ABSTRACT
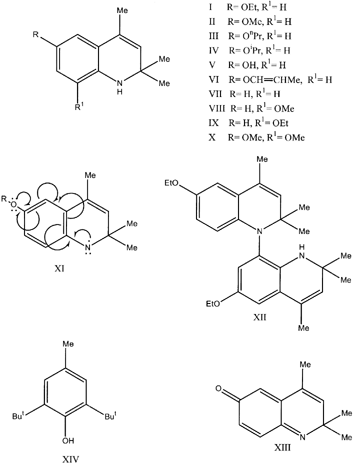
This review records original research carried out in our Institute since 1983 on the antioxidant ethoxyquin (1,2 dihydro-6-ethoxy-2,2,4-trimethylquinoline)(EQ, I) used for the protection of the valuable polyunsaturated fatty acids in fish meal. The residual lipids in South African fish meals have a high degree of unsaturation, which renders the meals prone to spontaneous combustion. Addition of EQ is therefore essential. Accurate physical constants have been determined for EQ, while over the years a number of analogues of EQ have been prepared and their antioxidant efficacy in fish meal compared to that of EQ. It became clear that the efficacy of an antioxidant intended for fish meal must be determined in fish meal and not in an accelerated test in fish oil. Only one analogue could compete with EQ in efficacy and price namely hydroquin (1,2-dihydro-2,2,4-trimethylquinoline)(VII). A patent on this antioxidant in fish meal has been taken out. On storage of fish meal the extractable EQ content diminishes and eventually reaches zero. Other workers have demonstrated that in fish meal EQ is converted into two oxidation products: a dimeric oxidative coupling product, 1,8 ′-di-(1,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline) (EQ-dimer, XII) and a qu i n olone (2,6-dihydro-2,2,4-trimethyl-6-quinolone)(XIII). Both compounds were shown in our Institute to be potent antioxidants in their own right. The “half life” of the dimer was considerably greater than that of EQ, implying that even if the EQ content of a meal were to drop to negligible levels there would still be sufficient EQ-dimer to protect the meal lipids against oxidation. This may explain why EQ is such a strong antioxidant. A method for assessing the purity of EQ and the analysis of EQ is described. It entails the use of methoxyquin (1,2-dihydro-6-methoxy-2,2,4-trimethylquinoline) (MQ, II) a close analogue of EQ. This method can also be employed for the simultaneous determination of the quinolone and using MQ-dimer for the analysis of the EQ-dimer.
INTRODUCTION
Ethoxyquin (l,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline) (EQ, I) was first used as an antioxidant in rubber to prevent it from cracking due to oxidation of isoprene,Citation[1] whilst the earliest mention of the use of EQ in feedstuffs was in 1957, when KyteCitation[2] treated herring meal with it at levels of 100 and 800 mg/kg to combat overheating of the meal. Since then it has been the antioxidant of choice for protection of the polyunsaturated fatty acids (PUFA: those fatty acids having two or more double bonds) of the lipids remaining in fish meal after processing. Worldwide the total amount of EQ used in the fish meal industry is approximately 2000 tonnes.Citation[3] The dosage level in South African and Namibian meals is 400 mg/kg, whereas in South American meals it can reach levels of 1000 mg/kg. After dosing the meal it becomes increasingly difficult to recover EQ as it is consumed in the reactions taking place in the meal. The International Maritime Organisation, however, insists that 100 mg/kg antioxidant remains detectable in the meal prior to shipment.Citation[3] Hence countries such as Chile and Peru, which produce meals with a very high PUFA content, must add more EQ in order to comply with shipping rules. Butylated hydroxytoluene (3,5-di-tert.butyl-4-hydroxy toluene) (BHT, XIV), the antioxidant largely used prior to EQ, has virtually been replaced by EQ, except on some Russian and Polish factory ships fishing off the coast of Namibia. In addition to using EQ in fish meals it is also added as a post-harvest dip for apples to inhibit the development of brown spots (scald) due to oxidation of alpha farnesene.Citation[4] Most countries do not permit EQ to be used in foodstuffs, although in the United States of America it is allowed for the preservation of colour in chilli powder and paprika at levels not exceeding 100 mg/kg.Citation[3] Pure EQ is a colourless, viscous, fluorescent liquid, which rapidly turns brown on exposure to air, this however does not affect its efficacy as an antioxidant. The reasons for its popularity in the fish meal industry are firstly its astonishing capacity to prevent oxidation of highly unsaturated fatty acids at very low dosage levels and secondly its rapid and easy distribution in meals because of its fluidity.
PREPARATION OF EQ AND A NUMBER OF PHYSICAL CONSTANTS
It is normally prepared on a large scale by refluxing acetone with p-phenetidine (p-ethoxyaniline) in the presence of a catalyst such as p-toluene-sulphonic acid or iodine. The crude product is simply washed with excess water and often sold without any further purification. Work carried out in our Institute showed that a sample of commercial EQ contained between 20 and 30% of material which was not EQ, while another commercial product had more than 99% EQ.Citation[5] This latter product was carefully distilled under high vacuum and served for the determination of some physical constants. This appeared necessary as literature data are unreliable; for example the refractive index at 25°C for EQ is quoted in the Merck Index to range from 1.569 to 1.672 at 25°C,Citation[1] whereas its real value is 1.5712.Citation[5] A number of physical constants of EQ and nine of its analogues are recorded in Table . The preparation of these analogues has been described elsewhere; essentially their synthesis was similar to that of EQ.Citation6-7 All compounds are fluorescent liquids or solids. The ultraviolet spectrum of EQ shows a maximum at 362 nm and in some spectrophotometric analyses this maximum is used.Citation8-9 The molar absorptivity of EQ in hexane at 362 nm determined at our Institute is 3254 moles−1 Lċcm−1, while Spark uses a value of 2936 moles−1 L·cm−1; clearly this author cannot have used pure EQ.Citation[9] Other analyses employ the absorption maximum of the HCl salt of EQ at 296 nm. The molar absorptivity of EQ in 0.5 M HCl was found to be 1734 moles−1 L·cm−1 which is in excellent agreement with the value of 1758 moles−1 L·cm−1 that can be calculated from the data of Choy et al.,Citation[10] although it must be mentioned here that their absorptivity in mg−1ċmLċcm−1 of 80.9 is out by a factor 10. Methoxyquin (1,2-dihydro-6-methoxy-2,2,4-trimethylquinoline) (MQ, II) was accidentally made crystalline in our Institute melting at 40–41°C, while EQ, although it is the higher homologue, has persistently refused to become crystalline. EQ therefore is a supercooled liquid at room temperature, its melting point is estimated to be about 45–46°C from the melting points of MQ and propoxyquin which are 40–41° and 49–50°C respectively. As a supercooled liquid EQ has a great advantage, as in its solid state it would be much more difficult to distribute in fish meal. But one day somebody may manage to obtain crystalline EQ and incur the wrath of all fish meal producers.
Table 1. Physical Constants of EQ and a Number of Its Analogues
ANTIOXIDANT EFFICACY OF EQ AND ITS ANALOGUES
The reason for preparing analogues of EQ was to compare their antioxidant efficacy with that of EQ. All nine analogues have been tested in our Institute in a refined fish oil and some of the most promising have subsequently also been tested in fish meal. A test in fish oil is completed in a few days, while a test in fish meal normally lasts a year. The test in fish oil is an accelerated one where air is blown into oil samples held in glass tubes at 50°C. The rate of air flow as determined by a soap bubble flow meter is kept constant at 5 mL/sec. Oxidation of the oil is followed by determining peroxide values at intervals of 4 h. An oil treated with EQ and a control oil without antioxidant are also included in the test. The efficacy of an antioxidant is obtained by determining the time it takes for the oil treated with the antioxidant to show a rapid increase in peroxide value. This time is known as the induction time. It was found convenient to express the efficacy relative to that of EQ. In an oil the level of the antioxidant tested is usually 0.01%.Citation[11] A test in fish meal is done by dosing a reactive meal such as anchovy (Engraulis japonicus) or pilchard (Sardinops ocellata), obtained from a local factory within a few hours of production, with 400 mg/kg of the antioxidant, while a meal treated with EQ and an untreated meal are also included. The meals are stored in polythene bags with ample air space at 25°C and analysed at regular intervals (e.g., bi-monthly) for PUFA content of the residual lipids by gas chromatography. The decrease in PUFA content serves to express the efficacy of the antioxidant, which is always expressed relative to EQ. No absolute value exists (as yet) to express the activity of an antioxidant in fish meal. This is because meals differ almost hourly in their oxidation rate and therefore no meal of standard reactivity can be produced. The efficacy of an antioxidant relative to EQ is calculated at each time of testing by the following expression:
Table 2. Efficacy Values of a Number of Antioxidants Relative to Ethoxyquin
It is curious to note that the results obtained in an accelerated test in fish oil do not always agree with those in fish meal. The reasons for this are not yet fully understood, especially as one antioxidant performs better in oil, while another for unknown reasons performs better in meal. Large differences were observed for hydroxyquin (V) and hydroquin (VII). In fish oil hydroxyquin was more than three times as effective as EQ, while in fish meal it showed only three-quarters of its efficacy. In contrast, hydroquin, which was only half as effective as EQ in fish oil, proved as effective as EQ in fish meal. In fact, hydroquin can compete with EQ as the antioxidant of choice in fish meal as it is more economical to produce than EQ since its preparation is based on aniline and acetone rather than p-phenetidine (a more expensive chemical than aniline) and acetone. For this reason hydroquin was patented as an antioxidant in fish meal and other animal feeds in 1997.Citation[12] MQ, propoxyquin and isopropoxyquin are as effective in fish oil as EQ, but their use as antioxidants to replace EQ is limited, as their method of production offers no advantage over that of EQ, because p-methoxyaniline, p-propoxyaniline and p-isopropoxyaniline are either similar in price or more expensive than p-ethoxyaniline. Virtually no antioxidant activity is shown by compounds VIII and IX, i.e., 1,2-dihydro-8-methoxy-2,2,4-trimethylquinoline and 1,2-di hydro-8-ethoxy-2,2,4-trimethylquinoline. This is rather unexpected because if the antioxidant properties of the dihydroquinolines substituted in the 6-position are due to their ability to delocalise the lone electron on the nitrogen atom of the free radical XI and thus stabilise it, a similar effect for alkoxy groups in the 8-position is expected. Since this is not the case, it may be concluded that a different mechanism is responsible for the antioxidant activity of EQ and its analogues. Allyloxyquin (VI) shows about 90% of the activity of EQ in both fish meal and oil, which, contrary to expectations, shows that the extra double bond in the allyl side chain does not enhance the activity of the molecule. This also does not support the theory of the extra delocalisation of the lone electron on the free radical XI. Further work seems required to establish exactly why the dihydroquinolines are such powerful antioxidants.
Unexpectedly, one possible reason for this has emerged from studying the oxidation products of EQ. Two oxidation products have been identified by Thorisson working in the laboratory of Gunstone.Citation[13] A fluorescent dimeric oxidative coupling product, 1,8 ′-di-(1,2dihydro-6-ethoxy-2,2,4-trimethylquinoline) (XII, EQ-dimer) formed in about 40% yield and a non-fluorescent quinolone 2,6-dihydro-2,2,4-trimethyl-6-quinolone (XIII) in about 15% yield. Both compounds were present in a fish meal treated with EQ after one week of storage at room temperature. Thorisson claimed that the EQ-dimer had no antioxidant activity, whereas the quinolone was only slightly less active than EQ.Citation[13] Work in our Institute, however, has shown that the EQ-dimer had 69% and the quinolone 80% of the efficacy of EQ in fish meal.Citation[14] In fish oil their efficacies were slightly lower but still substantial. The fact that the breakdown products of EQ are also antioxidants explains in some measure why EQ is such a strong antioxidant. The “half-life” of the EQ dimer is much greater than that of EQ so that when EQ is completely consumed the dimer is still present in substantial amounts to protect the meal.Citation[6] Similarly, all the analogues of EQ, with the exceptio n of dimethoxyquin (X), are theoretically able to form the quinolone and their respective dimers and thus exert their antioxidant activity.
ANALYSIS OF EQ
Analysis of commercial EQ is often done by titrating the compound in glacial acetic acid with standardised perchloric acid.Citation[15] This particular method is completely unreliable, as it includes other basic impurities in the titration and frequently yields far too high results for the purity of EQ. This of course makes it a favourite method for manufacturers of EQ. When faced with a sample of commercial EQ it is qualitatively an easy matter to distinguish between a pure EQ (i.e., a distilled product) and a crude product by adding a little hexane. The crude product yields a murky brown solution with a precipitate settling out, while pure EQ yields a clean transparent brown solution. The impurities consist of unidentified polymerisation products (dimers, trimers, polymers) together with starting material. An accurate and specific method is to analyse it by gas chromatography using MQ as internal standard.Citation[8] Gas chromatography on almost any column separates the two isomers beautifully and accurately yields the purity of EQ. Analysis of the EQ content of fish meals can also readily be carried out by gas chromatography using MQ as internal standard. To a fish meal is added a small amount of MQ, it is then extracted with hexane and filtered. The hexane extract is washed with 1 M HCl, which separates the EQ from the meal lipids into the aqueous phase as EQ is an organic base. The HCl extract is then made alkaline and the EQ extracted back into hexane. This solution, after concentration, is injected into the gas chromatograph. The procedure using MQ as internal standard can of course also be adapted to the high performance liquid chromatographic (HPLC) technique. Other gas chromatographic methods have been described in the literatureCitation[4], Citation[16] but none employ MQ as internal standard. Hobson-Frohock discontinued using 1,2-dihydro-2,2,4-trimethylquinoline (hydroquin) as internal standard in his work on residual EQ determination in poultry as, according to this author, its oxidation products interfered increasingly with the measurement of EQ.Citation[17]
The internal standard MQ has been distributed to several laboratories in Chile, Peru, Denmark and Norway and this is now the official method for determination of EQ in fish meal. There are still laboratories however that routinely use the method of separating EQ from lipids and protein by chromatography of a hexane extract of the meal on a column of alumina. The EQ is eluted with hexane diethyl ether (9:1,by volume), while the progress of elution is followed with a UV lamp, as EQ is fluorescent, and the EQ collected in a calibrated flask. The absorbance is read at 362 nm and using the molar absorptivity of 3254 moles −1 Lċcm −1 the content of EQ in the meal is calculated. This procedure is sufficiently accurate for meals having more than approximately 30 mg/kg EQ. Values obtained for meals with EQ contents below about 30 mg/kg are much too high and the results are to be regarded as unreliable. For these meals, a gas chromatographic or fluorescence method should be employed.[Citation[8]] Bruggemann and ZentzCitation[18] were the first to make use of the fluorescence of EQ in determining the EQ content of animal feeds. Subsequently others have employed this characteristic for determining residual EQ in chicken tissues and eggs in quantities of as little as 0.05 mg/kg.Citation[19]
However, laboratories serving the fishing industry have no need to analyse EQ at that level.
TOXICITY OF EQ
The toxicity of EQ remains an area of continuing debate and contro versy, and every country has its own legislation for the maximum permissible levels of it in Foodstuffs.Citation[3] For instance, in Belgium, Denmark, Greece, Italy, Luxembourg, The Netherlands and Spain, EQ is not allowed in any food for human consumption. Sweden, like the United States of America (USA), however, allows it at levels of as much as 100 mg/kg in spice blends and spice extracts, while in the United Kingdom (UK) only 3 mg/kg is allowed as a pesticide on apples and pears. Legislation on animal feedstuffs are more relaxed. In the USA, for instance, levels of 150 mg/kg are allowed, while in South America it is common to add as much as 1000 mg/kg EQ to fish meal.
It appears that the levels of EQ used in fish meals (400–1000 mg/kg) are low enough to ensure that no, or only minute, amounts of it are recovered in the flesh and eggs of chickens and in the muscle of salmon reared on a fish meal diet. For instance, tissues of broilers fed a diet containing 125 mg/kg EQ contained less than 0.005 mg/kg EQ, while eggs from hens in the UK gave an average value of 0.011 mg/kg.Citation[3] Concern about the safety of EQ in pet food, in particular dog food, has prompted a study of its toxicity in dogs, as a result “a safe tolerance level” of 150 mg/kg of feed was established.Citation[3]
CONCLUSIONS
Ethoxyquin (1,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline) (EQ, 1) has proved to be an ideal antioxidant in fish meal, in protecting the valuable highly unsaturated fatty acids of the residual lipids of the meal and therefore in preventing overheating and combustion of the meal. These fatty acids provide many benefits to animals, such as improved immunity towards infectious diseases, better bone formation and increased fertility. EQ therefore greatly enhances the value of the meal. Only one analogue of EQ can compete with it on the basis of cost and efficacy namely 1,2 dihydro-2,2,4 trimethylquinoline (hydroquin, VII). It is envisaged that in future this compound will be produced commercially in competition with EQ. The antioxidant butylated hydroxytoluene (BHT, XIV) has virtually disappeared in the fish meal industry as it has only about half the efficacy of EQ and sells at a similar price. The efficacy of an antioxidant in fish meal is often completely different from that in a fish oil. Therefore an antioxidant intended for fish meal should be tested in a meal and not an oil.
Acknowledgments
REFERENCES
- 1976 . The Merck Index , 9 494 Rahway, N.J. USA : M. .
- Kyte , R.M. 1957 . Bulk Handling of Alaska Herring Meal . Commercial Fisheries Review , 19 : 9 – 14 .
- 1993–7 . Anonymous. Present and Future Uses of Ethoxyquin and Alternative Anti oxidants for the Stabilisation of Fish Meal . International Fishmeal and Oil Manufacturers Association Research Report , : 1 – 25 .
- Winell , B. 1976 . Quantitative Determination of Ethoxyquin in Apples by Gas Chromatography . Analyst , 101 : 883 – 886 .
- De Koning , A.J. 1987 . Ethoxyquin – A Note on its Refractive Index and some of its Spectral Data . Fat Science Technology , 89 : 103 – 106 .
- De Koning , A.J. 1991 . The Synthesis of a Number of Ethoxyquin Analogues and their Evaluation as Antioxidants in Fish Oil . Fat Science Technology , 93 : 378 – 382 .
- De Koning , A.J. 1996 . The Synthesis of a Number of Ethoxyquin Analogues and their Evaluation as Antioxidants in Fish Oil, Part II . Fett/Lipid , 98 : 14 – 17 .
- De Koning , A.J and van der Merwe , G. 1992 . Determination of Ethoxyquin and Two of Its Oxidation Products in Fish Meal by Gas Chromatography . Analyst , 117 : 1571 – 1576 .
- Spark , A.A. 1982 . Ethoxyquin in Fish Meal . Journal of the American Oil Chemists' Society , 59 : 185 – 188 .
- Choy , T. , Alicino , N.J. , Klein , H.C. and Quatrone , J. 1963 . Determination of Ethoxyquin by Ultraviolet Spectrometry . Journal of Agricultural and Food Chemistry , 11 : 340 – 342 .
- Laubli , M.W. and Bruttel , A.P. 1986 . Determination of the Oxidative Stability of Fats and Oils: Comparison between the Active Oxygen Method (AOCS Cd 12–57) and The Rancimat Method . Journal of the American Oil Chemists' Society , 63 : 792 – 795 .
- De Koning , A.J. 1997 . An Antioxidant for Fish Meal . Republic of South Africa Patent , : 970894
- Thorisson, S. Antioxidant Properties of Ethoxyquin and some of Its Oxidation Products. Ph.D. Thesis, University of St. Andrews, Scotland.
- De Koning , A.J. 1998 . A New Method for Measuring Efficacies of Antioxidants in Fish Meal . International Journal of Food Properties , 1 : 255 – 261 .
- 1972 . Food Chemicals Codex , 2 270 Washington, D.C. : National Academy of Sciences .
- Dahle , H.K. and Skaare , J.U. 1975 . Gas Chromatographic Determination of Ethoxyquin in Feed and Food Products II . Journal of Agricultural and Food Chemistry , 23 : 1093 – 1095 .
- Hobson-Frohock , A. 1982 . Residues of Ethoxyquin in Poultry Tissues and Eggs . Journal of the Science of Food and Agriculture , 33 : 1269 – 1274 .
- Bruggemann , J. and Zentz , C. 1963 . Zur Bestimmung von Ethoxyquin (Santoquin) in Futtermischungen, Zeischrift fur Tierphysiologie, Tierernahrung und Futtermittelkunde . 18 : 99 – 110 .
- Van Deren , J.M. and Jaworski , E.G. 1966 . Ethoxyquin (Santonin) in Eggs, Chicken Muscle, and Chicken Liver . Journal of the Association of Official Agricultural Chemists , 49 : 712 – 714 .