ABSTRACT
There is conflicting evidence whether single-suture craniosynostosis (SSC), is linked to adversities of cognitive development. To assess the evidence for a link between SSC and cognition, a systematic literature search was conducted and eligible studies assessed for inclusion by two independent readers. Forty-eight studies met inclusion criteria. Small to medium but persistent effects on both general and some specific cognitive functions across age bands were found in higher quality studies for SSC overall. There was limited evidence for effects related to surgical correction. Methodologies varied substantially and there was a lack of longitudinal studies using broad assessment batteries.
Introduction
Craniosynostosis (CS) is a congenital condition in which the sutures of the cranial bones in the developing infant have fused in utero, restricting and altering the growth of the skull. The condition affects approximately one in every 1.300 to 2,500 births (DiRocco et al., Citation2009; Lee et al., Citation2012; Tarnow et al., Citation2022). Depending on the number and location of affected cranial sutures, the condition can be subdivided into single-suture (SSC) or complex CS. SSC affects either sagittal, metopic, unicoronal or lambdoid sutures (Governale, Citation2015), while complex CS signifies multi-sutural fusion and/or association with a craniofacial or genetic syndrome. Approximately 200 genetic conditions have been identified as involved in the etiology of CS, but a vast number of cases are still classified as non-syndromic craniosynostosis (NSC) without an as of yet identified genetic cause (Lee et al., Citation2012; Tarnow et al., Citation2022). In recent years, significant advances have been made with regards to genetic screening in the field and many cases formerly labeled NSC have been found to have a genetic cause though not yet deemed syndromic in nature and scope (Timberlake et al., Citation2019). However, empirical studies frequently simply refer to the condition as SSC without reference to a putative cause, a term which will be used in the present review since it is still the most inclusive and widespread term referring to the patient group of interest.
Apart from the esthetic effects of the skull deformity caused by CS, the condition may also restrict the space available for the growth of the infant’s developing brain. There is a well-known risk for raised intracranial pressure in some forms of CS, particularly in certain genetic syndromes such as Crouzon and Apert (Tamburrini et al., Citation2005). In SSC, raised intracranial pressure has mainly been associated with sagittal synostosis (Wall et al., Citation2014). Moreover, it has been suggested that the deformation of the skull and the underlying brain as such may be harmful for brain development, irrespective of intracranial hypertension. However, a link between either intracranial hypertension or deformity and delayed cognitive development has not been established (Knight et al., Citation2014). SSC is usually treated by surgical intervention during the infant’s first year of life, aiming to correct deformity and prevent any harmful effects on the growing brain, the balance of these two indications varying depending on the type of craniosynostosis (Governale, Citation2015).
Cognition is a complex phenomenon encompassing a host of intrapsychic processing in both humans and animals, affecting behavior in various ways (Lezak et al., Citation2012). Cognition can be conceptualized both as higher-order functions such as memory or general intelligence but also as a wide range of specific abilities such as focused attention, working memory or visuospatial reasoning that together comprise and enable these higher-order functions to operate (Kaplan & Sacuzzo, Citation2017). Traditionally, this distinction between higher-order and more specific abilities was established on psychometric grounds. However, since the advent of functional neuroimaging techniques, this model has also received an increasingly robust validation on structural grounds, with the mapping of cortical networks in the human brain serving higher-order functions and specific regions of the brain serving more specific functions (Tomasi & Volkow, Citation2011; Woolgar et al., Citation2010; Zuo et al., Citation2012).
Syndromic CS has been linked to a variety of adverse neurodevelopmental outcomes with cognitive underpinnings, such as an increased prevalence of intellectual disability (ID) and attention-deficit/hyperactivity disorder (ADHD) (Fernandes et al., Citation2016; Kruszka et al., Citation2016). The findings regarding SSC are contradictory: Some studies based on register data regarding clinical diagnoses across the lifespan or long-term follow-up of SSC patients have reported an increased prevalence of neurodevelopmental disorders similar to those found in syndromic CS (Becker et al., Citation2005; Chieffo et al., Citation2010; Tillman et al., Citation2020). However, direct assessment of general cognitive functions such as developmental or intelligence quotients in both younger as well as school-aged children have not found definitive evidence of global cognitive deficits in children with SSC compared to healthy controls or test norms (Osborn et al., Citation2019, Citation2021). In contrast, cognitive deficits in more specialized functions such as attention and visuospatial processing have been reported in SSC at least from school age (Chieffo et al., Citation2010; Kljajić et al., Citation2020; van der Meulen et al., Citation2008). Some studies have also suggested that factors related to treatment, such as the age at which corrective surgery was performed (Patel et al., Citation2014) or the type, amount and duration of anesthetics used during the operation (Naumann et al., Citation2012) may impact long-term cognitive outcomes in these patients.
As outlined above, the study of cognition in SSC has expanded substantially since the early 2000s, but with conflicting results. Studies in the field have utilized a wide range of data sources, designs and methods for data collection and several literature reviews as well as two meta-analyses have been conducted to provide more synthesized accounts of these data (Kapp-Simon et al., Citation2007; Knight et al., Citation2014; Osborn et al., Citation2019, Citation2021; Speltz et al., Citation2004). However, a thorough review that formally assesses the quality of these studies to encompass the contradictory findings is still lacking. The aim of the present review was therefore to assess the current state of knowledge regarding cognitive development in relation to SSC across the lifespan, in relation to sutural subtypes and surgical interventions, as well as to assess the quality of the basis of this knowledge.
Methods
Inclusion criteria and literature search
To address the issue of whether there is a link between SSC and deficient cognition, the inclusion criteria were formulated as follows: using the Population, Intervention, Comparison, Outcome, Study (PICOS) format (Page et al., Citation2021). Being mindful of the recent advances in genetics in the field, it was nonetheless decided to retain the SSC label in the search strategy since the term was deemed to best encompass the population of interest across the time period studied.
Population: Studies with human subjects with SSC as the main condition, excluding known craniofacial syndromes such as Crouzon/Pfeifer, Apert, Muenke and Saethre Chotzen.
Intervention: Studies employing cognitive measures using structured assessment including all forms of direct psychometric testing.
Comparison: Studies using control groups or test norms as comparisons.
Outcome: Studies reporting outcomes based on cognitive assessment or test scores in sufficient detail to enable thorough evaluation of quality (i.e., not a conference proceeding or brief report).
Study design: Empirical studies written in English, not considered a case report (i.e., studies based on samples with less than five participants were excluded).
The following databases were searched in March and August 2021: PubMed, CINAHL, PsycInfo and EmBase. The Medical Subject Heading (MESH)/index search terms were “craniosynostosis AND cognition.” In order to ascertain that the search terms did not limit the scope of this review, a repeated search of PubMed was performed in August 2021 using the MESH major topics “mental processes AND craniofacial abnormalities.” The initial search rendered 643 publications for screening after removal of duplicates.
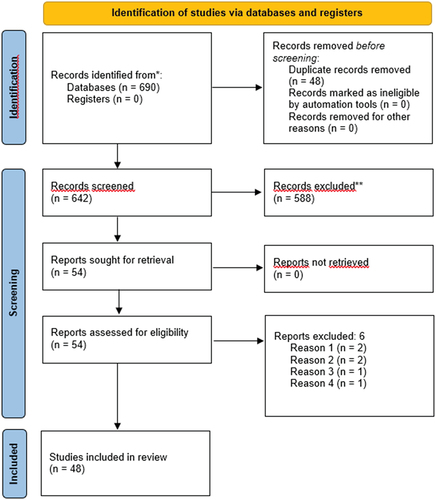
Two independent reviewers (KO, MF) assessed all articles considered for inclusion based on the PICOS using titles and abstracts, resulting in 54 studies being deemed eligible for full-text review. After independent full-text review by the same reviewers, 48 studies were included in the study. Studies based on overlapping samples were only excluded if the same set of measures was used in two articles. In both steps of article inclusion, disagreement between reviewers was resolved using consensus. A summary of the screening and inclusion process is displayed in . The screening and inclusion process was performed in Covidence software (Veritas International Ltd, Citation2014).
Data analysis
Relevant data from the 48 included articles was assessed independently and in full by the two reviewers using a standardized extraction form based on the National Institute of Mental Health (NIMH) Guidelines for assessment of quality in correlational studies (Se App. 1). The two reviewers then compared notes and resolved disagreements on assessment of individual items in the NIMH guidelines by consensus. Additionally, the same reviewers independently assigned a Grading of Recommendations, Assessment, Development and Evaluations (GRADE; see ) (Brozek et al., Citation2009) score for overall study quality from (+) to (++++) based on the NIMH guidelines assessment. In this final quality assessment, consensually high ratings of a clear definition of inclusion criteria and an adequate sample size were given a higher weight than other aspects of quality. For instance, two studies deemed to have equally clearly defined research questions and reliability of measures could be deemed of differing quality if one study used a larger sample size and included more stringent inclusion criteria. The items used to determine aspects of quality can be referenced in the extraction template in Appendix 1. Initial agreement between raters regarding GRADE scores was substantial (85.4%, Kappa = .76), with disagreements being resolved by consensus. As in the case of the screening and inclusion process, all quality assessments were performed, cross-checked and resolved using the Covidence software (Veritas International Ltd, Citation2014).
Table 1. Description of GRADE scores by level of confidence.
Results
An overview of key data on overall features extracted from the 48 included studies is summarized in . In total, the studies included data from 22 different cohorts.
Table 2. Summary of overall features of the included studies.
A majority of the included studies (n = 27) had been conducted in US populations with a substantial minority (n = 17) having been conducted in European settings. Only a few studies had been conducted in Asian (n = 2) or Australian (n = 2) populations, and no studies based on populations from other parts of the world were included. A substantial minority of the studies (n = 19) used data collected from individuals across several age bands in the same analyses. Key features regarding the included studies as well as the final GRADE scores and quality assessments based on the NIMH guidelines are summarized in . In studies that reported both means and standard deviations, and when corresponding data was available for control groups or population norms, Cohen’s d was calculated for effect size estimation. In these calculations, d was determined using the appropriate formulas for independent or matched samples expressing differences in means between SSC and control groups or population means taken from test norms as pooled or test norm standard deviations (Kotz et al., Citation1988).
Table 3. Summary of included studies with description of participants as well as cognitive measures used.
Table 4. Summary of quality assessments and final GRADE score of the included studies.
Summary of findings by subject areas
In the following sections, results are summarized according to developmental aspects (age), effects of surgery, factors related to synostosis (including suture subtypes), demographics, and assessment issues. Since studies on surgery primarily involve preschool participants, studies on this age group are mainly presented under the section on effects of surgery. In the text, studies are grouped by main findings and assessed quality and are individually referenced in .
Age effects
Pre-surgical infant development
A third of the studies (n = 16) included measurement of cognitive development during infancy, before surgery had been conducted. The larger set of these studies (n = 9) found significant effects of SSC on measures of DQ/IQ before surgery. However, the vast majority of these studies were of very low to low quality, GRADE score (+) to (++) (Arnaud et al., Citation2002; Becker et al., Citation2005; Bellew et al., Citation2005, Cohen et al., Citation2004; Da Costa et al., Citation2012; Fontana et al., Citation2017; Panchal et al., Citation2001; Shimoji et al., Citation2015) and a single study of moderate (+++) quality (Kapp-Simon et al., Citation2005). Effect sizes varied widely in that some studies, especially retrospective studies of clinical records using norm data for comparisons, found effect sizes of up to d = −2, implying IQ scores consistent with ID, while other studies found effect sizes of up to d = −1 in late operated children (after age 1 year). A smaller number of studies (n = 5) of low quality (++) found minimal to no effects of SSC on cognition (Kapp-Simon, Citation1998; Mathijssen et al., Citation2006; Shipster et al., Citation2007; Speltz et al., Citation1997; Warschausky et al., Citation2005). A small number of studies (n = 2) utilized a control group, controlled statistically for confounders and were assessed as high-quality studies (++++) (Speltz et al., Citation2007; Starr et al., Citation2007). Data in these two studies were both drawn from the same longitudinal multisite study in the US; the Infant Learning Project, suggesting small-to-medium effects on general cognition, effects that were further attenuated and becoming small to minimal when potential confounders were accounted for.
School age and adolescence development
A majority of the studies (n = 26) concerned school-aged individuals aged 6 to mid-adolescence. Twenty of these reported effect sizes, which permitted comparison of results.
Only one study in this age band, deemed as of low quality (++), reported substantial and global cognitive difficulties among subjects with SSC (Becker et al., Citation2005). In contrast, a large set of the studies in this age band (n = 11), of very low (+) to moderate (+++) quality, reported average global IQ, academic achievement or language scores when compared to test norms (Alperovich et al., Citation2021; Bellew & Chumas, Citation2015; Chieffo et al., Citation2010; Chuang et al., Citation2021; Da Costa et al., Citation2006; Hashim et al., Citation2014; Kljajić et al., Citation2019; Magge et al., Citation2002; van der Meulen et al., Citation2008, Shipster et al., Citation2003; R. T. Wu, Gabrick, et al., Citation2020).
One set of studies (n = 5), of moderate-to-high quality, (+++) to (++++), used data from the same US multicenter study and assessed both global and specific cognitive functions in a stringently defined age band of primary school-age children with SSC in comparison with healthy controls (Collett et al., Citation2017; Cradock et al., Citation2015; Kapp-Simon et al., Citation2016; Speltz et al., Citation2015; Wallace et al., Citation2016). The results from these studies indicated that SSC children had lower global IQ scores, with small overall effects, d = −0.16 to −0.26 (Cradock et al., Citation2015; Speltz et al., Citation2015). Similar effects were observed for visuomotor functions, memory, language, executive functions and attention (Collett et al., Citation2017; Kapp-Simon et al., Citation2016; Wallace et al., Citation2016). Comparable effects were also found in studies (n = 3) of low (++) to moderate (+++) quality regarding other specific cognitive functions such as conceptual reasoning and verbal short-term memory (Virtanen et al., Citation1999), aspects of attention (Kljajić et al., Citation2019) and visual motor integration and perception (R. T. Wu, Gabrick, et al., Citation2020) in this age band.
Adulthood cognitive functioning
Only a small number of studies (n = 4) included data from adult patients, and none of these reported data separately for this age group, primarily due to small samples (Becker et al., Citation2005; Boltshauser et al., Citation2003; Hashim et al., Citation2014; Patel et al., Citation2014).
Effects of surgery
Effects of surgery at infant and preschool ages
A substantial minority of the included studies (n = 10) examined cognitive development before and after surgery in infants and preschoolers. Results from the studies assessing post-surgical cognitive status in infants with SSC varied widely. One group of studies (n = 6) of very low (+) to low (++) quality yielded results that reflected vast improvements in at least one domain of DQ following surgery (Arnaud et al., Citation2002; Bellew et al., Citation2005, Citation2011; Cohen et al., Citation2004; Fontana et al., Citation2017; Shimoji et al., Citation2014). However, studies (n = 5) of higher overall quality, (++) to (++++), did not find similar improvements post-surgery but rather persistent small to moderate deficits in global cognition compared to controls or test norms, or no effect of surgery (Kapp-Simon, Citation1998; Speltz et al., Citation1997; Starr et al., Citation2007, Citation2012; van der Vlugt et al., Citation2017).
A small number of studies (n = 5) examined cognition in preschoolers after surgery but did not utilize longitudinal designs that enabled assessment of the effects of surgery per se. Most of these studies (n = 4), deemed of low quality (++), did not find any deficits in global cognition compared to test norms (Da Costa et al., Citation2012; Mathijssen et al., Citation2006; Shipster et al., Citation2003) and no differences in memory or response inhibition compared to controls in a study of high quality (++++) (Toth et al., Citation2008). Only one study of low quality (++) indicated moderate-to-severe deficits in global cognition in this age group post-surgery (Byun et al., Citation2018) and sampled late operated children (mean age at operation >2 years).
A small set of studies (n = 6) of very low (+) to low (++) quality were in part or entirely dedicated to the assessment of unoperated patients. Herein, one study (Boltshauser et al., Citation2003) found mild deficits in attention, processing speed, learning and memory in patients compared to sibling controls of varying ages. Similarly, mild effects on global cognition or no effect compared to test norms were seen in studies of infants and at preschool age (Bellew et al., Citation2005; Da Costa et al., Citation2012; Kapp-Simon, Citation1998; Warschausky et al., Citation2005). One study of low quality (++) found deficits in aspects of DQ in unoperated children with some indications of deterioration over time (Bellew et al., Citation2011).
Long-term effects of factors associated with surgical intervention
A substantial minority of studies (n = 19) reported associations between aspects related to surgery and cognitive outcomes. A smaller number of these studies (n = 6) indicated beneficial effects of early operation. Three of these, of low (++) to moderate (+++) quality reported greater cognitive deficits in preschool and school-aged individuals operated after age one (Arnaud et al., Citation2002; Byun et al., Citation2018; Patel et al., Citation2014) and another study of moderate quality (+++) found statistically significant but small to negligible effects on both IQ, attention and executive function in school age (Hashim et al., Citation2014), with another two studies of low quality (++) yielding similar but inconclusive results across the preschool and school age bands (Shipster et al., Citation2003; Speltz et al., Citation1997). A greater number of studies (n = 10), of variable quality (+) to (++++), did not report this association (Collett et al., Citation2017, Cohen et al., Citation2003; Da Costa et al., Citation2012; Kapp-Simon, Citation1998; Magge et al., Citation2002; Mathijssen et al., Citation2006; Starr et al., Citation2007, Citation2012; Toth et al., Citation2008; Virtanen et al., Citation1999).
Two studies of low (++) to moderate (+++) quality explored the effects of the duration of exposure to surgery and associated use of anesthesia on cognitive outcomes and reported lower test scores in individuals exposed to longer surgeries and greater exposure to anesthetics, with a stronger association to the latter (Naumann et al., Citation2012; J. van der Vlugt et al., Citation2021). One study of low quality (++) (Alperovich et al., Citation2021), found a beneficial effect of cranial vault remodeling when compared to spring-assisted surgery and another study of similar quality (++) did not find any negative effects on cognition from reoperation (Chuang et al., Citation2021).
Suture type and other factors related to synostosis
Type of suture – sagittal subtype
A majority of the studies (n = 28) included children with the sagittal suture type and reported data in a manner that enabled interpretation. In younger individuals (infants to preschoolers), several studies (n = 8) of varying quality, (+) to (++++), reported average general mental and motor development (Bellew et al., Citation2005, Citation2011; Kapp-Simon, Citation1998, Cohen et al., Citation2002; Shipster et al., Citation2003; Speltz et al., Citation1997; Starr et al., Citation2007, Citation2012). A smaller number of studies (n = 3) of similar varying quality, (++) to (++++), in the same age group reported moderate to strong effects (d = −0.5 to −1.0) of cognitive deficits in sagittal SSC compared to test norms, effects comparable to other subtypes (Byun et al., Citation2018; Da Costa et al., Citation2012; Speltz et al., Citation2007).
In school-age children, results were more unambiguous. A large set of the studies in this group (n = 11), of varying quality, (+) to (++++), reported less extensive cognitive deficits in subjects with sagittal SSC compared to other subtypes, or average cognitive functioning in comparison to test norms (Alperovich et al., Citation2021; Boltshauser et al., Citation2003; Chieffo et al., Citation2010; Chuang et al., Citation2021; Collett et al., Citation2017; Hashim et al., Citation2014, Junn et al., Citation2021; Kapp-Simon et al., Citation2016; Patel et al., Citation2014, Shipster et al., Citation2003; Speltz et al., Citation2015; Wallace et al., Citation2016). A smaller number of studies (n = 6), of very low (+) to moderate (+++), quality reported milder cognitive deficits in sagittal SSC in IQ (Da Costa et al., Citation2006), in specific functions such as language and auditory memory (Virtanen et al., Citation1999) and attention (Kljajić et al., Citation2020), or uneven IQ profiles in the presence of average general IQ (Bellew & Chumas, Citation2015; Kljajić et al., Citation2019; Magge et al., Citation2002).
A smaller set of studies (n = 6) of low (++) to moderate (+++) quality reported separate indices for verbal and perceptual reasoning on IQ tests in school age participants with sagittal synostosis (Alperovich et al., Citation2021; Bellew & Chumas, Citation2015; Chuang et al., Citation2021; Hashim et al., Citation2014, Junn et al., Citation2021; Kljajić et al., Citation2019; Patel et al., Citation2014). In all but one of these studies, there was a pattern of higher verbal than perceptual function, albeit with a small overall effect (mean d = 0.21).
Type of suture – metopic subtype
A substantial minority of the included studies (n = 20) reported data on individuals with the metopic subtype of SSC. One set of studies (n = 8), of varying quality, (++) to (++++), reported general cognitive difficulties in children with metopic SSC comparable to those found in other subtypes across infant to school age (Bellew & Chumas, Citation2015; Kapp-Simon et al., Citation2005; Speltz et al., Citation2007; Starr et al., Citation2012), or mild deficits in specific functions such as attention, memory or visuomotor function, again similar to other sutural subtypes (Kljajić et al., Citation2020, Speltz et al., Citation2017; Toth et al., Citation2008; Wallace et al., Citation2016). Meanwhile, several studies (n = 6) of low to moderate quality, (++) to (+++), found average overall DQ or IQ scores compared to test norms in both preschool and school aged children (Collett et al., Citation2017; Da Costa et al., Citation2006, Citation2012, Junn et al., Citation2021; Kljajić et al., Citation2019; J. J. B. van der Vlugt et al., Citation2017; Warschausky et al., Citation2005), though Kljajić et al. (Citation2019) found higher verbal than perceptual IQ in a manner similar to findings reported for sagittal SSC.
A few studies (n = 3) of lower quality, (+) or (++), found developmental delay in metopic SSC both in relation to SSC individuals in general and more than −1 d compared to test norms or controls when examining preschool and school age individuals (Byun et al., Citation2018; Kapp-Simon, Citation1998; Shimoji et al., Citation2014). Two studies of low quality (++) reported higher than expected prevalence of “low IQ” or mental retardation in individuals with metopic SSC of predominately school age (Bottero et al., Citation1998; J. J. B. van der Vlugt et al., Citation2012). One study of low quality (++) found no similar effect in comparison to other subtypes of SSC (Becker et al., Citation2005).
Type of suture – unicoronal subtype
A substantial minority of studies (n = 13) reported data on individuals with unicoronal SSC. Studies on infants (n = 3), assessed as moderate (+++) to high (++++) quality, and originating from the same US cohort reported lower DQ scores in right-sided unicoronal SSC compared to other subtypes in the later preschool years but not in infancy (Kapp-Simon et al., Citation2005; Speltz et al., Citation2007; Starr et al., Citation2007). Similar results were found in two studies of lower quality, (+) to (++) (Cohen et al., Citation2003; Da Costa et al., Citation2012). Two studies of low (++) quality found average DQ/IQ in this group (Arnaud et al., Citation2002; Mathijssen et al., Citation2006) in the infant and preschool age bands.
In the preschool and school-age bands, findings were more ambiguous. Some studies (n = 4) of moderate (+++) to high (++++) quality, again from the same US cohort study, reported DQ/IQ and attention and executive function for unicoronal SSC as comparable to other subtypes, though lower than for the sagittal subtype in the case of DQ/IQ (Collett et al., Citation2017; Speltz et al., Citation2015; Starr et al., Citation2012; Wallace et al., Citation2016). One study of high quality (++++) reported lower scores on measures of language, learning and memory compared to other subtypes (Kapp-Simon et al., Citation2016). In school aged and adolescent children with unicoronal SSC, general IQ was reported as average with specific difficulties noted in visuospatial function, selective attention or significantly uneven IQ profiles with associated learning problems in a few studies (n = 3) deemed of low to moderate quality (++) or (+++) (Bellew & Chumas, Citation2015; Chieffo et al., Citation2010; R. T. Wu, Gabrick, et al., Citation2020). These studies reported findings consistent with general knowledge of hemispherical specialization in that low perceptual IQ was associated with right-sided sutures while lower verbal IQ scores were found in left-sided sutures.
Type of suture – lambdoid subtype
As expected, given that it is the rarest subtype of SSC, data from individuals with the lambdoid subtype was reported in a minority of the studies (n = 8). Data was reported in infants, preschool and school-age children, all from the same US longitudinal cohort study. Some studies (n = 4) deemed as moderate-to-high quality, (+++) or (++++), reported the lowest test scores in infants with the lambdoid subtype, with DQ more than −1 d compared to test norms as well as control group means (Kapp-Simon et al., Citation2005; Speltz et al., Citation2007; Starr et al., Citation2007, Citation2012). At school age in the same cohort (n = 4), mean test scores in the lambdoid subtype were average for general IQ as well as for more specific cognitive abilities but remained in the lower end of the spectrum across SSC subtypes, again reported in higher quality studies, (+++) to (++++) (Collett et al., Citation2017; Kapp-Simon et al., Citation2016; Speltz et al., Citation2015; Wallace et al., Citation2016).
Severity of synostosis
A small number of studies (n = 3) examined associations between severity of synostosis measured by skull shape or by the presence of extracranial malformations and cognitive outcome. Two of these were of varying quality, (++) and (++++), and did not find any association between severity of synostosis and cognitive outcome (Starr et al., Citation2012; Virtanen et al., Citation1999). In contrast, one study of low quality (++) found an association between severity of synostosis and motor DQ in infants with metopic SSC (Warschausky et al., Citation2005).
Cerebral and neurophysiological factors
Several studies (n = 7) examined associations between cerebral anatomy or neurophysiological measures and cognitive function measured by testing. Neither intracranial pressure (ICP) nor the presence of beaten-copper pattern (BCP) on the cerebral cortex was significantly associated with cognitive measures, according to studies (n = 4) of very low to low quality (+) or (++) (Shimoji et al., Citation2014; van der Meulen et al., Citation2008; Mathijssen et al., Citation2006; Shipster et al., Citation2003). However, ventricular width was found to be a significant predictor of cognitive outcome in a study of low quality (++) on preschool to school age participants with metopic synostosis (J. J. B. van der Vlugt et al., Citation2017). Two studies of low quality (++) in school age children with SSC also found that cognitive test scores but not school performance at this age could be accurately predicted by Electroencephalogram (EEG) recordings taken pre- and postoperatively during the individuals’ first year of life (Junn et al., Citation2021; R. Wu et al., Citation2021).
Sex, parental influences and assessment issues related to design
Sex
A few studies (n = 3) of moderate-to-high quality, (+++) to (++++), using data from the same US cohort examined the association between sex and cognitive functions in individuals with SSC. None found any significant association, and only Cradock et al. (Citation2015) found associations similar to those found in the general population, in that males scored lower than females on all measures (Cradock et al., Citation2015; Kapp-Simon et al., Citation2005; Speltz et al., Citation2007).
Parental IQ/SES/educational level influence
A majority of the included studies (n = 27) reported parental socio-economic status (SES), educational level, health insurance coverage or parental IQ. However, only a smaller set (n = 13) of these studies reported actual associations between these constructs and cognitive outcomes in the participants or used these measures as covariates in statistical analyses. In a set of these studies (n = 8), of low to moderate quality, (++) to (+++), no such associations were found (Alperovich et al., Citation2021; Chuang et al., Citation2021; Da Costa et al., Citation2012; Hashim et al., Citation2014, Junn et al., Citation2021; Panchal et al., Citation2001; R. T. Wu, Gabrick, et al., Citation2020; R. T. Wu, Timberlake, et al., Citation2020). However, in the case of a set of studies from the US infant learning project (n = 4), all of moderate-to-high quality, (+++) to (++++), parental SES had a significant impact on cognitive outcome (Kapp-Simon et al., Citation2005; Speltz et al., Citation2007; Starr et al., Citation2010; Toth et al., Citation2008). One study of low quality (++) also reported an association between maternal education and language DQ (Warschausky et al., Citation2005).
Global DQ/IQ
A majority of the studies (n = 35) used global measures of cognitive functioning such as DQ or IQ scores as outcome measures, frequently in combination with global measures of language. When summed across all age bands, a large set of these studies (n = 23) of varying quality, (+) to (++++), found little to no effect on general cognition in SSC, with test scores in the average range and/or small effect sizes of less than 0.2 d in comparison to controls (Alperovich et al., Citation2021, Arnaud et al., Citation2002; Bellew et al., Citation2011; Bellew & Chumas, Citation2015; Boltshauser et al., Citation2003; Chieffo et al., Citation2010; Chuang et al., Citation2021; Da Costa et al., Citation2006; Cradock et al., Citation2015; Hashim et al., Citation2014; Kapp-Simon, Citation1998; Kljajić et al., Citation2019; Magge et al., Citation2002; Mathijssen et al., Citation2006; van der Meulen et al., Citation2008; Patel et al., Citation2014, Shipster et al., Citation2003; Speltz et al., Citation1997; J. J. B. van der Vlugt et al., Citation2012, Citation2017; Warschausky et al., Citation2005; R. T. Wu, Gabrick, et al., Citation2020; R. T. Wu, Timberlake, et al., Citation2020).
A smaller number of studies (n = 5) of low to high quality, (++) to (++++), found small (d = 0.2 to 0.4) effects on DQ/IQ (Bellew et al., Citation2005; Da Costa et al., Citation2012; Speltz et al., Citation2015; Starr et al., Citation2007, Citation2012). A substantial minority of studies (n = 8) across all age bands, of varying quality (+) to (++++), reported moderate-to-large effects (d = 0.5 to 1.5) regarding deficits in general DQ/IQ (Byun et al., Citation2018, Cohen et al., Citation2003; Fontana et al., Citation2017; Kapp-Simon et al., Citation2005; Naumann et al., Citation2012, Shimoji et al., Citation2014; Speltz et al., Citation2007; Starr et al., Citation2010). All of the studies that reported effects >1 d were of very low to low quality, (+) to (++).
Visuospatial/motor function
A substantial minority of studies (n = 12) reported data on visuospatial or visuomotor function independent of general IQ, all sampling school-age individuals. Two studies of moderate-to-high quality, (+++) and (++++), reported small to large significant negative effects (d = 0.2 to 0.9) on visuomotor abilities in SSC generally and in a group with sagittal and metopic SSC, respectively (Wallace et al., Citation2016; R. T. Wu, Gabrick, et al., Citation2020). A third study of low quality (++) found a small and non-significant effect (d = 0.17) in metopic SSC (J. van der Vlugt et al., Citation2021) and a fourth study of similar quality (++) did not find any effect (Chieffo et al., Citation2010) in unicoronal and sagittal SSC. Another study of low quality (++) reported lower scores in SMAD6 genetic carriers but not in SSC children without this genotype (R. T. Wu, Timberlake, et al., Citation2020). Three studies of low to moderate quality, (++) to (+++), found effects on visuomotor function favoring operation before age 1 year, even in children having undergone reoperation (Alperovich et al., Citation2021, Junn et al., Citation2021; Patel et al., Citation2014). Two studies of low to moderate quality, (++) to (+++), found effects on visuomotor function favoring cranial vault reconstruction compared to spring-assisted surgery and whole-vault cranioplasty compared to strip craniectomy (Chuang et al., Citation2021; Hashim et al., Citation2014).
Memory
A few studies (n = 5) reported effects of SSC on memory, independent of general IQ. One study of high quality (++++), on memory and response inhibition in infants found no difference in performance between individuals with SSC and matched controls (Toth et al., Citation2008). Two studies of low and high quality, (++) to (++++), on school-aged children found small to moderate effects on memory (d = 0.1 to 0.4) in comparison to matched controls (Kapp-Simon et al., Citation2016; Virtanen et al., Citation1999). Similar effects were also reported in a similar study of low quality (++) (Chieffo et al., Citation2010). One study of very low quality (+) reported a higher than expected prevalence of low test scores in their sample, especially for visual memory (Boltshauser et al., Citation2003).
Attention/vigilance
A few studies (n = 4) reported data on attention or vigilance in SSC. Two studies of low and moderate quality, (++) and (+++), reported small but significant effects on both these functions in school-aged children with SSC (Collett et al., Citation2017; Kljajić et al., Citation2020). Two studies of very low and low quality (+) and (++) in the same age group found a high prevalence of pathologically low test scores on attention (Boltshauser et al., Citation2003; Chieffo et al., Citation2010).
Presence of comorbid neuropsychiatric conditions
A few of the included studies (n = 3) had directly assessed the presence of neuropsychiatric conditions in their samples. Two of these studies, of low quality (++), reported an increased prevalence of such disorders, with ASD or ADHD reported in up to 24% of cases and intellectual disability (ID) reported in 10% of cases in two Dutch samples of school age children (J. J. B. van der Vlugt et al., Citation2012, Citation2017) and an above 80% prevalence of features of autism in a Japanese study of very low quality (+), with a significant decrease of such symptoms post-surgery (Shimoji et al., Citation2015).
Domain of individual recruitment (single clinic/multi-site/clinical referral)
Half (n = 24) of the studies sampled individuals from a single surgical institute, either by prospective testing or by review of medical records or similar post hoc data gathering. Results for cognitive outcomes in these studies were mixed. Several studies (n = 7) across all age bands, generally of very low to low quality (+) or (++), one study (+++), found substantial effects with mean test scores 1 to 2 d below the comparison mean on at least one cognitive measure (Arnaud et al., Citation2002; Becker et al., Citation2005, Bottero et al., Citation1998; Byun et al., Citation2018; Naumann et al., Citation2012; Shimoji et al., Citation2015; J. J. B. van der Vlugt et al., Citation2012). A smaller number of studies (n = 5) of low quality, (++), found small-to-medium effects on cognition (Bellew et al., Citation2005; Da Costa et al., Citation2012; Panchal et al., Citation2001; van der Meulen et al., Citation2008; Virtanen et al., Citation1999). A larger set of studies (n = 12) of very low to low quality, (+) or (++), found no significant effect on cognition (Bellew et al., Citation2011; Bellew & Chumas, Citation2015; Boltshauser et al., Citation2003; Da Costa et al., Citation2006; Kapp-Simon, Citation1998; Magge et al., Citation2002; Mathijssen et al., Citation2006, Shipster et al., Citation2003; Speltz et al., Citation1997; Warschausky et al., Citation2005; J. J. B. van der Vlugt et al., Citation2017; J. van der Vlugt et al., Citation2021).
A substantial minority of the studies (n = 21) sampled individuals from several surgical centers and reported data in a way that enabled direct comparison. Among these studies, only one of very low quality (+) found effects of comparable magnitude to the majority of the studies mentioned above with main effects of 1 d below the mean (Cohen et al., Citation2003). A set of studies in this category (n = 6) of moderate-to-high quality, (+++) to (++++), out of which five sampled infant individuals from the infant learning project, reported moderate effects (d = 0.4 to 0.7) (Cradock et al., Citation2015; Kapp-Simon et al., Citation2005; Patel et al., Citation2014; Speltz et al., Citation2007; Starr et al., Citation2010). Only a single study of infants in this category, of high quality (++++), reported no effect on cognition (Toth et al., Citation2008). A set of studies (n = 9) in this category mainly sampled individuals of school age and of low to high quality (++) to (++++) reported small yet significant effects on cognition in SSC of up to 0.3 d below the mean (Chieffo et al., Citation2010; Collett et al., Citation2017; Kapp-Simon et al., Citation2016; Kljajić et al., Citation2020; Speltz et al., Citation2015; Starr et al., Citation2007, Citation2012; Wallace et al., Citation2016; R. T. Wu, Gabrick, et al., Citation2020). Another set of studies (n = 5) in this category deemed of low to moderate quality (++) to (+++) sampled school age children and reported no significant effects (Alperovich et al., Citation2021; Chuang et al., Citation2021; Hashim et al., Citation2014; Kljajić et al., Citation2019; R. T. Wu, Timberlake, et al., Citation2020).
Use of control groups/test norms for comparison
A majority of the studies (n = 35) did not include a control group and instead compared cognitive outcomes to test norms or other measures of normative development such as developmental rating scales. Of these, a large set (n = 28) could be interpreted and categorized by type of cognitive outcome in a comparable way.
A set of studies (n = 6) of very low to low quality (+) or (++) found substantial effects on general cognition with mean results for infant and school-aged SSC children more than 1 d below test norm means (Arnaud et al., Citation2002; Becker et al., Citation2005; Byun et al., Citation2018, Cohen et al., Citation2003; Fontana et al., Citation2017; Shimoji et al., Citation2015). A smaller number of studies (n = 4) of low to moderate quality (++) to (+++) sampled individuals from similarly heterogenous age bands and found effects in the moderate range between d 0.4 and 0.8 below test norm means on general as well as specific cognitive measures (Da Costa et al., Citation2012; Kapp-Simon et al., Citation2005; Kljajić et al., Citation2020; Panchal et al., Citation2001). A large set of studies (n = 18) in this category, chiefly sampling school-age individuals and of very low to moderate quality (+) to (+++), found little to no effect on cognition (Alperovich et al., Citation2021; Bellew & Chumas, Citation2015; Chuang et al., Citation2021; Da Costa et al., Citation2006; Hashim et al., Citation2014; Kapp-Simon, Citation1998; Kljajić et al., Citation2019; Magge et al., Citation2002; Mathijssen et al., Citation2006; van der Meulen et al., Citation2008; Patel et al., Citation2014, Shipster et al., Citation2003; Warschausky et al., Citation2005; J. J. B. van der Vlugt et al., Citation2012, Citation2017; J. van der Vlugt et al., Citation2021; R. T. Wu, Gabrick, et al., Citation2020; R. T. Wu, Timberlake, et al., Citation2020).
A substantial minority of the studies (n = 11) included a control group for comparisons and reported results in a way that enabled direct comparison. Of these, a few studies (n = 3) were of moderate-to-high quality (+++) or (++++) and sampled children of infant to school age from the same US cohort, finding moderate to strong effects, generally in the d = 0.4 to 0.7 range on at least one measure of general or specific function of cognition (Collett et al., Citation2017; Cradock et al., Citation2015; Speltz et al., Citation2007). A similar number of studies (n = 7) in this category sampled a wide age range from infancy to adolescence and were generally of comparable quality, (++) to (++++), found small effects on both general and specific cognitive functions of under d = 0.5 (Chieffo et al., Citation2010; Gray et al., Citation2015; Speltz et al., Citation2015; Starr et al., Citation2007, Citation2012; Virtanen et al., Citation1999; Wallace et al., Citation2016). One study of high quality (++++) sampled infants and found no effect on memory (Toth et al., Citation2008). No studies in this category reported large effects on cognition.
Within-group variations in test scores
A substantial minority of the studies (n = 18) of low to high quality, (++) to (++++), measured DQ in infant or preschool age participants and reported standard deviation (sd) scores in a way that permitted comparison (Bellew et al., Citation2005, Citation2005; Byun et al., Citation2018; Da Costa et al., Citation2012; Kapp-Simon, Citation1998; Mathijssen et al., Citation2006; Naumann et al., Citation2012; Speltz et al., Citation1997, Citation2007, Citation2015; Starr et al., Citation2007, Citation2010, Citation2012; Toth et al., Citation2008; van der Meulen et al., Citation2008; J. J. B. van der Vlugt et al., Citation2012, Citation2017; Warschausky et al., Citation2005). SD across these studies varied widely (m = 19; range = 6.8 to 21.3) with a higher SD than test norms.
Another substantial minority of the studies (n = 13) of varying quality, (++) to (++++), measured IQ in participants from late preschool age upwards and reported SD scores in a comparable fashion (Alperovich et al., Citation2021; Bellew et al., Citation2005, Citation2011; Chuang et al., Citation2021; Da Costa et al., Citation2006; Cradock et al., Citation2015; Hashim et al., Citation2014, Junn et al., Citation2021; Kapp-Simon et al., Citation2016; Kljajić et al., Citation2019; Patel et al., Citation2014; J. van der Vlugt et al., Citation2021; R. T. Wu, Gabrick, et al., Citation2020). In these studies, sd scores also varied widely (m = 15.5; range = 10.7–20.9), but overall without significant deviation from norm data. However, when studies that used IQ < 70 as an exclusion criterium were not included (n = 4), this discrepancy increased slightly (m = 16.5).
Discussion
Overall findings
The present review aimed to describe the current state of evidence regarding cognition in individuals with SSC by performing a systematic literature review. Overall, there was substantial evidence for small but possibly clinically significant negative effects on both general and specific cognitive functions across development, with limited evidence for differences across subtypes of the condition. There was some evidence for effects related to surgical correction. In line with previous reviews and meta-analyses (Kapp-Simon et al., Citation2007; Knight et al., Citation2014; Osborn et al., Citation2019, Citation2021; Speltz et al., Citation2004), the results show high variability both in terms of study design and obtained results. However, the results also show signs of better methodology across time and several promising directions for future research. The present review, being the first of its kind to formally assess quality as a central aspect, is uniquely able to shed light on this progression within the field.
A central finding is a consistent pattern of variability in findings across studies that is associated with quality and rigor of design: Our findings show a tendency in studies of less rigorous design to report dramatic effects on cognition when the sample is recruited from a single surgical center or when no control group or formal measure to ensure reliability of testing is in place. When more strenuous designs are utilized, effects on cognition remain significant but decrease in magnitude. Studies using a control group were more likely to show significant effects on cognition in SSC, even when the mean score in the SSC group was within the average range (e.g., IQ = 85–115) compared to test norms. In line with these findings, we advocate greater use of multi-center studies using control groups for comparison. In contrast, most studies included in this review used small, single-center samples compared by test performance solely in comparison to test norms, frequently using only test data gathered in clinical practice without measurement of inter-rater reliability.
Inclusion and exclusion criteria
A further observation that can be made from the present review is related to issues of score distributions within studies: An inflated number of individuals with very high scores may result in average mean scores in subject groups, but simultaneously obscuring that there may be an inflated prevalence of subjects with very low scores due to higher variance in SSC samples compared to the population. This is consistent with the higher-than-expected variability regarding general cognition found in some studies, especially in the infant and preschool age bands. In light of such findings, studies in the field using ID diagnosis as an exclusion criterion should be called into question. Again, the generally small sample sizes utilized in most studies hamper further progress in this regard since small samples, by statistical necessity, are more sensitive to outliers. This problem is not remedied by systematic exclusion of participants expected to receive test scores at a specific extreme of the spectrum as becomes the case of DQ/IQ measures when ID is used as an exclusion criterion.
A similar line of argument can be made regarding the highly variable inclusion and exclusion criteria observed among the included studies regarding comorbid conditions, both in terms of associated somatic illnesses and syndromes as well as neuropsychiatric conditions. The fact that only three of all the studies involved made a formal attempt to categorize patients in terms of ADHD, ASD and similar conditions implies that it is likely a substantial influence of undiagnosed conditions on the data discussed in the present review, hampering conclusions that can be drawn from the findings. In addition, there is a persistent problem in the data regarding uncertainties of genetic causes for SSC in many samples, which may be more feasibly addressed in the future as access to genetic screening is more and more readily available in clinical practice.
Age and suture location effects
Overall, the findings in studies regarding pre-school SSC children indicate small to moderate effects on cognition. In school age and adolescence, there is also indications of some persistence of these effects although they seem to become more subtle over time. There are also some trends of these effects becoming isolated to more specific cognitive functions such as attention, visuomotor function and language rather than general IQ over time. More studies are needed to validate this observation further.
Regarding the different subtypes of SSC, there is some evidence, particularly in school-aged individuals, of better cognitive prognosis in sagittal SSC compared to the other subtypes. Tentative evidence exists for particular vulnerability in the metopic and lambdoid subtypes, but more studies are needed to validate these findings. This will be difficult in the case of lambdoid SSC given the exceptional rarity of this condition, again pointing to the need for multi-center studies in the field.
In a further line of inquiry regarding specific cognitive deficits, the evidence is tentative for a particular vulnerability in unicoronal CS for deficits in attention, visuomotor function and language that merit further investigation. In addition, the verbal/visuospatial discrepancy found in a number of studies on sagittal synostosis warrants further investigation as it may provide insights into typical brain development. Based on current understanding of cortical network reliance in higher cognitive function, sagittal synostosis could be understood as a condition disrupting dorsal parieto-frontal network connections across development that can be directly linked to the location of the sagittal suture and, possibly, the mechanical effects associated with it on the developing brain. This observation could be further validated if more studies finding a mediating effect of age at surgery on this link were to be conducted.
Effects of surgery
On the subject of interventions, there was some limited evidence for early surgery as a component in positive prognosis and a potential dose–response relation with more dramatic effects of a better cognitive prognosis in early operated children than in those operated after 6 and 12 months of age. More research is needed here given how crucial this issue is for clinical decision-making. Also, the findings indicating possible substantial effects of anesthesia and surgical methods on cognitive prognosis warrant further study. Overall, the lack of assessment of SES in many studies, which has previously been found to be highly correlated with age of diagnosis and treatment in SSC (Gray et al., Citation2015.) and an important contributor to cognition, hampers further progress in this line of inquiry.
Regarding prognostic demographic factors, there is inconclusive evidence about the influence of sex on cognition in SSC, though commendable efforts have been made to address this subject. In this context, it is striking that so few studies have included parental IQ as a covariate in analyses, given the well-established high heritability of cognitive abilities, an observation that has also been made in the SSC field (Bergen et al., Citation2007; Gray et al., Citation2015; Polderman et al., Citation2006). Better control of background variables will enable more powerful study designs, by eliminating a substantial portion of noise.
Assessment issues
In a wider context, we argue that the results of this review point toward two important assessment issues not usually raised in the field. Firstly, our results indicate an over-reliance on global measures of cognition. We argue that cognition in SSC individuals from school age and upwards is too narrowly conceptualized and advocate the use of broader neuropsychological test batteries to assess this population in future studies. The findings of the present review support this position in the sense that they reflect an attenuated effect on global cognitive measures, such as IQ, over time while indicating persistent effects on more specific abilities such as visuospatial abilities or attention.
Our second point of argument is regarding the age bands under study in the field, where a wide literature search was unable to find a single study that had specifically examined cognition in adults with SSC. Humans spend most of their lifetimes in adulthood, and prediction of cognitive function at this age should be the ultimate goal of any assessment of cognitive function in SSC children, underscoring the need for more studies on this age group.
Directions for future research
Overall, the present review must reiterate the recommendations given in previous reviews of the field regarding the need for multicenter, longitudinal studies using appropriate control groups (Kapp-Simon et al., Citation2007; Knight et al., Citation2014; Speltz et al., Citation2004). Apart from including parental IQ as a covariate, formal checks of interrater reliability and, when applicable, procedures for double-blind assessment are under-utilized in the field. To date, the US Infant Learning Project remains the only cohort that fulfill all these criteria (Kapp-Simon et al., Citation2005; Gray et al., Citation2015; Speltz et al., Citation2007; Starr et al., Citation2012, Citation2007, Citation2010, Citation2012; Toth et al., Citation2008; Cradock et al., Citation2015; Wallace et al., Citation2016 among others).
It must be stated that several progressions in the field hold substantial promise. This includes the use of EEG for direct assessment of brain function in SSC that has attracted some attention in recent years, as well as the renewed interest in genetics in connection with the SMAD6 genotype (R. T. Wu, Timberlake, et al., Citation2020; Junn et al., Citation2021). Non-invasive procedures for cortical mapping hold the promise of becoming a paradigm for direct assessment of network-based brain functioning in SSC individuals. When correlated with behavioral measures such as cognitive tests, these types of biomarkers could lead to a new understanding of the still largely unknown mechanisms underpinning the neurodevelopmental effects in SSC.
Strengths and limitations of the present review
The present study reviewed cognition in SSC using an ambitious search strategy including repeated searches with higher-order definitions and across several databases. We are confident that we have been able to achieve a high degree of coverage of relevant studies in the field. This review is the first in the field to incorporate a formal assessment of quality using independent raters as a central outcome, which lends further confidence to the results.
Another strength of the current review is its relatively narrow definition of cognition as test-based data rather than parental or professional rating scales. This explains why studies performed prior to 2014 included in the current review are not fully overlapping with the studies included in the review by Knight et al. (Citation2014).
The chief limitation of the present review is inherent to the differing terminology across the field and concerns the inclusion criteria of the studies included regarding NSC/SSC, with several studies using scant exclusion criteria and a focus on suture presentation in inclusion. This infers that a substantial proportion of the included studies probably contain a set of subjects with genetic causes for their SSC. Direct comparisons between studies using more and less stringent inclusion criteria might be the focus of a future review of the field.
Conclusions
The present review describes the current state of evidence regarding cognition in SSC by using a systematic literature search according to the PRISMA statement, combined with a stringent procedure for assessment of quality using the GRADE system. The results show that there is growing evidence for small to moderate overall effects on cognition in SSC that seem to be persistent across infancy and childhood, but more research is needed to determine its more precise nature and to ascertain whether these effects persist into adulthood. We postulate that the findings point to SSC as a disorder of cortical network organization of higher-order cognition of substantial interest to neuroscience as a whole beyond the scope of craniosynostosis if the pathophysiology underlying the cognitive effects of SSC were to be understood more fully. A more thorough understanding of these effects is also critical to informing clinical judgment regarding surgical intervention as well as to providing correct information to parents at the critical junction when the decision on whether or not to operate on synostosis is taken in the child’s infancy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alperovich, M., Runyan, C. M., Gabrick, K. S., Wu, R. T., Morgan, C., Park, S. E., Chapman, L. A., Couture, D. E., David, L. R., & Persing, J. A. (2021). Long-term neurocognitive outcomes of spring-assisted surgery versus cranial vault remodeling for SagittalSynostosis. Plastic and Reconstructive Surgery, 147(3), 661–671. https://doi.org/10.1097/PRS.0000000000007640
- Arnaud, E., Meneses, P., Lajeunie, E., Thorne, J. A., Marchac, D., & Renier, D. (2002). Postoperative mental and morphological outcome for nonsyndromic brachycephaly. Plastic and Reconstructive Surgery, 110(1), 6–13. https://doi.org/10.1097/00006534-200207000-00002
- Becker, D. B., Petersen, J. D., Kane, A. A., Cradock, M. M., Pilgram, T. K., & Marsh, J. L. (2005). Speech, cognitive, and behavioral outcomes in nonsyndromic craniosynostosis. Plastic and Reconstructive Surgery, 116(2), 400–407. https://doi.org/10.1097/01.prs.0000172763.71043.b8
- Bellew, M., & Chumas, P. (2015). Long-term developmental follow-up in children with nonsyndromic craniosynostosis. Journal of Neurosurgery Pediatrics, 16(4), 445–451. https://doi.org/10.3171/2015.3.PEDS14567
- Bellew, M., Chumas, P., Mueller, R., Liddington, M., & Russell, J. (2005). Pre- and postoperative developmental attainment in sagittal synostosis. Archives of Disease in Childhood, 90(4), 346–350. https://doi.org/10.1136/adc.2003.035824
- Bellew, M., Liddington, M., Chumas, P., & Russell, J. (2011). Preoperative and postoperative developmental attainment in patients with sagittal synostosis: 5-year follow-up. Journal of Neurosurgery Pediatrics, 7(2), 121–126. https://doi.org/10.3171/2010.11.PEDS10216
- Bergen, S. E., Gardner, C. O., & Kendler, K. S. (2007). Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: A meta-analysis. Twin Research and Human Genetics: The Official Journal of the International Society for Twin Studies, 10(3), 423–433. https://doi.org/10.1375/twin.10.3.423
- Boltshauser, E., Ludwig, S., Dietrich, F., & Landolt, M. A. (2003). Sagittal craniosynostosis: Cognitive development, behaviour, and quality of life in unoperated children. Neuropediatrics, 34(6), 293–300. https://doi.org/10.1055/s-2003-44667
- Bottero, L., Lajeunie, E., Arnaud, E., Marchac, D., & Renier, D. (1998). Functional outcome after surgery for trigonocephaly. Plastic and Reconstructive Surgery, 102(4), 952–960. https://doi.org/10.1097/00006534-199809020-00002
- Brozek, J. L., Akl, E. A., Alonso-Coello, P., Lang, D., Jaeschke, R., Williams, J. W., Phillips, B., Lelgemann, M., Lethaby, A., Bousquet, J., Guyatt, G. H., Schünemann, H. J., & GRADE Working Group. (2009). Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy, 64(5), 669–677.
- Byun, I. H., Hong, J. W., Hussein, M. A., & Kim, Y. O. (2018). Demographic characteristics of craniosynostosis patients in Asia. Journal of Cranio-Maxillo-Facial Surgery: Official Publication of the European Association for Cranio-Maxillo-Facial Surgery, 46(4), 674–678. https://doi.org/10.1016/j.jcms.2018.02.008
- Chieffo, D., Giovanni, S. D., Rocco, C. D., DiGiovanni, S., Giansanti, C., Caldarelli, M., & DiRocco, C. (2010). Long-term neuropsychological development in single-suture craniosynostosis treated early. Journal of Neurosurgery, 5(3), 232–237. https://doi.org/10.3171/2009.10.PEDS09231
- Chuang, C., Chaunzwa, T. L., Wu, R., Singh, A., Patel, A., Yang, J. F., Hashim, P. W., Travieso, R., Terner, J. S., Mayes, L. C., Duncan, C. C., Jane, J. A., Jr., Lin, K. Y., Bridgett, D. J., & Persing, J. A. (2021). Long-term neurocognitive outcomes in sagittal synostosis: The impact of reoperation. The Journal of Craniofacial Surgery, 32(1), 58–61. https://doi.org/10.1097/SCS.0000000000006909
- Cohen, S. R., Cross, K. P., Nichols, S. L., Simms, C., Cross, K. P., & Burstein, F. D. (2004). American Society of Maxillofacial Surgeons Outcome Study. Preoperative and Postoperative Neurodevelopmental Findings in Single-Suture Craniosynostosis. PLASTIC and RECONSTRUCTIVE SURGERY, 114(4), 841–847. https://doi.org/10.1097/01.PRS.0000132854.14237.A8
- Collett, B. R., Kapp-Simon, K. A., Wallace, E., Cradock, M. M., Buono, L., & Speltz, M. L. (2017). Attention and executive function in children with and without single-suture craniosynostosis. Child Neuropsychology: A Journal on Normal & Abnormal Development in Childhood & Adolescence, 23(1), 83–98. https://doi.org/10.1080/09297049.2015.1085005
- Cradock, M. M., Gray, K. E., Kapp-Simon, K. A., Collett, B. R., Buono, L. A., & Speltz, M. L. (2015). Sex differences in the neurodevelopment of school-age children with and without single-suture craniosynostosis. Child’s Nervous System: ChNs: Official Journal of the International Society for Pediatric Neurosurgery, 31(7), 1103–1111. https://doi.org/10.1007/s00381-015-2671-0
- Da Costa, A. C., Anderson, V. A., Savarirayan, R., Wrennall, J. A., Chong, D. K., Holmes, A. D., Greensmith, A. L., & Meara, J. G. (2012). Neurodevelopmental functioning of infants with untreated single-suture craniosynostosis during early infancy. Child’s Nervous System: ChNs: Official Journal of the International Society for Pediatric Neurosurgery, 28(6), 869–877. https://doi.org/10.1007/s00381-011-1660-1
- Da Costa, A. C., Walters, I., Savarirayan, R., Anderson, V. A., Wrennall, J. A., & Meara, J. G. (2006). Intellectual outcomes in children and adolescents with syndromic and non-syndromic craniosynostosis. Plastic and Reconstructive Surgery, 118(1), 175–181. https://doi.org/10.1097/01.prs.0000221009.93022.50
- DiRocco, F., Arnaud, E., & Renier, D. (2009 Retrieved January 11, 2022). Evolution in the frequency of non-syndromic craniosynostosis. Journal of Neurosurgery: Pediatrics PED, 4(1), 21–25. from. https://thejns-org.ezproxy.its.uu.se/pediatrics/view/journals/j-neurosurg-pediatr/4/1/article-p21.xml https://doi.org/10.3171/2009.3.PEDS08355
- Fernandes, M. B., Maximino, L. P., Perosa, G. B., Abramides, D. V., Passos-Bueno, M. R., & Yacubian-Fernandes, A. (2016). Apert and Crouzon syndromes - Cognitive development, brain abnormalities, and molecular aspects. American Journal of Medical Genetics Part A, 170(6), 1532–1537. https://doi.org/10.1002/ajmg.a.37640
- Fontana, S. C., Belinger, S., Daniels, D., Tuttle, M., Camarata, P. J., & Andrews, B. T. (2017). Longitudinal assessment of developmental outcomes in infants undergoing late craniosynostosis repair. Journal of Craniofacial Surgery, 29(1), 25–28. https://doi.org/10.1097/SCS.0000000000004024
- Governale, L. S. (2015). Craniosynostosis. Pediatric Neurology, 53(5), 394–401. https://doi.org/10.1016/j.pediatrneurol.2015.07.006
- Gray, K. E., Kapp-Simon, K. A., Starr, J. R., Collett, B. R., Wallace, E. R., & Speltz, M. L. (2015). Predicting developmental delay in a longitudinal cohort of preschool children with single-suture craniosynostosis: Is neurobehavioral assessment important? Developmental Medicine and Child Neurology, 57(5), 456–462. https://doi.org/10.1111/dmcn.12643
- Hashim, P. W., Patel, A., Yang, J. F., Travieso, R., Terner, J., Losee, J. E., Pollack, I., Jane, J., Sr., Jane, J., Jr., Kanev, P., Mayes, L., Duncan, C., Bridgett, D. J., & Persing, J. A. (2014). The effects of whole-vault cranioplasty versus strip craniectomy on long-term neuropsychological outcomes in sagittal craniosynostosis. Plastic and Reconstructive Surgery, 134(3), 491–501. https://doi.org/10.1097/PRS.0000000000000420
- Junn, A., Dinis, J., Park, K. E., Hauc, S., Yang, J. F., Chuang, C., Han, G., McPartland, J. C., Persing, J. A., & Alperovich, M. (2021). Long-term Follow-up of Preoperative Infant Event-related Potentials in School-age Children with Craniosynostosis. Plastic and Reconstructive Surgery Global Open, 9(10), e3844. https://doi.org/10.1097/GOX.0000000000003844
- Kaplan, R. M., & Sacuzzo, D. P. (2017). Psychological assessment: Principles, Issues and applications. Cengage Learning.
- Kapp-Simon, K. A. (1998). Mental Development and Learning Disorders in Children with Single Suture Craniosynostosis. 30 october 2020 från https://journals.sagepub.com/doi/10.1597/1545-1569_1998_035_0197_mdaldi_2.3.co_2#articleCitationDownloadContainer
- Kapp-Simon, K. A., Leroux, B., Cunningham, M., & Speltz, M. L. (2005). Multisite Study of infants with single-suture craniosynostosis: preliminary report of presurgery development. 8.
- Kapp-Simon, K. A., Speltz, M. L., Cunningham, M. L., Patel, P. K., & Tomita, T. (2007). Neurodevelopment of children with single suture craniosynostosis: A review. Child’s Nervous System: ChNs: Official Journal of the International Society for Pediatric Neurosurgery, 23(3), 269–281. https://doi.org/10.1007/s00381-006-0251-z
- Kapp-Simon, K. A., Wallace, E., Collett, B. R., Cradock, M. M., Crerand, C. E., & Speltz, M. L. (2016). Language, learning, and memory in children with and without single-suture craniosynostosis. Journal of Neurosurgery: Pediatrics, 17(5), 578–588. https://doi.org/10.3171/2015.9.PEDS15238
- Kljajić, M., Maltese, G., Tarnow, P., Sand, P., & Kölby, L. (2019). The cognitive profile of children with non-syndromic craniosynostosis. Plastic and Reconstructive Surgery, 143(5), 1037e–1052e. https://doi.org/10.1097/PRS.0000000000005515
- Kljajić, M., Maltese, G., Tarnow, P., Sand, P., & Kölby, L. (2020). [Formula: See text] Sustained attention and vigilance of children treated for sagittal and metopic craniosynostosis. Child Neuropsycholog: A Journal on Normal and Abnormal Development in Childhood and Adolescence, 26(4), 475–488. https://doi.org/10.1080/09297049.2019.1682130
- Knight, S. J., Anderson, V. A., Spencer-Smith, M. M., & Da Costa, A. C. (2014). Neurodevelopmental outcomes in infants and children with single-suture craniosynostosis: A systematic review. Developmental Neuropsychology, 39(3), 159–186. https://doi.org/10.1080/87565641.2014.886690
- Kotz, S., Johnson, N. L., & Read, C. B. (Eds.). (1988). Encyclopedia of Statistical Sciences (Vol. 9, 1st ed.). Wiley-Interscience.
- Kruszka, P., Addissie, Y. A., Yarnell, C. M., Hadley, D. W., Guillen Sacoto, M. J., Platte, P., Paelecke, Y., Collmann, H., Snow, N., Schweitzer, T., Boyadjiev, S. A., Aravidis, C., Hall, S. E., Mulliken, J. B., Roscioli, T., & Muenke, M. (2016). Muenke syndrome: An international multicenter natural history study. American Journal of Medical Genetics Part A, 170A(4), 918–929. https://doi.org/10.1002/ajmg.a.37528
- Lee, H. Q., Hutson, J. M., Wray, A. C., Lo, P. A., Chong, D. K., Holmes, A. D., & Greensmith, A. L. (2012). Changing epidemiology of non-syndromic craniosynostosis and revisiting the risk factors. The Journal of Craniofacial Surgery, 23(5), 1245–1251. https://doi.org/10.1097/SCS.0b013e318252d893
- Lezak, M. D., Howieson, D. B., Bigler, E. D., & Tranel, D. (2012). Neuropsychological Assessment. Oxford Univ Press:.
- Magge, S. N., Westerveld, M., Pruzinsky, T., & Persing, J. A. (2002). Long-term neuropsychological effects of sagittal craniosynostosis on child development. Journal of Craniofacial Surgery, 13(1), 99–104. https://doi.org/10.1097/00001665-200201000-00023
- Mathijssen, I., Marchac, D., Lajeunie, E., Marchac, D., & Renier, D. (2006). Postoperative cognitive outcome for synostotic frontal plagiocephaly. Journal of Neurosurgery, 105(1), 16–20. https://doi.org/10.3171/ped.2006.105.1.16
- Naumann, H. L., Haberkern, C. M., Pietila, K. E., Birgfeld, C. B., Starr, J. R., Kapp-Simon, K. A., Hopper, R. A., & Speltz, M. L. (2012). Duration of exposure to cranial vault surgery: Associations with neurodevelopment among children with single-suture craniosynostosis: Anesthesia exposure and neurodevelopment. Pediatric Anesthesia, 22(11), 1053–1061. https://doi.org/10.1111/j.1460-9592.2012.03843.x
- Osborn, A. J., Roberts, R. M., Dorstyn, D. S., Grave, B. G., & David, D. J. (2021). Sagittal synostosis and its association with cognitive, behavioral, and psychological functioning: A meta-analysis. JAMA Network Open, 4(9), e2121937. https://doi.org/10.1001/jamanetworkopen.2021.21937
- Osborn, A. J., Roberts, R. M., Mathias, J. L., Anderson, P. J., & Flapper, W. J. (2019). Cognitive, behavioral and psychological functioning in children with metopic synostosis: A meta-analysis examining the impact of surgical status. Child Neuropsychology: A Journal on Normal & Abnormal Development in Childhood & Adolescence, 25(2), 263–277. https://doi.org/10.1080/09297049.2018.1441821
- Page, M. J., J E, M., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E. … Whiting, P. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372, n71. https://doi.org/10.1136/bmj.n71
- Panchal, J., Amirsheybani, H., Gurwitch, R., Cook, V., Francel, P., Neas, B., & Levine, N. (2001). Neurodevelopment in children with single-suture craniosynostosis and plagiocephaly without synostosis. Plastic and Reconstructive Surgery, 108(6), 1492–1500. https://doi.org/10.1097/00006534-200111000-00007
- Patel, A., Yang, J. F., Hashim, P. W., Travieso, R., Terner, J., Mayes, L. C., Kanev, P., Duncan, C., Jane, J., Jane, J., Pollack, I., Losee, J. E., Bridgett, D. J., & Persing, J. A. (2014). The impact of age at surgery on long-term neuropsychological outcomes in sagittal craniosynostosis. The Impact of Age at Surgery on Long-Term Neuropsychological Outcomes in Sagittal Craniosynostosis: Plastic and Reconstructive Surgery, 134(4), 608e–617e. https://doi.org/10.1097/PRS.0000000000000511
- Polderman, T. J., Gosso, M. F., Posthuma, D., Van Beijsterveldt, T. C., Heutink, P., Verhulst, F. C., & Boomsma, D. I. (2006). A longitudinal twin study on IQ, executive functioning, and attention problems during childhood and early adolescence. Acta Neurologica Belgica, 106(4), 191–207.
- Shimoji, T., Tominaga, D., Shimoji, K., Miyajima, M., & Tasato, K. (2015). Analysis of pre- and post-operative symptoms of patients with mild trigonocephaly using several developmental and psychological tests. Child’s Nervous System: ChNs: Official Journal of the International Society for Pediatric Neurosurgery, 8(3), 433–440. https://doi.org/10.1007/s00381-014-2595-0
- Shipster, C., Stackhouse, J., & Wade, A. (2007). Speech, language, and cognitive development in children with isolated sagittal synostosis. Developmental Medicine & Child Neurology, 45(1), 34–43. https://doi.org/10.1111/j.1469-8749.2003.tb00857.x
- Speltz, M. L., Collett, B. R., Wallace, E. R., Starr, J. R., Cradock, M. M., Buono, L., Cunningham, M., & Kapp-Simon, K. (2015). Intellectual and academic functioning of school-age children with single-suture craniosynostosis. Pediatrics, 135(3), e615–e623. https://doi.org/10.1542/peds.2014-1634
- Speltz, M. L., Endriga, M. C., & Mouradian, W. E. (1997). Presurgical and postsurgical mental and psychomotor development of infants with sagittal synostosis—Matthew LC. Endriga, Wendy. Speltz, Marya E. Mouradian, 1997. Hämtad 30 oktober 2020, från. https://journals-sagepub-com.ezproxy.its.uu.se/doi/10.1597/1545-1569_1997_034_0374_papmap_2.3.co_2#articleCitationDownloadContainer.
- Speltz, M. L., Kapp-Simon, K., Collett, B., Keich, Y., Gaither, R., Cradock, M. M., Buono, L., & Cunningham, M. L. (2007). Neurodevelopment of Infants with single-suture craniosynostosis: Presurgery comparisons with case-matched controls. Plastic and Reconstructive Surgery, 119(6), 1874–1881. https://doi.org/10.1097/01.prs.0000259184.88265.3f
- Speltz, M. L., Kapp-Simon, K. A., Cunningham, M., Marsh, J., & Dawson, G. (2004). Single-suture craniosynostosis: A review of neurobehavioral research and theory. Journal of Pediatric Psychology, 29(8), 651–668. https://doi.org/10.1093/jpepsy/jsh068
- Starr, J. R., Collett, B. R., Gaither, R., Kapp-Simon, K. A., Cradock, M. M., Cunningham, M. L., & Speltz, M. L. (2012). Multicenter study of neurodevelopment in 3-Year-old children with and without single-suture craniosynostosis. Archives of Pediatrics & Adolescent Medicine, 166(6). https://doi.org/10.1001/archpediatrics.2011.1800
- Starr, J. R., Kapp-Simon, K. A., Cloonan, Y. K., Collett, B. R., Cradock, M. M., Buono, L., Cunningham, M. L., & Speltz, M. L. (2007). Presurgical and postsurgical assessment of the neurodevelopment of infants with single-suture craniosynostosis: Comparison with controls. Journal of Neurosurgery: Pediatrics, 107(2), 103–110. https://doi.org/10.3171/PED-07/08/103
- Starr, J. R., Lin, H. J., Ruiz-Correa, S., Cunningham, M. L., Ellenbogen, R. G., Collett, B. R., Kapp-Simon, K. A., & Speltz, M. L. (2010). Little evidence of association between severity of trigonocephaly and cognitive development in infants with single-suture metopic synostosis. Neurosurgery, 67(2), 408–416. https://doi.org/10.1227/01.NEU.0000371992.72539.8B
- Tamburrini, G., Caldarelli, M., Massimi, L., Santini, P., & DiRocco, C. (2005). Intracranial pressure monitoring in children with single suture and complex craniosynostosis: A review. Child’s Nervous System: ChNs: Official Journal of the International Society for Pediatric Neurosurgery, 21(10), 913–921. https://doi.org/10.1007/s00381-004-1117-x
- Tarnow, P., Kölby, L., Maltese, G., Söfteland, M. B., Lewén, A., Nilsson, P., Enblad, P., & Nowinski, D. (2022). Incidence of non-syndromic and syndromic craniosynostosis in Sweden. The Journal of Craniofacial Surgery, 33(5), 1517–1520. https://doi.org/10.1097/SCS.0000000000008457
- Tillman, K. K., Höijer, J., Ramklint, M., Ekselius, L., Nowinski, D., & Papadopoulos, F. C. (2020). Non-syndromic craniosynostosis is associated with increased risk for psychiatric disorders. Plastic and Reconstructive Surgery, 146(2), 355–365. https://doi.org/10.1097/PRS.0000000000007009
- Timberlake, A. T., Jin, S. C., Nelson-Williams, C., Wu, R., Furey, C. G., Islam, B., Haider, S., Loring, E., Galm, A., Larysz, D., Staffenberg, D. A., Flores, R. L., Rodriguez, E., Boggon, T. J., Persing, J. A., & Lifton, R. P. (2019). Mutations in TFAP2B and previously unimplicated genes of the BMP, Wnt, and Hedgehog pathways in syndromic craniosynostosis. Proceedings of the National Academy of Sciences of the United States of America (pp. 15116–15121). https://doi.org/10.1073/pnas.1902041116
- Tomasi, D., & Volkow, N. D. (2011). Functional connectivity hubs in the human brain. NeuroImage, 57(3), 908–917. https://doi.org/10.1016/j.neuroimage.2011.05.024
- Toth, K., Collett, B., Kapp-Simon, K. A., Cloonan, Y. K., Gaither, R., Cradock, M. M., Buono, L., Cunningham, M. L., Dawson, G., Starr, J., & Speltz, M. L. (2008). Memory and response inhibition in young children with single-suture craniosynostosis. Child Neuropsychology, 14(4), 339–352. https://doi.org/10.1080/09297040701594888
- van der Meulen, J., van der Vlugt, J., Okkerse, J., & Hofman, B. (2008). Early beaten-copper pattern: Its long-term effect on intelligence quotients in 95 children with craniosynostosis. Journal of Neurosurgery: Pediatrics, 1(1), 25–30. https://doi.org/10.3171/PED-08/01/025
- van der Vlugt, J., Coebergh van den Braak, R., van der Meulen, J., Hovius, S., Verhulst, F. C., Okkerse, J., Wierdsma, A. I., Kushner, S. A., & Klimek, M. (2021). Prolonged surgical duration in open craniofacial surgery: Detrimental to cognitive functioning? Journal of Plastic, Reconstructive & Aesthetic Surgery: JPRAS, 74(12), 3443–3476. https://doi.org/10.1016/j.bjps.2021.08.017
- van der Vlugt, J. J. B., van der Meulen, J. J. N. M., Creemers, H. E., Verhulst, F. C., Hovius, S. E. R., & Okkerse, J. M. E. (2012). Cognitive and behavioral functioning in 82 patients with trigonocephaly. Plastic and Reconstructive Surgery, 130(4), 885–893. https://doi.org/10.1097/PRS.0b013e318262f21f
- van der Vlugt, J. J. B., van der Meulen, J. J. M. N., van den Braak, R. R. J. C., Vermeij-Keers, C., Horstman, E. G. C., Hovius, S. E. R., Verhulst, F. C., Wierdsma, A. I., Lequin, M. H., & Okkerse, J. M. E. (2017). Insight into the pathophysiologic mechanisms behind cognitive dysfunction in trigonocephaly. Plastic and Reconstructive Surgery, 139(4), 954e–964e. https://doi.org/10.1097/PRS.0000000000003179
- Veritas International Ltd. (2014). Covidence. www.covidence.org.
- Virtanen, R., Korhonen, T., Fagerholm, J., & Viljanto, J. (1999). Neurocognitive sequelae of scaphocephaly. Pediatrics, 103, 791–795. https://doi.org/10.1542/peds.103.4.791
- Wallace, E. R., Collett, B. R., Kapp-Simon, K., Starr, J. R., Birgfeld, C., & Speltz, M. L. (2016). Visuomotor function in school-age children with single-suture craniosynostosis. Journal of Developmental & Behavioral Pediatrics: JDBP, 37(6), 483–490. https://doi.org/10.1097/DBP.0000000000000319
- Wall, S. A., Thomas, G. P., Johnson, D., Byren, J. C., Jayamohan, J., Magdum, S. A., McAuley, D. J., & Richards, P. G. (2014). The preoperative incidence of raised intracranial pressure in nonsyndromic sagittal craniosynostosis is underestimated in the literature. Journal of Neurosurgery Pediatrics, 14(6), 674–681. https://doi.org/10.3171/2014.8.PEDS1425
- Warschausky, S., Angobaldo, J., Kewman, D., Buchman, S., Muraszko, K. M., & Azengart, A. (2005). Early development of infants with untreated metopic craniosynostosis. Plastic and Reconstructive Surgery, 115(6), 1518–1523. https://doi.org/10.1097/01.PRS.0000160270.27558.64
- Woolgar, A., Parr, A., Cusack, R., Thompson, R., Nimmo-Smith, I., Torralva, T., Roca, M., Antoun, N., Manes, F., & Duncan, J. (2010). Fluid intelligence loss linked to restricted regions of damage within frontal and parietal cortex. Proceedings of the National Academy of Sciences of the United States of America, 107(33), 14899–14902. https://doi.org/10.1073/pnas.1007928107
- Wu, R. T., Gabrick, K. S., Singh, A., Landi, N., Taylor, J. A., Bartlett, S. P., Persing, J. A., & Alperovich, M. (2020). Comparison of neurocognitive outcomes in postoperative adolescents with unilateral coronal synostosis. Plastic and Reconstructive Surgery, 146(3), 614–619. https://doi.org/10.1097/PRS.0000000000007067
- Wu, R., Nie, J., Abraham, P., Halligan, T., Gabrick, K., Peck, C. J., Sawh-Martinez, R., Steinbacher, D. M., Alperovich, M., McPartland, J., & Persing, J. A. (2021). Neurologic characterization of craniosynostosis: Can direct brain recordings predict language development? The Journal of Craniofacial Surgery, 32(1), 78–82. https://doi.org/10.1097/SCS.0000000000007004
- Wu, R. T., Timberlake, A. T., Abraham, P. F., Gabrick, K. S., Lu, X., Peck, C. J., Sawh-Martinez, R. F., Steinbacher, D. M., Alperovich, M. A., & Persing, J. A. (2020). SMAD6 genotype predicts neurodevelopment in non-syndromic craniosynostosis. Plastic and ReconstructiveSurgery, 145(1), 117e–125e. https://doi.org/10.1097/PRS.0000000000006319
- Zuo, X. N., Ehmke, R., Mennes, M., Imperati, D., Castellanos, F. X., Sporns, O., & Milham, M. (2012). Network centrality in the human functional connectome. Cerebral Cortex (New York, N.Y.: 1991), 22(8), 18621875. https://doi.org/10.1093/cercor/bhr269
Appendix
Table A1. A standardized extraction form based on the National Institute of Mental Health (NIMH) Guidelines for assessment of quality in correlational studies.