, and Safety of the Traditional Food of Medicinal Split-Gill Fugus Schizophyllum Commune Fr.: Fr. (Schizophyllaceae). A Literature Review. Int. J. Med. Mushrooms. 2005, 7(1&2), 127. DOI: 10.1615/IntJMedMushr.v7.i12.130.
- Zhai, X.; Zhao, A.; Geng, L.; Xu, C. Fermentation Characteristics and Hypoglycemic Activity of an Exopolysaccharide Produced by Submerged Culture of Stropharia Rugosoannulata #2. Ann. Microbiol. 2013, 63(3), 1013. DOI: 10.1007/s13213-012-0555-z.
- Hou, Y.; Ding, X.; Hou, W.; Zhong, J.; Zhu, H.; Ma, B.; Xu, T.; Li, J. Anti-Microorganism, Anti-Tumor, and Immune Activities of a Novel Polysaccharide Isolated from Tricholoma Matsutake. Pharmacogn. Mag. 2013, 9(35), 244. DOI: 10.4103/0973-1296.113278.
- Yin, X.; You, Q.; Jiang, Z. Immunomodulatory Activities of Different Solvent Extracts from Tricholoma Matsutake (S. Ito Et S. Imai) Singer (Higher Basidiomycetes) on Normal Mice. Int. J. Med. Mushrooms. 2012, 14(6), 549. DOI: 10.1615/IntJMedMushr.v14.i6.20.
- Li, H.; Lee, H. -S.; Kim, S. -H.; Moon, B.; Lee, C. Antioxidant and Anti-Inflammatory Activities of Methanol Extracts of Tremella Fuciformis and Its Major Phenolic Acids. J. Food Sci. 2014, 79(4), C460. DOI: 10.1111/1750-3841.12393.
- Liu, J.; Meng, C. -G.; Yan, Y. -H.; Shan, Y. -N.; Kan, J.; Jin, C. -H. Structure, Physical Property and Antioxidant Activity of Catechin Grafted Tremella Fuciformis Polysaccharide. Int. J. Biol. Macromol. 2016, 82, 719. DOI: 10.1016/j.ijbiomac.2015.11.027.
- Paul, N.; Slathia, P. S.; Vaid, A.; Kumar, R. Traditional Knowledge of Gucchi, Morchella Esculenta (Ascomycetes), in Doda District, Jammu and Kashmir, India. Int. J. Med. Mushrooms. 2018, 20(5), 445. DOI: 10.1615/IntJMedMushrooms.2018025995.
- Vital, A. C. P.; Croge, C.; Gomes-da-Costa, S. M.; Matumoto-Pintro, P. T. Effect of Addition of Agaricus Blazei Mushroom Residue to Milk Enriched with Omega-3 on the Prevention of Lipid Oxidation and Bioavailability of Bioactive Compounds After in vitro Gastrointestinal Digestion. Int. J. Food Sci. Technol. 2017, 52(6), 1483. DOI: 10.1111/ijfs.13413.
- Stoffel, F.; Santana, W. D. O.; Fontana, R. C.; Gregolon, J. G. N.; Kist, T. B. L.; De Siqueira, F. G.; Mendonça, S.; Camassola, M. Chemical Features and Bioactivity of Grain Flours Colonized by Macrofungi as a Strategy for Nutritional Enrichment. Food Chem. 2019, 297, 124988. DOI: 10.1016/j.foodchem.2019.124988.
- Lu, X.; Brennan, M. A.; Narciso, J.; Guan, W.; Zhang, J.; Yuan, L.; Serventi, L.; Brennan, C. S. Correlations Between the Phenolic and Fibre Composition of Mushrooms and the Glycaemic and Textural Characteristics of Mushroom Enriched Extruded Products. LWT. 2020, 118, 108730. DOI: 10.1016/j.lwt.2019.108730.
- Olawuyi, I. F.; Lee, W. Y. Quality and Antioxidant Properties of Functional Rice Muffins Enriched with Shiitake Mushroom and Carrot Pomace. Int. J. Food Sci. Technol. 2019, 54(7), 2321. DOI: 10.1111/ijfs.14155.
- Spim, S. R. V.; Castanho, N. R. C. M.; Pistila, A. M. H.; Jozala, A. F.; Oliveira Júnior, J. M.; Grotto, D. Lentinula Edodes Mushroom as an Ingredient to Enhance the Nutritional and Functional Properties of Cereal Bars. J. Food Sci. Technol. 2021, 58(4), 1349. DOI: 10.1007/s13197-020-04646-5.
- Sabaratnam, V.; Kah-Hui, W.; Naidu, M.; David, P. R. Neuronal Health – Can Culinary and Medicinal Mushrooms Help? J. Traditional Complementary Med. 2013, 3(1), 62. DOI: 10.4103/2225-4110.106549.
- Chung, D. J.; Yang, M. Y.; Li, Y. R.; Chen, W. J.; Hung, C. Y.; Wang, C. J. Ganoderma Lucidum Repress Injury of Ethanol-Induced Steatohepatitis via Anti-Inflammation, Anti-Oxidation and Reducing Hepatic Lipid in C57BL/6J Mice. J. Funct. Foods. 2017, 33, 314. DOI: 10.1016/j.jff.2017.03.059.
- Wang, H.; Yu, Q.; Nie, S. P.; Xiang, Q. D.; Zhao, M. M.; Liu, S. Y.; Xie, M. Y.; Wang, S. Q. Polysaccharide Purified from Ganoderma Atrum Induced Activation and Maturation of Murine Myeloid-Derived Dendritic Cells. Food Chem. Toxicol. 2017, 108, 478. DOI: 10.1016/j.fct.2017.02.026.
- Jin, M.; Zhu, Y.; Shao, D.; Zhao, K.; Xu, C.; Li, Q.; Yang, H.; Huang, Q.; Shi, J. Effects of Polysaccharide from Mycelia of Ganoderma Lucidum on Intestinal Barrier Functions of Rats. Int. J. Biol. Macromol. 2017, 94, 1. DOI: 10.1016/j.ijbiomac.2016.09.099.
- Wu, Y.; Choi, M. -H.; Li, J.; Yang, H.; Shin, H. -J. Mushroom Cosmetics: The Present and Future. Cosmetics. 2016, 3(3), 22. DOI: 10.3390/cosmetics3030022.
- Taofiq, O.; González-Paramás, A. M.; Martins, A.; Barreiro, M. F.; Ferreira, I. C. F. R. Mushrooms Extracts and Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics—A Review. Ind. Crops Prod. 2016, 90, 38. DOI: 10.1016/j.indcrop.2016.06.012.
- Yang, S. -H.; Liu, H. -I.; Tsai, S. -J. Edible Tremella Polysaccharide for Skin Care. Patent US20060222608A1, 2006.
- Hui, L.; He, L. Comparison of the Moisture Retention Capacity of Tremella Polysaccharides and Hyaluronic Acid. Journal of Anhui Agricultural Sciences. 2012, 40, 13093.
- Kim, S. Y.; Go, K. C.; Song, Y. S.; Jeong, Y. S.; Kim, E. J.; Kim, B. J. Extract of the Mycelium of T. Matsutake Inhibits Elastase Activity and TPA-Induced MMP-1 Expression in Human Fibroblasts. Int. J. Mol. Med. 2014, 34(6), 1613. DOI: 10.3892/ijmm.2014.1969.
- Zhang, Y.; Mills, G. L.; Nair, M. G. Cyclooxygenase Inhibitory and Antioxidant Compounds from the Mycelia of the Edible Mushroom Grifola Frondosa. J. Agric. Food Chem. 2002, 50(26), 7581. DOI: 10.1021/jf0257648.
- Parvez, S.; Kang, M.; Chung, H. -S.; Bae, H. Naturally Occurring Tyrosinase Inhibitors: Mechanism and Applications in Skin Health, Cosmetics and Agriculture Industries. Phytotherapy Res. 2007, 21(9), 805. DOI: 10.1002/ptr.2184.
- Chang, T. -S. An Updated Review of Tyrosinase Inhibitors. Int. J. Mol. Sci. 2009, 10(6), 2440. DOI: 10.3390/ijms10062440.
- Nagasaka, R.; Ishikawa, Y.; Inada, T.; Ohshima, T. Depigmenting Effect of Winter Medicinal Mushroom Flammulna Velutipes (Higher Basidiomycetes) on Melanoma Cells. Int. J. Med. Mushrooms. 2015, 17(6), 511. DOI: 10.1615/IntJMedMushrooms.v17.i6.20.
- Kim, D.; Park, J.; Kim, J.; Han, C.; Yoon, J.; Kim, N.; Seo, J.; Lee, C. Flavonoids as Mushroom Tyrosinase Inhibitors: A Fluorescence Quenching Study. J. Agric. Food Chem. 2006, 54(3), 935. DOI: 10.1021/jf0521855.
- Chien, C. -C.; Tsai, M. -L.; Chen, C. -C.; Chang, S. -J.; Tseng, C. -H. Effects on Tyrosinase Activity by the Extracts of Ganoderma Lucidum and Related Mushrooms. Mycopathologia. 2008, 166(2), 117. DOI: 10.1007/s11046-008-9128-x.
- Rai, S. N.; Mishra, D.; Singh, P.; Vamanu, E.; Singh, M. P. Therapeutic Applications of Mushrooms and Their Biomolecules Along with a Glimpse of in silico Approach in Neurodegenerative Diseases. Biomed. Pharmacother. 2021, 137, 111377. DOI: 10.1016/j.biopha.2021.111377.
- Valverde, M. E.; Hernández-Pérez, T.; Paredes-López, O. Edible Mushrooms: Improving Human Health and Promoting Quality Life. Int. J. Microbiol. 2015, 2015, 376387. DOI: 10.1155/2015/376387.
- Spelman, K.; Sutherland, E.; Bagade, A. Neurological Activity of Lion’s Mane (Hericium Erinaceus). J. Restorative Med. 2017, 6(1), 19. DOI: 10.14200/jrm.2017.6.0108.
- Panda, S. K.; Luyten, W. Medicinal Mushrooms: Clinical Perspective and Challenges. Drug Discovery Today. 2022, 27(2), 636. DOI: 10.1016/j.drudis.2021.11.017.
- Tsai, M.; Hung, Y. C.; Chen, Y.; Huang, Y. C.; Kao, C. W.; Su, Y. L.; Chiu, H. E.; Rau, K. M. A Preliminary Randomised Controlled Study of Short-Term Antrodia Cinnamomea Treatment Combined with Chemotherapy for Patients with Advanced Cancer. BMC Complementary Altern. Med. 2016, 16.
- Sivanesan, I.; Muthu, M.; Gopal, J.; Oh, J. -W. Mushroom Polysaccharide-Assisted Anticarcinogenic Mycotherapy: Reviewing Its Clinical Trials. Molecules. 2022, 27(13), 4090. DOI: 10.3390/molecules27134090.
- Yu, Y.; Liu, Z.; Song, K.; Li, L.; Chen, M. Medicinal Value of Edible Mushroom Polysaccharides: A Review. J. Future Foods. 2023, 3(1), 16. DOI: 10.1016/j.jfutfo.2022.09.003.
- Wang, J. -L.; Bi, Z.; Zou, J. -W.; Gu, X. -M. Combination Therapy with Lentinan Improves Outcomes in Patients with Esophageal Carcinoma. Mol. Med. Rep. 2012, 5(3), 745.
- Zhang, M.; Zhang, Y.; Zhang, L.; Tian, Q. Mushroom Polysaccharide Lentinan for Treating Different Types of Cancers: A Review of 12 Years Clinical Studies in China. Prog. mol. biol. transl. sci. 2019, 163, 297.
- Kim, H. S.; Hong, J. T.; Kim, Y.; Han, S. -B. Stimulatory Effect of β-Glucans on Immune Cells. Immun. net. 2011, 11(4), 191. DOI: 10.4110/in.2011.11.4.191.
- Sun, M.; Bu, R.; Zhang, B.; Cao, Y.; Liu, C.; Zhao, W. Lentinan Inhibits Tumor Progression by Immunomodulation in a Mouse Model of Bladder Cancer. Integr. Cancer Ther. 2020, 19, 1534735420946823. DOI: 10.1177/1534735420946823.
- Zhang, Z.; Zha, Z.; Zhao, Z.; Liu, W.; Li, W. Lentinan Inhibits AGE-Induced Inflammation and the Expression of Matrix-Degrading Enzymes in Human Chondrocytes. Drug Des. Dev. Ther. 2020, Volume 14, 2819. DOI: 10.2147/DDDT.S243311.
- Landi, N.; Ragucci, S.; Culurciello, R.; Russo, R.; Valletta, M.; Pedone, P. V.; Pizzo, E.; Di Maro, A. Ribotoxin-Like Proteins from Boletus Edulis: Structural Properties, Cytotoxicity and in vitro Digestibility. Food Chem. 2021, 359, 129931. DOI: 10.1016/j.foodchem.2021.129931.
- Landi, N.; Ragucci, S.; Russo, R.; Valletta, M.; Pizzo, E.; Ferreras, J. M.; Di Maro, A. The Ribotoxin-Like Protein Ostreatin from Pleurotus Ostreatus Fruiting Bodies: Confirmation of a Novel Ribonuclease Family Expressed in Basidiomycetes. Int. J. Biol. Macromol. 2020, 161, 1329. DOI: 10.1016/j.ijbiomac.2020.07.267.
- Tayyrov, A.; Azevedo, S.; Herzog, R.; Vogt, E.; Arzt, S.; Lüthy, P.; Müller, P.; Rühl, M.; Hennicke, F.; Künzler, M. Heterologous Production and Functional Characterization of Ageritin, a Novel Type of Ribotoxin Highly Expressed During Fruiting of the Edible Mushroom Agrocybe Aegerita. Appl. Environ. Microbiol. 2019, 85(21), e01549. DOI: 10.1128/AEM.01549-19.
- Landi, N.; Grundner, M.; Ragucci, S.; Pavšič, M.; Mravinec, M.; Pedone, P. V.; Sepčić, K.; Di Maro, A. Characterization and Cytotoxic Activity of Ribotoxin-Like Proteins from the Edible Mushroom Pleurotus Eryngii. Food Chem. 2022, 396, 133655. DOI: 10.1016/j.foodchem.2022.133655.
- Ragucci, S.; Hussain, H. Z. F.; Bosso, A.; Landi, N.; Clemente, A.; Pedone, P. V.; Pizzo, E.; Di Maro, A. Isolation, Characterization, and Biocompatibility of Bisporitin, a Ribotoxin-Like Protein from White Button Mushroom (Agaricus Bisporus). Biomolecules. 2023, 13(2), 237. DOI: 10.3390/biom13020237.
- Landi, N.; Pacifico, S.; Ragucci, S.; Iglesias, R.; Piccolella, S.; Amici, A.; Di Giuseppe, A. M.; Di Maro, A. Purification, Characterization and Cytotoxicity Assessment of Ageritin: The First Ribotoxin from the Basidiomycete Mushroom Agrocybe Aegerita. Biochim Et Biophys. Acta (BBA)-General Subj. 2017, 1861(5), 1113. DOI: 10.1016/j.bbagen.2017.02.023.
- Citores, L.; Ragucci, S.; Ferreras, J. M.; Di Maro, A.; Iglesias, R. Ageritin, a Ribotoxin from Poplar Mushroom (Agrocybe Aegerita) with Defensive and Antiproliferative Activities. ACS Chem. Biol. 2019, 14(6), 1319. DOI: 10.1021/acschembio.9b00291.
- Ragucci, S.; Pacifico, S.; Ruocco, M. R.; Crescente, G.; Nasso, R.; Simonetti, M.; Masullo, M.; Piccolella, S.; Pedone, P. V.; Landi, N. Ageritin from Poplar Mushrooms: Scale-Up Purification and Cytotoxicity Towards Undifferentiated and Differentiated SH-SY5Y Cells. Food & Function. 2019, 10(10), 6342. DOI: 10.1039/C9FO01483G.
- Ragucci, S.; Landi, N.; Russo, R.; Valletta, M.; Pedone, P. V.; Chambery, A.; Di Maro, A. Ageritin from Pioppino Mushroom: The Prototype of Ribotoxin-Like Proteins, a Novel Family of Specific Ribonucleases in Edible Mushrooms. Toxins. 2021, 13(4), 263. DOI: 10.3390/toxins13040263.
- Patinho, I.; Selani, M. M.; Saldaña, E.; Bortoluzzi, A. C. T.; Rios-Mera, J. D.; da Silva, C. M.; Kushida, M. M.; Contreras-Castillo, C. J. Agaricus Bisporus Mushroom as Partial Fat Replacer Improves the Sensory Quality Maintaining the Instrumental Characteristics of Beef Burger. Meat Sci. 2021, 172, 108307. DOI: 10.1016/j.meatsci.2020.108307.
- Wang, L.; Guo, H.; Liu, X.; Jiang, G.; Li, C.; Li, X.; Li, Y. Roles of Lentinula Edodes as the Pork Lean Meat Replacer in Production of the Sausage. Meat Sci. 2019, 156, 44. DOI: 10.1016/j.meatsci.2019.05.016.
- Wang, M.; Zhao, R. A Review on Nutritional Advantages of Edible Mushrooms and Its Industrialization Development Situation in Protein Meat Analogues. J. Future Foods. 2023, 3(1), 1. DOI: 10.1016/j.jfutfo.2022.09.001.
- Kurek, M. A.; Onopiuk, A.; Pogorzelska-Nowicka, E.; Szpicer, A.; Zalewska, M.; Półtorak, A. Novel Protein Sources for Applications in Meat-Alternative Products—Insight and Challenges. Foods. 2022, 11(7), 957. DOI: 10.3390/foods11070957.
- Yuan, X.; Jiang, W.; Zhang, D.; Liu, H.; Sun, B. Textural, Sensory and Volatile Compounds Analyses in Formulations of Sausages Analogue Elaborated with Edible Mushrooms and Soy Protein Isolate as Meat Substitute. Foods. 2021, 11(1), 52. DOI: 10.3390/foods11010052.
- Stephan, A.; Ahlborn, J.; Zajul, M.; Zorn, H. Edible Mushroom Mycelia of Pleurotus Sapidus as Novel Protein Sources in a Vegan Boiled Sausage Analog System: Functionality and Sensory Tests in Comparison to Commercial Proteins and Meat Sausages. Eur. Food Res. Technol. 2018, 244(5), 913. DOI: 10.1007/s00217-017-3012-1.
- Santos, M. V.; Lespinard, A. R. Numerical Simulation of Mushrooms During Freezing Using the FEM and an Enthalpy: Kirchhoff Formulation. Heat Mass Transfer. 2011, 47(12), 1671. DOI: 10.1007/s00231-011-0831-7.
- Salehi, F. Characterization of Different Mushrooms Powder and Its Application in Bakery Products: A Review. Int. J. Food Prop. 2019, 22(1), 1375. DOI: 10.1080/10942912.2019.1650765.
- Cirlincione, F.; Venturella, G.; Gargano, M. L.; Ferraro, V.; Gaglio, R.; Francesca, N.; Rizzo, B. A.; Russo, G.; Moschetti, G.; Settanni, L. Functional Bread Supplemented with Pleurotus Eryngii Powder: A Potential New Food for Human Health. Int. J. Gastronomy Food Sci. 2022, 27, 100449. DOI: 10.1016/j.ijgfs.2021.100449.
- Amerikanou, C.; Tagkouli, D.; Tsiaka, T.; Lantzouraki, D. Z.; Karavoltsos, S.; Sakellari, A.; Kleftaki, S. -A.; Koutrotsios, G.; Giannou, V.; Zervakis, G. I. Pleurotus Eryngii Chips—Chemical Characterization and Nutritional Value of an Innovative Healthy Snack. Foods. 2023, 12(2), 353. DOI: 10.3390/foods12020353.
- Khoshnoudi-Nia, S.; Sharif, N.; Jafari, S. M. Loading of Phenolic Compounds into Electrospun Nanofibers and Electrosprayed Nanoparticles. Trends Food Sci. Technol. 2020, 95, 59. DOI: 10.1016/j.tifs.2019.11.013.
- Singhal, S.; Rasane, P.; Singh, J.; Kaur, S.; Kumar, V.; Dhawan, K.; Gurumayum, S.; Kaur, N.; Gupta, N.; Kaur, D. Effect of Processing on Vital Chemical Components of Button Mushroom. J. Food Process Eng. 2020, 43(1), e13229. DOI: 10.1111/jfpe.13229.
- Faridi Esfanjani, A.; Jafari, S. M. Biopolymer Nano-Particles and Natural Nano-Carriers for Nano-Encapsulation of Phenolic Compounds. Colloids and Surfaces B: Biointerfaces. 2016, 146, 532. DOI: 10.1016/j.colsurfb.2016.06.053.
- Soleymani, S.; Iranpanah, A.; Najafi, F.; Belwal, T.; Ramola, S.; Abbasabadi, Z.; Momtaz, S.; Farzaei, M. H. Implications of Grape Extract and Its Nanoformulated Bioactive Agent Resveratrol Against Skin Disorders. Arch. Dermatol. Res. 2019, 311(8), 577. DOI: 10.1007/s00403-019-01930-z.
