Abstract
The effects of sucrose, NaCl, and arabinoxylan on the α-relaxation of wheat doughs with different water contents were investigated using Dynamic Mechanical Thermal Analysis (DMTA). DMTA measurements were made at the heating rate of 2°C/min from at least 30°C below the observed onset of the α-relaxation (glass transition) to at least 30°C above the transition. The glass transition temperature, Tg , was taken from the onset temperature of the decrease in storage modulus (G′). The frequencies used were 0.1, 1, and 5 Hz and amplitude was 16 μm. The storage modulus, G′, showed α-relaxation in all doughs with added ingredients. Added ingredients decreased the glass transition temperature of dough. The Tg of doughs with different ingredients decreased with increasing water content of doughs over the whole aw range used (0.113–0.753). Also, the Tg increased with increasing frequency.
Keywords:
INTRODUCTION
The glass transition behavior of various cereal materials has often been studied using Differential Scanning Calorimetry (DSC). Zeleznak and Hoseney Citation[1] studied the glass transition temperature behavior of wheat starch, Kalichevsky et al. Citation[2] and Kalichevsky and Blanshard Citation[3] of amylopectin, de Graaf et al. Citation[4] of gliadin, Kalichevsky et al. Citation[5], Noel et al. Citation[6], and Cherian et al. Citation[7] of wheat gluten. Baked products consist of polymer mixtures, mainly starch and gluten, showing a broad glass transition with a small change in heat capacity. Laaksonen and Roos Citation8-9 showed very clearly that DSC, as used in their studies, is not sensitive enough to observe state transitions, such as the glass transition, in frozen wheat doughs. Moreover, Wetton Citation[10] and Rotter and Ishida Citation[11] reported that DMA/DMTA is about 1000 times more sensitive in observing thermal transitions than DSC. The importance of the glass transition temperature (Tg ) in determining the stability and dynamic-mechanical properties of cereal materials has been recently investigated Citation8-9, Citation12-16.
Dynamic Mechanical Thermal Analysis (DMTA) is a technique for measuring mechanical properties of materials, including foods, as they are deformed under varying stress as a function of time, frequency, or temperature. The analysis measures three major properties of the material: 1) the storage modulus, G′, which measures the amount of energy stored in the material per cycle; 2) the loss modulus, G″, which is proportional to the amount of energy dissipated per cycle; and 3) the loss tanδ, which corresponds to the ratio of energy lost to the energy stored per cycle. The glass transition results in a structural mechanical event related to decreasing viscoelasticity. Therefore, the DMA is a very sensitive method for detecting second-order transitions resulting in large changes in viscoelastic properties occurring over the transition temperature region Citation[17].
Water is an important plasticizer of amorphous polymers. It is well established that the plasticization by water affects the glass transition temperature (Tg ) of many biopolymers, including amorphous food materials, resulting in depression of Tg Citation18-22. The glass transition related relaxations of various cereal polymers have been widely studied as a function of water content using the DMA/DMTACitation[3], Citation13-15, Citation23-24.
Sugars added to cereal foods can also have a significant plasticization effect, because of their low glass transition temperature and low molecular weight Citation[7], Citation[25]. There are also opposite results concerning the effects of sucrose on the Tg Citation[9], Citation[26]. Salts increase the Tg of polymers, including food materials, as has been observed by a number of researchers Citation[9], Citation27-29.
Water extractable arabinoxylans (WEA) are polysaccharides and form a part of pentosans fraction in cereals. The pentosan content of wheat flour amounts to 2–3% about which 30% is water extractable Citation30-32. The pentosans are particularly important in bread making because of their physical properties. It is well established that the pentosans are extremely hydrophilic. Even though the same farinograph method was used, different values for water-binding capacity of water extractable pentosans can be found in the literature, e.g. Kulp Citation[30] found it was 11 times the mass of the water for extractable pentosans. Jelaca and Hlynca Citation[33] showed that the water extractable pentosans adsorbed 9.2 times the mass of water. Kim and D'Appolonia Citation[34] obtained a value of 4.4 for the water-binding capacity of water extractable pentosans. It is well established that addition of water extractable pentosans into baking systems result in a pronounced increase in bread volume Citation35-38. Pentosans have been shown to decrease the rate of recrystallization of starch by reducing the amount of starch components available for recrystallization Citation[34], and therefore reduce staling rate of baked bread Citation[34], Citation[39]. However, no studies have been reported on the effects of pentosans on the glass transition on wheat doughs.
The objective of the present study was to investigate the effects sucrose, NaCl, and water extractable arabinoxylan (WEA) on the glass transition of wheat dough at different water contents using Dynamic Mechanical Thermal Analysis (DMTA).
MATERIALS AND METHODS
Flour
Wheat (Torfrida, harvest 1997) was obtained from AVEVE (Landen, Belgium) and milled using a Buhler MLU-202 laboratory mill (Uzwill, Switzerland), according to AACC Method 26–31. Milling yield, protein content (% of dry matter), ash content (% of dry matter) and Farinograph water absorption (14% moisture basis) were 69%, 9.2%, 0.54%, and 62.0%, respectively. A micro-Kjeldahl procedure, according to AACC Method 46–13 (N × 5.7), was used for protein assessment. Ash content was measured according to AACC Method 08–01. Farinograph water absorption was determined according to AACC Method 54–21, and was based on dough consistency at the 500 BU (Brabender Unit) line.
Sample Preparation
Table shows the composition of the various doughs. Sucrose and NaCl were dissolved in distilled water giving a proper concentration. The resulting solutions were added into a mixing bowl with the flour. The WEA was mixed with flour and water as a powder. Wheat flour doughs were mixed 3 min with the solutions to optimum consistency using the 10 g bowl Micro-Mixer (National Mfg. Co., Lincoln, Nebraska).
Table 1. Compositions of Various Wheat Doughs
Dimensions of dough samples were 9 mm width, 0.9 mm thickness, and length varied from 15 to 20 mm. Samples were humidified at room temperature (24–25°C) in desiccators without vacuum over 11.3, 22.5, 32.8, 43.2, 57.6, 65.4, and 75.3% RH, as controlled by saturated solutions of LiCl, CH3COOK, MgCl2, K2CO3, NaBr, NaNO2, and NaCl, respectively Citation40-41. Equilibrium occurred in about 3 weeks. The equilibrium water contents of the doughs were determined according to AACC Method 44–15A (at 130°C for 60 min).
Dynamic Mechanical Thermal Analysis (DMTA)
A Dynamic Mechanical Thermal Analyzer (Mark III DMTA, Polymer Laboratories, Loughborough, U.K.) was used to determine the α-relaxation of different doughs. At least triplicate samples were analyzed using DMTA at a heating rate of 2°C/min from at least 30°C below the observed onset temperature of the α-relaxation to at least 30°C above the transition temperature range. The glass transition temperature, Tg , was determined from the onset temperature of the α-relaxation, which resulted in a sharp decrease in storage modulus (G″). During dynamic heating, the samples were analyzed at frequencies of 0.1, 1, and 5 Hz and a strain setting was 0, which corresponded to an amplitude of 16 μm. The instrument measured each frequency for about 2 to 3 min and then changed to the next frequency during heating.
RESULTS AND DISCUSSION
The water content of the different dough samples as a function of water activity are given in Table . As seen, they varied from 1.73 to 10.40 g H2O/100 g dm over aw range of 0.113–0.753 for the plain dough (flour + water). Figure shows water sorption isotherms for the different doughs. The water sorption isotherm is an important tool in the characterization of relationships between water content and water activity of food materials. Water contents in the doughs with added sucrose were higher over the whole aw range used when compared to the water contents of the plain dough (Table and Figure ). The higher water-binding capacity of dough with added sucrose was probably due to more OH-groups available for same total weight of dry matter. Water contents of doughs with added NaCl were slightly higher than water contents of doughs with added sucrose (Table ). This was probably due to capability of NaCl to partially open the gluten network and therefore allowing more water to be adsorbed. Figure and Table also show that doughs with added sucrose + NaCl had the highest water contents among the doughs studied. These water contents were from 2.98 to 12.64 g H2O/1002g dm over the whole aw range used (0.113–0.753). Although it is well known that pentosans have a high water-binding capacity, Table shows that addition of 1% WEA (% of flour weight) did not increase the water content of the doughs over the aw range used. This shows that either the amount of WEA was too minor to increase the water-binding capacity of dough or that the WBC Constant Dough Farinograph method for water binding capacity does not truly measured adsorbed water at lower water activities. If it were so, each one gram of pentosan would acount for an additional 9 grams of water. As seen, the addition of 3% of WEA increased the water contents at the same level as doughs with added sucrose and NaCl (Table ).
Table 2. Water Contents (g H2O/100 g Dry Matter) for Wheat Doughs with Different Ingredients
Figure 1. Comparison of water isotherms at 24–25°C for dough (flour + water) (•), dough + sucrose (+), dough + NaCl (▵), dough + sucrose + salt (○), dough + 1% WEA (♦), and dough + 3 WEA (□).
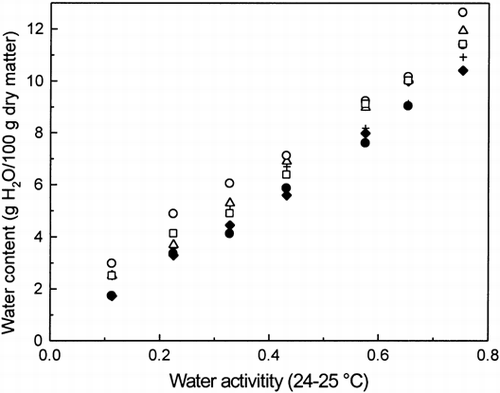
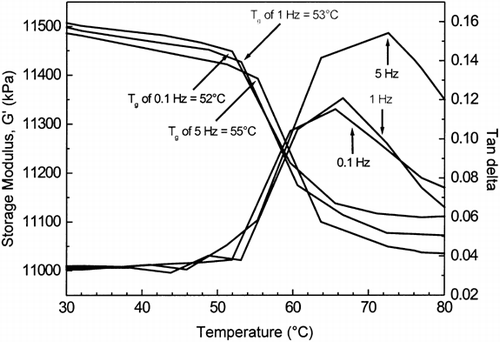
Figure shows typical changes in the dynamic-mechanical properties of the wheat dough (flour + water) stored at aw of 0.225, as a function of temperature measured by DMTA at 0.1, 1, and 5 Hz. A sharp drop in the G′ can be observed depending on frequency. This change in G′ corresponded to the Tg of the material. Moreover, at the glass transition, a material becomes softened and its ability to store energy is partially lost resulting in a decrease in G′. The Tg was also frequency dependent as seen in Figure . Dependence on frequency of the Tg is well known Citation[8], Citation42-44, but its significance to dough rheology is unclear.
Figure 2. The storage modulus (G′) and loss tan delta of the wheat dough (flour + water) stored at aw of 0.225, as a function of temperature, as measured by DMTA at 0.1, 1, and 5 Hz.
The plastization effect of water observed on the Tg is very clear as seem from the decrease in Tg (Table , Figures and ). The Tg dropped 5–10°C per 1% water (dm), which is typical for various food materials Citation[5], Citation[15], Citation[45]. The Tg values decreased with increasing water contents of the samples at all frequencies, from 57 to −10°C, from 58 to −8°C, and from 60 to −5°C at 0.1, 1, and 5 Hz, respectively. Both water contents and Tg values of the samples agreed very well with the results of Nikolaidis and Labuza Citation[15] for cracker dough measured by DMTA with a heating rate of 3°C/min at 1 Hz. Hoseney et al. Citation[46] and Noel et al. Citation[6] reported higher Tg values for gluten and gluten proteins, respectively, than observed in the present study. Also, native and gelatinized starch had higher Tg values Citation[1]. It should be noted that all these prior studies were done by DSC with a heating rate of 10°C and Tg was taken from the midpoint of the glass transition. It is well known that the faster heating rate and midpoint of the glass transition gives higher Tg values. Anyhow, it seems that the Tg of gluten dominated the glass transition of dough given the closest Tg values.
Table 3. Glass Transition Temperatures (Tg ) of Wheat Dough with Different Ingredients Measured by DMTA as Storage Modulus (G′) at 0.1, 1, and 5 Hz
Figure 3. Comparison of the glass transition temperatures (Tg ) as a function of water activity (aw) at 24–25°C for dough (flour + water) (•), dough + sucrose (+), dough + NaCl (▵), dough + sucrose + salt (○), dough + 1% WEA (♦), and dough + 3% WEA (□), as measured by DMTA at 1 Hz.
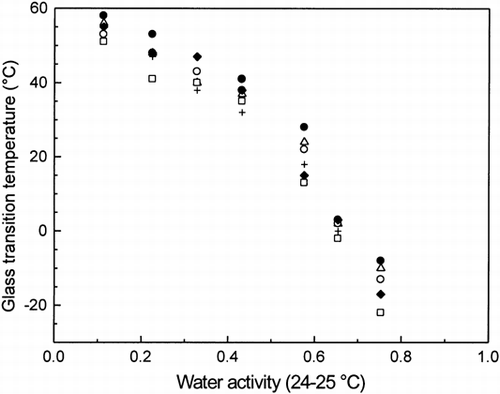
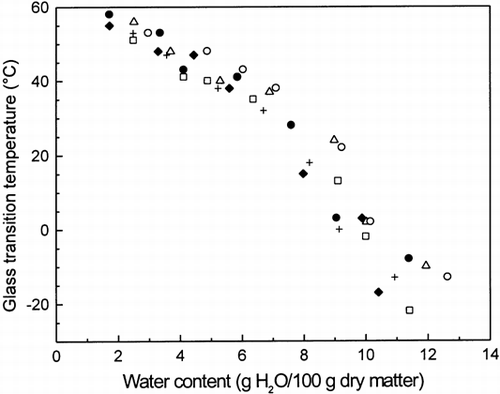
Figure 4. Comparison of the glass transition temperatures (Tg ) as a function of water content (g H2O/100 g dry matter) for dough (flour + water) (•), dough + sucrose (+), dough + NaCl (▵), dough + sucrose + salt (○), dough + 1% WEA (♦), and dough + 3% WEA (□), as measured by DMTA at 1 Hz.
Effects of Sucrose
Added sucrose decreased the Tg of the dough 5 to 10°C, over the aw range of 0.113–0.753, compared to the plain dough (flour + water) as seen in Table . Similar results have obtained by Cherian et al. Citation[7] for wheat gluten films with added sucrose, and Georget and Smith Citation[47] for wheat flakes with added fructose and sucrose. Moreover, according to Levine and Slade Citation[48] and Slade and Levine Citation[49] small molecule weight carbohydrates can plasticize food materials, resulting in a drop in Tg. The Tg increased with increasing frequency, as observed in the plain dough. The Tg values decreased with increasing water contents of the samples over aw range of 0.113–0.753 (2.48–10.93 g H2O/100 g dm, Table ) at all frequencies, from 52 to −15°C, from 53 to −13°C, and from 55 to −10°C at 0.1, 1, and 5 Hz, respectively.
Effects of NaCl
The effect of frequency on the Tg was clearly observed in doughs with added NaCl, with an increase in the Tg with increasing frequency. The Tg values were slightly higher than those in the doughs with added sucrose, however it was lower than those in the plain doughs over the whole aw range used. Thus, added NaCl had a plasticization effect on the wheat dough. This was mostly due to the higher water contents of the dough samples with added NaCl as compared to the plain dough. There are several studies, which reported that added salts increase the Tg of polymers. James et al. Citation[27] have seen increased in the Tg of poly(propylene oxide) with the addition of cobalt chloride. Cowie and Martin Citation[28] have observed that with the addition of lithium perchlorate and LiT to poly(vinyl methyl ether). Both groups have reported that the elevation in Tg levels off beyond a certain salt concentration. Moreover, Olsen and Koksbang Citation[29] showed that the Tg increased dramatically with increasing LiAsF6 concentration for propylene carbonate-and hybrid polymer electrolyte.
Effects of Sucrose-NaCl-Mixture
The Tg values of the dough with added sucrose and NaCl were intermediate, compared to doughs with added sucrose or NaCl separately. At some water activities and frequencies, the Tg was the same as in doughs with added sucrose. While the addition of sucrose had the plasticization effect on doughs decreasing the Tg , NaCl conversely increased the Tg (Table ). The Tg values of the doughs with added sucrose + NaCl decreased with increasing water contents of the samples over aw range of 0.113–0.753 at all frequencies, from 52 to −15°C, from 53 to −13°C, and from 55 to −10°C at 0.1, 1, and 5 Hz, respectively.
Effects of WEA
Addition of 1% of WEA decreased the Tg at all frequencies and over the whole aw range used. The decrease in the Tg was approximately at the same level or lower than in doughs with other added ingredients. Addition of 3% of WEA decreased the Tg giving the lowest Tg values of all doughs with or without added ingredients. Doughs with WEA had a decrease in the Tg 10–15°C compared to the plain dough. Addition of 3% of WEA caused lower Tg values for dough than addition of 1% WEA, over whole aw range used, probably because of higher water contents in doughs with 3% WEA (Tables and ).
CONCLUSION
The present study of effects of different ingredients on the Tg of wheat doughs confirmed that doughs are plasticized by water. Dynamic Mechanical Thermal Analysis (DMTA) was appropriate and sensitive enough to observe the α-relaxation (glass transition) of all doughs studied. When the water sorption isotherm and state diagram of various doughs are known, processing and environmental conditions during storage can be controlled. The lowering effect of added sucrose and NaCl on the Tg of dough was similar as reported for other polymers in a literature. Even though, addition of WEA decreased the Tg more than expected. The change in water binding capacity was small.
ACKNOWLEDGMENTS
This study has been carried out with financial support from the Finnish Food Research Foundation, the Foundation of Tiura, the Commission of the European Communities, Agriculture and Fisheries (FAIR) specific RTD program, CT97–3609, Use of Wheat Water Extractable Arabinoxylans (WEA) to Improve Stability of Frozen Doughs and Quality of Bread, the University of Minnesota, Agriculture Experiment Station, Project 18–72, and the Institute of Food Technologist Marcel Loncin Research Prize. The study does not necessarily reflect the Commission's views and in no way anticipates the EC Commission's future policy in this area. The flour and flour analysis were kindly provided by Professor Jan Delcour, Katholieke Universiteit, Leuven, Belgium.
REFERENCES
- Zeleznak , K. J. and Hoseney , R. C. 1987 . The Glass Transition in Starch . Cereal Chem. , 64 ( 2 ) : 121 – 124 .
- Kalichevsky , M. T. , Jaroszkiewicz , E. M. , Ablett , S. , Blanshard , J. M.V. and Lillford , P. J. 1992 . The Glass Transition of Amylopectin Measured by DSC, DMTA and NMR . Carbohydr. Polym. , 18 : 77 – 88 .
- Kalichevsky , M. T. and Blashard , J. M.V. 1993 . The Effect of Fructose and Water on the Glass Transition of Amylopectin . Carbohydr. Polym. , 20 : 107 – 113 .
- De Graaf , E. M. , Madeka , H. , Cocero , A. M. and Kokini , J. L. 1993 . Determination of the Effect of Moisture on Gliadin Glass Transition using Mechanical Spectrometry and Differential Scanning Calorimetry . Biotechnol. Prog. , 9 : 210 – 213 .
- Kalichevsky , M. T. , Jaroszkiewicz , E. M. and Blanshard , J. M.V. 1992 . Glass Transition of Gluten. 1: Gluten and Gluten-sugar Mixtures . Int. J. Biol. Macromol. , 14 : 257 – 266 .
- Noel , T. R. , Parker , R. , Ring , S. G. and Tatham , A. S. 1995 . The Glass-transition Behaviour of Wheat Gluten Proteins . Int. J. Biol. Macromol. , 17 ( 2 ) : 81 – 85 .
- Cherian , G. , Gennadios , A. , Weller , C. and Chinachoti , P. 1995 . Thermomechanical Behavior of Wheat Gluten Films: Effect of Sucrose, Glycerin, and Sorbitol . Cereal Chem. , 72 ( 1 ) : 1 – 6 .
- Laaksonen , T. J. and Roos , Y. H. 2000 . Thermal, Dynamic-mechanical, and Dielectric Analysis of Phase and State Transitions of Frozen Wheat Doughs . J. Cereal Sci. , 3 ( 2 ) : 281 – 292 .
- Laaksonen , T. J. and Roos , Y. H. 2001 . Thermal and Dynamic-mechanical Analysis of Transitions of Frozen Wheat Doughs with Sucrose, NaCl, Ascorbic Acid, and Their Mixtures . Int. J. Food Prop. , 4 ( 2 ) : 25 – 35 .
- Wetton , R. E. 1986 . “ Dynamic Mechanical Thermal Analysis of Polymers and Related Systems ” . In Developments in Polymer Characteristics Edited by: Dawkins , J. V. Vol. 5 , 179 – 222 . New York : Elsevier Applied Science .
- Rotter , G. and Ishida , H. 1992 . Dynamic Mechanical Analysis of the Glass Transition. Curve Resolving Applied to Polymers . Macromolecules , 25 : 2170 – 2176 .
- Kalichevsky , M. T. and Blashard , J. M.V. 1992 . A Study of the Effect of Water on the Glass Transition of 1:1 Mixtures of Amylopectin, Casein and Gluten using DSC and DMTS . Carbohydr. Polym. , 19 : 271 – 278 .
- Nicholls , R. J. , Appelqvist , I. A.M. , Davies , A. P. , Ingman , S. J. and Lillford , P. J. 1995 . Glass Transition and the Fracture Behaviour of Gluten and Starches within the Glassy State . J. Cereal Sci. , 21 : 25 – 36 .
- Vodovotz , Y. and Chinachoti , P. 1996 . Thermal Transitions in Gelatinized Wheat Starch at Different Moisture Contents by Dynamic Mechanical Analysis . J. Food Sci. , 61 ( 5 ) : 932 – 941 .
- Nikolaidis , A. and Labuza , T. P. 1996 . Use of Mechanical Thermal Analysis (DMTA): Glass Transition of a Cracker and its Dough . J. Thermal Anal. , 47 : 1315 – 1328 .
- Räsänen , J. , Blanshard , J. M.V. , Mitchell , J. R. , Derbyshire , W. and Autio , K. 1998 . Properties of Frozen Wheat Doughs at Subzero Temperatures . J. Cereal Sci. , 28 : 1 – 14 .
- Ma , C. Y. , Harwalker , V. R. and Maurice , T. J. 1990 . “ Instrumentation and Techniques of Thermal Analysis in Food Research ” . In Thermal Analysis of Food Edited by: Harwalker , V. R. and Ma , C. Y. 1 – 15 . New York : Elsevier Applied Science .
- Herrington , T. M. and Branfield , A. C. 1984 . Physico-chemical Studies on Sugar Glasses. II. Glass Transition Temperature . J. Food Technol. , 19 : 427 – 435 .
- Karel , M. 1985 . “ Effects of Water Activity and Water Content on Mobility of Food Components, and their Effects on Phase Transitions in Food Systems ” . In Properties of Water in Foods, ISOPOW 3 Edited by: Simatos , D. and Multon , J. L. 153 – 169 . Netherlands : Martinus Nijhoff Publishers .
- Roos , Y. H. 1987 . Effect of Moisture on the Thermal Behavior of Strawberries Studied using Differential Scanning Calorimetry . J. Food Sci. , 52 : 146 – 149 .
- Levine , H. and Slade , L. 1988 . “ Water as a Plasticizer: Physico-chemical Aspects of Low-moisture Polymeric Systems ” . In Water Science Reviews, Water Dynamics Edited by: Franks , F. Vol. 3 , 79 – 185 . England : Cambridge University Press .
- Roos , Y. H. and Karel , M. 1990 . Differential Scanning Calorimetry Study of Phase Transitions Affecting the Quality of Dehydrated Materials . Biotechnol. Prog. , 6 : 159 – 163 .
- Hallberg , L. M. and Chinachoti , P. 1992 . Dynamic Mechanical Analysis for Glass Transitions in Long Shelf-life Bread . J. Food Sci. , 57 ( 5 ) : 1201–1204 – 1229 .
- Nikolaidis , A. and Labuza , T. P. 1996 . Glass Transition State Diagram of a Baked Cracker and its Relationship to Gluten . J. Food Sci. , 61 ( 4 ) : 803 – 806 .
- Fan , J. , Mitchell , J. R. and Blanshard , J. V.M. 1996 . The Effect of Sugars on the Extrusion of Maize Grits: II. Starch Conversion . Int. J. Food Sci. Technol. , 31 : 67 – 76 .
- Papageorgiou , M. , Kasapis , R. K. and Richardson , R. K. 1994 . Glassy State Phenomena in Gellan-sucrose-corn Syrup Mixtures . Carbohydr. Polym. , 25 : 101 – 109 .
- James , D. B. , Wetton , R. E. and Brown , D. S. 1979 . Polyether-metal Salt Complexes: 1. The Elevation of the Glass Transition Temperature of Polyethers by Metal Salts . Polymer , 20 : 187 – 195 .
- Cowie , J. M.G. and Martin , A. C.S. 1987 . Ionic Conductivities and Glass Transition Temperatures in Poly(vinyl methyl ether)-salt mixtures . Polym. Bull. , 17 : 113 – 117 .
- Olsen , I. I. and Koksbang , R. 1996 . A Temperature Study of the Ionic Conductivity of a Hybrid Polymer Electrolyte . J. Electrochem. Soc. , 143 ( 2 ) : 570 – 574 .
- Kulp , K. 1968 . Pentosans of Wheat Endosperm . Cereal Sci. Today , 13 ( 11 ) : 414–417 – 426 .
- D'Appolonia , B. L. 1971 . Role of Pentosans in Bread and Dough. A Review . Bakers Dig. , 12 : 20 – 23 .
- Hoseney , R. C. 1984 . Functional Properties of Pentosans in Baked Foods . Food Technol. , 1 : 114 – 117 .
- Jelaca , S. L. and Hlynka , I. 1971 . Water-binding Capacity of Wheat Flour Crude Pentosans and their Relation to Mixing Characteristics of Dough . Cereal Chem. , 48 : 211 – 222 .
- Kim , S. K. and D'Appolonia , B. L. 1977 . Bread Staling Studies. III. Effect of Pentosans on Dough, Bread, and Bread Staling Rate . Cereal Chem. , 54 ( 2 ) : 225 – 229 .
- Pence , J. W. , Elder , H. A. and Mecham , D. K. 1950 . Preparation of Wheat Flour Pentosans for use in Reconstituted Doughs . Cereal Chem. , 27 : 60 – 66 .
- Hoseney , R. C. , Finney , K. F. , Shogren , M. D. and Pomeranz , Y. 1969 . Functional (breadmaking) and Biochemical Properties of Wheat Flour Components. II. Role on Water Solubles . Cereal Chem. , 46 : 117 – 125 .
- Jelaca , S. L. and Hlynka , I. 1972 . Effect of Wheat-flour Pentosans in Dough, Gluten, and Bread . Cereal Chem. , 49 : 489 – 495 .
- Michniewicz , J. , Biliaderis , C. G. and Bushuk , W. 1992 . Effect of Added Pentosans on Some Properties of Wheat Bread . Food Chem. , 43 : 251 – 257 .
- Jankiewicz , M. and Michniewicz , J. 1987 . The Effect of Soluble Pentosans Isolated from Rye Grain on Staling of Bread . Food Chem. , 25 : 241 – 249 .
- Greenspan , L. 1977 . Humidity Fixed Points of Binary Saturated Aqueous Solutions . J. Res. Natl. Bur. Stand., Sect. A , 81A : 89 – 96 .
- Resnik , S. L. and Chirife , J. 1988 . Proposed Theoretical Water Activity Values at Various Temperatures for Selected Solutions to be Used as Reference Sources in the Range of Microbial Growth . J. Food Prot. , 51 : 419 – 423 .
- Sperling , L. H. 1992 . Introduction to Physical Polymer Science 594 USA : John Wiley & Sons, Inc. .
- Kalichevsky , M. T. , Blanshard , J. M.V. and Marsh , R. D.L. 1992 . “ Applications of Mechanical Spectroscopy to the Study of Glassy Biopolymers and Related Systems ” . In The Glassy State in Foods Edited by: Blanshard , J. M.V. and Lillford , P. J. 133 – 156 . Loughborough, , UK : Nottingham University Press .
- Laaksonen , T. J. and Roos , Y. H. 2001 . Dielectric Relaxations of Frozen Wheat Doughs Containing Sucrose, NaCl, Ascorbic Acid, and their Mixtures . J. Cereal Sci. , 33 : 331 – 340 .
- Levine , H. and Slade , L. 1990 . “ Influences of the Glassy and Rubbery States on the Thermal, Mechanical, and Structural Properties of Doughs and Baked Products ” . In Dough Rheology and Baked Product Texture Edited by: Faridi , H. and Faubion , J. M. 157 – 330 . New York : Van Nostrand Reinhold .
- Hoseney , R. C. , Zeleznak , K. and Lai , C. S. 1986 . Wheat Gluten: A Glassy Polymer . Cereal Chem. , 63 ( 3 ) : 285 – 286 .
- Georget , D. M.R. and Smith , A. C. 1996 . The Effects of Sugars on the Mechanical Properties of Processed Cereals . J. Thermal Anal. , 47 : 1377 – 1389 .
- Levine , H. and Slade , L. 1988 . Thermomechanical Properties of Small-carbohydrate-water Glasses and “Rubbers” . J. Chem. Soc., Faraday Trans. , 84 ( 8 ) : 2619 – 2633 .
- Slade , L. and Levine , H. 1988 . Non-equilibrium Behavior of Small Carbohydrate-water Systems . Pure Appl. Chem. , 60 ( 12 ) : 1841 – 1864 .