ABSTRACT
A mixture of starch and guar gum was extruded with citric acid, sodium stearoyl-2-lactylate (SSL) and diacetyl tartaric acid ester of monoglyceride (DATEM). Water solubility, blue value, extrudate expansion and viscosity were measured for the different additives. Incorporation of hydrocolloids caused a synergistic increase in viscosity indicating a strong interaction between starch amylose and gums. Extrusion with chemical agent affected the physical properties of starch. Citric acid decreased viscosity and increased water solubility and expansion volume. Sodium stearoyl-2-lactylate and diacetyl tartaric acid ester of monoglyceride increased viscosity while reducing expansion and water solubility. This indicated that even at extrusion temperatures, these surfactants maintained their complexing power. Analysis of variance showed that addition of chemical agents significantly affected the physical and rheological properties of the extrudates.
INTRODUCTION
Starch represents the major source of available carbohydrates in human diets. It is made up of polymers of glucose, which consists essentially of two components- amylose, a linear straight chain,α-1,4 glucopyran ose polymer and amylopectin, a random branched configuration of α-1,4 glucopyranose with periodic branching of side chains with 1,6 linkages.[Citation[1]] Starches are known to participate in synergistic interactions with hydrocolloid.Citation2-3 These interactions have been shown to take place between amylose of solubilized starch and the hydrophilic portion of the hydrocolloid.Citation4-5
Galactomannans, a kind of hydrocolloid, are fairly hydrophilic polysacc harides with a polymanose backbone and grafted galactose unit with a fairly rigid structure.Citation[6] Guar gum is a kind of galactomannan obtained from seeds of Cyamopsis tetragonolobus. It is a water-soluble polysaccharide, which essentially consists of 1-4 linked β-D-mannopyran osyl backbone partially substituted at 0–6 sides with D-galactopyranosy l chains.Citation7-8
In many instances, starch behaves differently from galactomannan. Their properties are complementary and this is used to achieve special rheological properties. Starch/hydrocolloid mixtures are widely employed in the food industries to control water mobility and maintain overall product stabilityCitation[9] and modify texture.Citation10-13
Launey et al.Citation[14] and DoublierCitation[15] studied comprehensively the factors affecting rheological behavior of starch. Similarly, Henna et al.Citation[16] noted the effect of certain chemical agents on physical and rheological behavior of extruded starch. Lipids have been known to affect gelatinization, texture and retrogradation behavior of starch-thickened foodsCitation17-19 by formation of complex with amylose.Citation20-21 Physical modification has been shown to alter the structural arrangement of starch chains.Citation[22]
Work had been done on starch-gum interaction at a high concentration.Citation[10] Similarly, Sudhakar et al.Citation[7] investigated the functional and rheo logical properties of starch-gum combination and more recently, Rojas et al.Citation[23] studied the pasting of wheat flour in hydrocolloid solutions at concentration usually applied in food. Information on the effect of citric acid and emulsifier/surfacta nt on a mixed starch-hydrocolloid system in an extruder is limited. The objective of this study was to determine any changes in physical and rheologi cal properties of extruded starch-guar mixtures before and after addition of chemical agents.
MATERIALS AND METHODS
Materials
A commercial cornstarch (13.5% moisture, 23% amylose) was obtained from Wuxi starch factory, China. The hydrocolloids tested included guar gum, which was a kind donation from Dr. Shui Xue Ming and gum arabic and locust bean gum were obtained from Shanghai food gum company Ltd. Food grade emulsifiers Sodium stearoyl lactylate (GRISTEDTM SSL P70) and diacetyl tartaric acid ester of monoglyceride (PANODANTM DATEM 517) were obtained gratis from Danisco Cultor (China) Ltd, food grade Citric acid was purchased from Roche (China) Ltd. Amyloglucosidase AMG 300L from Aspergillus niger, Alcalase 2.4L (food grade) from Bacillus licheniformis and Termamyl 120L (Takalite) from Bacillu s lincheniformis were a gift from Novo Nordisk Shanghai Company Ltd, China. All other chemicals were of analytical grade.
Methods
Experimental Plan
The study was divided into two parts. The first experiment was conducte d using an L9(34) factorial experimental design in which, the combined effect of gum type (Arabic, Guar, Locust Bean), moisture content (18, 24, 30%), temperature (140, 155, 170°C) and gum concentration (0, 5, 10%) were investigated. The second experiment investigated the effect of citric acid and emulsifiers at 2% (w/w) on physical and functional properties of extruded starch. Statistical analyses of results were conducted using the GLM procedure.Citation[24]
Extrusion
Starch-gum mixtures were prepared by mixing gum and starch in a Hobart blender for two minutes. The moisture content was adjusted to the desired level and the chemical agents were added at concentration of 2% (w/w) and the mixture was mixed for further 5 min. The samples were put into plastic bags and sealed overnight to equilibrate. Extrusion was performed on a co-rotating twin-screw extruder (model BC 45 Creusot Loire Firminy, France) 500 mm screws, a module with a reverse flight located on the terminal position of each screw just before the two dies of 50 mm length and 5 mm diameter. An induction belt heated the terminal section of the extruder while running water, cooled the feeding section. Samples were collected after 10 min equilibration period.
Analytical Methods
The capacity of the sample to dissolve in water under specific condition was determined as described by Hanna et al.Citation[16] and expressed as water solubility index (WSI). Expansion of extrudate was expressed as the ratio of the cross sectional area of the diameter of rod shaped extrudate over the cross sectional area of the diameter of the die and was measured as described by Colonna et al.Citation[25] Averages of ten readings were taken.
The amylose extracted in aqueous solution was estimated by the absorbance value at 680 nm upon addition of tri-iodide as described by Gilbert and Spragg.Citation[26] The reading is defined as blue value and is expressed as BV=(Absorption×4/C) where C is the concentration of carbohydrate solution.
Apparent viscosity was measured by the method of Christianson and Bagley,Citation[27] with a slight modification, using a Haake Rotovisco viscometer with a coaxial cylinder design (Haake, Saddlebrook, NJ). Slurry 200 g was prepared by adding ground samples to distilled water at a 9% (w/w) concentration. The slurry was stirred for 2 min, kept for another 2 min and then poured into the viscometer cup and viscosity measurements were made at 60°C. Readings were made progressively from 0–256 sec−1. All measurements were done using the MV-I cup.
Viscosity changes with time on extrudate were measured in a Brabender Viscoamylograph. Briefly, a 9% (w/w d.b) aqueous suspension of the samples were heated from 30 to 95°C for 45 min, held at that temperature for 10 min and then cooled back to 50°C in 35 mins and held there for 10 min. Viscosities were recorded in brabender units (BU).
RESULT AND DISCUSSION
Effect of Chemical Agents on the Viscosity of Extruded Cornstarch-Guar Mixture
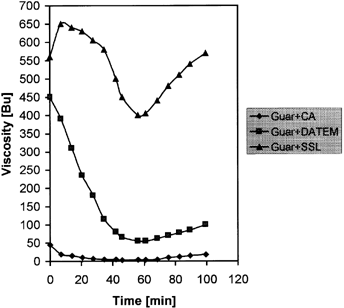
The effect of chemical agents on paste viscosity (BU) for control samples and guar-treated samples are shown in Table . Sodium stearoyl-2-lactylate complexed sample had the highest value for both hot and cold paste viscosities followed by diacetyl tartaric acid ester of monoglyceride complexed samples and the control samples. The addition of citric acid produced the least effect on viscosity of paste. Viscosity changes were not observed for starch extrudate containing chemical agents but without guar gum probably due to insolubility of complexed products after extrusion. Also, Fig. shows the viscosity curves for extruded cornstarch-guar paste. Citric acid addition resulted in total breakdown of polymer while diacetyl tartaric acid ester of monoglyceride-complexed sample exhibited the characteristic curve of a gelatinized product. Sodium stearoyl-2-lactylate complexed sample showed a peak at 50°C corresponding to 10 min cooking time in the viscoamylograph.
Table 1. Effect of Chemical Additives on Paste Viscosity (BU)
Apparent viscosity increased with the addition of chemical agents. Sodium stearoyl-2-lactylate had the greatest increase followed by diacetyl tartaric acid ester of monoglyceride and extruded starch containing guar gum. Apparent viscosity for citric acid-treated sample was extremely low compared to the control and other complexed samples.
Extrusion processing decreased the viscosity of the extrudate possibly due to conditions used. Hot and cold paste viscosities increased with the addition of guar gum (Table ). This was attributed to the inherent visco elastic properties of guar and its synergistic interaction with starch due to its increased hydration capacityCitation[28] as a result of interference on the formation of crystalline region by the presence of evenly spaced side chainsCitation[29], Citation[8] and to stearic effect of linear polysaccharide in solution.Citation[30] There was a trend for hot paste viscosity to decrease compared to cold paste viscosity. As shear stress increased, viscosity decreased for all samples. This was attributed to pseudo plastic flow behavior of galactomanna n whereby viscosity decreases with shear and regains its initial viscosity on removal of shear. This was similar to that reported for xanthan gum.[Citation[16]]
The entire chemical agents tested had significant effect on brabender viscosity. Citric acid reduced the viscosity considerably compared to sodium stearoyl-2-lactylate and diacetyl tartaric acid ester of monogylceride. This drop in viscosity was attributed to depolymerization in presence of acid and heat.Citation31-32 The measurable viscosity recorded by guar gum-treated samples was due to the additive effect of gum, which reduces the extent of depolymerization. The addition of citric acid might have produced a charge on solution viscosity and alteration of electrostatic charge,Citation[33] resulting in reduced ionization, more compact volume and drop in solution viscosity. Similarly, at low pH, extensive hydrolysis of starch occurs yielding non-thickening dextrins that gave reduced viscosity.Citation[34]
Fatty acids and emulsifiers/surfactants have been reported to form water-insoluble complexes with amyloseCitation[20] and possibly with layer of outer chain of amylopectin.Citation[34] If this complex takes place inside the granule, it resist water entry and swelling and thus reduce the amount of amylose leaking from the granule.Citation[34], Citation[19], Citation[35] Between the two emulsifiers investigated, sodium stearoyl-2-lactylate showed the strongest effect on viscosity with an astronomical increase while diacetyl tartaric acid ester of monoglyceride increased viscosity slightly over non-surfactant treated samples. EvansCitation[19] and Hahn and HoodCitation[36] reported that at low temperature, amylose-surfactant complex is strong and amylose leaching is minimal but at high temperature above 95°C, more amylose (30%) is leached out due to granular destruction or increase in thermal stress on the week interaction holding the complex. The increase in viscosity of sodium stearoyl-2-lactylate treated samples was attributed to the susceptibility of sodium stearoyl-2-lactylate to dissociation at high temperature[18] resulting in increased leaching of amylose, which forms a 3-dimensional structure that enhanced viscosity.
The presence of guar also promoted the release of more solubles as suggested by Christianson et al.Citation[37] Also, the adsorption of emulsifier on the hydrophobic part of the hydrocolloid might have resulted in formation of a mixed interfacial film of gum and emulsifier, which increased visco elastic properties.[38] The difference in hot and cold paste viscosities for SSL and DATEM-complexed samples was attributed to potential of SSL to dissociate at high temperature. Diacetyl tartaric acid ester of monoglyceride is stable at high temperature and the complex formed between amylose and DATEM retards amylose leaching thereby resulting in reduced solution viscosity.
Sodium stearoyl-2-lactylate lost its effectiveness as a complexing agent at high temperature leading to increase release of amylose, which produced enhancement of viscosity.Citation[18] Also, SSL a strong surface active ionic emulsifier,Citation[39] can form stable crystalline gel structure in water that can hydrate easily as described by Krog,[Citation[38] while DATEM exhibited limited swelling in water as a result of formation of lamellar liquid crystal as suggested by Nawar.Citation[39]
Figure shows the viscosity curves of extruded corn starch-guar paste with added chemical agents. It was observed that citric acid resulted in total breakdown of polymer while DATEM treated sample exhibited the characteristic curve of a gelatinized product. SSL-treated sample showed a peak at 50°C, which indicated that SSL complexed in such a way that intact starch granules were shielded from the action of extrusion.
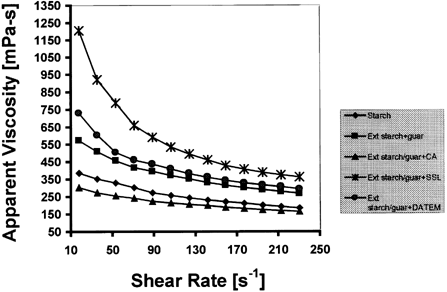
Figure shows the apparent viscosity curves (25°C) of extruded starch-gum system. A trend was observed in which the viscosity of the mixture increased as with the addition of the surfactants. The citric acid samples had lower apparent viscosity in comparison to the starch control and starch-guar blend. The fairly high viscosity for starch control could be due to the low moisture content at which starch was extruded. The high viscosity observed for guar-starch blend could also be related to the synergistic interaction of starch with gum.Citation[4], Citation[11]
Extrusion cooking is known to facilitate gelatinization. When starch-gum mixtures gelatinize, the swollen starch granules disintegrate and disperse in a macromolecular medium while guar gum occupies the continuous phase. This phase available to guar gum becomes reduced with time leading to increase in concentration and thus high viscosity.Citation[7] Guar gum and starch interaction is accomplished because of the non-ionic nature of guar gum, which inhibits H-bond formation. This tends to keep the molecule of gum in an extended form and facilitated its interaction with amylose released from disintegration of starch.Citation[8] Similarly, starch sample extruded without gum or citric acid showed a high viscosity value. This may be due to gelatinization, granular swelling and release of amylose during extrusion followed by re-association of linear amylose giving rise to “set back” and consequently, increased viscosity.[Citation[40]
A high degree of reduction in the viscosity of starch-guar gum mixtures was observed for all the samples extruded with 2% citric acid notwithstanding the report indicating that guar gum is not charged and thus less affected by acidic medium.Citation[40] A probable explanation as suggested by Peder sen,Citation[31] could be that as polysaccharides depolymerizes at lower pH and condition of heat, the addition of critic acid might have reduced the pH of the system leading to drop in viscosity.
Effect of Starch Treatment on Water Solubility Index
The Water solubility indices (WSI) of cornstarch and guar-treated samples to which chemical agents were added is given in Table . The solubility varied from 9.88 to 87.47%. Extrusion of guar with starch increased the solubility of the extrudate. Addition of CA increased the WSI of extrudate without guar gum and guar treated sample significantly from 56.26 and 61.45% to 74.82 and 87.47% respectively. The addition of DATEM caused a decrease in WSI while the addition of SSL further reduced the WSI from 56.26 and 61.45% for control sample without guar gum and guar-added samples to 9.88 and 14.97% respectively. The ANOVA for the data showed a significant effect of chemical agents and guar gum and indicated that WSI of the extrudate is more affected by the agents than by the gum.
Table 2. Effect of Chemical Additives on Water Solubility (%)a,b
All solubility values for starch containing guar gum were higher than that of pure starch (Table ). This is expected because pure starch is insoluble in water. Shearing of starch granule in presence of a viscous material promoted the release of more soluble as suggested by Christianson et al.Citation[37] Hanna et al.,Citation[16] made similar observation. The analysis of variance result of combined effect of gum and chemical agents showed a significant effect on water solubility indices, however, the additive effect was more significant compared to the effect contributed by the gum (Table ).
Table 3. Analysis of Variance Results for Effect of Chemical Agents and Gum on Water Solubility
Sodium stearoyl-2-lactylate-treated samples were most affected in their solubility due to its strong complexing ability as it resists water uptake, retard swelling and thereby reducing solubility. It is well known that SSL looses its effectiveness at high temperature,Citation[18] thus it is expected that at 140°C in the extruder, SSL should have lost its effectiveness completely leading to release of solubles and increased viscosity, however, this was not the case in this work due to possible reformation upon release from the extruder and subsequent cooling as reported by Ghiasi et al.Citation[18] and Evans.Citation[19] The addition of gum increased WSI slightly due to reasons suggested by Christianson et al.Citation[37] Water solubility index (WSI) for DATEM-complexed sample was higher than WSI for SSL-completed sample due to difference in structure and complexing ability. Addition of citric acid increased WSI markedly and it varied from 74.82 to 87.47%. The increase in WSI was attributed to increased starch solubility at high temperature by citric acid. Hanna et al.Citation[16] reported similar findings for the effect of xanthan gum and adipoyl chloride on starch.
Effect of Gum and Chemical Additives Interaction on Blue Value
The interaction of amylose with iodine is known to generate a helical inclusion complex in which iodine occupies the central cavity of the helix,Citation[41] resembling the complex of amylose and surfactant. Result of Table shows the effect of added chemical agents on the iodine affinity for polysaccharide otherwise known as blue value (BV). Blue value varied from 1.60 to 11.14. Addition of gum to starch before extrusion slightly increased the BV. SSL samples gave the least BV value at 1.60, followed by DATEM sample at 7.92, which were well below the value for the control. Among the entire chemical agent tested, CA addition produced the most pronounced effect on BV at 11.14, which was slightly higher than the control sample. The ANOVA showed a significant effect for the agents on BV than does the gum.
Table 4. Effect of Chemical Additives on Blue Valuea,b
It is well documented,Citation20-21, Citation[42], Citation[18] that complexes of amylose with surfactants greatly reduce iodine affinity values. Blue value, which is based on iodine affinity and linear polysaccharide, was relatively high for the control sample due to its solubility, which released amylose into solution for complexing. Furthermore, the addition of gum increased slightly the BV due to extra force exerted on granules during shearing. Analysis of variance results showed a very significant effect of the emulsifier and citric acid on blue value of samples (Table ).
Table 5. Analysis of Variance Results for Effect of Chemical Agents and Gum on Blue Value
Sodium stearoyl-2-lactylate-treated samples gave the lowest BV value among all the additives used. It has been reported that SSL readily forms complex with starchCitation[39] however, at high temperature, this complex becomes unstable, dissociates and reassociate very fast on cooling to form a complex again.Citation[18] The complexed material formed after leaving the extruder prevented the association of iodine and amylose. The observations made in this work are in agreement with the findings of the authors above. Similar low values were recorded for samples with added gums, indicating non-interference by hydrophilic gum on amylose in the complex.
BV values for DATEM-treated samples showed a marked increase compared to the SSL-treated samples. This was attributed to the ability of DATEM to imbibe water in presence of sodium hydroxide (NaOH) used in the determination.Citation[39] BV values for citric acid-treated samples were high than those for control, DATEM and SSL. This is expected as citric acid enhanced starch hydrolysis,Citation[16], Citation[43] which gave rise to more soluble amylose in solution for iodine to bind.
Effect of Additives on Expansion of Extruded Products
Expansion ratio is an indicator of dimensional changes taking place during extrusion. With the exception of guar blended extrudate to which CA was added, the expansion ratios of samples given in Table indicated that the co-extrusion of guar gum and starch brought about a reduction in the expansion of the extrudate for control and DATEM treated samples. There was a general trend for surfactants to reduce the expansion of extrudate as typified by the effect of SSL addition. Similarly, the ANOVA indicate a significant effect of chemical agents.
Table 6. Effect of Chemical Agents on Expansion Ratioa,b
A lot of factors have been put forward to explain the increase or decrease in expansion ratios of extruded product. These include starch types,Citation[44] amylose content, structure molecular weight of amylopectin.Citation[45] In the samples with no additives, expansion ratio was higher for control compared to gum added samples. The analysis of variance result showed that expansion of samples was more affected by the chemical agents added than by the gum (Table ). The difference in control and gum treated samples was due to the viscous properties of the gum. Launay and LischCitation[46] reported that viscosity changes influence expansion volume of starches. An explanation could be that as viscosity of starch slurry increases with the addition of gum, gelatinization is delayed thereby hindering starch swelling and subsequent release of amylose and amylopectin thus lowering the ability of the material to expand. Furthermore, the screw speed (180 rpm) at which the samples were extruded may have encouraged faster movement through the extruder and thus a decrease in the residence time of the material in the extruder.Citation[42]
Chinaswamy and HannaCitation[42] have reported that increased amylose content enhanced expansion of extrudate. Expansion ratio for CA-treated samples was higher than surfactant treated. This was attributed to the hydrolysis effect of the acid on starch, which led to increased solubility and higher concentration of amylose. Expansion volume decreased for samples containing SSL because of the strong complexing ability of SSL, which made amylose-surfactant interaction resistant to amylose leaching. However, DATEM treated samples exhibited higher expansions than SSL samples.
Table 7. Analysis of Variance Results for Effect of Chemical Agents and Gum on Expansion Ratio
CONCLUSION
The present results showed that citric acid and surfactant and/or emulsifiers behave differently when extruded with starch in presence of hydrocolloid. It indicates that viscosity of extruded starch-surfactant system is enhanced in gum environment. Addition of citric acid decreased viscosity even in the presence of gums, however, WSI and expansion increased. This information would be of importance in the production of extruded foods as emulsifiers, hydrocolloids and acids are increasingly being used in the food industry.
Acknowledgments
REFERENCES
- Glicksman , M. 1979 . “ Gelling Hydrocolloids in Food Product Applications ” . In Polysaccharides in Food Edited by: Blanchard , J.V.M. and Mitchell , J.R. Vol. 27 , 185 – 204 . London : Butterworths .
- Crossland , L.B. and Favor , H.H. 1948 . Starch Gelatinization Studies II. A Method for Showing the Stages of Swelling of Starch During Heating in the Amylograph . Cereal Chem. , 25 : 213 – 221 .
- Sandstedt , R.M. and Abbott , R.C. 1964 . A Comparison of Methods for Studying the Course of Starch Gelatinization . Cereal Sci. Today. , 9 ( 1 ) : 13 – 18 .
- Christianson , D.D. 1981 . “ Hydrocolloid Interaction with Starches ” . In Food Carbohydrate Edited by: Lineback , D.R. and Inglett , G.E. 399 – 419 . Westport. CT : Avi Publ. Co. Inc. .
- Sajijan , S.U. and Rao , M.R. 1987 . Effect of Hydrocolloid on the Rheological Properties of Wheat Starch . Carbohydrate Polymer , 7 : 395 – 402 .
- Garti , N. and Reichman , D. 1994 . Surface Properties and Emulsification Activity of Galactomannans . Food Hydrocoll. , 8 ( 2 ) : 155 – 173 .
- Sudhakar , V. , Singal , R.S. and Kulkani , P.R. 1995 . Starch-Galactomannan Interaction: Functionality and rheological aspect . Food Chem. , 55 ( 3 ) : 259 – 264 .
- Nussinovitch , A. 1997 . “ Hydrocolloid applications ” . In Gum Technology in the Food and Other Industries 140 – 153 . London : Blackie Academic & Professional .
- Glicksman , M. 1974 . “ Hydrocolloids ” . In Ingredient Technology for Product Development Edited by: Twiggs , B.A . Chicago : Inst. of Food Tech. . Chap.2
- Tye , R.J. 1988 . The Rheology of Starch-Carageenan System . Food Hydrocoll. , 2 : 259 – 266 .
- Alloncle , M. , Lefebve , J. , Llamas , G. and Doublier , J.L. 1989 . Rheological Characterization of Cereal Starch-Galactomannan Mixtures . Cereal Chem. , 66 : 90 – 93 .
- Alloncle , M. and Doublier , J.L. 1991 . Viscoelastic Properties of Maize Starch-Hydrocolloid Pastes and Gels . Food Hydrocoll. , 5 : 455 – 467 .
- Amero , E. and Collar , C. 1996b . Antistaling Additive, Flour Type and Sour Dough Process Effect of Functionality of Wheat Dough . J. Food Sci. , 61 ( 12 ) : 299 – 303 .
- Launay , B. , Doublier , J.L. and Cuvelier , G. 1986 . “ Flow Properties of Aqueous Solution and Dispersions of Polysaccharides ” . In Functional Properties of Food Macromolecules Edited by: Mitchell , J.R. and Ledward , D.A. 157 – 162 . London : Elsevier .
- Doublier , J.L. 1990 . “ Rheological Properties of Cereal Carbohydrates ” . In Dough Rheology and Baked Product Texture Edited by: Faridi , H. and Faubion , J.M. 111 – 155 . New York : Van Nostrand Reinhold .
- Hanna , A.M. , Chinnaswamy , R. , Gray , D.R. and Milladinov , V.D. 1997 . Extrudate of Starch-Xanthan Gum Mixtures as Affected by Chemical Agents and Irradiation . J. Food Sci. , 62 ( 4 ) : 816 – 820 .
- Goering , K.J. , Jackson , L.L. and DeHaas , B.W. 1975 . Effect of some Non-Starch Components in Corn and Barley Starch Granules on the Viscosity of Heated Starch-Water Suspensions . Cereal Chem. , 52 : 493 – 500 .
- Ghiasi , K. , Varriano-Marston , E. and Hoseney , R.C. 1982 . Gelatinization of Wheat Starch II. Starch-Surfactant interaction . Cereal Chem. , 59 ( 2 ) : 86 – 88 .
- Evans , I.D. 1986 . An Investigation of Starch/Surfactant Interactions Using Viscometry and Differential Scanning Calorimetry . Starch/Starke , 38 : 227 – 235 .
- Osman , E.M. , Leith , S.J. and Fles , M. 1961 . Complexes of Amylose with Surfactants . Cereal Chem. , 38 : 449 – 463 .
- Krog , N. 1971 . Amylose Complexing Effect of Food Grade Emulsifier . Starch/Starke , 23 : 206 – 210 .
- Hoover , R. and Vasanthan , T. 1994 . Effect of Heat-Moisture Treatment on the Structure and Physiochemical Properties of Cereal, Legume and Tuber Starches . Carbohydr. Res. , 252 : 33 – 40 .
- Rojas , J.A. , Rosell , C.M. and Benedito , C. de Barber . 1999 . Pasting of Different Wheat Flour Hydrocolloid System . Food Hydrocoll. , 13 : 27 – 33 .
- 1996 . SAS Institute. SAS Programme for Microcomputers. Version 6.11; The Institute Cary; N.C
- Colonna , P. , Melcion , J.P. , Vergnes , B. and Mercier , C. 1983 . Flow, Mixing and Residence-Time Distribution of Maize Starch Within a Twin-Screw Extruder With a Longitudinally Split Barrel . J. Cereal Sci. , 1 : 115 – 121 .
- Gilbert , G.A. and Spragg , S.P. 1964 . Methods in Carbohydrate Chemistry 168 – 174 . New York : Academic Press .
- Christianson , D.D. and Bagley , E.B. 1983 . Apparent Viscosities of Dispersion of Swollen Starch Granules . Cereal Chem. , 60 ( 2 ) : 116 – 121 .
- Glicksman , M. 1969 . Gum Technology in the food industry New York : Academic Press .
- Maier , H. , Anderson , M. , Karl , C. , Magnuson , K. and Whistler , R.L. Guar . 1993 . Industrial Gums Edited by: Whistler , R.L. and BeMiller , J.N. 181 – 226 . San Diego : Academic Press .
- Glicksman , M. 1983 . “ Functional properties of hydrocolloid ” . In Food Hydrocolloid Edited by: Glicksman , M. Vol. 1 , 47 – 99 . Boca Raton, FL : CRC Press .
- Pedersen , J.K. 1979 . “ The Selection of Hydrocolloid to Meet Functional Requirement ” . In Polysaccharide in Food Edited by: Blanchard , J.V.M. and Mitchell , J.R. 219 – 228 . London : Butterworths .
- BeMiller , J.N. and Whistler , R.L. 1996 . “ Carbohydrate ” . In Food Chemistry , 3rd edition; Edited by: Fennema , O.R. 157 – 223 . New York : Marcel Dekker Inc. .
- William , P.A. , Phillips , G.O. and Randall , R.C. 1990 . “ Structure-Function Relationships of Gum Arabic ” . In Gum and Stabilizers for the Food Industry Edited by: Phillips , G.O. , Wedlock , D.J. and Williams , P.A. Vol. 5 , 25 – 36 . Oxford : Oxford University Press .
- Whistler , R.L. and Daniels , J.R. 1985 . “ Carbohydrates ” . In Food Chemistry , 2nd edition Edited by: Fennema , O.R. 69 – 137 . New York : Maercel Dekker Inc. .
- Czuchajowska , Z. , Sievert , D. and Pomeranz , Y. 1991 . Enzyme Resistant Starch IV: Effects of Complexing Lipids . Cereal Chem. , 68 : 537 – 542 .
- Hahn , D.E. and Hood , L.F. 1986 . Factors Influencing Corn Starch-Lipid Complexing . Cereal Chem. , 64 ( 2 ) : 81 – 85 .
- Christianson , D.D. , Hodge , J.E. , Osborne , D. and Detroy , R.W. 1991 . Gelatinization of Wheat Starch as Modified by Xanthan Gum, Guar Gum and Cellulose Gum . Cereal Chem. , 58 : 513 – 517 .
- Krog , N. 1995 . “ Interaction of Emulsifier With Other Components in Food ” . In Ingredient Interaction, Effect on Food Quality Edited by: Gaonkar , A.G. 377 – 392 . New York : Marcel Dekker Inc. .
- Nawar , W.W. 1985 . “ Lipids ” . In Food Chemistry Edited by: Fennemma , O.R. 139 – 243 . New York : Marcel Dekker Inc. .
- Sanderson , G.R. 1981 . Polysaccharides in Food . Food Tech. , : 50 – 57 83 .
- Rundle , R.E. , Foster , J.F. and Baldwin , R.R. 1944 . On the Nature of Starch-Iodine Complex . J. Am. Chem. Soc. , 66 : 2116 – 2120 .
- Chinnaswamy , R. and Hanna , M.A. 1988 . Relationship Between Amylose Content and Extrusion-Expansion Properties of Cornstarches . Cereal Chem. , 65 ( 2 ) : 138 – 143 .
- Emine , U. and Faller , J.F. 1998 . Formation of Resistant Starch by a Twin-Screw Extruder . Cereal Chem. , 75 ( 3 ) : 346 – 350 .
- Mercier , C. and Fillet , P. 1975 . Modification of Carbohydrate Components by Extrusion Cooking of Cereal Products . Cereal Chem. , 52 : 283 – 297 .
- Chinnaswamy , R. and Hanna , M.A. 1990 . Relationship Between Amylose Content and Extrusion Expansion Properties of Cornstarch . Cereal Chem. , 65 ( 2 ) : 138 – 143 .
- Launay , B. and Lisch , J.M. 1983a . Twin Screw Extrusion of Starches . Flow Behavior of Paste, Expansion and Mechanical Properties of Extrudates. J. Food Eng. , 2 : 259.