ABSTRACT
Chilling injury (CI) causes significant losses in fruits and vegetables during cold storage. CI symptoms exhibited as browning, off-flavour and sunken spots, reduced juice content, uneven ripening and softening in fresh horticultural produce. Application of melatonin (MT) effectively mitigates CI in cold-stored fruits and vegetables. This comprehensive review focuses at discussing symptoms, the mechanism of CI, regulation of melatonin-mediated chilling tolerance, and meta-analysis of CI reduction in horticultural produce. Melatonin mitigates CI, maintains quality of cold-stored horticultural produce by upregulating early hydrogen peroxide signalling, activities of antioxidant enzymes after inhibition of reactive oxygen species (ROS) accumulation, mitochondrial dysfunction, and membrane leakage.
Introduction
Fruits and vegetables are an integral part of the human diet and are a rich source of health-promoting compounds such as phenolics, flavonoids, protein, dietary fibres, antioxidants, vitamins, and minerals[Citation1]. However, fresh horticultural produce is highly perishable and deteriorates with the extension of the storage period. The improper harvest, postharvest handling, and storage conditions during the supply chain result in reduced shelf life and higher postharvest losses in different fruits and vegetables. Overall, up to 44% of postharvest losses have been reported in fresh horticultural produce.[Citation2] Cold temperature during storage and transportation of horticultural produce to distant markets ensure longer storage life and better quality. Nevertheless, tropical and subtropical fruits and vegetables are prone to CI, therefore the cold storage technology cannot be exploited to its full potential to extend storage life, maintain quality, and minimise postharvest losses.[Citation2,Citation3] CI commences from the plasma membrane where it transforms the liquid crystalline phase into the gel phase, increasing membrane permeability by robust electrolyte leakage. Dysfunction of membrane protein lowers membrane integrity and upregulates oxidative stress via the accumulation of malondialdehyde (MDA), which subsequently results in CI and the deterioration of fresh horticultural produce.[Citation4]
Previously, various techniques have been reported to alleviate CI in fruits and vegetables such as modified atmosphere packaging (MAP), hypobaric storage, edible coatings, controlled atmosphere (CA), heat treatment and use of anti-ripening chemicals including nitric oxide (NO), 1-methylcyclopropene and methyl jasmonate.[Citation5] There are increasing concerns about the deleterious effects of residual fumigants and chemical dip treatments on human health and the environment despite their beneficial effects on delaying senescence and controlling pathogens. This presents an opportunity to develop food-safe alternative substitutes to be used in the horticulture industry on a commercial scale.[Citation6]
MT is an indoleamine signalling molecule and has a structural resemblance to indole-3-acetic acid (IAA), serotonin and tryptophan. The biosynthesis of MT takes place in plants by serotonin and tryptophan which are the basic precursors of MT.[Citation7] Exogenous MT application induces MT biosynthesis in fruits and vegetables as it passes through the phytomelatonin receptor from the plasma membrane to the inside of cell. The first phytomelatonin receptor, CAND2/PMTR1, was discovered in Arabidopsis thaliana which regulates MT-mediated functions in a cell through AtCand2 expression.[Citation8] MT has been identified to mediate stress response, increase the activities of the antioxidant system and extend the postharvest storage life of fruits and vegetables. The exogenous application of MT effectively attenuates CI via activation of the early ROS signalling pathway, polyamine-dependent arginine metabolic pathway and the antioxidant system. MT has been reported to reduce CI and maintain the quality of fresh horticultural produce.[Citation9] MT application effectively suppressed CI symptoms in fruits and vegetables however, the part of high variable magnitude was associated with the fact that different methods of application, concentrations and duration of treatment were used in different studies. For instance, plum fruit treated with 0.1 mmol L−1 MT for 100 min reduced nearly 2-fold CI symptoms[Citation10] as compared to the fruit dipped for 15 min.[Citation11] Likewise, the preharvest spray application of MT (0.1 mmol L−1) to apricot fruit exhibited less CI tolerance than the postharvest dip application.[Citation12]
The application of MT to improve postharvest quality management of horticultural crops, notably fruits and vegetables, has become a vibrant research area, and the resulting findings are of much interest across a broad spectrum of stakeholders. The mechanistic approach of melatonin application in relation to CI in fruits and vegetables has been described in different studies.[Citation9,Citation13] These studies focused on the quality inference of melatonin in CI tolerance of horticultural produce. The present work aimed to describe the quantitative relationships between different quality parameters during CI in control and MT-treated fruits and vegetables. This was achieved through a comprehensive meta-analytic review which focused on describing CI symptoms and provided evidence of recent advancements in the mechanistic role of MT in mitigating CI and maintaining the quality of cold-stored fruits and vegetables. We have provided first-hand exhaustive evidence of the effects of MT applications and estimated pooled effect estimates of the quantitative relationships across different studies and parameters for robust inference in the postharvest CI management of fruits and vegetables.[Citation14,Citation15] Additionally, we have highlighted the dynamic responses of MT applications in fruits and vegetables and provided detailed exposition on the optimal concentrations of MT applications across several fruits and vegetables. To the best of our knowledge, this is the first study that has synthesised results of several studies to estimate the odds of the effects of MT application on the postharvest quality parameters in both fruits and vegetables.
CI symptoms
The symptoms of CI can be in fruits include abnormal ripening, sunken spots, pitting, hardening of flesh and browning of peel and pulp in cold-stored fruits,[Citation2,Citation5,Citation16,Citation17] while browning of tissues, lignification of the stalk, hardening, limp tips, russet formation, pitting, translucency and water-soaked lesions are expressed in vegetables.[Citation18–20] These symptoms vary among species and cultivars of different fruits and vegetables (). For instance, banana fruits express a loss of aroma and develop off-flavour,[Citation24] red blotches and abnormal flavedo in lemon and oranges[Citation37,Citation70] and water-soaked sunken lesions in papaya fruit.[Citation48] Symptoms of CI usually become more noticeable after the removal of cold-stored produce to ambient temperature. The average storage temperature for subtropical fruits and vegetables usually ranges from 4–8°C while 10–20°C is optimum to avoid CI during cold storage in tropical fruits and vegetables. The development of CI symptoms depends upon exposure time to chilling temperature, type of fruit or vegetable, cultivar, and maturity stage (). Wang et al.[Citation71] have reported that red flesh loquat cultivars are more sensitive to CI than white-flesh cultivars. Likewise, the ‘Okrong’ mango fruit is more chilling tolerant than the ‘Red’ mango.[Citation72] Early maturing peach cultivars often show less sensitivity to CI than late maturing ones.[Citation73] However, it could be argued that fruit harvested at earlier maturity stages is more susceptible to CI. More mature or riper fruit has already developed most organoleptic characteristics when exposed to chilling, so that is why they seem more ‘tolerant’ than less mature fruit.[Citation74] Leafy vegetables such as spinach are most susceptible to CI and develop CI symptoms in very short exposure to low temperature[Citation67] while tomato delays CI for many days during cold storage.[Citation69] Similarly, avocado is very sensitive to low temperature and develops CI symptoms even after one hour of cold storage.[Citation75] Apart from tropical and subtropical fruits, some apple, plum and apricot cultivars are also chilling sensitive and can develop CI symptoms following two to four weeks of cold storage.[Citation21]
Table 1. Chilling injury symptoms expressed on cold-stored fruits and vegetables.
CI mechanism and MT-induced chilling tolerance
CI is the major abiotic stress in subtropical and tropical horticultural produce during cold storage which downgrades the cosmetic quality and disturbs their biochemical kinetics.[Citation49] CI significantly lowers the storage potential of cold-stored fruits and vegetables by increasing electrolyte leakage, oxidative stress and mitochondrial dysfunction, while decreasing cellular energy and membrane integrity.[Citation5] Two hypotheses for CI mechanism have been proposed in horticultural produce; one is the membrane fluidity hypothesis which describes the dysfunction of lipid molecules in the plasma membrane due to the formation of a gel phase from a liquid state while the second one is the accumulation of ROS in a cell.[Citation4,Citation5] Membrane damage is the primary event in chilling stress which is often accompanied by electrolyte leakage and metabolic disorders due to the activities of polyphenol oxidase (PPO), lipoxygenase (LOX) and phospholipase D (PLD) enzymes. PPO upregulates ROS accumulation, and PLD promotes the hydrogenation of membranous unsaturated fatty acids (UFA) to form saturated fatty acids (SFA) that are further oxidised to MDA by LOX activity, leading to visual CI symptoms in fruits and vegetables.[Citation65,Citation76] In addition, these oxidative enzymes modulate the eating quality of horticultural produce by reducing the soluble solid content (SSC) due to the oxidation of non-reducing sugars by ROS activity.[Citation77,Citation78]
The chilling tolerance mechanism could be possibly regulated using an exogenous MT application which effectively alleviated CI symptoms in cold-stored fruits and vegetables (, ). Exogenous MT application has been found to significantly extend cold storage life and maintain fruit quality by lowering fruit weight loss, retaining firmness and titratable acidity (TA), and reducing CI in guava,[Citation83] mango,[Citation87] nectarine,[Citation89] peach,[Citation98] pineapple,[Citation92] plum,[Citation11] bitter melon,[Citation19] eggplant[Citation56] and cucumber.[Citation61] MT treatments induced chilling tolerance primarily due to the reduction of oxidative stress by lowering the accumulation of hydrogen peroxide (H2O2), superoxide anion (O2•‒) and MDA, and delayed activities of PPO, PLD and LOX enzymes.[Citation9] MT treatment also upregulated the activity of phenylalanine ammonia-lyase (PAL) in pomegranate,[Citation53] kiwifruit[Citation36] and peach[Citation90] involved in the phenolic biosynthesis which helps in lowering CI. MT application was found to conserve higher activities of antioxidant enzymes such as ascorbate peroxidase (APX), peroxidase (POD), superoxide dismutase (SOD) and catalase (CAT) in cold-stored fruit and vegetables.[Citation53,Citation54] MT application conserved higher levels of ascorbic acid, total antioxidants in terms of free radical scavenging 2,2-diphenyl-1-picrylhydrazyl (DPPH) activities, and phenolic concentrations which helps in reducing CI as earlier reported in apricot fruit during cold storage.[Citation79] Conversely, the application of MT led to higher GABA, polyamines and proline contents in peach,[Citation91] tomato[Citation95] and cucumber.[Citation61]
Figure 1. Possible mechanism of melatonin-mediated chilling tolerance in the fruit and vegetables. .
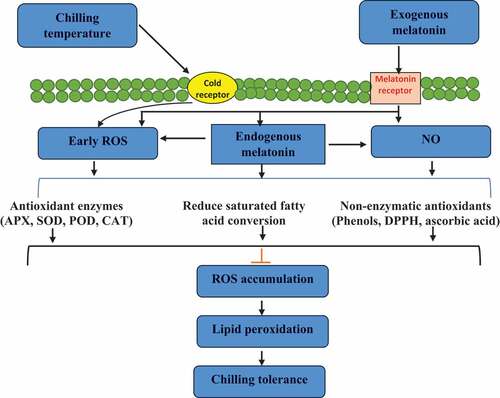
Table 2. Effects of postharvest application of melatonin, storage condition and duration on CI and quality parameters in different fruits and vegetables.
The role of MT application in the downregulation of CI among different fruits and vegetables during cold storage has been well-reported in the literature. The variable results associated with cultivar, genotype and storage conditions emphasized the need for meta-analysis to identify the quantitative relationship of MT treatment with CI, quality parameters, oxidative stress, antioxidative capacity and the polyamine-dependent arginine pathway.
MT-induced ROS signalling and scavenging
ROS production is often associated with the degradation of membrane integrity that elevates CI susceptibility.[Citation36,Citation99] However, the time of ROS accumulation is very important, and it induces a defence-oriented signalling pathway if produced at an earlier stage of storage while promoting oxidative stress when accumulates at a later stage.[Citation91] The antioxidant levels increased by exogenous MT application were due to upregulation of initial H2O2 content with subsequent increase in SOD and APX enzymes which are two basic molecular quenchers against ROS accumulation at the later stage of storage. A higher H2O2 level at the initial stage triggers the formation of antioxidant enzymes and induces phenolic biosynthesis, a major secondary metabolite for the normal functioning of cytoplasmic macromolecules.[Citation100] Cao et al.[Citation91] reported that exogenous MT application induced higher H2O2 content at the start of cold storage which upregulated endogenous MT. Endogenous MT suppressed ROS accumulation at a later stage via a negative feedback mechanism and induced the ascorbate-glutathione pathway. This modulated signalling pathway is helpful to reduce CI symptoms and CI incidence in peach fruit.[Citation90] MT delayed transcription expression for PPO coding genes, which is a main oxidative enzyme and promotes lipids catabolism by inducing ROS accumulation resulting in ion leakage and membrane degradation in litchi fruit.[Citation39] The other possible pathway of the ROS-induced chilling tolerance pathway is the possible crosstalk between MT and other phytohormones including NO, jasmonic acid (JA) and salicylic acid (SA). Exogenous MT induced NO signalling in tomato fruit by increasing the expression of nitric oxide synthase (NOS) genes which upregulated endogenous NO accumulation. Endogenous NO accumulation catalysed the formation of proline and polyamine through early H2O2 accumulation.[Citation101] MT has been reported to upregulate the endogenous levels of JA and SA during cold stress in plum fruit which reduces the fruit firmness loss during chilling stress.[Citation102] However, the information regarding MT crosstalk with other phytohormones in the chilling tolerance of fresh fruits and vegetables is limited and warrants to be further investigated.
MT-induced antioxidant defence system
Production of ROS such as H2O2, O2•‒, hydroxyl radical (OH¯) and singlet oxygen (1O2) are major reasons for membrane dysfunctions which induce lipid peroxidation.[Citation76] ROS scavenging is an important physiological aspect to retain better fruit quality. Fruits have a natural antioxidant defence mechanism against ROS production including antioxidative enzymes like APX, CAT, SOD, glutathione reductase (GR), dehydroascorbate reductase (DHAR) and monodehydroascorbate reductase (MDHAR). SOD is the first line of defence against ROS and causes the reduction of superoxide to H2O2 which further hydrolyse into water molecules either by the activity of CAT or via the Helliwell-Asada pathway by APX activity.[Citation103,Citation104] MT is a strong antioxidant in plants and induces CI tolerance by upregulating the activities of antioxidant enzymes.[Citation39,Citation54] Wang et al.[Citation81] have reported that exogenous MT-treated banana fruit exhibited higher activities of SOD and CAT, and reduced CI incidence by downregulating membrane leakage and MDA content. Similarly, the activities of GR, MDHAR, APX, CAT and SOD were also observed higher in MT-treated ‘Hyayou’ kiwifruit fruit during low-temperature storage, while O2•‒ and H2O2 content were alleviated during storage. Higher antioxidant enzymes downregulated CI in MT treatment than in control.[Citation105] Browning is the major indicator of CI in fruits and exogenous application of MT suppressed browning incidence in pomegranate[Citation53] and litchi[Citation39] during cold storage which indicates its efficacy to lessen the activities of oxidative enzymes such as LOX, PPO and PLD.
Besides antioxidant enzymes, the non-enzymatic antioxidants are present in fruits and vegetables including ascorbic acid, phenolics, anthocyanins, tocopherols, carotenoids, flavonoids, and glutathione, and significantly contribute to the total antioxidant system.[Citation99,Citation103,Citation104] Exogenous application of MT markedly regulated the biosynthesis of phenolic content, flavonoids and anthocyanins in table grapes.[Citation106] MT treatment induced higher ascorbic acid and glutathione content, and lessened H2O2 and MDA content thereby reducing CI in cold-stored kiwifruit[Citation36] and orange.[Citation47] Higher ascorbic acid content can initiate ROS scavenging and helps the APX enzyme to maintain membrane integrity by providing energy to APX in the ascorbate-glutathione cycle, thus taking an active part in CI management. Phenolics are one of the most important antioxidants in horticultural commodities that are produced via the shikimate pathway by the activities of glucose-6-phosphate dehydrogenase (G6PDH), PAL and shikimate dehydrogenase (SKDH). Higher phenolic levels were observed along with less CI incidence in MT-treated cherimoya,[Citation27] nectarine,[Citation89] mango,[Citation85] peach,[Citation49] apricot,[Citation79] pomegranate[Citation93] and plum fruit,[Citation11] and bitter melon.[Citation19]
MT-induced polyamine regulation
Polyamines are among the most important compounds associated with plant stress tolerance and have efficacy not only to reduce CI but also to maintain membrane integrity and reduce ROS accumulation at a later stage of storage.[Citation107] Polyamines have a natural tendency to bind with membrane protein molecules instead of oxidative enzymes and convert them into stabilized molecules.[Citation108] Exogenous MT application induced endogenous polyamine in peach fruit resulted in higher activities of ornithine decarboxylase (ODC) and arginine decarboxylase (ADC) enzymes. Similarly, higher activities of ODC and ADC were observed in response to exogenous MT that upregulated polyamine biosynthesis in cucumber.[Citation61] MDA content, a major by-product of lipid peroxidation, was also lowered by endogenous polyamines due to less ROS production after MT dip treatment. This positive correlation upregulated the CI defence mechanism in ‘Langra’ mango fruit.[Citation84] The role of polyamines in CI reduction can be estimated by calculating the conjugated polyamines that bind with negatively charged reactive protein on the plasma membrane and convert them into stabilizing components making them unavailable to bind with ROS and reduce electrolyte leakage.[Citation10] The level of conjugated covalently bonded polyamines in the ‘Queen Rose’ plum was negatively associated with CI incidence. Similarly, MT-treated apricot fruit exhibited higher levels of conjugated spermidine and putrescine, and non-covalently bonded spermidine and spermine contents in plasma membrane.[Citation80] A significant decline in membrane permeability and higher polyamine biosynthesis was observed when exogenous MT was applied to the grapes.[Citation106] Exogenous MT application increased GABA, polyamines and proline content thus activating the defence mechanism against CI in sapota.[Citation54] Moreover, polyamines and GABA tend to dismutase H2O2 either by increasing the activity of CAT or APX via the ascorbate-glutathione cycle.[Citation91]
In the presence of ROS, diamine oxidase (DAO) catalyses the oxidation of polyamines into pyrroline-5-carboxylase that further hydrolyse into proline and GABA molecules by the activities of Δ1-pyrroline-5-carboxylate synthetase (P5CS) and glutamate decarboxylase (GAD) enzymes, respectively.[Citation109] MT activated the GABA shunt pathway by the activities of polyamine oxidase (PAO) and DAO maintained higher GABA content leading to less oxidative damage and CI symptoms in mango.[Citation84] The degradation of polyamines catalysis the formation of H2O due to the reduction of H2O2 either by the activity of CAT or by the ascorbic acid cycle via APX in the presence of ascorbic acid. Hence, polyamine and GABA make a strong defence mechanism where polyamine acts as a primary antioxidant while GABA works as a secondary antioxidant in chilling tolerance.[Citation110] MT application in sapota increased GABA, polyamines and proline content thus reducing CI.[Citation54] On the other hand, PDH-activated proline catabolism produces glucose molecules that take part in the citric acid cycle and provide cellular energy in the form of ATP and NADPH via the electron transport chain. MT treatment upregulated the activities of P5CS and OAT while downregulated PDH activity resulting in higher proline content, reduced CI and maintained better fruit quality in ‘Keitt’ mango[Citation43] ‘Baitangying’ litchi,[Citation39] ‘Langra’ mango,[Citation87] peach[Citation91] and cucumber.[Citation61] Lipid metabolism and conversion to gel phase during low-temperature stress were effectively reduced by MT-induced higher endogenous proline thereby suppressing an ion leakage and lipid peroxidation, free superoxide radicals (O2•‒) and H2O2 accumulation, thus reducing CI in tomato.[Citation69]
MT regulated cellular energy metabolism
The cellular energy level is one of the critical factors to delay senescence and maintain membrane fluidity. Adenosine triphosphate (ATP) is the major energy currency of cells and it is synthesized via photocatalytic phosphorylation reaction in mitochondria.[Citation111] A higher ATP level is necessary for major cellular activities especially the maintenance of cellular homeostasis. The formation of ATP molecules is catalysed by the activities of cytochrome C oxidase (CCO), succinate dehydrogenase (SDH), H+-ATPase and Ca2+-ATPase enzymes. ROS and other free radicals produce H+ and Ca2+ resulting in an endogenous increase in lipid metabolizing enzymes including PLD, LPS and LOX. Ca2+-ATPase is a natural exosmotic substance to remove higher Ca2+ out of the cell through active transport via calcium pumps in the plasma membrane at the expense of ATP hydrolysis producing ADP and AMP.[Citation112] Similarly, the extracellular movement of H+ is catalysed by H+-ATPase. Both these reactions use ATP as the energy molecule for the active transport of ions that mitigate energy levels during chilling stress.[Citation113] MT treatment mitigated CI symptoms and elicited higher ATP and ADP while lowered AMP contents along with higher activities of SDH, CCO, H+-ATPase and Ca2+-ATPase in tomato[Citation96] and litchi fruit.[Citation39]
Higher respiration and ethylene rates are another cause of reduced ATP with higher AMP. ROS-oriented stress pathway starts ethylene burst with subsequent reduction in lipid contents and enhanced postharvest senescence.[Citation114] Further, an increase in respiration is indispensable for the ripening of non-climacteric fruits while a respiration peak is an indication of ripening in climacteric fruits. Crosstalk between respiration and fruit senescence is well known physiological effect in postharvest. A higher respiration rate lowers ATP levels in the plant cells leading to the earlier senescence of the plants.[Citation115] However, exogenous MT suppressed and delayed ethylene peak on ‘Friar’ plum with less ROS production, MDA content and higher ATP level.[Citation11] The other important energy source in plant cells is NADH which is synthesized during the Krebs cycle and electron transport chain, both are interlinked due to the SDH complex and CCO is responsible to activate proton pumps in the mitochondrial outer membrane to initiate the ATP synthesis in aerobic respiration energy. Oxidative stress due to CI causes structural and functional dysfunction of mitochondria, thus NADH production decreases which leads to a subsequent decline in cellular energy.[Citation116] However, higher SDH activity promoted NADH biosynthesis in MT-treated lotus, which might be due to the transfer of free electrons from hydrolysis reaction in the cytosol, reduced mitochondrial dysfunction, ROS and activities of lipid catabolizing enzymes (PLD, LPS and LOX). The overall energy change before and after storage was proliferated in control fruits while ATP content was directly correlated with NADH content.[Citation117]
MT-induced regulation of membrane integrity
Chilling tolerance in horticultural produce is mainly regulated by lower or delayed activities of lipid metabolizing enzymes (LPS, LOX, PLD) and MT as strong antioxidant that inhibit their endogenous accumulation. Inhibition of later stage ROS lower lipid peroxidation and this is the consequence of MT application in lotus fruit during cold storage with less activities of LSP, LOX and PLD and higher UFA to SFA ratio, thus higher lipid stability resulting in less membrane degradation.[Citation117] MT has a cascading influence on phospholipid catalysing enzymes endeavouring to mitigate ester bond hydrolysis in membranous phosphoglyceride macromolecule during cold stress and hampering SFA production by LPS and LOX enzymes while reducing catabolic reaction of phospholipids by inhibiting PLD. ATP is the main source of energy for this cascading reaction in fruit.[Citation49] In addition, chilling tolerance in MT dip mango retained better phosphatidylinositol and phosphatidylglycerol, basic components of membranous lipid bilayer necessary for its structural and functional physiology. These contents are directly associated with less membrane hardening due to gel formation in cells and induce chilling tolerance in fruits and vegetables.[Citation118]
Linolenic and linoleic acids are major UFA in the cell membrane and their higher value directly correlates with a higher UFA/SFA ratio while oleic, palmitic and stearic acids are major SFA, indicating the degradation level in the cell membrane. MT-treated fruits demonstrated higher UFA content during low-temperature storage. MT-treated fruit maintained higher phospholipid content resulting in lower lipid peroxidation and electrolyte leakage in banana.[Citation81] Likewise, the UFA profile was also improved in MT-treated tomato fruit, alleviating chilling symptoms throughout cold storage which demonstrated a higher UFA/SFA ratio.[Citation96] In addition, MT application induced chilling tolerance by delaying ion leakage, MDA content and ROS accumulation, and maintained higher membrane integrity in bitter melon,[Citation19] peach[Citation49] and bell pepper.[Citation18] Besides lipids, the other important constituent of the membrane are proteins that are very sensitive to a minute change in temperature. Chilling alleviation is not possible without protecting protein contents from denaturation.[Citation119] The only known possible pathway of MT-induced protein stability is related to polyamine biosynthesis (explained in polyamine section) which has the capacity to bind with protein molecules to make them unavailable for ROS and alleviate their degradation.[Citation10] influx and high fluctuations in its magnitude alter membranous protein.[Citation120] There is limited information about protein variation and the possible mechanism of MT on its stability in terms of cold tolerance. There is a need to explore the mechanism of protein degradation in relation to the CI in MT-treated fruits and vegetables.
MT and postharvest quality
CI significantly downregulates the quality of fruits and vegetables which lowers their acceptability among consumers. Multiple studies have reported that MT application shown great potential in maintaining the external and internal quality attributes in addition to CI reduction during storage. Xin et al.[Citation121] revealed that postharvest MT application delayed reduction in ascorbic acid, soluble protein, and TA, and inhibited the degradation of chlorophyll content in cold stored cucumbers. Additionally, the exogenous MT application retained higher total phenolics and anthocyanins in litchi and strawberry fruits.[Citation122] Exogenous MT application in strawberries cv. ‘Hongyan’ improved fruit firmness, colour, and TA during entire period of storage.[Citation123] As reported by Bal[Citation89] postharvest MT treatment also enhanced total flavonoids, total antioxidants, and ascorbic acid content in nectarine cv. ‘Fantasia’. Similarly, MT application in mango cv. ‘Dashehri’ resulted in higher fruit firmness, SSC, TA, SSC: TA, ascorbic acid, total flavonoids, and total phenolics along with alleviation of CI symptoms.[Citation84] Similar effect has been observed by Chen et al.[Citation83] where MT application in guava cv. ‘Xiguahong’ resulted in maintenance of fruit firmness, SSC, TA, and ascorbic acid content. Furthermore, MT treatment to sweet cherries cv. ‘Sunburst’ markedly reduced physiological weight loss, relative electrolyte leakage, and showed higher fruit firmness, SSC and TA during storage period.[Citation124] Moreover, postharvest MT application has exhibited higher phenolics and antioxidant activity in grapes,[Citation125] pomegranate,[Citation53] and jujubes.[Citation126] Accordingly, MT treatment not only ameliorates symptoms of but also improves overall fruit quality.
Literature search, data selection and analysis for meta-analysis
For gathering data, several web databases including ‘Google’, ‘Google Scholar’, ‘Web of Science’ and ‘Scopus’ were used to search the articles by using the ‘melatonin’, ‘postharvest physiology’, ‘chilling injury’ and ‘storage’ keywords in two rounds on July 06, 2022, and December 14, 2022. A total of 92 articles were found and downloaded to a specific folder. Eligible articles (23) were selected for the meta-analysis of CI and MT by screening all articles where CI incidence or CI index was determined in fruits and vegetables, while all other articles were excluded from the study. The values for all parameters from selected articles were noted for the control and MT-treated group, whereas numerical values from bar or line graphs were extracted by using the web-based Get-Data Graph Digitizer (http://www.getdata-graph-digitizer.com/) previously described by Zhang et al.[Citation14] The selected parameters included CI, electrolyte leakage, weight loss, fruit firmness, soluble solid contents, titratable acidity, hydrogen peroxide, malondialdehyde content, superoxide anion, lipoxygenase, phospholipase D, polyphenol oxidase, total phenolics, ascorbic acid, 2,2-diphenyl-1-picrylhydrazyl, phenylalanine ammonia-lyase, catalase, superoxide dismutase, putrescine, spermidine, spermine, γ-aminobutyric acid and proline content were categorised into five groups; quality attributes, oxidative stress, antioxidants and polyamines. The final data were analysed in R software[Citation127] using the ‘meta’ package.[Citation128] In accessing the combined impact of the exogenous application of MT across multiple fruits and vegetables, random-effects models were fit based on the ratio of means (ROM) for the control and treatment groups.
CI and meta-analysis in MT-treated fruits and vegetables
In recent years, exogenous application of MT has been found effective in downregulating CI in several fruits and vegetables during cold storage. The mechanism of MT in mitigating CI is through a ROS-induced defence mechanism that activates antioxidants including enzymatic and non-enzymatic compounds. The biosynthesis of phenolics and ascorbic acid, both major non-enzymatic antioxidants, show a positive correlation with MT treatment and in return are helpful in maintaining better fruit quality including firmness, SSC, and TA during cold storage. The metabolism of secondary metabolites such as polyamines, proline and GABA make a strong free radical scavenging system in addition to antioxidant enzymes.
CI and quality attributes
Overall, the exogenous application of MT reduced CI in several fruits and vegetables (). The inhibition of CI with the application of MT ranged between 21–42% compared to the controls across all fruits and vegetables, based on the random effect model (ROM = 0.49, 95%-CI − 0.38-0.63). As expected, CI sensitivity varied among different fruits and vegetables. For example, in assessing the levels of suppression of CI with the exogenous application of MT in fruits, we observed that ‘Langra’ mango, orange and peach fruit exhibited higher suppression as compared to kiwifruit, litchi, pomegranate, and banana during cold storage. A similar trend of suppression of CI (~1–24%) was observed in studied vegetables such as bell pepper, bitter melon and cucumber but generally, the efficacy of exogenously applied MT in suppression of CI in vegetables was noted as lower when compared to fruits (~4–72%). The efficacy of MT to minimise CI varied across different cultivars of mango and plum, emphasising genotypic differences among different cultivars. Compared to the control groups, there was 10% and 62% suppression of CI during cold storage in cultivars ‘Dashehari’ and ‘Langra’ mangoes, respectively. Similar variations were noted in plum cultivars (‘Queen Rose’ ~39% vs ‘Friar’ ~6%). On the other hand, peach cultivars did not exhibit such great variations in the suppression of CI with the exogenous application of MT. Significant heterogeneity score (100%) across the studies (τ2 = 0.2853, p < 0.001) was observed, highlighting the uniqueness of the suppression of CI in response to the exogenous application of MT across several fruits and vegetables ().
Figure 2. Summary of implications indicating the role of exogenous application of MT on chilling injury, electrolyte leakage, fruit firmness, weight loss, SSC and TA in different fruits and vegetables. Change in all parameters across individual genotypes is given by the values in the ‘Ratio of Means’ column. Where, Cl = confidence level; ROM = ratio of means. .
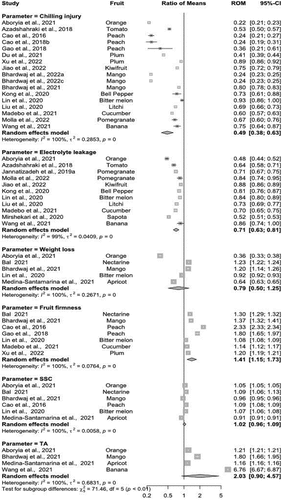
MT treatment generally reduced electrolyte leakage in numerous fruits and vegetable crops, with an overall ratio of means (ROM) of 0.71 (95% CI − 0.63, 0.81). This indicates that the exogenous application of MT suppressed electrolyte leakage by ~20% for most fruits and vegetables as compared to control during storage. There was significant heterogeneity (I2 = 99%) across the studies (τ2 = 0.0409, p < 0.001), indicating a higher level of uniqueness in the response of electrolyte leakage in fruits and vegetables after the application of MT (). The random-effects model revealed that MT-treated fruits and vegetables maintained higher firmness across all studies with an overall ROM of 1.41 (95% CI − 1.15, 1.73) than control groups (). MT treatment reduced~13% firmness loss in all fruits and vegetables than control treatments. Comparatively, the application of MT was more effective in reducing the loss of firmness in fruits than vegetables. Moreover, the reduction in loss of firmness was dependent on the cultivar among the same species. For example, the peach cultivar ‘Qimni’ showed 26% while ‘Hujing’ experienced 37% higher firmness after MT application. There was a significantly higher heterogeneity (I2 = 100) across the studies (τ2 = 0.0764, p < 0.001) among all species of fruits and vegetables.
Compared to the control groups, MT-treated fruits and vegetables generally showed a decline in weight loss except for nectarine and mango. In comparison, orange fruit maintained the lowest weight loss (~44%) while nearly ~8 and 9% higher weight loss in mango and nectarine, respectively than in control (). Exogenous MT application maintained marginally better SSC in most of the fruits and vegetables ranging between ~2–4% (ROM = 1.02, 95%-CI 0.96–1.09) as compared to their respective controls (), while reverse influence was observed in mango and apricot where MT treatment downregulated almost ~1.7 and 4.1% respectively. Similarly, a higher TA level was exhibited in MT-treated fruits as compared to untreated control during cold storage (). The random-effects model demonstrated about~5–66% higher TA among different fruits (ROM = 2.03, 95%-CI − 0.90-4.57). The efficacy of MT treatment varied by species, as there was a higher heterogeneity score (I2 = 100) in fruits for TA content. The results were significantly based on cross studies (τ2 = 0.6831, p < 0.001) for all fruits in contrast to all respective control treatments. The maximum TA was observed in bananas (~83%).
CI and oxidative stress
There was a reduction in H2O2 content ranging from 12% to 22% in all MT-treated fruits and vegetables than in control (ROM = 0.68, 95%-CI − 0.61-0.76). The level of H2O2 inhibition was unique to the individual species, resulting in a higher heterogeneity score (I2 = 100) among different studies (τ2 = 0.0243, p < 0.001). In another measurement, MDA production was inhibited (~12–29%) by the application of MT treatment across different fruits and vegetables (ROM = 0.62, 95%-CI − 0.51-0.75) than their respective untreated groups. The level of MDA content suppression varied greatly among fruits and vegetables across the studies (I2 = 100) (τ2 = 0.1065, p < 0.001). Among the different fruits and vegetables assessed, the highest inhibition was noted in orange (~42%), however, MDA content was increased (~4%) in bananas than in control fruit (). Likewise, superoxide anion accumulation was downregulated by MT treatment in all fruits ranging between~7.5% to 20% as compared to their respective controls. Nevertheless, the efficiency of MT to suppress superoxide anion accumulation was comparatively higher in fruits than vegetables, except for kiwifruit where no significant effect was observed between MT treated and control fruit (). Higher heterogeneity levels (I2 = 100%) across different studies were observed (τ2 = 0.0331, p < 0.001), indicating the genotype-dependent efficacy of MT on the inhibition of superoxide accumulation.
Figure 3. Summary of implications indicating the role of exogenous application of MT on the inhibition of H2O2, MDA content, superoxide anion, lipoxygenase, phospholipase D and PPO activity in different fruits and vegetables. Change in all parameters across individual genotypes is given by the values in the ‘Ratio of Means’ column. Where, Cl = confidence level; ROM = ratio of means.
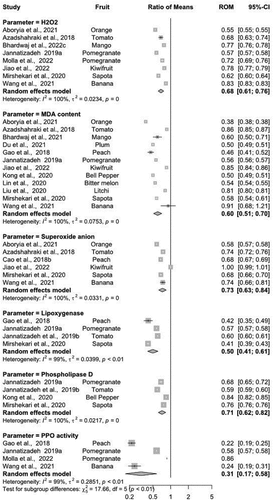
Postharvest application of MT suppressed the activity of the PLD enzyme involved in ROS accumulation and lipid peroxidation. The highest PLD inhibition was observed in tomatoes, while the minimum was recorded in bell peppers (). The random-effects model indicated nearly~9 to 20% lower PLD activity in MT-treated fruits and vegetables. The PLD inhibition was correlated with genotypes as shown by higher heterogeneity index scores (I2 = 100%) across the different studies (τ2 = 0.0217, p < 0.001). Likewise, the inhibition of LOX activity was observed in all MT-treated fruits and vegetables ranging between 21% to 39% to the corresponding control (ROM = 0.50, 95%-CI − 0.41-0.61) (). Maximum LOX inhibition was noted in peach (~38%) while minimum inhibition was observed in tomato (~22%) during storage. The exogenous MT treatment delayed PPO activity in all fruits varying from~11% to 68% as compared to control (). However, the higher inhibition of PPO activity was observed in peach (~66%) while the lower (~6.5%) was noted in pomegranate fruit.
CI and antioxidants
The random-effects model revealed higher phenolic content in most of the MT-treated fruits and vegetables (ROM = 1.34, 95%-CI − 1.12-1.60), except for plum fruit where phenolic content was adversely affected (). Overall, the MT effect varied among different genotypes (I2 = 100%, τ2 = 0.0644, p < 0.001). A similar effect was observed for levels of ascorbic acid among different genotypes (I2 = 100%, τ2 = 0.0859, p < 0.001). The exogenous application of MT induced higher ascorbic acid levels (~7–27%) in all fruit and vegetables, with an overall ROM of 1.48 (95%-CI − 1.17-1.87) as compared to controls. MT-treated fruits showed higher ascorbic acid levels than vegetables (). In general, the MT treatment upregulated DPPH-radical scavenging activity ranging from~5.3–33.2% in all fruits, with an overall ROM of 0.71 (95% CI − 0.63, 0.81). This indicates that the application of MT was found to be effective in increasing DPPH radical activity in fruits depending upon genotype as compared to the control groups. There was significant heterogeneity (I2 = 99%) across the studies (τ2 = 0.0409, p < 0.001), indicating a higher level of uniqueness in the response of DPPH assay in fruits after the application of MT ().
Figure 4. Summary of implications indicating the role of exogenous application of MT on phenolics, ascorbic acid, DPPH, PAL, CAT and SOD activity in different fruits and vegetables. Change in all parameters across individual genotypes is given by the values in the ‘Ratio of Means’ column. Where, Cl = confidence level; ROM = ratio of means.
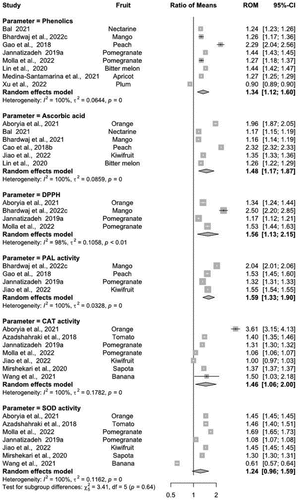
Exogenous MT treatment upregulated PAL activity in all fruits varying from~12% to 28% with an overall ROM of 1.59 (95%-CI − 1.33-1.90) (). PAL activity was observed to be higher in mango (~31%), while the lowest activity was recorded in pomegranate fruit (~12%). Similarly, the postharvest application of MT increased~3 to 30% CAT activity in all fruits and vegetables (). The highest CAT activity was exhibited by MT-treated orange (~58%). In addition, CAT activity was associated with genotype as higher heterogeneity index scores (I2 = 100%) were noted across the different studies (τ2 = 0.1782, p < 0.001). Furthermore, it has been revealed that fruits and vegetables treated with exogenous MT maintained higher SOD activity (~2–20%), with an overall ROM of 1.24 (95%-CI − 0.96-1.59) (). However, the SOD activity in bananas was negatively correlated with the MT application, indicating a 22% reduction. A significantly high heterogeneity score (100%) was noted across the studies (τ2 = 0.1162, p < 0.001), highlighting the uniqueness of the expression of SOD activity in response to MT application across the various fruits and vegetables.
CI and polyamine dependent arginine metabolic pathway
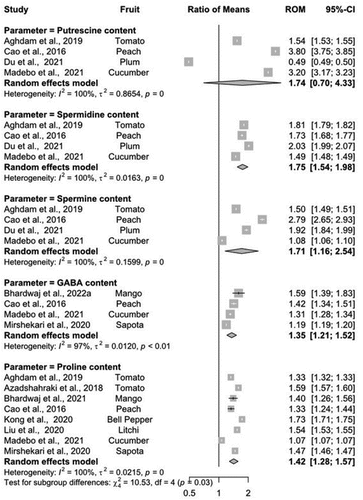
There was a ~ 16–64% increase in putrescine content among different MT-treated fruits and vegetables (ROM = 1.74, 95%-CI − 0.70-4.33), except for plums where a nearly~31% reduction was observed (). Similarly, the spermidine content was also upregulated by MT treatment in all fruits and vegetables ranging between~19% to 30%. The effectiveness of MT for the maintenance of spermidine content was comparatively higher in fruits than in vegetables (). In the same way, the spermine content was also increased in MT-treated fruits and vegetables by ~ 6 to 41%, with an overall ROM of 1.71 (95%-CI − 1.16-2.54). Moreover, the MT treatment significantly exhibited higher GABA (ROM = 1.35, 95%-CI − 1.21-1.52) and proline content (ROM = 1.42, 95%-Cl 1.28–1.57) in vegetables than for fruits, excluding cucumber where no significant difference was observed between MT treatment and control (). Overall, the MT treatment-induced about~11 to 20% increase in proline content depending upon species, which presented higher a heterogeneity index score (I2 = 100, τ2 = 0.0215, p < 0.001) across different reports.
Figure 5. Summary of implications indicating the role of MT treatment on putrescine, spermidine, spermine, GABA and proline content in different fruits and vegetables. Change in all parameters across individual genotypes is given by the values in the ‘Ratio of Means’ column. Where, Cl = confidence level; ROM = ratio of means.
Conclusion and future prospects
CI causes serious economic losses in fresh horticultural produce and is particularly more pronounced in tropical and subtropical fruits and vegetables during cold storage. CI symptoms, sensitivity and optimum cold storage temperature vary among different fruits and vegetables. MT has been widely used to improve chilling tolerance in cold-stored horticultural produce. It alleviates CI by regulating fatty acid metabolism and enhancing the levels of enzymatic and non-enzymatic antioxidant levels, resulting in ROS scavenging. Our meta-analysis results highlighted the following;
The efficacy of MT in inducing cold tolerance varies among genotypes and cultivars.
MT application was more effective in downregulating CI, loss of firmness and superoxide anion accumulation in fruits as compared to vegetables.
MT treatment reduced weight loss in fruits and vegetables except for mangos and nectarines.
MT application delayed the activities of PLD, LOX, and PPO while inhibiting H2O2 and MDA in fruits and vegetables.
MT increased the levels of ascorbic acids, phenolics (except plum), spermidine, spermine, putrescine, GABA, proline, DPPH scavenging, and activities of PAL, CAT, SOD (except banana).
Our results enhance the understanding of the mechanism of CI, MT-mediated cold tolerance in subtropical and tropical fruits and vegetables during cold storage and the quantitative relationship between melatonin treatment and CI.
The effectiveness of MT chilling tolerance in tropical and subtropical horticultural produce during cold storage is well documented, however, the exact physiological and molecular pathway of CI alleviation warrants future investigations. Whilst exogenous MT-treated fruits and vegetables elicit lower CI symptoms, the influence of exogenous MT on protein molecules in the plasma membrane during cold stress is still lacking. Additionally, the exact role of MT application on the mitochondrial structure and cell wall degrading enzymes during cold stress deserves to be examined on a molecular basis concerning ATP synthesis and associated enzymes in horticultural produce. The molecular crosstalk between MT and other phytohormones like NO, H2S, ethylene and salicylic acid in cold stress is yet unknown and justifies future investigations.
Author contributions
H. M. S. Shah; conceptualization, data acquisition, writing original draft, visualization, Z. Singh: conceptualization, review and editing, project administration, supervision, E. Afrifa-Yamoah; Statistical analysis, review and editing, visualization, supervision; M. U. Hasan; review and editing, J. Kaur; review and editing; A. Woodward; review and editing, supervision
Acknowledgments
The first author is very thankful to Edith Cowan University (ECU), Joondalup Western Australia, Australia, to grant the Higher Degree by Research Scholarship during the Ph. D degree in Horticulture at the School of Science, ECU. We are also thankful to Michael Stein, HDR Communication Adviser, Edith Cowan University, Joondalup, Western Australia, Australia for editing this manuscript.
Disclosure statement
All the authors have reviewed the paper and agreed to publish it without any financial or personal interest in the work.
Additional information
Funding
References
- Bellavia, A.; Larsson, S. C.; Bottai, M.; Wolk, A.; Orsini, N. Fruit and Vegetable Consumption and All-Cause Mortality: A Dose-Response Analysis. Am. J. Clin. Nut. 2013, 98(2), 454–459. DOI: 10.3945/ajcn.112.056119.
- Singh, Z. Interventions to Minimise Postharvest Losses, Ensure the Quality and Safety of Tropical and Subtropical Fruits: An Overview. Acta Hortic. 2022, 1340, 1–12. DOI: 10.17660/ActaHortic.2022.1340.1.
- Singh, S. P.; Singh, Z.; Swinny, E. E. Postharvest Nitric Oxide Fumigation Delays Fruit Ripening and Alleviates Chilling Injury During Cold Storage of Japanese Plums (Prunus salicina Lindell). Postharvest. Biol. Technol. 2009, 53(3), 101–108. DOI: 10.1016/j.postharvbio.2009.04.007.
- Shewfelt, R. Response of Plant Membranes to Chilling and Freezing. In The Plant Membrane: A Biophysical Approach; Leshem, Y.Y., Ed.; Springer: Kluwer: Dordrecht, The Netherlands, 1992; pp. 192–219.
- Zhang, W.; Jiang, H.; Cao, J.; Jiang, W. Advances in Biochemical Mechanisms and Control Technologies to Treat Chilling Injury in Postharvest Fruits and Vegetables. Trends Food Sci. Technol. 2021, 113, 355–365. DOI: 10.1016/j.tifs.2021.05.009.
- Droby, S.; Wisniewski, M.; Teixidó, N.; Spadaro, D.; Jijakli, M. H. The Science, Development, and Commercialization of Postharvest Biocontrol Products. Postharvest. Biol. Technol. 2016, 122, 22–29. DOI: 10.1016/j.postharvbio.2016.04.006.
- Back, K.; Tan, D. X.; Reiter, R. J. Melatonin Biosynthesis in Plants: Multiple Pathways Catalyze Tryptophan to Melatonin in the Cytoplasm or Chloroplasts. J. Pin. Res. 2016, 61(4), 426–437. DOI: 10.1111/jpi.12364.
- Wei, J.; Li, D. X.; Zhang, J. R.; Shan, C.; Rengel, Z.; Song, Z. B.; Chen, Q. Phytomelatonin Receptor PMTR1-Mediated Signaling Regulates Stomatal Closure in Arabidopsis thaliana. J. Pin. Res. 2018, 65(2), e12500. DOI: 10.1111/jpi.12500.
- Madebo, M. P.; Hu, S.; Zheng, Y.; Jin, P. Mechanisms of Chilling Tolerance in Melatonin Treated Postharvest Fruits and Vegetables: A Review. J. Fut. Foods. 2021, 1(2), 156–167. DOI: 10.1016/j.jfutfo.2022.01.005.
- Du, H.; Liu, G.; Hua, C.; Liu, D.; He, Y.; Liu, H.; Kurtenbach, R.; Ren, D. Exogenous Melatonin Alleviated Chilling Injury in Harvested Plum Fruit via Affecting the Levels of Polyamines Conjugated to Plasma Membrane. Postharvest. Biol. Technol. 2021, 179, 111585. DOI: 10.1016/j.postharvbio.2021.111585.
- Xu, R.; Wang, L.; Li, K.; Cao, J.; Zhao, Z. Integrative Transcriptomic and Metabolomic Alterations Unravel the Effect of Melatonin on Mitigating Postharvest Chilling Injury Upon Plum (Cv. Friar) Fruit. Postharvest. Biol. Technol. 2022, 186, 111819. DOI: 10.1016/j.postharvbio.2021.111819.
- Medina-Santamarina, J.; Serrano, M.; Lorente-Mento, J. M.; García-Pastor, M. E.; Zapata, P. J.; Valero, D.; Guillén, F. Melatonin Treatment of Pomegranate Trees Increases Crop Yield and Quality Parameters at Harvest and During Storage. Agron. 2021, 11(5), 861. DOI: 10.3390/agronomy11050861.
- Jayarajan, S.; Sharma, R. Melatonin: A Blooming Biomolecule for Postharvest Management of Perishable Fruits and Vegetables. Trends Food Sci. Technol. 2021, 116, 318–328. DOI: 10.1016/j.tifs.2021.07.034.
- Zhang, J.; Ma, Y.; Dong, C.; Terry, L. A.; Watkins, C. B.; Yu, Z.; Cheng, Z. M. Meta-Analysis of the Effects of 1-Methylcyclopropene (1-MCP) Treatment on Climacteric Fruit Ripening. Hortic. Res. 2020, 7(1), 208. DOI: 10.1038/s41438-020-00405-x.
- Borenstein, M.; Hedges, L. V.; Higgins, J. P.; Rothstein, H. R. Introduction to Meta-Analysis, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021.
- Chen, J.; He, L.; Jiang, Y.; Wang, Y.; Joyce, D. C.; Ji, Z.; Lu, W. Role of Phenylalanine Ammonia‐lyase in Heat Pretreatment‐induced Chilling Tolerance in Banana Fruit. Physiol. Planta. 2008, 132(3), 318–328. DOI: 10.1111/j.1399-3054.2007.01013.x.
- Zaharah, S.; Singh, Z. Postharvest Nitric Oxide Fumigation Alleviates Chilling Injury, Delays Fruit Ripening and Maintains Quality in Cold-Stored ‘Kensington Pride’ Mango. Postharvest. Biol. Technol. 2011, 60(3), 202–210. DOI: 10.1016/j.postharvbio.2011.01.011.
- Kong, X.; Ge, W.; Wei, B.; Zhou, Q.; Zhou, X.; Zhao, Y.; Ji, S. Melatonin Ameliorates Chilling Injury in Green Bell Peppers During Storage by Regulating Membrane Lipid Metabolism and Antioxidant Capacity. Postharvest. Biol. Technol. 2020, 170, 111315. DOI: 10.1016/j.postharvbio.2020.111315.
- Lin, X.; Wang, L.; Hou, Y.; Zheng, Y.; Jin, P. A Combination of Melatonin and Ethanol Treatment Improves Postharvest Quality in Bitter Melon Fruit. Foods. 2020, 9(10), 1376. DOI: 10.3390/foods9101376.
- Park, M. H.; Sangwanangkul, P.; Choi, J. W. Reduced Chilling Injury and Delayed Fruit Ripening in Tomatoes with Modified Atmosphere and Humidity Packaging. Sci. Hortic. 2018, 231, 66–72. DOI: 10.1016/j.scienta.2017.12.021.
- Leisso, R. S.; Buchanan, D. A.; Lee, J.; Mattheis, J. P.; Sater, C.; Hanrahan, I.; Watkins, C. B.; Gapper, N.; Johnston, J. W.; Schaffer, R. J. Chilling-Related Cell Damage of Apple (Malus × domestica Borkh.) Fruit Cortical Tissue Impacts Antioxidant, Lipid and Phenolic Metabolism. Physiol. Planta. 2015, 153(2), 204–220. DOI: 10.1111/ppl.12244.
- Stanley, J.; Prakash, R.; Marshall, R.; Schröder, R. Effect of Harvest Maturity and Cold Storage on Correlations Between Fruit Properties During Ripening of Apricot (Prunus armeniaca). Postharvest. Biol. Technol. 2013, 82, 39–50. DOI: 10.1016/j.postharvbio.2013.02.020.
- Pesis, E.; Ackerman, M.; Ben-Arie, R.; Feygenberg, O.; Feng, X.; Apelbaum, A.; Goren, R.; Prusky, D. Ethylene Involvement in Chilling Injury Symptoms of Avocado During Cold Storage. Postharvest. Biol. Technol. 2002, 24(2), 171–181. DOI: 10.1016/S0925-5214(01)00134-X.
- Nguyen, T. B. T.; Ketsa, S.; Van Doorn, W. G. Effect of Modified Atmosphere Packaging on Chilling-Induced Peel Browning in Banana. Postharvest. Biol. Technol. 2004, 31(3), 313–317. DOI: 10.1016/j.postharvbio.2003.09.006.
- Molimau-Samasoni, S.; Vaavia, V.; Wills, R. B. Effect of Low Temperatures on the Storage Life of Two Samoan Breadfruit (Artocarpus altilis) Cultivars. J. Hortic. Postharvest Res. 2020, 3, 91–96. DOI: 10.22077/JHPR.2019.2912.1106.
- Ali, Z. M.; Chin, L. H.; Marimuthu, M.; Lazan, H. Low Temperature Storage and Modified Atmosphere Packaging of Carambola Fruit and Their Effects on Ripening Related Texture Changes, Wall Modification and Chilling Injury Symptoms. Postharvest. Biol. Technol. 2004, 33(2), 181–192. DOI: 10.1016/j.postharvbio.2004.02.007.
- Medina-Santamarina, J.; Guillén, F.; Ilea, M. I. M.; Ruiz-Aracil, M. C.; Valero, D.; Castillo, S.; Serrano, M. Melatonin Treatments Reduce Chilling Injury and Delay Ripening, Leading to Maintenance of Quality in Cherimoya Fruit. Int. J. Mol. Sci. 2023, 24(4), 3787. DOI: 10.3390/ijms24043787.
- Fan, Z.; Lin, B.; Lin, H.; Lin, M.; Chen, J.; Lin, Y. γ-Aminobutyric Acid Treatment Reduces Chilling Injury and Improves Quality Maintenance of Cold-Stored Chinese Olive Fruit. Food Chem X. 2022, 13, 100208. DOI: 10.1016/j.fochx.2022.100208.
- Wang, C. Y.; Wang, S. Y. Effect of Storage Temperatures on Fruit Quality of Various Cranberry Cultivars. Acta Hortic. 2009, 810(810), 853–862. DOI: 10.17660/ActaHortic.2009.810.114.
- Prasanna, V. K.; Sudhakar, R. D.; Krishnamurthy, S. Effect of Storage Temperature on Ripening and Quality of Custard Apple (Annona squamosa L.) Fruits. J. Hortic. Sci. Biotechnol. 2000, 75(5), 546–550. DOI: 10.1080/14620316.2000.11511283.
- Tongdee, S. C.; Suwanagul, A.; Neamprem, S.; Bunruengsri, U. Effect of Surface Coatings on Weight Loss and Internal Atmosphere of Durian (Durio zibethinus Murray) Fruit. ASEAN Food J. 1990, 5, 103–107.
- McCollum, T.; McDonald, R. Electrolyte Leakage, Respiration, and Ethylene Production as Indices of Chilling Injury in Grapefruit. HortScience. 1991, 26(9), 1191–1192. DOI: 10.21273/HORTSCI.26.9.1191.
- Alba-Jiménez, J.; Benito-Bautista, P.; Nava, G.; Rivera-Pastrana, D.; Vázquez-Barrios, M. E.; Mercado-Silva, E. Chilling Injury is Associated with Changes in Microsomal Membrane Lipids in Guava Fruit (Psidium guajava L.) and the Use of Controlled Atmospheres Reduce These Effects. Sci. Hortic. 2018, 240, 94–101. DOI: 10.1016/j.scienta.2018.05.026.
- Kaur, J.; Singh, Z.; Shah, H. M. S.; Mazhar, M. S.; Hasan, M. U.; Woodward, A. Insights into Phytonutrient Profile and Postharvest Quality Management of Jackfruit: A Review. Crit. Rev. Food Sci. Nutr. 2023, 1–27. DOI: 10.1080/10408398.2023.2174947.
- Zhang, J.; Wu, Z.; Ban, Z.; Li, L.; Chen, C.; Kowaleguet, M. G. G. M.; Chen, F.; Fei, L.; Wang, L. Exogenous Polyamines Alleviate Chilling Injury of Jujube Fruit (Zizyphus jujuba Mill). J. Food Proc. Pres. 2020, 44(10), e14746. DOI: 10.1111/jfpp.14746.
- Jiao, J.; Jin, M.; Liu, H.; Suo, J.; Yin, X.; Zhu, Q.; Rao, J. Application of Melatonin in Kiwifruit (Actinidia chinensis) Alleviated Chilling Injury During Cold Storage. Sci. Hortic. 2022, 296, 110876. DOI: 10.1016/j.scienta.2022.110876.
- Obenland, D.; Margosan, D.; Houck, L.; Aung, L. Essential Oils and Chilling Injury in Lemon. HortScience. 1997, 32(1), 108–111. DOI: 10.21273/HORTSCI.32.1.108.
- Khan, A. S.; Singh, Z. Harvesting and postharvest management. In The Lime Botany: Production and Uses; Khan, Mumtaz M., Al-Yahyal, R., Al-Said, F. Eds. CAB International: Wallingford, UK, 2017; pp. 186–205.
- Liu, G.; Zhang, Y.; Yun, Z.; Hu, M.; Liu, J.; Jiang, Y.; Zhang, Z. Melatonin Enhances Cold Tolerance by Regulating Energy and Proline Metabolism in Litchi Fruit. Foods. 2020, 9(4), 454. DOI: 10.3390/foods9040454.
- Jaitrong, S.; Rattanapanone, N.; Manthey, J. A. Analysis of the Phenolic Compounds in Longan (Dimocarpus longan Lour.) Peel. Acta Hortic. 2006, 119, 371–375. DOI: 10.17660/ActaHortic.2005.682.208.
- Shah, H. M. S.; Khan, A. S.; Singh, Z.; Ayyub, S. Postharvest Biology and Technology of Loquat (Eriobotrya japonica Lindl.). Foods. 2023, 12(6), 1329. DOI: 10.3390/foods12061329.
- Ghasemnezhad, M.; Marsh, K.; Shilton, R.; Babalar, M.; Woolf, A. Effect of Hot Water Treatments on Chilling Injury and Heat Damage in ‘Satsuma’ Mandarins: Antioxidant Enzymes and Vacuolar ATPase, and Pyrophosphatase. Postharvest. Biol. Technol. 2008, 48(3), 364–371. DOI: 10.1016/j.postharvbio.2007.09.014.
- Kebbeh, M.; Dong, J.; Chen, H.; Shen, S.; Yan, L.; Zheng, X. Melatonin Treatment Alleviates Chilling Injury in Mango Fruit ‘Keitt’ by Modulating Proline Metabolism Under Chilling Stress. J. Integ. Agric. 2023, 22(3), 935–944. DOI: 10.1016/j.jia.2023.02.008.
- Kondo, S.; Jitratham, A.; Kittikorn, M.; Kanlayanarat, S. Relationships Between Jasmonates and Chilling Injury in Mangosteens are Affected by Spermine. HortScience. 2004, 39(6), 1346–1348. DOI: 10.21273/HORTSCI.39.6.1346.
- Yang, C.; Han, B.; Zheng, Y.; Liu, L.; Li, C.; Sheng, S.; Zhang, J.; Wang, J.; Wu, F. The Quality Changes of Postharvest Mulberry Fruit Treated by Chitosan- g -Caffeic Acid during Cold Storage. J. Food Sci. 2016, 81(4), C881–888. DOI: 10.1111/1750-3841.13262.
- Nanos, G.; Kiritsakis, A.; Sfakiotakis, E. Preprocessing Storage Conditions for Green ‘Conservolea’ and ‘Chondrolia’ Table Olives. Postharvest. Biol. Technol. 2002, 25(1), 109–115. DOI: 10.1016/S0925-5214(01)00164-8.
- Aboryia, M.; Loay, A.; Omar, A. S. Reduction of Chilling Injury of ‘Washington’ Navel Orange Fruits by Melatonin Treatments During Cold Storage. Folia Hortic. 2021, 33(2), 343–353. DOI: 10.2478/fhort-2021-0026.
- Pan, Y. G.; Yuan, M. Q.; Zhang, W. M.; Zhang, Z. K. Effect of Low Temperatures on Chilling Injury in Relation to Energy Status in Papaya Fruit During Storage. Postharvest. Biol. Technol. 2017, 125, 181–187. DOI: 10.1016/j.postharvbio.2016.11.016.
- Gao, H.; Lu, Z.; Yang, Y.; Wang, D.; Yang, T.; Cao, M.; Cao, W. Melatonin Treatment Reduces Chilling Injury in Peach Fruit Through Its Regulation of Membrane Fatty Acid Contents and Phenolic Metabolism. Food Chem. 2018, 245, 659–666. DOI: 10.1016/j.foodchem.2017.10.008.
- Salvador, A.; Arnal, L. A.; Monterde, A.; Cuquerella, J. N. Reduction of Chilling Injury Symptoms in Persimmon Fruit Cv. ‘Rojo Brillante’ by 1-MCP. Postharvest. Biol. Technol. 2004, 33(3), 285–291. DOI: 10.1016/j.postharvbio.2004.03.005.
- Nilprapruck, P.; Pradisthakarn, N.; Authanithee, F.; Keebjan, P. Effect of Exogenous Methyl Jasmonate on Chilling Injury and Quality of Pineapple (Ananas comosus L.) Cv. Pattavia. Sci. Eng. Health Stud. 2008, 33–42. DOI: 10.14456/sustj.2008.9.
- Freitas, S. T. D.; Mitcham, E. J. Quality of Pitaya Fruit (Hylocereus undatus) as Influenced by Storage Temperature and Packaging. Sci. Agric. 2013, 70(4), 257–262. DOI: 10.1590/S0103-90162013000400006.
- Jannatizadeh, A. Exogenous Melatonin Applying Confers Chilling Tolerance in Pomegranate Fruit During Cold Storage. Sci. Hortic. 2019, 246, 544–549. DOI: 10.1016/j.scienta.2018.11.027.
- Mirshekari, A.; Madani, B.; Yahia, E. M.; Golding, J. B.; Vand, S. H. Postharvest Melatonin Treatment Reduces Chilling Injury in Sapota Fruit. J. Sci. Food Agric. 2020, 100(5), 1897–1903. DOI: 10.1002/jsfa.10198.
- Perkins-Veazie, P.; Collins, J.; McCollum, T.; Motes, J. Comparison of Asparagus Cultivars During Storage. HortTechnol. 1993, 3(3), 330–331. DOI: 10.21273/HORTTECH.3.3.330.
- Song, L.; Zhang, W.; Li, Q.; Jiang, Z.; Wang, Y.; Xuan, S.; Zhao, J.; Luo, S.; Shen, S.; Chen, X. Melatonin Alleviates Chilling Injury and Maintains Postharvest Quality by Enhancing Antioxidant Capacity and Inhibiting Cell Wall Degradation in Cold-Stored Eggplant Fruit. Postharvest. Biol. Technol. 2022, 194, 112092. DOI: 10.1016/j.postharvbio.2022.112092.
- Liu, Z.; Li, L.; Luo, Z.; Zeng, F.; Jiang, L.; Tang, K. Effect of Brassinolide on Energy Status and Proline Metabolism in Postharvest Bamboo Shoot During Chilling Stress. Postharvest. Biol. Technol. 2016, 111, 240–246. DOI: 10.1016/j.postharvbio.2015.09.016.
- Raseetha, S.; Nadirah, S. Effect of Different Packaging Materials on Quality of Fresh-Cut Broccoli and Cauliflower at Chilled Temperature. Int. Food Res. J. 2018, 25(4), 1559–1565.
- Xu, C. C.; Liu, D. K.; Guo, C. X.; Wu, Y. Q. Effect of Cooling Rate and Super-Chilling Temperature on Ice Crystal Characteristic, Cell Structure, and Physicochemical Quality of Super-Chilled Fresh-Cut Celery. Int. J. Refrig. 2020, 113, 249–255. DOI: 10.1016/j.ijrefrig.2020.01.024.
- Sullivan, K. M.; Bramlage, W. J. Chilling Injury of Chile Peppers (Capsicum annuum L.). HortScience. 2000, 35(5), 829. DOI: 10.21273/HORTSCI.35.5.829B.
- Madebo, M. P.; Luo, S.; Li, W.; Zheng, Y.; Peng, J. Melatonin Treatment Induces Chilling Tolerance by Regulating the Contents of Polyamine, γ-Aminobutyric Acid, and Proline in Cucumber Fruit. J. Integ. Agric. 2021, 20(11), 3060–3074. DOI: 10.1016/S2095-3119(20)63485-2.
- Kaushal, M.; Gupta, A.; Vaidya, D.; Gupta, M. Postharvest Management and Value Addition of Ginger (Zingiber officinale Roscoe): A Review. Int. J. Environ. Agric. Biotech. 2017, 2(1), 397–412. DOI: 10.22161/ijeab/2.1.50.
- Wang, J.; Mao, L.; Li, X.; Lv, Z.; Liu, C.; Huang, Y.; Li, D. Oxalic Acid Pretreatment Reduces Chilling Injury in Hami Melons (Cucumis melo Var. Reticulatus Naud.) by Regulating Enzymes Involved in Antioxidative Pathways. Sci. Hortic. 2018, 241, 201–208. DOI: 10.1016/j.scienta.2018.06.084.
- Phornvillay, S.; Pongprasert, N.; Aree, W. C.; Uthairatanakij, A.; Srilaong, V. Exogenous Putrescine Treatment Delays Chilling Injury in Okra Pod (Abelmoschus esculentus) Stored at Low Storage Temperature. Sci. Hortic. 2019, 256, 108550. DOI: 10.1016/j.scienta.2019.108550.
- Lukatkin, A. S.; Brazaityte, A.; Bobinas, C.; Duchovskis, P. Chilling Injury in Chilling-Sensitive Plants: A Review. Agric. 2012, 99, 111–124.
- Zuo, X.; Cao, S.; Zhang, M.; Cheng, Z.; Cao, T.; Jin, P.; Zheng, Y. High Relative Humidity (HRH) Storage Alleviates Chilling Injury of Zucchini Fruit by Promoting the Accumulation of Proline and ABA. Postharvest. Biol. Technol. 2021, 171, 111344. DOI: 10.1016/j.postharvbio.2020.111344.
- Karim, N. U.; Yusof, N. L. Effect of Vacuum Impregnation with Sucrose and Plant Growth Hormones to Mitigate the Chilling Injury in Spinach Leaves. Appl. Sci. 2021, 11(21), 10410. DOI: 10.3390/app112110410.
- Pan, Y.; Chen, L.; Chen, X.; Jia, X.; Zhang, J.; Ban, Z.; Li, X. Postharvest Intermittent Heat Treatment Alleviates Chilling Injury in Cold‐stored Sweet Potato Roots Through the Antioxidant Metabolism Regulation. J. Food Proc. Pres. 2019, 43(12), e14274. DOI: 10.1111/jfpp.14274.
- Azadshahraki, F.; Jamshidi, B.; Mohebbi, S. Postharvest Melatonin Treatment Reduces Chilling Injury and Enhances Antioxidant Capacity of Tomato Fruit During Cold Storage. Adv. Hortic. Sci. 2018, 32, 299–310. DOI: 10.13128/ahs-22260.
- Habibi, F.; Ramezanian, A.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Valero, D. Susceptibility of Blood Orange Cultivars to Chilling Injury Based on Antioxidant System and Physiological and Biochemical Responses at Different Storage Temperatures. Foods. 2020, 9(11), 1609. DOI: 10.3390/foods9111609.
- Wang, P.; Zhang, B.; Li, X.; Xu, C.; Yin, X.; Shan, L.; Ferguson, I.; Chen, K. Ethylene Signal Transduction Elements Involved in Chilling Injury in Non-Climacteric Loquat Fruit. J. Exp. Bot. 2010, 61(1), 179–190. DOI: 10.1093/jxb/erp302.
- Phakawatmongkol, W.; Ketsa, S.; Van Doorn, W. G. Variation in Fruit Chilling Injury Among Mango Cultivars. Postharvest. Biol. Technol. 2004, 32(1), 115–118. DOI: 10.1016/j.postharvbio.2003.11.011.
- Crisosto, C. H.; Mitchell, F. G.; Ju, Z. Susceptibility to Chilling Injury of Peach, Nectarine, and Plum Cultivars Grown in California. HortScience. 1999, 34(6), 1116–1118. DOI: 10.21273/HORTSCI.34.6.1116.
- Biswas, P.; East, A. R.; Hewett, E. W.; Heyes, J. A. Chilling Injury in Tomato Fruit. Hortic. Rev. 2016, 44, 229–278. DOI: 10.1002/9781119281269.ch5.
- Woolf, A. B.; Watkins, C. B.; Bowen, J. H.; Lay-Yee, M.; Maindonald, J. H.; Ferguson, I. B. Reducing External Chilling Injury in Stored ‘Hass’ Avocados with Dry Heat Treatments. J. Am. Soc. Hortic. Sci. 1995, 120(6), 1050–1056. DOI: 10.21273/JASHS.120.6.1050.
- Shewfelt, R.; Del Rosario, B. The Role of Lipid Peroxidation in Storage Disorders of Fresh Fruits and Vegetables. HortScience. 2000, 35(4), 575–579. DOI: 10.21273/HORTSCI.35.4.575.
- Aghdam, M. S.; Fard, J. R. Melatonin Treatment Attenuates Postharvest Decay and Maintains Nutritional Quality of Strawberry Fruits (Fragaria × anannasa Cv. Selva) by Enhancing GABA Shunt Activity. Food Chem. 2017, 221, 1650–1657. DOI: 10.1016/j.foodchem.2016.10.123.
- Savchenko, T.; Tikhonov, K. Oxidative Stress-Induced Alteration of Plant Central Metabolism. Life. 2021, 11(4), 304. DOI: 10.3390/life11040304.
- Medina-Santamarina, J.; Zapata, P. J.; Valverde, J. M.; Valero, D.; Serrano, M.; Guillén, F. Melatonin Treatment of Apricot Trees Leads to Maintenance of Fruit Quality Attributes During Storage at Chilling and Non-Chilling Temperatures. Agron. 2021, 11(5), 917. DOI: 10.3390/agronomy11050917.
- Dong, Q.; Liu, H.; Kurtenbach, R. Polyamines in Plasma Membrane Function in Melatonin-Mediated Tolerance of Apricot Fruit to Chilling Stress. Czech J. Food Sci. 2022, 40(4), 313–322. DOI: 10.17221/74/2022-CJFS.
- Wang, Z.; Pu, H.; Shan, S.; Zhang, P.; Li, J.; Song, H.; Xu, X. Melatonin Enhanced Chilling Tolerance and Alleviated Peel Browning of Banana Fruit Under Low Temperature Storage. Postharvest. Biol. Technol. 2021, 179, 111571. DOI: 10.1016/j.postharvbio.2021.111571.
- Wang, Z.; Zhang, L.; Duan, W.; Li, W.; Wang, Q.; Li, J.; Song, H.; Xu, X. Melatonin Maintained Higher Contents of Unsaturated Fatty Acid and Cell Membrane Structure Integrity in Banana Peel and Alleviated Postharvest Chilling Injury. Food Chem. 2022, 397, 133836. DOI: 10.1016/j.foodchem.2022.133836.
- Chen, H.; Lin, H.; Jiang, X.; Lin, M.; Fan, Z. Amelioration of Chilling Injury and Enhancement of Quality Maintenance in Cold-Stored Guava Fruit by Melatonin Treatment. Food Chem X. 2022, 14, 100297. DOI: 10.1016/j.fochx.2022.100297.
- Bhardwaj, R.; Pareek, S.; Mani, S.; Domínguez-Avila, J. A.; González-Aguilar, G. A.; Aguayo, E. A Melatonin Treatment Delays Postharvest Senescence, Maintains Quality, Reduces Chilling Injury, and Regulates Antioxidant Metabolism in Mango Fruit. J. Food Qual. 2022, 2022, 2022. DOI: 10.1155/2022/2379556.
- Bhardwaj, R.; Pareek, S.; Saravanan, C.; Yahia, E. M. Contribution of Pre-Storage Melatonin Application to Chilling Tolerance of Some Mango Fruit Cultivars and Relationship with Polyamines Metabolism and γ-Aminobutyric Acid Shunt Pathway. Environ. Exp. Bot. 2022, 194, 104691. DOI: 10.1016/j.envexpbot.2021.104691.
- Bhardwaj, R.; Pareek, S.; Domínguez-Avila, J. A.; Gonzalez-Aguilar, G. A.; Valero, D.; Serrano, M. An Exogenous Pre-Storage Melatonin Alleviates Chilling Injury in Some Sango Fruit Cultivars, by Acting on the Enzymatic and Non-Enzymatic Antioxidant System. Antioxidants. 2022, 11(2), 384. DOI: 10.3390/antiox11020384.
- Bhardwaj, R.; Pareek, S.; González-Aguilar, G. A.; Domínguez-Avila, J. A. Changes in the Activity of Proline-Metabolising Enzymes is Associated with Increased Cultivar-Dependent Chilling Tolerance in Mangos, in Response to Pre-Storage Melatonin Application. Postharvest. Biol. Technol. 2021, 182, 111702. DOI: 10.1016/j.postharvbio.2021.111702.
- Xu, P.; Huber, D. J.; Gong, D.; Yun, Z.; Pan, Y.; Jiang, Y.; Zhang, Z. Amelioration of Chilling Injury in ‘Guifei’ Mango Fruit by Melatonin is Associated with Regulation of Lipid Metabolic Enzymes and Remodeling of Lipidome. Postharvest. Biol. Technol. 2023, 198, 112233. DOI: 10.1016/j.postharvbio.2022.112233.
- Bal, E. Effect of Melatonin Treatments on Biochemical Quality and Postharvest Life of Nectarines. J. Food Meas. Charact. 2021, 15(1), 288–295. DOI: 10.1007/s11694-020-00636-5.
- Cao, S.; Shao, J.; Shi, L.; Xu, L.; Shen, Z.; Chen, W.; Yang, Z. Melatonin Increases Chilling Tolerance in Postharvest Peach Fruit by Alleviating Oxidative Damage. Sci. Rep. 2018, 8(1), 1–11. DOI: 10.1038/s41598-018-19363-5.
- Cao, S.; Song, C.; Shao, J.; Bian, K.; Chen, W.; Yang, Z. Exogenous Melatonin Treatment Increases Chilling Tolerance and Induces Defense Response in Harvested Peach Fruit During Cold Storage. J. Agric. Food. Chem. 2016, 64(25), 5215–5222. DOI: 10.1021/acs.jafc.6b01118.
- Guillén, F.; Medina-Santamarina, J.; García-Pastor, M. E.; Chen, N. J.; Uruu, G.; Paull, R. E. Postharvest Melatonin Treatment Delays Senescence and Increases Chilling Tolerance in Pineapple. LWT. 2022, 169, 113989. DOI: 10.1016/j.lwt.2022.113989.
- Molla, S. M. H.; Rastegar, S.; Omran, V. G.; Khademi, O. Ameliorative Effect of Melatonin Against Storage Chilling Injury in Pomegranate Husk and Arils Through Promoting the Antioxidant System. Sci. Hortic. 2022, 295, 110889. DOI: 10.1016/j.scienta.2022.110889.
- Wang, L.; Shen, X.; Chen, X.; Ouyang, Q.; Tan, X.; Tao, N. Exogenous Application of Melatonin to Green Horn Pepper Fruit Reduces Chilling Injury During Postharvest Cold Storage by Regulating Enzymatic Activities in the Antioxidant System. Plants. 2022, 11(18), 2367. DOI: 10.3390/plants11182367.
- Aghdam, M. S.; Luo, Z.; Jannatizadeh, A.; Sheikh-Assadi, M.; Sharafi, Y.; Farmani, B.; Fard, J. R.; Razavi, F. Employing Exogenous Melatonin Applying Confers Chilling Tolerance in Tomato Fruits by Upregulating ZAT2/6/12 Giving Rise to Promoting Endogenous Polyamines, Proline, and Nitric Oxide Accumulation by Triggering Arginine Pathway Activity. Food Chem. 2019, 275, 549–556. DOI: 10.1016/j.foodchem.2018.09.157.
- Jannatizadeh, A.; Aghdam, M. S.; Luo, Z.; Razavi, F. Impact of Exogenous Melatonin Application on Chilling Injury in Tomato Fruits During Cold Storage. Food Bioproc. Technol. 2019, 12(5), 741–750. DOI: 10.1007/s11947-019-2247-1.
- Luo, Y.; Wang, R.; Lei, X.; Ren, Y.; Yuan, C. Melatonin Treatment Delays Senescence and Alleviates Chilling Injury in Spaghetti Squash During Low-Temperature Storage. Sci. Hortic. 2023, 310, 111778. DOI: 10.1016/j.scienta.2022.111778.
- Cao, S.; Bian, K.; Shi, L.; Chung, H.; Chen, W.; Yang, Z. Role of Melatonin in Cell-Wall Disassembly and Chilling Tolerance in Cold-Stored Peach Fruit. J. Agric. Food. Chem. 2018, 66(22), 5663–5670. DOI: 10.1021/acs.jafc.8b02055.
- Singh, S. P.; Singh, Z. Controlled and Modified Atmospheres Influence Chilling Injury, Fruit Quality and Antioxidative System of Japanese Plums (Prunus salicina Lindell). Int. J. Food Sci. 2013, 48(2), 363–374. DOI: 10.1111/j.1365-2621.2012.03196.x.
- Gao, H.; Zhang, Z. K.; Chai, H. K.; Cheng, N.; Yang, Y.; Wang, D. N.; Yang, T.; Cao, W. Melatonin Treatment Delays Postharvest Senescence and Regulates Reactive Oxygen Species Metabolism in Peach Fruit. Postharvest. Biol. Technol. 2016, 118, 103–110. DOI: 10.1016/j.postharvbio.2016.03.006.
- Sun, Q.; Liu, L.; Zhang, L.; Lv, H.; He, Q.; Guo, L.; Zhang, X.; He, H.; Ren, S.; Zhang, N. Melatonin Promotes Carotenoid Biosynthesis in an Ethylene-Dependent Manner in Tomato Fruits. Plant Sci. 2020, 298, 110580. DOI: 10.1016/j.plantsci.2020.110580.
- Arabia, A.; Munne-Bosch, S.; Muñoz, P. Melatonin Triggers Tissue-Specific Changes in Anthocyanin and Hormonal Contents During Postharvest Decay of Angeleno Plums. Plant Sci. 2022, 320, 111287. DOI: 10.1016/j.plantsci.2022.111287.
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7(9), 405–410. DOI: 10.1016/S1360-1385(02)02312-9.
- Shah, H. M. S.; Khan, A. S.; Ali, S. Pre-Storage Kojic Acid Application Delays Pericarp Browning and Maintains Antioxidant Activities of Litchi Fruit. Postharvest. Biol. Technol. 2017, 132, 154–161. DOI: 10.1016/j.postharvbio.2017.06.004.
- Wang, X.; Liang, D.; Xie, Y.; Lv, X.; Wang, J.; Xia, H. Melatonin Application Increases Accumulation of Phenol Substances in Kiwifruit During Storage. Emir. J. Food Agric. 2019, 361–367. DOI: 10.9755/ejfa.2019.v31.i5.1954.
- Wang, L.; Luo, Z.; Yang, M.; Li, D.; Qi, M.; Xu, Y.; Abdelshafy, A. M.; Ban, Z.; Wang, F.; Li, L. Role of Exogenous Melatonin in Table Grapes: First Evidence on Contribution to the Phenolics-Oriented Response. Food Chem. 2020, 329, 127155. DOI: 10.1016/j.foodchem.2020.127155.
- Gupta, K.; Dey, A.; Gupta, B. Plant Polyamines in Abiotic Stress Responses. Acta Physiol. Plant. 2013, 35(7), 2015–2036. DOI: 10.1007/s11738-013-1239-4.
- Galston, A. W.; Kaur-Sawhney, R. Polyamines as Endogenous Growth Regulators. In Plant Hormones; Davis, P.J., Ed.; Springer: Dordrecht, Netherlands, 1995; pp. 158–178.
- Zhang, X.; Ji, N.; Zhen, F.; Ren, P.; Li, F. Metabolism of Endogenous Arginine in Tomato Fruit Harvested at Different Ripening Stages. Sci. Hortic. 2014, 179, 349–355. DOI: 10.1016/j.scienta.2014.09.045.
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of Amine Oxidases in Plant Development and Defence. Trends Plant Sci. 2006, 11(2), 80–88. DOI: 10.1016/j.tplants.2005.12.009.
- Bogoyevitch, M. A.; Fairlie, D. P. A New Paradigm for Protein Kinase Inhibition: Blocking Phosphorylation Without Directly Targeting ATP Binding. Drug Discov. Tod. 2007, 12(15–16), 622–633. DOI: 10.1016/j.drudis.2007.06.008.
- Jin, P.; Zhu, H.; Wang, J.; Chen, J.; Wang, X.; Zheng, Y. Effect of Methyl Jasmonate on Energy Metabolism in Peach Fruit During Chilling Stress. J. Sci. Food Agric. 2013, 93(8), 1827–1832. DOI: 10.1002/jsfa.5973.
- Alves, G.; Ameglio, T.; Guilliot, A.; Fleurat-Lessard, P.; Lacointe, A.; Sakr, S.; Petel, G.; Julien, J. L. Winter Variation in Xylem Sap pH of Walnut Trees: Involvement of Plasma Membrane H+-ATPase of Vessel-Associated Cells. Tree Physiol. 2004, 24(1), 99–105. DOI: 10.1093/treephys/24.1.99.
- Hartman, S.; Sasidharan, R.; Voesenek, L. A. The Role of Ethylene in Metabolic Acclimations to Low Oxygen. N. Phytol. 2021, 229(1), 64–70. DOI: 10.1111/nph.16378.
- Bailey-Serres, J.; Fukao, T.; Gibbs, D. J.; Holdsworth, M. J.; Lee, S. C.; Licausi, F.; Perata, P.; Voesenek, L. A.; van Dongen, J. T. Making Sense of Low Oxygen Sensing. Trends Plant Sci. 2012, 17(3), 129–138. DOI: 10.1016/j.tplants.2011.12.004.
- Chaban, Y.; Boekema, E. J.; Dudkina, N. V. Structures of Mitochondrial Oxidative Phosphorylation Supercomplexes and Mechanisms for Their Stabilisation. Biochim. Biophys. Acta Bioenerg. 2014, 1837(4), 418–426. DOI: 10.1016/j.bbabio.2013.10.004.
- Luo, S.; Hu, H.; Wang, Y.; Zhou, H.; Zhang, Y.; Zhang, L.; Li, P. The Role of Melatonin in Alleviating the Postharvest Browning of Lotus Seeds Through Energy Metabolism and Membrane Lipid Metabolism. Postharvest. Biol. Technol. 2020, 167, 111243. DOI: 10.1016/j.postharvbio.2020.111243.
- Dong, J.; Kebbeh, M.; Yan, R.; Huan, C.; Jiang, T.; Zheng, X. Melatonin Treatment Delays Ripening in Mangoes Associated with Maintaining the Membrane Integrity of Fruit Exocarp During Postharvest. Plant Physiol. Biochem. 2021, 169, 22–28. DOI: 10.1016/j.plaphy.2021.10.038.
- Sabehat, A.; Lurie, S.; Weiss, D. Expression of Small Heat-Shock Proteins at Low Temperatures: A Possible Role in Protecting Against Chilling Injuries. Plant Physiol. 1998, 117(2), 651–658. DOI: 10.1104/pp.117.2.651.
- Bush, D. S. Regulation of Cytosolic Calcium in Plants. Plant Physiol. 1993, 103(1), 7. DOI: 10.1104/pp.103.1.7.
- Xin, D.; Si, J.; Kou, L. Postharvest Exogenous Melatonin Enhances Quality and Delays the Senescence of Cucumber. Acta Hortic. Sin. 2017, 44, 891–901.
- Zhang, Y.; Huber, D. J.; Hu, M.; Jiang, G.; Gao, Z.; Xu, X.; Jiang, Y.; Zhang, Z. Delay of Postharvest Browning in Litchi Fruit by Melatonin via the Enhancing of Antioxidative Processes and Oxidation Repair. J. Agric. Food. Chem. 2018, 66(28), 7475–7484. DOI: 10.1021/acs.jafc.8b01922.
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of Melatonin Treatment on the Postharvest Quality of Strawberry Fruit. Postharvest. Biol. Technol. 2018, 139, 47–55. DOI: 10.1016/j.postharvbio.2018.01.016.
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous Melatonin Delays Postharvest Fruit Senescence and Maintains the Quality of Sweet Cherries. Food Chem. 2019, 301, 125311. DOI: 10.1016/j.foodchem.2019.125311.
- Meng, J. F.; Xu, T. F.; Song, C. Z.; Yu, Y.; Hu, F.; Zhang, L.; Zhang, Z. W.; Xi, Z. M. Melatonin Treatment of Pre-Veraison Grape Berries to Increase Size and Synchronicity of Berries and Modify Wine Aroma Components. Food Chem. 2015, 185, 127–134. DOI: 10.1016/j.foodchem.2015.03.140.
- Wang, L.; Luo, Z.; Ban, Z.; Jiang, N.; Yang, M.; Li, L. Role of Exogenous Melatonin Involved in Phenolic Metabolism of Zizyphus jujuba Fruit. Food Chem. 2021, 341, 128268. DOI: 10.1016/j.foodchem.2020.128268.
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on December 14, 2022).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to Perform a Meta-Analysis with R: A Practical Tutorial. Evid. Based Ment. Health. 2019, 22(4), 153–160. DOI: 10.1136/ebmental-2019-300117.
