?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The D-enantiomers of L-amino acids are non-proteinogenic but widely present in foods. This is due to spontaneous racemization or processing, such as heating or alkali treatment, leading to substantial dietary exposure. Additional exposure to D-amino acids (D-AAs) comes from the human microbiota; D-AAs are present in bacterial surface proteoglycans, essential for bacterial competition and growth. Humans and other mammals have a complex set of genes for D-AA transport and degradation, and capacity to synthesize several D-AAs. Free D-AAs are present at low levels in human tissues and body fluids, yet they are apparently of considerable physiological and pathological importance. Amino acid transport regulates their presence and favors specific D-AAs, e.g. D-serine, D-aspartate, D-cysteine, and D-glutamate, over many others. Some of these D-AAs interact with the ubiquitous glutamate-gated Ca2+ channels, affecting signaling functions in most organs, especially the intestine, kidney, and brain. Consequently, the exposures, synthesis, local and systemic transport of D-AAs could be much more biologically important in humans than previously assumed, likely playing a role in gut-organ signaling and in many degenerative diseases.
Introduction
The non-proteinogenic D-amino acids (D-AA), are enantiomers of the common L-amino acids (L-AAs). They are present at low levels in tissues and body fluids in humans. D-AAs were previously thought to be of minor importance,[1,Citation2] however they have been well known to be commonly present in foods.[Citation3] They are formed in several food industrial processes and by microbial synthesis and fermentation in microbial ecosystems in soil, foods, and domestic animals. D-Aspartate was discovered in asparagus in 1889.[Citation4] The next landmark was not until ~50 years later, when Hans Adolf Krebs[Citation5] in 1935 identified D-amino acid oxidase (DAAO, EC 1.4.3.3), the enzyme that deaminates and oxidizes non-acidic D-AAs. Humans and other mammals have additional capacity for endogenous D-AA synthesis,[Citation6] although the exact genes and proteins involved are still not fully elucidated. D-AA levels are tightly regulated in mammals. This includes the metabolism of D-AAs of dietary origin,[Citation7] those synthesized by bacteria in the intestines[Citation8] and those originating from endogenous racemization.[Citation9]
The sources for the resulting local levels of D-AAs in humans have not been studied to any large extent but could be of considerable physiological importance. D-AAs have multiple actions in the body with both physiological and pathophysiological impacts.[Citation10–12] Despite this, the effects of physiological levels of D-AAs in humans have not received much attention until recently. In the 1980s, a peptide containing D-alanine with an opioid effect was discovered in frog skin secretions,[Citation13] and in the 1990s, D-serine was identified in rat brains[Citation14] indicating their wider involvement in biology. With the development of faster and more precise identification and quantification of D-AAs,[Citation15,Citation16] it is now apparent that D-AA are found at measurable levels in all human tissues and bio-fluids[Citation17,Citation18] and that they have several functions in the body. For instance, some D-AAs function as neurotransmitters,[Citation19,Citation20] and they interact with the immune system,[Citation21] where they regulate cellular integrity.[Citation22]
Analytical methods were developed in the early 20th century for studying the chemical properties of AAs. Studies on their chemical changes during food fermentation and alkali treatment began to emerge. Dakin and Dudley[Citation23,Citation24] observed racemization of AAs in proteins already in 1912–13 by using strong alkaline solutions and moderate temperatures, and in 1919 Dakin and Dale[Citation25] found that alkali treatment of eggs resulted in racemization of AAs, extracted from polypeptides.
D-AAs are present in protein-rich foods and beverages that have been fermented, alkali treated, or heated for extended periods.[Citation26–28] D-AA contents increase substantially during processing due to racemization.[Citation29]
The increasing consumption of fast foods and ultra-processed products with a high content of D-AAs may result in increased systemic exposures. This might affect systemic D-AA levels as well as the chemical environment within the gastrointestinal (GI) tract. To better understand the importance of D-AAs in nutrition, we have reviewed the current literature on D-AAs in foods and the intestines, their formation by microorganisms, and their function in the body. This includes mammalian metabolism, disposition, local biological functions, and systemic interplay; the intestines, kidney and brain are used as examples to illustrate their known and potential effects on human physiology and pathogenesis,[Citation30] however many other organs are also involved.
D-amino acids in foods and beverages
For a long time, D-AAs were generally considered to be rare in food products including plant- and animal-based foods and beverages.[Citation31,Citation32] More recently D-AAs have been identified at measurable levels in a variety of common food products.[Citation33] There are several possible explanations for their presence in foods. For instance, D-AAs are quite common in bacteria,[Citation34] especially in the bacterial cell wall,[Citation35] from where they are released in considerable amounts into the ambient environment.[Citation22] For example, D-AAs in soil derive from several sources, including lysis of bacterial cell walls, droppings, and eukaryotic biomass such as algae,[Citation36] plants,[Citation37] and fungi.[Citation38] D-AAs are found in all plants at low levels, either originating from de novo synthesis[Citation39] or taken up from soil.[Citation40]
In the marine ecosystems, bacteria account for a major fraction of the oceanic biomass,[Citation41] one of the greatest reservoirs of organic matter on Earth.[Citation42] Incorporation of D-AAs from the bacterial biomass therefore makes them a natural part of the classic food chain, also in the ocean.[Citation43] D-AAs have been measured in bracken waters in a fjord, and the concentration of D-alanine, D-serine, D-aspartate, and D-glutamate represent up to 3.6% of the dissolved free AA.[Citation44] Therefore, oysters, mussels, and scallops have relatively high contents of D-AA,[Citation45] whereas most other raw and minimally processed foods contain moderate D-AA levels (see and ).
Figure 1. D-Amino acids in the diet. The outer ring shows examples of natural sources of D-amino acids (D-AAs) in foods. The relative D-AA contribution as a fraction of the total AA concentration in the foods is illustrated by the thickness of the arrows. Raw meat, poultry, and fish contain small amounts of D-AA, whereas raw shellfish have a higher content of D-AA. The second ring shows examples of foods with higher D-AA after cooking, fermentation, or other processing, as colour coded in the legend and as illustrated by thicker arrows.
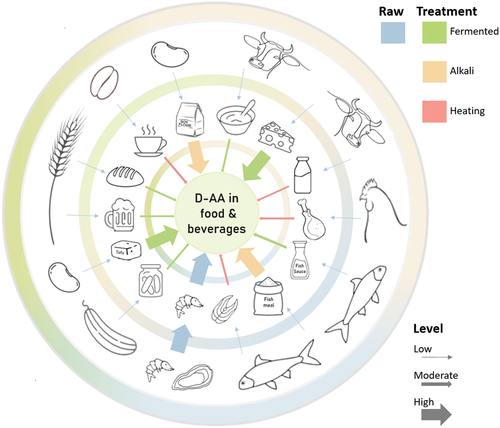
Table 1. The levels of D-alanine, D-aspartate, and D-glutamate, reported in foods and beverages.
During bacterial fermentation, high levels of D-AAs are formed by the action of AA racemases (EC 5.1.1.1), in particular by starter cultures,[Citation68] or from spontaneous racemization.[Citation69] Especially lactic acid bacteria, commonly used as starter cultures, enhance racemization.[Citation68] Other bacterial phyla and even some fungal species, such as Saccharomyces cerevisiae (i.e., bakers’ yeast), widely utilized in the production of fermented foods, are also D-AA producers.[Citation51] The formation, composition, and final level of D-AAs in the fermented product depend on the microbial starter cultures and several other processing parameters.[Citation70]
Raw cow’s milk and milk from other ruminants also contain D-AA,[Citation71] but lower than other dairy products matured by the activity of fermenting bacteria. D-Alanine, D-aspartate, D-glutamate, D-glutamine, and D-serine, in particular, are present in relatively high amounts in cheese,[Citation61] due to racemases present during fermentation. Some free D-AAs may even be present at higher concentrations compared to the corresponding free L-AAs.[Citation72] In fermented products with low protein content, such as sauerkraut, juices, vinegars, Japanese sake,[Citation73] beer,[Citation65] and wine, the relative amounts of D-AAs in a serving are higher than in the raw materials. Bacterial or yeast fermentation of sourdough before baking also results in higher levels of D-alanine, D-asparagine, and D-glutamine in the dough compared to doughs fermented only by yeast,[Citation58] see also .
Other sources of D-AAs in food are alkali treatment and long-term heating that also markedly increase AA racemization rates.[Citation26,Citation28] Food producers are generally aware of the need to produce at a more neutral pH and reduce heating time to minimize food safety risks and keep protein quality. However, extreme procedures such as strong acid or alkali treatments are sometimes required in food technology[Citation74,Citation75] to grind and mill byproducts and oilseeds, and in extrusion of plant, milk, and fish proteins used for example in tofu, sausages, infant formulas, and coffee creamers.[Citation76] Consequently, the racemization rate increases in alkaline processes with increased hydroxide ion concentration.[Citation77,Citation78] Protein-bound serine racemases (EC 5.1.1.16) are particularly sensitive to high pH, but even short periods of alkaline treatment of soy protein generates D-serine in significant amounts.[Citation79] This has consequences for the utilization of dietary protein. Even low racemization rates of AAs from alkaline processing affect the conformation of the peptide chains around the newly formed D-AA residues. Therefore, peptides containing D-AAs cannot be degraded efficiently by mammalian proteases and result in a decrease in proteolysis and under-utilization of the proteins,[Citation80] and consequently higher loss of peptides to bacterial fermentation in the large intestine.
Furthermore, many industrially produced foods contain significant amounts of D-glutamate because mono-sodium L-glutamate is added to products to accentuate the umami taste, and the increased amount of free L-glutamate increases racemization into D-glutamate.[Citation81]
Widespread ultra-processing by heating and treatments at extreme pH, aiming to give foods specific flavors, textures, or solubility, is common in many high-volume, low-price product and also used to satiate the increasing demand for products acceptable for vegetarian or vegan customers. Ultra-processed foods are now forming a substantial part of the food supplies in most high- and middle-income countries.[Citation74,Citation82]
shows concentrations of D-AAs relative to the total AA concentration (i.e., D-AAs divided by the sum of D- and L-AAs) as a percentage for D-alanine, D-aspartate, and D-glutamate, which are the most abundant D-AAs in processed food products.[Citation61] Studies on foods and beverages, where only the relative values were published, are presented in Table S1.
As seen from , natural unprocessed foods usually contain relatively low concentrations of D-AAs, originating from plant sources and soil.[Citation31] The exception is marine invertebrates, such as oysters with relatively high D-AA concentrations from the aquatic food chains. The D-AA concentrations in foods and beverages increase significantly during food processing. D-AA concentrations are higher even with simple processing, e.g. in juices, vinegars, and alcoholic beverages, as well as in fermented dairy products and doughs. The differences in D-AA concentrations in ripened cheeses may be due to variations in fermentation processes, e.g. the use and nature of starter cultures as well as the duration of fermentations.[Citation83] Baking of sourdough breads did not appreciably alter the D-AA levels, however the free L-AAs decreased leading to a change in the D:L-AA ratio.[Citation58] The racemization of AA residues in alkali-treated food products, such as certain soy and meat products, increases with pH, temperature, and storage. Dietary protein sources, such as fresh dairy, cooked beans, fish, or meat, all contribute to similar levels of D-AAs, whereas protein concentrates tend to have very high levels.
Overall, the D-AAs in food we consume are formed during food processing but also originate from microbial sources in aqueous, soil-, and other environments.
Dietary intakes of D-amino acids
The dietary intake of D-AA depends heavily on the diet and especially on the protein source and intake of fermented and processed foods. Apart from the three D-AAs in , D-phenylalanine, D-leucine, D-valine, and D-methionine have been observed in some foods at comparable levels while other D-AAs were mostly below detection limits.[Citation61] The lowest dietary intakes would result from an unprocessed diet without molluscs and with beans, dairy or possibly meat as protein source, i.e. foods with 3–10 mg of the three D-AAs per kg, and probably 2–3 times as much of all D-AAs. Based on such a diet, it may be estimated that minimal human dietary D-AA intake would be around 10–30 mg/d. This diet would contain very little processed food and would perhaps be possible on a raw food diet; but D-AA intakes from recommended healthy diets are likely much higher. Alkali-treated, ripened, and fried or baked foods, as in a typical Western diet may contain foods with levels of 30–100 mg/kg or more, thereby increasing the estimated intakes to 50–100 mg/d. On top of this, many beverages, snacks, and heavily processed products add to the exposure, and so would excess food intake.
In a recent study the D-AA levels in whole meals, a breakfast, a lunch and a dinner akin to WHO dietary guidelines, were measured [Citation67] and also a pooled sample from an intervention with diets of varying concordance to these guidelines.[Citation84] A fully validated method allowed sensitive quantitation of 17 D-AAs in the meals. The summed levels of D-AA in the healthy meals were found to equal around 10 mg/d, however this was mainly due to D-histidine being by far the most abundant D-AA with an estimated contribution of ~7 mg/d, while the remaining D-AAs were found to contribute around 3 mg/d. For the average of pooled healthy and unhealthy diets, the content of D-AAs was found to be 30 mg/d, again with D-histidine explaining 70%. There is no separate data for the unhealthy diets alone, but they would clearly be somewhat higher.
Comparing our calculated intakes based on food analyses and the experimental measurements for whole diets the agreement is fair, although the measured values are in the lower end of the calculated intake range. Based on published analyses of foods and of pooled diets, an approximate daily dietary intake of 10–100 mg D-AAs may therefore be realistic on different diets. However, most of the studies on D-AA in foods, listed in , are some 30 years old and many current foods have never been analyzed. Estimates of free and bound D-AA using more modern techniques would be needed to validate or improve the estimated intakes of D-AA.
The source of the high levels of D-histidine in the pooled diets was not identified, except for a 4–50 times higher presence in the lunch meals compared to dinner and breakfast. The standardized healthy lunch meal contained steamed salmon[Citation67] and since farmed salmon is substantially supplemented with DL-histidine, it may be speculated that the source of D-histidine might be farmed salmon in this study, however a targeted study is needed to verify this.
Importance of bacterial formation of D-amino acids in the intestine
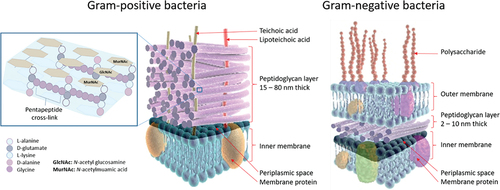
Bacteria are, among the many life forms, unique in having the capacity to produce a wide range of D-AAs. Bacteria have co-evolved in interaction with their host environments making the microbial-derived D-AAs potential inter-domain signaling molecules and regulators of bacterial interactions and biofilm formation. In mammals, enormous numbers of bacteria inhabit the intestine,[Citation67] resulting in considerable amounts of D-AAs being synthesized.[Citation22] Besides bacteria, the intestine is also colonized by fungi, archaea, viruses, and protozoans, collectively called the gut microbiota,[Citation85] with trillions of microorganisms belonging to thousands of species.[Citation86] Along the intestine, a multitude of bacterial species live in polymicrobial communities, where competitive stress forces members to manage metabolism in order to optimize competition, interaction and survival. D-AAs are essential for synthesis of certain bacterial structures, in particular as integrated components in the peptidoglycan layer of the bacterial cell wall, in both Gram-negative[Citation87] and Gram-positive bacteria.[Citation88] The peptidoglycans are typically single layered in Gram-negative bacteria and multilayered in Gram-positive bacteria, see . D-Alanine and D-glutamate in particular, are integral in the peptidoglycan stem framework.[Citation89] Various other D-AAs, such as D-tyrosine, D-methionine, D-tryptophan, and D-leucine, are also produced by bacteria in the intestine,[Citation90] however, the amounts and composition of D-AAs ultimately depend on the individual’s gut microbiome composition, which is highly unique and person-specific.
Figure 2. Stem peptide framework of peptidoglycan in Gram-positive bacteria and cell wall components in Gram-positive and Gram-negative bacteria. Left: The peptidoglycan layer of most Gram-positive bacteria consists of linear glycan strands made of repeating disaccharide units, where MurNAc and GlcNAc are cross-linked by peptides containing L-alanine, D-glutamate, L-lysine, and D-alanine. The pentapeptide cross-links consist of glycine strands. Peptidoglycans are an integrated part of bacterial cell walls. In the middle: a cross-section of the cell wall for Gram-positive bacteria is shown; Right: a similar cross-section for Gram-negative bacteria.
Presence and function of D-amino acids in bacteria
D-AAs contribute to the formation of short cross-linking glycan chains in the peptidoglycan in Gram-positive bacteria,[Citation87] thereby providing a protective barrier against physical, chemical, and enzymatic cell injury.[Citation1] Gram-positive bacteria can have up to 40 peptidoglycan layers, conferring high mechanical strength. In contrast, Gram-negative bacteria only have a single layer, so cross-linking glycan chains are rarely formed.[Citation88] In Gram-positive bacteria, the peptidoglycan layers are about 15–80 nm thick and account for 50–60% of the weight of the cell wall in comparison to Gram-negative bacteria, in which peptidoglycan layers are about 2–10 nm thick and account for only 5–10% of the total weight of the cell walls.[Citation88] The peptidoglycan layer consists of repeated β-1-4 linked disaccharides, N-acetyl glucosamine (GlcNAc), and N-acetylmuramic acid (MurNAc), linking linear glycan chains. In Gram-negative bacteria, meso-diaminopimelic acid (m-DAP) is present primarily in the peptide stem framework whereas m-DAP is mainly replaced by L-lysine in most Gram-positive bacteria, see .
Despite a typical homogeneity of the stem peptide in peptidoglycan architecture, the mature peptidoglycan is highly heterogeneous due to variable patterns of cross-linking peptides.[Citation88] D-AAs are likely to increase metabolic fitness and for adapting to changes in the environment.[Citation2] For instance, the zwitterionic properties of D-alanine is thought to be of crucial importance for the stiffness and physical resistance of the cell wall, and for providing sites for bacterial attachment in biofilm formation.[Citation91] A wide range of side chains in the peptidoglycan indicates that various D-AAs can regulate the amount and structure of peptidoglycan during bacterial growth, thereby affecting cell wall dynamics.[Citation92]
D-AAs produced by bacteria in the intestine can be incorporated into the peptidoglycan, typically replacing D-alanine in the stem peptide, thereby modifying the bacterial cell wall.[Citation93] In some bacteria, accumulation of D-AAs such as D-phenylalanine, D-methionine, and D-tryptophan are observed in modified muropeptides, e.g. accumulating during the growth of E-coli cells in medium. The newly synthesized peptidoglycan does not have cross-links in the 4th and 5th positions of the peptide chain. When cross-linked peptide chains are formed, the terminal D-alanine is cleaved from the peptide and recycled. Bacteria remodel their peptidoglycan during growth by replacing old fragments in exchange for newly synthesized peptidoglycans.[Citation94] The intestinal concentrations of different D-AAs are, therefore, important factors affecting bacterial growth and interactions.
The small intestine contains high levels of acids, antimicrobial components, and oxygen, which limits bacterial growth.[Citation95] In the small intestine, food- and microbial-derived D-AAs may provide additional protection of the thin mucus layer and epithelial cell lining as they may prevent pathogens from producing bacteriocins and forming biofilm.[Citation8] This way, the levels of the D-AAs in the small intestine could be essential for intestinal barrier function. The possible influence of D-AA from foods on this gut microbial environment has not yet been studied. Intestinal pathogens must compete with the resident bacteria for a favorable site of colonization regarding nutrient availability. D-AAs have been shown also to strongly affect gene expression in the complex gut ecosystem.
Recently the 19 proteinogenic L-AAs and their corresponding D-AAs have been quantified to examine chiral homeostasis in human feces. A variety of D-AAs were found, including D-serine, D-arginine, D-aspartate, D-glutamate, D-alanine, D-proline, D-leucine, and D-lysine, ranging from 10 to 800 nmol/g. Despite lack of dietary uniformity, the D:L-AA ratios were comparable between subjects with a total D:L-AA ratio within some 18% in feces.[Citation96] However, others recently reported D:L-ratios of more than 50% for aspartate, alanine and proline in human feces.[Citation96] Assuming steady state formation by gut bacteria, this level in stool would indicate a daily exposure of up to 30 mg D-AA derived from gut bacteria, most of which is excreted with the stool.
In a study on free D-AA concentrations in the colonic lumen of mice, 12 different D-AAs were detected, including D-allo-isoleucine having two chiral centers. There was a significant difference in the colonic luminal D-AA concentrations of germ-free (GF) mice and conventionalized germ-free (Ex-GF) mice. Dietary D-AA content of the diet was also measured, and estimates of the concentrations of D-AAs in the colonic lumen were calculated for both GF and ex-GF mice. All D-AAs observed (D-alanine, D-aspartate, D-glutamate, D-glutamine, D-serine, D-arginine, D-methionine, D-leucine, D-lysine, D-phenylalanine, D-tryptophan, D-allo-isoleucine) originated mainly from intestinal bacteria and much less from the diet.[Citation97] For D-alanine, D-aspartate, and D-glutamate, the ratios of L:D were a factor of 2–8, while for most other amino acids measures, the ratio was more often >100. In addition, the ability of symbiotic bacteria to synthesize D-AAs has recently been investigated in adult wt and GF mice fed a stereospecific AA diet with > 99.9% L-AAs. Only D-serine and D-proline at trace levels were observed in GF mouse feces compared to 11 D-AAs detected in wt mice, where D-serine, D-arginine, D-aspartate, D-glutamate, D-alanine, and D-proline ranged from 30–500 nmol/g, while all other D-AAs were at trace levels.[Citation96]
DAAO is a posttranslationally modified peroxisomal enzyme that oxidizes neutral and basic D-AAs into hydrogen peroxide (H2O2), ammonia, and α-keto acids. Only a few bacteria exhibit DAAO activity. H2O2 is toxic to most bacteria, and the release of H2O2 by DAAO is suggested to be important in host defense. In mice, bacterially produced D-AAs stimulate the release of DAAO from the goblet and other epithelial cells from the host, thereby causing an increase in H2O2 production and protecting the mucosal surface against microbial attack.[Citation8]
Epithelial DAAO expression has also been found to modify the Immunoglobulin A (IgA)-induced effects on various intestinal commensal bacteria. It is, therefore, possible that DAAO limits bacterial growth close to the absorptive epithelial surface and controls bacterial growth in the small intestine.[Citation8]
It has been suggested that DAAO restricts the availability of D-AAs, influences bacterial community composition, regulates bacterial virulence, and promotes the production of host defense peptides and immunity.[Citation8]
Effects of D-amino acids in the gut
Today, only few studies have investigated the impact of D-AAs concentrations on intestinal bacterial communities and colonization dynamics in the gut.
Differences in colonic luminal D-AA levels have been investigated in mice where at least 12 different D-AAs were identified and produced by bacteria belonging to the Firmicutes phylum.[Citation97] In another study, D-AA-producing bacterial strains were isolated from the colon of rats. A total of 19 strains (50% of the isolates) were identified as D-AA producing and belong to four genera, Bacillus, Staphylococcus, Enterococcus, and Paenibacillus, all from the phylum, Firmicutes. In individual bacterial strains, high relative amounts of D-aspartate, D-alanine, D-glutamate, and D-serine amount to 30–75% of total free AAs. D-Alanine is particularly abundant in eight of the AA-producing stains. Large inter- and intra-species variation of D-AA profiles have also been observed, i.e. the ratio of D-aspartate ranges from ~3–~74% among the different bacterial strains. Bacillus species were characterized as high producers of D-AAs.[Citation98]
Supplementation of mice with D-tryptophan has been shown to affect the gut microbiota composition by inducing an increase in gut regulatory T-cells, which are known to play a crucial role in immune regulation and tolerance; the supplementation increased bacterial diversity and promoted the restoration of a healthy microbial community in mice with allergic airway disease.[Citation21]
It is known from in vitro biofilm studies that replacing D-alanine in the peptidoglycan by other D-AAs is one of the critical interactions affecting biofilms.[Citation90] D-AAs are associated with the bacterial amyloid fibers that provide structural integrity to a biofilm. These fibers and fragments that contain D-alanine are recognized by the host as a bacterial signature, a pathogen-associated molecular pattern, which stimulates immune responses.[Citation99,Citation100]
D-AAs influence the biofilm life cycle in vitro for many species, including Bacillus subtilis, Staphylococcus aureus, and Pseudomonas aeruginosa.[Citation90] D-AAs are specially produced in mature biofilm, when nutrients become limiting, and waste products accumulate. The biofilm disassembly allows the microorganisms to return to the planktonic stage.[Citation90] Some D-AAs have highly synergistic effects on biofilm inhibition and disassembly.[Citation90,Citation101] It has been suggested that the release of D-AAs in mixed biofilms acts as paracrine signals to control the architecture of the biofilm communities.[Citation22]
Whether this mechanism is also active in complex biological environments such as the human gut needs additional study. As far as we know, it has also yet to be investigated whether D-AAs in the human diet affect the microbiota in the small or large intestine. Whether D-AAs are crucial for bacteria to alternate between multi-bacterial biofilm communities and planktonic states in the GI is presently unknown. In the upper GI tract, the diet may be the most important source of D-AAs; human metabolism and the increasing bacterial loads along the GI-tract likely tip this balance towards bacterially produced D-AA.
Absorption and metabolism of D-amino acids in bacteria
Since D-AAs may have both dietary and bacterial origins and potentially affect the gut microbiome and the host immune response, it is important to understand their disposition in the intestine. Expression of enzymes regulating D-AAs is common in the microbiota, and several intestinal bacterial species absorb and utilize D-AAs. This could affect D-AA levels, absorption, and their biological effects.
Transport of D-amino acids across bacterial membranes
Several amino acid transporters exist in bacteria with partial homology to mammalian transporters, from the amino acid-polyamine-organocation (APC) superfamily of transporters.[Citation102] Their folding structure forms the basis for a general amino acid transporter mechanism. The transporters have partially overlapping affinities with several of the human solute carrier (SLC) transporters i.e., the L-type amino acid transporters (LATs)[Citation103] and the proton-coupled amino acid transporter 2 (PAT2).[Citation104] In contrast, most amino acid transporters in bacteria are driven by H+ gradient pumps. The stereoselectivity of the different transporters has not been extensively investigated, but fluxes likely depend on the relative abundance of D- and L-AAs.
While the intestinal content of total and specific D-AAs has not been studied extensively, one study reported levels of D-alanine, D-aspartate, and D-glutamate in the contents of the small intestine and cecum as well as in feces of mice. The luminal levels were found to be up to 2 nmol/g in the small intestine, 500 nmol/g in the cecum, and 300 nmol/g in faeces, indicating microbial formation in caecum as the most important source in mice.[Citation8] There is a marked difference between isolated caecal bacterial strains in their production of D-serine, D-proline, D-alanine, D-glutamate, and D-aspartate.[Citation98]
Bacterial metabolism of D-amino acids
D-AAs are formed from L-AA by microbial AA racemases to provide substrates for cell wall homeostasis and local antibacterial responses against competitors. Bacteria convert L-AA to D-AA through stereo-chemical inversion of the asymmetric carbon.
The diversity of the microbial D-AAs is mainly a result of available bacterial racemases, which in the gut depend on the composition and abundance of the various bacterial species affecting the resulting metabolic functionality.[Citation105] The functions and mechanisms of AA racemases have been reviewed,[Citation106] and numerous racemases have been identified in bacteria. Alanine and glutamine racemases are expressed in most Gram-negative bacteria,[Citation22] while aspartate racemases are expressed in lactic acid bacteria.[Citation107] Alanine racemases are especially widespread and present in both Gram-negative and Gram-positive bacteria[Citation108,Citation109] and are found to play a key role for their growth.[Citation109] For instance, alanine racemases are encoded in S. aureus, and D-alanine dominates the structural protein in teichoic acid, a cell surface component in Gram-positive bacteria. D-AAs are not only produced by specific racemases; they are also produced by non-specific “broad spectrum racemases” (bsr), producing D-AAs from a wide range of both proteinogenic and non-proteinogenic L-AAs.[Citation22,Citation110]These enzymes seem to greatly influence microbial ecology; bsr activity has been suggested to have a key function in maintaining dietary D- and L-AA exposures below a detrimental threshold.[Citation22] Especially Gram-negative bacteria produce bsr to generate D-AA, which can affect the mammalian host and result in specific stress reactions,[Citation2] including diarrhea.[Citation22,Citation105]
It has been reported that cystathionine β-lyase (MetC, EC 4.4.1.8) and other L-AA-metabolizing enzymes are involved in D-AA synthesis.[Citation111] Two racemases, YgeA in Escherichia coli and RacX in Bacillus subtilis, have been found to have high substrate specificity toward 15 and 16 out of 25 AAs tested, respectively[Citation112] In severeal other bacteria, D-AAs are also syntesized, especially in stationary phases where D-AAs may accumulate at mM concentrations.[Citation105] Each of the 19 D-AAs has been identified in specific bacterial cultures. The total amount of D-AAs was found to be highest in Gram-positive bacteria, mainly due to a much higher production of D-alanine, with levels ranging up to 4 mM compared to maximally 0.87 mM in Gram-negative bacteria.[Citation105]
Bacterial abundance in human stool, urine and plasma has been found to positively correlate with fecal total D:L-AA ratio.[Citation96] Particularly high ratios were seen for D:L-alanine and -serine, but they were not correlated with the abundance of specific bacterial classes, indicating that chirality is controlled more broadly by the gut microbial density. It has been suggested that humans have a chiral equilibrium of AAs in body fluids independent of gut bacteria.[Citation96]
Overall, AAs production by gut bacteria is extensive due to the need for D-AAs in the bacterial wall and for regulating bacterial interaction, virulence, and growth. Therefore, D-AA containing peptides of dietary or microbial origin are potential triggers of the mammalian host immune, inflammatory, and enzymatic responses to regulate the gut bacterial community. D-AA transporters in bacteria have partial homology to human transporters. Each of the AAs can be formed by some bacterial strain. The common bacterial cell wall components, D-alanine, D-aspartate, and D-glutamine are particularly abundant in the large intestinal contents and specific bacterial racemases are known to form these molecules, while bsr can produce the other D-AAs. Dietary D-AAs may be the most important source in the upper GI tract, while bacterial production seems far more important for large intestinal D-AA content. It is known that especially in neonates, D-AA content in AA-tracers lead to an up-concentration in plasma and urine caused by lower D-AA metabolism rates.[Citation113] However, the net importance of D-AA influx into the human circulation and whether bacterial D-AA production or high dietary intake of D-AAs affects long-term health in humans is an important, but unanswered question at this time.
D-amino acids in mammalian tissues and body fluids
D-AAs are found at relatively high levels in the mammalian body, and overall levels are tightly regulated; in the brain by racemases (primarily for serine and aspartate), and in tissues and fluids by the widely expressed peroxisomal enzymes, DAAO and D-aspartate oxidase (DDO EC 1.4.3.1). DAAO catalyzes the oxidative deamination of neutral and basic D-AA,[Citation9] in particular with a high affinity for D-serine.[Citation114] In contrast, DDO catalyzes the oxidation of acidic D-AA, especially D-aspartate[Citation115] and, to a lesser extent D-glutamate.[Citation116]
D-AAs are widely present in mammals, including humans. Some of the most abundant and most frequently measured D-AAs are D-serine, D-aspartate, D-alanine, D-glutamate, and D-proline.[Citation117,Citation118] D-Serine[Citation119] and D-aspartate[Citation120] are found in particularly high levels in regions of the brain.
Almost all D-AAs have been found in human urine.[Citation121–123] For example, all D-AAs, except for D-glutamate, D-glutamine, and D-leucine, were found recently in fasting spot urine samples from five healthy subjects. The highest levels were of D-serine > D-alanine > D-aspartate > D-lysine > D-arginine, with wide inter-individual variation in concentrations. Many free D-AAs and especially D-alanine, D-serine, and D-aspartate are found in plasma and serum,[Citation118,Citation121] saliva,[Citation124] gastric juice,[Citation125] colostrum,[Citation126] and CSF,[Citation127] with the highest levels reported in the pancreas and jejunum[Citation128] and the brain[Citation117] especially in the pituitary and pineal glands.[Citation128,Citation129] Levels of the corresponding L-AAs are up to 200-fold higher. In mammalian protein, D-AA presence is low. D-AAs are found more abundantly in long-lived proteins, e.g. in teeth and in the lens of the eye, where they are formed de novo at a slow but constant rate by spontaneous racemization of L-AAs.[Citation130]
Levels of D-alanine and D-serine in urine, serum, and cerebrospinal fluid (CSF), reported in human studies, are shown in . The levels observed indicate that D-serine concentrations are generally higher than those of D-alanine, indicating specific mechanisms of formation, independent of gut bacterial synthesis. The table also shows that urine levels of the two D-AAs are higher than in plasma and cerebrospinal fluid, indicating that glomerular filtration plays a role in removing D-AAs from the circulation.
Table 2. D-Alanine and D-serine levels in human urine, plasma, and cerebrospinal fluid.
In humans, the relative amount of D-AA (median percentage of D/(D + L)) has been found to be markedly higher in urine than in plasma. Relative D-serine, D-alanine, and D-asparagine/aspartate levels, were much higher in urine (42.2, 18.2, and 7.9 D%, respectively) than in plasma (<0.5%).[Citation16] In a study with 24 males and females (20–45 years) it was confirmed that serine, alanine and asparagine in urine have D:L ratios of 40–100 while most other ratios are <10%.[Citation96] These ratios are far below 0.5% in plasma, except for serine (1.8%) while proline and alanine were in the range 0.2–0.4%. In the stools, proline, alanine, and aspartate ratios are >40%, while glutamate and serine are around 5–15%, again indicating that systemic D-serine has a different source. In a pilot study with five healthy young males with normal circadian rhythm the 24-hour course of urine and plasma D-alanine profiles were evaluated. The highest D-alanine levels were found in the late evening, and the lowest levels were seen in the morning.[Citation121] These 24-hour patterns of D-alanine have been confirmed in rodent studies,[Citation140] indicating that circadian rhythms, or simply resting or active states, may be important for D-alanine excretion. In another study, the levels of D-alanine and D-serine were measured and compared in urine from 33 subjects, aged 3 days to 94 years. Levels were lower in newborns and children up to 10 years and then apparently remained more constant up to 30–40 years, where the contents were slightly increased and then quite stable at higher ages.[Citation126]
In humans, the equilibrium evaluated by total D:L-ratios of the 19 chiral AAs have been measured in urine and plasma; ratios vary a bit between studies, but are much higher in urine. Several D:L-AA ratios were positively correlated between AAs in urine, e.g. for alanine, asparagine, glutamine, and particularly serine. However, in plasma, only D-serine, D-alanine, and D-proline were at trace levels and with a weak positive correlation.[Citation96] These results suggest that strict chiral homeostasis is maintained in humans.[Citation96] Urinary excretion is part of this regulation, also during the neonatal period, but human enzymatic catabolism has been suggested to be the most important way to remove D-AAs.[Citation96]
D-serine and D-aspartate are widely, but heterogeneously present in different regions of the brain in rodents,[Citation18] as well as humans.[Citation141,Citation142] D-alanine has been found in the human brain, but D-cysteine,[Citation143] D-leucine,[Citation144] D-proline,[Citation144] and D-glutamate[Citation145] have so far only been detected in rodent brains.
In the human brain, the highest levels of D-serine are found in the cerebrum, including the cerebral hemispheres, and in subcortical structures such as the hippocampus and basal ganglia. The highest level of D-serine has been found in the hippocampus.[Citation119] In the human prefrontal cortex (cerebral cortex covering the foremost area of the frontal lobe), persistent high D-serine levels at 0.13 ± 0.01 µmol/g were identified in 11 subjects at different ages (from birth to 101 years old). The same subjects had more variable D-aspartate levels in the prefrontal cortex of 0.008 ± 0.004 µmol/g.[Citation146]
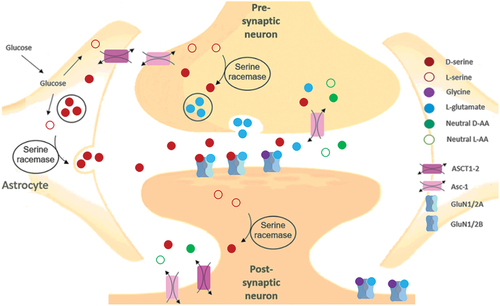
In rats, more than 25% of total free serine throughout the body has been identified as D-serine present in brain regions.[Citation14] In the brain of adult rats, D-serine has been found primarily to be produced in glial cells for the neuronal N-Methyl-D-Aspartate Receptors (NMDARs),[Citation147] and within hippocampal astrocytes surrounding the neurons and capillaries.[Citation148]
In humans, D-aspartate levels are higher in the embryonic and perinatal states; thereafter it declines with age.[Citation141] D-Serine levels in the cerebellum are persistently high during the embryotic and postnatal stages, while concentrations in adolescents and older subjects are about half of that found in the fetus.[Citation146] A temporal shift of D-cysteine has recently been identified in mouse brain.[Citation143] The regional distribution of D-cysteine in the adult mouse brain is closely but inversely related to that of D-serine.[Citation6,Citation149]] D-Aspartate shows a similar developmental shift in expression as D-cysteine.[Citation146] These temporal shifts in D-aspartate, D-serine, and D-cysteine levels correlate with major milestones in forebrain development.[Citation143]
Metabolism of D-amino acids in mammalian tissues and body fluids
A seminal review by Clarence Berg collated the findings of comparative feeding studies conducted in humans and rodents in the first half of the twentieth century.[Citation149] Data from these studies demonstrate that rodents utilize D-AAs nutritionally.[Citation149,Citation150] Comparable data from human studies was limited.
Early human studies compared urinary excretion of N-containing compounds, including urea, and AAs after intake of equimolar doses of D- and L-AAs (~1 g of each). D-Arginine and D-threonine seemed to be well utilized in humans.[Citation151,Citation152] Approximately 3 hours after intake, 25% of D-tryptophan and D-cystine were excreted in the urine, whereas <3% of the L-AAs were excreted.[Citation151,Citation152] D-Kynurenine levels have also been identified in urine after intake of D-tryptophan.[Citation149] Intake of 2 g D-glutamate has been found to elevate plasma D-glutamate levels immediately.[Citation153] These studies show that oral D-AAs are absorbed and partially utilized, and that high intakes can affect plasma and urine levels of several bioactive metabolites.
Formation of D-amino acids in mammalian tissues
Some racemase processes similar to those known from the microbiota occur in the brain.[Citation6] Despite high racemase activity[Citation106] only two specific racemases have been found in mammalian tissues: serine racemase and aspartate racemase.[Citation6,Citation154] Racemization of serine and aspartate is not restricted to the brain but is also widely expressed in the intestine, pancreas, liver, and kidney.[Citation155] D-Serine is predominantly synthesized locally in the tissues from L-serine, which is formed from glucose.[Citation100] In studies with mouse models, only 10–20% of the D-serine in the brain originate from exogenous synthesis.[Citation156] The overall pathways by which serine racemase regulates D-serine homeostasis in both the rodent and the human brain seem highly plastic.[Citation157,Citation158] Due to numerous ligands, compounds interacting with the enzyme as well as post-translational modifications, the potential to modulate serine racemase activity is significant,[Citation159] indicating that its function is tightly regulated. Serine racemase has been shown to be involved also in D-aspartate synthesis in some organs, but there is evidence that additional enzymes for D-aspartate synthesis must exist in mammals.[Citation160,Citation161] Serine racemase is also effectively forming D-cysteine.[Citation143]
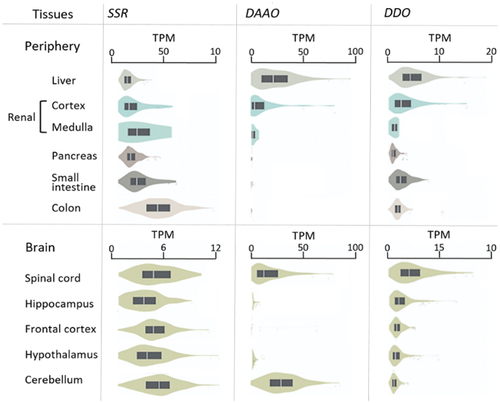
Serine racemase expression at the gene and protein levels in the kidney is found in renal tubular cells but not in renal glomerular cells. The presence of serine racemase in the tubular cells suggests that D-serine is also synthesized in the kidney. Serine racemases presumably participate in renal physiological mechanisms via modulation of the NMDAR, which is present in the kidney parenchymal tissue. Both GluN1 and GluN2C subunits of NMDAR are expressed in kidney[Citation162] and this is the subunit combination for which D-serine has demonstrated the highest potency to activate the receptor.[Citation163] The gene expression of human serine racemase, DAAO, and DDO in selected peripheral tissues and brain regions is shown in , based on data obtained from the Genotype-tissue expression GTEx project (www.gtexportal.org).[Citation164]
DAAO and DDO are widely expressed in mammalian organisms and ensure that D-AAs remain at non-toxic levels. DAAO has broad substrate specificity and oxidizes both neutral and basic D-AAs[Citation165] as well as the D-tryptophan derivative, D-kynurenine.[Citation166] However, DDO oxidizes only the acidic D-AAs: D-aspartate and D-glutamate.[Citation12] During the oxidative deamination of D-AAs by DDO and DAAO, H2O2 and NH4+ ions are generated,[Citation167] with the formation of α-keto-acids.[Citation167–169] High rates of substrate transformation can lead to toxicity due to increased oxidative stress by H2O2 and a more alkaline pH due to rising levels of NH4+.
The gene and protein expression levels are generally much higher for DAAO than DDO or serine racemase. DAAO is locally highly abundant but with a very heterogeneous expression in different brain regions.[Citation156] Serine racemase is predominantly expressed in the cerebellum[Citation119] and in glial cells,[Citation170] where it primarily metabolizes D- and L-serine.[Citation171] The expression patterns are further characterized by low expression of DDO in the cerebellum as opposed to DAAO and serine racemase, indicating the importance of tight regulation of D-serine and D-cysteine. All three enzymes are expressed at high levels in the spinal cord.
In the abdominal area, DDO is widely expressed; DAAO is predominantly expressed in the liver and kidneys, especially in distal tubular cells in segment 3 (S3), where it metabolizes D-AAs to prevent nephrotoxicity.[Citation172] There is less information on intestinal expression. In mice, DAAO activity has been identified in the small intestine.[Citation8] DAAO is also detected in the human small intestine.[Citation8] This indicates an important action of DAAO in handling dietary D-AA and/or in regulating bacterial growth and immune response in the small intestine. In a recent review it is concluded that in healthy subjects, catabolism of D-AA by mammalian enzymes is the most important mechanism for keeping a low abundance of D-AAs, thereby maintaining homochirality of the systemic AA pool.[Citation96]
Sources and function of D-amino acids in mammalian tissues and body fluids
Physiological concentrations of D-AAs in rodents and humans are only about 1–100 µM with few exceptions. Preliminary data shows that sources of D-AAs in body fluids are both exogenous and endogenous.[Citation173,Citation174]
As mentioned above, bacterial formation of specific D-AAs makes these compounds a target for our immune system. A human G-protein-coupled receptor with affinity to D-tryptophan and D-phenylalanine has been identified in white blood cells.[Citation175] Through this receptor activity, D-tryptophan was found to elicit a chemotactic response in human neutrophils.[Citation175]
D-AA formation in the large intestine is of importance for systemic levels. In rodents physiological levels of D-AAs in body fluids derive to a large extent from intestinal bacteria.[Citation8,Citation140,Citation176] The comparison of GF and wild-type mice has shown that systemic D-alanine is mainly coming from intestinal bacteria.[Citation8,Citation140] Significant levels of D-aspartate, D-glutamine, and D-proline were also observed to be of gut microbial origin.[Citation8]
In comparing GF and Ex-GF mice, circulating D-AA levels were driven mainly by the microbiota, while host racemases may contribute to a small extent. The much lower D-AA levels observed in the GF mice may be formed by host racemases from dietary L-AAs.[Citation97] In humans, D-serine, and D-aspartate in particular, have been found to be of both endogenous and exogenous origin.[Citation61,Citation160]
In the rat brain, D-serine is found at 0.27 µmol/g, which is about 25% of the L-serine level.[Citation14] D-Aspartate and D-cysteine are found at high levels[Citation143,Citation177] even compared to their L-forms in the fetal brain, but after birth, levels plummet, and high DDO and DAAO expression is observed to reduce these brain levels.[Citation177,Citation178] The D-aspartate concentration in the insulin-containing secretory granules in β-cells in rats is about 8% of total aspartate.[Citation179] D-Aspartate is co-secreted with insulin by beta-cells[Citation179] and similar observations are seen in other endocrine organs,[Citation177] indicating important and specific functions. Still, the origin of D-aspartate in human tissues is not well understood. In rodents, D-cysteine is also known to be of endogenous origin.[Citation143] No endogenous synthetic pathway of D-alanine has been found in mammals. However, possible endogenous formation of D-alanine from alanine racemase or D-alanine transaminase activity is suspected in animals.[Citation180] There is little data on the relative contribution of dietary or bacterially produced D-AAs to the levels found in mammals, except for the ability of oral doses to reach plasma and urine and the documented local endogenous production by specific racemases. The impact of dietary sources of D-AAs and their interplay with the gut microbial formation of D-AA is not well understood, neither in rodents nor in humans.
Oral D-amino acid intake and systemic levels
Studies predominantly conducted in rodents show that absorption rates of oral D-AA depend on the dosing schedule (i.e., single, or continuous doses). It has been a general assumption that dietary D-AAs do not contribute to the accumulation of D-AAs in the body due to the high stereoselectivity of amino acid transporters for L-AAs and because DAAO and DDO would effectively deaminate absorbed D-AAs. However, many common foods have relatively high contents of D-AAs, but it has not been investigated whether meals with high levels of various D-AAs impact absorption or how dietary D-AAs may interact with and affect the gastrointestinal D-AA production by microbiota.
Levels of D-AA in tissues and body fluids have been compared between mice with and without DAAO activity. In these studies, D-alanine concentrations in urine correlate more strongly with formation by intestinal bacteria than with contents in the diet.[Citation8,Citation176,Citation181] In contrast, D-methionine levels in urine seem to originate from the diet,[Citation176,Citation181] and D-proline levels in urine may be derived primarily from endogenous production, however, the pathway is unknown.[Citation182] High D-proline excretion into urine has been observed in gnotobiotic mice and GF ddY/DAO− mice without DAAO. Four strains of commensal bacteria were not influencing the excreted amounts of D-proline in the gnotobiotic mice. During a four-day fasting period, the mean value of urinary D-proline decreased to 25%, indicating that the substrate for D-proline formation could be dietary.[Citation182] It may be speculated that proline biosynthesis from arginine leads to both D- and L-proline formation and that the fraction of the resulting D-proline that is not racemized into L-proline by DAAO may be the source of urinary D-proline.
Large intravenous doses of D-serine are required to increase D-serine concentrations in the brain of WT mice, whereas dietary exposure was less effective. Overall, current evidence indicates that DAAO is important for regulating the presence of D-AAs in mammalian brains, and dietary sources have limited effect. Several studies have been conducted in transgenic mice lacking serine racemase activity to clarify the importance of D-serine synthesis, and it has been found that 80–90% of the D-serine in the mouse brain originates from endogenous synthesis.[Citation156,Citation183] The likely take-home message is that under normal conditions, dietary D-AAs are contributing to systemic D-AA levels. They may also be essential regulators of upper GI functionality; therefore, further human studies are needed to assess their quantitative contribution in these processes.
Effect of gut microbial and endogenous sources on systemic D-amino acids
Preliminary data has shown that host–microbiome interactions may be associated with D-AA metabolism. It is suggested that D-serine in the colonic lumen exerts an agonistic effect by binding to receptors on epithelial cells and lymphocytes in the lamina propia, similar to D-serine in the brain.[Citation97] D-Serine is detected in the colon in EX-GF mice and wt mice[Citation97] but not in GF mice.[Citation184,Citation185] Higher motor activity and less anxiety have been observed in GF mice in comparison to mice with regular bacterial activity.[Citation184,Citation185] It is speculated that this might be due to the lack of bacterial influence on upper GI functionality and D-AA transport.
Bacterially produced D-serine is found in the brain GF mice,[Citation97] and we know that endogenously produced D-serine levels are high in the cerebrum humans,[Citation119] but oral sources of D-serine do not seem to strongly affect levels in these brain regions.[Citation186] It has been proposed that endogenous D-serine is mainly found in the intracellular compartment and that exogenous D-serine therefore might have less impact on D-serine levels in the hippocampus and other areas with high levels of endogenous D-serine synthesis, while D-serine concentrations in the pineal and pituitary glands are more susceptible to exogenous D-serine.
Multiple metabolically inert tissues of elderly subjects, such as arteries, eye lenses, cartilage, dentine, and brain, contain long-lived proteins that undergo long-term exposures to factors that lead to AA racemization. It is suggested that age-related decline in organ function may partially be due to the resulting denaturation of long-lived proteins.[Citation187] Association between physiological aging and changes in the content of D-AAs in the brain has been observed for D-serine[Citation188] and D-aspartate[Citation20] since their L-forms are among the more vulnerable AAs to racemization.[Citation130,Citation189] L-Aspartate residues are more frequently converted into D-aspartate when the neighboring AA residue has a small side chain such as glycine, alanine, and serine, because aspartate-mediated stereoinversion occurs specifically via succinimide-intermediates. D-Aspartate residues in “long-lived” proteins have been associated with Alzheimer’s disease (AD).[Citation190] Regardless of brain region, the changes in AA chirality cause changes in protein structures, including the formation of alpha-helical and beta-sheet structures that change metabolic functions.[Citation191]
Overall, endogenous synthesis appears to be an important source of D-AA in the brain. Numerous studies have also reported that D-AAs from exogenous sources, including diet, can be found in the brain in rodents. Conversely, it has been suggested that D-AAs in rodent body fluids originate mainly from intestinal bacteria.[Citation8,Citation97,Citation140]
This is supported by more extensive transport and metabolism of D-AAs in the bacterial cell wall[Citation22] Dietary D-AA has been shown to affect levels of some D-AAs in body fluids and in some regions of the brain in rodents.[Citation136,Citation192] We do not know whether this may also occur in humans and whether dietary D-AAs could have any systemic functional effects. Spontaneous racemization also occurs but the contribution to endogenous levels of free D-AA is unknown.
Mammalian absorption and reabsorption of D-amino acids
The AA absorption and reabsorption occurs by transport systems having several specialized functions. They are selective in terms of charge and highly stereospecific, favoring L-AAs but some amino acid transporters also transport D-AAs, and a few have a significant affinity. D-AA transporters are expressed in the mammalian intestine, kidney, and brain. Different isoforms are often expressed in these organs, indicating that D-AAs have specialized functions in mammals. Overall, the different transporter proteins facilitate the transfer between compartments within cells, between cells, and into the circulation for transport to other organs.[Citation193]
The absorption of AAs and peptides through the apical membrane occurs mainly by active transport processes, whereas the absorption across the basolateral membrane occurs mostly by facilitated diffusion. The overall characteristics of the known transporters with at least some affinity for D-AAs or D-AA-containing peptides in the intestine, in the kidney, across the blood-brain barrier (BBB), and in the brain are listed in .
Table 3. Characteristics and function of amino acid transporters with stereospecific (re)absorption of D-amino acids in humans
The SLC transporters are localized on the cell surface and in organellar membranes, transferring AAs intracellularly as well as over the apical and basolateral membranes in the intestine, kidney, brain, and other tissues. Members of the SLC6 family share the folding mode of the APC superfamily.[Citation206] The SLC6 family includes the X transporter protein 3 (XTRP3), the sodium-dependent broad substrate selectivity neutral amino acid transporter 1–2 (B0AT1-2), and the amino acid transporter responsible for the activity of the system termed B0,+ (ATB0,+). These transporters are highly related in structure and function and highly expressed in the intestinal and kidney apical membranes with affinity to D-AAs.[Citation207,Citation208]
Proton-dependent transporters are ubiquitous in bacteria, and some are also important in AA transport in mammals. Peptide transporter 1 (PEPT1) is a known proton-dependent transporter,[Citation209] having broad selectivity, also for small peptides containing D-AAs,[Citation210] albeit at a reduced level. Substitution of NH2-terminal L-alanine in an Ala-Gly peptide by D-alanine caused a 60-fold reduction in affinity; substitution of the COOH-terminal residue by a corresponding D-AA nearly stopped absorption while D/D dipeptides have no interaction with the carrier site. Hydrophilic dipeptides containing D-AAs have higher affinity than hydrophobic ones.[Citation211] It is known that PEPT1 is critical for the absorption, disposition, and effectiveness of several peptidomimetic medicines.[Citation212] The angiotensin-converting enzyme 2 (ACE2; EC 3.4.17.23) has a key function in the renin-angiotensin system in the kidney,[Citation213] but also identified as a key regulator of AA transport and homeostasis, innate immunity, and intestinal bacterial ecology.[Citation214] ACE2 is joining by collectrin in the transmembrane C-terminal domain and is highly expressed in the intestinal and kidney apical membrane[Citation215] in humans[Citation216] and rodents.[Citation217,Citation218] It is also co-expressed with the primary D-AA transporters, PEPT1,[Citation216] PAT1,[Citation219,Citation220] and B0AT1[Citation221] and influence their expression.[Citation221]
The transporters are not distributed uniformly along the intestines or the renal proximal tubules, indicating specialized physiological functions. The uptake of AA from digested dietary proteins at the apical membrane, and the efflux at the basolateral membrane in the intestine is similar to the reabsorption mechanism in the renal proximal tubular cells (see kidney section below). The forceful vectorial activity of apical transporters provides almost complete absorption of the AAs and peptides from the intestine as well as reabsorption in the kidney. The intestinal and renal amino acid transporters have much higher stereoselectivity for reabsorption of L-AAs (and LL-peptides) than for the corresponding D-AAs and (DL/LD-peptides).[Citation210] The satiable reabsorption of D-AAs is even further limited by competition from higher concentrations of L-AAs[Citation222,Citation223] and competitive intestinal absorption is also observed between free D-AAs.[Citation224] Human gene expressions of various amino acid transporters with known affinity for absorption of D-AAs are shown in . The expression levels are approximate estimates of RNA-seq data from the Human Protein Atlas (HPA), version 22.0.[Citation225] The amino acid transporter affinity for D-AAs is not fully understood, leading to uncertainty about the uptake for some individual D-AAs.
Most results originate from older feeding and injection studies examining the absorption of D-AA in rodents after very high doses. The older review conducted by Berg on the physiology of D-AAs, documented that L-AAs are absorbed actively and at a faster rate into the portal blood than the corresponding D-AAs.[Citation149]
Absorption and transport of amino acids in the gut
Most of the L-AA and small L-peptides from protein digestion are absorbed along the small intestine. The stereospecificity of absorption across the mucosa in the ileum has been studied in rabbits, where selectivity is especially strong for serine, alanine, and to a lesser extent for leucine, phenylalanine, and tryptophan, having bulky hydrocarbon side chains. Due to differences in hydrogen bonding, much higher affinity is observed for D-alanine than for D-serine. No or very low affinity has been found for D-valine. Thus, both the α-amino group, the α-carboxylate ion, the conformation, and the side chain seem to be involved in the absorption mechanism.[Citation226]
Transport of amino acids in the small intestine
In the small intestine, approximately half the AAs are absorbed as di- and tri-peptides via the peptide transporter 1 (PEPT1) and then hydrolyzed intracellularly into single AAs. Stereoselective AA transport mechanisms by PAT1 and transport of different D-AAs have been investigated in a human intestinal epithelial cell line. Here it was observed that the stereo-selectivity for D-AA absorption depends largely on the AA side chains; the absorption rates measured were in the order D-alanine > D-serine > D-cysteine. The PAT1 transporter has 6- to 8-fold higher affinity for these three D-AAs compared to their corresponding L-enantiomers.[Citation227] PAT1 mRNA is highly expressed along the entire length of the intestine and is also known to transport D-proline, and D-valine.[Citation227] The ATB0,+ transporter also has a high affinity for D-serine, as well as for D-alanine, D-tryptophan, and others.[Citation228]
Absorption of D-amino acids in the large intestine
In the large intestine, D-AAs are transported into the epithelial lining cells via PAT1[Citation227] where D-AAs are most abundant. This agrees with the previously mentioned studies pointing at the colonic microbiota as the primary source of systemic D-AAs in mice.[Citation228] AA absorption in the colon is controversial.[Citation229] However, colonic uptake may serve roles in colonocyte housekeeping or in the maintenance of systemic availability of specific D-AAs such as D-serine, D-cysteine, and D-aspartate. A substantial amount of peptides and proteins enter the colon, where they are degraded by bacterial enzymes, and absorption of AAs, di- and tri-peptides in colon has been recently reviewed.[Citation230] It has been suggested that AAs and small peptides are absorbed in animal cecum to some degree, but whether AAs are absorbed by the human colon is still uncertain.[Citation229] Current consensus is that although some absorption of AAs may occur, they do not contribute markedly to the supply of AAs in humans.[Citation230] It has been speculated that various transporters found in colonic tissues are upregulated when the dietary AA supply is insufficient.[Citation230] The inferred importance of microbial D-AA in the colon for systemic levels is therefore still controversial and needs direct investigation.
Several transporters have been identified at the gene expression level in the human colon but the transporter proteins have only been reported in other mammals.[Citation230] The PAT1 and ATB0,+ genes are expressed in the ascending, transverse, and descending parts of the human colon.[Citation231] The PEPT1 and −2 genes are also expressed in human colon.[Citation232,Citation233] In rodents, the ATB0,+ transporter is found to transport D-serine efficiently (Kt value for active transport of D- and L-serine were both ~150 µM) in the distal intestine with the strongest gene expression in the colon compared to the ileum.[Citation228] This localization of amino acid transporters in the colon could indicate that they are not primarily involved in the absorption of AAs arising from human protein digestion but that their physiological function may be to mediate the transport of bacterial D-serine and other D-AAs into colonocytes. This transport of bacterially produced D-AAs has been confirmed for ATB0,+ in the mouse colon.[Citation234]
The expression of colonic amino acid transporters, especially ATB0,+ which is expressed at very high levels may be important for regulating the microbiota by controlling levels of D-AA. D-Serine is an important co-agonist at the NMDAR, a subclass of ionotropic glutamate receptors that modulate the effects of the physiologic substrates for several neuronal functions. NMDAR is a central driver of synaptic plasticity in the brain, where serine racemase primarily synthesizes D-serine to regulate NMDAR neurotransmission. D-Serine could possibly act as a co-agonist via binding to receptors on epithelial cells and lymphocytes in the lamina propria similarly to its agonist function in the brain. Studies in mice lacking racemase activity indicate that 10–20% of the D-serine level in the brain is independent of serine racemases. Therefore, D-serine produced by the microbiota of the small or large intestine may be a source of the D-serine in the brain, and ATB0,+ is uniquely suited for the absorption of bacterially derived D-serine and other D-AAs.[Citation216]
Reabsorption of D-amino acids in the kidney
In the kidney, transporter-facilitated reabsorption of D- and L-AAs takes place along the length of the nephron, including tubular structures and surrounding capillaries. Most transporters have a stronger affinity for L-AAs, leading to a change in the ratio of D:L-AA along the tubuli. The structure of the nephron is shown in . Apart from facilitated transport paracellular diffusion also plays a role in the kidneys. Paracellular AA transport is a passive process that entirely depends on the local concentration gradients. The AAs are transferred through the intercellular spaces between epithelial cells, and the tight junctions seem essential for this process.[Citation235]
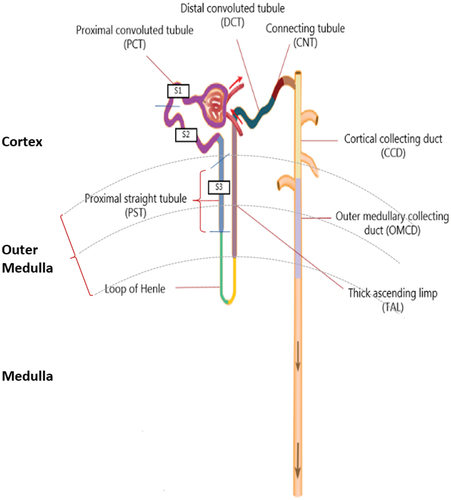
Figure 4. Structure of the nephron in relation to L-and D-amino acid absorption. The cortex is depicted in the top, the outer medulla is in the middle, and the medulla is at the bottom. The figure shows the location of various nephron segments involved in D- or L-AA resorption (proximal straight tubule, proximal convoluted tubule, loop of Henle, thick ascending limb, distal convoluted tubule, connecting tubule, cortical collecting duct, and outer medullary collecting duct). D- and L-AAs are filtered through the tubule of the nephron, and about 97% of the L-AA are reabsorbed in the proximal convoluted tubule (PCT; purple colour) (S1 and S2). D-AAs, mainly D-serine, are reabsorbed in the proximal straight tubule (PST; blue colour) (S3).
DAAO is expressed along the tubules ensuring that the D-AA content remains at low levels in the blood and the tubular fluid.[Citation236] Along the tubule, DAAO catalyzes D-AA oxidation at the apical membrane to prevent transitional efflux at the basolateral side, particularly in S3 where DAAO is most highly expressed, preventing D-AA reabsorption into the circulating blood flow. The D-AAs are metabolized into their corresponding α-keto acids, which can be used to form L-AAs.[Citation211]
Assuming that the filtration coefficient is uniform along the length of the capillary, glomerular filtration rate would be highest in S1 of the proximal tubule, where the blood flow is very high and lowest in S3 at the proximal straight tubules with lower blood flow. This results in a nearly complete renal reabsorption of all L-AAs,[Citation237] with only a very low L-AA concentration remaining after reabsorption from the proximal tubules when reaching S3. It is reported that about 80% of the total filtered load of AAs are reabsorbed at S1,[Citation238] and about 3% pass unabsorbed through S3, whereof more than half is reabsorbed in the collecting duct.[Citation238] However, when the blood flow falls moderately through the capillary network surrounding the renal tubules, the distal hydrostatic pressure is reduced. While the reabsorption of most L-AAs is still efficient, the relative D-AA concentration in the tubular capillary increases. Since D-AAs are filtered by the glomerulus and are therefore not reabsorbed in the proximal convoluted tubule, they are present at S3 in approximately 3-fold higher concentration at low than at high blood flow rates.[Citation211]
Apical amino acid transport in the tubule
Satiable reabsorption of AA into the cell lining is characteristic of amino acid transporters in S1 and S2 having high transport capacity and low affinity. In contrast amino acid transporters in S3 have low transport capacity and high affinity. This differential distribution of low- and high-affinity transporters along the tubule at the apical membrane optimizes the reabsorption of L-AAs against the small concentration gradient in S1 and S2, with a lesser amount transported against a larger concentration gradient in S3.[Citation210] This differential and complementary localization of AA transporters along the proximal tubule favors the reabsorption of D-AA in S3 against an overall AA concentration gradient.
Epithelial DAAO in S2 and S3 and basolateral antiporters, exchanging D-serine, D-aspartate, and D-methionine for L-AAs help to maintain an overall low D-AA reabsorption, except for selected D-AAs, possibly needed by the brain and other organs. Meanwhile, these transepithelial and transmembrane concentration gradients constitute driving forces for back leak passive AA fluxes, and this transport mechanism increases the risk of some transfer of L-AAs back into the tubular fluid in S3 by the resulting leaky paracellular pathway.[Citation239]
The transepithelial transport across the apical membrane is dominated by active transport mechanisms, which function to selectively reabsorb free AAs from the tubular fluid. The primary D-AA transporters and D-peptide transporter expressed in the apical membrane of S3 include PAT1, B0AT1, XTRP3, EAAT3,[Citation235,Citation240] and PEPT2.[Citation235]
In the kidney, the expression of B0AT1 depends on its association with collectrin, a tissue-specific protein with homologies to the intestinal ACE2.[Citation241] EAAT3 has relatively high stereoselectivity for transferring D-aspartate.[Citation242] It is so far the only anionic amino acid transporter detected at the apical side, expressed at S3 and in the distal convoluted tubule.[Citation243] The expression of D-amino acid transporters, peptide transporters, and accessory proteins are mainly observed in the straight proximal tubule (PST; S3) at the apical membrane, which applies to both the number of transporters and their expression level. D-Peptide transporters are only expressed at the apical membrane at S3, see .
Table 4. Expression levels of D-amino acid transporters along the nephron.
Basolateral amino acid transport in the tubule
The amino acid transporters at the basolateral membrane function similarly to the transporters at the apical membrane but also have housekeeping functions to ensure an adequate supply of AA to the epithelial cells, whereas apical transport is primarily involved in reabsorption. The reabsorption of AAs over the basolateral membrane is primarily passive.[Citation249] The few active transporters satisfy the need for nutritive compounds and homeostasis of AAs metabolized by kidney cells. The need for such nutritive basolateral uptake is limited in S1 and S2 because reabsorption of L-AAs already provides the requirements. In contrast, in S3, the AA concentration is extremely low, and peritubular AA transport constitutes the major source of these essential compounds for the lining cells.[Citation239] AAs are not only needed for local protein synthesis but also for renal metabolic pathways important for the whole body, e.g., reabsorption of glutamate to form ammonia used for the secretion of protons.[Citation250] The passive transport of AAs is driven by an electrochemical gradient. It seems to be a relatively slow process because high intracellular concentrations of AAs are apparently required to increase the transport rate sufficiently to ensure a steady state transepithelial flux.[Citation239]
Since some intracellular AAs are present at much higher concentrations in the cytosol than in the extracellular space, basolateral AA reabsorption is much more tightly regulated and controlled to maintain an equilibrium of essential AAs, ensuring basal cell functions.[Citation249] Absorption of essential AAs is only facilitated by uniporters, whereas antiporters facilitate the absorption of the non-essential AAs. To ensure sufficient intracellular levels, both kinds of transporters with the ability to absorb AAs must be expressed within the same membrane.[Citation159]
Several amino acid transporters at the basolateral membrane with stereoselectivity for transferring D-AAs are antiporters, i.e., they exchange D-AAs across the membrane for L-AAs, including LAT1, and Asc1.[Citation193,Citation235,Citation251] Asc-1 is expressed in the loop of Henle, distal convoluted tubule, cortical collecting duct, and outer medullary collecting duct with an affinity for D-alanine and D-cysteine and especially high affinity for transport of D-serine.[Citation251] Eleven other D-AAs have additionally been observed to be transported by Asc-1 in studies conducted with rodents, including D-glutamate and D-aspartate.[Citation173,Citation252] LAT4 is expressed in S3[Citation235] and functions as a uniporter with affinity for transferring D-leucine, D-phenylalanine, D-valine, and D-methionine.[Citation253,Citation254] It has an important role in controlling the reabsorption of essential AAs across the basolateral membrane.[Citation254] To avoid toxic concentrations of D-AA in the cell lining of the S3, there is a high peroxisomal abundance of DAAO,[Citation255] and with the favorable ratio of the enantiomers, the net change will be from D-AA to L-AA and support of housekeeping. The reabsorption across the membranes of D-AA and peptides containing D-AA in S3 are shown in .
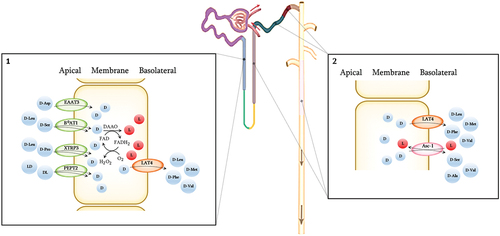
Figure 5. Renal transmembrane transport of D-AAs and peptides containing one D-AA.Transporters involved in the reabsorption of D-AAs present at the proximal straight tubule (S3), the Loop of Henle, the thick ascending limp, distal convoluted tubule, cortical connecting duct, cortical collecting duct, and outer medullary collecting duct. Through the utilization of flavin adenine dinucleotide (FAD) as a cofactor, D-amino acid oxidase (DAAO) exhibits stringent stereoselectivity in facilitating the oxidative deamination of D-AAs, concomitant with the reoxidation of FADH2 via molecular oxygen, resulting in the production of hydrogen peroxide. 1. D-Aspartate, D-leucine, D-serine, D-proline, and DL- and LD-peptides are transferred through the apical membrane at the S3 mainly or solely by symporters, whereas D-leucine, D-methionine, D-phenylalanine, and D-valine are reabsorbed at the basolateral membrane by the uniporter LAT4. The known symporters involved in D-AA transport include Excitatory amino acid transporter 3 (EAAT3), Broad amino acid transporter 1 (B0AT1), Sodium- and chloride dependent transporter 3 (XTRP3), and Peptide transporter 2 (PEPT2). Peroxisomal degradation of excess D-AA by DAAO takes place between the membranes. Uniporters exchange selected D-AAs (D) for L-AAs (L) at the basolateral membrane, thereby overall favouring reuptake into the blood of D-serine, D-asparagine, D-cysteine and likely some other D-AAs. 2. Potential mechanisms for basolateral transport in the more distal part of the nephrons. D-leucine, D-methionine and D-phenylalanine are known to be reabsorbed at the basolateral membrane by LAT4. D-Alanine, and D-serine are exchanged by the antiporter Asc-1, and D-valine by both LAT4 and Asc-1.
Overall, the kidneys are vital for maintaining a high L:D-AA ratio and specifically to reabsorb L-AAs in S1 and S2 while the resulting up-concentrated D-AAs in the tubular fluid are absorbed into the cells in S3, where DAAO assures a high rate of epimerization into L-AAs. Except for a few systemically important D-AAs, which are rescued back into the circulation, the kidneys are therefore the primary filter in the body to specifically remove, degrade or epimerize D-AAs.
Absorption and transport of D-amino acid in the brain
Regulation of the availability of D-AAs is an important part of AA metabolism in the brain. As outlined below, tight regulation of D-AA concentrations in the brain[Citation256] depends on saturable and competitive transport mechanisms.[Citation257]
A continued transport of essential AAs across the BBB is critical to supply the neurons, glial cells, and the interstitial space and help maintain brain function.[Citation258] Glycine and the small neutral D-AAs, such as D-serine, D-alanine, and D-proline can permeate through the BBB across both the luminal and abluminal membranes[Citation259]; but the larger D-AAs such as D-phenylalanine, D-valine, or D-histidine cannot. In analogy with intestine and kidney, D-AA uptake and release across brain barriers happens at a much lower rate compared to the corresponding L-AAs.[Citation260] LAT1,[Citation261] and EAAC1-3[Citation262] are also important D-AAs transporters in the brain, localized in neurons and astrocytes. LAT1 is crucial for homeostasis of the neurotransmitter glutamine through exchange with neutral AAs at the BBB,[Citation263] and brain LAT1 exhibits higher affinity for AAs compared to LAT1 in peripheral tissues.[Citation263] Also, neutral D-AAs are transferred by LAT1 and-2 across the BBB, in particular, the large neutral D-AAs[Citation264] through exchange with other neutral AAs.
The three D-AAs selectively reabsorbed in the kidneys, D-serine, D-aspartate, and D-cysteine are the ones most important for NMDAR mediated regulation. Asc-1[Citation265,Citation266] is specific to neurons and important for release of D-serine from the neurons. It mediates hetero-exchange of small neutral AA, especially alanine, serine, and cysteine, while D-serine is coupled to the transport in the opposite direction. Asc-1 has an affinity for the transport of D-alanine and D-cysteine as well.[Citation173] A study with Asc-1 knock-out mice resulted in a 65–80% decline in D-serine uptake in the cerebellum and forebrain, indicating that Asc1 is the primary D-serine transporter in the brain.[Citation266] Asc-1 is widely expressed in the brain,[Citation203] with particularly high levels in hippocampal CA1-3.[Citation252]
Fine-tuning of extracellular D-serine content is necessary to maintain proper NMDAR functions. D-Serine dynamics involve several cell types, including neurons and astrocytes, where the two antiporters, ASCT1-2,[Citation203] are expressed[Citation267] and catalyze D- and L-AA hetero-exchange; both uptake and release of D-serine is coupled to the transport in the opposite direction. The two ASCT family members are responsible for the uptake of D-serine,[Citation203] and therefore, despite their low affinity for D-serine,[Citation265] they are important regulators of D-serine levels in the extracellular space,[Citation265,Citation268] see also .
Physiological concentrations of hydrogen sulfide (H2S) selectively activate NMDAR neurotransmission and enhance long-term potentiation (LTP).[Citation269] In young rats, H2S increases intracellular Ca2+ in hippocampal astrocytes.[Citation270] Since D-cysteine is transported by Asc-1 mediated exchanges in the brain[Citation269] and produces H2S after DAAO-facilitated deamination, D-cysteine has been suggested to act as an additional agonist at the NMDAR.[Citation269]
LAT1 also regulates the glutamine exchange for other AAs in astrocytes and neurons.[Citation271] On the apical membranes, the transport of L-glutamate only occurs by a unidirectional mechanism, thereby achieving an equilibrium of AAs across the membrane.[Citation194] Because L-glutamine is transferred by exchange across the abluminal membrane, L-glutamate transport into the brain will not be restricted.[Citation271] The EAATs are responsible for the removal of the neurotransmitter L-glutamate from the synaptic cleft.[Citation272] EAAT1-3 have high stereoselectivity for uptake of D-aspartate with km around 50 µm, and they all have high stereoselectivity for L-glutamate.[Citation273]
In addition to facilitating transport across the BBB, D-asparagine is also a potent activator of glutamine-gated NMDAR and possibly other glutamate-gated regulators such as AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid). D-Asparagine in addition stimulates endocrine hormone production, including steroid and peptide hormones in the brain as well as peripherally.[Citation177]
D-amino acids in health and disease
D-AAs fulfil biological functions in the mammalian brain,[Citation274] from overall signaling and memory functions to specific regulation of processes in the synapses.[Citation9,Citation275] Specific functions are also reported for D-AAs in peripheral tissues and cells,[Citation39,Citation276] and recent reviews have confirmed that they may have both advantageous and unfavorable consequences for health.[Citation10,Citation180,Citation277] For instance, increased serum levels of D-AAs, including D-alanine, D-aspartate, D-glutamate, D-leucine, D-proline, and D-serine, have been identified as potential biomarkers for the early and sensitive diagnoses of diseases, such as chronic kidney disease (CKD) and[Citation132,Citation278] AD.[Citation132,Citation278]
D-amino acids and brain function
NMDAR is an important excitatory glutamate receptor having central functions in the brain, regulating non-selective cation channels, typically expressed in the post-synaptic areas. These receptors form hetero-complex structures and have a large number of receptor subtypes[Citation279] typically composed of four subunits, each usually one or two GluN subunits together with two or three subunits of GluN2 (A-D) or GluN3 (A-B).[Citation280] The NMDARs are responsible for the most common forms of synaptic plasticity, including strong depolarization to induce LTP and weaker depolarization facilitating long-term depression (LTD).[Citation279] During LTP processes, synaptic connections between neurons become stronger with frequent co-activation, and this is one of the mechanisms affecting learning and memory storage in the neuronal circuits.[Citation281]
The NMDARs require pre- and post-synaptic action potentials to be activated. If the post-synaptic cell is not depolarized when the receptor is activated, then Mg2+ remains in the receptor channel and blocks calcium flux. However, if an action potential in the post-synaptic cell causes depolarization of the membrane, the Mg2+ blockade is removed, and Ca2+ can flow through the channel, which has high permeability to Ca2+.[Citation282] Ca2+ permeability[Citation282] and Mg2+ blockade vary and depend on NMDAR subunit composition,[Citation279] see . L-Glutamate or D-aspartate[Citation283] is required as agonist on the GluN2 (A-D) subunits at the L-glutamate binding site for receptor activation. In addition, either D-serine or glycine always act as co-agonists on the GluN1 subunit.[Citation19] D-serine is the predominating co-agonist in the forebrain, which include most of the limbic system, while glycine predominates in the brainstem and cerebellum.[Citation284]
Figure 7. The NMDAR structure with its main binding sites. In addition to the binding of its agonist L-glutamate to the GluN2 unit, NMDAR signalling requires post-synaptic depolarization to remove Mg2+ blocking of the passage of cations as well as coagonistic binding of either D-serine or glycine at the GluN1. When the post-synaptic membrane is sufficiently depolarized, the NMDAR channels open, Ca2+ and Na2+ enter the neuron, and K+ exits. NMDARs may be composed of several subunits; GluN1 and GluN2A-D, as well as GluN3A-B. Functional NMDARs are di-heteromeric or tri-heteromeric receptors consisting of two GluN1 subunits or GluN1 combined with one or two different subunits, respectively.
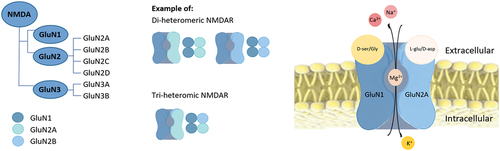
Tri-heteromeric receptors with GluN1/2A/2B subunit configurations are prevalent throughout the adult hippocampus and cerebral cortex[Citation285] but their properties are less well understood[Citation286] compared to the di-heteromeric receptor subunits of GluN1/2A or GluN1/2B,[Citation287] widely expressed in the adult brain.[Citation279] Heteromeric receptors containing GluN2A and GluN2B predominate in higher brain structures such as the hippocampus.[Citation288,Citation289]
D-serine serves especially as the main co-agonist for NMDAR,[Citation290,Citation291] in the hippocampal synapses between the Cornu Ammonis 1–3 (CA1-3) pyramidal neurons.[Citation292] It is also the most potent co-agonist at GluN1/2A-containing NMDARs, which have the highest gate-opening probability compared to the GluN1/2B-configured receptor.[Citation290] In addition, D-aspartate has[Citation293] been found to co-activate the NMDARs in hippocampal CA1 pyramidal and striatal neurons.[Citation294] D-serine has also affinity to NMDARs with GluN3 A or B subunits,[Citation295–297] expressed in neurons[Citation297] and glia cells.[Citation298]
Importance of D-amino acids for brain function and memory
Astrocyte signaling pathways through the hippocampus area play important roles in the consolidation of memory by moving information from short-term to long-term memory and enhancing spatial memory to enable navigation.[Citation194] Sufficient synapse depolarization is required to fulfill receptor activation, thereby maintaining dynamic rebuilding of the neural circuits. The specialized release and uptake areas of astrocytic and neuronal D-serine and its critical function as co-agonist of the NMDAR in synaptic transmission represent complex mechanisms, which are still not fully elucidated. The serine shuttle hypothesis,[Citation6] suggests that neuronal D-serine is dependent on astrocytic L-serine synthesis by 3-phosphoglycerate dehydrogenase (PHGDH; EC 1.1.1.95), which is exclusively expressed in astrocytes.[Citation6,Citation299] The L-serine released is taken up in neurons, where it is converted to D-serine by the enzyme, serine racemase;[Citation265] D-serine is subsequently released from neurons to activate NMDARs.[Citation265]
D-Serine released from neurons also enters astrocytes, where it is stored in glial vesicles for later release by stimulation of the astrocytes.[Citation6] ASCT1 is essential for regulating D-serine and shuttling L-serine between glia cells and neurons,[Citation300] as it exchanges L-serine for neutral AA,[Citation301] while Asc-1 is essential for neuronal transport of both D- and L-serine.[Citation265]
In addition to D-serine, D-aspartate, and D-cysteine are particularly important during fetal brain development where stimulation and inhibition of cell proliferation and dendritic development in the prefrontal cortex and other structures have been observed.[Citation143,Citation302] The effects and distribution of D-aspartate and D-cysteine change postpartum, whereas D-serine is much less affected. Along with the importance of D-serine and D-glutamate, D-AAs play central roles in normal brain development and in functions such as memory and regulation of endocrine secretion. Dysfunctional gut microbiota can affect circulating and brain levels of some D-AAs; however, food levels of D-AA have not yet been shown to affect levels or functions in the brain in humans.
Dysregulation of D-amino acids in glutamate-gated NMDAR function and signaling
The NMDARs are not only found in brain neurons. NMDAR subunits are also expressed in non-neuronal cells in the brain, such as glia cells[Citation303] and in peripheral tissues and cells,[Citation304,Citation305] including peripheral neurons, e.g., in the GI tract. These NMDARs have distinct functions and subunit compositions compared to those found in neurons in the brain.[Citation306,Citation307] Many subunit combinations exist, and they play important roles in the peripheral nervous system,[Citation308] such as in the communication between the central and peripheral nervous systems and between organs.[Citation308] NMDARs and D-AAs, including D-serine, D-alanine,[Citation309] D-glutamate, and D-aspartate are co-expressed in peripheral tissues and cells,[Citation277,Citation309,Citation310] but the functional relationship between D-AAs and NMDAR signaling in cells in the periphery remains largely unexplored, and the current understanding of the field is therefore limited.
Through the interaction between NMDARs in the periphery and in the brain and spinal cord, D-AA affects a large number of important physiological functions such as regulation of muscle contraction,[Citation311] cardiovascular[Citation312] and respiratory function,[Citation313] GI motility,[Citation314] and secretion,[Citation313] as well as regulation of flow in the vasculature[Citation312] and kidneys.[Citation315] In addition, NMDARs are involved in would healing[Citation316] and in responses to movement,[Citation317] pain, and sensation.[Citation318] They are also important for hormone regulation, including insulin secretion,[Citation319] cell growth and differentiation, and for bone resorption.[Citation320] Furthermore, they have a central role in immune responses and in inflammatory processes[Citation308] and are associated with the development of certain cancers, e.g., in tumor growth.[Citation321] See for an overview of the various functions of NMDARs in the human body as points of physiological effects of D-AAs.
The GluN3 subunits are robustly expressed in neurons[Citation297] and in glia cells[Citation307] and have roles in neuron-glia communication.[Citation322] In particular, GluN3A is expressed at high levels in many brain regions, including the hippocampus.[Citation323] GluN3 A and B are present in the BBB and in peripheral organs such as the kidney, lung, and stomach, while GluN3A is also found in lymphocytes and platelets.[Citation308]
D-serine or glycine GluN3 subunits, are not activated by L-glutamate.[Citation296,Citation323] GluN3 containing NMDARs have lower Ca2+ permeability, and particularly GluN3B has dramatically reduced Mg2+ blockade compared to the classical NMDARs.[Citation322] In addition, GluN3A has a shorter open time compared to those containing GluN3B subunits.[Citation323]
The GluN1/3A subunit combination is also more sensitive to redox changes, which profoundly affect agonist sensitivity and gating properties[Citation297] Because of the atypical biophysical properties of the GluN1/3 NMDARs, they seem to antagonize the functional and structural plasticity mediated by NMDARs containing GluN1/2 subunits.[Citation323] NMDARs containing GluN3A or B subunits may have important regulatory roles only during normal postnatal development.[Citation322] In adults, GluN3A has been shown to reduce plasticity and has been linked to negative functional outcomes, including psychiatric disorders and neurodegeneration.[Citation324,Citation325] This is also observed in peripheral tissues with GluN3A potentially increasing ischemia in the kidney.[Citation326] D-Serine and glycine seem therefore to be involved in normal as well as dysfunctional NMDAR activation.
In addition to the importance of D-serine for glutamate-gated NMDAR function, D-serine may also be important for other glutamine receptors, termed GluD2[Citation327] or GluRδ2[Citation328] that do not form ion channels[Citation329] but rather make a conformational change in the synaptic region upon binding by D-serine, thereby adding plasticity. Below we briefly outline examples of pathologies involving D-AA and NMDARs with a focus on the three organs previously covered in relation to D-AA metabolism, the intestine, the kidneys, and the brain.
D-amino acids in physiology and pathology of the gastrointestinal tract
The gut microbiota produces a range of small metabolites that can affect NMDAR signaling, including D-AAs, H2S, and metabolites from the kynurenic acid pathway;[Citation330,Citation331] these metabolites may also affect NMDARs locally in the gut lining and in other peripheral tissues. H2S is produced at low levels locally, both in the brain[Citation331] and gut. Other metabolites of gut origin may also affect L-glutamate signaling in the brain; for instance, D-glutamate is itself formed by gut microbial racemases or its levels are affected by microbial scavenging of glutamate precursors.[Citation332] Intraperitoneal injection of the gut metabolite, p-cresol has been shown to affect the expression of NMDAR subunits in different regions of the brain of rats leading to dysfunction,[Citation333] while feeding of rats with galacto-oligosaccharides (GOS) has been shown to stimulate brain NMDAR signaling and cognition[Citation334] while inhibiting olanzapine-induced weight gain;[Citation335] this may be secondary to increased acetate formation, although the role of microbial short chain fatty acid production for NMDAR function needs further study.[Citation335] Postnatal GOS administration also affects central and enteric NMDAR expression as well as long-term signaling in rats and has anxiolytic effects in mice.[Citation336,Citation337] While the examples outlined above underline that gut metabolites can affect neuronal function, the effects of gut microbial metabolites on L-glutamate signaling through NMDAR in the gut itself or other organs are not extensively studied. However, colonic dysbiosis is common in several brain conditions, including schizophrenia[Citation338] and (anti)-NMDAR auto-antibody related encephalitis; experimentally induced gut dysbiosis in rats using microbiota from encephalitis patients affects the brain as well as local inflammation and permeability in the gut.[Citation338] Dysbiosis and altered serum metabolite and cytokine profiles also strongly predict encephalitis.[Citation339] Dysfunctional gut microbial metabolism is therefore a likely player in the malfunctioning of distant organs. However, the involvement of NMDAR signaling in peripheral tissues as a function of gut-derived D-AA and other metabolites needs further elucidation.
NMDARs are abundantly expressed on epithelial cells in the colon of mice.[Citation340,Citation341] Macrophages also express NMDAR and are essential for anti-inflammatory signaling and suppression of cytokine expression in mice.[Citation340] In an experimental colitis mouse model, NMDAR expression on the epithelial cell surface of T-cells and neutrophils was markedly increased compared to the expression in controls.[Citation341] GluN1, GluN2A, and GluN2B subunits are expressed throughout intestinal neurons of the submucosal and myenteric plexuses, which play important roles in regulating gut motility and secretion. Excitotoxicity is seen after ischemia caused by NMDAR hyperactivity in enteric neurons after intestinal ischemia/reperfusion injury,[Citation342] similar to the mechanisms described for the brain below. NMDAR is widely found in the cholinergic myenteric plexuses of the GI tract, including the stomach,[Citation308] ileum,[Citation343] and colon.[Citation344] Inhibition of NMDAR signaling has been shown to stimulate gastric emptying in rats, thereby increasing meal size.[Citation345] NMDAR signaling is also central in gut motility and interacts with nitric oxide (NO) signaling in enteric reperfusion damage.[Citation343] It could therefore be speculated that compounds such as D-serine and D-aspartate that are able to modulate glutamate-gated stimulation of cholinergic signaling might affect food intake as well as gut transit time. NMDAR is also involved in gastric acid secretion through cholinergic signaling and in its inhibition by affecting NMDAR on H2-histamine receptors.[Citation346]
Irritable bowel syndrome (IBS) has been known for a long time to have a neuronal hypersensitivity component, likely mediated by NMDAR hyperactivity, either centrally or locally. In experimental colitis, inhibition of NMDAR by injection of kynurenic acid restored normal motility.[Citation314] NMDAR signaling has been shown to increase hypersensitivity of the gut epithelium in IBS patients and to increase brain-derived neurotrophic factor production and extracellular signal-regulated kinase (EC 2.712.2) activation in gut epithelial cells in vitro.[Citation318] NADPH oxidases (NOX EC 1.6.5.10) are important reactive oxygen species (ROS) generating defense systems in the gut, and they are chronically activated in irritable bowel syndrome models in mice.[Citation347] Inhibition of NMDAR in inflammatory conditions reduces ROS formation and restores motility.[Citation348] Antibodies to wheat gliadin found in patients with celiac disease cross-react with an NMDAR component, termed GRINA. These gluten antibodies may act as NMDAR auto-antibodies and increase gut permeability in mice and in humans.[Citation349] Antibodies to NMDAR also seem to cause inflammatory bowel disease (IBD) in a subset (one-third) of patients with schizophrenia,[Citation349] possibly by a similar mechanism. In a systematic review, high comorbidity is also reported between anxiety and depression in patients with IBD, particularly during active phases of the disease.[Citation350]
NMDARs are abundantly expressed on enteric cholinergic neurons co-expressed with the D-aspartate transporter EAAT3[Citation342,Citation351] which is highly expressed in the entire GI motor cortex.[Citation194,Citation273] In the enteric neurons, NMDAR containing GluN1 and GluN2 A and B subunits are co-expressed with EAAT3
Table 5. D-Alanine and D-serine levels in serum and cerebrospinal fluid.
D-amino acids in kidney function and disease
High D-serine plasma levels can induce nephrotoxicity caused by proximal tubular necrosis in rats, mice, and patients with chronic kidney disease (CKD). It has been reported that the toxic response is caused by the DAAO metabolism of D-serine to form H2O2, thereby increasing ROS levels. When a dose of 780 mg D-serine/kg BW was provided to rats, oxidative deamination resulted in a 70% reduction of renal glutathione (GSH) levels.[Citation355]
Epithelial damage caused loss of lining cells, resulting in lack of a functional epithelial barrier and tight junctional complexes. Elevated reabsorption of D-AAs happens during pathological processes when the normally low passive back flux into the tubular fluid is increased. Reabsorption of D-serine at similar rates as L-serine has been observed in rodents under these conditions,[Citation211] and even moderately increased plasma levels of D-serine might have a toxic effect on the tubular cells in S3, causing chronic renal failure.[Citation356]
In healthy humans, concentrations of D-serine of up to 3% of total serine in plasma have been reported. Chronic kidney disease (CKD) is defined as abnormalities of kidney structures or function being present for > 3 months, and increases in D-serine plasma levels have been observed in patients with CKD.[Citation11,Citation239] In contrast, the concentration of L-serine decreases in plasma in patients with CKD due to demands by other tissues;[Citation357,Citation358] a D/D + L fraction of total serine of up to 23% has been observed in patients with CKD. D-Alanine, D-asparagine, and D-proline have been associated with CKD,[Citation359] indicating that systemic changes affecting D-AA homeostasis may be an underlying cause of the complication. In patients with CKD at stages 3, 4, and 5, plasma levels of D-serine, D-asparagine, D-alanine, and D-proline are inversely correlated with kidney function.[Citation359]
Glutamate signaling in the kidneys
The kidney is an essential excretory organ, and NMDAR hyperactivity is associated with progressive functional loss during kidney disease due to increased cellular Ca2+ influx.[Citation360] D-Serine acts as a co-agonist of the NMDAR and it has been suggested that its receptor functions in the kidney are similar to those found in the brain.[Citation361] The GluN1-3 subunits are all identified in human proximal tubular cell lines. In rats, GluN1 and −2 subunit proteins are abundantly expressed in kidneys;[Citation360,Citation361] D-serine acts as co-agonist at GluN1[Citation361] and serine racemase has been found to be primarily co-expressed with GluN1 in renal vessels, apical membranes of proximal tubules, and collecting ducts.[Citation362] GluN1 plays an important role in maintaining sensoric function in the normal kidney.[Citation363]
D-Serine-induced NMDAR hyperactivation is associated with decreased urine osmolarity and elevated volume and consequently with pronounced proteinuria, glucosuria, and marked renal hypotrophy in rats.[Citation361,Citation364] The resulting intracellular overload of Ca2 causes activation of NOS, which also regulates renin release from the adjacent afferent arteriole. This process may elicit a vicious circle, where the tubular stress by renin and the NMDAR hyperactivity caused by D-serine enhances Ca2+ influx and ROS formation, further enhancing toxicity.[Citation279]
In Acute Kidney Injury (AKI), defined as an abrupt decrease in kidney function that occurs over a period of 7 days or less,[Citation365] hyperactivated NMDAR signaling is associated with kidney dysfunction.[Citation360]
The balance between D- and L-serine seems to be key to D-serine mediated toxicity. In human tubular cells, depletion of L-serine induces D-serine toxicity by upregulating pro-fibrotic factors leading to reduced cell proliferation rates and apoptosis, but re-supplementation with L-serine, negates the effect of D-serine on cell survival. D-Serine exposure upregulates the essentiel enzymes for L-serine synthesis,[Citation366] possibly as a biological feedback mechanism to restore the balance between the two enantiomers.
D-amino acids in brain pathology
In the brain, there are several common features in NMDAR-related pathologies and imbalanced NMDAR signaling may be seen as a common mechanism of many neuronal disease conditions increasing with age. Especially D-serine has an important role. In the healthy aging brain, reduced D-serine synthesis causes NMDAR hypoactivity and reduced neurotransmission. This leads to increased production of NO and S-nitrosylation of serine racemase,[Citation367] further inhibiting D-serine synthesis with progressive loss of neuronal plasticity and and higher cognitive deficits, commonly reflected during aging.[Citation368]
Elevated DAAO activity and lack of D-serine are also associated with age-related cognitive decline and NMDAR hypo-activation.[Citation134,Citation367]
Age-related memory impairment is also correlated with decreasing intracellular GSH levels in the brain, indicating involvement of oxidative damage.[Citation369]
Perturbed D-serine homeostasis seems central to several of these pathologies, although other D-AAs are also directly or indirectly involved, including D-aspartate,[Citation370] D-alanine,[Citation282] D-cysteine,[Citation178] and D-tryptophan.[Citation371] The mature disease states are often characterized by increased NMDAR signalling,[Citation361,Citation372,Citation373] but they may in some pathologies result from mechanisms countering too low NMDAR activation,[Citation374] e.g., from too low D-serine availability.[Citation375,Citation376] Conversely in AD, a high initial D-serine and NMDAR activation is followed by lower D-serine formation and hypoactivation of NMDAR in some studies,[Citation377] but this is still conctroversial.
Neuronal NMDARs act as detectors of multiple coincident effector molecules, thereby coordinating presynaptic neuronal activity with post-synaptic depolarization processes by opening their Ca2+ channels. Changes in the local levels of effector molecules over time change the neuronal activity and can lead to pathological changes. Excitatory Ca2+ influx and downstream signaling by neuro-activated mediators affect synaptic function, as well as the plasticity and oscillation of the neuronal network.[Citation378] In the brain synapses, NMDAR activity is very central; both synaptically and extra-synaptically, it tightly regulates Ca2+ influx and through its activation, a wide range of cellular functions are elicited, ranging from modulation of neuronal action to excitotoxicity. Optimal NMDAR signaling stimulates intracellular transduction cascades that regulate the strength of neural connectivity and neuroplasticity.
However, NMDAR localization in dendritic spines (protrusions that channel various post-synaptic inputs to the neuron) is essential for optimal functioning. Changes in subunit composition in the synapses can significantly affect the neuronal synaptic activity. NMDARs constantly cycle in and out of the postsynaptic protein complex (the so-called postsynaptic density), making studies on their function complex. Dysfunction of NMDARs, such as altered subunit expression or activation, is linked to several neurological diseases, injuries, or trauma, as well as psychiatric disorders.[Citation279]
Alzheimer´s disease and D-amino acids
AD is a highly complex neurodegenerative disorder characterized by memory loss and cognitive impairment, which usually starts slowly and then progressively becomes more severe. The initial pathology is likely caused by overloaded NMDAR signaling of neurons due to over-activation by stimuli[Citation379,Citation380] including increased D-serine levels. In the early stages, cortical and hippocampal brain signals are mainly affected. In comparative clinical studies, memory-related[Citation381] neuronal activity has been examined across the various states of memory deficiency, ranging from the normal aging brain to the late stages of AD. In one study, asymptomatic carriers of genes associated with increased risk of early-onset AD were found to have increased activation in the neuronal network in the hippocampus, compared to controls.[Citation382] In some studies, elevated NMDAR activation in hippocampus has been observed in subjects with mild cognitive impairment (MCI), a state between dementia and the average cognitive decline during aging.[Citation383] MCI may be differentiated into two states: hyperactivity has been described in less severe state of MCI and hypoactivity in the more severe. In the early stage of AD, both hyperactivated and hypo-activated signaling of the hippocampal network is observed, whereas hypoactivity predominates in the later stages of AD.[Citation377,Citation381,Citation383]
D-AAs, and in particular D-serine, are associated with these pathological conditions in AD.[Citation384,Citation385] Several studies reporting plasma levels of D-AAs are listed in but show almost 100-fold heterogeneity. A recent systematic review and meta-analysis, including seven studies with 1186 participants, evaluated the association of D-serine levels in serum and cerebrospinal fluid (CFS) in patients with AD and non-AD control subjects.[Citation385] The analysis showed that D-serine concentrations were significantly higher in serum and CSF of the diagnosed AD patients despite the high heterogeneity among measurements. An inverse association between D-serine levels and the mean mini-mental state examination scores was found in the patients with AD.[Citation385] Controls are younger in most comparative studies, which may bias the results.
The β-amyloid-containing plaques (Aβ) that generate and accumulate tau-containing neurofibrillary aggregates are histopathological hallmarks of AD.[Citation391] Aβ selectively activates signaling in GluN2B-containing NMDARs resulting in a shift towards GluN2A-containing receptors.[Citation303] This would result in dysfunction of D-serine dependent signaling, possibly leading to compensatory actions, increasing D-serine levels.
Associations of AD with plasma D-alanine, D-proline, D-aspartate, and D-glutamate have also been observed, indicating a common mechanism related to D-AA homeostasis. D-Alanine in gray matter of AD brains was present at 2-fold higher levels than in healthy control subjects. In a cross-sectional study of 305 women (65–80 years), MCI and dementia were associated with increased plasma D-proline levels.[Citation278] In contrast, D-aspartate has repeatedly been found at lower levels in brain tissue and CSF of patients with AD compared to healthy controls.[Citation127,Citation389,Citation392,Citation393]
Decreased plasma D-glutamate levels and reduced NMDAR activity have been associated with early symptoms of cognitive impairment in AD patients;[Citation385] D-glutamate was decreased by about 30% in MCI and by 60% in severe AD. The metabolic pathways and neurological function of D-glutamate remain unclear and also the relationship with D-serine and other D-AAs that change in AD.
Inflammatory processes, D-amino acids and involving D-amino acids in Alzheimer’s disease
Inflammatory processes can be stimulated by high NMDAR activity and lead to excitotoxicity, loss of cellular functionality, and cell death[Citation394] of likely importance to AD.[Citation395] Some underlying mechanisms and their relation to D-serine levels are described below. Astrocytes and microglia are neuro-epithelial cells[Citation396] surrounding the neurons and maintain a microenvironment that ensures functional neuronal circuits in the healthy adult brain.[Citation397] This happens by modulating synaptic activity,[Citation398] by taking up glucose, storing glycogen, and delivering D-serine and ATP.[Citation399] The microglia are phagocytic cells that sense neuronal hypo- and hyperexcitability in the healthy brain and support the ongoing neurogenesis to maintain homeostatic network activity.[Citation400] However, microglia are also identified as potential cellular mediators of synaptic dysfunction.[Citation401] Cytokines activate the cells to elicit rapid transcriptional responses to pathogens and stressors by releasing pro-inflammatory molecules.[Citation395] For example, after activation in mouse models, microglia can eliminate amyloid plaques. However, in hyperactivated NMDAR, the microglial excitotoxicity activates pro-inflammatory responses[Citation402,Citation403] such as tumor necrosis factor-α,[Citation404] and affect NMDAR signaling in neurons.[Citation405]
When neurons are injured, the astrocytes undergo morphological and functional changes that alter their core physiological state,[Citation406] and they gain neurotoxic functions, referred to as reactive astrocytes.[Citation407] The shift in astrocytic signaling from physiological to pathological reactive astrocytes is secondary to the NMDAR hyperactivity-mediated extrinsic signaling. This response is caused by increasing D-serine levels,[Citation407] resulting in excitotoxic responses in the tissue. The reactive astrocytes enhance oxidative stress and imbalanced phagocytosis and may lead to chronic inflammatory responses.[Citation402] Neuroinflammation has been confirmed in reactive mouse astrocytes by genomic profiling analysis. Markedly increased numbers of reactive astrocytes compared to controls in post-mortem cortex and increased immune activation in the hippocampus have been observed in subjects diagnosed with AD.[Citation408]
Loss of D-serine homeostasis in Alzheimer’s disease
Astrocytes are highly sensitive to glucose deprivation and depend on glycogen stores and available GSH.[Citation409]
From an ongoing cohort study, it has been suggested that impaired glucose metabolism due to reduced glycolytic flux may begin several years before the onset of cognitive impairment in AD.[Citation410]
In subjects with MCI or early-stage AD, brain glucose uptake was found to be reduced in parts of the limbic system by ~15 and ~7%, respectively, compared to cognitively healthy controls.[Citation411] L-Serine synthesis and derived biomolecules, in particular D-serine, are suggested to be essential for the survival of neurons and astrocytes, and in an AD mouse model, reduced L-serine levels were likely caused by reduced glucose uptake and decreased glycolysis.[Citation412] PHGDH, the first step of de novo synthesis of L-serine, is essential for the synthesis of D-serine and was reduced by ~62% in patients with intermediate AD and by ~82% in the hippocampus, parahippocampal gyrus, and fusiform gyrus (brain regions located in the temporal lobe of the cerebral cortex) in those with advanced AD in comparison to controls.[Citation413] It has therefore been suggested that gradually progressive impairment of serine synthetic pathways in astrocytes might be present in AD.[Citation413] However, when astrocytes become reactive, synthesis and release of D-serine in the hippocampus preferentially activates the destructive extrasynaptic NMDAR signaling.[Citation414] In addition,[Citation415] serine racemase is found to be highly expressed in hippocampal reactive astrocytes of post-mortem AD human brains and rat models of AD,[Citation416] indicating that over-production of D-serine may be a result of the transition of normal glia cells to reactive astrocytes in the progression of AD.
D-amino acids in other brain-related conditions
There is evidence for the involvement of D-AAs in several other pathological conditions affecting the brain with a few examples below, but it goes beyond the scope of this review to go into detail. For instance, D-serine in the spinal cord is associated with the pathology of amyotrophic lateral sclerosis,[Citation417] and low D-serine levels and NMDAR hypoactivation are also related to post-traumatic stress disorder[Citation418] and Parkinson’s disease.[Citation419]
Dysregulated D-aspartate metabolism in the processes of the embryonic brain is linked to schizophrenia,[Citation420] and reduced D-aspartate levels in the brain of schizophrenia-affected patients are associated with increased DDO mRNA expression or DDO activity.[Citation420] In post-mortem brains of schizophrenia-affected patients, D-aspartate levels have been found to be reduced by up to 70% in the prefrontal cortex compared to levels in the brains of individuals without schizophrenia. This decrease correlates with marked downregulation of GluN1, GluN2A and -B subunits.[Citation421]
It is widely accepted that low D-serine levels and NMDAR hypoactivation are associated with various psychiatric disorders, such as schizophrenia. The D-tryptophan derivatives, D-kynurenine and its metabolite, kynurenic acid, are also associated with schizophrenia[Citation422]; D-kynurenine is elevated in schizophrenic brains,[Citation422] and kynurenic acid stimulates pro-inflammatory processes via interleukin-6.[Citation371] However, kynurenic acid can also act as an anti-excitotoxic, non-competitive inhibitor at the glycine-binding site of NMDARs, indicating a highly complex interaction of D-AAs in brain disorders.
D-AAs in human disability and disease
D-AAs are involved in several human diseases due to their important roles as regulators of transport and signaling, see . While most D-AAs may be of little importance, except for toxicity or local sensing by the immune cells, some specific D-AAs and, in particular D-serine, D-aspartate and D-cysteine are important regulators of physiological function. These D-AAs are absorbed from food in the small intestine and possibly more importantly- produced by the gut microbiota and selectively absorbed into the portal blood. They are also re-absorbed by the kidneys and favored by antiporters at the BBB and at other sites. However, to maintain sufficient levels locally, they are also endogenously synthesized. For instance, to regulate NMDARs and other gateways, a considerable local production of D-serine is needed, and glucose utilization is central for such local D-serine production, implicating lack of glucose homeostasis as an effector of NMDAR dysfunction. Competition for glucose due to energy demands or decreasing insulin sensitivity may cause D-serine production to become too low. It is well recognized that type 2 diabetes predisposes to neurodegenerative disease but an increase in degenerative complications of other organs like skin, kidneys, liver, and intestines would also be predicted due to decreased availability of glucose for serine synthesis. Resulting feedback upregulation of L-serine conversion can end in local, chronic dysregulation of D-serine signaling, e.g., in IBD, in CKD, and in loss of memory and memory-processing. Similarly, D-aspartate production is central for many endocrine functions,[Citation177] and dysregulation, e.g. by altered DDO activity, may affect hormone secreting organs leading to chronic functional decline and even disease. Serine racemase is expressed in keratinocytes of the skin, where conversion of L-serine to D-serine takes place. Lower epidermal barrier function has been observed in serine racemase knockout mice compared to controls,[Citation423] indicating the potential involvement of D-serine or D-cysteine in eczema and allergic responses. High plasma levels of D-serine have been associated with renal damage suggesting a potential vicious circle, where D-serine levels gradually increase, leading to several organ-specific physiological responses.[Citation424] In pancreatic cells, D-serine has been found to stimulate insulin secretion,[Citation308] but concentrations above or below a crucial range have been reported to be inhibitory. Serine racemase is abundantly expressed in the pancreas of both humans and mice.[Citation425] L-serine concentration in plasma and tissues decreases significantly in both type 1 and 2 diabetes,[Citation357,Citation426] suggesting that the D:L-serine ratio may be critical for diabetic complications.
In addition, D-aspartate has been suggested to play a role in the maturation and functions of the pituitary, pineal, and adrenal glands by inducing the synthesis and secretion of different hormones. When D-aspartate has been administered intraperitoneally or orally, it accumulates in these endocrine glands and acts as an excitatory molecule, stimulating the production and release of hormones such as cortisol, testosterone, and growth hormone. This process may be important for regulating various physiological processes, such as stress response and reproductive function.
Dysregulation of D-AA synthesis, metabolism, and transport may therefore constitute a common denominator for many degenerative diseases and organ dysfunction.
Conclusion
D-AAs found in food come largely from bacteria or food processing. While we have racemases in the upper GI tract, dietary intake of D-AAs may still contribute to systemic levels. A variety of bacteria in the large intestine also produce substantial amounts of D-AAs. Although uptake rates from the healthy colon is quite limited, the constant flux is likely a significant source contributing to the circulating levels of D-AAs. Therefore, dysbiosis of the colon microbiota may affect D-AA homeostasis distally. Efficient human D-AA catabolism is likely the most important way of removing systemic D-AAs. The remaining D-AAs in the blood are filtered in the kidneys in a two-step process, where L-AAs are first recovered from the filtrate, and among the remaining D-AAs selective transporters allow specifically D-serine, D-aspartate, D-glutamate, and D-cysteine to be partially recovered into the circulation. These D-AAs coincide with those affecting the NMDAR glutamate-gated Ca2+ channels across all body organs, most notably in the brain, where also the BBB seems to have selective transport of these D-AAs. NMDARs are highly diverse receptors involved in integrating multiple tissue signals in neuronal tissue but also with important regulatory functions in many other cell types throughout the body. Most D-AAs interacting with these receptors are likely produced by supporting cells, such as glia in the brain. NMDARs relate to many organ functions, e.g. contractile functions of the gut and kidney tubules, memory, immune function, skin and intestinal barrier function, and muscle tonus; dysregulation of D-AA production, transport, and degradation may therefore affect their availability locally or systemically. This may, in turn, influence NMDAR signaling and affect the risk or progression of several human degenerative diseases, all characterized by a decline in organ function. These include diseases such as AD and several other neurodegenerative diseases, acute or progressive kidney failure, and inflammatory bowel syndrome. The contribution of dietary D-AAs and the gut microbial production of D-AAs in preventing or exacerbating degenerative diseases has not been studied in larger detail. Nevertheless, it is now clear that the D-AAs are crucial effectors of local cellular communication and of the function of many organs, also playing an important role in the gut-kidney-brain axis. D-AA dysregulation may therefore constitute a common denominator for many degenerative diseases, and the importance of diet, microbiota and physiological states for these conditions present an important window of opportunity for new treatment options and for reducing risk of disease.
Abbreviations
Aβ, β-amyloid; AβO, β-amyloid oligomer; ACE2, Angiotensin-converting enzyme 2; AD, Alzheimer’s disease; APC, Amino acid-polyamine-organocation; Asc-1, Alanine-serine-cysteine transporter 1; ASCT1-2, Alanine-serine-cysteine-threonine transporter 1-2; ATB0,+, Amino acid transporter B0,+; BBB, Blood-brain barrier; bsr, Broad spectrum racemases; B0AT1-2, Broad amino acid transporter 1-2; CA1-CA-3, Cornu Ammonis 1-3; CSF, Cerebrospinal fluid; D-AA, D-amino acid; DAAO, D-amino acid oxidase; DDO, aspartate oxidase; EAAT1-3, Excitatory amino acid transporter1-3; Ex-GF, re-colonized Germ-free (using wild-type microbiota); GOS, Galactooligosaccharides; GF, Germ-free; GI, Gastrointestinal; GSH, Glutathione; H2O2, Hydrogen peroxide; H2S, hydrogen sulfide; IBD, Inflammatory bowel disease; IBS, Irritable bowel syndrome; IgA, Immunoglobulin A; L-AA, L-amino acid; LAT1-2 and 4, L-type amino acid transporters 1-2 and 4; LTP, Long-term potentiation; m-DAP, meso-diaminopimelic acid; NMDAR, N-Methyl-D-Aspartate Receptor; NO, Nitric oxide; PAT1-2, Proton coupled amino acid transporter 1; PEPT1-2, Peptide transporter 1-2; PHGDH, 3-phosphoglycerate dehydrogenase; PST, Proximal straight tubule; ROS, Reactive oxygen species; SLC, Human solute carrier; S1, S2, S3, Segment 1, 2, 3; XTRP3, X transporter protein 3.
Author contributions
ABR conceived the idea and together with LOD drafted the manuscript. HMR added text on microbiomics. All authors read and approved the final manuscript.
Supplemental Material
Download MS Word (201.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/87559129.2024.2347472.
Additional information
Funding
References
- Li, H.; Anuwongcharoen, N.; Malik, A. A.; Prachayasittikul, V.; Wikberg, J. E.; Nantasenamat, C. Roles of D-Amino Acids on the Bioactivity of Host Defense Peptides. Int. J. Mol. Sci. 2016, 17(7), From NLM Medline. DOI: 10.3390/ijms17071023.
- Aliashkevich, A.; Alvarez, L.; Cava, F. New Insights into the Mechanisms and Biological Roles of D-Amino Acids in Complex Eco-Systems. Front. Microbiol. 2018, 9, 683. DOI: 10.3389/fmicb.2018.00683.
- Okuma, E.; Fujita, E.; Amano, H.; Noda, H.; Abe, H. Distribution of Free D-Amino Acids in the Tissues of Crustaceans. Fish. Sci. 1995, 61(1), 157–160. DOI: 10.2331/fishsci.61.157.
- Gal, J. The Discovery of Stereoselectivity at Biological Receptors: Arnaldo Piutti and the Taste of the Asparagine enantiomers-history and Analysis on the 125th Anniversary. Chirality. 2012, 24(12), 959–976. From NLM Medline. DOI: 10.1002/chir.22071.
- Krebs, H. A. C. X. C. V. I. I. Metabolism of Amino-Acids. III. Deamination of Amino-Acids. Biochem. J. 1935, 29(7), 1620–1644. DOI: 10.1042/bj0291620.
- Wolosker, H.; Blackshaw, S. S.; Snyder, S. H. Serine Racemase: A Glial Enzyme synthesizing D -serine to Regulate glutamate-N-Methyl-D-Aspartate Neurotransmission. Proc. National Academy Sci. 1999, 96(23), 13409–13414. DOI: 10.1073/pnas.96.23.13409.
- Konno, R.; Yasumura, Y. Involvement of D-Amino-Acid Oxidase in D-Amino Acid Utilization in the Mouse. J. Nutr. 1984, 114(9), 1617–1621. DOI: 10.1093/jn/114.9.1617.
- Sasabe, J.; Miyoshi, Y.; Rakoff-Nahoum, S.; Zhang, T.; Mita, M.; Davis, B. M.; Hamase, K.; Waldor, M. K. Interplay between microbial D-Amino Acids and host D-Amino Acid Oxidase Modifies Murine Mucosal Defence and Gut Microbiota. Nat. Microbiol. 2016, 1(10), 16125. From NLM Medline. DOI: 10.1038/nmicrobiol.2016.125.
- Pollegioni, L.; Piubelli, L.; Sacchi, S.; Pilone, M. S.; Molla, G. Physiological Functions of D-Amino Acid Oxidases: From Yeast to Humans. Cell. Mol. Life Sci. 2007, 64(11), 1373–1394. DOI: 10.1007/s00018-007-6558-4.
- Bastings, J. J. A. J.; van Eijk, H. M.; Olde Damink, S. W.; Rensen, S. D-Amino Acids in Health and Disease: A Focus on Cancer. Nutrients. 2019, 11(9), 2205. From NLM Medline. DOI: 10.3390/nu11092205.
- Hesaka, A.; Sakai, S.; Hamase, K.; Ikeda, T.; Matsui, R.; Mita, M.; Horio, M.; Isaka, Y.; Kimura, T. D-Serine Reflects Kidney Function and Diseases. Sci. Rep. 2019, 9(1), 5104. From NLM Medline. DOI: 10.1038/s41598-019-41608-0.
- Ohide, H.; Miyoshi, Y.; Maruyama, R.; Hamase, K.; Konno, R. D-Amino Acid Metabolism in Mammals: Biosynthesis, Degradation and Analytical Aspects of the Metabolic Study. J. Chromatogr. B. 2011, 879(29), 3162–3168. DOI: 10.1016/j.jchromb.2011.06.028.
- Richter, K.; Egger, R.; Kreil, G. D-Alanine in the Frog Skin Peptide Dermorphin Is Derived from L-Alanine in the Precursor. Science. 1987, 238(4824), 200–202. DOI: 10.1126/science.3659910.
- Hashimoto, A.; Nishikawa, T.; Hayashi, T.; Fujii, N.; Harada, K.; Oka, T.; Takahashi, K. The Presence of Free D-serine in Rat Brain. FEBS Lett 1992, 296(1), 33–36. From NLM Medline. DOI: 10.1016/0014-5793(92)80397-y.
- Miyoshi, Y.; Koga, R.; Oyama, T.; Han, H.; Ueno, K.; Masuyama, K.; Itoh, Y.; Hamase, K. HPLC Analysis of Naturally Occurring Free D-Amino Acids in Mammals. J. Pharm. Biomed. Anal 2012, 69, 42–49. From NLM Medline. DOI: 10.1016/j.jpba.2012.01.041.
- Visser, W. F.; Verhoeven-Duif, N. M.; Ophoff, R.; Bakker, S.; Klomp, L. W.; Berger, R.; de Koning, T. J. A Sensitive and Simple ultra-high-performance-liquid chromatography-tandem Mass Spectrometry Based Method for the Quantification Of D-Amino Acids in Body Fluids. J. Chromatogr. A. 2011, 1218(40), 7130–7136. From NLM Medline. DOI: 10.1016/j.chroma.2011.07.087.
- Brückner, H.; Schieber, A. Determination of Amino Acid Enantiomers in Human Urine and Blood Serum by Gas chromatography-mass Spectrometry. Biomed. Chromatogr. 2001, 15(3), 166–172. From NLM Medline. DOI: 10.1002/bmc.57.
- Hashimoto, A.; Oka, T. Free D-aspartate And D-serine in the Mammalian Brain and Periphery. Prog. Neurobiol. 1997, 52, 325–353.
- Mothet, J. P.; Parent, A. T.; Wolosker, H.; Brady, R. O.; Linden, D. J.; Ferris, C. D.; Rogawski, M. A.; Snyder, S. H. D-serine Is an Endogenous Ligand for the Glycine Site of the N-methyl-D-aspartate Receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 4926–4931. DOI: 10.1073/pnas.97.9.4926.
- D’Aniello, A. D-Aspartic Acid: An Endogenous Amino Acid with an Important Neuroendocrine Role. Brain Res. Rev. 2007, 53(2), 215–234. From NLM Medline. DOI: 10.1016/j.brainresrev.2006.08.005.
- Kepert, I.; Fonseca, J.; Muller, C.; Milger, K.; Hochwind, K.; Kostric, M.; Fedoseeva, M.; Ohnmacht, C.; Dehmel, S.; Nathan, P. et al D-tryptophan from Probiotic Bacteria Influences the Gut Microbiome and Allergic Airway Disease. J. Allergy Clin. Immunol. 2017, 139(5), 1525–1535. From NLM Medline. DOI: 10.1016/j.jaci.2016.09.003.
- Cava, F.; Lam, H.; de Pedro, M. A.; Waldor, M. K. Emerging Knowledge of Regulatory Roles Of D-Amino Acids in Bacteria. Cell. Mol. Life Sci. 2011, 68(5), 817–831. From NLM Medline. DOI: 10.1007/s00018-010-0571-8.
- Dakin, H. D. The Racemization of Proteins and Their Derivatives Resulting from Tautomeric Change. J. Biol. Chem. 1912, 13(3), 357–362. DOI: 10.1016/s0021-9258(18)88648-3.
- Dakin, H. D.; Dudley, H. W. The Racemization of Proteins and Their Derivatives Resulting from Tautomeric Change. Part II. J. Biol. Chem. 1913, 15(2), 263–269. DOI: 10.1016/s0021-9258(18)88525-8.
- Dakin, H. D.; Dale, H. H., XXVI. Chemical Structure and Antigenic Specificity. A Comparison of the Crystalline Egg-Albumins of the Hen and the Duck. Biochem J. 1919, 13(3), 248–257. DOI: 10.1042/bj0130248.
- Friedman, M.; Gumbmann, M. L.; Masters, P. M. Protein-alkali Reactions: Chemistry, Toxicology, and Nutritional Consequences. Nutri & Toxicolo Aspects Food Saf. 1984, 367, 367–412.
- Mutaguchi, Y.; Ohmori, T.; Akano, H.; Doi, K.; Ohshima, T. Distribution Of D-Amino Acids in Vinegars and Involvement of Lactic Acid Bacteria in the Production Of D-Amino Acids. SpringerPlus. 2013, 2, 1–9.
- Hayase, F.; Kato, H.; Fujimaki, M. Racemization of Amino Acid Residues in Casein Roasted with Glucose And/Or Methyl Linoleate. Agric Biol Chem. 1979, 43(12), 2459–2465. DOI: 10.1080/00021369.1979.10863851.
- Csapo, J.; Varga-Visi, E.; Loki, K.; Albert, C.; Salamon, S. The Influence of Extrusion on Loss and Racemization of Amino Acids. Amino Acids. 2008, 34(2), 287–292. From NLM Medline. DOI: 10.1007/s00726-006-0484-x.
- Monteiro, C. A.; Cannon, G.; Moubarac, J. C.; Levy, R. B.; Louzada, M. L. C.; Jaime, P. C. The UN Decade of Nutrition, the NOVA Food Classification and the Trouble with ultra-processing. Public. Health. Nutr. 2018, 21(1), 5–17. From NLM Medline. DOI: 10.1017/S1368980017000234.
- Aldag, R. W.; Young, J. L.; Yamamoto, M. An Enzymatic Chromatograpic Procedure for the Determintion of D-Amino Acids in Plant and Soil Extracts. Phytochemistry. 1971, 10(1), 267–274.
- Kolukisaoglu, Ü.; Suarez, J. Amino Acids in Plants: New Insights and Aspects, but also More Open Questions. In Amino Acid - New Insights and Roles in Plant and Animal; Asao, T.; Asaduzzaman, M., Eds.; IntechOpen, 2017; p 155–164. DOI: 10.5772/66064.
- Cardoso, M. H.; Cândido, E. S.; Oshiro, K. G. N.; Rezende, S. B.; Franco, O. L. Peptides containing amino acids and retro-inverso peptides. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering, Eds.; Koutsopoulos, S. Woodhead Publishing, 2018; p 131–155. DOI: 10.1016/B978-0-08-100736-5.00005-3.
- Nagata, Y.; Fujiwara, T.; Kawaguchi, K.; Fukumori, Y.; Yamanaka, T. Occurrence of Peptidyl D-Amino Acids in Soluble Fractions of Several Eubacteria, Archaea and Eukaryotes. Biochim. Biophys. Acta. 1998, 1379, 76–82.
- Radkov, A. D.; Moe, L. A. Bacterial Synthesis Of D-Amino Acids. Appl. Microbiol. Biotechnol. 2014, 98(12), 5363–5374. From NLM Medline. DOI: 10.1007/s00253-014-5726-3.
- Uda, K.; Edashige, Y.; Nishimura, R.; Shikano, Y.; Matsui, T.; Radkov, A. D.; Moe, L. A. Distribution and Evolution of the serine/aspartate Racemase Family in Plants. Phytochemistry. 2020, 169, 112164. DOI: 10.1016/j.phytochem.2019.112164.
- Amelung, W.; Zhang, X.; Flach, K. W. Amino Acids in Grassland Soils: Climatic Effects on Concentrations and Chirality. Geoderma. 2006, 130(3–4), 207–217. DOI: 10.1016/j.geoderma.2005.01.017.
- Hoffmann, K.; Schneider-Scherzer, E.; Kleinkauf, H.; Zocher, R. Purification and Characterization of Eucaryotic Alanine Racemase Acting As Key Enzyme in Cyclosporin Biosynthesis. J. Biol. Chem. 1994, 269(17), 12710–12714. DOI: 10.1016/s0021-9258(18)99934-5.
- Baccari, C. G.; Falvo, S.; Santillo, A.; Russo, D. G. F.; Fiore, M. M. D-Amino Acids in Mammalian Endocrine Tissues. Amino Acids 2020, 52(9), 1263–1273. From NLM Medline. DOI: 10.1007/s00726-020-02892-7.
- Vranova, V.; Zahradnickova, H.; Janous, D.; Skene, K. R.; Matharu, A. S.; Rejsek, K.; Formanek, P. The Significance Of D-Amino Acids in Soil, Fate and Utilization by Microbes and Plants: Review and Identification of Knowledge Gaps. Plant Soil. 2011, 354(1–2), 21–39. DOI: 10.1007/s11104-011-1059-5.
- Azam, F.; Malfatti, F. Microbial Structuring of Marine Ecosystems. Nat. Rev. Microbiol. 2007, 5(10), 782–791. DOI: 10.1038/nrmicro1747.
- Hedges, J. I. Why Dissolved Oganics Matter. Biogeochemistry of Marine Dissolved Organic Matter, Eds.; Hansell, DA.; Carlson, CA. Academic Press, 2002, pp 1–32. DOI: 10.1016/B978-0-12-323841-2.50020-8.
- Dafne, E. V.; Wangersky, P. J. A Brief Overview of Modern Directions in Marine DOC Studies Part II-recent Progress in Marine DOC Studies. J. Environ. Monit. 2002, 4(1), 55–69. From NLM Medline. DOI: 10.1039/b107279j.
- Pedersen, A. U.; Thomsen, T. R.; Lomstein, B. A.; Jørgensen, N. O. G. Bacterial Influence on Amino Acid Enantiomerization in a Coastal Marine Sediment. Limnol. Oceanogr. 2001, 46(6), 1358–1369. DOI: 10.4319/lo.2001.46.6.1358.
- Felbeck, H.; Wiley, S. Free D-Amino Acids in the Tissues of Marine Bivalves. Biol. Bull. 1987, 173, 252–259.
- Brückner, H.; Westhauser, T. Chromatographic Determination of D-Amino Acids as Native Constituents of Vetetables and Fruits. Chromatographia. 1994, 39, 419–426.
- Brückner, H.; Langer, L.; Lüpke, M.; Westhauser, T.; Godel, H. Liquid Chromatographi Determination of Amino Acid Enantiomers by Derivatization with o-phthaldialdeyde and Chiral Thiols Applications with Reference to Food Science. J. Chromatogr. A. 1995, 697, 229–245.
- Brückner, H.; Westhauser, T. Chromatographic Determination of L- and D-Amino Acids in Plants. Amino Acids. 2003, 24(1–2), 43–55. From NLM Medline. DOI: 10.1007/s00726-002-0322-8.
- Gao, L.; Xu, P.; Ren, J. A Sensitive and Economical Method for Simultaneous Determination of D/L-Amino Acids Profile in Foods by HPLC-UV: Application in Fermented and Unfermented Foods Discrimination. Food Chem. 2023, 410, 135382. DOI: 10.1016/j.foodchem.2022.135382.
- Casado, F. J.; Sánchez, A. H.; Rejano, L.; Montaño, A. Amino Acid Formation in Sterilized Alkali-Treated Olives. J. Agric. Food. Chem. 2007, 55, 3503–3535–3507.
- Brückner, H.; Hausch, M. Gas Chromatographic Detection of D-Amino Acids as Common Constituents of Fermented Foods. Chromatographia. 1989, 28, 487–492.
- Brückner, H.; Hausch, M. Detection of Free Amino Acids in Food by Chiral Phase Capillary Gas Chromotography. J. High Reso. Chromato. 1989, 12, 680–684.
- Jin, D.; Miyahara, T. M.; Oe, T.; Toyo`Oka, T. Determination of D-Amino Acids Labeled with Fluorescent Chiral Reagents, R(2)- and S(1)-4-(3-Isothiocyanatopyrrolidin-1-yl)-7-(N,N-dimethylaminosulfonyl)-2,1,3-benzoxadiazoles, in Biological and Food Samples by Liquid Chromatography. Anal. Biochem. 1999, 269, 124–132.
- Danielsen, M.; Nebel, C.; Dalsgaard, T. K. Simultaneous Determination of L- and D-Amino Acids in Proteins: A Sensitive Method Using Hydrolysis in Deuterated Acid and Liquid Chromatography-Tandem Mass Spectrometry Analysis. Foods 2020, 9(3), From NLM PubMed-not-MEDLINE. DOI: 10.3390/foods9030309.
- Harada, M.; Karakawa, S.; Yamada, N.; Miyano, H.; Shimbo, K. Biaryl Axially Chiral Derivatizing Agent for Simultaneous Separation and Sensitive Detection of Proteinogenic Amino Acid Enantiomers Using Liquid chromatography-tandem Mass Spectrometry. J. Chromatogr. A. 2019, 1593, 91–101. From NLM Medline. DOI: 10.1016/j.chroma.2019.01.075.
- Mori, M.; Ito, Y.; Nagasawa, T. Content of Free D-Ala and D-Glu in Traditional Asian Fermented Seasonings. J. Nurt. Sci. Vitaminol. 2010, 56, 428–435.
- Sakamoto, T.; Onozato, M.; Uekusa, S.; Ichiba, H.; Umino, M.; Shirao, M.; Fukushima, T. Development of Derivatization Reagents Bearing Chiral 4-Imidazolidinone for Distinguishing Primary Amines from Other Amino Acids and Application to the Liquid Chromatography-Tandem Mass Spectrometric Analysis of Miso. J. Chromatogr. A. 2021, 1652, 462341. DOI: 10.1016/j.chroma.2021.462341.
- Gobbetti, M.; Simonetti, M. S.; Rossi, J.; Cossignani, L.; Corsetti, A.; Damiani, P. Free D- and L-Amino Acid Evolution During Sourdough Fermentation and Baking. J. Food Sci. 1994, 59(4), 881–884. DOI: 10.1111/j.1365-2621.1994.tb08149.x.
- Rubio-Barroso, S.; Santos-Delgado, M. J.; Martin-Olivar, C.; Polo-Diez, L. M. Indirect Chiral HPLC Determination and Fluorimetric Detection of D-Amino Acids in Milk and Oyster Samples. J. Dairy. Sci. 2006, 89, 82–89.
- Nakano, Y.; Taniguchi, M.; Fukusaki, E. High-Sensitive Liquid Chromatography-Tandem Mass Spectrometry-Based Chiral Metabolic Profiling Focusing on Amino Acids and Related Metabolites. J. Biosci. Bioeng. 2019, 127(4), 520–527. DOI: 10.1016/j.jbiosc.2018.10.003.
- Csapó, J.; Albert, C.; Csapó-Kiss, Z. The D-Amino Acid Content of Foodstuffs (A Review). Acta Univ. Sapientiae, Alimentaria. 2009, 2, 5–30.
- Csapo, J.; Varga-Visi, E.; Loki, K.; Albert, C. The Influence of Manufacture on the Free D-Amino Acid Content of Cheddar Cheese. Amino Acids. 2006, 32(1), 39–43. From NLM Medline. DOI: 10.1007/s00726-006-0355-5.
- Abe, H.; Park, J. N.; Fukumoto, Y.; Fujita, E.; Tanaka, T.; Washio, T.; Otsuka, S.; Shimizu, T.; Watanabe, K. Occurrence of D-Amino Acids in Fish Sauces and Other Fermented Fish Products. Fish. Sci. 1999, 65(4), 637–641.
- Xu, Y.; Liu, Z.; Liu, Z.; Feng, Z.; Zhang, L.; Wan, X.; Yang, X. Identification of D-Amino Acids in Tea Leaves. Food Chem. 2020, 317, 126428. From NLM Medline. DOI: 10.1016/j.foodchem.2020.126428.
- Erbe, T.; Brückner, H. Chromatographic Determination of Amino Acid Enantiomers in Beers and Raw Materials Used for Their Manufacture. J. Chromatogr. A. 2000, 881(1–2), 81–91. DOI: 10.1016/S0021-9673(00)00255-7.
- Kato, M.; Fukushima, T.; Santa, T.; Homma, H.; Imai, K. Determination of D-Amino Acids, Derivatized with 4-fluoro-7-nitro-2,1,3-benzoxadiazole (NBD-F), in Wine Samples by high-performance Liquid Chromatography. Biomed. Chromatogr. 1995, 9(4), 193–194. From NLM Medline. DOI: 10.1002/bmc.1130090409.
- Barbas-Bernardos, C.; Garcia-Perez, I.; Lorenzo, M. P.; Alonso-Herranz, V.; Nicholson, J.; Garcia, A. Development and Validation of a High Performance Liquid chromatography-tandem Mass Spectrometry Method for the Absolute Analysis of 17 α-D-Amino Acids in Cooked Meals. J. Chromatogr. A. 2020, 1611, 460598. From NLM Medline. DOI: 10.1016/j.chroma.2019.460598.
- Brückner, H.; Becker, D.; Lupke, M. Chirality of Amino Acids of Microorganisms Used in Food Biotechnology. Chirality. 1993, 5(5), 385–392. DOI: 10.1002/chir.530050521.
- Martinez-Rodriguez, S.; Martinez-Gomez, A. I.; Rodriguez-Vico, F.; Clemente-Jimenez, J. M.; Las Heras-Vazquez, F. J. Natural Occurrence and Industrial Applications of D-Amino Acids: An Overview. Chem. Biodivers. 2010, 7(6), 1531–1548. From NLM Medline. DOI: 10.1002/cbdv.200900245.
- Friedman, M.; Levin, C. E. Nutritional and Medicinal Aspects of D-Amino Acids. Amino Acids. 2012, 42(5), 1553–1582. From NLM Medline. DOI: 10.1007/s00726-011-0915-1.
- Tian, H.; Zheng, N.; Li, S.; Zhang, Y.; Zhao, S.; Wen, F.; Wang, J. Characterization of Chiral Amino Acids from Different Milk Origins Using ultra-performance Liquid Chromatography Coupled to ion-mobility Mass Spectrometry. Sci. Rep. 2017, 7, 46289. From NLM Medline. DOI: 10.1038/srep46289.
- Palla, G.; Marchelli, R.; Dossena, A.; Casnati, G. Occurrence of D-Amino Acids in Food. Detection by Capillary Gas Chromatography and by reversed-phase high-performance Liquid Chromatography with L-phenylalanin amides as Chiral Selectors. J. Chromatogr. 1989, 475, 45–53.
- Fujii, T.; Yamauchi, T.; Ishiyama, M.; Gogami, Y.; Oikawa, T.; Hata, Y. Crystallographic Studies of Aspartate Racemase from Lactobacillus Sakei NBRC 15893. Acta Crystallogr F Struct. Biol. Commun. 2015, 71(Pt 8), 1012–1016. From NLM Medline. DOI: 10.1107/S2053230X15010572.
- Friedman, M. C. Nutrition, and Microbiology of D-Amino Acids. J. Agric. Food. Chem. 1999, 47, 3457–3479.
- Lüpke, M.; Brückner, H. Gas Chromatographic Evaluation of Amino Acid Epimerisation in the Course of Gelatin Manufacturing and Processing. Z Lebensm Unters Forsch A. 1998, 206(5), 323–328. DOI: 10.1007/s002170050266.
- Masters, P. M.; Friedman, M. Racemization of Amino Acids in Alkali-Treated Food Proteins. J. Agríe. Food Chem. 1979, 27, 508–511.
- Haysahi, R.; Kameda, I. Racemization of Amino Acid Residues during Alkali-Treatment of Protein and Its Adverse Effect on Pepsin Digestibility. Agric Biol Chem. 1980, 44(4), 891–895.
- Hayashi, R.; Kameda, I. Conditions of Lysinoalanine Formation during Ecposure of Protein to Alkali. Agric Biol Chem. 1980, 44(1), 175–181.
- Friedman, M.; Liardon, R. Racemization Kinetics of Amino Acid Residues in Alkali-Treated Soybean Proteins. J. Agric. Food. Chem. 1985, 33(4), 666–672. DOI: 10.1021/jf00064a025.
- Haysahi, R.; Kameda, I. Decreased Proteolysis of Alkali-Treated Protein: Consequences of Racemization in Food Processing. J. Food Sci. 1980, 45, 1430.
- Torii, K Brain Activation by the Umami Taste Substance Monosodium L-glutamate via Gustatory and Visceral Signaling Pathways, and Its Physiological Significance Due to Homeostasis after a Meal. J. Oral Biosci. 2012, 54(3), 144–150. DOI: 10.1016/j.job.2012.03.005.
- Popkin, B. Ultra-processed Foods´ Impacts on Health. 2030. Food, Agriculture and rural development in Latin America and the Caribbean, No. 34. Santiago de Chile. FAO 2020.
- Brückner, H.; Jaek, P.; Langer, M.; Godel, H. Liquid Chromatographic Determination of D-Amino Acids in Cheese and Wow Milk. Implication of Starter Cultures, Amino Acid Racemases, and Rumen Microorganisms on Formation, and Nutritional Considerations. Amino Acids. 1992, 2, 271–284.
- Garcia-Perez, I.; Posma, J. M.; Gibson, R.; Chambers, E. S.; Hansen, T. H.; Vestergaard, H.; Hansen, T.; Beckmann, M.; Pedersen, O.; Elliott, P., et al. Objective Assessment of Dietary Patterns by Use of Metabolic Phenotyping: A Randomised, Controlled, Crossover Trial. Lancet Diabetes Endocrinol. 2017, 5(3), 184–195. DOI: 10.1016/S2213-8587(16)30419-3. From NLM Medline.
- Carabotti, M.; Scirocco, A.; Maselli, M. A.; Serveri, C. The gut-brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterology. 2015, 28, 203–209.
- Human Microbiome Project C. Structure, Function and Diversity of the Healthy Human Microbiome. Nature. 2012, 486(7402), 207–214. From NLM Medline. DOI: 10.1038/nature11234.
- Hancock, R. The Amino Acid Composition of the Protein and Cell Wall of Staphylococcus Aureus. Biochim. Biophys. Acta. 1960, 37(1), 42–46. DOI: 10.1016/0006-3002(60)90076-7.
- Vollmer, W.; Blanot, D.; de Pedro, M. A. Peptidoglycan Structure and Architecture. FEMS Microbiol. Rev. 2008, 32(2), 149–167. DOI: 10.1111/j.1574-6976.2007.00094.x.
- Heijenoort, J. V. Formation of the Glycan Chains in the Synthesis of Bacterial Peptidoglycan. Glycobiology. 2001, 11(3), 25R–36R. DOI: 10.1093/glycob/11.3.25R.
- Kolodkin-Gal, I.; Romero, D.; Cao, S.; Clardy, J.; Kolter, R.; Losick, R. D-Amino Acids Trigger Biofilm Disassembly. Science. 2010, 328, 627–630.
- Latteyer, F.; Peisert, H.; Gohring, N.; Peschel, A.; Chasse, T. Vibrational and Electronic Characterisation of Staphylococcus Aureus Wall Teichoic Acids and Relevant Components in Thin Films. Anal. Bioanal. Chem. 2010, 397(6), 2429–2437. DOI: 10.1007/s00216-010-3832-3.
- Lupoli, T. J.; Tsukamoto, H.; Doud, E. H.; Wang, T. S.; Walker, S.; Kahne, D. Transpeptidase-mediated Incorporation of D-Amino Acids into Bacterial Peptidoglycan. J. Am. Chem. Soc 2011, 133(28), 10748–10751. From NLM Medline. DOI: 10.1021/ja2040656.
- Connolly, J. P.; Goldstone, R. J.; Burgess, K.; Cogdell, R. J.; Beatson, S. A.; Vollmer, W.; Smith, D. G.; Roe, A. J. The Host Metaboliteserine Contributes to Bacterial Niche Specificity through Gene Selection. ISME J. 2015, 9(4), 1039–1051. From NLM Medline. DOI: 10.1038/ismej.2014.242.
- Scheffers, D. J.; Pinho, M. G. Bacterial Cell Wall Synthesis: New Insights from Localization Studies. Microbiol. Mol. Biol. Rev. 2005, 69(4), 585–607. DOI: 10.1128/MMBR.69.4.585-607.2005.
- Donaldson, G. P.; Lee, S. M.; Mazmanian, S. K. Gut Biogeography of the Bacterial Microbiota. Nat. Rev. Microbiol. 2016, 14(1), 20–32. DOI: 10.1038/nrmicro3552.
- Gonda, Y.; Matsuda, A.; Adachi, K.; Ishii, C.; Suzuki, M.; Osaki, A.; Mita, M.; Nishizaki, N.; Ohtomo, Y.; Shimizu, T., et al. Mammals Sustain Amino Acid Homochirality Against Chiral Conversion by Symbiotic Microbes. Proc. Natl. Acad. Sci. U. S. A. 2023, 120(15), e2300817120.
- Matsumoto, M.; Kunisawa, A.; Hattori, T.; Kawana, S.; Kitada, Y.; Tamada, H.; Kawano, S.; Hayakawa, Y.; Iida, J.; Fukusaki, E. Free D-Amino Acids Produced by Commensal Bacteria in the Colonic Lumen. Sci. Rep. 2018, 8(1), 17915. From NLM Medline. DOI: 10.1038/s41598-018-36244-z.
- Lee, C. J.; Qiu, T. A.; Hong, Z.; Zhang, Z.; Min, Y.; Zhang, L.; Dai, L.; Zhao, H.; Si, T.; Sweedler, J. V. Profiling of D-Alanine Production by the Microbial Isolates of Rat Gut Microbiota. FASEB. J. 2022, 36(8), e22446. From NLM Medline. DOI: 10.1096/fj.202101595R.
- Sharma, A. Y. S. Biofilms: Microbes and Disease. The Braz. J. Infect. Dis. 2008, 12(6), 526–530.
- Ehmsen, J. T.; Ma, T. M.; Sason, H.; Rosenberg, D.; Ogo, T.; Furuya, S.; Snyder, S. H.; Wolosker, H. D-Serine in Glia and Neurons Derives from 3-phosphoglycerate Dehydrogenase. J. Neurosci. 2013, 33(30), 12464–12469. From NLM Medline. DOI: 10.1523/JNEUROSCI.4914-12.2013.
- Ramon-Perez, M. L.; Diaz-Cedillo, F.; Ibarra, J. A.; Torales-Cardena, A.; Rodriguez-Martinez, S.; Jan-Roblero, J.; Cancino-Diaz, M. E.; Cancino-Diaz, J. C. D-Amino Acids Inhibit Biofilm Formation in Staphylococcus Epidermidis Strains from Ocular Infections. J. Med. Microbiol. 2014, 63(Pt 10), 1369–1376. From NLM Medline. DOI: 10.1099/jmm.0.075796-0.
- Wong, F. H.; Chen, J. S.; Reddy, V.; Day, J. L.; Shlykov, M. A.; Wakabayashi, S. T.; Saier, M. H., Jr. The Amino acid-polyamine-organocation Superfamily. J. Mol. Microbiol. Biotechnol. 2012, 22(2), 105–113. From NLM Medline. DOI: 10.1159/000338542.
- Verrey, F.; Closs, E. I.; Wagner, C. A.; Palacin, M.; Endou, H.; Kanai, Y. CATs and HATs: The SLC7 Family of Amino Acid Transporters. Pflugers Arch. 2004, 447(5), 532–542. DOI: 10.1007/s00424-003-1086-z.
- Perez, C.; Koshy, C.; Ressl, S.; Nicklisch, S.; Kramer, R.; Ziegler, C. Substrate Specificity and Ion Coupling in the Na+/betaine Symporter BetP. EMBO J. 2011, 30(7), 1221–1229. DOI: 10.1038/emboj.2011.46.
- Lam, H.; Oh, D. C.; Cava, F.; Takacs, C. N.; Clardy, J.; Pedro, M. A. D.; Waldor, M. K. D-Amino Acids Govern Stationary Phase Cell Wall Remodeling in Bacteria. Science. 2009, 325, 1552–1556.
- Yoshimura, T.; Eskai, N. Amino Acid Racemases: Functions and Mechanisms. J. Biosci. Bioeng. 2003, 96, 103–109.
- Okada, H.; Yohda, M.; Giga-Hama, Y.; Ueno, Y.; Ohdo, S.; Kumagai, H. Distribution and Purification of Aspartatc Raccmase in Lactic Acid Bacteria. Biochim. Biophys. Acta. 1991, 1078, 377–382.
- Lambert, M. P.; Neuhaus, F. C. Factors Affecting the Level of Alanine Racemase in Escherichia Coli. J. Bacteriol. 1972, 109(3), 1156–1161. DOI: 10.1128/jb.109.3.1156-1161.1972.
- Wei, Y.; Qiu, W.; Zhou, X. D.; Zheng, X.; Zhang, K. K.; Wang, S. D.; Li, Y. Q.; Cheng, L.; Li, J. Y.; Xu, X., et al. Alanine Racemase Is Essential for the Growth and Interspecies Competitiveness of Streptococcus Mutans. Int. J. Oral Sci. 2016, 8(4), 231–238. From NLM Medline. DOI: 10.1038/ijos.2016.34.
- Espaillat, A.; Carrasco-Lopez, C.; Bernardo-Garcia, N.; Pietrosemoli, N.; Otero, L. H.; Alvarez, L.; de Pedro, M. A.; Pazos, F.; Davis, B. M.; Waldor, M. K., et al. Structural Basis for the Broad Specificity of a New Family of amino-acid Racemases. Acta Crystallogr. D Biol. Crystallogr. 2014, 70(Pt 1), 79–90. DOI: 10.1107/S1399004713024838. From NLM Medline.
- Miyamoto, T.; Katane, M.; Saitoh, Y.; Sekine, M.; Homma, H. Cystathionine β-lyase Is Involved in D-Amino Acid Metabolism. Biochem J. 2018, 475(8), 1397–1410. From NLM Medline. DOI: 10.1042/BCJ20180039.
- Miyamoto, T.; Katane, M.; Saitoh, Y.; Sekine, M.; Homma, H. Identification and Characterization of Novel Broad-Spectrum Amino Acid Racemases from Escherichia Coli and Bacillus Subtilis. Amino Acids. 2017, 49(11), 1885–1894. DOI: 10.1007/s00726-017-2486-2.
- Tomlinson, C.; Rafii, M.; Ball, R. O.; Pencharz, P. The Significance of isomers in Stable Isotope Studies in Humans Is Dependent on the Age of the Subject and the Amino Acid Tracer. Metabolism. 2010, 59(1), 14–19. From NLM Medline. DOI: 10.1016/j.metabol.2009.06.024.
- Verrall, L.; Burnet, P. W.; Betts, J. F.; Harrison, P. J. The Neurobiology of D-Amino Acid Oxidase and Its Involvement in Schizophrenia. Mol. Psychiatry. 2010, 15(2), 122–137. From NLM Medline. DOI: 10.1038/mp.2009.99.
- Yamada, R. H.; Nagasaki, H.; Wakabayashi, Y.; Iwashima, A. Presence of Aspartate Oxidase in Rat Liver and Mouse Tissues. Biochem. Biophys. Acta. 1988, 965, 202–205.
- Dixon, M.; Kenworthy, P. D-Aspartate Oxidase of Kidney. Biochem. Biophys. Acta. 1967, 146, 54–76.
- Hamase, K.; Konno, R.; Morikava, A.; Zaitsu, K. Sensitive Determination of D-Amino Acids in Mammals and the Effect of D-Amino-Acid Oxidase Activity on Their Amounts. Biol. Pharm. Bull 2005, 28(9), 1578–1584.
- Ishii, C.; Akita, T.; Mita, M.; Ide, T.; Hamase, K. Development of an Online Two-Dimensional High-Performance Liquid Chromatographic System in Combination with Tandem Mass Spectrometric Detection for Enantiomeric Analysis of Free Amino Acids in Human Physiological Fluid. J. Chromatogr. A. 2018, 1570, 91–98. DOI: 10.1016/j.chroma.2018.07.076.
- Suzuki, M.; Imanishi, N.; Mita, M.; Hamase, K.; Aiso, S.; Sasabe, J. Heterogeneity of D-Serine Distribution in the Human Central Nervous System. ASN neuro. 2017, 9(3), 1759091417713905. From NLM Medline. DOI: 10.1177/1759091417713905.
- Schell, M. J.; Cooper, O. B.; Snyder, S.H. D-Aspartate Localizations Imply Neuronal and Neuroendocrine Roles. Proc. Natl. Acad. Sci. USA. 1997, 94, 2013–2018.
- Morikawa, A.; Fukuoka, H.; Uezono, K.; Mita, M.; Koyanagi, S.; Ohdo, S.; Zaitsu, K.; Hamase, K. Sleep-Awake Profile Related Circadian D-Alanine Rhythm in Human Serum and Urine. Chromatography. 2017, 38(2), 53–58. DOI: 10.15583/jpchrom.2017.003.
- Armstrong, D. W.; Duncan, J. D.; Lee, S. H. Evaluation Of D-Amino Acid Levels in Human Urine and in Commercial L-amino Acid Samples. Amino Acids. 1991, 1, 97–106.
- Harada, M.; Karakawa, S.; Miyano, H.; Shimbo, K. Simultaneous Analysis of D,L-Amino Acids in Human Urine Using a Chirality-Switchable Biaryl Axial Tag and Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry. Symmetry. 2020, 12, 6. DOI: 10.3390/sym12060913.
- Nagata, Y.; Higashi, M.; Ishii, Y.; Sano, H.; Tanigawa, M.; Nagata, K.; Noguchi, K.; Urade, M. The Presence of High Concentrations of Free D-Amino Acids in Human Saliva. Life. sci. 2006, 78(15), 1677–1681. From NLM Medline. DOI: 10.1016/j.lfs.2005.08.009.
- Nagata, Y.; Sato, T.; Enomoto, N.; Ishii, Y.; Sasaki, K.; Yamada, T. High Concentrations Of D-Amino Acids in Human Gastric Juice. Amino Acids. 2007, 32(1), 137–140. From NLM Medline. DOI: 10.1007/s00726-006-0262-9.
- Huang, Y.; Nishikava, T.; Satoh, K.; Iwata, T.; Fykyshima, T.; Santa, T. H. H.; Imai, K. Urinary Excretion of D-Serine in Human: Comparison of Different Ages and Species. Biol. Pharm. Bull 1998, 21, 156–162.
- Fisher, G. H.; Petrucelli, L.; Gardner, C.; Emory, C.; Frey, W. H.; Amaducci, L.; Sorbi, S.; Sorrentino, G.; Borghi, M.; D`Aniello, A. Free D-Amino Acids in Human Cerebrospinal Fluid of Alzheimer Disease, Multiple Sclerosis, and Healthy Control Subjects. Mol & Chem. Neuropathology. 1994, 23, 115–124.
- Karakawa, S.; Shimbo, K.; Yamada, N.; Mizukoshi, T.; Miyano, H.; Mita, M.; Lindner, W.; Hamase, K. Simultaneous Analysis of D-Alanine, D-Aspartic Acid, and D-Serine Using Chiral high-performance Liquid chromatography-tandem Mass Spectrometry and Its Application to the Rat Plasma and Tissues. J. Pharm. Biomed. Anal 2015, 115, 123–129. From NLM Medline. DOI: 10.1016/j.jpba.2015.05.024.
- Kiriyama, Y.; Nochi, H. D-Amino Acids in the Nervous and Endocrine Systems. Scientifica (Cairo). 2016, 2016, 6494621. From NLM PubMed-not-MEDLINE. DOI: 10.1155/2016/6494621.
- Masters, P. M.; Bada, J. L.; Zigler, J. S. Aspartic Acid Racemisation in the Human Lens During Ageing and in Cataract Formation. Nature. 1977, 268(5615), 71–73. DOI: 10.1038/268071a0.
- Luykx, J. J.; Bakker, S. C.; Visser, W. F.; Verhoeven-Duif, N.; Buizer-Voskamp, J. E.; den Heijer, J. M.; Boks, M. P.; Sul, J. H.; Eskin, E.; Ori, A. P., et al. Genome-wide Association Study of NMDA Receptor Coagonists in Human Cerebrospinal Fluid and Plasma. Mol. Psychiatry. 2015, 20(12), 1557–1564. DOI: 10.1038/mp.2014.190. From NLM Medline.
- Kimura, T.; Hesaka, A.; Isaka, Y. D-Amino Acids and Kidney Diseases. Clin. Exp. Nephrol. 2020, 24(5), 404–410. From NLM Medline. DOI: 10.1007/s10157-020-01862-3.
- Luykx, J. J.; Bakker, S. C.; van Boxmeer, L.; Vinkers, C. H.; Smeenk, H. E.; Visser, W. F.; Verhoeven-Duif, N. M.; Strengman, E.; Buizer-Voskamp, J. E.; de Groene, L., et al. D-Amino Acid Aberrations in Cerebrospinal Fluid and Plasma of Smokers. Neuropsychopharmacology. 2013, 38(10), 2019–2026. DOI: 10.1038/npp.2013.103. From NLM Medline.
- Lin, C. H.; Yang, H. T.; Chiu, C. C.; Lane, H. Y. Blood Levels Of D-Amino Acid Oxidase Vs. D-Amino Acids in Reflecting Cognitive Aging. Sci. Rep. 2017, 7(1), 14849. From NLM Medline. DOI: 10.1038/s41598-017-13951-7.
- Hashimoto, K.; Fukushima, T.; Shimizu, E.; Kosatsu, N.; Watanabe, H.; Shinoda, N.; Nakazato, M.; Kumakiri, C.; Okada, S. I.; Hasegawa, H., et al. Decreased Serum Levels Of D-Serine in Patients with Schizophrenia. Arch. Gen. Psychiatry. 2003, 60, 572–576.
- Hashimoto, A.; Chiba, S. Effect of Systemic Administration Of D-Serine on the Levels D- of and L-Serine in Several Brain Areas and Periphery of Rat. Eur. J. Pharmacol. 2004, 495(2–3), 153–158. From NLM Medline. DOI: 10.1016/j.ejphar.2004.05.036.
- Grant, S. L.; Shulman, Y.; Tibbo, P.; Hampson, D. R.; Baker, G. B. Determination Of D-Serine and Related Neuroactive Amino Acids in Human Plasma by high-performance Liquid Chromatography with Fluorimetric Detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 844(2), 278–282. From NLM Medline. DOI: 10.1016/j.jchromb.2006.07.022.
- Nagata, Y.; Masui, R.; Akino, T. The Presence of Free D-Serine, D-Alanine And D-Proline in Human Plasma. Experientia. 1992, 48, 986–988.
- Biemans, E. A.; Verhoeven-Duif, N. M.; Gerrits, J.; Claassen, J. A.; Kuiperij, H. B.; Verbeek, M. M. CSF D-Serine Concentrations are Similar in Alzheimer’s Disease, Other Dementias, and Elderly Controls. Neurobiol. Aging 2016, 42, 213–216. From NLM Medline. DOI: 10.1016/j.neurobiolaging.2016.03.017.
- Karakawa, S.; Miyoshi, Y.; Konno, R.; Koyanagi, S.; Mita, M.; Ohdo, S.; Hamase, K. Two-dimensional high-performance Liquid Chromatographic Determination of day-night Variation Of D-Alanine in Mammals and Factors Controlling the Circadian Changes. Anal. Bioanal. Chem 2013, 405(25), 8083–8091. From NLM Medline. DOI: 10.1007/s00216-013-7071-2.
- Punzo, D.; Errico, F.; Cristino, L.; Sacchi, S.; Keller, S.; Belardo, C.; Luongo, L.; Nuzzo, T.; Imperatore, R.; Florio, E., et al. Age-Related Changes In D-Aspartate Oxidase Promoter Methylation Control Extracellular D-Aspartate Levels and Prevent Precocious Cell Death during Brain Aging. J. Neurosci. 2016, 36(10), 3064–3078.
- Kumashiro, S.; Hashimoto, A.; Nishikawa, T. Free D-Serine in post-mortem Brains And Spinal Cords of Individuals with and without Neuropsychiatric Diseases. Brain. res. 1995, 681, 117–125.
- Semenza, E. R.; Harraz, M. M.; Abramson, E.; Malla, A. P.; Vasavda, C.; Gadalla, M. M.; Kornberg, M. D.; Snyder, S. H.; Roychaudhuri, R. D-Cysteine is an Endogenous Regulator of Neural Progenitor Cell Dynamics in the Mammalian Brain. Proc. Natl. Acad. Sci. U. S. A 2021, 118, 39. From NLM Medline. DOI: 10.1073/pnas.2110610118.
- Hamase, K.; Inoue, T.; Morikawa, A.; Konno, R.; Zaitsu, K. Determination of Free D-Proline And D-Leucine in the Brains of Mutant Mice Lacking D-Amino Acid Oxidase Activity. Anal. Biochem. 2001, 298(2), 253–258. From NLM Medline. DOI: 10.1006/abio.2001.5382.
- Kera, Y.; Aoyama, H.; Matsumura, H.; Hasegawa, A.; Nagasaki, H.; Yamada, R. H. Presence of Free D-Glutamate And D-Aspartate in Rat Tissues. Biochem. Biophys. Acta. 1995, 1243, 282–286.
- Hashimoto, A.; Kumashiro, S.; Nishikawa, T.; Oka, T.; Takahashi, K.; Mito, T.; Takashima, S.; Doi, N.; Mizutani, Y.; Yamazaki, T., et al. Embryonic Development and Postnatal Changes in Free D-Aspartate And D-Serine in the Human Prefrontal Cortex. J. Neurochem. 1993, 61(1), 348–351. DOI: 10.1111/j.1471-4159.1993.tb03575.x. From NLM Medline.
- Wolosker, H.; Dumin, E.; Balan, L.; Foltyn, V. N. D-Amino Acids in the Brain: D-Serine in Neurotransmission and Neurodegeneration. FEBS J. 2008, 275(14), 3514–3526. From NLM Medline. DOI: 10.1111/j.1742-4658.2008.06515.x.
- Schell, M. J. B. R. O.; Moliver, M. E.; Snyder, S. H. D-Serine as a Neuromodulator: Regional and Developmental Localizations in Rat Brain Glia Resemble NMDA Receptors. J. Neurosci. 1997, 17(5), 1604–1615.
- Berg, C. P. Physiology of the D-Amino Acids. The Amer. Physio. Soc. 1953, 33, 145–189.
- Ota, N.; Shi, T.; Sweedler, J. V. D-Aspartate Acts as a Signaling Molecule in Nervous and Neuroendocrine Systems. Amino Acids. 2012, 43(5), 1873–1886. From NLM Medline. DOI: 10.1007/s00726-012-1364-1.
- Albanese, A. A. The Amino Acid Requirements of Man. Adv. Protein Chem. 1947, 3, 227–268. From NLM Medline. DOI: 10.1016/s0065-3233(08)60081-9.
- Albanese, A. A.; Irby, V.; Lein, M.; Frankston, J. E. The Utilization of D-Amino Acid by Man. III. Arginine. J. Biol. Chem. 1945, 160(1), 25–30. DOI: 10.1016/s0021-9258(18)43093-1.
- Raj, D.; Langford, M.; Krueger, S.; Shelton, M.; Welbourne, T. Regulatory Responses to an Oral D-Glutamate Load: Formation of D-Pyrrolidone Carboxylic Acid in Humans. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E214–E220.
- Foltyn, V. N.; Bendikov, I.; De Miranda, J.; Panizzutti, R.; Dumin, E.; Shleper, M.; Li, P.; Toney, M. D.; Kartvelishvily, E.; Wolosker, H. Serine Racemase Modulates Intracellular D-Serine Levels through an α,β-elimination Activity. J. Biol. Chem. 2005, 280(3), 1754–1763. From NLM Medline. DOI: 10.1074/jbc.M405726200.
- Xia, M.; Liu, Y.; Figueroa, D. J.; Chiu, C. S.; Wei, N.; Lawlor, A. M.; Lu, P.; Sur, C.; Koblan, K. S.; Connolly, T. M. Characterization and Localization of a Human Serine Racemase. Mol. Brain Res. 2004, 125(1–2), 96–104. DOI: 10.1016/j.molbrainres.2004.03.007.
- Horio, M.; Kohno, M.; Fujita, Y.; Ishima, T.; Inoue, R.; Mori, H.; Hashimoto, K. Levels Of D-Serine in the Brain and Peripheral Organs of Serine Racemase (Srr) knock-out Mice. Neurochem. Int. 2011, 59(6), 853–859. From NLM Medline. DOI: 10.1016/j.neuint.2011.08.017.
- Nong, Y.; Huang, Y. Q.; Ju, W.; Kalia, L. V.; Ahmadian, G.; Wang, Y. T.; Salter, M. W. Glycine Binding Primes NMDA Receptor Internalization. Nature. 2003, 422(6929), 302–307. DOI: 10.1038/nature01497.
- McKay, S.; Ryan, T. J.; McQueen, J.; Indersmitten, T.; Marwick, K. F. M.; Hasel, P.; Kopanitsa, M. V.; Baxter, P. S.; Martel, M. A.; Kind, P. C., et al. The Developmental Shift of NMDA Receptor Composition Proceeds Independently of GluN2 Subunit-Specific GluN2 C-Terminal Sequences. Cell Rep. 2018, 25(4), 841–851 e844. DOI: 10.1016/j.celrep.2018.09.089. From NLM Medline.
- Bauch, C.; Forster, N.; Loffing-Cueni, D.; Summa, V.; Verrey, F. Functional Cooperation of Epithelial Heteromeric Amino Acid Transporters Expressed in Madin-Darby Canine Kidney Cells. J. Biol. Chem. 2003, 278(2), 1316–1322. DOI: 10.1074/jbc.M210449200.
- Ito, T.; Hayashida, M.; Kobayashi, S.; Muto, N.; Hayashi, A.; Yoshimura, T.; Mori, H. Serine Racemase Is Involved In D-Aspartate Biosynthesis. J. biochem. 2016, 160(6), 345–353. From NLM Medline. DOI: 10.1093/jb/mvw043.
- Kim, P. M.; Duan, X.; Huang, A. S.; Liu, C. Y.; Ming, G. L.; Song, H.; Snyder, S. H. Aspartate Racemase, Generating Neuronal D-Aspartate, Regulates Adult Neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 2010, 107(7), 3175–3179. From NLM Medline. DOI: 10.1073/pnas.0914706107.
- Leung, J. C.; Travis, B. R.; Verlander, J. W.; Sandhu, S. K.; Yang, S. G.; Zea, A. H.; Weiner, I. D.; Silverstein, D. M. Expression and Developmental Regulation of the NMDA Receptor Subunits in the Kidney and Cardiovascular System. Am J. Physiol. Regulatory. Integrative Comp. Physiol. 2002, 283, R971–R971.
- Matsui, T.; Sekiguchi, M.; Hashimoto, A.; Tomita, U.; Nishikawa, T.; Wada, K. Functional Comparison Of D-Serine and Glycine in Rodents: The Effect on Cloned NMDA Receptors and the Extracellular Concentration. J. Neurochem. 1995, 65(1), 454–458. From NLM Medline. DOI: 10.1046/j.1471-4159.1995.65010454.x.
- Consortium, G. T. The Genotype-Tissue Expression (Gtex) Project. Nat. Genet. 2013, 45(6), 580–585. From NLM Medline. DOI: 10.1038/ng.2653.
- Zaar, K. Light and Electron Microscopic Localization Of D-Aspartate Oxidase in Peroxisomes of Bovine Kidney and Liver: An Immunocytochemical Study. The J. Histochem. & Cytochem. 1996, 44, 1013–1019.
- Sacchi, S.; Cappelletti, P.; Murtas, G. Biochemical Properties of Human D-Amino Acid Oxidase Variants and Their Potential Significance in Pathologies. Front. Mol. Biosci. 2018, 5, 55. From NLM PubMed-not-MEDLINE. DOI: 10.3389/fmolb.2018.00055.
- D’Aniello, A.; Vetere, A.; Petrucelli, L. Further Study on the Specificity Of D-Amino Acid Oxidase and of D-Aspartate Oxidase and Time Course for Complete Oxidation of D-Amino Acids. Comp. Biochem. Physiol. 1993, 105B, 731–734.
- Pollegioni, L.; Diederichs, K.; Molla, G.; Umhau, S.; Welte, W.; Ghisla, S.; Pilone, M. S. Yeast D-Amino Acid Oxidase: Structural Basis of Its Catalytic Properties. J. Mol. Biol. 2002, 324(3), 535–546. From NLM Medline. DOI: 10.1016/s0022-2836(02)01062-8.
- Fan, A.; Li, J. Y.; Yu, Y.; Zhang, D.; Nie, Y.; Xu, Y. Enzymatic Cascade Systems For D-amino acid Synthesis: Progress and Perspectives. Syst. Microbiol. Biomanufacturing. 2021, 1(4), 397–410. DOI: 10.1007/s43393-021-00037-9.
- Verrall, L.; Walker, M.; Rawlings, N.; Benzel, I.; Kew, J. N.; Harrison, P. J.; Burnet, P. W. D-Amino Acid Oxidase and Serine Racemase in Human Brain: Normal Distribution and Altered Expression in Schizophrenia. Eur. J. Neurosci. 2007, 26(6), 1657–1669. From NLM Medline. DOI: 10.1111/j.1460-9568.2007.05769.x.
- Wolosker, H.; Balu, D. T.; Coyle, J. T. The Rise and Fall of the D-serine-Mediated Gliotransmission Hypothesis. Trends Neurosci. 2016, 39(11), 712–721. From NLM Medline. DOI: 10.1016/j.tins.2016.09.007.
- Sasabe, J.; Suzuki, M.; Miyoshi, Y.; Tojo, Y.; Okamura, C.; Ito, S.; Konno, R.; Mita, M.; Hamase, K.; Aiso, S., James, L R. Ischemic Acute Kidney Injury Perturbs Homeostasis of Serine Enantiomers in the Body Fluid in Mice: Early Detection of Renal Dysfunction Using the Ratio of Serine Enantiomers. PLoS One. 2014, 9(1), e86504. DOI: 10.1371/journal.pone.0086504.
- Fukasawa, Y.; Segawa, H.; Kim, J. Y.; Chairoungdua, A.; Kim, D. K.; Matsuo, H.; Cha, S. H.; Endou, H.; Kanai, Y. Identification and Characterization of a Na+-independent Neutral Amino Acid Transporter that Associates with the 4F2 Heavy Chain and Exhibits Substrate Selectivity for Small Neutral and L-amino Acids. J. Biol. Chem. 2000, 275(13), 9690–9698. From NLM Medline. DOI: 10.1074/jbc.275.13.9690.
- Fujii, N. D-Amino Acids in Living Higher Organisms. Orig. Life & Evol. Biosphere. 2002, 32(2), 103–127. DOI: 10.1023/A:1016031014871.
- Irukayama-Tomobe, Y.; Tanaka, H.; Yokomizo, T.; Hashidate-Yoshida, T.; Yanagisawa, M.; Sakurai, T. Aromatic D-Amino Acids Act as Chemoattractant Factors for Human Leukocytes through a G protein-coupled Receptor, GPR109B. PNAS. 2009, 106, 3930–3934.
- Konno, R.; Niwa, A.; Yasumura, Y. Intestinal Bacterial Origin of D-alanine in Urine of Mutant Mice lacking D-Amino-Acid Oxidase. Biochem J. 1990, 268, 263–265.
- Usiello, A.; Di Fiore, M. M.; De Rosa, A.; Falvo, S.; Errico, F.; Santillo, A.; Nuzzo, T.; Chieffi Baccari, G. New Evidence on the Role of D-aspartate Metabolism in Regulating Brain and Endocrine System Physiology: From Preclinical Observations to Clinical Applications. Int. J. Mol. Sci. 2020, 21, 22. From NLM Medline. DOI: 10.3390/ijms21228718.
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A Novel Pathway for the Production of Hydrogen Sulfide From D-cysteine in Mammalian Cells. Nat. Commun. 2013, 4, 1366. From NLM Medline. DOI: 10.1038/ncomms2371.
- Iharada, K.; Hiasa, M.; Kobara, A.; Moriyama, Y. Exocytosis Of D-aspartate from INS-1E Clonal β Cells. Biol. Pharm. Bull 2007, 30(7), 1329–1331.
- Lee, C. J.; Qiu, T. A.; Sweedler, J. V. D-alanine: Distribution, Origin, Physiological Relevance, and Implications in Disease. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868(11), 140482. From NLM Medline. DOI: 10.1016/j.bbapap.2020.140482.
- Konno, R.; Yasumura, Y. Mouse Mutant Deficient In D-Amino Acid Oxidase Activity. Genetics. 1983, 103, 277–285.
- Hamase, K.; Takagi, S.; Morikawa, A.; Konno, R.; Niwa, A.; Zaitsu, K. Presence and Origin of Large Amounts Of D-proline in the Urine of Mutant Mice Lacking D-Amino Acid Oxidase Activity. Anal. Bioanal. Chem. 2006, 386(3), 705–711. From NLM Medline. DOI: 10.1007/s00216-006-0594-z.
- Basu, A. C.; Tsai, G. E.; Ma, C. L.; Ehmsen, J. T.; Mustafa, A. K.; Han, L.; Jiang, Z. I.; Benneyworth, M. A.; Froimowitz, M. P.; Lange, N., et al. Targeted Disruption of Serine Racemase Affects Glutamatergic Neurotransmission and Behavior. Mol. Psychiatry. 2009, 14(7), 719–727. DOI: 10.1038/mp.2008.130. From NLM Medline.
- Neufeld, K. M.; Kang, N.; Bienenstock, J.; Foster, J. A. Reduced anxiety-like Behavior and Central Neurochemical Change in germ-free Mice. Neurogastroenterol. Motility. 2011, 23(3), 255–264, e119. From NLM Medline. DOI: 10.1111/j.1365-2982.2010.01620.x.
- Heijtz, R. D.; Wang, S.; Anuar, F.; Qian, Y.; Bjorkholm, B.; Samuelsson, A.; Hibberd, M. L.; Forssberg, H.; Pettersson, S. Normal Gut Microbiota Modulates Brain Development and Behavior. Proc. Natl. Acad. Sci. U. S. A. 2011, 108(7), 3047–3052. DOI: 10.1073/pnas.1010529108.
- Morikawa, A.; Hamase, K.; Inoue, T.; Konno, R.; Zaitsu, K. Alterations In D-amino acid Levels in the Brains of Mice and Rats after the Administration of D-amino acids. Amino Acids. 2007, 32(1), 13–20. From NLM Medline. DOI: 10.1007/s00726-005-0357-8.
- Fujii, N. D-amino acid Biosystem. Biol. Pharm. Bull 2005, 28(9), 1585–1589.
- Balu, D. T.; Takagi, S.; Puhl, M. D.; Benneyworth, M. A.; Coyle, J. T. D-serine and Serine Racemase are Localized to Neurons in the Adult Mouse and Human Forebrain. Cell Mol Neurobiol. 2014, 34(3), 419–435. From NLM Medline. DOI: 10.1007/s10571-014-0027-z.
- Lyons, B.; Jamie, J. F.; Truscott, R. J. Separate Mechanisms for age-related Truncation and Racemisation of peptide-bound Serine. Amino Acids. 2014, 46(1), 199–207. From NLM Medline. DOI: 10.1007/s00726-013-1619-5.
- Fisher, G. H.; D`Aniello, A.; Vetere, A.; Cysano, G.; Chávez, M.; Petrucelli, L. Quantification of D-Aspartate in Normal and Alzheimer Brains. Neurosci. Lett. 1992, 143, 215–218.
- Zheng, Y.; Mao, K.; Chen, S.; Zhu, H. Chirality Effects in Peptide Assembly Structures. Front. Bioeng. Biotechnol. 2021, 9, 703004. DOI: 10.3389/fbioe.2021.703004.
- Aso, K.; Nishigawa, T.; Nagamachi, S.; Takakura, M.; Furuse, M. Orally Administrated D-Arginine Exhibits Higher Enrichment in the Brain and Milk than L-Arginine in ICR Mice. J. Vet. Med. Sci. 2020, 82(3), 307–313. From NLM Medline. DOI: 10.1292/jvms.19-0630.
- Broer, S. Amino Acid Transport Across Mammalian Intestinal and Renal Epithelia. Physiol. Rev. 2008, 88(1), 249–286. DOI: 10.1152/physrev.00018.2006.
- Kanai, Y.; Clemencon, B.; Simonin, A.; Leuenberger, M.; Lochner, M.; Weisstanner, M.; Hediger, M. A. The SLC1 High-Affinity Glutamate and Neutral Amino Acid Transporter Family. Mol. Aspects Med. 2013, 34(2–3), 108–120. DOI: 10.1016/j.mam.2013.01.001.
- Shayakul, C. K. Y.; Lee, W. S.; Brown, D.; Rothstein, J. D.; Hediger, M. A. Localization of the high-affinity Glutamate Transporter EAAC1 in Rat Kidney. The Amer. Physio. Soc. 1998, 273(6), F1023–F1029.
- Takanaga, H.; Mackenzie, B.; Peng, J. B.; Hediger, M. A. Characterization of a Branched-Chain Amino-Acid Transporter SBAT1 (SLC6A15) That Is Expressed in Human Brain. Biochem. Biophys. Res. Commun. 2005, 337(3), 892–900. DOI: 10.1016/j.bbrc.2005.09.128.
- Angelopoulou, E.; Bougea, A.; Paudel, Y. N.; Georgakopoulou, V. E.; Papageorgiou, S. G.; Piperi, C. Genetic Insights into the Molecular Pathophysiology of Depression in Parkinson’s Disease. Medicina (Kaunas) 2023, 59(6), From NLM Medline. DOI: 10.3390/medicina59061138.
- Zhao, D.; Lu, K. Substrates of the Human Oligopeptide Transporter hPept2. Biosci. Trends. 2015, 9(4), 207–213. DOI: 10.5582/bst.2015.01078.
- Ramamoorthy, S.; Liu, W.; Ma, Y. Y.; Yang-Feng, T. L.; Ganapathy, V.; Leibach, F. H. Proton/Peptide Cotransporter (PEPT 2) from Human Kidney: Functional Characterization and Chromosomal Localization. Biochem. Biophys. Acta. 1995, 1240(1), 1–4. DOI: 10.1016/0005-2736(95)00178-7.
- Anderson, C. M. H.; Thwaites, D. T. Hijacking Solute Carriers for proton-coupled Drug Transport. Physiology (Bethesda). 2010, 25(6), 364–377. From NLM Medline. DOI: 10.1152/physiol.00027.2010.
- Nielsen, C. U.; Pedersen, M.; Muller, S.; Kaestel, T.; Bjerg, M.; Ulaganathan, N.; Nielsen, S.; Carlsen, K. L.; Nohr, M. K.; Holm, R. Inhibitory Effects of 17-α-Ethinyl-Estradiol and 17-β-Estradiol on Transport via the Intestinal Proton-Coupled Amino Acid Transporter (PAT1) Investigated in vitro and in vivo. J. Pharm. Sci. 2021, 110(1), 354–364. DOI: 10.1016/j.xphs.2020.08.010.
- Scalise, M.; Console, L.; Cosco, J.; Pochini, L.; Galluccio, M.; Indiveri, C. ASCT1 and ASCT2: Brother and Sister? SLAS Discov. 2021, 26(9), 1148–1163. From NLM Medline. DOI: 10.1177/24725552211030288.
- Maucler, C.; Pernot, P.; Vasylieva, N.; Pollegioni, L.; Marinesco, S. In Vivo D-Serine hetero-exchange through alanine-serine-cysteine (ASC) Transporters Detected by Microelectrode Biosensors. ACS Chem. Neurosci. 2013, 4(5), 772–781. From NLM Medline. DOI: 10.1021/cn4000549.
- Chien, H. C.; Colas, C.; Finke, K.; Springer, S.; Stoner, L.; Zur, A. A.; Venteicher, B.; Campbell, J.; Hall, C.; Flint, A., et al. Reevaluating the Substrate Specificity of the L-Type Amino Acid Transporter (LAT1). J. Med. Chem. 2018, 61(16), 7358–7373. DOI: 10.1021/acs.jmedchem.8b01007. From NLM Medline.
- Del Amo, E. M.; Urtti, A.; Yliperttula, M. Pharmacokinetic Role of L-Type Amino Acid Transporters LAT1 and LAT2. Eur. J. Pharm. Sci. 2008, 35(3), 161–174. DOI: 10.1016/j.ejps.2008.06.015.
- Kandasamy, P.; Gyimesi, G.; Kanai, Y.; Hediger, M. A. Amino Acid Transporters Revisited: New Views in Health and Disease. Trends Biochem. Sci. 2018, 43(10), 752–789. From NLM Medline. DOI: 10.1016/j.tibs.2018.05.003.
- Romeo, E.; Dave, M. H.; Bacic, D.; Ristic, Z.; Camargo, S. M. R.; Loffing, J.; Wagner, C. A.; Verrey, F. Luminal Kidney and Intestine SLC6 Amino Acid Transporters of B0AT-cluster and Their Tissue Distribution in Mus Musculus. Am. J. Physiol. Renal Physiol. 2006, 290(2), F376–383. From NLM Medline. DOI: 10.1152/ajprenal.00286.2005.
- Takanaga, H.; Mackenzie, B.; Suzuki, Y.; Hediger, M. A. Identification of Mammalian Proline Transporter SIT1 (SLC6A20) with Characteristics of Classical System Imino. J. Biol. Chem. 2005, 280(10), 8974–8984. DOI: 10.1074/jbc.M413027200.
- Daniel, H.; Herget, M. Cellular and Molecular Mechanisms of Renal Peptide Transport. Am. J. Physiol (Renal Physiol. 42). 1997, 273, F1–F8.
- Daniel, H.; Rubio-Aliaga, I. An Update on Renal Peptide Transporters. Am. J. Physiol. Renal Physiol. 2003, 284(5), F885–F892. DOI: 10.1152/ajprenal.00123.2002.
- Silbernagl, S.; Völker, K.; Dantzler, W. H. D-Serine Is Reabsorbed in Rat Renal Pars Recta. the Ame. Physio. Soc. 1999, 276(6), F857–F863.
- Brandsch, M. Transport of Drugs by proton-coupled Peptide Transporters: Pearls and Pitfalls. Expert Opin. Drug Metab. Toxicol. 2009, 5(8), 887–905. From NLM Medline. DOI: 10.1517/17425250903042292.
- Wysocki, J.; Ye, M.; Rodriguez, E.; Gonzalez-Pacheco, F. R.; Barrios, C.; Evora, K.; Schuster, M.; Loibner, H.; Brosnihan, K. B.; Ferrario, C. M., et al. Targeting the Degradation of Angiotensin II with Recombinant angiotensin-converting Enzyme 2: Prevention of Angiotensin II-dependent Hypertension. Hypertension. 2010, 55(1), 90–98. DOI: 10.1161/HYPERTENSIONAHA.109.138420. From NLM Medline.
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S., et al. ACE2 Links Amino Acid Malnutrition to Microbial Ecology and Intestinal Inflammation. Nature. 2012, 487(7408), 477–481. DOI: 10.1038/nature11228. From NLM Medline.
- Camargo, S. M. R.; Vuille-Dit-Bille, R. N.; Meier, C. F.; Verrey, F. ACE2 and Gut Amino Acid Transport. Clin. Sci. (Lond.). 2020, 134(21), 2823–2833. From NLM Medline. DOI: 10.1042/CS20200477.
- Vuille-Dit-Bille, R. N.; Camargo, S. M. R.; Emmenegger, L.; Sasse, T.; Kummer, E.; Jando, J.; Hamie, Q. M.; Meier, C. F.; Hunziker, S.; Forras-Kaufmann, Z., et al. Human Intestine Luminal ACE2 and Amino Acid Transporter Expression Increased by ACE-inhibitors. Amino Acids. 2015, 47(4), 693–705. DOI: 10.1007/s00726-014-1889-6. From NLM Medline.
- Danilczyk, U.; Eriksson, U.; Oudit, G. Y.; Penninger, J. M. Physiological Roles of angiotensin-converting Enzyme 2. Cell. Mol. Life Sci. 2004, 61(21), 2714–2719. From NLM Medline. DOI: 10.1007/s00018-004-4241-6.
- Camargo, S. M. R.; Singer, D.; Makrides, V.; Huggel, K.; Pos, K. M.; Wagner, C. A.; Kuba, K.; Danilczyk, U.; Skovby, F.; Kleta, R., et al. Tissue-specific Amino Acid Transporter Partners ACE2 and Collectrin Differentially Interact with Hartnup Mutations. Gastroenterol. 2009, 136(3), 872–882. DOI: 10.1053/j.gastro.2008.10.055. From NLM Medline.
- Boll, M.; Foltz, M.; Rubio-Aliaga, I.; Kottra, G.; Daniel, H. Functional Characterization of Two Novel Mammalian Electrogenic Proton-Dependent Amino Acid Cotransporters. J. Biol. Chem. 2002, 277(25), 22966–22973. DOI: 10.1074/jbc.M200374200.
- Thwaites, D. T.; McEwan, G. T. A.; Simmons, N. L. The Role of the Proton Electrochemical Gradient in the Transepithelial Absorption of Amino Acids by Human Intestinal Caco-2 Cell Monolayers. J Membrane Biol. 1995, 145(3), 245–256. DOI: 10.1007/BF00232716.
- Broer, A.; Klingel, K.; Kowalczuk, S.; Rasko, J. E.; Cavanaugh, J.; Broer, S. Molecular Cloning of Mouse Amino Acid Transport System B0, a Neutral Amino Acid Transporter Related to Hartnup Disorder. J. Biol. Chem. 2004, 279(23), 24467–24476. From NLM Medline. DOI: 10.1074/jbc.M400904200.
- Jervis, E.; Smyth, D. H. Competition between Enantiomorphs of Amino Acids during Intestinal Absorption. J. Physiol. 1958, 145, 57–65.
- Silbernagel, S.; Völkl, H. Amino Acid Reabsorption in the Proximal Tubule of Rat Kidney: Sterospecificity and Passive Diffusion Studied by Continous Microperfusion. Pflugers Arch. 1977, 367, 221–227.
- Agar, W. T.; Hird, F. J. R.; Sidhu, G. S. The Absorption, Transer and Uptake of Amino Acids by Intestinal Tissue. Biochim. Biophys. Acta. 1956, 22, 21–30.
- Uhlen, M.; Fagerberg, L.; Hallstrom, B. M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A., et al. Proteomics. Tissue-based Map of the Human Proteome. Science. 2015, 347(6220), 1260419. DOI: 10.1126/science.1260419. From NLM Medline.
- Munck, B. G.; Munck, L. K. Effects of pH Changes on Systems ASC and B in Rabbit Ileum. The Amer. Physio. Soc. 1999, 276(1), G173–G184.
- Chen, Z.; Fei, Y. J.; Anderson, C. M. H.; Wake, K. A.; Miyauchi, S.; Huang, W.; Thwaites, D. T.; Ganapathy, V. Structure, Function and Immunolocalization of a proton-coupled Amino Acid Transporter (hPAT1) in the Human Intestinal Cell Line Caco-2. J. Physiol. 2003, 546(Pt 2), 349–361. From NLM Medline. DOI: 10.1113/jphysiol.2002.026500.
- Hatanaka, T.; Huang, W.; Nakanishi, T.; Bridges, C. C.; Smith, S. B.; Prasad, P. D.; Ganapathy, M. E.; Ganapathy, V. Transport Of D-Serine via the Amino Acid Transporter ATB0,+ Expressed in the Colon. Biochem. Biophys. Res. Commun 2002, 291(2), 291–295. From NLM Medline. DOI: 10.1006/bbrc.2002.6441.
- van der Wielen, N.; Moughan, P. J.; Mensink, M. Amino Acid Absorption in the Large Intestine of Humans and Porcine Models. J. Nutr. 2017, 147(8), 1493–1498. DOI: 10.3945/jn.117.248187.
- Fuller, M. F.; Tomé, D. In Vivo Determination of Amino Acid Bioavilability in Humans and Model Animals. J. AOAC Int. 2005, 88, 923–933.
- Anderson, C. M. H.; Howard, A.; Walters, J. R.; Ganapathy, V.; Thwaites, D. T. Taurine Uptake across the Human Intestinal brush-border Membrane Is via Two Transporters: H+-coupled PAT1 (SLC36A1) and Na+ and Cl− dependent TauT (SLC6A6). J. Physiol. 2009, 587(Pt 4), 731–744. From NLM Medline. DOI: 10.1113/jphysiol.2008.164228.
- Zhang, E. Y.; Emerick, R. M.; Pak, Y. A.; Wrighton, S. A.; Hillgren, K. M. Comparison of Human and Monkey Peptide Transporters: PEPT1 and PEPT2. Mol. Pharm. 2004, 1(3), 201–210. DOI: 10.1021/mp0499712.
- Ford, D.; Howard, A.; Hirst, B. H. Expression of the Peptide Transporter hPept1 in Human Colon: A Potential Route for Colonic Protein Nitrogen and Drug Absorption. Histochem. Cell Biol. 2003, 119(1), 37–43. DOI: 10.1007/s00418-002-0479-y.
- Ugawa, S.; Sunouchi, Y.; Ueda, T.; Takahashi, E.; Saishin, Y.; Shimada, S. Characterization of a Mouse Colonic System B0+ Amino Acid Transporter Related to Amino Acid Absorption in Colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281(2), G365–G370. DOI: 10.1152/ajpgi.2001.281.2.G365.
- Makrides, V.; Camargo, S. M. R.; Verrey, F. Transport of Amino Acids in the Kidney. Compr. Physiol. 2014, 4(1), 367–403. From NLM Medline. DOI: 10.1002/cphy.c130028.
- Kawazoe, T.; Park, H. K.; Iwana, S.; Tsuge, H.; Fukui, K. Human D-Amino Acid Oxidase: An Update and Review. Chem. Rec. 2007, 7(5), 305–315. From NLM Medline. DOI: 10.1002/tcr.20129.
- Verrey, F.; Ristic, Z.; Romeo, E.; Ramadan, T.; Makrides, V.; Dave, M. H.; Wagner, C. A.; Camargo, S. M. R. Novel Renal Amino Acid Transporters. Annu. Rev. Physiol 2005, 67, 557–572. From NLM Medline. DOI: 10.1146/annurev.physiol.67.031103.153949.
- Eisenbach, G. M.; Weise, M.; Stolte, H. Amino Acid Reabsorption in the Rat Nephron. Free Flow Micropuncture Study. Pflugers Arch. 1975, 357(1–2), 63–76. DOI: 10.1007/BF00584545.
- Silbernagel, S. The Renal Handling of Amino Acids and Oligopeptides. Physiol. Rev. 1988, 68, 912–971.
- Thwaites, D. T.; Anderson, C. M. The SLC36 Family of Proton-Coupled Amino Acid Transporters and Their Potential Role in Drug Transport. Br. J. Pharmacol. 2011, 164(7), 1802–1816. DOI: 10.1111/j.1476-5381.2011.01438.x.
- Danilczyk, U.; Sarao, R.; Remy, C.; Benabbas, C.; Stange, G.; Richter, A.; Arya, S.; Pospisilik, J. A.; Singer, D.; Camargo, S. M. R., et al. Essential Role for Collectrin in Renal Amino Acid Transport. Nature. 2006, 444(7122), 1088–1091. DOI: 10.1038/nature05475. From NLM Medline.
- Kanai, Y.; Hediger, M. A. Primary Structure and Functional Characterization of a High-Affinity Glutamate Transporter. Nature. 1992, 360(6403), 467–471. DOI: 10.1038/360467a0.
- Matsuo, H.; Kanai, Y.; Kim, J. Y.; Chairoungdua, A.; Kim, D. K.; Inatomi, J.; Shigeta, Y.; Ishimine, H.; Chaekuntode, S.; Tachampa, K., et al. Identification of a Novel Na+-Independent Acidic Amino Acid Transporter with Structural Similarity to the Member of a Heterodimeric Amino Acid Transporter Family Associated with Unknown Heavy Chains. J. Biol. Chem. 2002, 277(23), 21017–21026.
- Vanslambrouck, J. M.; Broer, A.; Thavyogarajah, T.; Holst, J.; Bailey, C. G.; Broer, S.; Rasko, J. E. Renal Imino Acid and Glycine Transport System Ontogeny and Involvement in Developmental Iminoglycinuria. Biochem J. 2010, 428(3), 397–407. DOI: 10.1042/BJ20091667.
- Peters, T.; Thaete, C.; Wolf, S.; Popp, A.; Sedlmeier, R.; Grosse, J.; Nehls, M. C.; Russ, A.; Schlueter, V. A Mouse Model for Cystinuria Type I. Hum. Mol. Genet. 2003, 12(17), 2109–2120. From NLM Medline. DOI: 10.1093/hmg/ddg189.
- Pineda, M.; Font, M.; Bassi, M. T.; Manzoni, M.; Borsani, G.; Marigo, V.; Fernandez, E.; Rio, R. M.; Purroy, J.; Zorzano, A., et al. The Amino Acid Transporter Asc-1 is not Involved in Cystinuria. Kidney Int. 2004, 66(4), 1453–1464. DOI: 10.1111/j.1523-1755.2004.00908.x. From NLM Medline.
- Guetg, A.; Mariotta, L.; Bock, L.; Herzog, B.; Fingerhut, R.; Camargo, S. M. R.; Verrey, F. Essential Amino Acid Transporter Lat4 (Slc43a2) Is Required for Mouse Development. J. Physiol. 2015, 593(5), 1273–1289. From NLM Medline. DOI: 10.1113/jphysiol.2014.283960.
- Rossier, G.; Meier, C.; Bauch, C.; Summa, V.; Sordat, B.; Verrey, F.; Kuhn, L. C. LAT2, a New Basolateral 4F2hc/CD98-Associated Amino Acid Transporter of Kidney and Intestine. J. Biol. Chem. 1999, 274(49), 34948–34954. DOI: 10.1074/jbc.274.49.34948.
- Verrey, F.; Singer, D.; Ramadan, T.; Vuille-dit-Bille, R. N.; Mariotta, L.; Camargo, S. M. R. Kidney Amino Acid Transport. Pflugers Arch. 2009, 458(1), 53–60. From NLM Medline. DOI: 10.1007/s00424-009-0638-2.
- Moret, C.; Dave, M. H.; Schulz, N.; Jiang, J. X.; Verrey, F.; Wagner, C. A. Regulation of Renal Amino Acid Transporters during Metabolic Acidosis. Am. J. Physiol. Renal Physiol. 2007, 292(2), F555–566. From NLM Medline. DOI: 10.1152/ajprenal.00113.2006.
- Suzuki, M.; Gonda, Y.; Yamada, M.; Vandebroek, A. A.; Mita, M.; Hamase, K.; Yasui, M.; Sasabe, J. Serum D-Serine Accumulation after Proximal Renal Tubular Damage Involves Neutral Amino Acid Transporter Asc-1. Sci. Rep. 2019, 9(1), 16705. From NLM Medline. DOI: 10.1038/s41598-019-53302-2.
- Helboe, L.; Egebjerg, J.; Moller, M.; Thomsen, C. Distribution and Pharmacology of alanine-serine-cysteine Transporter 1 (Asc-1) in Rodent Brain. Eur. J. Neurosci. 2003, 18(8), 2227–2238. From NLM Medline. DOI: 10.1046/j.1460-9568.2003.02966.x.
- Bodoy, S.; Martin, L.; Zorzano, A.; Palacin, M.; Estevez, R.; Bertran, J. Identification of LAT4, a Novel Amino Acid Transporter with System L Activity. J. Biol. Chem. 2005, 280(12), 12002–12011. DOI: 10.1074/jbc.M408638200.
- To, V.; Masagounder, K.; Loewen, M. E. Critical Transporters of Methionine and Methionine Hydroxyl Analogue Supplements across the Intestine: What We Know so Far and What Can Be Learned to Advance Animal Nutrition. Comp. Biochem. Physiol. A Mol. Integr. Physiol 2021, 255, 110908. From NLM Medline. DOI: 10.1016/j.cbpa.2021.110908.
- Usuda, N.; Yokota, S.; Hashimoto, T.; Nagata, T. Immunocytochemical Localization of D-Amino Acid Oxidase in the Central Clear Matrix of Rat Kidney Peroxisomes. The J. Histochem. & Cytochem. 1986, 34, 1709–1718.
- Chernobrovkin, M. G.; Ananéva, I. A.; Shapovalova, E. N.; Shpigun, O. A. Determination of Amino Acid Enantiomers in Pharmaceuticals by Reversed-Phase High-Performance Liquid Chromatography. J. Anal. Chem. 2004, 59(1), 55–63. DOI: 10.1023/B:JANC.0000011669.08932.d8.
- Smith, Q. M. S.; Aoyagi, M.; Rapoport, S. I. Kinetics of Neurtal Amino Acid Transport across the Blood-Brain Barrier. J. Neurochem. 1987, 49, 1651–1658.
- Prasad, P. D.; Wang, H.; Huang, W.; Kekuda, R.; Rajan, D. P.; Leibach, F. H.; Ganapathy, V. Human LAT1, a Subunit of System L-Amino Acid Transporter: Molecular Cloning and Transport Function. Biochem. Biophys. Res. Commun. 1999, 255(2), 283–288. DOI: 10.1006/bbrc.1999.0206.
- Hawkins, R. A.; Peterson, D. R.; Vina, J. R. The Complementary Membranes Forming the blood-brain Barrier. IUBMB Life. 2002, 54(3), 101–107. DOI: 10.1080/15216540214541.
- Oldendorf, W. H. Sterospecificity of blood-brain Barrier Permeability to Amino Acids. Am. J. Physiol. 1973, 224, 967–969.
- Sagne´, C.; Agulhon, C.; Ravassard, P.; Darmon, M.; Hamon, M.; Mestikawy, S. E.; Gasnier, B.; Giros, B. Identification and Characterization of a Lysosomal Transporter for Small Neutral Amino Acids. PNAS. 2001, 98, 7206–7211.
- Kugler, P.; Schmitt, A. Glutamate Transporter EAAC1 Is Expressed in Neurons and Glial Cells in the Rat Nervous System. Glia. 1999, 27(2), 129–142. DOI: 10.1002/(sici)1098-1136(199908)27:2<129::Aid-glia3>3.0.Co;2-y.
- Albrecht, J.; Zielinska, M. Exchange-mode Glutamine Transport across CNS Cell Membranes. Neuropharmacology. 2019, 161, 107560. DOI: 10.1016/j.neuropharm.2019.03.003.
- Miller, L. P.; Pardridge, W. M.; Braun, L. D.; Oldendorf, W. H. Kinetic Constants for blood-brain Barrier Amino Acid Transport in Conscious Rats. J. Neurochem. 1985, 45(5), 1427–1432. From NLM Medline. DOI: 10.1111/j.1471-4159.1985.tb07209.x.
- Rosenberg, D.; Artoul, S.; Segal, A. C.; Kolodney, G.; Radzishevsky, I.; Dikopoltsev, E.; Foltyn, V. N.; Inoue, R.; Mori, H.; Billard, J. M., et al. Neuronal D-Serine and Glycine Release via the Asc-1 Transporter Regulates NMDA receptor-dependent Synaptic Activity. J. Neurosci. 2013, 33(8), 3533–3544. From NLM Medline. DOI: 10.1523/JNEUROSCI.3836-12.2013.
- Rutter, A. R.; Fradley, R. L.; Garrett, E. M.; Chapman, K. L.; Lawrence, J. M.; Rosahl, T. W.; Patel, S. Evidence from Gene Knockout Studies Implicates Asc-1 as the Primary Transporter Mediating D-Serine Reuptake in the Mouse CNS. Eur. J. Neurosci. 2007, 25(6), 1757–1766. From NLM Medline. DOI: 10.1111/j.1460-9568.2007.05446.x.
- Gliddon, C. M.; Shao, Z.; LeMaistre, J. L.; Anderson, C. M. Cellular Distribution of the Neutral Amino Acid Transporter Subtype ASCT2 in Mouse Brain. J. Neurochem. 2009, 108(2), 372–383. DOI: 10.1111/j.1471-4159.2008.05767.x.
- Ribeiro, C. S.; Reis, M.; Panizzutti, R.; Miranda, J. D.; Wolosker, H. Glial Transport of the Neuromodulator D-Serine. Brain. res. 2002, 929, 202–209.
- Abe-Dohmae, S.; Takagi, Y.; Harada, N. Neurotransmitter-Mediated Regulation of Brain Aromatase: Protein Kinase C- and G-Dependent Induction. J. Neurochem. 1996, 67(5), 2087–2095. DOI: 10.1046/j.1471-4159.1996.67052087.x.
- Nagai, Y.; Tsugane, M.; Oka, J.; Kimura, H. Hydrogen Sulfide Induces Calcium Waves in Astrocytes. Faseb. J. 2004, 18(3), 557–559. DOI: 10.1096/fj.03-1052fje.
- Meier, C.; Ristic, Z.; Klauser, S.; Verrey, F. Activation of System L Heterodimeric Amino Acid Exchangers by Intracellular Substrates. EMBO J. 2002, 21(4), 580–589. From NLM Medline. DOI: 10.1093/emboj/21.4.580.
- Takahashi, K.; Foster, J. B.; Lin, C. L. Glutamate Transporter EAAT2: Regulation, Function, and Potential as a Therapeutic Target for Neurological and Psychiatric Disease. Cell. Mol. Life Sci. 2015, 72(18), 3489–3506. From NLM Medline. DOI: 10.1007/s00018-015-1937-8.
- Arriza, J. L.; Fairman, W. A.; Wadiche, J. I.; Murdoch, G. H.; Kavanaugh, M. P.; Amara, S. G. Functional Comparisons of Three Glutamate Transporter Subtypes Cloned from Human Motor Cortex. J. Neurosci. 1994, 14(9), 5559–5569. DOI: 10.1523/JNEUROSCI.14-09-05559.1994.
- Tsai, G. E.; Yang, P.; Chang, Y. C.; Chong, M. Y. D-Aalanine Added to Antipsychotics for the Treatment of Schizophrenia. Biol. Psychiatry. 2006, 59(3), 230–234. From NLM Medline. DOI: 10.1016/j.biopsych.2005.06.032.
- Pilone, M. S. D-Amino Acid Oxidase: New Findings. Cell. Mol. Life Sci. 2000, 57, 1732–1747.
- Hesaka, A.; Tsukamoto, Y.; Nada, S.; Kawamura, M.; Ichimaru, N.; Sakai, S.; Nakane, M.; Mita, M.; Okuzaki, D.; Okada, M., et al. D-Serine Mediates Cellular Proliferation for Kidney Remodeling. Kidney360. 2021, 2(10), 1611–1624. DOI: 10.34067/KID.0000832021. From NLM Medline.
- Seckler, J. M.; Lewis, S. J. Advances in D-Amino Acids in Neurological Research. Int. J. Mol. Sci. 2020, 21, 19. From NLM Medline. DOI: 10.3390/ijms21197325.
- Kimura, R.; Tsujimura, H.; Tsuchiya, M.; Soga, S.; Ota, N.; Tanaka, A.; Kim, H. Development of a Cognitive Function Marker Based On D-Amino Acid Proportions Using New Chiral Tandem LC-MS/MS Systems. Sci. Rep. 2020, 10(1), 804. From NLM Medline. DOI: 10.1038/s41598-020-57878-y.
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA Receptor Subunit Diversity: Impact on Receptor Properties, Synaptic Plasticity and Disease. Nat. Rev. Neurosci. 2013, 14(6), 383–400. DOI: 10.1038/nrn3504.
- Carta, M.; Srikumar, B. N.; Gorlewicz, A.; Rebola, N.; Mulle, C. Activity-Dependent Control of NMDA Receptor Subunit Composition at Hippocampal Mossy Fibre Synapses. J. Physiol. 2018, 596(4), 703–716. DOI: 10.1113/JP275226.
- Dringenberg, H. C. The History of long-term Potentiation as a Memory Mechanism: Controversies, Confirmation, and Some Lessons to Remember. Hippocampus. 2020, 30(9), 987–1012. DOI: 10.1002/hipo.23213.
- Traynelis, S. F.; Wollmuth, L. P.; McBain, C. J.; Menniti, F. S.; Vance, K. M.; Ogden, K. K.; Hansen, K. B.; Yuan, H.; Myers, S. J.; Dingledine, R., et al. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev. 2010, 62(3), 405–496. DOI: 10.1124/pr.109.002451.
- Cristino, L.; Luongo, L.; Squillace, M.; Paolone, G.; Mango, D.; Piccinin, S.; Zianni, E.; Imperatore, R.; Iannotta, M.; Longo, F. et al D-Aspartate Oxidase Influences Glutamatergic System Homeostasis in Mammalian Brain. Neurobiol. Aging. 2015, 36(5), 1890–1902. From NLM Medline. DOI: 10.1016/j.neurobiolaging.2015.02.003.
- Wolosker, H. NMDA Receptor Regulation By D-Serine: New Findings and Perspectives. Mol. Neurobiol. 2007, 36(2), 152–164. From NLM Medline. DOI: 10.1007/s12035-007-0038-6.
- Hansen, K. B.; Ogden, K. K.; Yuan, H.; Traynelis, S. F. Distinct Functional and Pharmacological Properties of Triheteromeric GluN1/GluN2A/GluN2B NMDA Receptors. Neuron. 2014, 81(5), 1084–1096. DOI: 10.1016/j.neuron.2014.01.035.
- Hansen, K. B.; Yi, F.; Perszyk, R. E.; Furukawa, H.; Wollmuth, L. P.; Gibb, A. J.; Traynelis, S. F. Structure, Function, and Allosteric Modulation of NMDA Receptors. J. Gen. Physiol. 2018, 150(8), 1081–1105. DOI: 10.1085/jgp.201812032.
- Lu, W.; Du, J.; Goehring, A.; Gouaux, E. Cryo-EM Structures of the Triheteromeric NMDA Receptor and Its Allosteric Modulation. Science 2017, 355, 6331. From NLM Medline. DOI: 10.1126/science.aal3729.
- Akazawa, C.; Shigemoto, R.; Bessho, Y.; Nakanishi, S.; Mizuno, N. Differential Expression of Five N-Methyl-D-Aspartate Receptor Subunit mRNAs in the Cerebellum of Developing and Adult Rats. J. Comp. Neurol. 1994, 347(1), 150–160. From NLM Medline. DOI: 10.1002/cne.903470112.
- Money, H.; Burnashev, N.; Laurie, D. J.; Sakmann, B.; Seeburg, P. H. Developmental and Regional Expression in the Rat Brain and Functional Properties of Four NMDA Receptors. Neuron. 1994, 12, 529–540.
- Martineau, M.; Parpura, V.; Mothet, J. P. Cell-type Specific Mechanisms Of D-Serine Uptake and Release in the Brain. Front. Synaptic Neurosci 2014, 6, 12. From NLM PubMed-not-MEDLINE. DOI: 10.3389/fnsyn.2014.00012.
- Orzylowski, M.; Fujiwara, E.; Mousseau, D. D.; Baker, G. B. An Overview of the Involvement of D-Serine in Cognitive Impairment in Normal Aging and Dementia. Front. Psychiatry 2021, 12, 754032. From NLM PubMed-not-MEDLINE. DOI: 10.3389/fpsyt.2021.754032.
- Lüscher, C.; Malenka, R. C. NMDA receptor-dependent long-term Potentiation and long-term Depression (LTP/LTD). Cold Spring Harb Perspect Biol 2012, 4, 6. From NLM Medline. DOI: 10.1101/cshperspect.a005710.
- Yamamoto, T.; Nishizaki, I.; Furuya, S.; Hirabayashi, Y.; Takahashi, K.; Okuyama, S.; Yamamoto, H. Characterization of Rapid and High-Affinity Uptake of L-Serine in Neurons and Astrocytes in Primary Culture. FEBS Lett. 2003, 548(1–3), 69–73. DOI: 10.1016/s0014-5793(03)00742-7.
- Errico, F.; Nuzzo, T.; Carella, M.; Bertolino, A.; Usiello, A. The Emerging Role of Altered D-Aspartate Metabolism in Schizophrenia: New Insights from Preclinical Models and Human Studies. Front. Psychiatry 2018, 9, 559. From NLM PubMed-not-MEDLINE. DOI: 10.3389/fpsyt.2018.00559.
- Awobuluyi, M.; Yang, J.; Ye, Y.; Chatterton, J. E.; Godzik, A.; Lipton, S. A.; Zhang, D. Subunit-specific Roles of glycine-binding Domains in Activation of NR1/NR3 N-Methyl-D-Aspartate Receptors. Mol. Pharmacol. 2007, 71(1), 112–122. DOI: 10.1124/mol.106.030700.
- Otsu, Y.; Darcq, E.; Pietrajtis, K.; Matyas, F.; Schwartz, E.; Bessaih, T.; Abi Gerges, S.; Rousseau, C. V.; Grand, T.; Dieudonne, S., et al. Control of Aversion by glycine-gated GluN1/GluN3A NMDA Receptors in the Adult Medial Habenula. Science. 2019, 366(6462), 250–254. DOI: 10.1126/science.aax1522. From NLM Medline.
- Grand, T.; Gerges, S. A.; David, M.; Diana, M. A.; Paoletti, P. Unmasking GluN1/GluN3A Excitatory Glycine NMDA Receptors. Nat. Commun. 2018, 9(1), 4769. DOI: 10.1038/s41467-018-07236-4.
- Verkhratsky, A.; Chvatal, A. NMDA Receptors in Astrocytes. Neurochem. Res. 2020, 45(1), 122–133. DOI: 10.1007/s11064-019-02750-3.
- Yang, J. H.; Wada, A.; Yoshida, K.; Miyoshi, Y.; Sayano, T.; Esaki, K.; Kinoshita, M. O.; Tomonaga, S.; Azuma, N.; Watanabe, M., et al. Brain-specific Phgdh Deletion Reveals a Pivotal Role for L-serine Biosynthesis in Controlling the Level of D-Serine, an N-Methyl-D-Aspartate Receptor co-agonist, in Adult Brain. J. Biol. Chem. 2010, 285(53), 41380–41390. DOI: 10.1074/jbc.M110.187443. From NLM Medline.
- Foster, A. C.; Farnsworth, J.; Lind, G. E.; Li, Y. X.; Yang, J. Y.; Dang, V.; Penjwini, M.; Viswanath, V.; Staubli, U.; Kavanaugh, M. P. D-Serine is a Substrate for Neutral Amino Acid Transporters ASCT1/SLC1A4 and ASCT2/SLC1A5, and is Transported by Both Subtypes in Rat Hippocampal Astrocyte Cultures. PLoS One. 2016, 11(6), e0156551. From NLM Medline. DOI: 10.1371/journal.pone.0156551.
- Wolosker, H. Serine Racemase and the Serine Shuttle Between Neurons and Astrocytes. Biochim. Biophys. Acta. 2011, 1814(11), 1558–1566. DOI: 10.1016/j.bbapap.2011.01.001.
- Seki, T.; Sato, M.; Konno, A.; Hirai, H.; Kurauchi, Y.; Hisatsune, A.; Katsuki, H. D-Cysteine Promotes Dendritic Development in Primary Cultured Cerebellar Purkinje Cells via Hydrogen Sulfide Production. Mol. Cell. Neurosci. 2018, 93, 36–47. From NLM Medline. DOI: 10.1016/j.mcn.2018.10.002.
- Kessels, H. W.; Nabavi, S.; Malinow, R. Metabotropic NMDA Receptor Function is Required for β-amyloid-induced Synaptic Depression. Proc. Natl. Acad. Sci. U. S. A. 2013, 110(10), 4033–4038. From NLM Medline. DOI: 10.1073/pnas.1219605110.
- Valdivielso, J. M.; Eritja, A.; Caus, M.; Bozic, M. Glutamate-Gated NMDA Receptors: Insights into the Function and Signaling in the Kidney. Biomolecules 2020, 10(7), From NLM Medline. DOI: 10.3390/biom10071051.
- Huang, X. T.; Yue, S. J.; Li, C.; Huang, Y. H.; Cheng, Q. M.; Li, X. H.; Hao, C. X.; Wang, L. Z.; Xu, J. P.; Ji, M., et al. A Sustained Activation of Pancreatic NMDARs is A Novel Factor of β-Cell Apoptosis and Dysfunction. Endocrinology. 2017, 158(11), 3900–3913. DOI: 10.1210/en.2017-00366. From NLM Medline.
- Burzomato, V.; Frugier, G.; Perez-Otano, I.; Kittler, J. T.; Attwell, D. The Receptor Subunits Generating NMDA Receptor Mediated Currents in Oligodendrocytes. J. Physiol. 2010, 588(Pt 18), 3403–3414. From NLM Medline. DOI: 10.1113/jphysiol.2010.195503.
- Palygin, O.; Lalo, U.; Pankratov, Y. Distinct Pharmacological and Functional Properties of NMDA Receptors in Mouse Cortical Astrocytes. Br. J. Pharmacol. 2011, 163(8), 1755–1766. DOI: 10.1111/j.1476-5381.2011.01374.x.
- Hogan-Cann, A. D.; Anderson, C. M. Physiological Roles of Non-Neuronal NMDA Receptors. Trends Pharmacol. Sci. 2016, 37(9), 750–767. DOI: 10.1016/j.tips.2016.05.012.
- Du, S.; Sung, Y. S.; Wey, M.; Wang, Y.; Alatrash, N.; Berthod, A.; MacDonnell, F. M.; Armstrong, D. W. Roles of N-Methyl-D-Aspartate Receptors and D-Amino Acids in Cancer Cell Viability. Mol. Biol. Rep. 2020, 47(9), 6749–6758. From NLM Medline. DOI: 10.1007/s11033-020-05733-8.
- Lin, C. H.; Chiu, C. C.; Huang, C. H.; Yang, H. T.; Lane, H. Y. pLg72 Levels Increase in Early Phase of Alzheimer’s Disease but Decrease in Late Phase. Sci. Rep. 2019, 9(1), 13221. DOI: 10.1038/s41598-019-49522-1.
- Anaparti, V.; Ilarraza, R.; Orihara, K.; Stelmack, G. L.; Ojo, O. O.; Mahood, T. H.; Unruh, H.; Halayko, A. J.; Moqbel, R. NMDA Receptors Mediate Contractile Responses in Human Airway Smooth Muscle Cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308(12), L1253–1264. From NLM Medline. DOI: 10.1152/ajplung.00402.2014.
- McGee, M. A.; Abdel-Rahman, A. A. N-Methyl-D-Aspartate Receptor Signaling and Function in Cardiovascular Tissues. J. Cardiovasc. Pharmacol. 2016, 68(2), 97–105. DOI: 10.1097/FJC.0000000000000398.
- Dong, Y. N.; Hsu, F. C.; Koziol-White, C. J.; Stepanova, V.; Jude, J.; Gritsiuta, A.; Rue, R.; Mott, R.; Coulter, D. A.; Panettieri, R. A., Jr, et al. Functional NMDA Receptors are Expressed by Human Pulmonary Artery Smooth Muscle Cells. Sci. Rep. 2021, 11(1), 8205. DOI: 10.1038/s41598-021-87667-0. From NLM Medline.
- Varga, G.; Erces, D.; Fazekas, B.; Fulop, M.; Kovacs, T.; Kaszaki, J.; Fulop, F.; Vecsei, L.; Boros, M. N-Methyl-D-aspartate Receptor Antagonism Decreases Motility and Inflammatory Activation in the Early Phase of Acute Experimental Colitis in the Rat. Neurogastroenterol. Motility. 2010, 22(2), 217–225, e268. From NLM Medline. DOI: 10.1111/j.1365-2982.2009.01390.x.
- Slomowitz, L. G.; Khang, S. J.; Satriano, J.; Thareau, S.; Deng, A.; Thomson, S. C.; Blantz, R. C.; Munger, K. A. Protein Intake Regulates the Vasodilatory Function of the Kidney and NMDA Receptor Expression. Am J. Physiol. Regulatory. Integrative Comp. Physiol. 2004, 287(5), R1184–R1189.
- Kalev-Zylinska, M. L.; Green, T. N.; Morel-Kopp, M. C.; Sun, P. P.; Park, Y. E.; Lasham, A.; During, M. J.; Ward, C. M. N-ethyl-D-Aspartate Receptors Amplify Activation and Aggregation of Human Platelets. Thromb. Res. 2014, 133(5), 837–847. From NLM Medline. DOI: 10.1016/j.thromres.2014.02.011.
- Duan, B. C.; Weng, W. C.; Lin, K. L.; Wong, L. C.; Li, S. T.; Hsu, M. H.; Lin, J. J.; Fan, P. C.; Lin, M. I.; Chiu, N. C., et al. Variations of Movement Disorders in anti-N-methyl-D-aspartate Receptor Encephalitis: A Nationwide Study in Taiwan. Medicine. 2016, 95(37), e4365. DOI: 10.1097/MD.0000000000004365. From NLM Medline.
- Qi, Q.; Chen, F.; Zhang, W.; Wang, P.; Li, Y.; Zuo, X. Colonic N-Methyl-D-Aspartate Receptor Contributes to Visceral Hypersensitivity in Irritable Bowel Syndrome. J. Gastroenterol. Hepatol. 2017, 32(4), 828–836. DOI: 10.1111/jgh.13588.
- Molnar, E.; Varadi, A.; McIlhinney, R. A.; Ashcroft, S. J. Identification of Functional Ionotropic Glutamate Receptor Proteins in Pancreatic β-cells and in Islets of Langerhans. FEBS Lett 1995, 371(3), 253–257. From NLM Medline. DOI: 10.1016/0014-5793(95)00890-l.
- Patton, A. J.; Genever, P. G.; Birch, M. A.; Suva, L. J.; Skerry, M. T. Expression of an N-Methyl-D-Aspartate-Type Receptor by Human and Rat Osteoblasts and Osteoclasts Suggests a Novel Glutamate Signaling Pathway in Bone. Bone. 1998, 22, 645–649.
- Du, S.; Wang, Y.; Alatrash, N.; Weatherly, C. A.; Roy, D.; MacDonnell, F. M.; Armstrong, D. W. Altered Profiles and Metabolism of L- and D-amino acids in Cultured Human Breast Cancer Cells Vs. non-tumorigenic Human Breast Epithelial Cells. J. Pharm. Biomed. Anal 2019, 164, 421–429. From NLM Medline. DOI: 10.1016/j.jpba.2018.10.047.
- Pankratov, Y.; Lalo, U. Calcium Permeability of Ligand-Gated Ca2+ Channels. Eur. J. Pharmacol. 2014, 739, 60–73. DOI: 10.1016/j.ejphar.2013.11.017.
- Perez-Otano, I.; Larsen, R. S.; Wesseling, J. F. Emerging Roles of GluN3-containing NMDA Receptors in the CNS. Nat. Rev. Neurosci. 2016, 17(10), 623–635. DOI: 10.1038/nrn.2016.92.
- Crawley, O.; Conde-Dusman, M. J.; Perez-Otano, I. GluN3A NMDA Receptor Subunits: More Enigmatic than Ever? J. Physiol. 2022, 600(2), 261–276. DOI: 10.1113/JP280879.
- Chen, J.; Zhang, J.; Yang, D. D.; Li, Z. C.; Zhao, B.; Chen, Y.; He, Z. Clonidine Ameliorates Cerebral ischemia-reperfusion Injury by up-regulating the GluN3 Subunits of NMDA Receptor. Metab. Brain Dis. 2022, 37(6), 1829–1841. From NLM Medline. DOI: 10.1007/s11011-022-01028-y.
- Dryer, S. E. Glutamate Receptors in the Kidney. Nephrol. Dial. Transplant. 2015, 30(10), 1630–1638. DOI: 10.1093/ndt/gfv028.
- Tapken, D.; Steffensen, T. B.; Leth, R.; Kristensen, L. B.; Gerbola, A.; Gajhede, M.; Jorgensen, F. S.; Olsen, L.; Kastrup, J. S. The Low Binding Affinity of D-Serine at the Ionotropic Glutamate Receptor GluD2 Can Be Attributed to the Hinge Region. Sci. Rep. 2017, 7, 46145. From NLM Medline. DOI: 10.1038/srep46145.
- Araki, K.; Meguro, H.; Kushiya, E.; Takayama, C.; Inoue, Y.; Mishina, M. Selective Expession of the Glutamate Receptor Channel δ2 Subunit in Cerebellar Purkinje Cells. Biochem. Biophys. Res. Commun 1993, 197, 1267–1276.
- Yuzaki, M. The δ2 Glutamate Receptor: 10 Years Later. Neurosci. Res. 2003, 46(1), 11–22. DOI: 10.1016/s0168-0102(03)00036-1.
- Maqsood, R.; Stone, T. W. The Gut-Brain Axis, BDNF, NMDA and CNS Disorders. Neurochem. Res. 2016, 41(11), 2819–2835. DOI: 10.1007/s11064-016-2039-1.
- Kamat, P. K.; Kalani, A.; Tyagi, N. Role of Hydrogen Sulfide in Brain Synaptic Remodeling. Methods Enzymol. 2015, 20150113. 555. 207–229.
- Chang, C. H.; Lin, C. H.; Lane, H. Y, D-Glutamate and Gut Microbiota in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21(8), From NLM Medline. DOI: 10.3390/ijms21082676.
- Tevzadze, G.; Zhuravliova, E.; Barbakadze, T.; Shanshiashvili, L.; Dzneladze, D.; Nanobashvili, Z.; Lordkipanidze, T.; Mikeladze, D. Gut Neurotoxin p-cresol Induces Differential Expression of GLUN2B and GLUN2A Subunits of the NMDA Receptor in the Hippocampus and Nucleus Accumbens in Healthy and Audiogenic seizure-prone Rats. AIMS Neurosci. 2020, 7(1), 30–42. From NLM PubMed-not-MEDLINE. DOI: 10.3934/Neuroscience.2020003.
- Gronier, B.; Savignac, H. M.; Di Miceli, M.; Idriss, S. M.; Tzortzis, G.; Anthony, D.; Burnet, P. W. J. Increased Cortical Neuronal Responses to NMDA and Improved Attentional set-shifting Performance in Rats following Prebiotic (B-GOS((R))) Ingestion. Eur. Neuropsychopharmacol. 2018, 28(1), 211–224. From NLM Medline. DOI: 10.1016/j.euroneuro.2017.11.001.
- Kao, A. C.; Chan, K. W.; Anthony, D. C.; Lennox, B. R.; Burnet, P. W. Prebiotic Reduction of Brain Histone Deacetylase (HDAC) Activity and olanzapine-mediated Weight Gain in Rats, are Acetate Independent. Neuropharmacology 2019, 150, 184–191. From NLM Medline. DOI: 10.1016/j.neuropharm.2019.02.014.
- Williams, S.; Chen, L.; Savignac, H. M.; Tzortzis, G.; Anthony, D. C.; Burnet, P. W. Neonatal Prebiotic (BGOS) Supplementation Increases the Levels of Synaptophysin, GluN2A-subunits and BDNF Proteins in the Adult Rat Hippocampus. Synapse. 2016, 70(3), 121–124. DOI: 10.1002/syn.21880.
- Spitzer, S. O.; Tkacz, A.; Savignac, H. M.; Cooper, M.; Giallourou, N.; Mann, E. O.; Bannerman, D. M.; Swann, J. R.; Anthony, D. C.; Poole, P. S., et al. Postnatal Prebiotic Supplementation in Rats Affects Adult Anxious Behaviour, Hippocampus, Electrophysiology, Metabolomics, and Gut Microbiota. iScience. 2021, 24(10), 103113. From NLM PubMed-not-MEDLINE. DOI: 10.1016/j.isci.2021.103113.
- Patrono, E.; Svoboda, J.; Stuchlik, A. Schizophrenia, the Gut Microbiota, and New Opportunities from Optogenetic Manipulations of the gut-brain Axis. Behav. Brain Funct. 2021, 17(1), 7. DOI: 10.1186/s12993-021-00180-2.
- Gong, X.; Liu, Y.; Liu, X.; Li, A.; Guo, K.; Zhou, D.; Hong, Z. Disturbance of Gut Bacteria and Metabolites are Associated with Disease Severity and Predict Outcome of NMDAR Encephalitis: A Prospective Case-Control Study. Front. Immunol. 2021, 12, 791780. From NLM Medline. DOI: 10.3389/fimmu.2021.791780.
- Mantuano, E.; Azmoon, P.; Brifault, C.; Banki, M. A.; Gilder, A. S.; Campana, W. M.; Gonias, S. L. Tissue-type Plasminogen Activator Regulates Macrophage Activation and Innate Immunity. Blood. 2017, 130(11), 1364–1374. DOI: 10.1182/blood-2017-04-780205.
- Das, L.; Banki, M. A.; Azmoon, P.; Pizzo, D.; Gonias, S. L. Enzymatically Inactive Tissue-Type Plasminogen Activator Reverses Disease Progression in the Dextran Sulfate Sodium Mouse Model of Inflammatory Bowel Disease. Am. J. Pathol. 2021, 191(4), 590–601. DOI: 10.1016/j.ajpath.2021.01.001.
- Kirchgessner, A. L.; Liu, M. T.; Alcantara, F. Excitotoxicity in the Enteric Nervous System. J. Neurosci. 1997, 17(22), 8804–8816. DOI: 10.1523/JNEUROSCI.17-22-08804.1997.
- Filpa, V.; Carpanese, E.; Marchet, S.; Prandoni, V.; Moro, E.; Lecchini, S.; Frigo, G.; Giaroni, C.; Crema, F. Interaction Between NMDA Glutamatergic and Nitrergic Enteric Pathways During in vitro Ischemia and Reperfusion. Eur. J. Pharmacol. 2015, 750, 123–131. DOI: 10.1016/j.ejphar.2015.01.021.
- Giaroni, C.; Zanetti, E.; Chiaravalli, A. M.; Albarello, L.; Dominioni, L.; Capella, C.; Lecchini, S.; Frigo, G. Evidence for a Glutamatergic Modulation of the Cholinergic Function in the Human Enteric Nervous System via NMDA Receptors. Eur. J. Pharmacol. 2003, 476(1–2), 63–69. DOI: 10.1016/s0014-2999(03)02147-2.
- Covasa, M.; Ritter, R. C.; Burns, G. A. NMDA Receptor Participation in Control of Food Intake by the Stomach. Am J. Physiol. Regulatory. Integrative Comp. Physiol. 2000, 278, R1362–R1368.
- Golovynska, I.; Beregova, T. V.; Falalyeyeva, T. M.; Stepanova, L. I.; Golovynskyi, S.; Qu, J.; Ohulchanskyy, T. Y. Peripheral N-Methyl-D-Aspartate Receptor Localization and Role in Gastric Acid Secretion Regulation: Immunofluorescence and Pharmacological Studies. Sci. Rep. 2018, 8(1), 7445. DOI: 10.1038/s41598-018-25753-6.
- Yu, T.; Wan, P.; Zhu, X. D.; Ren, Y. P.; Wang, C.; Yan, R. W.; Guo, Y.; Bai, A. P. Inhibition of NADPH Oxidase Activities Ameliorates DSS-Induced Colitis. Biochem Pharmacol. 2018, 158, 126–133. DOI: 10.1016/j.bcp.2018.10.010.
- Kaszaki, J.; Erces, D.; Varga, G.; Szabo, A.; Vecsei, L.; Boros, M. Kynurenines and Intestinal Neurotransmission: The Role of N-methyl-D-aspartate Receptors. J. Neural. Transm (Vienna). 2012, 119(2), 211–223. From NLM Medline. DOI: 10.1007/s00702-011-0658-x.
- Cihakova, D.; Eaton, W. W.; Talor, M. V.; Harkus, U. H.; Demyanovich, H.; Rodriguez, K.; Feldman, S.; Kelly, D. L. Gut Permeability and Mimicry of the Glutamate Ionotropic Receptor NMDA Type Subunit Associated with Protein 1 (GRINA) as Potential Mechanisms Related to a Subgroup of People with Schizophrenia with Elevated Antigliadin Antibodies (AGA IgG). Schizophr. Res 2019, 208, 414–419. From NLM Medline. DOI: 10.1016/j.schres.2019.01.007.
- Mikocka-Walus, A.; Knowles, S. R.; Keefer, L.; Graff, L. Controversies Revisited: A Systematic Review of the Comorbidity of Depression and Anxiety with Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2016, 22(3), 752–762. DOI: 10.1097/MIB.0000000000000620.
- Lui, M. T.; Rothstein, J. D.; Gershon, M. D.; Kirchgessner, A. Glutamatergic Enteric Neurons. J. Neurosci. 1997, 17(12), 4764–4784.
- Umeda, S.; Sujino, T.; Miyamoto, K.; Yoshimatsu, Y.; Harada, Y.; Nishiyama, K.; Aoto, Y.; Adachi, K.; Hayashi, N.; Amafuji, K., et al. D-Amino Acids Ameliorate Experimental Colitis and Cholangitis by Inhibiting Growth of Proteobacteria: Potential Therapeutic Role in Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. Hepatol. 2023. DOI: 10.1016/j.jcmgh.2023.08.002.
- Ikeda, Y.; Matsuda, S. Gut Protective Effect from D-Methionine or Butyric Acid against DSS and Carrageenan-Induced Ulcerative Colitis. Molecules 2023, 28(11), From NLM Medline. DOI: 10.3390/molecules28114392.
- Asakawa, T.; Onizawa, M.; Saito, C.; Hikichi, R.; Yamada, D.; Minamidate, A.; Mochimaru, T.; Asahara, S. I.; Kido, Y.; Oshima, S., et al. Oral Administration of D-Serine Prevents the Onset and Progression of Colitis in Mice. J. Gastroenterol. 2021, 56(8), 732–745. DOI: 10.1007/s00535-021-01792-1. From NLM Medline.
- Krug, A. W.; Volker, K.; Dantzler, W. H.; Silbernagl, S. Why is D-Serine Nephrotoxic and alpha-Aminoisobutyric Acid Protective? Am. J. Physiol. Renal Physiol. 2007, 293(1), F382–390. From NLM Medline. DOI: 10.1152/ajprenal.00441.2006.
- Kaltenbach, J. P.; Ganote, C.; Carone, F. A. Renal Tubular Necrosis Induced by Compounds Structurally Related to D-Serine. Exp. Mol. Pathol. 1979, 30, 209–214.
- Ceballos, I.; Chauveau, P.; Guerin, V.; Bardet, J.; Parvy, P.; Kamoun, P.; Jungers, P. Early Alterations of Plasma Free Amino Acids in Chronic Renal Failure. Clin. Chim. Acta. 1990, 188(2), 101–108. DOI: 10.1016/0009-8981(90)90154-K.
- van de Poll, M. C.; Soeters, P. B.; Deutz, N. E.; Fearon, K. C.; Dejong, C. H. Renal Metabolism of Amino Acids: Its Role in Interorgan Amino Acid Exchange. Am. J. Clin. Nutr. 2004, 79(2), 185–197. DOI: 10.1093/ajcn/79.2.185.
- Furusho, A.; Koga, R.; Akita, T.; Mita, M.; Kimura, T.; Hamase, K. Three-Dimensional High-Performance Liquid Chromatographic Determination of Asn, Ser, Ala, and Pro Enantiomers in the Plasma of Patients with Chronic Kidney Disease. Anal. Chem. 2019, 91(18), 11569–11575. DOI: 10.1021/acs.analchem.9b01615.
- Leung, J. C.; Marphis, T.; Craver, R. D.; Silverstein, D. M. Altered NMDA Receptor Expression in Renal Toxicity: Protection with a Receptor Antagonist. Kidney Int. 2004, 66(1), 167–176. DOI: 10.1111/j.1523-1755.2004.00718.x.
- Tseng, Y. S.; Liao, C. H.; Wu, W. B.; Ma, M. C. N-Methyl-D-aspartate Receptor Hyperfunction Contributes to D-Serine-mediated Renal Insufficiency. Am. J. Physiol. Renal Physiol. 2021, 320(5), F799–F813. From NLM Medline. DOI: 10.1152/ajprenal.00461.2020.
- Lin, C. S.; Hung, S. F.; Huang, H. S.; Ma, M. C., Seguro, A C. Blockade of the N-Methyl-D-Aspartate Glutamate Receptor Ameliorates Lipopolysaccharide-Induced Renal Insufficiency. PLoS One. 2015, 10(7), e0132204. DOI: 10.1371/journal.pone.0132204.
- Ma, M. C.; Huang, H. S.; Chen, Y. S.; Lee, S. H. Mechanosensitive N-methyl-D-aspartate Receptors Contribute to Sensory Activation in the Rat Renal Pelvis. Hypertension. 2008, 52(5), 938–944. DOI: 10.1161/HYPERTENSIONAHA.108.114116.
- Esposito, S.; Pristera, A.; Maresca, G.; Cavallaro, S.; Felsani, A.; Florenzano, F.; Manni, L.; Ciotti, M. T.; Pollegioni, L.; Borsello, T., et al. Contribution of Serine racemase/D-serine Pathway to Neuronal Apoptosis. Aging Cell. 2012, 11(4), 588–598. From NLM Medline. DOI: 10.1111/j.1474-9726.2012.00822.x.
- Eknoyan, G.; Kasiske, B.; Wheeler, D. C.; Winkelmayer, W. C. K. D. I. G. O. Clinical Practice Guideline for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease. Kidney International Supplements. 2018, 8(3), 91–165. From NLM PubMed-not-MEDLINE. DOI: 10.1016/j.kisu.2018.06.001.
- Okada, A.; Nangaku, M.; Jao, T. M.; Maekawa, H.; Ishimono, Y.; Kawakami, T.; Inagi, R. D-Serine, a Novel Uremic Toxin, Induces Senescence in Human Renal Tubular Cells via GCN2 Activation. Sci. Rep. 2017, 7(1), 11168. From NLM Medline. DOI: 10.1038/s41598-017-11049-8.
- Potier, B.; Turpin, F. R.; Sinet, P. M.; Rouaud, E.; Mothet, J. P.; Videau, C.; Epelbaum, J.; Dutar, P.; Billard, J. M. Contribution of the D-Serine-Dependent Pathway to the Cellular Mechanisms Underlying Cognitive Aging. Front. Aging Neurosci. 2010, 2, 1. From NLM PubMed-not-MEDLINE. DOI: 10.3389/neuro.24.001.2010.
- von Engelhardt, J.; Coserea, I.; Pawlak, V.; Fuchs, E. C.; Kohr, G.; Seeburg, P. H.; Monyer, H. Excitotoxicity in Vitro by NR2A- and NR2B-containing NMDA Receptors. Neuropharmacology. 2007, 53(1), 10–17. DOI: 10.1016/j.neuropharm.2007.04.015.
- Droge, W.; Schipper, H. M. Oxidative Stress and Aberrant Signaling in Aging and Cognitive Decline. Aging Cell. 2007, 6(3), 361–370. DOI: 10.1111/j.1474-9726.2007.00294.x.
- Molla, G.; Chaves-Sanjuan, A.; Savinelli, A.; Nardini, M.; Pollegioni, L. Structure and Kinetic Properties of Human D-Aspartate Oxidase, the enzyme-controlling D-Aspartate Levels in Brain. FASEB. J. 2020, 34(1), 1182–1197. From NLM Medline. DOI: 10.1096/fj.201901703R.
- DiNatale, B. C.; Murray, I. A.; Schroeder, J. C.; Flaveny, C. A.; Lahoti, T. S.; Laurenzana, E. M.; Omiecinski, C. J.; Perdew, G. H. Kynurenic Acid Is a Potent Endogenous Aryl Hydrocarbon Receptor Ligand that Synergistically Induces Interleukin-6 in the Presence of Inflammatory Signaling. Toxicol. Sci. 2010, 115(1), 89–97. DOI: 10.1093/toxsci/kfq024.
- Lichtman, S. W.; Pisaska, K.; Berman, E. R.; Pestone, M.; Dowling, H.; Offenbacher, E.; Weisel, H.; Heshka, S.; Matthews, D. E.; Heymsfield, S. B. Disprepancy between self-reported and Actural Caloric Intake and Exercise in Obese Subjects. New Engl. J. Med. 1992, 327, 1893–1898.
- Ma, T.; Cheng, Q.; Chen, C.; Luo, Z.; Feng, D. Excessive Activation of NMDA Receptors in the Pathogenesis of Multiple Peripheral Organs via Mitochondrial Dysfunction, Oxidative Stress, and Inflammation. SN Comprehen. Clini. Med. 2020, 2(5), 551–569. DOI: 10.1007/s42399-020-00298-w.
- Bitanihirwe, B. K.; Woo, T. U. Oxidative Stress in Schizophrenia: An Integrated Approach. Neurosci. Biobehav. Rev. 2011, 35(3), 878–893. From NLM Medline. DOI: 10.1016/j.neubiorev.2010.10.008.
- MacKay, M. B.; Kravtsenyuk, M.; Thomas, R.; Mitchell, N. D.; Dursun, S. M.; Baker, G. B. D-Serine: Potential Therapeutic Agent and/or Biomarker in Schizophrenia and Depression? Front. Psychiatry 2019, 10, 25. From NLM PubMed-not-MEDLINE. DOI: 10.3389/fpsyt.2019.00025.
- Iwama, H.; Takahashi, K.; Kure, S.; Hayashi, F.; Narisawa, K.; Tada, K.; Mizoguchi, M.; Takashima, S.; Tomita, U.; Nishikawa, T. Depletion of Cerebral D-Serine in Non-Ketotic Hyperglycinemia: Possible Involvement of Glycine Cleavage System in Control of Endogenous D-Serine. Biochem. Biophys. Res. Commun 1997, 231, 793–796.
- Targa Dias Anastacio, H.; Matosin, N.; Ooi, L. Neuronal Hyperexcitability in Alzheimer’s Disease: What Are the Drivers Behind This Aberrant Phenotype? Transl. Psychiatry. 2022, 12(1), 257. DOI: 10.1038/s41398-022-02024-7.
- Semyanov, A.; Henneberger, C.; Agarwal, A. Making Sense of Astrocytic Calcium Signals - from Acquisition to Interpretation. Nat. Rev. Neurosci. 2020, 21(10), 551–564. From NLM Medline. DOI: 10.1038/s41583-020-0361-8.
- Lerdkrai, C.; Asavapanumas, N.; Brawek, B.; Kovalchuk, Y.; Mojtahedi, N.; Olmedillas Del Moral, M.; Garaschuk, O. Intracellular Ca2+ Stores Control in vivo Neuronal Hyperactivity in a Mouse Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. U. S. A. 2018, 115(6), E1279–E1288. DOI: 10.1073/pnas.1714409115.
- Hanson, J. E.; Ma, K.; Elstrott, J.; Weber, M.; Saillet, S.; Khan, A. S.; Simms, J.; Liu, B.; Kim, T. A.; Yu, G. Q., et al. GluN2A NMDA Receptor Enhancement Improves Brain Oscillations, Synchrony, and Cognitive Functions in Dravet Syndrome and Alzheimer’s Disease Models. Cell Rep. 2020, 30(2), 381–396 e384. DOI: 10.1016/j.celrep.2019.12.030. From NLM Medline.
- Celone, K. A.; Calhoun, V. D.; Dickerson, B. C.; Atri, A.; Chua, E. F.; Miller, S. L.; DePeau, K.; Rentz, D. M.; Selkoe, D. J.; Blacker, D., et al. Alterations in Memory Networks in Mild Cognitive Impairment and Alzheimer’s Disease: An Independent Component Analysis. J. Neurosci. 2006, 26(40), 10222–10231. DOI: 10.1523/JNEUROSCI.2250-06.2006. From NLM Medline.
- Bassett, S. S.; Yousem, D. M.; Cristinzio, C.; Kusevic, I.; Yassa, M. A.; Caffo, B. S.; Zeger, S. L. Familial Risk for Alzheimer’s Disease Alters fMRI Activation Patterns. Brain. 2006, 129(Pt 5), 1229–1239. DOI: 10.1093/brain/awl089.
- Dickerson, B. C.; Salat, D. H.; Greve, D. N.; Chua, E. F.; Rand-Giovannetti, E.; Rentz, D. M.; Bertram, L.; Mullin, K.; Tanzi, R. E.; Blacker, D., et al. Increased Hippocampal Activation in Mild Cognitive Impairment Compared to Normal Aging and AD. Neurology. 2005, 65(3), 404–411. DOI: 10.1212/01.wnl.0000171450.97464.49. From NLM Medline.
- Morris, J. C. Clinical Dementia Rating: A Reliable and Valid Diagnostic and Staging Measure for Dementia of the Alzheimer Type. Int. Psychogeriatr. 1997, 9(1), 173–176. From NLM Medline. DOI: 10.1017/s1041610297004870.
- Chang, C. H.; Kuo, H. L.; Ma, W. F.; Tsai, H. C. Cerebrospinal Fluid and Serum D-Serine Levels in Patients with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 12. From NLM PubMed-not-MEDLINE. DOI: 10.3390/jcm9123840.
- Hashimoto, K.; Fukushima, T.; Shimizu, E.; Okada, S.; Komatsu, N.; Okamura, N.; Koike, K.; Koizumi, H.; Kumakiri, C.; Imai, K., et al. Possible Role of D-Serine in the Pathophysiology of Alzheimer’s Disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004, 28(2), 385–388. From NLM Medline. DOI: 10.1016/j.pnpbp.2003.11.009.
- Nuzzo, T.; Miroballo, M.; Casamassa, A.; Mancini, A.; Gaetani, L.; Nistico, R.; Eusebi, P.; Katane, M.; Homma, H.; Calabresi, P., et al. Cerebrospinal Fluid and Serum D-Serine Concentrations are Unaltered across the Whole Clinical Spectrum of Alzheimer’s Disease. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868(12), 140537. DOI: 10.1016/j.bbapap.2020.140537. From NLM Medline.
- Kimura, T.; Hesaka, A.; Isaka, Y. Utility of D-Serine Monitoring in Kidney Disease. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868(9), 140449. From NLM Medline. DOI: 10.1016/j.bbapap.2020.140449.
- Fisher, G. H.; Lorenzo, N.; Abe, H.; Fujita, E.; Frey, W. H.; Emory, C.; Fiore, M. M. D.; DÀniello, A. Free D- and L-Amino Acids in Ventricular Cerebrospinal Fluid from Alzheimer and Normal Subjects. Amino Acids. 1998, 15(3), 263–269. DOI: 10.1007/BF01318865.
- Madeira, C.; Lourenco, M. V.; Vargas-Lopes, C.; Suemoto, C. K.; Brandao, C. O.; Reis, T.; Leite, R. E.; Laks, J.; Jacob-Filho, W.; Pasqualucci, C. A. D-Serine Levels in Alzheimer’s Disease: Implications for Novel Biomarker Development. Transl. Psychiatry. 2015, 5(5), e561. From NLM Medline. DOI: 10.1038/tp.2015.52.
- Abramov, A. Y.; Duchen, M. R. The Role of an Astrocytic NADPH Oxidase in the Neurotoxicity of Amyloid Beta Peptides. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360(1464), 2309–2314. From NLM Medline. DOI: 10.1098/rstb.2005.1766.
- Fisher, G. H.; DÀniello, A.; Vetere, A.; Padula, L.; Cusano, G. P.; Man, E. H. Free D-Aspartate and D-Alanine in Normal and Alzheimer Brain. Brain Reserch Bulletin. 1991, 26, 983–985.
- D’Aniello, A.; Lee, J. M.; Petrucelli, L.; Fiore, M. M. D. Regional Decreases of Free D-Aspartate Levels in Alzheimer´s Disease. Neurosci. Lett. 1998, 250, 131–134.
- Pole, A.; Dimri, M.; Dimri, G. P. Oxidative Stress, Cellular Senescence and Ageing. AIMS Molecular Science. 2016, 3(3), 300–324. DOI: 10.3934/molsci.2016.3.300.
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D., et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging. 2018, 13, 757–772. DOI: 10.2147/CIA.S158513.
- Pestana, F.; Edwards-Faret, G.; Belgard, T. G.; Martirosyan, A.; Holt, M. G. No Longer Underappreciated: The Emerging Concept of Astrocyte Heterogeneity in Neuroscience. Brain Sci. 2020, 10(3), From NLM PubMed-not-MEDLINE. DOI: 10.3390/brainsci10030168.
- Guerra-Gomes, S.; Sousa, N.; Pinto, L.; Oliveira, J. F. Functional Roles of Astrocyte Calcium Elevations: From Synapses to Behavior. Front. Cell. Neurosci. 2017, 11, 427. From NLM PubMed-not-MEDLINE. DOI: 10.3389/fncel.2017.00427.
- Araque, A.; Carmignoto, G.; Haydon, P. G.; Oliet, S. H.; Robitaille, R.; Volterra, A. Gliotransmitters Travel in Time and Space. Neuron. 2014, 81(4), 728–739. From NLM Medline. DOI: 10.1016/j.neuron.2014.02.007.
- Deitmer, J. W.; Theparambil, S. M.; Ruminot, I.; Noor, S. I.; Becker, H. M. Energy Dynamics in the Brain: Contributions of Astrocytes to Metabolism and pH Homeostasis. Front. Neurosci. 2019, 13, 1301. DOI: 10.3389/fnins.2019.01301.
- Brown, G. C.; Neher, J. J. Microglial Phagocytosis of Live Neurons. Nat. Rev. Neurosci. 2014, 15(4), 209–216. DOI: 10.1038/nrn3710.
- Hong, S.; Baja-Glasser, V. F.; Nfonoyim, B. M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K. M.; Shi, Q.; Rosenthal, A.; Barres, B. A., et al. Complement and Microglia Mediate Early Synapse Loss in Alzheimer Mouse Models. Science 2016, 352, 712–717. DOI: 10.7910/DVN/C2VYNM.
- Salter, M. W.; Stevens, B. Microglia Emerge As Central Players in Brain Disease. Nat. Med. 2017, 23(9), 1018–1027. DOI: 10.1038/nm.4397.
- Matejuk, A.; Ransohoff, R. M. Crosstalk between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11(1416). DOI: 10.3389/fimmu.2020.01416.
- Sedel, F.; Bechade, C.; Vyas, S.; Triller, A. Macrophage-derived Tumor Necrosis Factor Alpha, an Early Developmental Signal for Motoneuron Death. J. Neurosci. 2004, 24(9), 2236–2246. From NLM Medline. DOI: 10.1523/JNEUROSCI.4464-03.2004.
- Santello, M.; Bezzi, P.; Volterra, A. TNFα Controls Glutamatergic Gliotransmission in the Hippocampal Dentate Gyrus. Neuron. 2011, 69(5), 988–1001. DOI: 10.1016/j.neuron.2011.02.003.
- Ortinski, P. I.; Dong, J.; Mungenast, A.; Yue, C.; Takano, H.; Watson, D. J.; Haydon, P. G.; Coulter, D. A. Selective Induction of Astrocytic Gliosis Generates Deficits in Neuronal Inhibition. Nat. Neurosci. 2010, 13(5), 584–591. DOI: 10.1038/nn.2535.
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J. P.; Petzold, G. C.; Serrano-Pozo, A.; Steinhauser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A., et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24(3), 312–325. DOI: 10.1038/s41593-020-00783-4. From NLM Medline.
- Price, B. R.; Johnson, L. A.; Norris, C. M. Reactive Astrocytes: The Nexus of Pathological and Clinical Hallmarks of Alzheimer’s Disease. Ageing Res. Rev. 2021, 68, 101335. DOI: 10.1016/j.arr.2021.101335.
- Kussmaul, L.; Hamprecht, B.; Dringen, R. The Detoxification of Cumene Hydroperoxide by the Glutathione System of Cultured Astroglial Cells Hinges on Hexose Availability for the Regeneration of NADPH. J. Neurochem. 1999, 73(3), 1246–1253. DOI: 10.1046/j.1471-4159.1999.0731246.x.
- An, Y.; Varma, V. R.; Varma, S.; Casanova, R.; Dammer, E.; Pletnikova, O.; Chia, C. W.; Egan, J. M.; Ferrucci, L.; Troncoso, J., et al. Evidence for Brain Glucose Dysregulation in Alzheimer’s Disease. Alzheimers Dement. 2018, 14(3), 318–329. DOI: 10.1016/j.jalz.2017.09.011. From NLM Medline.
- Croteau, E.; Castellano, C. A.; Fortier, M.; Bocti, C.; Fulop, T.; Paquet, N.; Cunnane, S. C. A Cross-Sectional Comparison of Brain Glucose and Ketone Metabolism in Cognitively Healthy Older Adults, Mild Cognitive Impairment and Early Alzheimer’s Disease. Exp Gerontol [Internet]. 2018, 107, 18–26. DOI: 10.1016/j.exger.2017.07.004.
- Maugard, M.; Vigneron, P. A.; Bolanos, J. P.; Bonvento, G. L-Serine Links Metabolism with Neurotransmission. Prog. Neurobiol. 2021, 197, 101896. DOI: 10.1016/j.pneurobio.2020.101896.
- Le Douce, J.; Maugard, M.; Veran, J.; Matos, M.; Jego, P.; Vigneron, P. A.; Faivre, E.; Toussay, X.; Vandenberghe, M.; Balbastre, Y., et al. Impairment of Glycolysis-Derived L-Serine Production in Astrocytes Contributes to Cognitive Deficits in Alzheimer’s Disease. Cell Metab. 2020, 31(3), 503–517 e508. DOI: 10.1016/j.cmet.2020.02.004. From NLM Medline.
- Perez, E. J.; Tapanes, S. A.; Loris, Z. B.; Balu, D. T.; Sick, T. J.; Coyle, J. T.; Liebl, D. J. Enhanced Astrocytics D-Serine Underlies Synaptic Damage after Traumatic Brain Injury. J. Clin. Invest. 2017, 127(8), 3114–3125. From NLM Medline. DOI: 10.1172/JCI92300.
- Coyle, J. T.; Balu, D.; Wolosker, H. D-Serine, the Shape-Shifting NMDA Receptor Co-agonist. Neurochem. Res. 2020, 45(6), 1344–1353. From NLM Medline. DOI: 10.1007/s11064-020-03014-1.
- Balu, D. T.; Pantazopoulos, H.; Huang, C. C. Y.; Muszynski, K.; Harvey, T. L.; Uno, Y.; Rorabaugh, J. M.; Galloway, C. R.; Botz-Zapp, C.; Berretta, S., et al. Neurotoxic Astrocytes Express The D-Serine Synthesizing Enzyme, Serine Racemase, in Alzheimer’s Disease. Neurobiol. Dis. 2019, 130, 104511. From NLM Medline. DOI: 10.1016/j.nbd.2019.104511.
- Paul, P.; de Belleroche, J. The Role of D-Amino Acids in Amyotrophic Lateral Sclerosis Pathogenesis: A Review. Amino Acids. 2012, 43(5), 1823–1831. From NLM Medline. DOI: 10.1007/s00726-012-1385-9.
- Inoue, R.; Talukdar, G.; Takao, K.; Miyakawa, T.; Mori, H. Dissociated Role of D-Serine in Extinction during Consolidation vs. Reconsolidation of Context Conditioned Fear. Front. Mol. Neurosci 2018, 11, 161. From NLM PubMed-not-MEDLINE. DOI: 10.3389/fnmol.2018.00161.
- Coyle, J. T.; Tsai, G.; Goff, D. Converging Evidence of NMDA Receptor Hypofunction in the Pathophysiology of Schizophrenia. Ann. N Y Acad. Sci. 2003, 1003, 318–327. From NLM Medline. DOI: 10.1196/annals.1300.020.
- De Rosa, A.; Mastrostefano, F.; Di Maio, A.; Nuzzo, T.; Saitoh, Y.; Katane, M.; Isidori, A. M.; Caputo, V.; Marotta, P.; Falco, G., et al. Prenatal Expression of D-Aspartate Oxidase Causes Early Cerebralaspartate Depletion and Influences Brain Morphology and Cognitive Functions at Adulthood. Amino Acids. 2020, 52(4), 597–617. DOI: 10.1007/s00726-020-02839-y. From NLM Medline.
- Errico, F.; Napolitano, F.; Squillace, M.; Vitucci, D.; Blasi, G.; de Bartolomeis, A.; Bertolino, A.; D’Aniello, A.; Usiello, A. Decreased Levels ofD-Aspartate and NMDA in the Prefrontal Cortex and Striatum of Patients with Schizophrenia. J. Psychiatr. Res. 2013, 47(10), 1432–1437. From NLM Medline. DOI: 10.1016/j.jpsychires.2013.06.013.
- Miller, C. L.; Llenos, I. C.; Dulay, J. R.; Barillo, M. M.; Yolken, R. H.; Weis, S. Expression of the Kynurenine Pathway Enzyme Tryptophan 2,3-Dioxygenase is Increased in the Frontal Cortex of Individuals with Schizophrenia. Neurobiol. Dis. 2004, 15(3), 618–629. DOI: 10.1016/j.nbd.2003.12.015.
- Inoue, R.; Yoshihisa, Y.; Tojo, Y.; Okamura, C.; Yoshida, Y.; Kishimoto, J.; Luan, X.; Watanabe, M.; Mizuguchi, M.; Nabeshima, Y., et al. Localization of Serine Racemase and Its Role in the Skin. J. Invest. Dermatol. 2014, 134(6), 1618–1626.
- Nakade, Y.; Iwata, Y.; Furuichi, K.; Mita, M.; Hamase, K.; Konno, R.; Miyake, T.; Sakai, N.; Kitajima, S.; Toyama, T., et al. Gut microbiota-derived D-Serine Protects against Acute Kidney Injury. JCI insight. 2018, 3, 20. From NLM Medline. DOI: 10.1172/jci.insight.97957.
- Lockridge, A. D.; Baumann, D. C.; Akhaphong, B.; Abrenica, A.; Miller, R. F.; Alejandro, E. U. Serine Racemase Is Expressed in Islets and Contributes to the Regulation of Glucose Homeostasis. Islets. 2016, 8(6), 195–206. DOI: 10.1080/19382014.2016.1260797.
- Drabkova, P.; Sanderova, J.; Kovarik, J.; Kandar, R. An Assay of Selected Serum Amino Acids in Patients with Type 2 Diabetes Mellitus. Adv. Clin. Exp. Med. 2015, 24(3), 447–451. DOI: 10.17219/acem/29223.