Abstract
Background: Treatment of hyperhomocysteinemia in patients with end‐stage renal disease (ESRD) can be performed with the oral application of vitamins. However, this therapy rarely normalizes total homocysteine (tHcy) levels. Frequently, a rebound is observed after the end of treatment. Currently, no data are available about intravenous combination therapy with folic acid, pyridoxine (B6), and cyanocobalamin (B12). Methods: We conducted a prospective pilot study comprising 13 patients on chronic hemodialysis treatment (63.7 ± 4.9 years; 6 female, 7 male) for 27 weeks. The patients received 10 mg folic acid and 100 mg pyridoxine intravenously (IV) after each dialysis plus 1000 µg vitamin B12 IV once a week for 9 weeks. Between weeks 10 and 18 the patients received 10 mg folic acid, 100 mg vitamin B6 once a week, and 1000 µg vitamin B12 bimonthly IV. Results: The therapy regimen decreased tHcy concentration (baseline: 30.5 ± 2.2 µmol/L) significantly to 17.4 ± 1.2 µmol/L, 15.6 ± 1.0 µmol/L, and 16.4 ± 0.1 µmol/L after 3, 6, and 9 weeks, respectively (p < 0.01 vs. baseline concentration). The maximum reduction (− 47.5 ± 3.3%) of tHcy concentration was measured after 6 weeks of therapy. During the following maintenance therapy, tHcy‐levels did not increase and no rebound of tHcy was detected during follow‐up (week 27:16.5 ± 1.97 µmol/L). Conclusion: The concept of a short, high‐dose induction therapy with intravenous folic acid, pyridoxine, cyanocobalamin, and a subsequent low‐dose maintenance regimen is effective in the treatment of hyperhomocysteinemia in patients with ESRD.
Introduction
Hyperhomocysteinemia is an established independent predictor of cardiovascular mortality in end‐stage renal disease (ESRD).Citation[1] The relative risk for cardiovascular events is increased by 1% per µmol/L increase in total homocysteine (tHcy) concentration in these patients.Citation[2] Despite the lack of interventional endpoint studies, a treatment of hyperhomocysteinemia is recommendedCitation[3] and usually performed as oral supplementation of vitamins (folic acid, pyridoxine, vitamin B12), which are critically involved in the homocysteine metabolism.Citation[4] Homocysteine can either be remethylated to methionine in a folate and vitamin B12–dependent reaction or be transsulfurated to cysteine. The enzymes of the transulfation require vitamin B6 as a cofactor.Citation[5] An alteration of the remethylation pathway has been verified in hemodialysis patients as one cause of hyperhomocysteinemia.Citation[6]
In patients on maintenance dialysis, various oral supplementation regimens failed to normalize homocysteine levels and the best treatment strategy for these patients is still under debate.Citation[3], Citation[7], Citation[8], Citation[9] Usually, a rebound is observed shortly after cessation of treatment.Citation[10], Citation[11], Citation[12] Recently, encouraging data on intravenous treatment of hyperhomocysteinemia with both folinic acid and pyridoxineCitation[13] or folic acid and methylcobalamin were published.Citation[14] Touam et al. showed that intravenous administration of folinic acid and pyridoxine reduced tHcy levels by nearly 70% after an average treatment time of 11 months.Citation[15] Since to date no study has investigated the effect of an intravenous combination therapy consisting of folic acid, vitamin B6, and cyanocobalamin, we conducted a pilot trial to evaluate the efficacy of an intravenous triple combination therapy on tHcy levels in patients with ESRD.
Subjects and Methods
Patients
Thirteen patients with end‐stage renal disease (63.7 ± 4.9 years; 6 female, 7 male) were included in the study. These patients underwent hemodialysis for 42.8 ± 44.6 months on average and received high‐flux dialysis for a mean duration of 13.3 ± 1.6 hours per week with constant blood flow (260 ± 74 mL/min). The primary renal diseases were chronic glomerulonephritis (n = 5), diabetic nephropathy (n = 2), rapidly progressive glomerulonephritis (n = 2), analgesic nephropathy (n = 1), and unknown (n = 3). Informed consent was obtained from all patients and the study conformed to the ethical guidelines of the 1983 Declaration of Helsinki. During the study, five patients were lost for follow‐up. Eight patients completed the 27‐week trial.
Throughout the dialysis treatment all patients received daily oral vitamin supplementation of water‐soluble vitamins, including 0.16 mg folic acid and 10 mg pyridoxine, continued through the study.
Study Protocol
The study protocol comprised the following dose schedule:
During period 1 (weeks 1–9), patients received 10 mg folic acid and 100 mg pyridoxine intravenously (IV) after each dialysis and 1000 µg cyanocobalamin once a week IV as induction therapy. For the second period (weeks 10–18), the dosing interval was extended to a weekly schedule for folic acid and pyridoxine, whereas cyanocobalamin was given bimonthly. The follow‐up without treatment (third period) was performed for a duration of nine weeks (weeks 19–27).
Analytical Procedures
Blood samples were drawn prior to the hemodialysis session. tHcy, folic acid, and vitamin B12 levels were measured every three weeks using a commercially available immunoassay (Abbott, Wiesbaden, Germany) (normal range 5–15 µmol/L).
Statistics
Data are expressed as mean ± standard error of the mean. Statistical analysis (Pearson correlation coefficient, t‐test) was performed using MedCalc® version 4.20.021 for Windows 95 (Medcalc® Software, Mariakerke, Belgium). p values less than 0.05 were considered significant.
Results
In all patients, mean baseline concentrations of cyanocobalamin (382.3 ± 38.5 pg/mL) and folic acid (9.6 ± 1.5 ng/mL) were within the normal range (cyanocobalamin: 180–920 pg/mL, folic acid: 2,7–17 ng/mL).
During therapy, baseline tHcy levels (mean ± SEM) decreased significantly from 30.5 ± 2.2 µmol/L to 17.4 ± 1.2 µmol/L (95%CI: 8.58–17.53) at 3 weeks, to 15.6 ± 1.0 µmol/L (95%CI: 10.8–19.02) at 6 weeks and to 16.4 ± 0.9 µmol/L (95% CI: 10.69–20.56) at 9 weeks (p < 0.001 for each vs. baseline concentration; t‐test; ). This resembled a mean reduction rate of tHcy of 40.7 ± 4.2%, 47.5 ± 3.3%, and 47.2 ± 3.3%, after 3, 6, and 9 weeks, respectively. A reduction of more than 40% was achieved in nearly half of patients after 3 weeks (46%) and about 70% of patients after 6 and 9 weeks, respectively. During the following maintenance therapy, tHcy‐levels did not increase (data not shown).
Figure 1. Homocysteine levels during period 1. Data are given as box plots. The box indicates median and 25th to 75th percentile, the whiskers minimum and maximum. Significant differences versus baseline are indicated by asterisks (*).
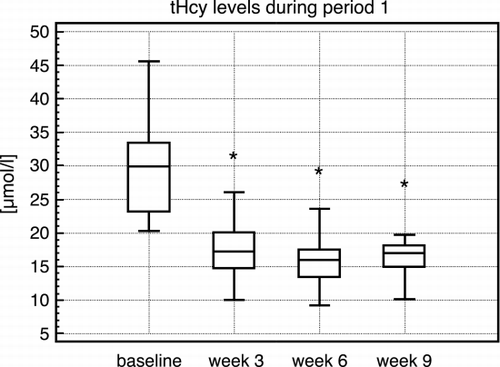
During a 9‐week follow‐up period without treatment, the tHcy levels remained stable without any rebound (16.5 ± 1.97 µmol/L, 95% CI: − 4.95–4.38).
Discussion
In our patients with ESRD undergoing regular hemodialysis, we found a mean decrease of 47% in tHcy levels after a short‐term intravenous treatment with folic acid, pyridoxine, and cyanocobalamin. During the following maintenance period and a further follow‐up of 9 weeks without therapy, no rebound of tHcy levels was detected.
Homocysteine enhances lipoprotein oxidation and influences endothelial activation of factor V and protein C.Citation[16] Although experimental studies suggest that homocysteine may increase smooth muscle cell proliferation and induce endothelial dysfunction, the underlying mechanism is not well understood. Nonetheless, hyperhomocysteinemia, which is highly prevalent in patients with ESRD, appears to promote arteriosclerosis.Citation[2] Therefore, it is believed that hyperhomocysteinemia contributes to the high incidence of cardiovascular morbidity in ESRD.Citation[17]
Contrary to the normal population, an oral application of vitamins reduced tHcy levels only by 20–40% in patients with ESRD.Citation[3], Citation[18] Due to altered folate metabolism and vitamin losses during dialysis sessions, supraphysiologic doses of folic acid are required to promote homocysteine metabolism in ESRD.Citation[8]
Recently, a more effective reduction of homocysteine concentration could be observed as a result of intravenous folic/folinic acid application. The currently available data on trials testing an intravenous or a combination of oral and intravenous vitamin supplementation to normalize tHcy levels in patients with ESRD are shown in .
Table 1. Demographic Characteristics of the Cohort
In this context, the impact of pyridoxine and vitamin B12 remained uncertain. While the oral addition of vitamin B12 to folic acid showed no benefit,Citation[19] the groups of Kaplan and Trimarchi found a slight beneficial effect for additional intravenous vitamin B12.Citation[12], Citation[20] By contrast, the major effect in the study of Koyoma et al. was achieved by the addition of intravenous methylcobalamin to folic acid.Citation[14]
Therapy regimen that contained intravenous pyridoxine could normalize tHcy levels in a high percentage of patients on continuous intermittent hemodialysis therapy.Citation[13], Citation[15] However, supplementation of oral pyridoxine showed only minimal improvement of homocysteine levels.Citation[14] It is conceivable that the deficiency of pyridoxal 5′‐phosphate, which occurs as a result of uremia, were restored by pyridoxine.Citation[21] One explanation for these conflicting results may be an insufficient or altered intestinal absorption of vitamins in uremic patients when applied orally.Citation[22] By contrast, our results show that intravenous combination therapy of folic acid, pyridoxine, and cyanocobalamin results in a substantial reduction of homocysteine serum levels. Furthermore, it prevented a rebound after cessation of treatment. Despite a mean tHcy reduction of 40–47%, only 5 of 13 patients (38.5%) reached a normal tHcy level in our pilot study. This is in contrast to previously published results: several investigators reported a normalization in up to 75% of patients.Citation[14], Citation[15] Since different dosage and application intervals may contribute to these discrepancies (), our results may be confounded by oral vitamin supplementation including folic acid and pyridoxine prior to our study. Furthermore, a bias due to the small cohort size cannot be excluded. This applies also for the role of the homozygous MTHFR 677 genotype (TT), which was found to predict excellently tHcy reduction,Citation[11] since in our study no homozygous MTHFR 677 TT genotype was detected. Thus, we noted no differences in treatment responses between patients with homozygous wild type (CC) and heterozygotes (CT allele) (data not shown).
Table 2. Survey of the Effects of Intravenous Treatment of Hyperhomocysteinemia in ESRD
Interestingly, the strong reduction of homocysteine was reached after only 3 weeks of treatment, demonstrating a rapid response to the intravenous vitamin supplementation. Therefore, it seems reasonable to perform an intravenous sequence treatment of hyperhomocysteinemia instead of an oral daily vitamin substitution. Furthermore, using this intravenous therapy approach, difficulties with patient compliance could be circumvented.
In conclusion, the concept of a short, high‐dose intravenous induction therapy with folic acid, vitamin B6, and vitamin B12 followed by a subsequent low‐dose regimen seems to be an effective treatment of hyperhomocysteinemia and may prevent a rebound of tHcy after the end of therapy in patients on chronic hemodialysis.
| Abbreviations | ||
| tHcy: | = | Homocysteine |
| ESRD: | = | End stage renal disease |
References
- Mallamaci F., Zoccali C., Tripepi G., Fermo I., Benedetto F. A., Cataliotti A., Bellanuova I., Malatino L. S., Soldarini A. Hyperhomocysteinemia predicts cardiovascular outcomes in hemodialysis patients. Kidney Int. 2002; 61(2)609–614, [PUBMED], [INFOTRIEVE]
- Moustapha A., Naso A., Nahlawi M., Gupta A., Arheart K. L., Jacobsen D. W., Robinson K., Dennis V. W. Prospective study of hyperhomocysteinemia as an adverse cardiovascular risk factor in end‐stage renal disease. Circulation 1998; 97(2)138–141, [PUBMED], [INFOTRIEVE]
- Woodside J. V., Yarnell J. W., McMaster D., Young I. S., Harmon D. L., McCrum E. E., Patterson C. C., Gey K. F., Whitehead A. S., Evans A. Effect of B‐group vitamins and antioxidant vitamins on hyperhomocysteinemia: a double‐blind, randomized, factorial‐design, controlled trial. Am. J. Clin. Nutr. 1998; 67(5)858–866, [PUBMED], [INFOTRIEVE]
- Bostom A. G., Culleton B. F. Hyperhomocysteinemia in chronic renal disease. J. Am. Soc. Nephrol. 1999; 10(4)891–900, [PUBMED], [INFOTRIEVE]
- Look M. P., Riezler R., Reichel C., Brensing K. A., Rockstroh J. K., Stabler S. P., Spengler U., Berthold H. K., Sauerbruch T. Is the increase in serum cystathionine levels in patients with liver cirrhosis a consequence of impaired homocysteine transsulfuration at the level of gamma‐cystathionase?. Scand. J. Gastroenterol. 2000; 35(8)866–872, [PUBMED], [INFOTRIEVE], [CROSSREF]
- van Guldener C., Kulik W., Berger R., Dijkstra D. A., Jakobs C., Reijngoud D. J., Donker A. J., Stehouwer C. D., De Meer K. Homocysteine and methionine metabolism in ESRD: a stable isotope study. Kidney Int. 1999; 56(3)1064–1071, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Dierkes J., Domrose U., Ambrosch A., Bosselmann H. P., Neumann K. H., Luley C. Response of hyperhomocysteinemia to folic acid supplementation in patients with end‐stage renal disease. Clin. Nephrol. 1999; 51(2)108–115, [PUBMED], [INFOTRIEVE]
- Bostom A. G., Shemin D., Lapane K. L., Hume A. L., Yoburn D., Nadeau M. R., Bendich A., Selhub J., Rosenberg I. H. High dose‐B‐vitamin treatment of hyperhomocysteinemia in dialysis patients. Kidney Int. 1996; 49(1)147–152, [PUBMED], [INFOTRIEVE]
- Thambyrajah J., Landray M. J., McGlynn F. J., Jones H. J., Wheeler D. C., Townend J. N. Does folic acid decrease plasma homocysteine and improve endothelial function in patients with predialysis renal failure?. Circulation 2000; 102(8)871–875, [PUBMED], [INFOTRIEVE]
- Sombolos K., Fragia T., Natse T., Bartholomatos G., Karagianni A., Katsaris G., Christidou F., Bamichas G., Stangou M., Papagalanis N. The effect of long‐term intravenous high dose B‐complex vitamins with or without folic acid on serum homocysteine in hemodialysis patients. J. Nephrol. 2002; 15(6)671–675, [PUBMED], [INFOTRIEVE]
- Sunder‐Plassmann G., Fodinger M., Buchmayer H., Papagiannopoulos M., Wojcik J., Kletzmayr J., Enzenberger B., Janata O., Winkelmayer W. C., Paul G., Auinger M., Barnas U., Horl W. H. Effect of high dose folic acid therapy on hyperhomocysteinemia in hemodialysis patients: results of the Vienna multicenter study. J. Am. Soc. Nephrol. 2000; 11(6)1106–1116
- Kaplan L. N., Mamer O. A., Hoffer L. Parenteral vitamin B12 reduces hyperhomocysteinemia in end‐stage renal disease. J. Clin. Invest. Med. 2001; 24(1)5–11
- Ducloux D., Aboubakr A., Motte G., Toubin G., Fournier V., Chalopin J. M., Drueke T., Massy Z. A. Hyperhomocysteinemia therapy in hemodialysis patients: folinic versus folic acid in combination with vitamin B6 and B12. Nephrol. Dial. Transplant. 2002; 17(5)865–870, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Koyama K., Usami T., Takeuchi O., Morozumi K., Kimura G. Efficacy of methylcobalamin on lowering total homocysteine plasma concentrations in hemodialysis patients receiving high‐dose folic acid supplementation. Nephrol. Dial. Transplant. 2002; 17(5)916–922, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Touam M., Zingraff J., Jungers P., Chadefaux‐Vekemans B., Drueke T., Massy Z. A. Effective correction of hyperhomocysteinemia in hemodialysis patients by intravenous folinic acid and pyridoxine therapy. Kidney Int. 1999; 56(6)2292–2296, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Lentz S. R. Homocysteine and vascular dysfunction. Life Sci. 1997; 61(13)1205–1215, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Bostom A. G., Shemin D., Verhoef P., Nadeau M. R., Jacques P. F., Selhub J., Dworkin L., Rosenberg I. H. Elevated fasting total plasma homocysteine levels and cardiovascular disease outcomes in maintenance dialysis patients. A prospective study. Arterioscler. Thromb. Vasc. Biol. 1997; 17(11)2554–2558, [PUBMED], [INFOTRIEVE]
- Arnadottir M., Brattstrom L., Simonsen O., Thysell H., Hultberg B., Andersson A., Nilsson‐Ehle P. The effect of high‐dose pyridoxine and folic acid supplementation on serum lipid and plasma homocysteine concentrations in dialysis patients. Clin. Nephrol. 1993; 40(4)236–240, [PUBMED], [INFOTRIEVE]
- Billion S., Tribout B., Cadet E., Queinnec C., Rochette J., Wheatley P., Bataille P. Hyperhomocysteinemia, folate and vitamin B12 in unsupplemented hemodialysis patients: effect of oral therapy with folic acid and vitamin B12. Nephrol. Dial. Transplant. 2002; 17(3)455–461, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Trimarchi H., Schiel A., Freixas E., Diaz M. Randomized trial of methylcobalamin and folate effects on homocysteine in hemodialysis patients. Nephron 2002; 91(1)58–63, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Lacour B., Parry C., Drueke T., Touam M., Kreis H., Bailly M., Durand D. Pyridoxal 5′‐phosphate deficiency in uremic undialyzed, hemodialyzed, and non‐uremic kidney transplant patients. Clin. Chim. Acta 1983; 127(2)205–215, [PUBMED], [INFOTRIEVE]
- Said H. M., Vaziri N. D., Kariger R. K., Hollander D. Intestinal absorption of 5‐methyltetrahydrofolate in experimental uremia. Acta Vitaminol. Enzymol. 1984; 6(4)339–346, [PUBMED], [INFOTRIEVE]
- Tremblay R., Bonnardeaux A., Geadah D., Busque L., Lebrun M., Ouimet D., Leblanc M. Hyperhomocysteinemia in hemodialysis patients: effects of 12‐month supplementation with hydrosoluble vitamins. Kidney Int. 2000; 58(2)851–858, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Buccianti G., Bamonti C. F., Patrosso C., Corghi E., Novembrino C., Baragetti I., Lando G., De Franceschi M., Maiolo A. T. Reduction of the homocysteine plasma concentration by intravenously administered folinic acid and vitamin B(12) in uraemic patients on maintenance haemodialysis. Am. J. Nephrol. 2001; 21(4)294–299, [PUBMED], [INFOTRIEVE], [CROSSREF]
- Corghi E., Patrosso C., Bamonti F., Baragetti E., Novembrino C., Lando G., De Franceschi M., Buccianti G. Intravenous folinic acid and vitamin B12 supplementation and homocysteine concentration in hemodialysis patients. G. Ital. Nefrol. 2002; 19(3)301–307, [PUBMED], [INFOTRIEVE]