Abstract
Liposomes encapsulating hemoglobin (LEHs) surface-conjugated with 2000 and 550 Da poly(ethylene glycol) (PEG) were produced via extrusion through 400, 200 and 100 nm pore diameter membranes in two types of phosphate buffer with different ionic strengths. The lipid bilayers were composed of dimyristoyl-phosphatidylcholine (DMPC), cholesterol, dimyristoyl-phosphoethanolamine-PEG (DMPE-PEG), dimyristoyl-phosphatidylglycerol (DMPG), and α-tocopherol (in a 43:40:10:5:2 mole ratio). N-acetyl-L-cysteine was coencapsulated in order to suppress hemoglobin (Hb) oxidation. Various physical properties of PEG-LEH dispersions were determined: size distribution, encapsulation efficiency, P50 (partial pressure of O2 where half of the oxygen binding sites are saturated with O2), cooperativity coefficient, and encapsulated methemoglobin (MetHb) level. In order to study the stabilization mechanism of these dispersions, the effective bending constant (KB) and the spontaneous radius of curvature (R0) of PEG-LEHs were extracted by fitting a mathematical model describing the size distribution of a liposome dispersion to the experimentally measured size distributions [Citation]. We observed that liposome dispersions extruded in phosphate buffer (PB) were more monodisperse than liposomes extruded in phosphate buffered saline (PBS), and higher molecular weight PEG promoted the formation of narrower size distributions. Moreover, extrusion in PB and lipid conjugation with higher molecular weight PEG imparted higher bilayer rigidity (high KB), and stabilized the liposome dispersions by the spontaneous curvature mechanism, whereas the other liposome dispersions were stabilized by thermal undulations (low KB). The P50 and cooperativity coefficient of PEG-LEHs extruded in PBS and PB was comparable to that of human blood, and the encapsulated MetHb levels were less than 5%. The highest encapsulation efficiencies obtained were 27%–36% (82–109 mg Hb/mL) for LEH dispersions extruded in PBS and grafted with 2000 Da PEG. These dispersions yielded KBs’ ranging from 7 kT to 27 kT, which indicated that these dispersions were stabilized by spontaneous curvature. Whereas the same lipid combination extruded in PBS, however, instead conjugated with 550 Da PEG resulted in KBs’ ranging from 2 kT to 2.7 kT, which indicated that these dispersions were stabilized by thermal undulations. Thermal undulations permitted Hb leakage through the lipid bilayers, which in turn lowered the encapsulation efficiency to 1%–10.7% (3–32 mg Hb/mL). Taken together, the experimentally measured size distributions and encapsulation efficiencies of PEG-LEH dispersions can be readily explained through analysis of the magnitude of KB, which dictates the stability mechanism of the liposome dispersion.
INTRODUCTION
Liposome encapsulated hemoglobin (LEH) is one strategy that is currently being used to create artificial blood substitutes. This strategy entails encapsulating potentially toxic stroma-free hemoglobin (Hb) inside the aqueous core of a lipid membrane shell, which is composed of phospholipids and cholesterol. LEHs are universal blood substitutes, and can readily be mass-produced with guaranteed sterility [Citation[2-5]]. Their oxygen binding properties have been shown to be comparable to those of human red blood cells (P50 ∼ 26 mmHg and cooperativity coefficient ∼ 2.3–2.4 [Citation[3], Citation[4]]). Reductants or catalases can be coencapsulated inside the liposome aqueous core to suppress methemoglobin (MetHb) formation [Citation[3], Citation[6], Citation[7]]. However, the circulation half life of LEHs is short (∼ 12–18 hours [Citation[8], Citation[9]]), and they tend to aggregate and fuse together after several days of storage [Citation[10]]. LEHs are primarily eliminated from the intravascular circulation by the cells of the reticuloendothelial system (RES) via a complement-mediated phenomenon [Citation[11]]. The surfaces of LEHs have been previously modified with ganglioside GM1 and anionic lipids in order to increase their circulatory half-life [Citation[12], Citation[13]]. However, previous studies have shown that GM1 surface modification of LEHs did not increase the intravascular persistence of LEH dispersions [Citation[13]].
The most promising strategy to improve the circulation half–life and stability of LEHs is through surface modification with poly(ethylene glycol) or PEG [Citation[3], Citation[14]]. PEG is a biologically inert polymer, which has been used extensively in drug delivery systems and is declared to be clinically safe by the Food and Drug Administration (FDA) [Citation[3], Citation[4], Citation[14]]. It was proposed that PEG conjugation creates a steric hydrophilic barrier surrounding each LEH, protecting LEHs from opsonizing plasma proteins, and thus increasing their intravascular persistence [Citation[14], Citation[15]]. The circulation half-life of LEH dispersions is dependent on vesicle size, molecular weight of PEG, and molar percentage of PEG incorporated into the bilayers.
Awasthi et al. [Citation[16]] studied the size dependence of empty PEG-conjugated liposomes on their circulation half-life in rabbits. The bilayers of these PEG-liposomes were composed of phospholipids, cholesterol, α-tocopherol and 0.025 mol% PEG (molecular weight = 5000Da). They observed that the optimum diameter of PEG-liposomes corresponding to maximum liposome circulation was 160–220 nm, with circulation half-lives ranging between 25–27 hours. In this size range, liver uptake was minimized, and spleen uptake was moderate. Beyond this size range, PEG's ability to camouflage LEHs was compromised, and high accumulation in the RES was observed. Zheng et al. [Citation[17]] measured the circulation half-life of LEHs grafted with 5 mol% of 1900 Da PEG in rabbits, and observed a half-life of 15–20 hours, similar to the circulation half-life of non-modified LEHs [Citation[8], Citation[9]]. Increasing the PEG mole percentage in lipid bilayers up to 10 mol% and using 5000 Da PEG, Phillips et al. [Citation[18]] demonstrated that PEG-conjugation could indeed extend the circulatory half-life of LEH dispersions in rabbits up to 65 hours.
The steric barrier created by PEG conjugation also prevents LEH aggregation and fusion and thus, stabilizes LEH dispersions during storage [Citation[10]]. PEG-LEH dispersions stored in a deoxygenated state at 4 and 23°C were stable without observable Hb leakage for one year, and showed only a slight decrease in pH and P50, provided that the P50 of LEH dispersions was regulated by coencapsulating the allosteric effector pyridoxal 5′-phosphate (PLP) [Citation[10]]. PEG-LEH dispersions stored at 40°C were stable for 6 months, before liposome aggregation and Hb leakage were observed. MetHb formation of these LEH dispersions was reversed from an initial MetHb level of 3% to 1% after one month of storage via coencapsulation of the reductant homocysteine [Citation[10]].
PEG surface modification improved the rheology and hemodynamic properties of LEH dispersions, as demonstrated by Sakai et al. [Citation[19]]. Specifically, it was observed that the viscosity of non-modified LEH dispersions suspended in an albumin solution was 8 cP due to LEH aggregation and interaction with albumin. In contrast, the viscosity of PEG-LEHs suspended in albumin was 3.5 cP, comparable to that of human blood (∼ 4 cP) [Citation[19]]. In an additional study using artificial capillaries, it was shown that both non-modified and PEG-LEH dispersions could easily permeate through capillaries with diameters of 3 µm and less, while erythrocytes could hardly traverse these capillaries. It was observed that PEG-LEH dispersions had a higher permeation rate due to the suppression of liposomes aggregation [Citation[19]]. In humans, the capillary diameter ranges from ∼ 3 µm to 15 µm, and during circulatory failure such as in the case of hemorrhagic shock, the capillary constricts [Citation[19]]. This study demonstrates quite convincingly that LEHs can easily permeate through capillary blockages, and are suitable oxygen carriers in cases of trauma and routine surgery.
The biocompatibility of PEG-conjugated LEHs was tested in several studies. Wakamoto et al. [Citation[20]] studied the effect of PEG-LEHs on human platelet functions in vitro. Neither platelet aggregation nor platelet activation was observed, which suggested that PEG-LEHs had no adverse effect on platelet function in plasma. Sakai et al. [Citation[19]] showed that erythrocytes exhibited no signs of aggregation or deformation in the presence of PEG-LEHs, indicating that these liposomes did not affect the structural integrity of erythrocytes. Sherwood et al. [Citation[21]] infused PEG-LEH and non-modified LEH dispersions into Listeria monocytogenes-infected mice, and concluded that PEG-LEHs had significantly less of an adverse impact on host immunity compared to non-modified LEHs. Further animal studies demonstrated that while infusion of PEG-conjugated and non-modified LEH dispersions into laboratory animals resulted in normal mean arterial pressure, heart rate and blood gas parameters, PEG-conjugation prevented vasoconstriction [Citation[22]] and significantly improved the hemodynamic state of the laboratory animals compared to non-modified LEHs [Citation[23-25]].
In this study, PEG-LEH dispersions composed of dimyristoyl-phosphatidylcholine, cholesterol, α-tocopherol, dimyristoyl-phosphatidylglycerol, and dimyristoyl-phosphoethanolamine-PEG were prepared via extrusion in phosphate buffered saline (PBS) at pH 7.3 through 100, 200 and 400 nm pore diameter membranes with an initial bovine Hb concentration of 300 mg/mL. PEG-LEHs were formed with two different PEG molecular weights (550 and 2000 Da). PBS was used as the extrusion buffer since the allosteric effector of bovine Hb is chloride ions [Citation[2]]. A physiological concentration of chloride ions was used to solvate Hb molecules, in order to obtain P50s’ close to that of human red blood cells. The effect of using different molecular weight PEGs on LEH: size distribution, encapsulation efficiency, oxygen affinity, and encapsulated methemoglobin (MetHb) level was measured. The size distribution and encapsulation efficiency of each PEG-LEH dispersion was measured using an asymmetric flow field-flow fractionator (AFFF) coupled with a multi-angle static light scattering (MASLS) photometer, and a differential interferometric refractometer (DIR) [Citation[26]]. The oxygen affinity of PEG-LEHs was determined by evaluating their P50s and cooperativity coefficients. α-tocopherol was incorporated into the lipid bilayers to suppress lipid oxidation, and a reductant, N-acetyl-L-cysteine (NAC) was coencapsulated inside the liposome aqueous core to suppress Hb oxidation [Citation[8], Citation[27]]. Since it was previously observed that chloride ions can also increase Hb oxidation [Citation[26], Citation[28]], LEHs were also extruded in phosphate buffer (PB, pH 7.3). Moreover, the ionic strength of the extrusion buffers also affected the physical conformation of the PEG brushes.
Having established that LEH surface modification with PEG could prolong LEH circulation half-lives [Citation[18]], the mechanism which stabilized PEG-conjugated LEHs was investigated. We undertook this effort by evaluating the effective bending constants, KB, and spontaneous radii of curvatures, R0, extracted by fitting Jung et al.'s size distribution model of liposome dispersions [Citation[1]] to PEG-LEH size distributions measured using AFFF-DIR-MASLS. The magnitude of KB reflected the energy required to bend a bilayer away from its spontaneous radius of curvature (R0), or it could be thought of as the minimum energy needed to maintain a vesicle of radius R0. The mechanism governing LEH stabilization either by thermal undulations or spontaneous curvature was determined by examining the magnitude of KB [Citation[1], Citation[29]]. Both mechanisms only permitted formation of unilamellar liposomes. The only known similar study done on PEG-conjugated vesicles was performed by Kang et al. [Citation[30]] on cetyltrimethylammonium bromide/sodium perfluorooctanoate (FC7) and CTAB/sodium perfluorohexanoate (FC5) surfactant mixtures. Another method that is currently being used to probe the stability mechanism of liposomes is micropipette aspiration, which can only be used to probe the mechanical properties of giant liposomes (∼ 10 µm in diameter) [Citation[31]]. However, it is important to note that the size of the liposomes to be probed will influence [Citation[32]] the elucidated vesicles’ stability mechanism, which can cause discrepancies between the stability mechanism elucidated for giant liposomes (diameter ∼ 10 µm) and sub-micron sized vesicle dispersions. In this study, the experimentally measured size distributions and encapsulation efficiencies of PEG-LEH dispersions will be explained in light of the magnitude of KB, which will determine the stability mechanism for liposome dispersions. In addition, the effect of PEG molecular weight and ionic strength of the extrusion buffers on the elucidated stabilization mechanism of PEG-LEH dispersions will be explored.
MATERIALS AND METHODS
Materials
Dimyristoyl-phosphatidylcholine (DMPC), dimyristoyl-phosphatidylglycerol (DMPG), 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DMPE-PEG 2000), 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-550] (DMPE-PEG 550), cholesterol and polycarbonate membranes were purchased from Avanti Polar Lipids, Inc. (Birmingham, AL). Phosphate buffered saline (PBS) and phosphate buffer (PB) at physiological pH 7.3, were made using phosphate salts and NaCl obtained from Sigma-Aldrich (St. Louis, MO). Spectra/Por® Cellulose Ester dialysis bags with 100,000 MWCO were purchased from Spectrum Laboratories, Inc. (Rancho Dominguez, CA). HEMOX-solution, additive-A and anti-foaming agent were purchased from TCS Scientific Corp (New Hope, PA). Octyl-β-D-glucopyranoside (OBG), N-acetyl-L-cysteine, and α-tocopherol were obtained from Sigma-Aldrich (St. Louis, MO).
Hb Preparation
Tetrameric Hb was extracted from freshly drawn bovine erythrocytes and assayed by UV visible spectrophotometry (Varian, Inc., Palo Alto, CA) according to previous publications [Citation[26], Citation[32-36]]. Bovine Hb has been shown to function as a compatible and convenient replacement to human Hb for use in formulating Hb-based blood substitutes [Citation[37]]. Bovine erythrocytes were extracted, centrifuged and collected in 3.8% sodium citrate by Animal Technologies, Inc. (Tyler, Texas). The purity of extracted tetrameric Hb was established by SDS-PAGE at 99%.
Lipid Composition
A combination of DMPC, cholesterol, DMPE-PEG, DMPG and α-tocopherol was used in a 43:40:10:5:2 mole ratio to produce LEHs. Unsaturated phosphatidylcholine (PC) is capable of oxidizing encapsulated Hb within a few days, therefore saturated PC, DMPC in this study, was found to be suitable in limiting Hb oxidation [Citation[8]]. Cholesterol was added to the LEH bilayer to overcome several known problems. Intravascularly, cholesterol in erythrocyte membranes transferred to pure phospholipid liposome membranes due to the cholesterol concentration gradient, resulting in osmotically fragile erythrocytes [Citation[38]]. Addition of cholesterol to liposomes reduced this concentration gradient. Moreover, cholesterol enhanced the liposome's resistance to fusion and lysis [Citation[8], Citation[39]], and the liposome's impermeability to small ions [Citation[40]]. DMPG was included to impart a slight negative charge to the vesicle bilayers, since neutral liposomes composed of phospholipids and cholesterol displayed a tendency to aggregate [Citation[8]]. Also, it was shown [Citation[8]] that DMPG was safe when infused into mice, and that addition of DMPG in the amount of 5% mole fraction produced the most optimum encapsulation efficiency. Incorporation of DMPE-PEG into bilayers membranes is able to increase the circulation half-life of LEHs [Citation[3], Citation[4], Citation[18]] by masking the LEHs from being recognized by the RES. Two molecular weights of PEG were used in this study: 550 and 2000 Da. Lipid bilayers are susceptible to oxidation, thus α-tocopherol was incorporated in the bilayers to suppress bilayer oxidation [Citation[18]]. Hb oxidation was suppressed by inclusion of 200 mg/L of the reductant N-acetyl-L-cysteine (NAC) in the extrusion buffer [Citation[27]].
LEH Preparation
Liposomes were extruded through polycarbonate membranes with the following membrane pore diameters: 400 nm, 200 nm, and 100 nm with an initial Hb concentration of 300 mg Hb per mL of buffer. Twenty milligrams of the lipid mixture was dried using a Buchi R-205 rotary evaporator (Buchi Analytical, Inc., New Castle, DA) for at least 4 hours at 40°C [Citation[41]]. The resultant lipid film was rehydrated with one mL of 300 mg/mL Hb solution (diluted in either PB or PBS at pH = 7.3, accordingly). Extrusion was done in steps starting from a larger pore diameter successively down to the final pore diameter [Citation[8]]. Ten passes were executed for each successive step, and 25 passes were completed at the final step to achieve a homogenous dispersion [Citation[26], Citation[32], Citation[42-47]]. Empty liposomes were extruded in both PB and PBS with 200 mg/L of NAC to serve as control dispersions. Liposome dispersions were used immediately after preparation.
Size Distribution and Encapsulation Efficiency
LEH size distributions and encapsulation efficiencies (E%) were measured simultaneously using an Eclipse asymmetric flow field-flow fractionator coupled in series to a 18-angle Dawn EOS multi-angle static light scattering photometer equipped with a linearly polarized 30 mW gallium-arsenide laser operating at 690 nm, and an Optilab DSP differential interferometric refractometer (Wyatt Technology Corp., Santa Barbara, CA) [Citation[26], Citation[43], Citation[45], Citation[46]]. Light scattering spectra were analyzed using the ASTRATM software (Wyatt Technology Corp.). The mobile phase for all experiments was PBS at physiological pH = 7.3, filtered through 0.2 micron filters.
Oxygen Affinity
Freshly extruded LEH dispersions were dialyzed overnight in PBS (pH 7.3) at 2–3°C using dialysis bags with a 100,000 Da MWCO (Spectrum Labs, Rancho Dominguez, CA) to separate unencapsulated Hb from LEHs. In preparation for oxygen binding measurements, 0.2 mL of dialyzed LEHs was lightly mixed with 4.8 mL of HEMOX solution, 20 µL of additive-A and 10 µL of anti-foaming agent. The P50 and cooperativity coefficient of LEH dispersions were measured using a HemoxTM-Analyzer from TCS Scientific Corp (New Hope, PA) at 37°C [Citation[26], Citation[32-36]]. The P50 and cooperativity coefficient were both calculated by fitting the oxygen binding curve to the Adair equation [Citation[48]].
MetHb Level
Protein adsorption to phospholipid membranes confers resistance to detergent lysis. Octyl-β-D-Glucopyranoside (OBG) is a detergent known to transform LEH dispersions into micelles [Citation[8]]. To measure the MetHb level inside the LEH particles, first freshly extruded LEH was dialyzed overnight in PBS (pH 7.3) at 2–3°C, using dialysis bags with 100,000 Da MWCO membranes (Spectrum Labs, Rancho Dominguez, CA) to separate unencapsulated Hb from the LEHs dispersion. LEH particles were then lysed with OBG to release the encapsulated Hb into solution. The MetHb concentration encapsulated inside the LEH dispersion was then assayed according to a previously published procedure [Citation[26]].
THEORETICAL BACKGROUND
Background theories of light scattering, Hb encapsulation efficiency determination, and oxygen binding equilibria, were described in a previous publication [Citation[26], Citation[43], Citation[45]].
Size Distribution Model of Liposome Dispersions
Previous studies [Citation[1], Citation[29], Citation[30], Citation[49]] have proposed that the polydispersity of a vesicle dispersion is controlled by the natural fluctuations of the lipid bilayer's curvature around a certain spontaneous curvature, and hence, the size distribution of this suspension depends on the bilayer fluctuations. It is possible to apply the thermodynamic theory of aggregation to a liposome dispersion, and calculate the effective bending constant and radius of curvature of the liposomes’ bilayers [Citation[49], Citation[50]]. The size distribution model presented here is taken from Denkov et al.'s derivation [Citation[49]].
Applying thermodynamic theory, each liposome is considered as an aggregate composed of N lipid units (assuming that DMPC, DMPG, DMPE-PEG, α-tocopherol, and cholesterol molecules are uniformly distributed in the bilayer, we can equally divide the bilayer into N segments or lipid units consisting of a fixed composition of the 5 molecules). At equilibrium, the chemical potential of the molecules in all aggregates must be equal:where μN is the chemical potential of a lipid unit in a vesicle composed of N lipid units,
is standard chemical potential, which is independent of the aggregate concentration (however, it depends on the temperature, pressure and radius of the liposomes), kT is the thermal energy (k is Boltzmann's constant and T is the absolute temperature of suspension), and XN is the total molar concentration of the lipid units in all aggregates composed of N lipid units. M is an arbitrary reference state, but in this case M is taken as the number of lipid units in an aggregate having the mean size with XM as the molar concentration. The probability of finding an aggregate of N lipid units is [Citation[49], Citation[50]] determined by the balance between the entropy and curvature energy:
Since the temperature and pressure are kept constant throughout the experiments, the standard chemical potential depends solely on the liposome radius.
For small deviations from the spontaneous curvature, and assuming that the radius of spontaneous curvature, R0, is the mean liposome radius, the bending energy can be presented as [Citation[49-51]]:where WB is the bending elasticity energy per unit area of the bilayer, and K is the average bending elasticity constant. The total surface area of the liposomes is proportional to the number of lipid units:
where A0 is the area of one lipid unit. Combining the previous Equations and substituting CN = XN/N, the liposome size distribution model can be derived. Assuming that the liposome suspension is fairly homogeneous or (1 − R/R0)2 < < 1, the model can be simplified by expansion to yield:
where C(R) and C(R0) are the molar or number concentrations of liposomes of radius R and R0, respectively. This equation predicts a Gaussian size distribution around the average radius R0. Note that the bending energy expressed in Eq. 5 is in agreement with Israelachvili's definition [Citation[50]], which is four times larger than Helfrich's effective bending energy (KB) [Citation[51]]; hence, consequently, K is four times larger than Helfrich's effective bending constant [Citation[49], Citation[51]]. Jung et al. [Citation[1]] used a similar size distribution model based on Helfrich's definition of the bending energy (KB) [Citation[51]]:
A mathematical tool, Scientist Software (Micromath Scientific Software, Inc., St. Louis, MO), was used to fit Eq. 6 to the measured differential number size distribution, to extract KB and R0 as well as their respective standard deviations [Citation[32]].
Since Jung et al.'s model [Citation[1]] was developed for spontaneously formed vesicles at equilibrium, it is pertinent to discuss the validity of applying this model to our liposome dispersions, which were created via extrusion. It is very difficult to prove that liposome dispersions are at equilibrium [Citation[1]]. However, if the composition and phase of the dispersion are reproducible regardless of the sample history (i.e. the liposome size distribution is stable for a long period of time on the order of several months), the dispersion can be safely assumed to be at equilibrium [Citation[1]]. Based on our observations, our PEG-liposome size distributions were stable for at least two months. Sakai et al. have shown that PEG-LEHs created via extrusion can be stable for as long as one year [Citation[10]]. In addition, we observed that when our liposomes were prepared at room temperature, followed by storage at 2–3°C for a day and then the temperature was raised back to room temperature, the size distribution of the originally extruded liposomes was recovered regardless of temperature cycling. Hence, we concluded that our liposome dispersions are at equilibrium.
RESULTS AND DISCUSSION
Size Distribution
The size distributions of PEG-LEH dispersions extruded with an initial Hb concentration of 300 mg/mL and their corresponding controls (empty liposomes) are displayed in and , respectively. Tables and contain the number- (Rn), weight- (Rw), z-average radii (Rz), and polydispersity indices (Rw/Rn and Rz/Rn) of PEG-LEH and PEG-control dispersions. For convenience, PEG-LEHs (PEG molecular weight = 2000Da) extruded in PB are referred to as PB-PL2000, and PEG-LEHs (PEG molecular weight = 550Da) extruded in PB are referred to as PB-PL550. Likewise, PEG-LEHs extruded in PBS are referred to as PBS-PL2000 and PBS-PL550, and the corresponding PEG-conjugated controls are referred to as PB-C2000, PB-C550, PBS-C2000, and PBS-C550.
Figure 1 Size distributions of PEG-conjugated LEH dispersions extruded with an initial Hb concentration of 300 mg/mL. Differential and cumulative size distributions of 2000 Da PEG-conjugated LEH dispersions extruded in PB are displayed in panels A and B respectively; 2000 Da PEG-conjugated LEH dispersions extruded in PBS are displayed in panels C and D, respectively; 550 Da PEG-conjugated LEH dispersions extruded in PB are displayed in panels E and F, respectively; and 550 Da PEG-conjugated LEH dispersions extruded in PBS are displayed in panels G and H, respectively. Dashed lines represent PEG-LEHs extruded through 400 nm pore diameter membranes, solid lines represent extrusion through 200 nm pore diameter membranes, and the dotted lines represent extrusion through 100 nm pore diameter membranes.
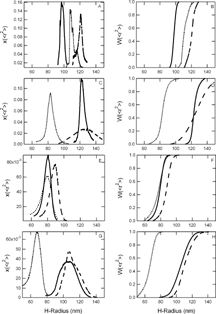
Figure 2 Size distributions of PEG-conjugated control (empty liposome) dispersions. Differential and cumulative size distributions of 2000 Da PEG-conjugated control dispersions extruded in PB are displayed in panels A and B, respectively; 2000 Da PEG-conjugated control dispersions extruded in PBS are displayed in panels C and D, respectively; 550 Da PEG-conjugated control dispersions extruded in PB are displayed in panels E and F, respectively; and 550 Da PEG-conjugated control dispersions extruded in PBS are displayed in panels G and H, respectively. Dashed lines represent PEG-controls extruded through 400 nm pore diameter membranes, solid lines represent extrusion through 200 nm pore diameter membranes, and dotted lines represent extrusion through 100 nm pore diameter membranes.
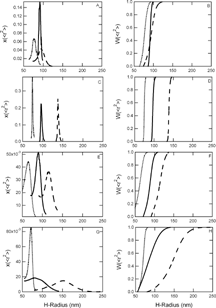
Table 1 Number-, weight-, z-average radii, and polydispersity indices of PEG-LEH and PEG-control dispersions extruded in PB. Part A corresponds to PEG-liposomes with a PEG molecular weight = 2000 Da, and part B corresponds to PEG-liposomes with a PEG molecular weight = 550 Da
Table 2. Number-, weight-, z-average radii, and polydispersity indices of PEG-LEH and PEG-control dispersions extruded in PBS. Part A corresponds to PEG-liposomes with a PEG molecular weight = 2000 Da, and part B corresponds to PEG-liposomes with a PEG molecular weight = 550 Da
PEG-LEHs and controls extruded through 100 nm pore diameter membranes exhibited a mean diameter that was larger than the membrane pore diameter, while liposomes extruded through 200 nm pore diameter membranes exhibited a mean diameter that was close to the membrane pore diameter, and liposomes extruded through 400 nm membranes exhibited a mean diameter that was smaller than the membrane pore diameter. Non-modified LEHs (non-PEGylated) and their controls’ size distributions’ also exhibited a similar trend [Citation[26]]. Although the polydispersity indices of our dispersions indicated that PEG-LEH and PEG-control dispersions were monodisperse (indices were close to one in value), closer inspection revealed that the size distributions of liposomes extruded in PB were narrower, compared to those of liposomes extruded in PBS, regardless of the PEG molecular weight. The distribution widths of PB-PL2000 and PB-PL550 dispersions extruded through 100, 200 and 400 pore diameter membranes were approximately 10–20 nm and 40 nm, respectively. In contrast, the distribution widths of PBS-PL2000 and PBS-PL550 extruded through 100, 200 and 400 pore diameter membranes were 20–60 nm and 30–60 nm, respectively. Electrostatic repulsion between the chloride ions in the buffer, and the negatively charged DMPG and DMPE-PEG in the bilayers could disrupt the liposomes’ dispersion state. Moreover, conjugation with higher molecular weight PEG resulted in narrower size distributions for PEG-LEH dispersions extruded in the same buffer. It appeared that higher molecular weight PEG chains were more effective in suppressing aggregation and fusion between liposomes, hence creating a more monodisperse suspension. A similar trend was also observed with PEG-controls. While the distribution widths of both PB-C2000 and PBS-C2000 extruded through 100, 200 and 400 pore diameter membranes were almost similar, ranging from 15 nm to 40 nm, those of PB-C550 and PBS-C550 ranged from 35 nm to 50 nm and 40 nm to 150 nm, respectively. In summary, we observed that PEG-conjugated liposome dispersions extruded in PB were more monodisperse than those extruded in PBS, and higher molecular weight PEG promoted the formation of narrower size distributions.
Since this is the first study to measure the absolute size distribution of PEG-LEHs, we could only compare the average size of PEG-LEH and PEG-control dispersions with results obtained from other groups. Extrusion though 220 nm pore diameter membranes yielded 222±62 nm [Citation[10]], 230±81 nm [Citation[20]], 259±82 nm [Citation[23]], and 250±80 nm [Citation[19]] PEG-LEHs, while microfluidization yielded 193 nm diameter PEG-LEHs [Citation[18]]. The best comparison was with Awasthi et al. [Citation[16]] who prepared 136.2, 165.5, 209.2, 275 and 318 nm diameter empty PEG-liposomes via extrusion. Awasthi [Citation[52]] experienced difficulties in preparing PEG-liposomes with diameters less than 100 nm via extrusion, while microfluidization yielded liposomes with sizes ranging from 80 nm to 120 nm. If anionic lipids were used (in our case, DMPG is negatively charged), liposomes with diameters larger than 320 nm could not be prepared via extrusion [Citation[52]]. This observation confirmed our finding that only PEG-liposomes with diameters ranging from 160 nm to 240 nm could easily be prepared by extrusion.
Encapsulation Efficiency and Oxygen Affinity
Hb Encapsulation efficiencies, estimated entrapped Hb concentrations, P50s and Hill coefficients (n) of PEG-LEHs are displayed in Tables and . The encapsulated MetHb levels for all samples were less than 5%. Although, it was reported that chloride ions promoted Hb oxidation [Citation[26], Citation[28]], we did not observe MetHb level differences in PEG-LEHs extruded in PB and those extruded in PBS. The P50s of PEG-LEHs extruded in PBS were higher than those of PEG-LEHs extruded in PB, which was expected since chloride ions are an allosteric effector of bovine Hb [Citation[2]]. Regardless of the extrusion buffer, PEG molecular weight, and extrusion membrane pore size, all PEG-LEHs exhibited P50s and cooperativity coefficients close to the measured P50 and cooperativity coefficient of bovine Hb (28 torr and 2.4–2.5, respectively), and were comparable to that of human blood (26 torr and 2.3–2.4, respectively) [Citation[4]]. Surface modification with PEG clearly did not compromise the oxygen binding properties of PEG-LEHs. However, we found that the encapsulation efficiencies of PB-PL2000, PB-PL550 and PBS-PL550 were very low (less than 11%) and the estimated entrapped Hb concentrations were significantly less than the Hb concentration of human erythrocytes (150 mg Hb/mL of blood) [Citation[4]]. Note that in our PEG-LEHs preparation scheme, PEG chains were incorporated into both the inner and outer leaflets of the liposomes; and consequently, PEG brushes in the inner leaflet reduced the core volume available for Hb encapsulation. Strangely, the encapsulation efficiencies of PBS-PL2000 were high (27–36%), where the highest encapsulation efficiency (36.31%) was obtained by extruding PEG-LEHs with the largest pore diameter membrane (400 nm). However, we did not expect this result, since incorporation of lower molecular weight PEG should have increased the entrapped Hb concentration. Moreover, we observed that lowering the ionic strength of the extrusion buffer generally lowered the Hb encapsulation efficiency.
Table 3. Hb encapsulation efficiencies, estimated entrapped Hb concentrations, P50s and Hill coefficients (n) of PEG-LEHs extruded in PB. Part A corresponds to PEG-liposomes with a PEG molecular weight = 2000 Da, and part B corresponds to PEG-liposomes with a molecular weight = 550 Da
Table 4. Hb encapsulation efficiencies, estimated entrapped Hb concentrations, P50s and Hill coefficients (n) of PEG-LEHs extruded in PBS. Part A corresponds to PEG-liposomes with a PEG molecular weight = 2000 Da, and part B corresponds to PEG-liposomes with a molecular weight = 550 Da
Phillips et al. [Citation[18]] reported that PEG-LEHs with an average diameter of 193 nm could encapsulate 1.2 g/dL or 12 mg/mL of Hb (with a lipid bilayer composed of 10 mol% of DSPE-conjugated 5000 Da PEG). Note that in these dispersions [Citation[18]], PEG brushes were also incorporated into the inner leaflet of the liposomes, thus, a low encapsulated Hb concentration was expected. Our estimated entrapped Hb concentrations of PEG-LEHs extruded through 200 nm pore diameter membranes in PB and PBS were 31.62 and 92.70 mg/mL, respectively, for LEHs conjugated with 2000 Da PEG, and 11.67 and 32.19 mg/mL, respectively, for LEHs conjugated with 550 Da PEG. We expected to obtain higher Hb encapsulation efficiencies than Phillips et al. [Citation[18]] since we employed shorter PEG chains. Hence, it was surprising to observe that PB-PL550 (extruded through 200 nm pore diameter membranes) exhibited an encapsulated Hb concentration close to the value obtained by Phillips et al. [Citation[18]]. Sakai et al. [Citation[10], Citation[19], Citation[23]] managed to increase the entrapped Hb concentration to 10 g/dL or 100 mg/mL by exclusively incorporating 5000 Da PEG on the outer surface of the PEG-LEHs (220–250 nm in diameter). Note that although the lower limit of Hb concentration in a Hb-based blood substitute has never been established, for acellular, Hb-based artificial blood substitutes, 5 g/dL (50 mg/mL) of Hb is theoretically sufficient to deliver adequate amounts of oxygen at rest without taxing cardiac output, provided that vasoconstriction does not occur [Citation[53]]. For cellular-based blood substitutes, the required Hb concentration should be higher because oxygen has to first diffuse out of the artificial membranes, before it is transported to tissues.
Stability Mechanisms
In order to understand the stabilization mechanism of our dispersions, we extracted effective bending constants, KB, and spontaneous radius of curvatures, R0, by fitting Jung et al.'s liposome size distribution model [Citation[1]] to our differential number size distributions measured by AFFF-MASLS-DIR. The results of these curve fittings and their corresponding standard deviations are displayed in Tables and . Note that KB and its standard deviation, ΔK, are presented in units of kT, where k is the Bolztmann's constant and T is the absolute temperature. The magnitude of KB dictates the stabilization mechanism of liposome dispersion. If KB ≈ kT then the liposome dispersion is stabilized by thermal undulations, otherwise, if KB > > kT then the stabilization mechanism is spontaneous curvature, and the bilayers are more rigid [Citation[54], Citation[55]].
Table 5. Effective bending constants, KB, and spontaneous radii of curvature, R0, and their corresponding standard deviations, ΔK and ΔRo of PEG-LEH and PEG-control dispersions extruded in PB. Note that KB and its standard deviation, ΔK, are presented in units of kT, where k is Bolztmann's constant and T is the absolute temperature. Part A corresponds to PEG-LEHs with a PEG molecular weight = 2000 Da, part B corresponds to PEG-LEHs with a PEG molecular weight = 550 Da, part C corresponds to PEG-controls with a PEG molecular weight = 2000 Da, and part D corresponds to PEG-controls with a PEG molecular weight = 550 Da
Table 6. Effective bending constants, KB, and spontaneous radii of curvature, R0, and their corresponding standard deviations, ΔK and ΔRo of PEG-LEH and PEG-control dispersions extruded in PBS. Note that KB and its standard deviation, ΔK, are presented in units of kT, where k is the Bolztmann's constant and T is the absolute temperature. Part A corresponds to PEG-LEHs with a PEG molecular weight = 2000 Da, part B corresponds to PEG-LEHs with a PEG molecular weight = 550 Da, part C corresponds to PEG-controls with a PEG molecular weight = 2000 Da, and part D corresponds to PEG-controls with a PEG molecular weight = 550 Da
PB-PL2000 dispersions were stabilized by spontaneous curvature, regardless of the membranes pore diameters used for extrusion. By changing the extrusion buffer to PBS, the bilayers rigidity decreased (KBs decreased), but the PBS-PL2000 dispersions were still stabilized by spontaneous curvature, except for PEG-LEHs extruded through 400 nm pore diameter membrane. Conjugation with lower molecular weight PEG (PB-PL550) decreased the bilayers rigidities, but, changing the extrusion buffer to PBS, decreased KB further and shifted the stabilization mechanism of PBS-PL550 dispersions from spontaneous curvature to thermal undulations. The size distributions of liposome dispersions with high KBs’ are expected to be stabilized by spontaneous curvature and are expected to be narrower [Citation[1], Citation[55]], which was the trend observed with the measured size distributions in this study. Moreover, the decrease in bilayer rigidity with decreasing PEG molecular weight explained the trend previously observed with the measured Hb encapsulation efficiencies. Highly flexible (less rigid) bilayers permitted Hb leakage [Citation[56]]. However, it did not explain why the presence of salt in the extrusion buffer increased Hb encapsulation, while decreasing bilayer rigidity. We propose that extrusion in PBS increased the ionic strength inside the liposomes. Consequently, these ions screened the charges on the PEG molecules, which caused the PEG tails to collapse into a globular conformation to shield the hydrophobic layers of the membranes from the highly charged aqueous core [Citation[56]], and hence permitting more Hb to be encapsulated, such as the case with PBS-PL2000. For PBS-PL550, the fluctuating bilayers (stabilized by thermal undulations) caused extensive Hb leakage, and consequently, exhibited low Hb encapsulation efficiencies, despite the favorable extrusion buffer. Comparing the effective bending constants of PEG-LEHs and non-modified LEHs (data was taken from a previous study [Citation[32]], refer to ), we found that conjugation with 2000 Da and 550 Da PEG increased the bilayers’ rigidity. A more significant increase in bilayer rigidity was observed with 2000 Da PEG-conjugation compared to 500 Da PEG-conjugation. The incorporation of wedge-shaped PEG molecules into bilayers composed of tubular-shaped phospholipid molecules induced surface lateral expansion in the bilayers, and as a result rigidified the bilayers [Citation[57]]. Moreover, the extrusion buffer had a prominent effect on the stabilization mechanism, specifically PEG-LEH bilayers were more rigid (and the stabilization mechanism was via spontaneous curvature) when the extrusion buffer was PB.
Table 7. Effective bending constants, KB, and corresponding standard deviations, ΔK of non-modified LEHs dispersions, extruded with an initial Hb concentration of 300 mg/mL (data taken from a previous study [Citation[32]]). Note that KB and its standard deviation, ΔK, are presented in units of kT, where k is the Bolztmann's constant and T is the absolute temperature
There was a mixture of both types of stabilization mechanisms for PB-C2000 dispersions. The PEG-control dispersion extruded through 200 nm pore diameter membranes was stabilized by spontaneous curvature, while the other dispersions were stabilized by thermal undulations. Opposite to the trend observed with PEG-LEH dispersions, the bilayer rigidity increased with extrusion in PBS, and the PBS-C2000 dispersions were stabilized by spontaneous curvature, regardless of the vesicles’ size. However, when the PEG molecular weight was decreased to 550 Da, KBs’ decreased and the stabilization mechanisms of PEG-control dispersions extruded in both PB and PBS shifted to thermal undulations. Based on the theory presented earlier for the stabilization mechanism of a liposome dispersion, the magnitude of KB determines the polydispersity of the liposome dispersion. In our study, we observed that KB for PEG-control dispersions increased with increasing PEG molecular weight. This correlated with a narrowing of the size distribution as KB increased. However, we observed that if the molecular weight of the PEG-control dispersion was held constant and the ionic strength of the extrusion buffer varied, no difference was detected in the widths of the PEG-control size distributions. Taken together, these results showed that although the stability mechanism (determined from the magnitude of KB) can explain the measured size distribution and encapsulation efficiency of PEG-LEH dispersions, it could not predict the behavior of the PEG-control dispersions. Overall, it appeared that incorporation of higher molecular weight PEG molecules into the bilayer imparted higher bilayer rigidity, which agreed with reported observations that PEG-LEH circulation half-life increased with increasing PEG molecular weight [Citation[18]].
To examine the effect of Hb encapsulation on the liposome dispersion stabilization mechanism, we compared KB values between PEG-LEH and PEG-control dispersions. For liposomes conjugated with 2000 Da PEG and extruded in PB, KBs’ increased regardless of the vesicle's size with Hb encapsulation, and the stabilization mechanism shifted from thermal undulations to spontaneous curvature for liposomes extruded through 400 and 100 nm pore diameter membranes. The opposite trend occurred with PEG 2000-liposomes extruded in PBS, KBs’ decreased with Hb encapsulation, but spontaneous curvature was still maintained as the preferred stabilization mechanism. For liposomes conjugated with 550 Da PEG and extruded in PB, KBs’ also increased with Hb encapsulation and shifted the stabilization mechanism from thermal undulations to spontaneous curvature. KBs’ slightly increased with Hb encapsulation for PEG 550-liposomes extruded in PBS, but the stabilization mechanisms of these dispersions were predominantly thermal undulations. In conclusion, KBs’ generally increased with Hb encapsulation. It was previously observed that lipid membranes and Hb molecules could initiate complex formation, followed by intercalation of Hb into the bilayers [Citation[58]]. Farmer et al. [Citation[8]] previously observed that this interaction manifested itself as an increase in the bilayers’ mechanical strength.
Using a similar method as ours to measure the stability mechanism, Kang et al. [Citation[30]] reported that when DMPE-PEG ( PEGMw = 5000Da) was mixed with spontaneously formed vesicle dispersions composed of cetyltrimethylammonium bromide/sodium perfluorooctanoate (FC7) and CTAB/sodium perfluorohexanoate (FC5) mixtures, the DMPE-PEG did not insert into the vesicle bilayers, leading to macro-phase separation. Incorporation of lower molecular weight PEG (2000 Da) into CTAB/FC7 did not induce this phenomenon, but shifted the stabilization mechanism from spontaneous curvature to thermal undulations (KB decreased from 6 kT to 0.3 kT with incorporation of PEG). As for the CTAB/FC5 system, the vesicles were already stabilized by thermal undulations, however, addition of PEG favored the formation of a narrower size distribution. Although the values of KB measured did not match our values, the trend exhibited by these vesicle dispersions agreed with our observations. Taken together, these results show that the influence of PEG on the stability of vesicle dispersions depends on the molecular weight of PEG and the composition of the vesicle bilayers.
The effective bending constants of liposomes was shown to vary as DMPE-PEG of different degrees of polymerization were inserted into the bilayers. KBs’ for DMPE-PEG molecules of polymerization degree 114, 45, 17 and 8 were about ∼ 113 kT, 30 kT, 25 kT and 25 kT, respectively, for 0.1 mole fraction of PEG-lipids incorporated into the bilayers [Citation[57]]. DMPE-PEG used in our study has a polymerization degree of 45 for 2000 Da PEG and 12 for 550 Da PEG. KBs’ for PB- and PBS-PL2000, and PB- and PBS-C2000 dispersions extruded through 200 nm pore diameter membranes were close to values obtained by Marsh [Citation[57]], while the values for liposomes grafted with 550 Da PEG were lower than those obtained by Marsh [Citation[57]]. These differences could be attributed to the different extrusion buffers and liposome sizes employed. Nevertheless, the trend that KB decreased with decreasing degree of PEG polymerization was similar to what we observed. In addition, it was observed that short polymers’ (degree of polymerization ≤ 8) contribution to effective bending constants never exceeded the KB for non-modified liposomes [Citation[59]]. For long polymers (2000 Da or 5000 Da PEG), the polymer contribution could exceed that of non-modified liposomes because of the cubic dependence of KB on the degree of polymerization [Citation[59]].
Optimized Design Criteria for LEHs
In summary, extrusion in PB imparted higher bilayer rigidity (high KB) and promoted the formation of narrower PEG-LEH size distributions. Taken together, we recommend preparing PEG-LEHs in PB to create stronger and more homogeneous artificial Hb-based blood substitutes, which correlates directly with their circulation half-life. However, the encapsulation efficiencies of PEG-LEHs extruded in PB were very low (less than 11%) and their estimated entrapped Hb concentrations (less than 33 mg/mL of buffer) were less than that of human erythrocytes (150 mg/ml of blood [Citation[4]]). This can be solved by exclusively conjugating PEG onto the outer surface of LEHs, as demonstrated by Sakai et al. [Citation[10], Citation[19], Citation[60]]. Using this preparation method, even PEG brushes longer than 2000 Da can be incorporated into the outer leaflet of the bilayers. Another method to solve this problem is to increase the total lipid concentration used in the PEG-LEH dispersions. While we employed a total lipid concentration of 20 mg/mL, Sakai et al. used a total lipid concentration of 62 mg/mL [Citation[60]] or 57 mg/mL [Citation[19], Citation[23]] to produce PEG-LEH dispersions with encapsulated Hb concentrations of 100 mg/mL. If sufficient encapsulated Hb concentrations cannot be achieved with either of these methods, PBS can be used as an extrusion buffer, however, the size distributions of PEG-LEH dispersions extruded in PBS are expected to be more polydisperse, and their bilayers are expected to be less rigid.
CONCLUSIONS
We observed that PEG-conjugated liposome dispersions extruded in PB were more monodisperse than those extruded in PBS, and higher molecular weight PEG promoted the formation of narrower size distributions. Moreover, extrusion in PB and conjugation with higher molecular weight PEG imparted higher bilayer rigidity and stabilized liposome dispersions by the spontaneous curvature mechanism. The experimentally measured size distributions and encapsulation efficiencies of PEG-LEH dispersions can be readily explained through analysis of the magnitude of the effective bending constant, which dictates the stability mechanism of the liposome dispersion. Despite previous findings that sodium chloride promotes Hb oxidation [Citation[26]], the oxygen binding properties of PBS- and PB-PEG-LEHs were not significantly different. The P50s and cooperativity coefficients of both PBS- and PB-PEG-LEHs were comparable to that of human blood, indicating that PEG-LEHs display good potential as an artificial blood substitute. Taking these results together, we recommend preparing PEG-LEHs in PB to create stronger and more homogeneous artificial Hb-based blood substitutes, which correlates directly with their circulation half-life. However, the encapsulation efficiencies of PEG-LEHs extruded in PB were very low (less than 11%). This can be solved by exclusively conjugating PEG onto the outer surface of LEHs or by increasing the total lipid concentration used in the PEG-LEH dispersions.
We acknowledge support from the National Science Foundation BES-0196432 (Arlington, VA). We would also like to thank Dennis Birdsell and Rian Galloway from the Center for Environmental Science and Technology (University of Notre Dame, South Bend, IN) for the use of the Center's facilities.
REFERENCES
- Jung, H.T., Coldren, B., Zasadzinski, J.A., Iampietro, D.J., Kaler, E.W. (2001). The origins of stability of spontaneous vesicles. Proc. Natl. Acad. Sci. USA 98: 1353–1357. [PUBMED], [INFOTRIEVE], [CSA]
- Stowell, C.P. (2002). Hemoglobin-based oxygen carriers. Curr. Opin. Hematol. 9: 537–543. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Riess, J.G. (2001). Oxygen carriers (“blood substitutes”)-raison d'etre, chemistry and some physiology. Chem. Rev. 101: 2797–2919. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Fournier, R.L. (1999). In Basic Transport Phenomena in Biomedical Engineering, Taylor & Francis: Philadelphia.
- Winslow, R.M. (1999). New transfusion strategies: Red cell substitutes. Annu. Rev. Med. 50: 337–353. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Teramura, Y., Kanazawa, H., Sakai, H., Takeoka, S., Tsuchida, E. (2003). Prolonged oxygen-carrying ability of hemoglobin vesicles by coencapsulation of catalase in vivo. Bioconjugate Chem. 14: 1171–1176. [CSA], [CROSSREF]
- Rudolph, A.S. (1994). Encapsulated hemoglobin: Current issues and future goals. Art. Cells, Blood Subs., and Immob. Biotech. 22: 347–360. [CSA]
- Farmer, M.C., Gaber, B.P. (1987). Liposome-encapsulated hemoglobin as an artificial oxygen-carrying system. Methods Enzymol. 149: 184–200. [PUBMED], [INFOTRIEVE]
- Rudolph, A.S., Klipper, R.W., Goins, B.A., Phillips, W.T. (1991). In vivo biodistribution of a radiolabeled blood substitute: 99 m Tc-labeled liposome-encapsulated hemoglobin in an anesthetized rabbit. Proc. Natl. Acad. Sci. USA 88: 10976–10980. [PUBMED], [INFOTRIEVE], [CSA]
- Sakai, H., Tomiyama, K., Sou, K., Takeoka, S., Tsuchida, E. (2000). Poly(ethylene glycol)-conjugation and deoxygenation enable long-term preservation of hemoglobin-vesicles as oxygen carriers in a liquid state. Bioconjugate Chem. 11: 425–432. [CSA], [CROSSREF]
- Szebeni, J., Alving, C.R. (1999). Complement-mediated acute effects of liposome-encapsulated hemoglobin. Art. Cells, Blood Subs., and Immob. Biotech. 27: 23–41. [CSA]
- Juliano, R.L., Stamp, D. (1975). The effect of particle size and charge on the clearance rates of liposomes and liposome encapsulated drugs. Biochem. Biophys. Res. Commun. 63: 651–658. [CROSSREF]
- Goins, B., Ligler, F.S., Rudolph, A.S. (1994). Inclusion of ganglioside GM1 into liposome encapsulated hemoglobin does not extend circulation persistence at clinically relevant doses. Art. Cells, Blood Subs., and Immob. Biotech. 22: 9–25.
- Bhadra, D., Bhadra, S., Jain, P., Jain, N.K. (2002). Pegnology: A review of PEG-ylated systems. Pharmazie. 57: 5–29. [PUBMED], [INFOTRIEVE]
- Torchilin, V.P., Papisov, M.I. (1994). Why do polyethylene glycol-coated liposomes circulate so long? Liposome Res. 4: 725–739.
- Awasthi, V.D., Garcia, D., Goins, B.A., Phillips, W.T. (2003). Circulation and biodistribution profiles of long-circulating PEG-liposomes of various sizes in rabbits. Int. J. Pharm. 253: 121–132. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Zheng, S., Zheng, Y., Beissinger, R.L., Martin, F.J. (1994). Efficacy, physical properties and pharmacokinetics of sterically-stabilized liposomes-encapsulated hemoglobin. Art. Cells, Blood Subs., and Immob. Biotech. 22: 487–501.
- Phillips, W.T., Klipper, R.W., Awasthi, V.D., Rudolph, A.S., Cliff, R., Kwasiborski, V., Goins, B.A. (1998). Polyethylene glycol-modified liposome-encapsulated hemoglobin: a long circulating red cell substitute. J. Pharmacol. Exp. Ther. 288: 665–670. [CSA]
- Sakai, H., Takeoka, S., Park, S.I., Kose, T., Nishide, H., Izumi, Y., Yoshizu, A., Kobayashi, K., Tsuchida, E. (1997). Surface modification of hemoglobin vesicles with poly(ethylene glycol) and effects on aggregation, viscosity and blood flow during 90% exchange transfusion in anesthesized rats. Bioconjugate Chem. 8: 23–30. [CSA], [CROSSREF]
- Wakamoto, S., Fujihara, M., Abe, H., Sakai, H., Takeoka, S., Tsuchida, E., Ikeda, H., Ikebuchi, K. (2001). Effects of poly(ethylenelglycol)-modified hemoglobin vesicles on agonist-induced platelet aggregation and RANTES release in vitro. Art. Cells, Blood Subs., and Immob. Biotech. 29: 191–201. [CSA], [CROSSREF]
- Sherwood, R.L., McCormick, D., Zheng, S., Beissinger, R.L. (1995). Influence of steric sabilization of liposome-encapsulated hemoglobin on listeria monocytogenes host defense. Art. Cells, Blood Subs., and Immob. Biotech. 23: 665–679. [CSA]
- Nakai, K., Usuba, A., Ohta, T., Kuwabara, M., Nakazato, Y., Motoki, R., Takahashi, T.A. (1998). Coronary vascular bed perfusion with a polyethylene glycol-modified hemoglobin-encapsulated liposome, neo red cell, in rats. Art. Organs. 22: 320–325. [CSA], [CROSSREF]
- Sakai, H., Tsai, A.G., H.K. Park, S.I., Takeoka, S., Nishide, H., Tsuchida, E., Intaglietta, M. (1997). Subcutaneous microvascular responses to hemodilution with a red cell substitute consisting of polyethyleneglycol-modified vesicles encapsulating hemoglobin. J. Biomed. Mater. Res. 40: 66–78. [CSA], [CROSSREF]
- Takahashi, A. (1995). Characterization of neo red-cells (NRCs), their function and safety in-vivo tests. Art. Cells, Blood Subs., and Immob. Biotech. 23: 347–354. [CSA]
- Utkhede, D.R., Tilcock, C.P. (1998). Studies upon the toxicity of polyethylene glycol coated lipid vesicles: acute hemodynamic effects, pyrogenicity and complement activation. J. Liposome Res. 8: 537–550. [CSA]
- Arifin, D.R., Palmer, A.F. (2003). Determination of size distribution and encapsulation efficiencies of liposomes encapsulated hemoglobin by asymmetric flow field-flow fractionation and multi-angle light scattering. Biotech. Prog. 19: 1798–1811. [CSA], [CROSSREF]
- Wright, R.O., Magnani, B., Shannon, M.W., Woolf, A.D. (1996). N-acetylcysteine reduces methemoglobin in vitro. Ann. of Emergency Med. 28: 499–503. [CSA]
- Wallace, W.J., Maxwell, J.C., Caughey, W.S. (1974). A role for chloride in the autoxidation of hemoglobin under conditions similar to those in erythrocytes. FEBS Lett. 43: 33–36. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Jung, H.T., Lee, S.Y., Kaler, E.W., Coldren, B., Zasadzinski, J.A. (2002). Gausssian curvature and the equilibrium among bilayer cylinders, spheres, and discs. Proc. Natl. Acad. Sci. USA 99: 15318–15322. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Kang, S.Y., Seong, B.S., Han, Y.S., Jung, H.T. (2002). Self-organization of amphiphilic polymer in vesicle bilayers composed of surfactant mixtures. Biomacromolecules 4: 360–365. [CSA], [CROSSREF]
- Rawicz, W., Olbrich, K.C., McIntosh, T., Needham, D., Evans, E. (2000). Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 79: 328–339. [PUBMED], [INFOTRIEVE], [CSA]
- Arifin, D.R., Palmer, A.F. (2005). Stability of liposome encapsulated hemoglobin dispersions. Art. Cells, Blood Subs., and Biotech. 33((2)): 113–136. [CSA]
- Eike, J.H., Palmer, A.F. (2005). The effect of NaBH4 concentration and reaction time on the physical properties of glutaraldehyde polymerized hemoglobin. Biotech. Prog. 20: 946–952. [CSA], [CROSSREF]
- Eike, J.H., Palmer, A.F. (2004). Effect of glutaraldehyde concentration on physical properties of polymerized hemoglobin. Biotech. Prog. 20: 1225–1232. [CROSSREF]
- Eike, J.H., Palmer, A.F. (2004). Effect of Cl− and H+ on the oxygen binding properties of gluteraldehyde-polymerized bovine hemoglobin-based blood substitutes. Biotech. Prog. 20: 1543–1549.
- Eike, J.H., Palmer, A.F. (2004). Oxidized mono-, di-, tri-, and polysaccharides as potential hemoglobin cross-linking reagents for the synthesis of high oxygen affinity artificial blood substitutes. Biotech. Prog. 20: 953–962. [CSA], [CROSSREF]
- Sakai, H., Masada, Y., Takeoka, S., Tsuchida, E. (2002). Characteristics of bovine hemoglobin as a potential source of hemoglobin-vesicles for an artificial oxygen carrier. J. Biochem. 131: 611–617. [PUBMED], [INFOTRIEVE]
- Bruckdorfer, K.R., Demel, R.A., DeGier, J., Van Deenen, L.L.M. (1969). The effect of partial replacements of membrane cholesterol by other steroids on the osmotic fragility and glycerol permeability of erythrocytes. Biochem. Biophys. Acta. 183: 334–345. [PUBMED], [INFOTRIEVE]
- Papahadjopoulos, D., Poste, G., Schaeffer, B.E., Vail, W.J. (1974). Membrane fusion and molecular segregation in phospholipid vesicles. Biochem. Biophys. Acta. 352: 10–28. [PUBMED], [INFOTRIEVE]
- Papahadjopoulos, D., Kimelberg, H.K. (1973). Phospholipid vesicles (liposomes) as models for biological membranes: their properties and interactions with cholesterol and proteins. Prog. Surf. Sci. 4: 141–144. [CROSSREF]
- Korgel, B.A., VanZanten, J.H., Monbouquette, H.G. (1998). Vesicle size distributions measured by flow field-flow fractionation coupled with multiangle light scattering. Biophys. J. 74: 3264–3272. [PUBMED], [INFOTRIEVE], [CSA]
- Li, S.L., Palmer, A.F. (2004). Structure of small actin-containing liposomes probed by atomic force microscopy: Effect of actin concentration and liposome size. Langmuir. 20: 7917–7925. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Li, S.L., Palmer, A.F. (2004). Effect of actin concentration on the structure of actin-containing liposomes. Langmuir. 20: 4629–4639. [CROSSREF]
- Li, S.L., Nickels, J., Palmer, A.F. (2005). Liposome-encapsulated actin-hemoglobin (LEAcHb) artificial blood substitutes. Biomaterials. 26: 3759–3769. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Patton, J.N., Palmer, A.F. (2005). Photopolymerization of bovine hemoglobin entrapped nanoscale hydrogel particles within liposomal reactors for use as an artificial blood substitute. Biomacromolecules. 6: 414–424. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Nickels, J., Palmer, A.F. (2003). Changes in liposomes morphology induced by actin polymerization in submicrometer liposomes. Langmuir. 19: 10581–10587. [CROSSREF]
- Palmer, A.F., Wingert, P., Nickels, J. (2003). Atomic force microscopy and light scattering of small unilamellar actin-containing liposomes. Biophys. J. 85: 1233–1247. [PUBMED], [INFOTRIEVE], [CSA]
- Voet, D., Voet, J.G. (1995). In Biochemistry, John Wiley & Sons, Inc.: New York, 241–242.
- Denkov, N.D., Yoshimura, H., Kouyama, T., Walz, J., Nagayama, K. (1998). Electron cryomicroscopy of bacteriorhodopsin vesicles: mechanism of vesicle formation. Biophys. J. 74: 1409–1420. [PUBMED], [INFOTRIEVE], [CSA]
- Israelachvili, J. (1995). In Intermolecular & Surface Forces, Academic Press Inc.: San Diego.
- Helfrich, W. (1973). Elastic properties of lipid bilayers: theory and possible experiments. Z. Naturforsch. 28: 693–703.
- Awasthi, V.D. (2003). ( personal communication).
- Winslow, R.M. (2003). Current status of blood substitute research: Towards a new paradigm. J. Intern. Med. 253: 508–517. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Lasic, D.D., Joannic, R., Keller, B.C., Frederik, P.M., Auvray, L. (2001). Spontaneous vesiculation. Adv. Colloid Interface Sci. 89–90: 337–349. [CROSSREF]
- Gradzielski, M. (2003). Vesicles and vesicle gels-structure and dynamics of formation. J. Phys.: Condens. Matter 15: R655–R697.
- Lasic, D.D. (1998). Novel applications of liposomes. Reviews 16: 307–321.
- Marsh, D. (2001). Elastic constants of polymer-grafted lipid membranes. Biophys. J. 81: 2154–2162. [PUBMED], [INFOTRIEVE], [CSA]
- Szebeni, J., Hauser, H., Eskelson, C.D., Watson, R.R., Winterhalter, K.H. (1988). Interaction of hemoglobin derivatives with liposomes. Membrane cholesterol protects against the changes of hemoglobin. Biochemistry 27: 6425–6434. [PUBMED], [INFOTRIEVE], [CROSSREF]
- Marsh, D., Bartucci, R., Sportelli, L. (2003). Lipid membranes with grafted polymers: physicochemical aspects. Biochim. Biophys. Acta. 1615: 33–59. [PUBMED], [INFOTRIEVE]
- Sakai, H., Hamada, K., Takeoka, S., Nishide, H., Tsuchida, E. (1996). Physical propeties of hemoglobin vesicles as red cell substitutes. Biotechnol. Prog. 12: 119–125. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]