ABSTRACT
A significant percentage of the world population suffers from non-communicable diseases (NCDs) such as diabetes, cardiovascular diseases, cancers, and obesity due to unhealthy food habits. There is an association between the ingestion of carbohydrate-dense food products and diabetes and obesity. Resistant (RS) starch is chemically tolerable to the digestion process in the human gut. RS has several health benefits such as hypoglycemic effects, hypocholesterolemic effects, acting as a prebiotic, prevention of colonic cancers. Most of the inherent characteristics of RS such as high gelatinization temperature, favorable color, prebiotic properties, and good extrusion qualities make it suitable to use as a functional ingredient. Incorporating RS into food products is one of the strategies food scientists implement to lower the Glycemic Index (GI) and Glycemic Load (GL) of the food products. When carefully scrutinizing the plant-based bio-sphere, many potential food sources are enriched in resistant starches. Different processing techniques can be used to alter RS characteristics, such as granule morphologies, crystalline patterns, changes in the organizational groups, and increase the amount of RS. Therefore, this review focused on resistant starch sources, their health benefits, the effect of processing techniques on resistant starch, potential applications in the dynamic food industry, and future trends.
Introduction
Resistant starch (RS) is a type of starch that cannot be digested by digestive enzymes in the small intestine and instead passes to the colon to be fermented by bacteria. [Citation1] In 1982 Englyst and colleagues discovered a portion of starch that remained after enzymatic hydrolysis when developing an in vitro test for non-starch polysaccharides.[Citation2] Further research found that these starches might act as a substrate for microbial fermentation resulting in the formation of hydrogen, carbon dioxide, methane, and short-chain fatty acids (SCFA) such as acetate, propionate, and butyrate.[Citation3] Following that, a group of European Union-funded investigators defined RS as the total quantity of starch that resists digestion in the small intestine of healthy people.[Citation4] The digestion fate of RS is influenced by the size and shape of starch granules, quantity, relationships between starch and other dietary components, temperature, food processing methods, and the physical form of the grains or seeds (whole or partially disrupted).[Citation5] For instance, slower digestion rates are associated with higher amylose levels and higher RS levels in the starch.[Citation6]
According to the World Health Organization, three-quarters of global deaths related to NCDs occur in low- and middle-income countries because they are inadvertently adapted to unhealthy food patterns, which is one of the main reasons for NCDs globally.[Citation7,Citation8] A healthy digestive system is critical for overall quality of life amidst the fast change in eating choices and demanding lifestyles of consumers. As a result, innovative diets have been developed to boost the health of the digestive system while providing indirect protection for overall health.[Citation9] The use of RS to reduce the calorie value and accessible carbohydrate content in meals is gaining prominence, minimizing NCDs. RS has a reduced calorific value (8 kJ/g) as a dietary component as compared to completely digested starch (15 kJ/g).[Citation4] The rate of digestion of meals containing RS is much slower in the small intestine when compared to food that simply contains easily digested starch. Therefore, consuming such food results in prolonged and lower glucose release.[Citation10] Carbohydrate foods with low GI and calorific values are always better for health than high GI foods with high calorific value because it helps to prevent type 2 diabetes.[Citation11,Citation12]
The small particle size, bland flavor, and pure white appearance of RS make it suitable for a wide variety of food products since it does not negatively affect the final product’s taste or texture.[Citation3,Citation13,Citation14] RS consists of low swelling power, high water absorption capacity, higher thermal stability, and higher gelatinization temperature.[Citation3] Different processing techniques such as heat moisture treatment, acid methanol treatment, roasting, and drying techniques significantly affect the amount of resistant starch by affecting the digestibility of starch.[Citation15–18] RS has been incorporated in low-moisture items (pasta, bread, biscuits, cake, muffins, and morning cereals), certain moderate-moisture food products (ice cream, milk desserts, cheese), and even in high-moisture food products (yogurt and fermented yogurt drink).[Citation19,Citation20] This review focuses on types of RS, rich food sources of RS, health benefits, functional properties, the effect of processing techniques on RS, and the processing of RS-incorporated food.
Types of resistant starch
There are five types (RS1, RS2, RS3, RS4, RS5) of resistant starch, as shown in .[Citation21,Citation22] Types 1, 2, 3, and 5 are naturally found in foods as fibers, whereas types 2, 3, and 4 can be added to meals as functional ingredients.[Citation25] Type 1 RS is unfractionated or unrefined starch that is physically inaccessible in starchy meals.[Citation23] Wholly or partially milled grains, legumes, raw fruits, and vegetables contain this type of RS.[Citation21,Citation26] RS 1 is accumulated chiefly inside the plant cells. Human enzymes cannot degrade the cell walls, and these starches remain inaccessible.[Citation21,Citation27] Type 2 RS is inadequately gelatinized or uncooked starch, mainly in green bananas and raw potatoes.[Citation28] The major component of this type of RS is linear amylose chains.[Citation26,Citation29] These starch structures are resistant to enzymatic hydrolysis due to their crystallinity and compact nature.[Citation3] Retrograded starch is the third category of RS, and it is produced as a result of different food processing techniques in the food industry or by processing unmodified starch.[Citation20,Citation29] Certain molecular structural forms of starch are referred to as retrograded starches. When starch is cooked in water above its gelatinization temperature and then cooled, it undergoes retrogradation.[Citation30] Type 4 RS represents starch that has been physically or chemically altered to resist enzymatic digestion.[Citation20] For example, some starch ethers and cross-linked starches can be given.[Citation28] These chemical modifications either substitute different functional groups like hydroxypropyl, acetyl, and octenyl succinic anhydride groups or make linkages between amylose chains and make starch inaccessible to digestive enzymes.[Citation31] RS5 is known as Amylose-lipid complexes and can be present in some starch sources.[Citation21,Citation31] Hydrothermal treatment, such as baking, can induce the formation of amylose lipid complexes in the presence of lipid sources.[Citation31]
Table 1. Resistant starch types, sources, and rate of digestion.
Food sources rich in resistant starch
Potatoes, cereals, entire grains, pulses, beans, legumes, and fruits such as bananas contain naturally occurring RS. However, the variety, preparation or cooking method, storage temperature and duration, and serving temperature environment affect the RS amount in these foods.[Citation25]
The most RS-dense non-processed food is unripe bananas. According to Tribess et al.,[Citation32] the RS content of green banana flour ranges from 40.9–58.5%. Unripe banana flour was recently described as having a concentration of dietary fiber and resistant starch.[Citation32–34] Although bananas are an alternative supply of indigestible carbohydrates, primarily RS and dietary fiber, it is crucial to remember that the natural RS of the unripe fruit is rendered digestible when cooked.[Citation35] According to Yang et al.,[Citation36] RS content in bananas decreases by 61% at the final ripening stage compared to the initial ripening stage.
Raw potato starch has the highest RS concentration (65.20% − 95.32%) as a percentage of total starch.[Citation37] Starches from tubers, such as potatoes, have β-type crystallinity patterns, which make them difficult to digest. Amylomaize is primarily made up of amylose, which has been found in humans to decrease not only digestibility but also blood insulin and glucose levels.[Citation38]
When considering grain flour, protein and starch are the two main ingredients. Cereal grains contain lipid and fiber-rich pericarp, aleurone layers, and germ sections.[Citation39] RS is found naturally in cereal grains due to cell starch encapsulation or a protein matrix that protects against enzymatic attack.[Citation40]
The quantity of RS in leguminous plant seeds varies significantly. Legumes are noted for having a high dietary fiber content, both soluble and insoluble. Pulses are high in RS and retain functionality after cooking.[Citation14] Legume starches have higher amylose levels than cereal and pseudocereal starches.[Citation41] Depending on the processing conditions and the legume variety, hydrothermal processing can increase or decrease the proportion of resistant starch.[Citation42] Since cooked legumes have a higher tendency to retrograde, the effect itself contributes to slowing down the digestive process.[Citation43] Moreover, the quantity of RS3 in processed legumes is relatively high, resulting in a lower digestibility rate than in cereals. Legumes’ low digestion may be due to their high amylose content, which leads to a greater RS level.[Citation44] RS levels of different types of food sources are listed in the following table ().
Table 2. Different types of RS sources and their RS levels.
Benefits of having resistant starch in low-calorie foods
Resistant starch acts as a prebiotic
RS functions as a prebiotic and are suggested for use in probiotic compositions to promote the growth of probiotic microorganisms such as Bifidobacterium and Lactobacillus.[Citation28,Citation55,Citation56] Prebiotics are non-digestible food components that benefit the host by selectively boosting the development and activity of probiotics in the gastrointestinal system.[Citation57] Prebiotics are only functional after they reach the colon, where they are used by a particular group of bacteria, such as Bifidobacterium and Lactobacillus, among other gut microbes.[Citation58] Prebiotics will only be regarded as effective if they exclusively encourage the development and activity of beneficial bacteria.[Citation59,Citation60] Microbial fermentation of RS by probiotic bacteria in the large intestine results in short-chain fatty acids (SCFA), which are helpful for human health.[Citation61,Citation62] Wang et al.[Citation63] conducted a study to investigate the effect of resistant starch nanoparticles on the growth and proliferation of the probiotic Lactiplantibacillus plantarum. The results suggested that resistant starch nanoparticles were continuously fermented by probiotic bacteria compared to other carbon sources. Furthermore, the fermentation products had significant levels of butyric acid. This substance is healthy for humans, suggesting that the resistant starch nanoparticles encouraged the growth of gut probiotics as a prebiotic.[Citation63]
Prevention of colonic cancers
RS is fermented by the colonic microflora and results in SCFA and gases such as CO2, CH4, and H2.[Citation62,Citation64] The fermentation of dietary fiber and RS in the colon produces a high quantity of butyric acid and its salts.[Citation61] RS has also been shown to play a function in boosting butyric acid-producing bacteria. Butyrate is one of the vital energy sources for large intestinal epithelial cells, and it prevents them from becoming cancerous.[Citation65,Citation66] Butyrate can inhibit the growth and proliferation of tumor cells by stopping one phase of the cell cycle.[Citation67]
Hypocholesterolemic effects
RS has been demonstrated to reduce a lot of lipid metabolism parameters.[Citation68] In rats, RS diets (25% raw potato) significantly increased the pool of SCFA and SCFA absorption and decreased plasma cholesterol and triglyceride levels.[Citation69] In addition, all lipoprotein fractions, particularly high-density lipoproteins, had lower cholesterol concentrations, and the triglyceride-rich lipoprotein fraction had lower triglyceride concentrations.[Citation70] A similar study showed that hamsters fed diets containing cassava starch combined with 9.9% oat fiber or 9.7% RS had hypocholesterolemic properties, suggesting their ability to use in foods to improve cardiovascular health.[Citation71] RS coIn multiple trials, RS consumption has also been shown lower blood cholesterol levels in rats fed with a cholesterol-free diet.[Citation72]
Hypoglycemic effects
The GI approach for diabetes and weight control has sparked a lot of public and commercial attention in recent years.[Citation73,Citation74] Meals with a high GI release glucose into the bloodstream quickly and vice versa.[Citation75] However, it is important to realize that RS-enriched foods lower glycemic response merely because there’s less digestible starch available, not because of any additional physiological consequences.[Citation76] As a result, foods with highly resistant starch cause a lower glycemic index, and their delayed digesting rate has implications for more controlled glucose release applications.[Citation77] It was found that RS consumption resulted in lower blood glucose and insulin levels than other carbohydrates. In clinical trials, RS-rich meals were found to lower postprandial blood glucose levels in humans, suggesting that they may help metabolic management in diabetes II.[Citation78] Mitra et al. reported that when individuals with type 2 diabetes were fed rice-resistant starch cooked in various ways, their blood glucose levels did not rise.[Citation79]
Reduction of gallstone formation
Digestible starch promotes the production of bilestones by increasing insulin secretion, which boosts cholesterol synthesis. In turn, indigestible RS lowers the risk of bilestone formation.[Citation80] In communities that consume high-starch meals, such as India and China, RS intake is 2–4 times higher than in cultures that consume low-starch foods, such as the United States, Europe, and Australia, which may explain why the latter nations have a higher rate of bilestone instances.[Citation81]
Mineral absorption
Many studies have revealed that RS promotes the ileal absorption of various minerals. The ileal absorption of elements such as calcium, magnesium, zinc, iron, and copper was enhanced in rats fed a resistant starch-rich diet.[Citation82–84] SCFA results from the fermentation of resistant starch and reduces intestinal pH. Calcium is ionized from negatively-charged molecules when the pH is reduced, allowing calcium to be absorbed into the bloodstream.[Citation83]
Inhibition of fat accumulation
RS has been shown in several studies to have a high potential for modifying lipid oxidation. Work has illustrated that consuming a RS-rich diet significantly improved fat mobilization and utilization due to reduced insulin production.[Citation84]
Functional properties of resistant starch and the effect of processing techniques on the resistant starch
RS possesses physicochemical qualities such as low swelling power, high water absorption capacity, higher thermal stability, and higher gelatinization temperature, making it suitable for a wide range of food items.[Citation3] According to the latest findings, RS has low solubility and swelling power values compared to native starches.[Citation3,Citation85] The reasons behind these lower values are increased interactions between starch chains within the starch molecules, increased granular stability, and crystallite perfection. When comparing native starches and RS, the crystallinity of RS is higher than native starches. As a result of that, the interactions between starch chains are increased, and granular stability increases.[Citation13,Citation86] Liu et al.[Citation85] compared the water absorption capacities of native tartary buckwheat and sorghum starches with their annealed modified starches. The modified tartary buckwheat flour showed a higher water absorption capacity (1.34 ± 0.07) when compared with the native starch (1.10 ± 0.01). Modified sorghum starch also showed a higher water absorption capacity (0.95 ± 0.21) when compared with native starch (0.60 ± 0.01). Giuberti et al.[Citation13] conducted a study to compare the water absorption capacity (WAC) of native sorghum starch and resistant sorghum starch and concluded that resistant starch has higher WAC values than native sorghum starch. The reason for this phenomenon is the high amount of water binding sites in resistant starches compared to native starches.[Citation87] Compared to native starches, RS has greater thermal stability and higher gelatinization temperature.[Citation13,Citation88] The reason is higher transition temperatures and high enthalpies due to stronger hydrogen bonds in the starch structure, crystallite perfection, and granule stability.[Citation13,Citation89] Sun et al.[Citation88] compared the gelatinization values of sorghum starch and sorghum starch modified by heat moisture treatment and concluded that modified starch showed higher gelatinization temperatures (81.83 ± 0.03 °C) compared to native starches (74.26 ± 0.10 °C).
In general, processing methods used to manufacture cereal products tend to disrupt the food matrix and cause the starch to gelatinize. These activities make starch resistant to digestive enzymes.[Citation90] Heat Moisture Treatment (HMT) is a processing technique that enhances the RS content. This treatment is carried out using low moisture content of<35%, high temperature (84–120°C), and heating duration varying from 15 minutes to 16 hours.[Citation91] Faridah et al.[Citation91,Citation92]conducted a meta-analysis to investigate the effect of HMT on the RS amount of various sources, including cereals. It was concluded that HMT increases the RS amount in grains, especially wheat. Li et al.[Citation93] conducted a study on the effect of HMT on rice flour and discovered that HMT increased the RS content of rice flour and decreased its digestibility. Ng et al.[Citation15] reported that HMT increased the RS content in raw and gelatinized sago starch. Furthermore, it stated that combining Acid Methanol Treatment (AMT) with HMT was more efficient in reducing starch digestibility. Hung et al. (94) studied the impact of acid and HMT combination on RS amount in sweet potato and yam starches. Finally, it was concluded that exposing these starches to a combination of acid and HMT might increase the RS content significantly. The amorphous and crystalline regions of the starch undergo structural changes when subjected to HMT and convert starch into more resistant.[Citation13]
Roasting can be done to enhance the RS content, for instance. Verma et al.,[Citation18] illustrated that roasting ground nuts at 160 ºC for 12 minutes increased the RS amount in the groundnuts. Kanagaraj et al.[Citation17] reported that out of convective microwave heating, fluidized bed drying, pan roasting, and tray drying, microwave heating was the best dry heat treatment process in enhancing the RS amounts in rice and barnyard millet. RS increment was 22% and 14%, in rice and barnyard millet respectively. Simsek et al.[Citation16] discovered that roasting chickpeas could increase the RS content. When subjected to different roasting processes amylose/amylopectin ratio of starch samples increases and the digestibility of starch decrease. Gulzar et al.[Citation94] conducted a study investigating the effect of extrusion on the resistant starch amount in modified rice flour and concluded that the RS amount significantly increased under optimized extrusion conditions. High heat, high pressure, and shear forces are applied during the thermal process of extrusion to an uncooked bulk, such as cereal meals.[Citation90] Increased thermal stability of the retrograded starch generated in modified rice flour after being subjected to extrusion showed higher gelatinization transition temperatures, which was further confirmed by analyses of the ultrastructure of modified rice flour. Additionally, the starch digestibility and functional characteristics such as water absorption capacity, foaming capacity, least gelation concentration, swelling capacity, and emulsion activity of modified rice flour were significantly impacted by extrusion. Water absorption capacity increased significantly after extrusion because of the greater gelatinization of starch with extrusion conditions. Foaming capacity decreased significantly due to protein unfolding and aggregation. Swelling capacity decreased significantly because of the higher interactions between amylose and amylopectin molecules which leads to more ordered crystallites.[Citation90]
According to Alsaffar,[Citation90] tempering can increase the amount of RS. Cooked grains go through time-dependent modifications throughout the tempering phase, including the rearrangement of starch which is known as retrogradation. Gelatinized starch molecules start to reassociate (retrograde) when the starch gels are cooled, which increases crystallinity. Therefore, starch becomes more resistant to enzyme digestion.[Citation90] Acevedo et al.[Citation35] conducted a study to investigate the effect of drying techniques on the resistant starch amount. According to Hagenimana et al.[Citation95] granular particle size, starch – protein interactions, and degree of crystallinity play an important role in the rate of starch digestion. When drying, the removal of water facilitates the partial re-arrangement of starch granules, and this has the effect of decreasing starch digestibility. Also, it stated that a suitable drying method with hydrothermal treatment can increase the RS amount in starches.
Falodun et al.[Citation96] conducted a study to investigate the effect of different drying techniques namely sun drying, cabinet drying and freeze drying on Cardaba banana flour. Starch fractions of banana flour after subjecting to different drying techniques were determined. Freeze-dried samples had the overall best physicochemical properties and best soluble and insoluble indigestible starch fractions. Sun-dried samples had the best crude fibre and total ash contents. According to the results, freeze-drying was the best technique to dry while retaining the physicochemical properties. The efficient inactivation of endogenous amylases during the freeze-drying process results in a larger quantity of resistant starch when compared with conventional drying techniques.[Citation97]
Zeng et al.[Citation98] investigated the effect of different drying techniques on the structure and digestibility of short-chain amylose crystals. Freeze drying, air drying, and spray drying were used as the drying techniques. According to the results spray dried crystals had the highest content of slowly digestible starch while air-dried crystals had the highest amount of RS content. These results imply that different drying techniques can alter the molecular structure of crystals with differing digestibility.
Therefore, it is very clear that different processing techniques can be used to increase the amount of RS in starches. Selecting the most appropriate processing technique is crucial when developing food products with high RS content.
Processing of resistant starch-incorporated foods
As aforementioned small particle size, bland flavor, and pure white appearance make RS suitable for various food applications.[Citation3,Citation13] RS can be successfully incorporated into the following food products without considerable decremental changes in texture and sensory properties.
Yoghurt
RS can be incorporated into yogurt without significantly altering sensory and textural attributes. A considerable amount of studies have investigated the potential of using RS as an ingredient in yoghurt.[Citation99–101] A recent study discovered that the gel network of RS2-incorporated yogurts holds more serum than the control one (). It also showed that RS increases viscosity while maintaining an optimum post-fermentation acidity suitable for probiotic microorganisms.[Citation102] Saleh et al.[Citation103] reported that RS-enriched starches, specially modified starches, can be used as a syneresis controller to increase the firmness in yogurts. The reduction of syneresis happens due to the formation of a more robust network associated with the high amylose content in the starches.[Citation103]
Figure 1. Scanning electron micrographs of the set yogurt: (a) 1.5% (wt/wt) resistant starch 3 (RS3; physically modified starch); (b) 1.5% (wt/wt) resistant starch 2 (RS2; native starch granules); (c) 1.5% (wt/wt) sucrose; and (d) blank control. Scale bar = 10 µm. (102).
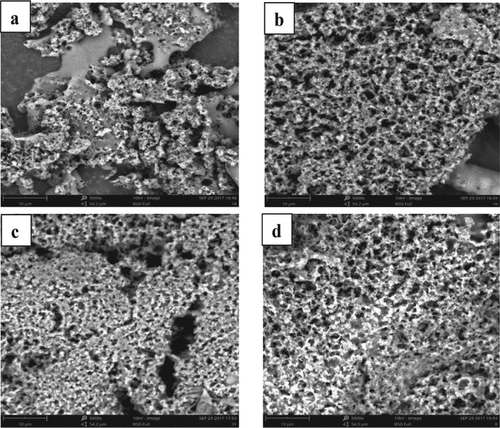
He et al.[Citation102] investigated the changes in the gelation process and microstructure of yogurt with the addition of resistant starch. They concluded that adding RS to yogurt improves qualitative characteristics such as serum separation, viscosity, flow behavior, and viscoelastic properties.
A network of casein micelles and fat was present in the yogurt containing RS3 (Figure a), which was distinguished by its very compact structure. This was consistent with earlier research on starch-based dairy. The image (figure b) revealed that RS2 was inserted between the protein aggregates, resulting in the formation of a fibrous structure and a reduction in the number of voids while an increase in their diameter. After this study, it was concluded that RS might be distributed throughout the casein micelles, interfering with the development of the protein matrix and resulting in a softer gel network while retaining more serum than the control one.[Citation102]
Mwizerwa et al.[Citation104] conducted a study to investigate the effect of adding RS-enriched cassava starch on the sensory attributes of yogurt. The results have shown that RS-enriched cassava starch did not affect sensory attributes such as mouthfeel, color, smell, and taste since it was applied in small amounts, less than 1%. Also, it stated that adding more than 1% of resistant starch-enriched cassava would adversely affect the sensory properties.[Citation104] Agyemang et al.[Citation105] showed that adding cassava starch during yogurt preparation may reduce the incidence of syneresis. A recent study reported that the physicochemical and sensory qualities of yogurt were enhanced by adding up to 5% green banana flour. It also improved the nutritional value of yogurt by increasing iron and fiber levels.[Citation106] Modified potato starch can be successfully used as a fat replacer in low-fat yogurt while achieving a wide range of physical textures because it reduces the syneresis successfully when reducing the fat level in yogurt.[Citation107] So, the addition of RS into yoghurts helps to retain more serum in the yoghurt structure while maintaining the sensory attributes. In contrast, Williams et al. found that the fermentation temperature plays a significant role towards the sensorial attributes of modified starch included milk yoghurts. For instance, fermentation at 43°C led to increased viscosity, increased graininess and whey drainage while fermentation at 35°C exhibited low viscous gels with decreased graininess and whey drainage. They further concluded the importance of the solids nonfat amount of the initial yoghurt formulation.[Citation100]
Inclusion of RS in yoghurt has positive impacts on the yoghurt gels such as higher serum holding capacity, increased viscosity, optimum post-fermentation acidity, and reduction of syneresis, softer gel network but also negatives such as affecting sensory attributes when a higher concentration of RS is used. All these changes depend on the concentration of RS incorporated. Therefore, it is critical to determine the correct percentage of RS.
Cheese
In the 1990s, new low-fat cheese products were introduced by cheese manufacturers.[Citation108] Noronha et al.[Citation108] conducted a study to investigate the possibility of replacing fat with functional fiber in imitation cheese and discovered that 90% of the fat in imitation cheese could be replaced by functional fibers such as RS at 60% moisture content. Typically imitation cheese contains 22% fat content and can be reduced to 2%.[Citation108]
Mozzarella cheese, which is popular in pizzas, typically contains about 20–27% fat and can be substituted with RS.[Citation108] Noronha et al.[Citation109] showed that RS helps to entrap the water molecules physically within the imitation cheese matrix as a result of the low water-holding capacity of RS. As a result, there is no competition to absorb water molecules between RS and casein. This property allows the water to remain available and maintain the desired texture of cheese. Adding ingredients that would compete with the protein for moisture might result in poor functionality of the product. Therefore, according to the study RS is an ideal ingredient to replace fat in cheese. Bi et al.[Citation19] conducted a study to investigate the effect of replacing fat from inulin or RS in imitation mozzarella cheese. According to the results when inulin or RS levels were increased to 12% (w/w), the resulting imitation cheeses were harder, less cohesive, and springier, as well as having less meltability and stretchability due to the lack of fat in the cheese matrix. The pH values of imitation mozzarella cheese were increased when incorporated with inulin or RS due to the increased moisture content. According to microscopic findings, interactions between casein and inulin or RS in imitation cheese were unfavorable and led to their mutual exclusion, notably for RS. According to Noronha et al.,[Citation109] RS is an ideal ingredient to replace the fat in imitation cheese whereas Bi et al.[Citation19] concluded that the replacement of fat with RS causes unfavorable characteristics in imitation mozzarella cheese.
Montesinos-Herrero et al.[Citation110] discovered that Novelose240 (RS2) or Novelose330 (RS3) could use to lower the fat content of Mozzarella cheese by up to 50% while maintaining good cheese functionality. Arimi et al.[Citation111] produced a low-fat cheese by substituting fat with 15.3% Hi-maize-240 (RS2), a type of resistant starch. Also, Hi-maize-240 acts as a crisping agent in imitation of heated expanded cheese.[Citation111]
The incorporation of RS helps to manufacture cheeses with a moisture content of 60% with increased hardness.[Citation112] Also, according to Noronha et al.,[Citation112] the melting temperature of cheese was not significantly affected by incorporating cheese because starch does not interact with the protein matrix. The melting temperature of cheese is defined as the temperature at which the protein matrix dissociates.[Citation112] Murtaza et al.[Citation113] investigated the impact of resistant starch on the quality parameters of low-fat cheddar cheese and discovered that textural, functional, and sensory properties could be increased positively by adding resistant starch. Therefore, RS is an ideal fat replacer in cheese.
Bakery products
There is a high demand for healthy bakery items, and product reformulation is critical when producing healthy bakery items.[Citation114] RS is a great ingredient when reformulating bakery products. RS can be incorporated into a large variety of baked foods, including batter systems, such as cakes, muffins, brownies, biscuits, and cookies.[Citation114–118]
RS was utilized as a low-calorie flour replacer in bakery items. It has baking qualities similar to wheat flour, such as golden brown color, cookie spread, surface cracking, and a pleasant aroma.[Citation114] RS functions as a texture modifier, giving the crumb a desirable crispness/tenderness.[Citation118] According to Spiller,[Citation119] when different fiber sources are incorporated into the bread, it produces undesirable characteristics such as reduced loaf volume, dark color, poor mouthfeel, and flavor masking. When resistant starch is incorporated instead of fiber, there is no increment in the water absorption capacity. When the bread was incorporated with the fibre, it showed a considerable increment in water-holding capacity. Because of its low water absorption capacity, RS had a less negative impact on dough rheology and was rheologically closest to the control dough. Compared to typical fibers, bread enriched with 40% RS had a higher loaf volume and better cell structure.[Citation119] The addition of RS to bread might lead to the formation of functional bread with significant benefits for consumers. Another study revealed that it is technologically feasible to manufacture bread that is acceptable to customers by substituting maize (20%) or green banana (10%) flour for wheat flour. However high quantities of maize or green banana flour had a detrimental effect on the creation of the gluten network, the retention of gas, and the quality of the finished loaf.[Citation115]
Laguna et al.[Citation117] researched to investigate the effect of adding resistant starch to short dough biscuits. Three percentages of resistant starch were used, namely 20%, 40%, and 60%. According to the results, sensory attributes of the 20% substitution did not differ from the control significantly. The taste, sweetness, and general acceptability were unaffected in 40% substitution while decreasing the acceptability of the color, look, and texture. However, biscuits made with 60% substitution had decreased sensory acceptance and significantly lower consumption intentions. According to another study, RS addition softened the texture of the dough and biscuits and helped to reduce dough stickiness, which was a crucial factor in regulating the best production.[Citation114] Khan et al.[Citation120] investigated the sensory properties of RS-incorporated cookies. They discovered that properties of RS, such as low water and oil absorption capacity and low water binding ability, provide better crispiness and crunchiness to the cookies.
RS can be substituted for bakery products successfully while reducing the GI value of the products and adding different functional properties. But it is crucial to determine the correct substitution percentage because high percentages can lead to lower consumption rates. According to Sanz et al.[Citation29] selecting the most appropriate RS type according to the final product is also very important.
Pasta
Pasta products are famous because they can be easily produced and stored for a long time. Compared to starchy meals like white bread, it has a lower GI.[Citation80] Fewer starch granules are available for amylase to hydrolyze due to the compact protein network formed by the extrusion process.[Citation121] Many studies have been demonstrated to investigate the effect of incorporating resistant starch into pasta.[Citation121–125] Aravind et al.[Citation122] showed that pasta enriched with RS2 and RS3 improved textural characteristics while reducing in vitro digestibility. Adding different forms of RS to pasta products might result in a further decreased glycemic response. The addition of RS affects the quality characteristics of the pasta, such as cooking time, water absorption, and texture.[Citation80]
Adding RS to pasta reduced the optimum cooking time and swelling index.[Citation122–126] According to Aravind et al.,[Citation122] it also increased the cooking loss allowing more carbohydrates to be leached out of pasta. High cooking loss happens due to the weakened protein matrix resulting from reduced gluten content.[Citation122] But Foschia et al.[Citation124] reported that RS can decrease cooking loss and stickiness while increasing firmness in pasta. Moreover, it stated that the cooking loss of the RS-enriched samples was 30% lower than the reference, indicating the development of a structure that is more resistant to disintegrating during boiling. Despite the higher price, RS fortification makes gluten-free pasta more effective for people with celiac disease.[Citation124] When considering literature some studies have stated opposite facts regarding cooking loss in pasta when incorporated with RS. However, when making gluten-free and low-GI pasta addition of RS is very important.
Noodles
Recently researchers are investigating the ability to incorporate noodles with RS and RS-rich sources.[Citation127–129] Li et al.[Citation130] studied the eating quality and glycemic index of RS-enriched flour incorporated (10–20%, w/w) noodles and discovered that it resulted in noodles with more nutrient composition and low GI value. Furthermore, it was found that 10–20% w/w of RS source flour generates a dough with appropriate strength and extensible texture required for noodles preparation. Hsieh et al.[Citation130] found that adding 40% RS4 to wheat noodles did not affect their hardness after cooking. A similar study showed that replacing 20% of wheat flour with barley-resistant starch (RS4) could increase the heat stability of noodles than using wheat starch alone.[Citation129] Higher hydrogen bonds in the starch structure, crystallite perfection, and granule stability are the reason for the higher transition temperatures, high RS enthalpies, and heat stability.[Citation13,Citation89] Hsieh et al.[Citation131] replace the wheat flour with cross-linked phosphorylated RS4 proportions and concluded that the extensibility, cohesion, and springiness of cooked noodles were also decreased when the amount of RS4 was raised, while the hardness of the noodles remained unaffected. Cooked noodles containing cross-linked RS4 appeared to have less cohesiveness and springiness while maintaining the same level of hardness, which was likely due to the RS4‘s low swelling capabilities. Menon et al.[Citation127] reported that adding banana, mung bean, or annealed cassava starch (ACS) which are rich in RS to noodles reduces the starch digestibility, resulting in the preservation of resistant starch. Therefore, we can conclude that RS is a very important ingredient when making low-GI, low-calorie and healthy noodles with functional properties.
Battered fried products
The thermal stability of RS3 is greater than that of the other types of RS. It permits a comparatively greater integration level without impairing the quality of several food items. The replacement of 20% RS3 (Novelose330) for wheat flour caused a significant increase in the total dietary fiber content.[Citation29] In battered fried foods, color is the leading property that represents the optimal time to control frying. The desirable color is light golden brown. The golden-brown color increases significantly with the increase of RS3 content. Maillard and caramelizing reactions are the main reason for intensified color.[Citation29] Boue et al.[Citation132] conducted a study using wheat flour batter and RS-rich fried rice batter (RS2 and RS3) in fried onions to discover the changes in the final product. It was found that when compared to wheat flour batter, RS-rich fried rice batter has a low oil absorption capacity without altering textural and sensory attributes. It also stated that adding RS to battered fried products as a functional fiber can promote the RS intake of consumers.
Sausages
Research has been carried out to investigate the ability to incorporate sausages with RS and RS-rich sources.[Citation62,Citation133,Citation134] Garcia-Santos et al.[Citation133] investigate the physicochemical characteristics and taste qualities after adding resistant starch up to 4% as a fat substitute in sausages. According to the results, using resistant starch as a fat substitute in sausages did not affect the overall composition, textural profile, or sensory qualities, suggesting that it may be used to make healthier meat products. Similar research indicates that a combination of resistant starch and β-glucan can be used to make prebiotic sausages.[Citation9] Salazar et al.[Citation135] developed a fiber-enriched Frankfurter-type sausage using underutilized green banana flour. According to the analysis results, sensory and textural properties (hardness, cohesiveness, springiness, chewiness, adhesiveness) did not differ significantly from the control one.
Future trends
Resistant starch possesses excellent potential in the future dynamic-food market, particularly in the health-food sector, because carbohydrate-related NCDs are increasing at an alarming rate globally, irrespective of economic growth. Under this circumstance, RS can play a vital role in the broad spectrum of the food industry, particularly in the bakery, pasta, dairy, and health food sectors.
In order to increase the utilization of RS within food products, preparing healthy flour by incorporating the flour of RS-rich sources and using them in food products such as bakery products, pasta, and noodles could be an effective strategy.[Citation19,Citation95,Citation100] Apart from that, flour from RS-rich sources can be incorporated directly into products such as dairy products and meat products. Also, symbiotic dairy products can be developed by incorporating RS-rich starch into set yogurts and drinking yogurts.[Citation102,Citation105,Citation136] In the future, RS will play a significant role in low-fat foods such as low-fat dairy and low-fat meat products.[Citation112,Citation133] These incorporated food products are developed on laboratory scales but not mass-scale productions. Further research studies should be carried out to investigate the adverse effects of using RS for mass-scale productions. Moreover, many resistant starch-rich sources are available yet to be discovered and incorporated into food. The interactions between food components and RS when incorporated into different types of food should be further investigated.
An attractive feature of RS is its relatively low oil holding capacity.[Citation132] This feature will also be a positive, tangible factor when making low-fat food products. Hence, scientists involved in conducting food research have a great potential to carry out their studies in the sphere of RS in the future while opening new business avenues for the investors involved in manufacturing new food products for the dynamic food market. Indeed, researchers are engaged in producing crops which have higher amounts of RS in their carbohydrate storage fraction for the specific purpose of increasing the non-digestible carbohydrate portions in foods.[Citation137–139] Furthermore, most of the beneficial attributes of certain food products were obtained mainly within lab-scale research. How these small-scale results be translated into pilot-scale outcomes is still a question. Thus, the results obtained through lab-scale research must be validated under pilot-scale operations to see if the same beneficial aspects can be obtained.
Conclusion
RS-rich sources can be used successfully to develop low-calorie food products due to the low calorific value of resistant starch. Also, RS can be incorporated into dairy, meat, and other types of starch-based products to make food healthier due to its various functional properties. It will contribute to societal well-being as well as the advancement of the food sector. Food processing techniques such as heat moisture treatment, acid methanol treatment, dry heat treatment, and extrusion can increase the amount of RS. However, identification of new resistant starch rich sources, their interactions with other components with a food matrix, their symbiotic benefits and pilot scale replication of lab scale experiments are still some of the challenges that needs to be addresses to fully understand the full nutritional potential. Hence, further research specific to a particular type of RS needs to be established.
Acknowledgments
This review article is based on the work supported by University Research Grants (Grant No. ASP/01/RE/SCI/2022/12), University of Sri Jayewardenepura, Gangodawila, Nugegoda, Sri Lanka.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Bojarczuk, A.; Skąpska, S.; Mousavi Khaneghah, A.; Marszałek, K. Health Benefits of Resistant Starch: A Review of the Literature. J. Funct. Foods. 2022, 93, 105094. DOI: 10.1016/j.jff.2022.105094.
- Lunn, J.; Buttriss, J. L. Carbohydrates and Dietary Fibre. Nutr. Bull. 2007, 32(1), 21–64. DOI: 10.1111/j.1467-3010.2007.00616.x.
- Ashwar, B. A.; Gani, A.; Shah, A.; Wani, I. A.; Masoodi, F. A. Preparation, Health Benefits and Applications of Resistant Starch—A Review. Starke. 2016, 68(3–4), 287–301. DOI: 10.1002/star.201500064.
- Nugent, A. P. Health Properties of Resistant Starch. Nutr. Bull. 2005, 30(1), 27–54. DOI: 10.1111/j.1467-3010.2005.00481.x.
- Slavin, J. Whole Grains and Human Health. Nutr. Res. Rev. 2004, 17(1), 99–110. DOI: 10.1079/NRR200374.
- Giuberti, G.; Gallo, A.; Masoero, F.; Ferraretto, L. F.; Hoffman, P. C.; Shaver, R. D. Factors Affecting Starch Utilization in Large Animal Food Production System: A Review. Starke. 2014, 66(1–2), 72–90. DOI: 10.1002/star.201300177.
- Olatona, F. A.; Onabanjo, O. O.; Ugbaja, R. N.; Nnoaham, K. E.; Adelekan, D. A. Dietary Habits and Metabolic Risk Factors for Non-Communicable Diseases in a University Undergraduate Population. J Health Popul. Nutr. 2018, 37(1), 1–9. DOI: 10.1186/s41043-018-0152-2.
- Al-Jawaldeh, A.; Abbass, M. S. Unhealthy Dietary Habits and Obesity: The Major Risk Factors Beyond Non-Communicable Diseases in the Eastern Mediterranean Region. Front Nutr. 2022, 9, 9. DOI: 10.3389/fnut.2022.817808.
- Amini Sarteshnizi, R.; Hosseini, H.; Bondarianzadeh, D.; Colmenero, F. J.; Khaksar, R. Optimization of Prebiotic Sausage Formulation: Effect of Using β-Glucan and Resistant Starch by D-Optimal Mixture Design Approach. LWT- Food Sci. Technol. 2015, 62(1), 704–710. DOI: 10.1016/j.lwt.2014.05.014.
- Cione, E.; Fazio, A.; Curcio, R.; Tucci, P.; Lauria, G.; Cappello, A. R.; Dolce, V. Resistant Starches and Non-Communicable Disease: A Focus on Mediterranean Diet. Foods. 2021, 10(9), 2062. DOI: 10.3390/foods10092062.
- Foroni, F.; Esmaeilikia, M.; Rumiati, R. I. What Makes a Food Healthy? Sex Differences in What is Associated to Healthiness Evaluations. Food Qual. Prefer. 2022, 96, 104438. DOI: 10.1016/j.foodqual.2021.104438.
- Cheng, M.; Cui, Y.; Yan, X.; Zhang, R.; Wang, J.; Wang, X. Effect of Dual-Modified Cassava Starches on Intelligent Packaging Films Containing Red Cabbage Extracts. Food Hydrocoll. 2022, 124, 107225. DOI: 10.1016/j.foodhyd.2021.107225.
- Giuberti, G.; Marti, A.; Gallo, A.; Grassi, S.; Spigno, G. Resistant Starch from Isolated White Sorghum Starch: Functional and Physicochemical Properties and Resistant Starch Retention After Cooking. A Comparative Study. Starch - Stärke. 2019, 71(7–8), 1800194. DOI: 10.1002/star.201800194.
- Rochfort, S.; Panozzo, J. Phytochemicals for Health, the Role of Pulses. J. Agric. Food. Chem. 2007, 55(20), 7981–7994. DOI: 10.1021/jf071704w.
- Ng, J. Q.; Siew, C. K.; Mamat, H.; Matanjun, P.; Lee, J. S. Effect of Acid Methanol Treatment and Heat Moisture Treatment on in-Vitro Digestibility and Estimated Glycemic Index of Raw and Gelatinized Sago (Metroxylon sagu) Starch. Starke. 2018, 70(9–10), 1700198. DOI: 10.1002/star.201700198.
- Simsek, S.; Herken, E. N.; Ovando‐martinez, M. Chemical Composition, Nutritional Value and in-Vitro Starch Digestibility of Roasted Chickpeas. J. Sci. Food Agric. 2016, 96(8), 2896–2905. DOI: 10.1002/jsfa.7461.
- Kanagaraj, S. P.; Ponnambalam, D.; Antony, U. Effect of Dry Heat Treatment on the Development of Resistant Starch in Rice (Oryza sativa) and Barnyard Millet (Echinochloa furmantacea). J. Food Process Preserv. 2019, 43(7), e13965. DOI: 10.1111/jfpp.13965.
- Verma, A.; Mahatma, M.; Thawait, L.; Singh, S.; Gangadhar, K.; Kona, P.; Singh, A. Processing Techniques Alter Resistant Starch Content, Sugar Profile and Relative Bioavailability of Iron in Groundnut (Arachis Hypogaea L.) Kernels. J. Food Compost. Anal. 2022, 112, 104653. DOI: 10.1016/j.jfca.2022.104653.
- Bi, W.; Zhao, W.; Li, D.; Li, X.; Yao, C.; Zhu, Y.; Zhang, Y. Effect of Resistant Starch and Inulin on the Properties of Imitation Mozzarella Cheese. Int. J. Food. Prop. 2016, 19(1), 159–171. DOI: 10.1080/10942912.2015.1013634.
- Homayouni, A.; Amini, A.; Keshtiban, A. K.; Mortazavian, A. M.; Esazadeh, K.; Pourmoradian, S. Resistant Starch in Food Industry: A Changing Outlook for Consumer and Producer. Starke. 2014, 66(1–2), 102–114. DOI: 10.1002/star.201300110.
- Walsh, S. K.; Lucey, A.; Walter, J.; Zannini, E.; Arendt, E. K. Resistant Starch—An Accessible Fiber Ingredient Acceptable to the Western Palate. Compr. Rev. Food Sci. Food Saf. 2022, 21(3), 2930–2955. DOI: 10.1111/1541-4337.12955.
- Li, C.; Hu, Y. New Definition of Resistant Starch Types from the Gut Microbiota Perspectives–A Review. Crit. Rev. Food Sci. Nutr. 2022, 1–11. DOI: 10.1080/10408398.2022.2031101.
- Sanz, T.; Salvador, A.; Baixauli, R.; Fiszman, S. M. Evaluation of Four Types of Resistant Starch in Muffins. Effects in Texture, Colour and Consumer Response. Eur. Food Res. Technol. 2009, 229(2), 197–204. DOI: 10.1007/s00217-009-1040-1.
- Mikulíková, D.; Benková, M.; Kraic, J. The Potential of Common Cereals to Form Retrograded Resistant Starch. Czech. J. Genet. Plant Breed. 2006, 42(3), 95. DOI: 10.17221/3648-cjgpb.
- Patterson, M. A.; Maiya, M.; Stewart, M. L. Resistant Starch Content in Foods Commonly Consumed in the United States: A Narrative Review. J. Acad. Nutr. Diet. 2020, 120(2), 230–244. DOI: 10.1016/j.jand.2019.10.019.
- Gill, S. K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary Fibre in Gastrointestinal Health and Disease. Nature Reviews Gastroenterology & Hepatology. 2021, 18(2), 101–116. DOI: 10.1038/s41575-020-00375-4.
- Dhital, S.; Brennan, C.; Gidley, M. J. Location and Interactions of Starches in Planta: Effects on Food and Nutritional Functionality. Trends Food Sci. Technol. 2019, 93, 158–166. DOI: 10.1016/j.tifs.2019.09.011.
- Fuentes‐zaragoza, E.; Sánchez‐zapata, E.; Sendra, E.; Sayas, E.; Navarro, C.; Fernández‐lópez, J.; Pérez‐alvarez, J. A. Resistant Starch as Prebiotic: A Review. Starke. 2011, 63(7), 406–415. DOI: 10.1002/star.201000099.
- Sanz, T.; Salvador, A.; Fiszman, S. M. Evaluation of Four Types of Resistant Starch in Muffin Baking Performance and Relationship with Batter Rheology. Eur. Food Res. Technol. 2008, 227(3), 813–819. DOI: 10.1007/s00217-007-0791-9.
- Matignon, A.; Tecante, A. Starch Retrogradation: From Starch Components to Cereal Products. Food Hydrocoll. 2017, 68, 43–52. DOI:10.1016/j.foodhyd.2016.10.032.
- Roman, L.; Martinez, M. M. Structural Basis of Resistant Starch (RS) in Bread: Natural and Commercial Alternatives. Foods. 2019, 8(7), 267. DOI: 10.3390/foods8070267.
- Tribess, T.; Hernández-Uribe, J.; Méndez-Montealvo, M.; Menezes, E.; Bello-Perez, L.; Tadini, C. Thermal Properties and Resistant Starch Content of Green Banana Flour (Musa cavendishii) Produced at Different Drying Conditions. LWT - Food Sci. Technol. 2009, 42(5), 1022–1025. DOI: 10.1016/j.lwt.2008.12.017.
- Das, M.; Rajan, N.; Biswas, P.; Banerjee, R. A Novel Approach for Resistant Starch Production from Green Banana Flour Using Amylopullulanase. LWT - Food Sci. Technol. 2022, 153, 112391. DOI: 10.1016/j.lwt.2021.112391.
- Sarawong, C.; Schoenlechner, R.; Sekiguchi, K.; Berghofer, E.; Ng, P. K. Effect of Extrusion Cooking on the Physicochemical Properties, Resistant Starch, Phenolic Content and Antioxidant Capacities of Green Banana Flour. Food Chem. 2014, 143, 33–39. DOI: 10.1016/j.foodchem.2013.07.081.
- Rodríguez-Ambriz, S.; Islas-Hernández, J.; Agama-Acevedo, E.; Tovar, J.; Bello-Pérez, L. Characterization of a Fibre-Rich Powder Prepared by Liquefaction of Unripe Banana Flour. Food Chem. 2008, 107(4), 1515–1521. DOI: 10.1016/j.foodchem.2007.10.007.
- Yang, J.; Bi, Y.; Liang, S.; Gu, Z.; Cheng, L.; Li, C.; Hong, Y.; Zhang, Y.; Hong, Y. The in vivo Digestibility Study of Banana Flour with High Content of Resistant Starch at Different Ripening Stages. Food Funct. 2020, 11(12), 10945–10953. DOI: 10.1039/D0FO02494E.
- Jiranuntakul, W.; Puttanlek, C.; Rungsardthong, V.; Puncha-Arnon, S.; Uttapap, D. Microstructural and Physicochemical Properties of Heat-Moisture Treated Waxy and Normal Starches. J. Food Eng. 2011, 104(2), 246–258. DOI: 10.1016/j.jfoodeng.2010.12.016.
- Bednar, G. E.; Patil, A. R.; Murray, S. M.; Grieshop, C. M.; Merchen, N. R.; Fahey, G. C., Jr. Starch and Fiber Fractions in Selected Food and Feed Ingredients Affect Their Small Intestinal Digestibility and Fermentability and Their Large Bowel Fermentability in-Vitro in a Canine Mode. Nutr. J. 2001, 131(2), 276–286. DOI: 10.1093/jn/131.2.276.
- Seal, C. J.; Courtin, C. M.; Venema, K.; de Vries, J. Health Benefits of Whole Grain: Effects on Dietary Carbohydrate Quality, the Gut Microbiome, and Consequences of Processing. Compr. Rev. Food Sci. Food Saf. 2021, 20(3), 2742–2768. DOI: 10.1111/1541-4337.12728.
- Nguyen, S. N.; Drawbridge, P.; Beta, T. Resistant Starch in Wheat, Barley, Rye, and Oat‐based Foods: A Review. Starke. 2022, 2100251. DOI: 10.1002/star.202100251.
- Mikulíková, D.; Masár, Š.; Kraic, J. Biodiversity of Legume Health‐promoting Starch. Starke. 2008, 60(8), 426–432. DOI: 10.1002/star.200700693.
- Giczewska, A.; Borowska, J. Nutritional Value of Broad Bean Seeds. Part 1: Starch and Fibre. Food/Nahrung. 2003, 47(2), 95–97. DOI: 10.1002/food.200390033.
- Palavecino, P. M.; Curti, M. I.; Bustos, M. C.; Penci, M. C.; Ribotta, P. D. Sorghum Pasta and Noodles: Technological and Nutritional Aspects. Plant Foods Hum. Nutr. 2020, 75(3), 326–336. DOI: 10.1007/s11130-020-00829-9.
- Tharanathan, R.; Mahadevamma, S. Grain Legumes—A Boon to Human Nutrition. Trends Food Sci. Technol. 2003, 14(12), 507–518. DOI: 10.1016/j.tifs.2003.07.002.
- Vijayakumar, V.; Samal, S. K.; Mohanty, S.; Nayak, S. K. Recent Advancements in Biopolymer and Metal Nanoparticle-Based Materials in Diabetic Wound Healing Management. Int. J. Biol. Macromol. 2019, 122, 137–148. DOI: 10.1016/j.ijbiomac.2018.10.120.
- Kim, M. J.; Oh, S. G.; Chung, H. J. Impact of Heat Moisture Treatment Applied to Brown Rice Flour on the Quality and Digestibility Characteristics of Korean Rice Cake. Food Sci. Biotechnol. 2017, 26(6), 1579–1586. DOI: 10.1007/s10068-017-0151-x.
- Chen, X.; He, X.; Fu, X.; Huang, Q. In Vitro Digestion and Physicochemical Properties of Wheat Starch/Flour Modified by Heat-Moisture Treatment. J. Cereal Sci. 2015, 63, 109–115. DOI: 10.1016/j.jcs.2015.03.003.
- Goel, C.; Semwal, A. D.; Khan, A.; Kumar, S.; Sharma, G. K. Physical Modification of Starch: Changes in Glycemic Index, Starch Fractions, Physicochemical and Functional Properties of Heat Moisture Treated Buckwheat Starch. J. Food Sci. Technol. 2020, 57(8), 2941–2948. DOI: 10.1007/s13197-020-04326-4.
- Amadou, I.; Gounga, M. E.; Shi, Y.; Le, G. Fermentation and Heat-Moisture Treatment Induced Changes on the Physicochemical Properties of Foxtail Millet (Setaria italica) Flour. Food Bioprod. Process. 2014, 92(1), 38–45. DOI: 10.1016/j.fbp.2013.07.009.
- Chung, H.; Liu, Q.; Hoover, R. Effect of Single and Dual Hydrothermal Treatments on the Crystalline Structure, Thermal Properties, and Nutritional Fractions of Pea, Lentil, and Navy Bean Starches. Food. Res. Int. 2010, 43(2), 501–508. DOI: 10.1016/j.foodres.2009.07.030.
- Van Hung, P.; Huong, N. T. M.; Phi, N. T. L.; Tien, N. N. T. Physicochemical Characteristics and in-Vitro Digestibility of Potato and Cassava Starches Under Organic Acid and Heat-Moisture Treatments. Int. J. Biol. Macromol. 2017, 95, 299–305. DOI: 10.1016/j.ijbiomac.2016.11.074.
- Trung, P. T. B.; Ngoc, L. B. B.; Hoa, P. N.; Tien, N. N. T.; Hung, P. V. Impact of Heat-Moisture and Annealing Treatments on Physicochemical Properties and Digestibility of Starches from Different Colored Sweet Potato Varieties. Int. J. Biol. Macromol. 2017, 105, 1071–1078. DOI: 10.1016/j.ijbiomac.2017.07.131.
- Li, H.; Wang, R.; Liu, J.; Zhang, Q.; Li, G.; Shan, Y.; Ding, S. Effects of Heat-Moisture and Acid Treatments on the Structural, Physicochemical, and in-Vitro Digestibility Properties of Lily Starch. Int. J. Biol. Macromol. 2020, 148, 956–968. DOI: 10.1016/j.ijbiomac.2020.01.181.
- Wu, Z.; Lu, J.; Wang, X.; Hu, B.; Ye, H.; Fan, J.; Abid, M.; Zeng, X. Optimization for Production of Exopolysaccharides with Antitumor Activity in-Vitro from Paecilomyces Hepiali. Carbohydr. Polym. 2014, 99, 226–234. DOI: 10.1016/j.carbpol.2013.08.010.
- Brown, I. L.; Yotsuzuka, M.; Birkett, A.; Henriksson, A. P. Synbiotics and Resistant Starch. Journal of Japanese Association for Dietary Fiber Research. 2006, 10(1), 1–10. DOI: 10.11217/jjdf2004.10.1.
- Shamloo, M.; Mollard, R.; Wang, H.; Kingra, K.; Tangri, N.; MacKay, D. A Randomized Double-Blind Cross-Over Trial to Study the Effects of Resistant Starch Prebiotic in Chronic Kidney Disease (ReSpeckd). Trials. 2022, 23(1), 1–12. DOI: 10.1186/s13063-022-06009-1.
- Scholz-Ahrens, K. E.; Ade, P.; Marten, B.; Weber, P.; Timm, W.; Aςil, Y.; Schrezenmeir, J. P.; Schrezenmeir, J. Prebiotics, Probiotics, and Synbiotics Affect Mineral Absorption, Bone Mineral Content, and Bone Structure1. J. Nutr. 2007, 137(3), 838S–846S. DOI: 10.1093/jn/137.3.838S.
- Cummings, J. H.; Macfarlane, G. T. Gastrointestinal Effects of Prebiotics. Br. J. Nutr. 2002, 87(S2), S145–151. DOI: 10.1079/BJN/2002530.
- Zaman, S. A.; Sarbini, S. R. The Potential of Resistant Starch as a Prebiotic. Crit. Rev. Biotechnol. 2016, 36(3), 578–584. DOI: 10.3109/07388551.2014.993590.
- Roberfroid, M. B. Prebiotics and Probiotics: Are They Functional Foods? Am. J. Clin. Nutr. 2000, 71(6), 1682S–1687S. DOI: 10.1093/ajcn/71.6.1682S.
- Liang, D.; Li, N.; Dai, X.; Zhang, H.; Hu, H. Effects of Different Types of Potato Resistant Starches on Intestinal Microbiota and Short‐chain Fatty Acids Under in-Vitro Fermentation. Int. J. Food Sci. Technol. 2021, 56(5), 2432–2442. DOI: 10.1111/ijfs.14873.
- Tiwari, U. P.; Singh, A. K.; Jha, R. Fermentation Characteristics of Resistant Starch, Arabinoxylan, and β-Glucan and Their Effects on the Gut Microbial Ecology of Pigs: A Review. Anim. Nutr. 2019, 5(3), 217–226. DOI: 10.1016/j.aninu.2019.04.003.
- Wang, C.; McClements, D. J.; Jiao, A.; Wang, J.; Jin, Z.; Qiu, C. Resistant Starch and Its Nanoparticles: Recent Advances in Their Green Synthesis and Application as Functional Food Ingredients and Bioactive Delivery Systems. Trends Food Sci. Technol. 2022, 119, 90–100. DOI: 10.1016/j.tifs.2021.11.025.
- Cui, W.; Ma, Z.; Li, X.; Hu, X. Structural Rearrangement of Native and Processed Pea Starches Following Simulated Digestion in-Vitro and Fermentation Characteristics of Their Resistant Starch Residues Using Human Fecal Inoculum. Int. J. Biol. Macromol. 2021, 172, 490–502. DOI: 10.1016/j.ijbiomac.2021.01.092.
- Bede, D.; Zaixiang, L. Recent Developments in Resistant Starch as a Functional Food. Starke. 2021, 73(3–4), 2000139. DOI: 10.1002/star.202000139.
- Nelson, B.; Cray, N.; Ai, Y.; Fang, Y.; Liu, P.; Whitley, E. M.; Birt, D. Effect of Dietary-Resistant Starch on Inhibition of Colonic Preneoplasia and Wnt Signaling in Azoxymethane-Induced Rodent Models. Nutr. Cancer. 2016, 68(6), 1052–1063. DOI: 10.1080/01635581.2016.1192203.
- Le Leu, R. K.; Hu, Y.; Brown, I. L.; Woodman, R. J.; Young, G. P. Synbiotic Intervention of Bifidobacterium Lactis and Resistant Starch Protects Against Colorectal Cancer Development in Rats. Carcinogenesis. 2010, 31(2), 246–251. DOI: 10.1093/carcin/bgp197.
- Ge, Y. F.; Wei, C. H.; Wang, W. H.; Cao, L. K. The Resistant Starch from Sorghum Regulates Lipid Metabolism in Menopausal Rats via Equol. J. Food Biochem. 2020, 44(8), e13295. DOI: 10.1111/jfbc.13295.
- Lopez, H. W.; Levrat-Verny, M. A.; Coudray, C.; Besson, C.; Krespine, V.; Messager, A.; Rémésy, C.; Rémésy, C. Class 2 Resistant Starches Lower Plasma and Liver Lipids and Improve Mineral Retention in Rats. J. Nutr. 2001, 131(4), 1283–1289. DOI: 10.1093/jn/131.4.1283.
- Sajilata, M. G.; Singhal, R. S.; Kulkarni, P. R. Resistant Starch– a Review. Compr. Rev. Food Sci. Food Saf. 2006, 5(1), 1–17. DOI: 10.1111/j.1541-4337.2006.tb00076.x.
- Martinez-Flores, H. E.; Kil Chang, Y.; Martinez-Bustos, F.; Sgarbieri, V. Effect of High Fiber Products on Blood Lipids and Lipoproteins in Hamsters. Nutr. Res. 2004, 24(1), 85–93. DOI: 10.1016/j.nutres.2003.08.016.
- Hashimoto, N.; Ito, Y.; Han, K. H.; Shimada, K. I.; Sekikawa, M.; Topping, D. L.; Fukushima, M.; NODA, T.; CHIJI, H.; FUKUSHIMA, M. Potato Pulps Lowered the Serum Cholesterol and Triglyceride Levels in Rats. J. Nutr. Sci. Vitaminol. 2006, 52(6), 445–450. DOI: 10.3177/jnsv.52.445.
- McKevith, B. Nutritional Aspects of Cereals. Nutr. Bull. 2004, 29(2), 111–142. DOI: 10.1111/j.1467-3010.2004.00418.x.
- Vlachos, D.; Malisova, S.; Lindberg, F. A.; Karaniki, G. Glycemic Index (GI) or Glycemic Load (GL) and Dietary Interventions for Optimizing Postprandial Hyperglycemia in Patients with T2 Diabetes: A Review. Nutrients. 2020, 12(6), 1561. DOI: 10.3390/nu12061561.
- Lal, M. K.; Singh, B.; Sharma, S.; Singh, M. P.; Kumar, A. Glycemic Index of Starchy Crops and Factors Affecting Its Digestibility: A Review. Trends Food Sci. Technol. 2021, 111, 741–755. DOI: 10.1016/j.tifs.2021.02.067.
- Jenkins, D. J.; Kendall, C. W.; Augustin, L. S.; Vuksan, V. High–Complex Carbohydrate or Lente Carbohydrate Foods? Am. j. med. 2002, 113(9), 30–37. DOI: 10.1016/S0002-9343(01)00989-5.
- Al-Tamimi, E. K.; Seib, P. A.; Snyder, B. S.; Haub, M. D. Consumption of Cross-Linked Resistant Starch (RS4XL) on Glucose and Insulin Responses in Humans. J Nutr Metab. 2010, 2010, 1–6. DOI: 10.1155/2010/651063.
- Yamada, Y.; Hosoya, S.; Nishimura, S.; Tanaka, T.; Kajimoto, Y.; Nishimura, A.; KAJIMOTO, O. Kajimoto, Effect of Bread Containing Resistant Starch on Postprandial Blood Glucose Levels in Humans. Biosci. Biotechnol., Biochem. 2005, 69(3), 559–566. DOI: 10.1271/bbb.69.559.
- Mitra, A.; Bhattacharya, D.; Roy, S. Role of Resistant Starches Particularly Rice Containing Resistant Starches in Type 2 Diabetes. J. Hum. Ecol. 2007, 21(1), 47–51. DOI: 10.1080/09709274.2007.11905950.
- Raigond, P.; Ezekiel, R.; Raigond, B. Resistant Starch in Food: A Review. J. Sci. Food Agric. 2015, 95(10), 1968–1978. DOI: 10.1002/jsfa.6966.
- Birkett, A. M.; Mathers, J. C.; Jones, G. P.; Walker, K. Z.; Roth, M. J.; Muir, J. G. Changes to the Quantity and Processing of Starchy Foods in a Western Diet Can Increase Polysaccharides Escaping Digestion and Improve in-Vitro Fermentation Variables. Br. J. Nutr. 2000, 84(1), 63–72. DOI: 10.1017/S0007114500001240.
- Younes, H.; Coudray, C.; Bellanger, J.; Demigné, C.; Rayssiguier, Y.; Rémésy, C. Effects of Two Fermentable Carbohydrates (Inulin and Resistant Starch) and Their Combination on Calcium and Magnesium Balance in Rats. Br. J. Nutr. 2001, 86(4), 479–485. DOI: 10.1079/BJN2001430.
- Whisner, C. M.; Castillo, L. F. Prebiotics, Bone and Mineral Metabolism. Calcif. Tissue Int. 2018, 102(4), 443–479. DOI: 10.1007/s00223-017-0339-3.
- Tapsell, L. C. Diet and Metabolic Syndrome: Where Does Resistant Starch Fit In? J. AOAC Int. 2004, 87(3), 756–760. DOI: 10.1093/jaoac/87.3.756.
- Liu, H.; Wang, L.; Shen, M.; Guo, X.; Lv, M.; Wang, M. Changes in Physicochemical Properties and In Vitro Digestibility of Tartary Buckwheat and Sorghum Starches Induced by Annealing. Starke. 2016, 68(7–8), 709–718. DOI: 10.1002/star.201500261.
- Singh, H.; Chang, Y. H.; Lin, J.; Singh, N.; Singh, N. Influence of Heat–Moisture Treatment and Annealing on Functional Properties of Sorghum Starch. Food. Res. Int. 2011, 44(9), 2949–2954. DOI: 10.1016/j.foodres.2011.07.005.
- Dundar, A. N.; Gocmen, D. Effects of Autoclaving Temperature and Storing Time on Resistant Starch Formation and Its Functional and Physicochemical Properties. Carbohydr. Polym. 2013, 97(2), 764–771. DOI: 10.1016/j.carbpol.2013.04.083.
- Sun, Q.; Han, Z.; Wang, L.; Xiong, L. Physicochemical Differences Between Sorghum Starch and Sorghum Flour Modified by Heat-Moisture Treatment. Food Chem. 2014, 145, 756–764. DOI: 10.1016/j.foodchem.2013.08.129.
- Pratiwi, M.; Faridah, D. N.; Lioe, H. N. Structural Changes to Starch After Acid Hydrolysis, Debranching, Autoclaving‐cooling Cycles, and Heat Moisture Treatment (HMT): A Review. Starke. 2018, 70(1–2), 1700028. DOI: 10.1002/star.201700028.
- Alsaffar, A. A. Effect of Food Processing on the Resistant Starch Content of Cereals and Cereal Products–A Review. Int. J. Food Sci. Technol. 2011, 46(3), 455–462. DOI: 10.1111/j.1365-2621.2010.02529.x.
- Faridah, D. N.; Damaiyanti, S.; Indrasti, D.; Jayanegara, A.; Afandi, F. A. Effect of Heat Moisture Treatment on Resistant Starch Content Among Carbohydrate Sources: A Meta‐aalysis. Int. J. Food Sci. Technol. 2022, 57(4), 1965–1974. DOI: 10.1111/ijfs.15276.
- Li, Z.; Guo, D.; Li, X.; Tang, Z.; Ling, X.; Zhou, T.; Zhang, B. Heat-Moisture Treatment Further Reduces In Vitro Digestibility and Enhances Resistant Starch Content of a High-Resistant Starch and Low-Glutelin Rice. Foods. 2021, 10(11), 2562. DOI: 10.3390/foods10112562.
- Faridah, D. N.; Anugerah, M. P.; Hunaefi, D.; Afandi, F. A.; Jayanegara, A. The Effect of Annealing on Resistant Starch Content of Different Crop Types: A Systematic Review and Meta‐analysis Study. Int. J. Food Sci. Technol. 2022, 57(4), 2026–2038. DOI: 10.1111/ijfs.15388.
- Gulzar, B.; Hussain, S. Z.; Naseer, B.; Naik, H. R. Enhancement of Resistant Starch Content in Modified Rice Flour Using Extrusion Technology. Cereal Chem. 2021, 98(3), 634–641. DOI: 10.1002/cche.10407.
- Hung, P. V.; My, N. T. H.; Phi, N. T. L. Impact of Acid and Heat–Moisture Treatment Combination on Physicochemical Characteristics and Resistant Starch Contents of Sweet Potato and Yam Starches. Starke. 2014, 66(11–12), 1013–1021. DOI: 10.1002/star.201400104.
- Falodun, A. I.; Ayo-Omogie, H. N.; Awolu, O. O. Effect of Different Drying Techniques on the Resistant Starch, Bioactive Components, Physicochemical and Pasting Properties of Cardaba Banana Flour. Acta Universitatis Cinbinesis, Series E: Food Technology. 2019, 23(1), 35–42. DOI: 10.2478/aucft-2019-0005.
- Pico, J.; Xu, K.; Guo, M.; Mohamedshah, Z.; Ferruzzi, M. G.; Martinez, M. M. Manufacturing the Ultimate Green Banana Flour: Impact of Drying and Extrusion on Phenolic Profile and Starch Boaccessibility. Food Chem. 2019, 297, 124990. DOI: 10.1016/j.foodchem.2019.124990.
- Zeng, F.; Zhu, S.; Chen, F.; Gao, Q.; Yu, S. Effect of Different Drying Methods on the Structure and Digestibility of Short Chain Amylose Crystals. Food Hydrocoll. 2016, 52, 721–731. DOI: 10.1016/j.foodhyd.2015.08.012.
- Rezaei, R.; Khomeiri, M.; Kashaninejad, M.; Mazaheri-Tehrani, M.; Aalami, M. Effect of Resistant Starch and Aging Conditions on the Physicochemical Properties of Frozen Soy Yogurt. J. Food Sci. Technol. 2015, 52(12), 8164–8171. DOI: 10.1007/s13197-015-1895-z.
- Williams, R. P. W.; Glagovskaia, O.; Augustin, M. A. Properties of Stirred Yogurts with Added Starch: Effects of Blends of Skim Milk Powder and Whey Protein Concentrate on Yogurt Texture. Aust. J. Dairy Technol. 2004, 59(3), 214.
- Aryana, K.; Greenway, F.; Dhurandhar, N.; Tulley, R.; Finley, J.; Keenan, M.; Martin, R.; Pelkman, C.; Olson, D.; Zheng, J., A Resistant-Starch Enriched Yogurt: Fermentability, Sensory Characteristics, and a Pilot Study in Children. F1000research. 2015, 4, 139–139. DOI:10.12688/f1000research.6451.1.
- He, J.; Han, Y.; Liu, M.; Wang, Y.; Yang, Y.; Yang, X. Effect of 2 Types of Resistant Starches on the Quality of Yogurt. J. Dairy. Sci. 2019, 102(5), 3956–3964. DOI: 10.3168/jds.2018-15562.
- Saleh, A.; Mohamed, A. A.; Alamri, M. S.; Hussain, S.; Qasem, A. A.; Ibraheem, M. A. Effect of Different Starches on the Rheological, Sensory and Storage Attributes of Non-Fat Set Yogurt. Foods. 2020, 9(1), 61. DOI: 10.3390/foods9010061.
- Mwizerwa, H.; Abong, G. O.; Okoth, M. W.; Ongol, M. P.; Onyango, C.; Pushparajah, T. Effect of Resistant Cassava Starch on Quality Parameters and Sensory Attributes of Yoghurt. Curr. Res. Nutr. Food Sci. 2017, 5(3), 353–367. DOI: 10.12944/CRNFSJ.5.3.21.
- Agyemang, P. N.; Akonor, P. T.; Tortoe, C.; Johnsona, P. T.; Manu-Aduening, J. Effect of the Use of Starches of Three New Ghanaian Cassava Varieties as a Thickener on the Physicochemical, Rheological and Sensory Properties of Yoghurt. Sci. Afr. 2020, 9, e00521. DOI: 10.1016/j.sciaf.2020.e00521.
- Harmayani, E.; Geraldo, N. F.; Murdiati, A. Physicochemical Nutritional and Sensory Properties of Kluklui Supplemented with Porang Glucomannan and Banana Flour. Indones. Food Nutr. Prog. 2021, 18(1), 9–15. DOI: 10.22146/ifnp.57223.
- Nikitina, E.; Ahmad Riyanto, R.; Vafina, A.; Yurtaeva, T.; Tsyganov, M.; Ezhkova, G. Effect of Fermented Modified Potato Starches to Low-Fat Yogurt. J. Food Nutr. Res. 2019, 7(7), 549–553. DOI: 10.12691/jfnr-7-7-10.
- Duggan, E.; Noronha, N.; O’Riordan, E.; O’Sullivan, M. Effect of Resistant Starch on the Water Binding Properties of Imitation Cheese. J. Food Eng. 2008, 84(1), 108–115. DOI: 10.1016/j.jfoodeng.2007.04.028.
- Noronha, N.; Duggan, E.; Ziegler, G. R.; O’Riordan, E. D.; O’Sullivan, M. Inclusion of Starch in Imitation Cheese: Its Influence on Water Mobility and Cheese Functionality. Food Hydrocoll. 2008, 22(8), 1612–1621. DOI: 10.1016/j.foodhyd.2007.11.007.
- Montesinos-Herrero, C.; Cottell, D. C.; Dolores O’Riordan, E.; O’Sullivan, M. Partial Replacement of Fat by Functional Fibre in Imitation Cheese: Effects on Rheology and Microstructure. Int. Dairy. J. 2006, 16(8), 910–919. DOI: 10.1016/j.idairyj.2005.08.008.
- Arimi, J.; Duggan, E.; O’Riordan, E.; O’Sullivan, M.; Lyng, J. Microwave Expansion of Imitation Cheese Containing Resistant Starch. J. Food Eng. 2008, 88(2), 254–262. DOI: 10.1016/j.jfoodeng.2008.02.021.
- Noronha, N.; O’Riordan, E.; O’Sullivan, M. Replacement of Fat with Functional Fibre in Imitation Cheese. Int. Dairy. J. 2007, 17(9), 1073–1082. DOI: 10.1016/j.idairyj.2007.01.011.
- Murtaza, M. S.; Sameen, A.; Rafique, S.; Shahbaz, M.; Gulzar, N.; Murtaza, M. A.; Hafiz, I.; Hafiz, I. Impact of Dietary Fiber (Inulin and Resistant Starch) on the Quality Parameters of Low Fat Cheddar Cheese from Buffalo Milk. Curr. Res. Nutr. Food Sci. 2022, 55(2). DOI: 10.17582/journal.pjz/20200630120620.
- Di Cairano, M.; Caruso, M. C.; Galgano, F.; Favati, F.; Ekere, N.; Tchuenbou-Magaia, F. Effect of Sucrose Replacement and Resistant Starch Addition on Textural Properties of Gluten-Free Doughs and Biscuits. Eur. Food Res. Technol. 2021, 247(3), 707–718. DOI: 10.1007/s00217-020-03659-w.
- Alcântara, R. G. D.; Fukumasu, H.; Raspantini, P. C. F.; Raspantini, L. E. R.; Steel, C. J.; Oliveira, L. D. C.; Vanin, F. M.; Vanin, F. M. Baking Effect on Resistant Starch Digestion from Composite Bread Produced with Partial Wheat Flour Substitution. J. Food Qual. 2020, 2020, 1–13. DOI: 10.1155/2020/9245035.
- Liljeberg, H.; Åkerberg, A.; Björck, I. Resistant Starch Formation in Bread as Influenced by Choice of Ingredients or Baking Conditions. Food Chem. 1996, 56(4), 389–394. DOI: 10.1016/0308-8146(95)00199-9.
- Laguna, L.; Salvador, A.; Sanz, T.; Fiszman, S. M. Performance of a Resistant Starch Rich Ingredient in the Baking and Eating Quality of Short-Dough Biscuits. LWT - Food Sci. Technol. 2011, 44(3), 737–746. DOI: 10.1016/j.lwt.2010.05.034.
- Kaimal, A. M.; Mujumdar, A. S.; Thorat, B. N. Resistant Starch from Millets: Recent Developments and Applications in Food Industries. Trends Food Sci. Technol. 2021, 111, 563–580. DOI: 10.1016/j.tifs.2021.02.074.
- Spiller, G. A. Handbook of Dietary Fiber in Human Nutrition; CRC Press: Boca Raton, FL, 2001.
- Khan, A.; Siddiqui, S.; Rahman, U.; Ali, H.; Saba, M.; Andleeb Azhar, F.; Khan, S.; Ali Shah, A.; Badshah, M.; Hasan, F., et al. Physicochemical Properties of Enzymatically Prepared Resistant Starch from Maize Flour and Its Use in Cookies Formulation. Int. J. Food. Prop. 2020, 23(1), 549–569. DOI: 10.1080/10942912.2020.1742736.
- Gelencsér, T.; Gál, V.; Hódsági, M.; Salgó, A. Evaluation of Quality and Digestibility Characteristics of Resistant Starch-Enriched Pasta. Food Bioproc. Tech. 2008, 1(2), 171–179. DOI: 10.1007/s11947-007-0040-z.
- Aravind, N.; Sissons, M.; Fellows, C. M.; Blazek, J.; Gilbert, E. P. Optimisation of Resistant Starch II and III Levels in Durum Wheat Pasta to Reduce in-Vitro Digestibility While Maintaining Processing and Sensory Characteristics. Food Chem. 2013, 136(2), 1100–1109. DOI: 10.1016/j.foodchem.2012.08.035.
- Vernaza, M. G.; Biasutti, E.; Schmiele, M.; Jaekel, L. Z.; Bannwart, A.; Chang, Y. K. Effect of Supplementation of Wheat Flour with Resistant Starch and Monoglycerides in Pasta Dried at High Temperatures. Int. J. Food Sci. Technol. 2012, 47(6), 1302–1312. DOI: 10.1111/j.1365-2621.2012.02974.x.
- Foschia, M.; Beraldo, P.; Peressini, D. Evaluation of the Physicochemical Properties of Gluten‐free Pasta Enriched with Resistant Starch. J. Sci. Food Agric. 2017, 97(2), 572–577. DOI: 10.1002/jsfa.7766.
- Makhlouf, S.; Jones, S.; Ye, S. H.; Sancho-Madriz, M.; Burns-Whitmore, B.; Li, Y. O. Effect of Selected Dietary Fibre Sources and Addition Levels on Physical and Cooking Quality Attributes of Fibre-Enhanced Pasta. Food Qual. Saf. 2019, 3(2), 117–127. DOI: 10.1093/fqsafe/fyz010.
- Bustos Shmidt, M. C.; Perez, G. T.; Leon, A. E. Effect of Four Types of Dietary Fiber on the Technological Quality of Pasta. Food Sci. Technol. Int. 2011, 17(3), 213–221. DOI: 10.1177/1082013210382303.
- Menon, R.; Padmaja, G.; Jyothi, A. N.; Asha, V.; Sajeev, M. S. Gluten-Free Starch Noodles from Sweet Potato with Reduced Starch Digestibility and Enhanced Protein Content. J. Food Sci. Technol. 2016, 53(9), 3532–3542. DOI: 10.1007/s13197-016-2330-9.
- Sharma, S.; Singh, N.; Katyal, M. Effect of Gelatinized-Retrograded and Extruded Starches on Characteristics of Cookies, Muffins and Noodles. J. Food Sci. Technol. 2016, 53(5), 2482–2491. DOI: 10.1007/s13197-016-2234-8.
- Punia, S.; Siroha, A. K.; Sandhu, K. S.; Kaur, M. Rheological Behavior of Wheat Starch and Barley Resistant Starch (Type IV) Blends and Their Starch Noodles Making Potential. Int. J. Biol. Macromol. 2019, 130, 595–604. DOI: 10.1016/j.ijbiomac.2019.03.009.
- Li, P. H.; Wang, C. W.; Lu, W. C.; Chan, Y. J.; Wang, C. C. R. Effect of Resistant Starch Sources on the Physical Properties of Dough and on the Eating Quality and Glycemic Index of Salted Noodles. Foods. 2022, 11(6), 814. DOI: 10.3390/foods11060814.
- Hsieh, C. F.; Wang, L. K.; Xu, B.; Seib, P. A.; Shi, Y. C. Preparation and Textural Properties of White Salted Noodles Made with Hard Red Winter Wheat Flour Partially Replaced by Different Levels of Cross‐linked Phosphorylated RS4 Wheat Starch. J. Sci. Food Agric. 2020, 100(15), 5334–5343.V. DOI: 10.1002/jsfa.10581.
- Boue, S. M.; Chen, M. H.; Daigle, K. W.; Lea, J. M.; Bett‐garber, K. L. Changes in Fried Rice Batter with Increased Resistant Starch and Effects on Sensory Quality of Battered Fried Onions. Cereal Chem. 2022, 99(3), 454–466. DOI: 10.1002/cche.10502.
- Garcia-Santos, M. D. S. L.; Conceição, F. S.; Villas Boas, F.; Salotti De Souza, B. M.; Barretto, A. C. D. S. Effect of the Addition of Resistant Starch in Sausage with Fat Reduction on the Physicochemical and Sensory Properties. Food Sci. Technol. 2019, 39(suppl 2), 491–497. DOI: 10.1590/fst.18918.
- Dos Santos, J. M.; Ignácio, E. O.; Bis-Souza, C. V.; da Silva-Barretto, A. C. Performance of Reduced Fat-Reduced Salt Fermented Sausage with Added Microcrystalline Cellulose, Resistant Starch and Oat Fiber Using the Simplex Design. Meat Sci. 2021, 175, 108433. DOI: 10.1016/j.meatsci.2021.108433.
- Salazar, D.; Arancibia, M.; Calderón, L.; López-Caballero, M. E.; Montero, M. P. Underutilized Green Banana (Musa Acuminata AAA) Flours to Develop Fiber Enriched Frankfurter-Type Sausages. Foods. 2021, 10(5), 1142. DOI: 10.3390/foods10051142.
- Geraldo, N. F. Physico-Chemical and Sensory Properties of Kluiklui Supplemented with Porang Glucomannan and Banana Flour. Foods (Basel, Switzerland). 2020, 10(5), 1142. DOI: 10.3390/foods10051142.
- Liu, T.; Zhou, Y.; Wu, D.; Chen, Q.; Shu, X. Germinated High‐resistant Starch Rice: A Potential Novel Functional Food. Int. J. Food Sci. Technol. 2022, 57(8), 5439–5449. DOI: 10.1111/ijfs.15876.
- Shen, H.; Xu, M.; Su, C.; Zhang, B.; Ge, X.; Zhang, G.; Li, W. Insights into the Relations Between the Molecular Structures and Physicochemical Properties of Normal and Waxy Wheat B‐starch After Repeated and Continuous Annealing. Int. J. Food Sci. Technol. 2021, 56(12), 6405–6419. DOI: 10.1111/ijfs.15302.
- Punia Bangar, S.; Sharma, N.; Singh, A.; Phimolsiripol, Y.; Brennan, C. S. Glycaemic Response of Pseudocereal‐based Gluten‐free Food Products: A Review. Int. J. Food Sci. Technol. 2022, 57(8), 4936–4944. DOI: 10.1111/ijfs.15890.
