ABSTRACT
Olive oil production and consumption has been increasing due to its organoleptic properties and health benefits. Consequently, waste generation also raised leading to environmental concerns. Nevertheless, this waste contains high amounts of bioactive compounds with potential to be extracted, recovered, and applied, following circular economy policies. New extractive technologies, especially green ones, are important for the success of this venture. This review focuses on the olive oil production chain, from the tree to olive oil and the respective waste/by-products, presenting and discussing emergent and green methodologies to recover and give new life to bioactive compounds that are usually discarded. Oleuropein, hydroxytyrosol, and other related compounds are molecules with important biological activities. They can be recovered in high concentrations through such technologies and used successfully in different industries to meet the sustainability and circular economy principles of the chain.
Introduction
The olive tree was one of the first tree species to be cultivated. Reports of cultivation date back to the 7th century BC, highlighting its symbolic significance as a representation of peace and friendship between nations.[Citation1] In Ancient Greece, a wreath of olive branches was awarded to the winners of the Olympic games.[Citation1] Later, the production of olive oil began, which is one of the oldest techniques practiced by humans to obtain fuel for lighting.[Citation1,Citation2]
Currently, olive oil is an essential ingredient in human daily life, leading to a constant increase on its production and consumption worldwide, not only due to its organoleptic characteristics, but also to the scientific evidence on its health-promoting properties.[Citation3,Citation4] This product is also important in other industries, such as cosmetics, pharmaceuticals, and others.[Citation3,Citation4] Olea europaea L., a species of tree belonging to the Oleaceae family, and commonly known as the olive tree, is the only species in that family that can produce fruits that are suitable to produce olive oil.[Citation2] Olive growing is one of the oldest and most important agricultural activities in countries belonging to the Mediterranean basin, thus having a high economic and social impact.[Citation2] Olive oil production is extremely important for those countries especially considering that most olive oil mills are concentrated in the Mediterranean area, where there is a high availability of olive growing areas. According to the Food and Agriculture Organization (FAO), it is estimated that the areas used for olive cultivation worldwide correspond to ~ 12 million ha, with the largest extension of these ha being observed in Spain, which is the world’s largest producer of olive oil.[Citation5] However, it is not only in Mediterranean countries that olive trees are present, such as Spain, Italy, Greece, Portugal, Albania, Montenegro, Egypt, Tunisia, Morocco, among others. They are also cultivated in Australia, Argentina, Brazil, Chile, China, and the United States, in a total of 41 nations.[Citation6] According to the International Olive Council (IOC), the European Union accounts for around 70% of all world olive oil production, generating a business value of around USD 10 billion in 2021/2022.[Citation7] In 2021/22, the global value was approximately USD 14 billion, noting that this market has tripled in the last 60 years, with a production of around 3.1 million tons of olive oil in this harvest.[Citation7] The world’s largest consumer of olive oil is the United States and consequently they are also the largest importer of this commodity, representing about 40% of all imports. However, a growing increase in the consumption and importation of olive oil by China has been documented since its first registration in 2009.[Citation7]
Despite the benefits for both health and economy, this sector has a high range of environmental problems arising from waste and by-products generated during the production process.[Citation8] Within these generated wastes, we can mention the biomass that is obtained from the maintenance of the olive tree (leaves and branches) as well as mill wastes such as stones, olive pomace (OP), and olive mill wastewaters (OMWW).[Citation9] In the past, these residues did not undergo any treatment process, being generally burned, or simply discarded in the field, with the intention of being used as fertilizers. Lately, such residues have undergone various treatment processes. These processes are typically expensive and inefficient, often involving storage or evaporation in lagoons, thereby posing significant environmental challenges in producing countries due to their high concentration of organic and phytotoxic compounds.[Citation9–11] Due to the seasonality of these products, another generated problem is the management of the high amount of waste obtained in a short period of time, representing a great economic burden for producers.[Citation12,Citation13] So, to overcome these problems, it is necessary to investigate new strategies for the management of waste and by-products of the olive oil industry, with the aim of reducing or mitigating the impacts caused by them on the environment.[Citation9,Citation12]
These residues and by-products have a large amount of chemical compounds with high added value, presenting high potential to be used as a low-cost source of these compounds.[Citation14,Citation15] Such bioactive compounds have attracted great interest for various sectors, including pharmaceutical, agrifood, and cosmetics, due to their antioxidant, biopesticide, anti-inflammatory, and anticancer potential, among other activities.[Citation16]
In recent years, many investigations have been carried out to obtain new uses for these residues and by-products compounds, regarding a highly efficient recovery. Amidst the array of strategies employed, one that has been having a great impact is the extraction through sustainable methodologies, mostly those that use green technologies and environmentally friendly solvents together.[Citation17] Within the spectrum of green extraction technologies, some can be mentioned: ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), pressurized liquid extraction (PLE), pulsed electric field (PEF), high voltage electrical discharge (HVED), supercritical fluid extraction (SFE), and others.[Citation18] Applying emerging technologies offers the advantage of mitigating the degradation of bioactive compounds. This results in increased stability over time and demonstrates good efficacy in extracting various food components such as lipids, minerals, vitamins, phenolic compounds, and enzymes.[Citation19,Citation20] This is observed because these methodologies do not use high temperatures, avoiding damage to the structure of the compound, concomitantly with a short extraction time, which leads to low energy consumption.[Citation21,Citation22] It is worth mentioning that in order to obtain high extractive yields, it is necessary to optimize the operational parameters, otherwise they may cause the degradation of macromolecules as well as the oxidation of labile compounds of interest.
Methodology
In this review, a systematic search was carried out in the literature in search of articles, books, dissertations, and theses published in the last 20 years with the requested themes. The search engines and databases used were mainly Elsevier’s Scopus and Clarivate Analytics’ Web of Science, with some publications also being obtained from consultations in SciFinder, PubMed and ResearchGate. All searches were carried out between March 2023 and April 2024, based on the interaction of different terms and their synonyms, including: “olive oil process”, “olive oil by-products”, “olive oil waste”, “phenolic compounds”, “green extraction”, “olive pomace”, “OMWW”, “phytotoxic”, “bioactive compounds”, and others. In the articles that served as a basis for observing the activities of bioactive compounds, several information was extracted, including: activity to be tested, target species, forms of application and purpose of application. The results of this evaluation and systematic categorization were synthesized and summarized in the following sections in text form and in tables.
Tree and fruit characterization
Olea europaea L. (commonly known as olive tree) is a plant that belongs to the botanical family of Oleaceae, which has tree species spread across tropical and temperate regions. The plants of this family, normally, present themselves in the form of a tree, however, some have the appearance of vines. Several of these plants are used for the extraction of essential oils from their flowers and fruits.[Citation1] The Oleaceae family is divided into 29 genera, the most important of which are Fraxinus, Jasminum, Phillyrea, Syringa and Olea.[Citation1] The genus Olea has 35 different species, with the species Olea europaea L. encompassing all cultivated and wild olive trees, including various varieties within this species.[Citation1]
The olive is the fruit of Olea europaea L., and it is characterized as a drupe with a fleshy mesocarp and rich in lipids, with a weight ranging from 2 to >8 g.[Citation1,Citation17] The olive fruit is divided into three parts: (1) the skin, called the epicarp; (2) the pulp or flesh, called mesocarp; (3) and the stone, called woody endocarp.[Citation1,Citation17] This endocarp is a hard shell, located at the center of the fruit, and it has a striated, relatively pointed, ovoid, or spheroid shape, which still contains the seed.[Citation1,Citation17] At the correct moment of maturation, the pulp represents between 65 and 90% of the weight of the fruit, with the stone having between 10 and 25%, the seed between 2 and 5% and the skin with a weight of 1–2% of the fruit.[Citation23] These described percentages will depend on some factors, such as variety, edaphoclimatic conditions, cultivation conditions, type of use and desired state of maturation. Most of the pulp is composed of water, between 70 and 75% in fresh weight, fatty acids (15–30%), carbohydrates (3–6%), proteins (1–3%), tannins (1.5–2%) and other substances such as organic acids, pectins, oleuropein, anthocyanins and minerals.[Citation1,Citation24]
The olives have two destinations: to be consumed as table olives or to produce olive oil. Their destination will depend on the variety of the olive and its state of maturation and, consequently, they can present different colors, from green to purple-black. The growth period of this fruit is longer when compared to other stone fruits, taking between 6 and 7 months for its growth and development, with a maturation period between 25 and 50 days.[Citation1] The olive harvest begins in October and extends until the onset of winter, with the destination of the product, and thus the required level of maturation, it is the parameter responsible for carrying out the harvest.[Citation1,Citation25]
Olive oil extraction: process, products and classification, and by-products
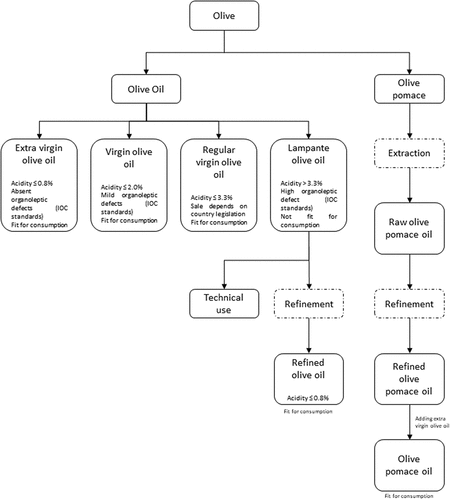
The activity of olive juice extraction aims to generate olive oil and also some bio-waste. This process, along with the definition of the generated products, is internationally regulated according to guidelines provided by the International Olive Council (IOC). In the European Union, it is further regulated by EU Regulation No. 1308/2013, wherein:[Citation26,Citation27]
Virgin olive oils are the oils obtained from the fruit of the olive tree solely by mechanical or other physical means under conditions, particularly thermal conditions, that do not lead to alterations in the oil, and which have not undergone any treatment other than washing, decantation, centrifugation and filtration.[Citation27]
The initial stage of verifying olive extraction products involves laboratory analysis to assess their quality. These products are classified based on their physicochemical and organoleptic characteristics, as well as the method of extraction, including extra virgin, virgin, regular virgin, or lampante olive oil, along with olive pomace oil, as shown in flowchart of .[Citation26]
The olive oil production process begins in the field, with the maintenance and care of the olive trees, from pruning, weeding, pesticide application and others.[Citation4,Citation28] At this stage, there is a high production of solid waste, including branches (from thin to thicker, as well as wood itself) and leaves. Note that the amount of waste generated will depend on weather conditions, the age of the olive tree and its conditions, among others.[Citation4,Citation28] The harvest stage will take place from the fruit maturation information to produce olive oil, thus being a condition for the yield and quality of the product.[Citation3,Citation4] Concomitantly with the harvest stage, a pre-cleaning stage of the olives can be carried out, generating solid residues (leaves and branches) that remain in the olive grove. This stage of the process is crucial to guarantee the quality of the olive oil, as between the harvest and the extraction of the olive oil it cannot take a long time as it can trigger a reduction in the olive’s sensory properties, an increase in acidity, and other undesirable effects.[Citation10,Citation17]
In the mills, the olives will go through a washing process again, in order to remove impurities and foreign materials, such as leaves, stones, damaged olives, earth, and other contaminants.[Citation3,Citation28] This removal takes place with the aid of water, as well as mechanical and pneumatic processes, using vibrating sieves to remove solids that are larger and materials that are lighter than the fruit (such as leaves) are removed with the aid of blowers.[Citation3,Citation28]
With the clean olives, they go through a milling process to reduce their size, breaking the stones, the skin cells and the pulp (hence the vacuoles that have small drops of oil).[Citation29] This process produces a homogeneous paste containing coarse-sized stone fragments. The milling and pitting operation can be carried out using various equipment, including single and double grid hammer mills, stone mills, disk mills, and other machinery.[Citation29] This step is important to aid in good oil drainage, recognizing that this paste can undergo a filtering process and removal of stone fragments, thereby producing a solid residue containing those fragments.[Citation4,Citation29] The paste is formed by a semi-liquid mixture of two different types of solids (hard fragments of the stones and soft fleshy parts of the pulp and peel) and by immiscible liquids (water and oil).[Citation4,Citation29]
The olive paste is taken to one of the most important stages of this process, as it is closely correlated with the quality and nutritional properties of the oil, since it allows a better separation of the oil in the next step of the process, which is the unit operation of malaxation.[Citation30] The malaxation step, the only discontinuous step, is necessary not only to generate oil coalescence, but also to carry out significant physical and chemical changes in the olive paste, responsible for imparting the oil its characteristics.[Citation30] Besides oil coalescence, leading to changes in the rheology of the olive paste, complex enzymatic activities (such as oxidases, hydrolases…) and chain reactions also take place.[Citation30,Citation31] Enzymatic actions generate a change in the balance and solubility of hydrophobic and hydrophilic substances. This occurs through the formation of monoglycerides and diglycerides, the production of carbonyl and short-chain aldehyde derivatives from polyunsaturated fatty acids, and a reduction in oxygen concentration with a concomitant increase in CO2 caused by respiratory metabolism.[Citation30,Citation31] The relationship between temperature and time are crucial parameters in this phase. High temperatures aid in the coalescence and diffusion of substances from the aqueous phase to the oil phase; however, they also lead to enzymatic degradation and loss of volatile compounds due to an increase in vapor pressure.[Citation30]
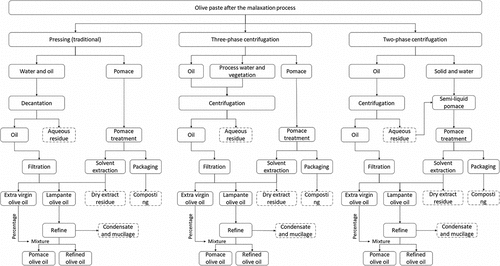
At the end of the malaxation process, the olive paste is sent to the separation stage, because this paste is composed of a phase of insoluble solids (stone pieces and organic semi-solids), aqueous phase (water and soluble compounds) and oil phase (triglycerides and minor compounds).[Citation32] This separation phase can occur by three distinct processes, namely: pressing or traditional, and centrifugation in two or three-phases (occurring in a continuous flow), as observed in the flowchart of .[Citation32]
The pressing method occurs discontinuously, where the olive paste enters into the process between the pressing mats, exerting pressure on them, releasing a solid phase (olive pomace) and a liquid phase, composed of a mixture of water and oil.[Citation1] This process is falling into disuse because, even with modern presses at high pressures, it presents a lower yield in the production of olive oil, still producing a product of inferior quality because it is more susceptible to oxidation and fermentation.[Citation1,Citation32]
The three-phase and two-phase centrifugation systems share the same operating principle, wherein the separation of the oil occurs due to differences in density between it and the other components present in the olive paste. In the three-phase system, the first stage of the process takes place in a decanter, where the three phases are a solid phase (pomace) and two liquid phases, one oily and one aqueous. In this process, it is necessary to add certain amounts of water to improve the separation and cleanliness of the oil, obtaining a more clarified oil and even impurities, both solid and liquid.[Citation1,Citation4] The oil phase centrifuged in this process has about 2–5% of water droplets and solids, so it is necessary to perform a new centrifugation.[Citation1,Citation4] Due to the addition of a certain amount of water, there is a substantial generation of wastewater (the OMWW) that is relatively toxic to the environment, requiring treatment.[Citation1,Citation4] Due to this amount of liquid waste generated, the three-phase system is being replaced by the two-phase system. In this system, the separation generated from the decanter is divided into two phases, an oily phase, and a semi-liquid pasty phase (also known as two-phase wet pomace). This pasty phase is an important residue because it contains high concentrations of organic substances (such as polyphenols).[Citation4,Citation6] As with the three-phase system, the oil phase still undergoes another centrifugation process, and it is necessary to add a small amount of water to improve cleaning and recovery of the oil.[Citation4,Citation6] Oils from extraction by two-phase centrifugation have better oxidative stability, due to a higher concentration of antioxidant compounds (such as oleuropein and ligstroside) when compared to other methods.[Citation33–35]
The traditional method generates the least amount olive pomace (OP), however, this solid waste has the highest concentration of residual oil, in addition to having a lower yield of extraction (). In turn, the two-phase centrifugation is the one that generates the largest amount of this residue.[Citation32,Citation36] Regarding OMWW, the extraction method through a three-phase centrifugation generates a much higher amount than the other methods due to the need to introduce water in this process.[Citation32,Citation36]
Table 1. Mass balance of olive oil extraction processes, from the olive to the oil and residues.[Citation32,Citation36]
With the extraction process carried out, the extract obtained is an extra virgin olive oil that has a certain turbidity which is caused by the presence of solid particles in suspension as well as by the presence of water microdroplets. Such water droplets and solid particles can affect the quality of the oil, promoting deterioration. Therefore, it is necessary to carry out a filtering step of the oil, through a pressure filtration, obtaining a clear liquid.[Citation4,Citation37] From the filtration stage, a small amount of solid waste is generated containing fine particles of solids and the filters, when they are already completely clogged.[Citation4,Citation37]
One of the main residues of olive oil production is OP. This by-product can still be used to obtain other products, namely refined oil, in the generation of energy through the form of biomass, animal feed and even for composting.[Citation4,Citation38]
The refining process is an important step as it removes, total or partially, certain chemical compounds that can affect the quality of the products, simultaneously producing a product that is safe for consumption.[Citation28] Some residues, both solid and liquid, are generated from the steps above described, such as mucilage, bad-smelling condensate from the deodorization operation, decanted material from the gravitational settler, soapstack, and others.[Citation4,Citation28,Citation38–41]
As shown in , based on the distribution of bioactive compounds present in olives, olive oil mainly contains compounds from the group of fatty acids, lignins, and triterpenoids, since the other bioactive compounds are distributed among the waste generated.[Citation28] It should be noted that does not consider the organic compounds present in the leaves and branches.
Table 2. Relative percentage of bioactive compounds present in olive oil and in bio-waste from its extraction, pomace, stones and OMWW.[Citation4,Citation28,Citation38–41]
Olive oil, particularly extra virgin olive oil (EVOO), is primarily composed of monounsaturated fatty acids (MUFAs), with oleic acid representing up to 80% of the total fatty acid content.[Citation42–45] Polyunsaturated fatty acids (PUFAs), with linoleic acid being the major representative, make up about 3–22% of olive oil, while saturated fatty acids (SFAs) constitute about 8–26%.[Citation42–45] In smaller proportions, as previously shown in , olive oil also contains phytochemical compounds that have important biological functions.[Citation42–45] Due to its high content in MUFA, especially oleic acid, this oil has important health effects and implications in dietary intake.[Citation42] MUFAs have been shown to modulate the immune response, a factor of great importance in the treatment of some autoimmune diseases, among various other functionalities.[Citation42] Besides these MUFAs’ properties, phenolic compounds and their antioxidant effects have also been correlated with several important health properties, including anti-atherogenic, anti-tumoral, anti-inflammatory, and antiviral effects.[Citation44,Citation45] One of the most commonly found phenolic compounds in EVOO is hydroxytyrosol, a compound also detectable in red wine, which possesses a strong antioxidant effect related to hydrogen donation.[Citation44,Citation45] The positive effects of hydroxytyrosol in humans and animals have been extensively investigated regarding different forms of ingestion beyond the commonly associated consumption through EVOO, as well as the extraction of hydroxytyrosol and other bioactive compounds from olive oil production residues.[Citation44,Citation45]
By-products from olive oil production
The physicochemical and microbiological characteristics of bioresidues from olive oil production are well known in literature. This includes reports of high phytotoxicity due to a pronounced concentration of phenolic compounds, alcohols, and lipids, alongside a large amount of organic matter ().[Citation31,Citation46,Citation53,Citation54] Based on that, this product cannot be discarded directly into the environment without prior treatment, and they still have an important commercial value, having the capacity to be upcycled. Even with technological evolution, olive oil extraction still produces high amounts of by-products/waste. Approximately 20% of the olive constitutes oil, while the remaining 80% comprises by-products/waste, primarily consisting of leaves and branches of the olive tree, OP, fragments of stones, and OMWW.[Citation31,Citation46,Citation53,Citation54]
Figure 3. Chemical structure of phenolic compounds mostly obtained from bio-waste from olive oil production.[Citation46–52]
![Figure 3. Chemical structure of phenolic compounds mostly obtained from bio-waste from olive oil production.[Citation46–52]](/cms/asset/19cc645c-3f9b-44bd-b5b7-b7ef7f61e779/lfri_a_2354331_f0003_b.gif)
Leaves and bio-waste from olive tree pruning
The batches of olives sent to the mills typically contain small and large branches, pieces of wood, leaves, dirt, and other impurities. It is estimated that annually, pruning or harvesting in olive groves generates approximately 25 kg of bio-waste per tree or between 1.5 and 3 tons per ha, resulting in a substantial amount of biomass.[Citation6,Citation55] This residue is the one that is generated in greater quantity in the olive oil production process, since it comes from the maintenance of the olive trees and the production in the olive oil mills.[Citation6,Citation55] Within all the constituents of this bio-waste, it is observed that about 50% of its weight is due to small branches, while 25% is due to leaves and the other 25% is due to large branches or pieces of wood.[Citation6]
A large part of this biomass, both leaves and branches, are burned in incinerators to generate electricity and are crushed to be used for animal feed, with these financially burdening producers and still being an environmental concern.[Citation28,Citation34] However, an alternative to this problem is the use of this bio-waste as a raw material for creating new products with high added value.[Citation28,Citation34] These products can be utilized in the food and pharmaceutical sectors, leveraging their content of carbohydrates, phenolic compounds, oil, crude protein, fiber, secondary metabolites, and other valuable constituents.[Citation28,Citation34]
The chemical characterization of olive leaves reveals a composition rich in lignocellulosic materials, with a high extraction percentage (between 36 and 52%).[Citation4,Citation28] The leaves typically contain 28–41% lignin, 5–7% cellulose, and 3–4% hemicellulose, with crude protein values for ground and unground leaves averaging 8.10% and 9.34%, respectively.[Citation4,Citation28] The values presented above, as well as the values of the other compounds, may vary in relation to the period of the year of collection, the cultivation conditions, the subspecies, the extractive methods, and the analysis methods.[Citation55] Despite their similarity to lignocellulosic materials, these bioresidues contain significant bioactive compounds in their composition. The compounds with the highest concentrations include oleuropein and its most important degradation product, hydroxytyrosol, as shown in .[Citation47,Citation56,Citation57] These two chemicals are found in high concentrations, particularly in leaves, as they provide a natural defense against predators.[Citation47] In addition to these two compounds, other phenolic/phenolic-related compounds were also identified. These include lignans (such as pinoresinol, 1-acetoxypinoresinol), secoiridoids (like ligstroside and its demethylated derivative), simple phenols (tyrosol), flavonoids (including luteolin-7-O-glucoside and apigenin-7-O-glucoside), flavonols (rutin, kaempferol, quercetin, among others), cinnamic acid derivatives (like verbascoside), and phenolic acids (such as caffeic acid, vanillic acid, cinnamic acid).[Citation4,Citation34,Citation47,Citation55–61]
Table 3. Variation in the quantification of the main phenolic compounds identified in olive oil by-products.
Oleuropein, like other phenolic compounds, is a substance that has important antioxidant and antimicrobial properties, thus being a good alternative to synthetic preservatives and antibiotics. Studies conducted with “taralli,” a typical food from southern Italy, indicate that enriching this food with dried olive leaves yielded a good quality index, both sensorial and physical, compared to the control.[Citation3] It exhibited even better antioxidant activity.[Citation3] Investigations carried out by Liu et al.[Citation77] against pathogens that can be transmitted by food revealed that olive branch extracts, at a concentration of 62.5 mg/mL, completely inhibited the growth of L. monocytogenes, E. coli and S. enteritidis. They also inhibited cell motility and further reduced the production of L. monocytogenes biofilm.[Citation77] In investigations carried out by Cappelin et al.[Citation78] the use of olive leaf extracts for the enrichment of a light craft beer was studied. These scientists observed that olive leaf extract led to an enrichment of beer samples due to its bioactive compounds, wherein the addition of 2% extract resulted on an increase in ABTS+● and DPPH● scavenging ability, and ferric reducing power (28.4%, 449.1%, and 120.5%, respectively).[Citation78] The phenolic acids cumaric, ferulic, and cinnamic were detected in appreciable quantities in the beers, being oleuropein the most abundant compound.[Citation78] The resulting beer possessed a low caloric content, a relatively reduced bitterness, and an enhanced EBC color; however, its foam stability was reduced.[Citation78] In turn, Said[Citation79] investigated the use of olive leaf extracts to maximize the bioactive compounds in EVOO. This researcher optimized the maceration period of the leaf to enhance the stabilization of bioactive compounds in the olive oil matrix, which served as the extracting solvent.[Citation79] With a maceration period of 7 days, this study demonstrated that EVOO can be used as an extracting solvent for olive leaves, aiding in the improvement of resistance and radio stability due to an increase in carotenoids and total chlorophylls.[Citation79]
Olive stones
In the milling step, the olives are crushed, generating an olive paste, noting that the olive stones can also be crushed, while some can be removed in this step of the process. Currently, the removal of fragments from olive stones, after two-phase centrifugation, is being increasingly implemented, as this separation is important to help generate added value for this bioresidue, separating them from the OP.[Citation28] This bioresidue constitutes between 10 and 25% of the weight of the olive fruit and can also be obtained from the table olive industry, being customarily used in domestic boilers and thermoelectric plants, due to its high calorific value, around 18 MJ/kg.[Citation4] They are an important source of dietary fiber and are lignocellulosic materials, having as main components cellulose (20–40%), lignin (25–35%), hemicelluloses (18–32%) and in smaller amounts xylose, galactose, and arabinose.[Citation4,Citation6,Citation28,Citation37] Apart from this lignocellulosic characteristic, it is worth mentioning that olive stones contain considerable concentrations of oil, primarily polyunsaturated fatty acids, notably oleic and linoleic acids.[Citation4,Citation6,Citation28,Citation37] They also contain a notable concentration of various phenolic compounds, including substances of the triterpenoid and secoiridoid class, as shown in .[Citation4,Citation6,Citation28,Citation37]
Due to the antioxidant activity of the phenolic compounds and the high concentration of fiber, studies carried out by Bolek[Citation80] have shown that the powder generated from olive stones can replace up to 15% of the wheat flour in the preparation of biscuits. This substitution does not induce any changes in sensorial properties but enhances antioxidant activity and fiber content.[Citation80] Another use of olive stones involved the creation of a biodegradable package using this bio-waste, named Oliplast, whereupon these investigations are part of a Spanish project called GO-OLIVA.[Citation81] In recent years, the use of activated carbon for adsorption has been under attention due to its potential application in various industrial sectors, including food, chemical, petroleum, nuclear, mining, or pharmaceutical industries.[Citation82] Consequently, several studies have demonstrated the potential use of olive stone as a sorbent for the removal of heavy metal ions from aqueous effluents. Investigations conducted by de Hoces et al.[Citation83] showed that the sorption of metal ions is correlated with the particle size of the olive stone and temperature. They demonstrated that the optimal point is related to particle sizes between 0.355 and 0.250 mm, at a temperature of 80°C, with a sorption of 9.72 mg Cd(II)/g of dried olive stone, during a 20-minute interval.[Citation83] Furfural is a chemical compound of great industrial importance for solvent production or as a basis for synthesizing its derivative solvent, being produced from the dehydration of pentoses present in lignocellulosic material.[Citation84] Montané et al.,[Citation84] showed that it is possible to produce furfural using olive stones as raw material (135 kg per ton of olive stone) through acid hydrolysis at high temperatures (220–240°C), in a short reaction time, using a tubing-bomb reactor system.[Citation84]
Olive mill wastewaters
OMWW (generated from the combination of different steps of the olive oil production process and known by various names such as águas-ruças in Portugal, alpechin in Spain, aqua reflue in Italy, among others) is a bio-waste generated from the combination of different steps of the olive oil production process.[Citation31,Citation34,Citation85,Citation86] This bio-waste is composed of water from the vegetation of the fruits and the fruits themselves.[Citation85,Citation86] It includes water added to the process, water used for washing the olives and the extra virgin olive oil, as well as water from washing equipment and installations, which may still contain residual amounts of olive oil.[Citation85,Citation86]
This liquid bio-waste has a color that varies from dark red to black, with slightly acidic characteristics (pH ≈ 5.0) and high conductivity.[Citation64,Citation85,Citation86] It consists mostly of water (83–92%) and presents a high concentration of phytotoxic compounds (4–16%), including organic acids, easily fermentable proteins, sugars, alcohols, tannins, pectins, and even phenolic compounds.[Citation64,Citation85,Citation86] This variety of compounds, and therefore the physicochemical characteristics, are correlated with the olive variety and the storage time of this bioresidue.[Citation1,Citation65,Citation87] The dispersion of these wastewaters in the environment, without prior treatment, is a major environmental problem. They contain a high concentration of these compounds, with a high chemical oxygen demand (COD varying between 50 and 200 g/L) and a high biochemical oxygen demand (BOD varying between 40 and 170 g/L).[Citation64,Citation65,Citation85–88] Such parameters can cause a change in the color and odor of natural waters, resulting in toxicity for aquatic organisms (with a lethal dose for fish around 8%) and even causing a change in soil quality.[Citation64,Citation65,Citation88]
Around 50 different phenolic compounds have already been identified in the OMWW.[Citation48,Citation64,Citation66] These compounds can be divided into 6 categories, namely benzoic acids and derivatives, cinnamic acids and derivatives, phenyl ethyl alcohols, flavones, secoiridoids, and other phenolic acids and derivatives.[Citation48,Citation64,Citation66] It’s important to highlight that the concentration of such substances varies greatly from article to article, as shown in .[Citation48,Citation64,Citation66] Within these classes of compounds, hydroxytyrosol and tyrosol stand out due to their highest concentration.[Citation49,Citation65,Citation67–71] However, it is still possible to mention other relevant compounds in OMWW. These include caffeic acid, p-coumaric acid, ferulic acid, vanillic acid, gallic acid, p-cresol, resorcinol, luteolin, luteolin-7-glucoside, luteolin-4’-O-glucoside, rutin, quercetin, oleuropein, demethyloleuropein, isoacteoside, ligstroside, and others.[Citation49,Citation65,Citation67–71] A fact worth noting is that the color of this bioresidue is linked to the presence of polymeric phenols, which have a structure like the one observed for lignin.[Citation49]
As previously reported, this bio-waste cannot be dumped directly into the environment due to its phytotoxic effect, thus there are several treatments mentioned in the literature, and the treatment is usually divided into two stages. The first stage is dedicated to the separation of solid and liquid phases through an evaporative process, typically in evaporation ponds.[Citation65–67] This is followed by stages of physical, chemical, and biological treatments, including decantation, sedimentation, coagulation/flocculation, anaerobic digestion, among others.[Citation65–67] However, evaporation pond technique is very slow and has some important disadvantages, like: attracting insects (contributing to the proliferation of pests) due to the odor released by the degradation of organic compounds and possible contamination of groundwater if the pond is not efficiently sealed.[Citation32]
One of the problems related to these processes is that the chemical compounds that have different activities are completely degraded, being transformed into substances of lower added value or with no value. In studies carried out by Yangui et al.[Citation89] it was observed that the use of the OMWW and its extracts, with high concentrations of hydroxytyrosol (around 53%), resulted in a decrease in the viability of the fungus Verticillium dahliae by 4 logarithmic units after 30 minutes of contact time. Therefore, with the use of these materials in tomato crops, an important reduction in the incidence of this fungus was observed, being around 86 and 83% when compared to those with plants without this treatment.[Citation89] It is worth mentioning that, with the removal of phenolic compounds, due to the considerable concentration of nitrogen, potassium, and phosphorus, as well as other inorganic atoms, this by-product can still be used as an important fertilizer for the soil.[Citation89,Citation90] It is observed that in some countries, like Portugal, its use is allowed within a limit of 80 m3/ha per year.[Citation89,Citation90] Another study focused on the use of OMWW as biopesticide for food cultivation was conducted by Boutaj et al.[Citation91] The authors used crude OMWW in toxicity bioassays, employing the spraying technique against Potosia opaca var. cardui Gyllenhal larva, achieving an efficacy against this pest comparable to that of commercial products.[Citation91] This biowaste generated a weight loss similar to Cordus (17% vs. 15%) and a mortality rate close to Kemaban (LT50 = 245.39 h vs. LT50 = 208.01 h) due to the high hydroxytyrosol and tyrosol concentrations.[Citation91] In investigations conducted by Foti et al.,[Citation67] a tangential membrane filtration system was applied to perform extraction followed by concentration of phenolic compounds present in OMWW for application in a commercial orange juice. It was demonstrated that, when compared to the control, the fortified juice (at a 2:250 v/v ratio) did not exhibit any strange taste or odor.[Citation67] Furthermore, a stable concentration of hydroxytyrosol was achieved, compliant with the daily intake recommended by EFSA, after a refrigerated storage period of 60 days.[Citation67]
Olive pomace
OP has the appearance of pinkish mud, quickly oxidizing to a brownish color, and has a slightly acidic pH (pH ≈ 5).[Citation36,Citation86] It is composed of pieces of olive skin, pulp, fragments of stones and seeds, oil, and water, being transported to other companies and sent to large storage containers for spontaneous evaporation, where it is conditioned for long periods.[Citation36,Citation86] Due to this long storage, the increase in olive oil production is correlated with the increase in its generation, causing problems for the olive oil industry and hindering the continuous production of olive oil with high quality standards.[Citation86] The amount of olive pomace generated results from the oil extraction process, producing about 0.80–0.87 kg/kg of olives in the two-phase system (commonly called alperujo) to 0.36–0.43 kg/kg of olives with the pressing system (traditional).[Citation3,Citation6,Citation40] Observing from a world perspective, in the 2021/2022 harvest, 3.1 million tons of olive oil were produced and, consequently, about 12.4 million tons of OP were generated.[Citation92,Citation93]
The chemical composition of OP, thus the concentration in hydrosoluble and liposoluble bioactive compounds, is directly associated to the oil production process, and the greatest difference is evident in the percentage of water.[Citation28,Citation94]
The industrial units that receive OP use this by-product to extract olive pomace oil.[Citation46,Citation87] Initially, it is submitted to an evaporation process, noting that this oil is usually extracted with the aid of organic solvents, generating a refined oil that can be used in food (when it is mixed with small amounts of virgin olive oil).[Citation46,Citation87] After oil removal from olive pomace, the resulting bio-waste is usually burned or used for animal feed.[Citation1,Citation23,Citation69] The high moisture content present in OP from the two-phase systems is the major limiting factor for this extraction.[Citation87,Citation95] It necessitates storage tanks specially made for this process, as well as pumps and tank trucks for proper handling, demanding high financial resources in the form of energy.[Citation87,Citation95] Another problem to be highlighted is that, depending on the temperature and time of the drying process, the formation of benzopyrenes can be promoted, which are limiting compounds for the use of olive pomace oil in a food matrix.[Citation87]
OP has a high range of lignocellulosic materials and an appreciable amount of fatty acids, minerals (being rich in potassium and with a low concentration of toxic heavy metals), and to a lesser extent a range of phenolic compounds, with a high C/N ratio.[Citation37,Citation41,Citation96] The lipid fraction consists mainly of monounsaturated fatty acids, with saturated and polyunsaturated fatty acids. Oleic acid corresponds to about 75% of the total, followed by palmitic, linoleic, and stearic acids.[Citation23,Citation38,Citation40,Citation97] Additionally, there is a high concentration of vitamin E (α-tocopherol, β-tocopherol, α-tocotrienol, and γ-tocopherol).[Citation23,Citation38,Citation40,Citation97]
The most important phytochemical compounds of OP are phenolic compounds, responsible for its phytotoxicity. The most abundant ones include hydroxytyrosol, tyrosol, and comselogoside, along with p-coumaric acid and vanillic acid. Several other compounds were also identified in small concentrations, such as oleacein, oleocanthal, verbascoside, oleuropein, demethyloleuropein, 11-methyloleoside, cinnamic acid, elenolic acid derivatives, caffeic acid, ferulic acid, gallic acid, oleoside, sinapic acid, luteolin, rutin, quercetin, apigenin, hydroxytyrosol-10-β-glucoside, ligstroside, and others, as shown in .[Citation34,Citation38,Citation40,Citation47,Citation56,Citation72] It is worth mentioning that pomace also contains squalene, phospholipids, and sterols.[Citation34,Citation38,Citation40,Citation47,Citation56,Citation72]
As previously mentioned, OP has a great impact on the environment and several investigations have been carried out to use it as an important source of value-added products. One of possibilities is to employ OP as an adsorbent of heavy metals and for sorption of certain herbicides and insecticides (such as imidacloprid) that are present in the soil, thus reducing the potential for leaching and contamination of groundwater.[Citation87] In studies carried out by Nunes et al.[Citation40] an extract of the lipid fraction with high oxidative stability was obtained for possible use in food and cosmetic formulations. This extract serves as a source of antioxidants such as vitamin E, primarily α-tocopherol, the most biologically active form, and monounsaturated fatty acids.[Citation40] These authors also obtained an extract of the aqueous fraction with the same antioxidant characteristics.[Citation40] This is attributed to the presence of phenolic compounds, with a high concentration of hydroxytyrosol (around 54% of the total) and secoiridoids and their derivatives.[Citation40] Lama-Muñoz et al.[Citation98] were able to isolate pectic and xyloglucans oligosaccharides, neutral xylo-oligosaccharides, and acids from alperujo, demonstrating a high possibility for the use of these compounds as stabilizers, emulsifiers, and even gelling agents in the agri-food industry. Studies conducted by Crizel et al.[Citation99] evaluated the potential of adding different concentrations of olive pomace (OP) flour and microparticles in chitosan-based films to create biodegradable packaging. The incorporation of OP into this matrix resulted in changes on its morphology, transforming the film into a more heterogeneous and rough material.[Citation99] With 10% OP, there was a significant increase in tensile strength (22.40 ± 0.22 MPa vs. 16.76 ± 3.51 MPa) of the films without modifications in their original properties.[Citation99] For films containing 30% OP, an effective protective packaging against nuts oxidation for 31 days was achieved, demonstrating the feasibility of these packages.[Citation99] Another work carried out by Lammi et al.[Citation100] explored the possibility of using OP dry fractionation in the production of high-purity cellulose- and stone-rich fractions. It was demonstrated that this fractionation has the potential to be employed for converting OP into valuable fractions through processes that avoid water consumption and the generation of effluents or by-products.[Citation100] Dry fractionation in a ball mill, operating under mild conditions (2 min at a frequency of 15 Hz), was found to be as efficient as wet fractionation, with total yields of 99.4% and 82.1%, respectively.[Citation100] Powders with contrasting biochemical compositions (rich in lignin or cellulose) were produced, and they were thermally stable up to at least 210°C, with potential applications for filler production, simultaneously contributing to the production of a range of biocomposites.[Citation100]
Emerging extraction methods for phenolic compounds
Phenolic compounds have this denomination because they have in their chemical structure one or more aromatic rings linked to one or more hydroxyls, may also contain other organic substrates and some minerals. These compounds normally have a polar characteristic, thus having greater hydrophilicity than lipophilicity, although this hydrophilicity/lipophilicity relationship is closely correlated with the number of phenolic groups and their conjugations.[Citation101,Citation102] These substances are available in plant matrices in different forms, ranging from a monomer to polymers, glycosylated species or aglycones, and even conjugated with proteins/carbohydrates.[Citation103,Citation104]
As demonstrated in the previous topics, bioresidues from the olive oil industry, as well as from several other agri-food industries, are rich in bioactive compounds, such as phenolics, being an important source for obtaining such substances. Currently, one of the major complications in obtaining them, with subsequent use in several other industries, is related to extractive methods. Such methods still require certain technological developments correlated to their economic factor, employability, and availability.
Extraction is a separation unit operation that is influenced by the specific mass diffusion coefficients, in which the chemical substances of interest diffuse from the sample to the extracting medium. Thus, in order to obtain good yields in this process, avoiding structural alterations or co-elution of unwanted species, it is necessary to select a suitable solvent for the substances of interest.[Citation105] Additionally, optimizing the physicochemical conditions of the medium (pH, temperature, time, and others) is essential.[Citation105]
This process only can extract the soluble parts; however, the elements of interest may still be present in the matrix in insoluble conjugates, requiring prior hydrolysis for their release.[Citation105]
The solvent used in the extraction process is one of its key steps, and its effectiveness is linked to the ability to solubilize the substances of interest. This efficiency is observed from the diffusion coefficient of each molecule in relation to the solvent. The polarity of the solvent, together with the samples of interest, is a critical parameter for this process, as it is one of the determinants for the selectivity of the partition system.[Citation106] Normally, polar protic solvents (capable of forming hydrogen bonds) have better results for the extraction of phenolic compounds, namely water and some organic solvents (methanol, ethanol, among others).[Citation34,Citation107] As phenolic compounds have a certain range of polarity, some of these substances may not solubilize very well in a single solvent.[Citation107] Therefore, it is necessary to make a mixture of two or more solvents for adequacy, as can be seen for benzoic and cinnamic acids that have low solubility in relation to organic solvents, but high for water.[Citation107] With growing environmental concerns, research into more sustainable and environmentally friendly extraction processes (e.g. using GRAS (Generally Recognized As Safe) solvents) has also been raising.
Along with the chosen solvent, the methodology used to carry out the extraction is of great importance, and lately many scientists are investigating new green techniques, as well as improving some others, as shown in . The operational parameters, such as time, temperature, and power, are also very relevant to the extraction process. It is necessary to optimize them to avoid deterioration of the target substances and protect the bioactive properties of the extracted phenolic compounds. A good example of this is the increase of temperature, raising the mass diffusivity concomitantly with the reduction of viscosity and surface tension of the medium, however, degradation or oxidation of the target compounds can occur.[Citation121]
Table 4. Green technologies used for the extraction of phenolic compounds from olive oil by-products.
Traditional extraction methods occur through solid-liquid systems (matrix-solvent) with samples in open or closed compartments, over a wide range of time (from minutes to days) and with the absence or presence of a heat source.[Citation122] One of the major problems with these methodologies is the high solvent/sample ratios, leading to potential environmental problems, and the elevated temperatures employed, resulting in the degradation of thermolabile soluble compounds.[Citation122] As a result, emerging methods aim to provide high-quality extracts through the use of alternative solvents, instead of conventional toxic organic solvents. They also focus on achieving high-efficiency extractions, concomitantly with reductions in energy consumption, minimization of waste generated in this process and costs associated with the methods, as shown in .
Table 5. Cost, advantages, and disadvantages of green technologies used to extract bioactive compounds.
Ultrasound-assisted extraction
Ultrasounds are sonic waves that occur in a frequency range between 20 and 2000 kHz, with wavelengths in the millimeter range.[Citation129] These wavelengths are larger than bioactive molecules and macromolecules, so their energy cannot be absorbed directly by these substances.[Citation129] This energy produced from sonic waves produces rapid high/low pressure oscillations in the system, generating the production of vacuum microbubbles (or even nanobubbles) at various points in the propagation directions.[Citation129,Citation130] During consecutive fluctuations of compression/rarefaction, these bubbles conserve energy until their resistance limit is exceeded and they collapse, generating violent explosions.[Citation130] Such explosions produce high shear forces and shock waves (velocity ≈280 m/s, 5000°C, 200 MPa) that propagate in the medium, wherein this phenomenon is known as cavitation.[Citation130] These shear forces and the collisions generated manage to break the cellular microstructure and even decrease the sample particle size.[Citation130] This increase in surface area of extraction helps generate better diffusion and mass transfer of the target molecules from the matrix to the solvent extractor.[Citation130]
The use of the UAE technique to carry out extraction of phenolic compounds is a good resource due to its low cost of implementation and operation. Also, it is only necessary to place the sample to be extracted in contact with the solvent into the equipment, which can be a bath or an ultrasonic probe, and this process can be carried out continuously or in batch.[Citation121,Citation123] This method still has the advantage of helping to protect the environment due to the use of small volumes of solvent. It is normally sustainable, such as water and/or ethanol, due to its ability to increase the diffusion of phenolic compounds to the extracting solvent. This statement is based on Nunes et al.[Citation121] that extracted phenolic compounds from olive pomace in a biphasic system using an ultrasonic probe and water as extractor solvent (5 min; frequency: 20.01 kHz; electrical input: 160 W), obtaining total phenolic content of 401.9 µg GAE/mL.
In addition to the low use of solvent compared to other methods, it has a fast work cycle, thereby minimizing the degradation of the target species and the use of energy and temperature.[Citation131] This enhances the quality of the obtained extracts, making it an equipment with ease of scale-up.[Citation131] This increase in vapor pressure generates a reduction in the efficiency of the UAE caused by the reduction of acoustic cavitation, due to this, this technique is usually carried out at moderate temperatures, with a variation between 20 and 70°C.[Citation132,Citation133] In this method, as the potency increases, an increase in cavitation is observed, thereby resulting in enhanced extraction of phenolic compounds.[Citation134] However, from a certain potency onwards, the formation of free radicals (hydroxyl) that can degrade the target species and reduce the extraction efficiency may occur.[Citation134]
One of the disadvantages of this methodology is that small amounts of samples or raw materials can be processed at the same time, as large volumes of materials require large operational areas, with the use of equipment in series.[Citation123,Citation124] Another disadvantage that is worth mentioning is that the ultrasonic bath has low reproducibility due to the water present in this equipment, reducing the dispersion of energy, caused by the absorption of a portion of it.[Citation123,Citation124] Therefore, to mitigate this problem, probes are gaining more and more importance in this technique.[Citation123,Citation124]
Microwave-assisted extraction
Microwave-assisted extraction uses the energy of non-ionizing microwave radiation (with a power between 100 and 900 W) to generate heating in the mixture between solvent and solute. This transmitted energy is absorbed in the form of thermal energy through varying degrees by these molecules. Absorption through thermal energy occurs because microwaves directly affect, through ionic conduction or dipole rotation, the dipole molecules.[Citation135–137] This causes a greater rotation in these molecules and, therefore, heating without the generation of a thermal gradient by the system and the cracking of hydrogen bonds.[Citation135–137] This increase in temperature helps to increase the porosity of the plant matrix and, consequently, the mass diffusion coefficient through a greater matrix desorption capacity/solvent absorption to bioactive compounds.[Citation135–137] The generation of this heating through the MAE technique is correlated mainly to two operational parameters, the frequency and power. As observed in the ultrasound technique, it’s important to note that this power has a maximum limit correlated with the mass of the sample and extracting conditions.[Citation129,Citation135,Citation138] Above this limit, the target substances in the sample matrix can be degraded due to the thermal effect and solvent evaporation.[Citation129,Citation135,Citation138] One of the advantages of this methodology is that the increase in temperature, aided using microwaves, reduces the need to use large volumes of solvent and still has short extraction times.[Citation138,Citation139]
In addition to optimizing the power used, the selection of the solvent is an extremely important operational parameter. It needs to be polar because microwaves affect the dipole of the molecules.[Citation140,Citation141] Additionally, the solvent should exhibit good heat absorption (as determined by its dielectric constant) and solvation ability for the target molecules.[Citation140,Citation141] It is known that the higher the dielectric constant, the greater the heat absorption as well as the speed of this absorption. Nevertheless, it is not recommended to use solvents with very high dielectric constants to prevent heating from occurring too quickly and extrapolating certain temperature values.[Citation140,Citation141] Thus, in investigations carried out by Macedo et al.[Citation142] an extraction of phenolic compounds from olive pomace was carried out using a system containing water with 2.34% of tannase, at 60°C, 1:15 pomace/water ratio, for 15 min and 175 W. Therefore, the authors obtained a content of total phenolic compounds of 7110.6 mg GAE/kg DW, mainly containing hydroxytyrosol, chlorogenic acid, 4-hydroxyphenylacetic acid, ferulic acid, among others.[Citation142]
Due to the potential of this methodology, a new application of it has been investigated to reduce the thermal effects of thermosensitive substances. This new method is known as vacuum microwave aqueous assisted extraction (VMAAE), which operates under low temperature and pressure conditions, eliminating the need for organic solvents.[Citation105,Citation123,Citation129] The solvent used in this technique is water, because microwaves increase the rotation and ionic conduction of water, thus increasing the diffusion of the target substances from the sample matrix to the solvent.[Citation105,Citation123,Citation129]
One of the disadvantages is that polymeric compounds that have several conjugated hydroxyls (such as tannins) and thermolabile ones (such as anthocyanins) are more susceptible to degradation generated by the action of these microwaves.[Citation105,Citation125]
Pressurized liquid extraction
Extraction with pressurized liquid, also known as accelerated solvent extraction (ASE), is a technique where phytochemical compounds are extracted using solvents at a temperature higher than their boiling temperature. This process is conducted at high pressures, allowing the solvents to remain in the liquid state. The extraction of the target substances present in the sample is normally performed using GRAS solvents with pressures ranging from 4 to 20 MPa and temperatures ranging from 50 to 300°C, resulting in increased capacity of matrix desorption/solvent absorption of bioactive compounds.[Citation123,Citation143] Regardless of the process used, continuous or discontinuous, this technique uses low volumes of a food-grade solvent.[Citation58,Citation144] It requires a short time for extraction and does not need a subsequent filtration step, with fully automated equipment improving reproducibility and quality control between batches.[Citation58,Citation144] This methodology can produce extracts with high purity, helping to improve the signal/noise ratio and the background noise of extract quantification methods, thereby demonstrating its potential for scaling.[Citation126] However, like all techniques, extraction with PLE also has disadvantages, such as: need for high initial investment since the equipment is expensive and low selectivity in several applications because there is a high degree of co-extraction of interfering compounds.[Citation126]
With the increase in temperature, many times above the boiling temperature of solvents, and pressure, to keep the solvent in the liquid phase, there is a reduction in viscosity and surface tension of the solvent. Consequently, the mass diffusion coefficient is increased, reducing the time needed to carry out high-performance extractions as well as preserving the degradation of thermolabile species.[Citation143,Citation145] This increase in mass transfer is also correlated with the improvement in solvent penetration into the matrix due to the formation of pores in it.[Citation143,Citation145] However, the increase in temperature also has a maximum limit, and above this limit the phenolic compounds, especially the thermosensitive ones, begin to degrade.[Citation146]
The operating parameters that have a high influence on this technique are temperature, pressure, and solvent, being these responsible for almost all the information necessary for the extraction of a wide range of bioactive compounds. The characteristics of the solvent, composed of a pure substance or a mixture, must be considered, wherein water has a good relevance because at high pressures and temperatures.[Citation145,Citation147] This effect is due to the reduction of its hydrogen bonds, therefore, becoming qualified to dissolve substances that have a lower polarity, like the potential that ethanol and methanol have in normal temperature and pressure conditions.[Citation145,Citation147] It is also worth emphasizing that water under these conditions has the capacity to break the internal connections of plant matrices, generating an increase in their extraction capacity.[Citation145,Citation147]
Extraction with pressurized liquid has been studied and improved in recent years and in the last two decades a variant of this method has emerged: the ultrahigh-pressure extraction (UHPE).[Citation148] This technique operates in cycles, generating very high-pressure pulses, varying between 100 and 1000 MPa, and subsequently with a pressure relaxation step, working at moderate temperatures (normally varying between 20 and 50°C).[Citation148–150] Because of this, damage to the cell wall is generated, which increases solvent penetration and mass transfer of compounds.[Citation148–150] This results in extracts with better quality and yield, mainly in relation to thermolabile substances, and reduces the time required for extraction.[Citation148–150] In studies carried out by Chen et al.[Citation149] it was observed that the large leaves of yellow tea underwent a modification in their surface and spatial morphology. This occurred after going through the extraction process at ultra-high pressure with a mixture of ethanol/water (75:25) at a temperature of 25°C for 5 minutes.[Citation149]
Supercritical fluid extraction
Supercritical fluids are fluids that have a single phase from the combination of the properties of its liquid and gaseous phases, from the thermodynamic critical point of each substance. This fluid has low surface tensions and viscosities, and high diffusion coefficients (both mass and heat). Therefore, they can protect thermolabile compounds, increasing their penetration possibility in the matrices to be extracted and facilitating the solvation and solubilization capacity of the target substances.[Citation151,Citation152] As noted for pressurized liquid extraction, this technique can be performed in a continuous or discontinuous, and it is performed in a dark vessel and an oxygen-free atmosphere, protecting substances that are more susceptible to oxidation.[Citation105,Citation123,Citation151]
One of the great advantages of using this extraction technique is that the solvents normally used don´t have an environmental impact and are innocuous to food components, therefore, being harmless to human health (being considered by both the FDA and the ESFA).[Citation153,Citation154] One of the most used solvents is CO2, known for its low temperature and critical pressure.[Citation122,Citation154–156] It is non-toxic, non-flammable, and corrosive, and chemically and thermodynamically stable, mitigating the degradation of thermosensitive substances and having a high capacity to solubilize a diverse range of biomolecules.[Citation154–156] CO2 can be easily removed through a decompression process and reused in future extractions, generating a relatively pure phenolic extract, avoiding later purification steps.[Citation154] Based on investigations carried out by Farías-Campomanes et al.[Citation157] for the extraction of polyphenols from pisco manufacturing lees in a supercritical CO2 system at 40°C and 20 MPa, an extractive yield of 10.5 g/100 g DW was obtained. The extraction yielded 10 polyphenols, including gallic acid, protocatechuic acid, vanillic acid, syringic acid, a p-coumaric acid derivative, ferulic acid derivatives, quercetin, and its derivatives, along with others.
Despite all the advantages described over extraction with supercritical CO2, its low polarity reduces its capacity for extracting polar compounds, such as phenolics, which are chemical substances that have hydroxyl and carboxyl groups.[Citation129,Citation158] Therefore, to mitigate this issue, increase the polarity of the solvent by adding relatively small percentages (1–10%) of polar solvents, known as modifiers or incorporators, such as water and organic solvents, can be a strategy.[Citation129,Citation158]
As previously demonstrated, this technique has many advantages, such as performing high-quality extractions in medium periods of time, and it is also possible to automate it, as noted for the technique with pressurized liquid.[Citation127,Citation128] However, this methodology also has some disadvantages, such as: the limitation for the extraction of whole phenolic fractions, causing a loss of polymeric species, a complex balance between solute and solvent.[Citation127,Citation128] Additionally, there is a need for high know-how to use this technique together with high-value instrumentation, as it involves several components.[Citation127,Citation128]
Contribution of emerging extraction methods to the circular economy
From the topics previously discussed, it can be settled that the use of green solvents and emerging methodologies to extract phenolic compounds from olive oil waste/by-products (as well as waste/by-products of other industries) are of great importance. This is to obtain bioactive compounds with a wide range of possible applications together with environmental and economic benefits.
It is still worth highlighting that there is no solvent and/or extractive methodology attributed as the best for obtaining phenolic compounds.[Citation101,Citation159] This is due to the wide variety of physicochemical properties of this vast class of bioactive compounds, as well as the properties of the matrices themselves to be used in this process.[Citation101,Citation159] All the methods discussed above are promising, having a shorter extraction time compared to the classical approach, thereby considerably reducing energy consumption, although this parameter is not normally reported in the literature. Also, there is also the possibility of combining the use of different methodologies.[Citation105,Citation123,Citation125,Citation147,Citation154]
A better knowledge of these technologies will enable progress in the development of new raw materials. These materials are based on the extraction of bioactive compounds from waste from the olive oil production industry and other industries, to be used in manufacturing industries. The use of such waste will prevent it from being discarded into landfills, or even incorrectly, mitigating environmental problems and the depletion of economic resources by producers.[Citation3,Citation39,Citation129,Citation160] This is in line with the concept of Circular Economy, based on the reuse, repair, renewal, and recycling of existing materials and products.[Citation3,Citation39,Citation129,Citation160] However, despite the importance of these Circular Economy values, there are currently a small number of industrial plants that are focused on the processing and recovery of waste.[Citation3,Citation39,Citation129,Citation160] This is due to the economic uncertainties inherent in the cost/benefit ratio of investments in these technologies, as well as the time required for their implementation.[Citation3,Citation39,Citation129,Citation160]
Conclusions
This review demonstrated that the valorization of by-products and bioresidues from the olive oil production industry is a promising solution for solving the problems generated by this industry, as well as generating profitability for these industries. Such profitability remains in the fact that these residues are an important and cheap source to obtain bioactive compounds with high potential for use in various industries. The use of green technologies to carry out the extractive process is an important approach to enable the full use of these products. However, some obstacles, both technical and economic, still need to be overcome. To overcome these challenges, further research into extractive processes is necessary to develop profitable, environmentally friendly, and scalable methods suitable for integration into various industries, aligning with the principles of the Circular Economy.
Author contributions
Conceptualization; T.F.S., R.C.A., M.B.P.P.O.; Data curation; T.F.S.; Funding acquisition; R.C.A., M.B.P.P.O.; Investigation; T.F.S.; Methodology; T.F.S.; Project administration; R.C.A., M.B.P.P.O.; Resources; R.C.A., M.B.P.P.O.; Supervision; R.C.A., M.B.P.P.O.; Validation; R.C.A.; Visualization; T.F.S.; Roles/Writing – original draft; T.F.S.; Writing – review & editing: R.C.A., M.B.P.P.O.
Acknowledgments
T.F. Soares thanks to FCT/MCTES and ESF (European Social Fund) through NORTE 2020 (Programa Operacional Região Norte) for his PhD Grant (ref. 2022.13829.BD). R. C. Alves (ref. CEECIND/01120/2017; DOI 10.54499/CEECIND/01120/2017/CP1427/CT0001) thanks to FCT for funding through the Scientific Employment Stimulus - Individual Call.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kapellakis, I. E.; Tsagarakis, K. P.; Crowther, J. C. Olive Oil History, Production and By-Product Management. Rev. Environ. Sci. Biotechnol. 2008, 7(1), 1–26. DOI: 10.1007/s11157-007-9120-9.
- Elkhateeb, W. A.; Noor, A.; Rashid, A.; Bilal, A.; Musharaf, G.; Akram, M.; Zafar, K.; Daba, G. Current Awareness and Knowledge of Olive Oil. Int. J. Pharm. Chem. Anal. 2022, 9(2), 64–70. DOI: 10.18231/j.ijpca.2022.011.
- Calvano, C. D.; Tamborrino, A. Valorization of Olive By-Products: Innovative Strategies for Their Production, Treatment and Characterization. Foods. 2022, 11(6), 768. DOI: 10.3390/foods11060768.
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M. A.; Simal-Gandara, J. Valorization of By-Products from Olive Oil Industry and Added-Value Applications for Innovative Functional Foods. Food Res. Int. 2020, 137(September), 109683. DOI: 10.1016/j.foodres.2020.109683.
- FAO. FAOSTAT - Statistical Database https://www.fao.org/faostat/en/#data/RT (accessed Jul 26, 2023).
- Selim, S.; Albqmi, M.; Al-Sanea, M. M.; Alnusaire, T. S.; Almuhayawi, M. S.; AbdElgawad, H.; Al Jaouni, S. K.; Elkelish, A.; Hussein, S.; Warrad, M., et al. Valorizing the Usage of Olive Leaves, Bioactive Compounds, Biological Activities, and Food Applications: A Comprehensive Review. Front Nutr. 2022, 9(November), 1008349. DOI: 10.3389/fnut.2022.1008349.
- International Olive Council. The World of Olive Oil. https://www.internationaloliveoil.org/the-world-of-olive-oil/ (accessed Apr 7, 2023).
- Manzanares, P.; Ballesteros, I.; Negro, M. J.; González, A.; Oliva, J. M.; Ballesteros, M. Processing of Extracted Olive Oil Pomace Residue by Hydrothermal or Dilute Acid Pretreatment and Enzymatic Hydrolysis in a Biorefinery Context. Renew. Energy. 2020, 145, 1235–1245. DOI: 10.1016/j.renene.2019.06.120.
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive Mill Wastes: Biochemical Characterizations and Valorization Strategies. Process Biochem. 2013, 48(10), 1532–1552. DOI: 10.1016/j.procbio.2013.07.010.
- Nunes, M. A.; Pimentel, F. B.; Costa, A. S. G.; Alves, R. C.; Oliveira, M. B. P. P. Olive By-Products for Functional and Food Applications: Challenging Opportunities to Face Environmental Constraints. Innovat. Food Sci. Emerg. Technol. 2016, 35, 139–148. DOI: 10.1016/j.ifset.2016.04.016.
- Goldsmith, C. D.; Vuong, Q. V.; Stathopoulos, C. E.; Roach, P. D.; Scarlett, C. J. Ultrasound Increases the Aqueous Extraction of Phenolic Compounds with High Antioxidant Activity from Olive Pomace. LWT. 2018, 89, 284–290. DOI: 10.1016/j.lwt.2017.10.065.
- La Scalia, G.; Micale, R.; Cannizzaro, L.; Marra, F. P. A Sustainable Phenolic Compound Extraction System from Olive Oil Mill Wastewater. J. Clean. Prod. 2017, 142, 3782–3788. DOI: 10.1016/j.jclepro.2016.10.086.
- Tamasi, G.; Baratto, M. C.; Bonechi, C.; Byelyakova, A.; Pardini, A.; Donati, A.; Leone, G.; Consumi, M.; Lamponi, S.; Magnani, A., et al. Chemical Characterization and Antioxidant Properties of Products and By‐Products from Olea Europaea L. Food Sci. Nutr. 2019, 7 (9), 2907–2920. DOI: 10.1002/fsn3.1142.
- da Rosa, G. S.; Vanga, S. K.; Gariepy, Y.; Raghavan, V. Comparison of Microwave, Ultrasonic and Conventional Techniques for Extraction of Bioactive Compounds from Olive Leaves (Olea Europaea L.). Innovat. Food Sci. Emerg. Technol. 2019, 58, 102234. DOI: 10.1016/j.ifset.2019.102234.
- Flamminii, F.; Di Mattia, C. D.; Difonzo, G.; Neri, L.; Faieta, M.; Caponio, F.; Pittia, P. From By-Product to Food Ingredient: Evaluation of Compositional and Technological Properties of Olive-Leaf Phenolic Extracts. J. Sci. Food Agric. 2019, 99(14), 6620–6627. DOI: 10.1002/jsfa.9949.
- Lama-Muñoz, A.; Del Mar Contreras, M.; Espínola, F.; Moya, M.; de Torres, A.; Romero, I.; Castro, E. Extraction of Oleuropein and Luteolin-7-O-Glucoside from Olive Leaves: Optimization of Technique and Operating Conditions. Food Chem. 2019, 293(January), 161–168. DOI: 10.1016/j.foodchem.2019.04.075.
- Rodrigues, F.; Pimentel, F. B.; Oliveira, M. B. P. P. Olive By-Products: Challenge Application in Cosmetic Industry. Ind. Crops Prod. 2015, 70, 116–124. DOI: 10.1016/j.indcrop.2015.03.027.
- Galanakis, C. M.; Aldawoud, T. M. S.; Rizou, M.; Rowan, N. J.; Ibrahim, S. A. Food Ingredients and Active Compounds Against the Coronavirus Disease (COVID-19) Pandemic: A Comprehensive Review. Foods. 2020, 9(11), 1171. DOI: 10.3390/foods9111701.
- Bursać Kovačević, D.; Barba, F. J.; Granato, D.; Galanakis, C. M.; Herceg, Z.; Dragović-Uzelac, V.; Putnik, P. Pressurized Hot Water Extraction (PHWE) for the Green Recovery of Bioactive Compounds and Steviol Glycosides from Stevia Rebaudiana Bertoni Leaves. Food Chem. 2018, 254, 150–157. DOI: 10.1016/j.foodchem.2018.01.192.
- Sarfarazi, M.; Jafari, S. M.; Rajabzadeh, G.; Galanakis, C. M. Evaluation of Microwave-Assisted Extraction Technology for Separation of Bioactive Components of Saffron (Crocus Sativus L.). Ind. Crops Prod. 2020, 145, 111978. DOI: 10.1016/j.indcrop.2019.111978.
- Otero, P.; Quintana, S. E.; Reglero, G.; Fornari, T.; García-Risco, M. R. Pressurized Liquid Extraction (PLE) as an Innovative Green Technology for the Effective Enrichment of Galician Algae Extracts with High Quality Fatty Acids and Antimicrobial and Antioxidant Properties. Mar. Drugs. 2018, 16(5), 156. DOI: 10.3390/md16050156.
- Nagarajan, J.; Krishnamurthy, N. P.; Nagasundara Ramanan, R.; Raghunandan, M. E.; Galanakis, C. M.; Ooi, C. W. A Facile Water-Induced Complexation of Lycopene and Pectin from Pink Guava Byproduct: Extraction, Characterization and Kinetic Studies. Food Chem. 2019, 296, 47–55. DOI: 10.1016/j.foodchem.2019.05.135.
- Obied, H. K.; Prenzler, P. D.; Robards, K. Potent Antioxidant Biophenols from Olive Mill Waste. Food Chem. 2008, 111(1), 171–178. DOI: 10.1016/j.foodchem.2008.03.058.
- Cioffi, G.; Pesca, M. S.; De Caprariis, P.; Braca, A.; Severino, L.; De Tommasi, N. Phenolic Compounds in Olive Oil and Olive Pomace from Cilento (Campania, Italy) and Their Antioxidant Activity. Food Chem. 2010, 121(1), 105–111. DOI: 10.1016/j.foodchem.2009.12.013.
- Shabir, S.; Ilyas, N.; Saeed, M.; Bibi, F.; Sayyed, R. Z.; Almalki, W. H. Treatment Technologies for Olive Mill Wastewater with Impacts on Plants. Environ. Res. 2023, 216(3), 114399. DOI: 10.1016/j.envres.2022.114399.
- European Parliament and Council of the European Union. Regulation (EU) No 1308/2013 of the European Parliament and of the Council of 17 December 2013 Establishing a Common Organisation of the Markets in Agricultural Products and Repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007. Offic. J. Eur. Comm. 2013, 347, 1–184.
- International Olive Council. Designations and Definitions of Olive Oils. https://www.internationaloliveoil.org/olive-world/olive-oil/ (accessed Apr 10, 2023).
- Otero, P.; Garcia-Oliveira, P.; Carpena, M.; Barral-Martinez, M.; Chamorro, F.; Echave, J.; Garcia-Perez, P.; Cao, H.; Xiao, J.; Simal-Gandara, J., et al. Applications of By-Products from the Olive Oil Processing: Revalorization Strategies Based on Target Molecules and Green Extraction Technologies. Trends Food Sci. Technol. 2021, 116, 1084–1104. DOI: 10.1016/j.tifs.2021.09.007.
- Leone, A. Olive Milling and Pitting. In The Extra-Virgin Olive Oil Handbook, Peri, C., (Ed.); Wiley-Blackwell: New Jersey, 2014; pp. 117–126. DOI: 10.1002/9781118460412.ch11.
- Tamborrino, A. Olive Paste Malaxation. In The Extra-Virgin Olive Oil Handbook, Peri, C., (Ed.); Wiley-Blackwell: New Jersey, 2014; pp. 127–137. DOI: 10.1002/9781118460412.ch12.
- Frankel, E.; Bakhouche, A.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Literature Review on Production Process to Obtain Extra Virgin Olive Oil Enriched in Bioactive Compounds. Potential Use of Byproducts as Alternative Sources of Polyphenols. J. Agric. Food. Chem. 2013, 61(22), 5179–5188. DOI: 10.1021/jf400806z.
- Muniz, F. G. Mapeamento De Resíduos E Subprodutos Derivados Da Extração De Azeites Da Região Do Alentejo. Master thesis, Universidade de Lisboa: Lisboa, 2021.
- Pavão, F. M. A.; de, S. A.; Reis, C. Avaliação e Sistematização de Subprodutos: Olivicultura; Centro Nacional de Competências dos Frutos Secos, Ed.; Valor +: Bragança, Portugal, 2020.
- Skaltsounis, A.-L.; Argyropoulou, A.; Aligiannis, N.; Xynos, N. Recovery of High Added Value Compounds from Olive Tree Products and Olive Processing Byproducts. In Olive and Olive Oil Bioactive Constituents, Boskou, D., (Ed.); Academic Press and AOCS Press: Cambridge, 2014; pp. 333–356. DOI: 10.1016/B978-1-63067-041-2.50017-3.
- Basso, C.; Uliana, G. C.; Richards, N. S. P. S. Compostos Bioativos Presentes No Azeite de Oliva e Seus Subprodutos: Revisão Bibliográfica. Res. Soc. Dev. 2022, 11(10), e196111032580. DOI: 10.33448/rsd-v11i10.32580.
- Doula, M. K.; Moreno-Ortego, J. L.; Tinivella, F.; Inglezakis, V. J.; Sarris, A.; Komnitsas, K. Olive Mill Waste: Recent Advances for the Sustainable Development of Olive Oil Industry. In Olive Mill Waste: Recent Advances for Sustainable Management, Galanakis, C.M., (Ed.); Elsevier Inc.: Chania, 2017; pp. 29–56. DOI: 10.1016/B978-0-12-805314-0.00002-9.
- Freitas, L.; Simões, R.; Miranda, I.; Peres, F.; Ferreira-Dias, S. Optimization of Autohydrolysis of Olive Pomaces to Obtain Bioactive Oligosaccharides: The Effect of Cultivar and Fruit Ripening. Catalysts. 2022, 12(7), 788. DOI: 10.3390/catal12070788.
- Nunes, M. A.; Palmeira, J. D.; Melo, D.; Machado, S.; Lobo, J. C.; Costa, A. S. G.; Alves, R. C.; Ferreira, H.; Oliveira, M. B. P. P. Chemical Composition and Antimicrobial Activity of a New Olive Pomace Functional Ingredient. Pharmaceuticals. 2021, 14(9), 923. DOI: 10.3390/ph14090913.
- Alcazar-Ruiz, A.; Garcia-Carpintero, R.; Dorado, F.; Sanchez- Silva, L. Valorization of Olive Oil Industry Subproducts: Ash and Olive Pomace Fast Pyrolysis. Food Bioprod. Process. 2020, 125, 37–45. DOI: 10.1016/j.fbp.2020.10.011.
- Nunes, M. A.; Costa, A. S. G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R. C.; Freitas, V.; Oliveira, M. B. P. P. Olive Pomace As a Valuable Source of Bioactive Compounds: A Study Regarding Its Lipid- and Water-Soluble Components. Sci. Total Environ. 2018, 644, 229–236. DOI: 10.1016/j.scitotenv.2018.06.350.
- Zhao, H.; Kim, Y.; Avena-Bustillos, R. J.; Nitin, N.; Wang, S. C. Characterization of California Olive Pomace Fractions and Their in vitro Antioxidant and Antimicrobial Activities. LWT - Food Sci. Technol. 2023, 180, 114677. DOI: 10.1016/j.lwt.2023.114677.
- Bilal, R. M.; Liu, C.; Zhao, H.; Wang, Y.; Farag, M. R.; Alagawany, M.; Hassan, F. U.; Elnesr, S. S.; Elwan, H. A. M.; Qiu, H., et al. Olive Oil: Nutritional Applications, Beneficial Health Aspects and Its Prospective Application in Poultry Production. Front. Pharmacol Aug 25, 2021, 12, 723040. DOI: 10.3389/fphar.2021.723040.
- Gurdeniz, G.; Ozen, B. Detection of Adulteration of Extra-Virgin Olive Oil by Chemometric Analysis of Mid-Infrared Spectral Data. Food Chem. 2009, 116(2), 519–525. DOI: 10.1016/j.foodchem.2009.02.068.
- Sánchez, R.; Beltrán Sanahuja, A.; Prats Moya, M. S.; Todolí, J.-L. Application of Dispersive Liquid–Liquid Aerosol Phase Extraction to the Analysis of Total and Individual Phenolic Compounds in Fried Extra Virgin Olive Oils. J. Agric. Food. Chem. 2023, 71(28), 10742–10750. DOI: 10.1021/acs.jafc.3c02634.
- Luque-Muñoz, A.; Tapia, R.; Haidour, A.; Justicia, J.; Cuerva, J. M. Direct Determination of Phenolic Secoiridoids in Olive Oil by Ultra-High Performance Liquid Chromatography-Triple Quadruple Mass Spectrometry Analysis. Sci. Rep. 2019, 9(1), 15545. DOI: 10.1038/s41598-019-52060-5.
- Abbattista, R.; Ventura, G.; Calvano, C. D.; Cataldi, T. R. I.; Losito, I. Bioactive Compounds in Waste By-Products from Olive Oil Production: Applications and Structural Characterization by Mass Spectrometry Techniques. Foods. 2021, 10(6), 1236. DOI: 10.3390/foods10061236.
- Araújo, M.; Pimentel, F. B.; Alves, R. C.; Oliveira, M. B. P. P. Phenolic Compounds from Olive Mill Wastes: Health Effects, Analytical Approach and Application As Food Antioxidants. Trends Food Sci. Technol. 2015, 45(2), 200–211. DOI: 10.1016/j.tifs.2015.06.010.
- El-Abbassi, A.; Saadaoui, N.; Kiai, H.; Raiti, J.; Hafidi, A. Potential Applications of Olive Mill Wastewater As Biopesticide for Crops Protection. Sci. Total Environ. 2017, 576, 10–21. DOI: 10.1016/j.scitotenv.2016.10.032.
- Leouifoudi, I.; Zyad, A.; Amechrouq, A.; Oukerrou, M. A.; Mouse, H. A.; Mbarki, M. Identification and Characterisation of Phenolic Compounds Extracted from Moroccan Olive Mill Wastewater. Food Sci. Technol. 2014, 34(2), 249–257. DOI: 10.1590/fst.2014.0051.
- Kisiriko, M.; Anastasiadi, M.; Terry, L. A.; Yasri, A.; Beale, M. H.; Ward, J. L. Phenolics from Medicinal and Aromatic Plants: Characterisation and Potential as Biostimulants and Bioprotectants. Molecules. 2021, 26(21), 6343. DOI: 10.3390/molecules26216343.
- Rueda, P.; Comino, M.; Aranda, F.; Domínguez-Vidal, V.; Ayora-Cañada, A.; J, M. Analytical Pyrolysis (Py-GC-MS) for the Assessment of Olive Mill Pomace Composting Efficiency and the Effects of Compost Thermal Treatment. J. Anal. Appl. Pyrolysis. 2022, 168(April), 105711. DOI: 10.1016/j.jaap.2022.105711.
- Suárez, M.; Romero, M. P.; Ramo, T.; Macià, A.; Motilva, M. J. Methods for Preparing Phenolic Extracts from Olive Cake for Potential Application As Food Antioxidants. J. Agric. Food. Chem. 2009, 57(4), 1463–1472. DOI: 10.1021/jf8032254.
- Leone, A.; Romaniello, R.; Tamborrino, A.; Beneduce, L.; Gagliardi, A.; Giuliani, M.; Gatta, G. Composting of Olive Mill Pomace, Agro‐Industrial Sewage Sludge and Other Residues: Process Monitoring and Agronomic Use of the Resulting Composts. Foods. 2021, 10(9), 2143. DOI: 10.3390/foods10092143.
- Orive, M.; Cebri, M.; Amayra, J.; Zufía, J.; Bald, C. Integrated Biorefinery Process for Olive Pomace Valorisation. Biomass Bioenergy. 2021, 149(March 2019), 106079. DOI: 10.1016/j.biombioe.2021.106079.
- Martínez-Navarro, M. E.; Cebrián-Tarancón, C.; Alonso, G. L.; Salinas, M. R. Determination of the Variability of Bioactive Compounds and Minerals in Olive Leaf Along an Agronomic Cycle. Agronomy. 2021, 11(12), 2447. DOI: 10.3390/agronomy11122447.
- Talhaoui, N.; Gómez-Caravaca, A. M.; León, L.; De la Rosa, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of Phenolic Compounds of “Sikitita” Olive Leaves by HPLC-DAD-TOF-MS. Comparison with Its Parents “Arbequina” and “Picual” Olive Leaves. LWT - Food Sci. Technol. 2014, 58(1), 28–34. DOI: 10.1016/j.lwt.2014.03.014.
- Herrero, M.; Temirzoda, T. N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibañez, E. New Possibilities for the Valorization of Olive Oil By-Products. J. Chromatogr. A. 2011, 1218(42), 7511–7520. DOI: 10.1016/j.chroma.2011.04.053.
- Lama-Muñoz, A.; Del Mar Contreras, M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Content of Phenolic Compounds and Mannitol in Olive Leaves Extracts from Six Spanish Cultivars: Extraction with the Soxhlet Method and Pressurized Liquids. Food Chem. 2020, 320(September 2019), 126626. DOI: 10.1016/j.foodchem.2020.126626.
- Abdel-Razek, A. G.; Badr, A. N.; Shehata, M. G. Characterization of Olive Oil By-Products: Antioxidant Activity, Its Ability to Reduce Aflatoxigenic Fungi Hazard and Its Aflatoxins. Annu. Res. Rev. Biol. 2017, 14(5), 1–14. DOI: 10.9734/ARRB/2017/35065.
- Dias, M. C.; Pinto, D. C. G. A.; Figueiredo, C.; Santos, C.; Silva, A. M. S. Phenolic and Lipophilic Metabolite Adjustments in Olea Europaea (Olive) Trees During Drought Stress and Recovery. Phytochemistry. 2021, 185(January), 112695. DOI: 10.1016/j.phytochem.2021.112695.
- Taamalli, A.; Arráez-Román, D.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Pérez-Sánchez, A.; Herrero, M.; Ibañez, E.; Micol, V.; Zarrouk, M.; Segura-Carretero, A., et al. Use of Advanced Techniques for the Extraction of Phenolic Compounds from Tunisian Olive Leaves: Phenolic Composition and Cytotoxicity Against Human Breast Cancer Cells. Food Chem. Toxicol. 2012, 50 (6), 1817–1825. DOI: 10.1016/j.fct.2012.02.090.
- Lama-Munoz, A.; Romero-García, J. M.; Cara, C.; Moya, M.; Castro, E. Low Energy-Demanding Recovery of Antioxidants and Sugars from Olive Stones as Preliminary Steps in the Biorefinery Context. Ind. Crops Prod. 2014, 60, 30–38. DOI: 10.1016/j.indcrop.2014.05.051.
- NakilcioğluTaş, E.; Ötleş, S. The Optimization of Solid – Liquid Extraction of Polyphenols from Olive Stone by Response Surface Methodology. J. Food Meas. Charact. 2019, 13(2), 1497–1507. DOI: 10.1007/s11694-019-00065-z.
- Cifuentes-Cabezas, M.; Galinha, C. F.; Crespo, J. G.; Cinta Vincent-Vela, M.; Antonio Mendoza-Roca, J.; Álvarez-Blanco, S. Nanofiltration of Wastewaters from Olive Oil Production: Study of Operating Conditions and Analysis of Fouling by 2D Fluorescence and FTIR Spectroscopy. Chem. Eng. J. 2023, 454, 140025. DOI: 10.1016/j.cej.2022.140025.
- Shabir, S.; Ilyas, N.; Saeed, M.; Bibi, F.; Sayyed, R. Z.; Almalki, W. H. Treatment Technologies for Olive Mill Wastewater with Impacts on Plants. Environ. Res. 2023, 216, 114399. DOI: 10.1016/j.envres.2022.114399.
- Bombino, G.; Andiloro, S.; Folino, A.; Lucas-Borja, M. E.; Zema, A. D. Short-Term Effects of Olive Oil Mill Wastewater Application on Soil Water Repellency. Agric. Water Manage. 2021, 244(August 2020), 106563. DOI: 10.1016/j.agwat.2020.106563.
- Foti, P.; Romeo, F. V.; Russo, N.; Pino, A.; Vaccalluzzo, A.; Caggia, C.; Randazzo, C. L. Olive Mill Wastewater as Renewable Raw Materials to Generate High Added-Value Ingredients for Agro-Food Industries. Appl. Sci. 2021, 11(16), 7511. DOI: 10.3390/app11167511.
- Azzam, M. O. J.; Hazaimeh, S. A. Olive Mill Wastewater Treatment and Valorization by Extraction/Concentration of Hydroxytyrosol and Other Natural Phenols. Process Saf. Environ. Prot. 2021, 148, 495–523. DOI: 10.1016/j.psep.2020.10.030.
- Agabo-García, C.; Repetto, G.; Albqmi, M.; Hodaifa, G. Evaluation of the Olive Mill Wastewater Treatment Based on Advanced Oxidation Processes (AOPs), Flocculation, and Filtration. J. Environ. Chem. Eng. 2023, 11(3), 109789. DOI: 10.1016/j.jece.2023.109789.
- Benincasa, C.; Pellegrino, M.; Romano, E.; Claps, S.; Fallara, C.; Perri, E. Qualitative and Quantitative Analysis of Phenolic Compounds in Spray-Dried Olive Mill Wastewater. Front Nutr. 2022, 8(January), 782693. DOI: 10.3389/fnut.2021.782693.
- Daâssi, D.; Lozano-Sánchez, J.; Borrás-Linares, I.; Belbahri, L.; Woodward, S.; Zouari-Mechichi, H.; Mechichi, T.; Nasri, M.; Segura-Carretero, A. Olive Oil Mill Wastewaters: Phenolic Content Characterization During Degradation by Coriolopsis Gallica. Chemosphere. 2014, 113, 62–70. DOI: 10.1016/j.chemosphere.2014.04.053.
- Bavaro, S. L.; D’Antuono, I.; Cozzi, G.; Haidukowski, M.; Cardinali, A.; Logrieco, A. F. Inhibition of Aflatoxin B1 Production by Verbascoside and Other Olive Polyphenols. World Mycotoxin J. 2016, 9(4), 545–553. DOI: 10.3920/WMJ2015.2018.
- Madureira, J.; Melgar, B.; Santos-Buelga, C.; Margaça, F. M. A.; Ferreira, I. C. F. R.; Barros, L.; Verde, S. C. Phenolic Compounds from Irradiated Olive Wastes: Optimization of the Heat-Assisted Extraction Using Response Surface Methodology. Chemosensors. 2021, 9(8), 231. DOI: 10.3390/chemosensors9080231.
- Cravotto, C.; Fabiano-Tixier, A. S.; Claux, O.; Rapinel, V.; Tomao, V.; Stathopoulos, P.; Skaltsounis, A. L.; Tabasso, S.; Jacques, L.; Chemat, F. Higher Yield and Polyphenol Content in Olive Pomace Extracts Using 2-Methyloxolane As Bio-Based Solvent. Foods. 2022, 11(9), 1357. DOI: 10.3390/foods11091357.
- Böhmer-Maas, B. W.; Otero, D. M.; Zambiazi, R. C.; Aranha, B. C. Optimization of the Extraction of Phenolic Compounds from Olive Pomace Using Response Surface Methodology. Revista. Ceres. 2020, 67(3), 181–190. DOI: 10.1590/0034-737X202067030003.
- Peralbo-Molina, Á.; Priego-Capote, F.; Luque De Castro, M. D. Tentative Identification of Phenolic Compounds in Olive Pomace Extracts Using Liquid Chromatography-Tandem Mass Spectrometry with a Quadrupole-Quadrupole-Time-Of-Flight Mass Detector. J. Agric. Food. Chem. 2012, 60(46), 11542–11550. DOI: 10.1021/jf302896m.
- Liu, Y.; McKeever, L. C.; Malik, N. S. A. Assessment of the Antimicrobial Activity of Olive Leaf Extract Against Foodborne Bacterial Pathogens. Front. Microbiol. 2017, 8(FEB), 113. DOI: 10.3389/fmicb.2017.00113.
- Cappelin, E.; Meneguzzi, D.; Hendges, D. H.; Oldoni, T. L. C.; Daltoé, M. L. M.; Marchioro, M. L. K.; da Cunha, M. A. A. Low-Alcohol Light Beer Enriched with Olive Leaves Extract: Cold Mashing Technique Associated with Interrupted Fermentation in the Brewing Process. Electron. J. Biotechnol. 2024, 68, 81–89. DOI: 10.1016/j.ejbt.2024.01.002.
- Said, H. H. UV–Visible Spectral Changes of Gamma Irradiated Olive Leaves Extract. Radiat. Phys. Chem. 2024, 217, 111506. DOI: 10.1016/j.radphyschem.2023.111506.
- Bolek, S. Olive Stone Powder: A Potential Source of Fi Ber and Antioxidant and Its E Ff Ect on the Rheological Characteristics of Biscuit Dough and Quality. Innovat. Food Sci. Emerg. Technol. 2020, 64(June), 102423. DOI: 10.1016/j.ifset.2020.102423.
- Putinja, I. Researchers Develop Compostable Plastic Packaging from Olive Waste. https://www.oliveoiltimes.com/world/researchers-develop-compostable-plastic-packaging-from-olivewaste/81373 (accessed Apr 4, 2023).
- Rodríguez, G.; Lama, A.; Rodríguez, R.; Jiménez, A.; Guillén, R.; Fernández-Bolaños, J. Olive Stone an Attractive Source of Bioactive and Valuable Compounds. Bioresour. Technol. September 2008, 99(13), 5261–5269. DOI: 10.1016/j.biortech.2007.11.027.
- De Hoces, M. C.; De Castro, F. H. B.; García, G. B.; Rivas, G. T. Equilibrium Modeling of Removal of Cadmium Ions by Olive Stones. Environ. Prog. 2006, 25(3), 261–266. DOI: 10.1002/ep.10151.
- Montanã, D.; Salvadã, J.; Torras, C.; Farriol, X. High-Temperature Dilute-Acid Hydrolysis of Olive Stones for Furfural Production. Biomass Bioenergy. 2002, 22(4), 295–304. DOI: 10.1016/S0961-9534(02)00007-7.
- Cayuela, M. L.; Millner, P. D.; Meyer, S. L. F.; Roig, A. Potential of Olive Mill Waste and Compost As Biobased Pesticides Against Weeds, Fungi, and Nematodes. Sci. Total Environ. 2008, 99(1–3), 11–18. DOI: 10.1016/j.scitotenv.2008.03.031.
- Medeiros, R. M. L.; Villa, F.; Silva, D. F.; Júlio, L. R. C. Destinação e Reaproveitamento de Subprodutos Da Extração Olivícola. Sci. Agrár. Parana. 2016, 15(2), 100–108. DOI: 10.18188/1983-1471/sap.v15n2p100-108.
- Moreno, A. D.; Ballesteros, M.; Negro, M. J. Biorefineries for the Valorization of Food Processing Waste. In The Interaction of Food Industry and Environment, Galanakis, C., (Ed.); Academic Press: London, 2019; pp. 1–36. DOI: 10.1016/B978-0-12-816449-5.00005-9.
- Huertas-Alonso, A. J.; Gonzalez-Serrano, D. J.; Hadidi, M.; Salgado-Ramos, M.; Orellana-Palacios, J. C.; Sánchez-Verdú, M. P.; Xia, Q.; Simirgiotis, M. J.; Barba, F. J.; Dar, B. N., et al. Table Olive Wastewater As a Potential Source of Biophenols for Valorization: A Mini Review. Fermentation. 2022, 8 (5), 215. DOI: 10.3390/fermentation8050215.
- Yangui, T.; Sayadi, S.; Gargoubi, A.; Dhouib, A. Fungicidal Effect of Hydroxytyrosol-Rich Preparations from Olive Mill Wastewater Against Verticillium Dahliae. Crop Prot. 2010, 29(10), 1208–1213. DOI: 10.1016/j.cropro.2010.04.016.
- García-Serrano, P.; Brenes, M.; Romero, C.; García-García, P. Reuse of KOH Solutions During Black Ripe Olive Processing, Effect on the Quality of the Final Product and Valorization of Wastewaters as Possible Fertilizer Product. Foods. 2022, 11(12), 1749. DOI: 10.3390/foods11121749.
- Boutaj, H.; Boutasknit, A.; Anli, M.; Ait Ahmed, M.; El Abbassi, A.; Meddich, A. Insecticidal Effect of Olive Mill Wastewaters on Potosia Opaca (Coleoptera: Scarabeidae) Larva. Waste Biomass Valorizat. 2020, 11(7), 3397–3405. DOI: 10.1007/s12649-019-00682-1.
- Sousa, D. A.; Ferreira, L. F. V.; Fedorov, A. A.; Rego, A. M. B.; Ferraria, A. M.; Cruz, A. B.; Berberan-Santos, M. N.; Prata, J. V. Luminescent Carbon Dots from Wet Olive Pomace: Structural Insights, Photophysical Properties and Cytotoxicity. Molecules. 2022, 27(19), 6768. DOI: 10.3390/molecules27196768.
- International Olive Oil. 2020/21 Crop Year: Production Down, Consumption Up. https://www.internationaloliveoil.org/2020-21-crop-year-production-down-consumption-up/ (accessed Feb 6, 2023).
- Duman, A. K.; Özgen, G. Ö.; Üçtuğ, F. G. Environmental Life Cycle Assessment of Olive Pomace Utilization in Turkey. Sustain. Prod. Consum. 2020, 22, 126–137. DOI: 10.1016/j.spc.2020.02.008.
- Muscolo, A.; Papalia, T.; Settineri, G.; Romeo, F.; Mallamaci, C. Three Different Methods for Turning Olive Pomace in Resource: Benefits of the End Products for Agricultural Purpose. Sci. Total Environ. 2019, 662, 1–7. DOI: 10.1016/j.scitotenv.2019.01.210.
- Expósito-Díaz, A.; Miho, H.; Ledesma-Escobar, C. A.; Moral, J.; Díez, C. M.; Priego-Capote, F. Influence of Genetic and Interannual Factors on Bioactive Compounds of Olive Pomace Determined Through a Germplasm Survey. Food Chem. 2022, 378, 132107. DOI: 10.1016/j.foodchem.2022.132107.
- Messad, S.; Bensmail, S.; Salhi, O.; Djouahra-Fahem, D. Effect of Extraction Method on Organoleptic, Physicochemical Properties and Some Biological Activities of Olive Oil from the Algerian Chemlal Variety. Eur. J. Biol. 2022, 81(1), 58–67. DOI: 10.26650/EurJBiol.2022.1109068.
- Lama-Muñoz, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Fernández-Bolaños, J. P. Production, Characterization and Isolation of Neutral and Pectic Oligosaccharides with Low Molecular Weights from Olive By-Products Thermally Treated. Food Hydrocoll. 2012, 28(1), 92–104. DOI: 10.1016/j.foodhyd.2011.11.008.
- de Moraes Crizel, T.; de Oliveira Rios, A.; Alves, V. D.; Bandarra, N.; Moldão-Martins, M.; Hickmann Flôres, S. Active Food Packaging Prepared with Chitosan and Olive Pomace. Food Hydrocoll. 2018, 74, 139–150. DOI: 10.1016/j.foodhyd.2017.08.007.
- Lammi, S.; Barakat, A.; Mayer-Laigle, C.; Djenane, D.; Gontard, N.; Angellier-Coussy, H. Dry Fractionation of Olive Pomace as a Sustainable Process to Produce Fillers for Biocomposites. Powder Technol. 2018, 326, 44–53. DOI: 10.1016/j.powtec.2017.11.060.
- Míguez, C.; Cancela, Á.; Álvarez, X.; Sánchez, Á. The Reuse of Bio-Waste from the Invasive Species Tradescantia Fluminensis as a Source of Phenolic Compounds. J. Clean. Prod. 2022, 336, 130293. DOI: 10.1016/j.jclepro.2021.130293.
- Dolma, S. K.; Singh, P. P.; Reddy, S. G. E. Insecticidal and Enzyme Inhibition Activities of Leaf/Bark Extracts, Fractions, Seed Oil and Isolated Compounds from Triadica Sebifera (L.) Small Against Aphis Craccivora Koch. Molecules. 2022, 27(6), 1967. DOI: 10.3390/molecules27061967.
- Schnarr, L.; Segatto, M. L.; Olsson, O.; Zuin, V. G.; Kümmerer, K. Flavonoids As Biopesticides – Systematic Assessment of Sources, Structures, Activities and Environmental Fate. Sci. Total Environ. 2022, 824, 153781. DOI: 10.1016/j.scitotenv.2022.153781.
- Hinchliffe, G. Novel Biopesticides Based on Recombinant Avidin for Protection of Crops Against Insect Pests. Master’s thesis, Durham University: Durham, 2012.
- Alara, O. R.; Abdurahman, N. H.; Ukaegbu, C. I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4(December 2020), 200–214. DOI: 10.1016/j.crfs.2021.03.011.
- Dossou, S. S. K.; Xu, F.; Cui, X.; Sheng, C.; Zhou, R.; You, J.; Tozo, K.; Wang, L. Comparative Metabolomics Analysis of Different Sesame (Sesamum Indicum L.) Tissues Reveals a Tissue-Specific Accumulation of Metabolites. BMC. Plant Biol. 2021, 21(1), 352. DOI: 10.1186/s12870-021-03132-0.
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients. 2010, 2(12), 1231–1246. DOI: 10.3390/nu2121231.
- İlbay, Z.; Şahin, S.; Büyükkabasakal, K. A Novel Approach for Olive Leaf Extraction Through Ultrasound Technology: Response Surface Methodology versus Artificial Neural Networks. Korean J. Chem. Eng. 2014, 31(9), 1661–1667. DOI: 10.1007/s11814-014-0106-3.
- Martínez-Patiño, J. C.; Gullón, B.; Romero, I.; Ruiz, E.; Brnčić, M.; Žlabur, J. Š.; Castro, E. Optimization of Ultrasound-Assisted Extraction of Biomass from Olive Trees Using Response Surface Methodology. Ultrason Sonochem. 2019, 51, 487–495. DOI: 10.1016/j.ultsonch.2018.05.031.
- Irakli, M.; Chatzopoulou, P.; Ekateriniadou, L. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds: Oleuropein, Phenolic Acids, Phenolic Alcohols and Flavonoids from Olive Leaves and Evaluation of Its Antioxidant Activities. Ind. Crops Prod. 2018, 124, 382–388. DOI: 10.1016/j.indcrop.2018.07.070.
- da Rosa, G. S.; Martiny, T. R.; Dotto, G. L.; Vanga, S. K.; Parrine, D.; Gariepy, Y.; Lefsrud, M.; Raghavan, V. Eco-Friendly Extraction for the Recovery of Bioactive Compounds from Brazilian Olive Leaves. Sus. Mater. Technol. 2021, 28, e00276. DOI: 10.1016/j.susmat.2021.e00276.
- Macedo, G. A.; Santana, Á. L.; Crawford, L. M.; Wang, S. C.; Dias, F. F. G.; de Mour Bell, J. M. L. N. Integrated Microwave- and Enzyme-Assisted Extraction of Phenolic Compounds from Olive Pomace. LWT. 2021, 138, 110621. DOI: 10.1016/j.lwt.2020.110621.
- Chanioti, S.; Tzia, C. Extraction of Phenolic Compounds from Olive Pomace by Using Natural Deep Eutectic Solvents and Innovative Extraction Techniques. Innovat. Food Sci. Emerg. Technol. 2018, 48, 228–239. DOI: 10.1016/j.ifset.2018.07.001.
- Fernandez-Pastor, I.; Fernandez-Hernandez, A.; Perez-Criado, S.; Rivas, F.; Martinez, A.; Garcia-Granados, A.; Parra, A. Microwave-Assisted Extraction versus Soxhlet Extraction to Determine Triterpene Acids in Olive Skins. J. Sep. Sci. 2017, 40(5), 1209–1217. DOI: 10.1002/jssc.201601130.
- Vásquez-Villanueva, R.; Plaza, M.; García, M. C.; Marina, M. L. Recovery and Determination of Cholesterol-Lowering Compounds from Olea Europaea Seeds Employing Pressurized Liquid Extraction and Gas Chromatography-Mass Spectrometry. Microchem. J. 2020, 156, 104812. DOI: 10.1016/j.microc.2020.104812.
- Martín-García, B.; Pimentel-Moral, S.; Gómez-Caravaca, A. M.; Arráez-Román, D.; Segura-Carretero, A. Box-Behnken Experimental Design for a Green Extraction Method of Phenolic Compounds from Olive Leaves. Ind. Crops Prod. 2020, 154. DOI: 10.1016/j.indcrop.2020.112741.
- Caballero, A. S.; Romero-García, J. M.; Castro, E.; Cardona, C. A. Supercritical Fluid Extraction for Enhancing Polyphenolic Compounds Production from Olive Waste Extracts. J. Chem. Technol. Biotechnol. 2020, 95(2), 356–362. DOI: 10.1002/jctb.5907.
- Schievano, A.; Adani, F.; Buessing, L.; Botto, A.; Casoliba, E. N.; Rossoni, M.; Goldfarb, J. L. An Integrated Biorefinery Concept for Olive Mill Waste Management: Supercritical CO2 Extraction and Energy Recovery. Green Chem. 2015, 17(5), 2874–2887. DOI: 10.1039/c5gc00076a.
- Ghoreishi, S. M.; Shahrestani, R. G. Subcritical Water Extraction of Mannitol from Olive Leaves. J. Food Eng. 2009, 93(4), 474–481. DOI: 10.1016/j.jfoodeng.2009.02.015.
- Durante, M.; Ferramosca, A.; Treppiccione, L.; Di Giacomo, M.; Zara, V.; Montefusco, A.; Piro, G.; Mita, G.; Bergamo, P.; Lenucci, M. S. Application of Response Surface Methodology (RSM) for the Optimization of Supercritical CO2 Extraction of Oil from Patè Olive Cake: Yield, Content of Bioactive Molecules and Biological Effects in vivo. Food Chem. 2020, 332, 127405. DOI: 10.1016/j.foodchem.2020.127405.
- Nunes, M. A.; Alves, R. C.; Costa, A. S. G.; Oliveira, M. B. P. P.; Puga, H. Olive Pomace Phenolics Extraction: Conventional Vs Emergent Methodologies. In WASTES - Solutions, Treatments and Opportunities II; Vilarinho, C., Castro, F. and de L Lopes, M., Eds.; CRC Press, Taylor & Francis Group: Porto, 2018; pp. 395–401.
- Barp, L.; Višnjevec, A. M.; Moret, S. Pressurized Liquid Extraction: A Powerful Tool to Implement Extraction and Purification of Food Contaminants. Foods. 2023, 12(10), 2017. DOI: 10.3390/foods12102017.
- Rodríguez De Luna, S. L.; Ramírez-Garza, R. E.; Serna Saldívar, S. O. Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties. Sci. World J. 2020, 2020, 1–38. DOI: 10.1155/2020/6792069.
- Machado, A. P. D. F.; Sumere, B. R.; Mekaru, C.; Martinez, J.; Bezerra, R. M. N.; Rostagno, M. A. Extraction of Polyphenols and Antioxidants from Pomegranate Peel Using Ultrasound: Influence of Temperature, Frequency and Operation Mode. Int. J. Food Sci. Technol. 2019, 54(9), 2792–2801. DOI: 10.1111/ijfs.14194.
- Alara, O. R.; Abdurahman, N. H. Kinetics Studies on Effects of Extraction Techniques on Bioactive Compounds from Vernonia Cinerea Leaf. J. Food Sci. Technol. 2019, 56(2), 580–588. DOI: 10.1007/s13197-018-3512-4.
- Sosa-Ferrera, Z.; Mahugo-Santana, C.; Santana-Rodríguez, J. J. Analytical Methodologies for the Determination of Endocrine Disrupting Compounds in Biological and Environmental Samples. Biomed. Chromatogr. 2013, 7, 23. DOI: 10.1155/2013/674838.
- Raks, V.; Al-Suod, H.; Buszewski, B. Isolation, Separation, and Preconcentration of Biologically Active Compounds from Plant Matrices by Extraction Techniques. Chromatographia. 2018, 81(2), 189–202. DOI: 10.1007/s10337-017-3405-0.
- Sapkale, G. N.; Patil, S. M.; Surwase, U. S.; Bhatbhage, P. K. Supercritical Fluid Extraction. Int. J. Chem. Sci. 2010, 8(2), 729–743. DOI: 10.1201/b17997-15.
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the Extraction Method on the Recovery of Bioactive Phenolic Compounds from Food Industry By-Products. Food Chem. 2022, 378, 131918. DOI: 10.1016/j.foodchem.2021.131918.
- Dai, J.; Mumper, R. J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules. 2010, 15(10), 7313–7352. DOI: 10.3390/molecules15107313.
- Yu, Q.; Li, C.; Duan, Z.; Liu, B.; Duan, W.; Shang, F. Ultrasonic Microwave-Assisted Extraction of Polyphenols, Flavonoids, Triterpenoids, and Vitamin C from Clinacanthus Nutans. Czech J. Food Sci. 2017, 35(1), 89–94. DOI: 10.17221/82/2016-CJFS.
- Poveda, J. M.; Loarce, L.; Alarcón, M.; Díaz-Maroto, M. C.; Alañón, M. E. Revalorization of Winery By-Products as Source of Natural Preservatives Obtained by Means of Green Extraction Techniques. Ind. Crops Prod. 2018, 112(January), 617–625. DOI: 10.1016/j.indcrop.2017.12.063.
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Vegetable Sources. Agronomy. 2017, 7(3), 47. DOI: 10.3390/agronomy7030047.
- Chemat, F.; Rombaut, N.; Sicaire, A. G.; Meullemiestre, A.; Fabiano-Tixier, A. S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason Sonochem. 2017, 34, 540–560. DOI: 10.1016/j.ultsonch.2016.06.035.
- Cassol, L.; Rodrigues, E.; Zapata Noreña, C. P. Extracting Phenolic Compounds from Hibiscus Sabdariffa L. Calyx Using Microwave Assisted Extraction. Ind. Crops Prod. 2019, 133(March), 168–177. DOI: 10.1016/j.indcrop.2019.03.023.
- Alara, O. R.; Abdurahman, N. H.; Ukaegbu, C. I.; Azhari, N. H. Vernonia Cinerea Leaves As the Source of Phenolic Compounds, Antioxidants, and Anti-Diabetic Activity Using Microwave-Assisted Extraction Technique. Ind. Crops Prod. 2018, 122(December 2017), 533–544. DOI: 10.1016/j.indcrop.2018.06.034.
- Chaves, J. O.; de Souza, M. C.; da Silva, L. C.; Lachos-Perez, D.; Torres-Mayanga, P. C.; Machado, A. P.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A. V.; Barbero, G. F., et al. Extraction of Flavonoids from Natural Sources Using Modern Techniques. Front. Chem. 2020, 8 (September), 507887. DOI: 10.3389/fchem.2020.507887.
- Wu, K.; Ju, T.; Deng, Y.; Xi, J. Mechanochemical Assisted Extraction: A Novel, Efficient, Eco-Friendly Technology. Trends Food Sci. Technol. 2017, 66, 166–175. DOI: 10.1016/j.tifs.2017.06.011.
- Pinela, J.; Prieto, M. A.; Barreiro, M. F.; Carvalho, A. M.; Oliveira, M. B. P. P.; Curran, T. P.; Ferreira, I. C. F. R. Valorisation of Tomato Wastes for Development of Nutrient-Rich Antioxidant Ingredients: A Sustainable Approach Towards the Needs of the Today’s Society. Innovat. Food Sci. Emerg. Technol. 2017, 41, 160–171. DOI: 10.1016/j.ifset.2017.02.004.
- Xie, X.; Zhu, D.; Zhang, W.; Huai, W.; Wang, K.; Huang, X.; Zhou, L.; Fan, H. Microwave-Assisted Aqueous Two-Phase Extraction Coupled with High Performance Liquid Chromatography for Simultaneous Extraction and Determination of Four Flavonoids in Crotalaria Sessiliflora L. Ind. Crops Prod. 2017, 95, 632–642. DOI: 10.1016/j.indcrop.2016.11.032.
- Saxena, V.; Arora, N.; Varghese, A.; Shandilya, K. Microwave Assisted Extraction of Moringa Oleifera Leaves and Their Phytochemical Analysis. Int. J. Curr. Res. 2016, 8(3), 27432–27433.
- Macedo, G. A.; Barbosa, P.; Dias, F. F. G.; Crawford, L. M.; Wang, S. C.; Bell, J. M. L. N. D. M. Optimizing the Integration of Microwave Processing and Enzymatic Extraction to Produce Polyphenol-Rich Extracts from Olive Pomace. Foods. 2023, 12(20), 3754. DOI: 10.3390/foods12203754.
- Zhang, Q. W.; Lin, L. G.; Ye, W. C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13(1), 20. DOI: 10.1186/s13020-018-0177-x.
- Tamkutė, L.; Liepuoniūtė, R.; Pukalskienė, M.; Venskutonis, P. R. Recovery of Valuable Lipophilic and Polyphenolic Fractions from Cranberry Pomace by Consecutive Supercritical CO2 and Pressurized Liquid Extraction. J. Supercrit. Fluids. 2020, 159, 104755. DOI: 10.1016/j.supflu.2020.104755.
- Plaza, M.; Turner, C. Pressurized Hot Water Extraction of Bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. DOI: 10.1016/j.trac.2015.02.022.
- Arapitsas, P.; Turner, C. Pressurized Solvent Extraction and Monolithic Column-HPLC/DAD Analysis of Anthocyanins in Red Cabbage. Talanta. 2008, 74(5), 1218–1223. DOI: 10.1016/j.talanta.2007.08.029.
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules. 2016, 21(7), 901. DOI: 10.3390/molecules21070901.
- Wang, Y.; Wang, Z.; Xue, Q.; Zhen, L.; Wang, Y.; Cao, J.; Liu, Y.; Khan, A.; Zhao, T.; Cheng, G. Effect of Ultra-High Pressure Pretreatment on the Phenolic Profiles, Antioxidative Activity and Cytoprotective Capacity of Different Phenolic Fractions from Que Zui Tea. Food Chem. 2023, 409(December 2022), 135271. DOI: 10.1016/j.foodchem.2022.135271.
- Chen, H.; Huang, Y.; Zhou, C.; Xu, T.; Chen, X.; Wu, Q.; Zhang, K.; Li, Y.; Li, D.; Chen, Y. Effects of Ultra-High Pressure Treatment on Structure and Bioactivity of Polysaccharides from Large Leaf Yellow Tea. Food Chem. 2022, 387(March), 132862. DOI: 10.1016/j.foodchem.2022.132862.
- Zhou, J.; Ma, Y.; Jia, Y.; Pang, M.; Cheng, G.; Cai, S. Phenolic Profiles, Antioxidant Activities and Cytoprotective Effects of Different Phenolic Fractions from Oil Palm (Elaeis Guineensis Jacq.) Fruits Treated by Ultra-High Pressure. Food Chem. 2019, 288(March), 68–77. DOI: 10.1016/j.foodchem.2019.03.002.
- Šulniūtė, V.; Pukalskas, A.; Venskutonis, P. R. Phytochemical Composition of Fractions Isolated from Ten Salvia Species by Supercritical Carbon Dioxide and Pressurized Liquid Extraction Methods. Food Chem. 2017, 224, 37–47. DOI: 10.1016/j.foodchem.2016.12.047.
- Torres-Ossandón, M. J.; Vega-Gálvez, A.; López, J.; Stucken, K.; Romero, J.; Di Scala, K. Effects of High Hydrostatic Pressure Processing and Supercritical Fluid Extraction on Bioactive Compounds and Antioxidant Capacity of Cape Gooseberry Pulp (Physalis Peruviana L.). J. Supercrit Fluids. 2018, 138(May), 215–220. DOI: 10.1016/j.supflu.2018.05.005.
- Salazar, M. A. R.; Costa, J. V.; Urbina, G. R. O.; Cunha, V. M. B.; Silva, M. P.; Bezerra, P. D. N.; Pinheiro, W. B. S.; Gomes-Leal, W.; Lopes, A. S.; Carvalho Junior, R. N. Chemical Composition, Antioxidant Activity, Neuroprotective and Anti-Inflammatory Effects of Cipó-Pucá (Cissus Sicyoides L.) Extracts Obtained from Supercritical Extraction. J. Supercrit. Fluids. 2018, 138(October 2017), 36–45. DOI: 10.1016/j.supflu.2018.03.022.
- Uwineza, P. A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules. 2020, 25(17), 3847. DOI: 10.3390/molecules25173847.
- Týskiewicz, K.; Konkol, M.; Rój, E. The Application of Supercritical Fluid Extraction in Phenolic Compounds Isolation from Natural Plant Materials. Molecules. 2018, 23(10), 2625. DOI: 10.3390/molecules23102625.
- Wrona, O.; Rafińska, K.; Możeński, C.; Buszewski, B. Supercritical Fluid Extraction of Bioactive Compounds from Plant Materials. J. AOAC Int. 2017, 100(6), 1624–1635. DOI: 10.5740/jaoacint.17-0232.
- Farías-Campomanes, A. M.; Rostagno, M. A.; Coaquira-Quispe, J. J.; Meireles, M. A. A. Supercritical Fluid Extraction of Polyphenols from Lees: Overall Extraction Curve, Kinetic Data and Composition of the Extracts. Bioresour. Bioprocess. 2015, 2(1), 45. DOI: 10.1186/s40643-015-0073-5.
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. New Perspective in Extraction of Plant Biologically Active Compounds by Green Solvents. Food Bioprod. Process. 2018, 109, 52–73. DOI: 10.1016/j.fbp.2018.03.001.
- Benvenutti, L.; Zielinski, A. A. F.; Ferreira, S. R. S. Which Is the Best Food Emerging Solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. DOI: 10.1016/j.tifs.2019.06.003.
- Mariatti, F.; Gunjević, V.; Boffa, L.; Cravotto, G. Process Intensification Technologies for the Recovery of Valuable Compounds from Cocoa By-Products. Innovat. Food Sci. Emerg. Technol. January, 2021, 68, 102601. DOI: 10.1016/j.ifset.2021.102601.