ABSTRACT
Texture is considered a key quality parameter determining the acceptability of many porous foods. Dynamic mechanical thermal analysis (DMTA) is commonly employed in food and polymer science research as a means of measuring the glass transition (Tg) of materials. In the field of food science, Tg measurements have been used to characterize the textural changes of food materials. However the effect of sample porosity and different sample preparation techniques on Tg measurement using a DMTA have not been investigated. The objectives of this study were to compare Tg measurements of identical materials varying only with porosity and to examine the effect of sample preparation technique on Tg measurements. Both starch based extrudates and synthetic polymers with different porosities were tested with DMTA. For comparison, representative samples from the porous specimens were obtained and subjected to one of three methods of Tg determination: 1) ground into a powder and compressed into tablets for testing with DMTA, 2) ground into a powder and tested by differential scanning calorimetry (DSC), and 3) ground into a powder and tested by controlled strain rheometry. Porosity was observed to affect the measurement of the glass transition using DMTA. The extrudate samples containing the lowest porosity exhibited a Tg range 22°C lower than the extrudate samples with the highest porosity. Also, the polymer samples with the lowest porosity showed a Tg range 20°C lower than the polymer samples with the highest porosity. The tablets (no pores) did not show any significant difference in Tg as measured by DMTA nor did the ground samples (no pores) measured by DSC and controlled strain rheometry. This study shows that porosity of the sample affects Tg measurement. Care should be taken when reporting Tg values as porosity may introduce an artifact in Tg measurements using DMTA.
INTRODUCTION
The importance of the glass transition of materials has long been recognized in determining the behaviour of amorphous synthetic polymers during processing and the concept of the glass transition has been adopted for a better understanding on the behaviour of biopolymers during processing.Citation[1] Measurements of glass transition have also received attention in the food science literature and research has been performed with the aim of relating the glass transition with textural quality.Citation[2]
There are a wide variety of techniques that can be used to measure the glass transition of materials and these include: differential scanning calorimetry (DSC), dynamic mechanical thermal analysis (DMTA), thermal mechanical analysis (TMA), and nuclear magnetic resonance (NMR).Citation[3] Interestingly, the study by Kalichevsky et al.Citation[3] indicated that glass transition temperature range measured depended upon the test method used. They attributed this fact to each different test method being sensitive to different degrees of molecular mobility which could be reasonably associated to the glass transition (Kalichevsky et al.Citation[3]). Therefore there are many methods available to food science researchers that are capable of measuring the glass transition range temperature and the measurement of the glass transition depends on the method used.
Notably, DMTA is a very common test method used by food science researchers to determine the glass transition temperature range of food materials.Citation[4] For example, Brent et al.Citation[1] used DMTA to characterize the glass transition of extruded cereal melts and Nichols et al.Citation[5] used DMTA to determine the glass transition of amorphous amylopectin sheets. The method by which DMTA measures the glass transition applies a small sinusoidal strain of known amplitude and thereby measures the resulting stress as a function of temperature.Citation[5] There are three mechanical properties that are obtained with DMTA and these include storage modulus (E′), loss modulus (E″) and tan δ. The glass transition can be characterized by different measured parameters, e.g., a sharp drop in E′, a peak in E″ or a peak in tan δ.Citation[5] Therefore explicitly stated, the measurement of the change in mechanical properties is paramount to determining glass transition of materials. With respect to DMTA, it also follows that factors that affect the mechanical properties of a material will also affect the measurement of the glass transition.
Regarding the measurement of mechanical properties of foods, there is much literature available concerned with characterizing the textural quality of food with measurements of mechanical properties.Citation6-11 Literature has indicated that the mechanical properties of a material are the direct consequences of the material microstructure and therefore they are affected by these microstructural characteristics.Citation6-7 Moreover, researchers have noted that the presence of pores and the degree of porosity affect the mechanical properties of the food materials.Citation12-14 Nicholls et al.Citation[5] indicated that cellular foam-like systems have been used to model crisp foods. They stated that mechanical failure depends on the cellular structure and the material properties of the matrix. It was noted by Attenburrow et al.Citation[15] that the structure of many cereal based foods consists of a porous structure and they may be described as food foams. It was stated that the key parameter used to characterize microstructure in relation to mechanical properties is the ratio of the bulk density of the foam to the density of the cell wall material.Citation[15] Furthermore, Attenburrow et al.Citation[15] indicated the relationship between the mechanical properties of porous cereal based foodstuffs and microstructure may be understood in terms of theoretical treatments that have been developed by Gibson et al.Citation[16] for a general class of cellular/porous materials. Work has been performed on extruded foods in order to characterize their mechanical properties.Citation[12], Citation17-19 It has been shown that the mechanical properties of extruded food products are influenced by moisture content, density, and porosity.Citation[11]
It follows that the justification of this research was motivated by the realization that the DMTA measures the glass transition temperature range via changes in mechanical properties. However, these mechanical properties are affected by porosity. Therefore the specific objective of this research was to examine the effect of porosity on the measurement of the glass transition temperatures.
MATERIALS AND METHODS
Sample Preparation-Corn Based Cheese Curl Extrudates
Samples of corn based cheese curl extrudates (i.e., cheezies) were obtained from a local grocery store (West Lafayette, IN) and cut into rectangular test specimens. The average dimensions of the rectangular specimens were 45±2.1 mm, 4±0.22 mm, and 2±0.1 mm for length, width and thickness respectively. The dimensions of the specimens were measured with a digital caliper (Mitsbushi, Japan).
Representative samples of the rectangular test specimens were subjected to one of five different relative humidity (RH) environments to alter the pore size of the test specimens. The salts used were lithium chloride (LiCl), potassium acetate (KAc), sodium chloride (NaCl), potassium chloride (KCl), and potassium sulfate (K2SO4) (Sigma Chemicals, St. Louis, MO) and they produced the following RH environments respectively, 11, 22, 75, 85, and 98% at 25°C. Also, representative samples of the pore modified rectangular test specimens were ground into powder using a mortar and pestle. All of the specimens (rectangular and powder) were dried in a vacuum oven (LabLine Instruments Inc., Melrose Park, IL) for 24 h at 70°C.Citation[20] This was done in order to reduce the samples moisture content. Additionally, all of the vacuum oven dried samples were equilibrated to the same moisture content (10.6%) by subjecting to a LiCl environment (11% RH) at 25°C and testing was performed on all samples after constant moisture content (10.6%) was achieved. The common moisture content of 10.6% was determined by obtaining representative samples of both the powder and the rectangular specimens that had been equilibrated in the common LiCl RH environment and analyzing for moisture content. The samples were collected and dried in a convection oven (Blue M, Blue Island, IL) for 24 h at 120°C.Citation[20] The mass of the samples before and after drying was read on an electronic (±0.01 g) balance (Denver Instruments Co., Denver, CO). The average of three readings was noted and moisture content was obtained on percent wet basis.
All of these steps were done to ensure that all of the pore modified samples had approximately the same moisture content and therefore this parameter was not considered a variable in the experiments. Also representative samples from each initial pore modified rectangular specimen containing 0.45 g of the ground “cheezie” powder were pressed into tablets using a pneumatic press (Carver Inc., Wabash, IN) under 4500 psi of pressure. After compression, the tablets were equilibrated again in the LiCl environment for 24 h at 25°C prior to testing. This was performed to ensure that the moisture content of the tablets did not change due to water absorption/desorption from ambient during the formation of the tablet.
Sample Preparation Polymer
Polyethylene polymer samples with varying degrees of porosity were obtained from the Porex Company (Georgia, AT). These samples came in three classes which are presented in increasing degree of porosity: fine, medium, and coarse with 15–45, 45–90, and 90–125 µm pore size ranges, respectively as specified by the company. It should be explicitly stated here that the samples were identical in composition and varied only in porosity. Samples were cut into rectangular test specimens. The average sample dimensions for the rectangular specimens were 45±1.8 mm, 4.1±0.9 mm, 2.0±0.82 mm for length, width and thickness respectively. Representative samples of the rectangular test specimens were ground into a powder.
Dynamic Mechanical Thermal Analysis (DMTA)
The rectangular “cheezie” test specimens, rectangular porous polyethylene test specimens, and “cheezie” tablets were tested in dynamic thermal mechanical analyser (DMTA) (Polymer Labs Mark I Dynamic Mechanical Spectrophotometer, Shropshire, England). The parameters utilized during testing were a temperature increase of 5°C/min, a temperature range of 5–130°C, a frequency of 1 Hertz and a strain level of 4 µm which caused deformations in the sample that had within the linear viscoelastic range. The test geometry used was single cantilever.
Differential Scanning Calorimetry (DSC)
The ground cheezie and polymer powders were tested with a differential scanning calorimeter operated on standard scanning mode (2920 Modulated DSC, TA Instruments, New Castle, DE). The parameters used for differential scanning calorimetry (DSC) were a temperature increase of 5°C/min and a temperature range of 5–140°C. The samples were analyzed in hermetically sealed aluminum pans. The DSC was calibrated by measuring the melting point of indium (157.44°C). An empty aluminum can was used as the reference holder. The average weight of ground cheezie sample tested was 8.21±0.42 mg and the average weight of synthetic sample analyzed was 5.354±0.31 mg.
Controlled Strain Rheometry
A controlled strain rheometer (Viscotech, Lund, Sweden) was used. The parameters for the controlled strain rheometry tests included a parallel plate geometry, a temperature increase of 5.0°C/min with a temperature range of 5–140°C for the synthetic polymer sample and a temperature range of 5–100°C for the extrudate sample.Citation[21] The amount of synthetic sample used for testing was 0.22 g whereas the amount of extrudate sample was 0.20 g.
Apparent and Substance Density of Biological Extrudates
The apparent density (ρ a ) of the cheezie was determined from the mass (m) and the apparent volume (v a ). The mass of all of the specimens was obtained after being equilibrated in the LiCl RH chamber. It was determined with an electronic balance, ±0.01 g, (Denver Instruments Co., Denver, CO). The mean apparent volume of the rectangular porous specimens was obtained by measuring the length, width, and thickness of the specimens with a digital caliper (Mitsbushi, Japan). The mean apparent volume was the average of 3 determinations. The apparent density was obtained from the following equation:
Extrudate samples were crushed into powder and the substance volume (v s ) of the extrudate powder was determined by gas displacement, using a helium stereo-pycnometer (SPY-2, Quatrochrome Corp., Syosset, NY). The gas pycnometer was operated at an absolute pressure of 33.3 psi. The substance volume values reported are the average of 3 determinations. The substance density was obtained from the following equation:
The apparent porosity (ϵ a ), defined as the fraction of air or void fraction in the sample, was estimated from the equation:
RESULTS AND DISCUSSION
Polymer
The results of Tg range measured with the DMTA, DSC, and the rheometer for the porous polyethylene polymer are given in Table . It should be stated that a synthetic polymer was chosen in order to reduce the effects of biological variability and as a control for testing the effect of porosity of measurement of the glass transition since it has been noted in the literature that biological materials are highly variable and may yield high standard deviations from experimental measurements.Citation22-23 It is acknowledged that the glass transition of a heterogeneous material (as the extruded biological material used in this research) can be expected to be broad and difficult to measure.Citation[24] In essence, it may be difficult to obtain meaningful glass transition measurements from multi-component materials. Additionally, physico-chemical changes may occur due to the method used for altering the porosity of the extruded biological material. Therefore, the use of a synthetic polymer serves as a good control that enables to eliminate most of the problems created by the sample preparation of biological materials.
Table 1. Glass Transition Measurements of Synthetic Polyethylene Polymer in Porous Form by DMTA and Powder Form by DSC and Controlled Strain Rheometer (n=3)
An attempt was made to press the polymer samples that had been ground to powder into tablets. This would have allowed for polymer specimens with pores (porous rectangular specimens) and polymer specimens without pores (tablets) to be tested with the same test (i.e., DMTA). The polymer tablets did not maintain their integrity upon sample loading in the DMTA. Therefore the only non-porous polymer specimens that could be tested were in powder form and by using DSC and a controlled strain rheometer.
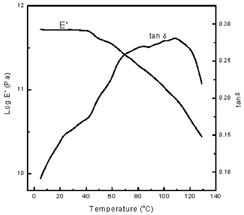
There is a significant difference in Tg temperature range measured by the DMTA. The largest average Tg value was 104.9°C for the coarse synthetic polymer sample. The fine synthetic polymer sample had the smallest average Tg value reported at 83.3°C. Statistical analysis (ANOVA) was performed on the data and it determined that the values were significantly different at p=0.05. The Tg range values (Tp) reported were taken as the tan delta peak from the experimental DMTA graphs. Kalichevsky et al.Citation[3] noted that in synthetic polymers the tan δ peak is commonly used as a convenient definition of the glass transition. Figure shows a representative DMTA plot containing both E′ and tan delta vs. temperature for the synthetic polymer.
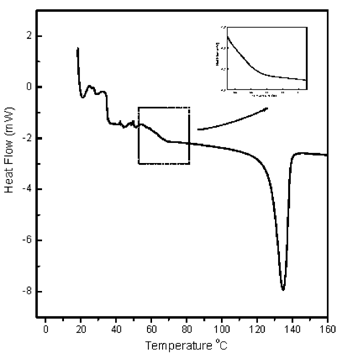
The Tg range values obtained by DSC were taken from the observed midpoint of the endothermic heat capacity change (T′m) which is in accord with the method used by Kalichevsky et al.Citation[3] Figure shows a representative DSC scan. The DSC results were subjected to statistical analysis (ANOVA) and it was determined that there is no significant difference (p=0.05) in Tg measured by DSC when the polymer sample is tested in powder form and the effect of porosity is eliminated.
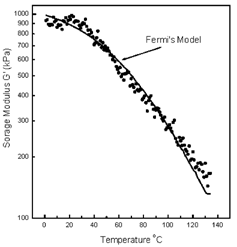
Tg range values obtained by the controlled strain rheometer (T″m) were determined from the midpoint of the change in slope of the G′ values plotted against temperature. The method was the same as that outlined by Sterling et al.Citation[21] Again, statistical analysis (ANOVA) of the powder form of the synthetic polymer showed no significant differences (p=0.05) in Tg as measured by controlled strain rheometry. Figure shows a representative plot containing values of storage modulus (G′) as a function of temperature. However, it must be acknowledged that the fitting of the G′ vs. temperature curve with the Fermi's model as suggested by PelegCitation[25] was included in the figure. PelegCitation[25] indicated that the change in mechanical properties in the Tg range of many biological and some synthetic materials can be described by the following equation:
The following Tc′ values were obtained by fitting the Fermi's model to the data for the synthetic polymers: coarse polymer, 64.7°C; medium polymer, 52.4°C; and fine polymer, 78.3°C.
The Tc′ for the coarse and medium polymer are similar to those obtained from the midpoint method of Sterling et al.Citation[21] but the fine polymer Tc′ values are nearly 20°C higher than the values obtained from the midpoint methodCitation[21] which are given in Table . The reason for these differences may be attributed to the shape of the G′ vs. temperature plots. PelegCitation[26] indicated that Tc′ specifies the inflection point of the curve G′ versus temperature, which may not coincide with the glass transition of the material. This explains the difference in values obtained with the method of Sterling et al.Citation[21] and the inflection point as determined by the fitting model suggested by PelegCitation[25] for the fine polymer. This further demonstrates the importance of not only acknowledging the method of sample preparation, the test method, and test conditions, but also the method of data analysis when discussing the Tg range of materials.
Extrudates
Table shows the apparent porosity and apparent density measurements of the porous extruded specimens after they were subjected to various relative humidity (RH) environments in order to alter their porosity. It was thought that higher RH environments would be a factor that may cause the sample to collapse, allowing the pores present to shrink and thereby decrease the porosity of the specimens. Research performed by Krokida et al.Citation[27] and Fan et al.Citation[28] support this idea, their works have shown that collapse and shrinkage of freeze-dried food material and extruded melts are influenced by the glass transition temperature. Krokida et al.Citation[27] showed that when freeze-dried food materials were subjected to a temperature above their glass transition temperature, their structure was observed to collapse, their bulk density increased, and porosity decreased. Fan et al.Citation[28] found that shrinkage and collapse of extruded melts ceased when the processing temperature was below Tg+30°C. For completeness it must be stated that the glass transition is not the only factor governing collapse and shrinkage and a full discussion of this phenomena in foods during drying is given by Rahman.Citation[29] Nevertheless it is known from literature that moisture content affects the glass transition temperature of biomaterials since moisture acts as a plasticizer that reduces glass transition temperature and encourages structural collapse and thereby reduces porosity.Citation[27], Citation[30] Thus, it was hypothesized that samples held over the highest RH environments should have shown the highest apparent density and the lowest porosity. This is what is shown in Table . However, it is noted that there is only a slight difference in the porosity measurements between the intermediate RH environments, notably in the 22–75% RH range. Nevertheless, there are significant differences, as determined by ANOVA (p=0.05) in porosity measurements between the samples conditioned in LiCl and KCl; LiCl and K2SO4; KAc and KCl; and KAc and K2SO4 environments. This fact will be further discussed in relation to Tg measurements of the extrudates given in Table .
Table 2. Porosity and Apparent Density Measurements of Porous Extruded Samples (n=3)
Table 3. Glass Transition Measurements of the Extruded Samples in Porous Form and Tablet Form by DMTA and Powder Form by DSC and Controlled Strain Rheometer (n=3)
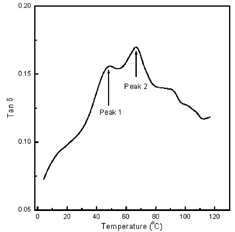
In DMTA measurements, two peaks were observed when porous extrudates were tested. However only for the second peak or main transition peak there appeared to be a correlation between porosity and Tg. Before discussing the presence of the two peaks, the relationship between porosity and Tg of the second peak will be discussed. Table shows the value of the two peaks observed in the tan delta data obtained for the porous extrudates. Figure shows a representative DMTA plot for the extrudate samples illustrating the two peaks. For the first peak or low temperature transition peak, there appeared to be no correlation between porosity and the temperature at which the peak occurs. However, the temperature associated with the second peak or the main transition peak appeared to change with an increase in the RH of the environment and a corresponding decrease in porosity. This is due to the fact that for the samples showing a statistically significant difference in porosity (e.g., LiCl and K2SO4) there is also a statistically significant difference in Tg. Conversely, for the samples in which the difference in porosity was not statistically significantly different there was no statistically significant difference in Tg. These results seem to indicate that there is a correlation between porosity and Tg measurements.
As previously mentioned two peaks were observed in the DMTA data generated for the porous extrudates. Work performed by Kalichevsky et al.Citation[3] also observed two peaks in their study using DMTA to examine the Tg of amorphous amylopectin. The first peak they observed was near 50°C which is in nearly the same temperature as the first peak temperatures obtained in this study. Kalichevsky et al.Citation[3] indicated the origin of the first peak or low temperature transitions was uncertain however, they hypothesized that its presence may be due to short range chain motions or a lipid melting transition. It should be stated that the ratio of the height of the first tan δ peak to the second tan δ peak obtained in these experiments was greater than the ratio of the first tan δ peak to the second tan δ peak obtained by Kalichevsky et al.Citation[3] This fact may be explained by indicating that the tan δ peak relates quantitatively to the volume fraction of the relaxing phase and therefore if the first peak was due to lipid melting, the extrudates in this experiment simply had more lipid present than the amorphous amylopectin in the experiment of Kalichevsky et al.Citation[3] This may be a plausible explanation since Kalichevsky et al.Citation[3] indicated that amylopectin contains relatively little lipid and vegetable oil was listed as a major ingredient of the extrudate utilized in this experiment. Interestingly, Kalichevsky et al.Citation[3] also stated that low temperature transitions were detectable by DSC experiments. However, it was noted that the low temperature transitions measured with DSC were not dependent on moisture content while the higher temperature transitions were. This helps to corroborate the idea that the low temperature transitions may be associated with the presence of lipid.
As mentioned, extrudates that had porosity modified were ground down and subjected to either testing with DSC in powder form, testing with controlled strain rheometry in powder form, or were pressed into tablets and tested with DMTA. The aim of all of these tests was to measure the Tg of the specimens without the presence/effect of porosity. It was deemed beneficial to test the specimens that had been pressed into tablets with DMTA because this allowed for the effect of porosity to be investigated using the same test equipment as the porous specimens. It can be seen in Table that the results for the powder tested with DSC, powder tested with controlled strain rheometry, and the tablets tested with DMTA show that there is no significant difference, as determined by ANOVA (p=0.05), between samples when the effect of porosity was eliminated. It is noted that there is a difference in the values obtained for the same specimens between test methods however this was somewhat expected on the basis of the findings of Kalichevsky et al.Citation[3] However there was only one transition associated with the tablets tested with DMTA, and powder tested by DSC and controlled strain rheometry. At first thought this appears to be inconsistent with the results showing two peaks for the porous extrudate samples measured with DMTA and the work by Kalichevsky et al.Citation[3] These findings may be due to the fact that the samples were ground with a mortar and pestle made of porcelain and it is possible that a majority of the oil/lipid in the native porous sample was released upon grinding or crushing and absorbed by the porcelain.
The Tg range values obtained by the controlled strain rheometer for the extrudate samples in powder form were determined from the midpoint of the G′ versus temperature curves (Tm″) which was the same procedure outlined by Sterling et al.Citation[21] It was also the same method used for the synthetic samples in this study. Table shows the results obtained by the controlled strain rheometer for the extrudate powder samples. Statistical analysis (ANOVA) of the extrudate powder samples showed no significant differences (p=0.05) in Tgbetween samples conditioned in the different RH environments. The rheometer data obtained for the extrudate powder samples was analyzed with the Fermi's model. The inflection points T′c obtained by fitting the Fermi's model to the data for the extrudate powder samples were: 78.3°C LiCl, 50.3°C; KAc, 54.1°C; NaCl, 53.9°C; KCl, 53.7°C; and K2SO4, 57.9°C.
The values obtained by the Fermi's model for the extrudate powder samples are all nearly 20°C lower than the Tg range values (given in Table ) obtained for the extrudate powder samples by using the midpoint method of Sterling et al.Citation[21] Again the reason for these results may be due to the shape of the G′ versus temperature plots.
For completeness, the final moisture content of the samples that were to be tested was examined to ensure that moisture content was within experimental error as it has been stated that moisture content affects the Tg.Citation[2], Citation[30] As can be seen in Table none of the specimens tested had statistically significantly different moisture contents. Also, the moisture loss from the sample during testing was examined. It was observed that there was a slight loss of moisture by the samples with different porosity during testing with DMTA, however there was no significant difference, as determined with ANOVA (p=0.05), in the amount of moisture loss among the sample of different porosity levels. Based on these results, it can be stated that the different Tg values measured was not due to differences in moisture contents or differences in moisture losses among the samples. For the synthetic polyethylene samples, the final moisture content and moisture loss from the samples during testing was not investigated by virtue of the nature of the specimens.
Table 4. Average Final Moisture Contents and Average Moisture Loss by the Extrudate Samples During Testing (n=3)
Hypotheses
Results of this research show that it is most likely that the effect of porosity on the measurement of the Tg is an artifact of the DMTA test. One plausible explanation for samples with greater porosity levels exhibiting higher Tg measurements may be due to the fact that porosity is inversely related to thermal conductivity. Marousis and SaravacosCitation[31] stated that thermal conductivity increases linearly with bulk density. Since thermal conductivity decreases with decreasing bulk density, samples with higher porosity will take a longer time to reach the desired temperature resulting in higher detected glass transition temperatures. Results of Tanaka and Tanaka,Citation[32] who measured the Tg temperature range of epoxy resin composites with silica microballoons, support the above explanation. Tanaka and TanakaCitation[32] indicated that the Tg of the composites was high when the resin and the filler were mixed prior to the addition of curing agent and thereby had a large amount of gas incorporated into the composite. However, the Tg of the composites was lower when the resin and the curing agent were mixed prior to the addition of the filler and this allowed for the amount of gas incorporated into the composite to be lessened. It was also noted that the Tg decreased with further degassing of the composites. A way to investigate the extent of the effect of decreased bulk density (i.e., less gas/air present) on thermal conductivity would be to run the DMTA test at a very slow heating rate to allow the temperature of the sample to reach its equilibrium value. However, a problem with this solution would be that a very slow heating rate would allow for moisture loss from the sample and this would incorporate a different artifact in glass transition measurements.
Also, the DMTA applies a constant oscillatory strain to the sample being tested whereas the temperature increases.Citation[4] This constant oscillatory strain supplies kinetic energy to the test material and therefore frictional forces arise. Presumably, more frictional forces arise in the sample with lower porosity due to the closer proximity of the solid structure on a macroscopic level. The increased frictional forces present in the less porous material may serve as an additional energy source to the thermal energy being supplied to the test material during the DMTA test. Thus, there is more energy associated with the less porous samples than the more porous sample at the same temperature. This may be an artifact and contribute to the less porous material exhibiting a lower Tg temperature range. This explanation may be strengthened by the fact that WeipertCitation[33] acknowledged that the presence of friction in DMTA tests should be investigated and if necessary should be quantified.
Alternatively, the effect of pore size may be a real result. Phase transitions of organic materials confined in controlled pore glasses have been studied and a relationship between pore size and phase transitions has been identified. Mansare et al.Citation[34] studied the phase transitions of 4-methoxy-benzilidene-4′-n-butylaniline in controlled porous solids and found that as the pore size decreased the primary phase transition of melting temperature and the secondary phase transition of Tg were both observed to decrease. Jackson and McKennaCitation[35] noted that crystalline melting, superfluid and solid-solid phase transitions shift to lower temperatures for materials confined to small pores. It has been shown by Jackson and McKennaCitation[36] that for ortho-terphenyl and benzyl alcohol glasses formed in pores, there was a reduction in the primary transition of melting temperature as pore size decreased. They ascribed the decrease in Tg with a decrease in pores size to the large surface to volume ratio of the material adsorbed in the capillary. Although melting behaviour is a primary phase transition and Tg is a secondary phase transition phenomenon a similar reasoning of preferential adsorption of water in smaller pores, which can be likened to capillaries, may account for the lowered Tg observed in the samples with smaller pores. Furthermore work done by Jackson and McKennaCitation[36] on ortho-terphenyl and benzyl alcohol glasses formed in pores indicated that there was a reduction in Tg as pore size decreased. Although, the work cited was concerned with measuring the phase transitions of a material confined in pores, they show that geometry can affect the measurement of the phase transitions and there is an effect of pore size on apparent material properties.
CONCLUSIONS
In all, it has been shown that the quality of porous foods is dictated by texture and the measurement of Tg has been noted in the literature to be a viable method of obtaining an index of texture and therefore an index of quality. DMTA is commonly used in food science research and is a preferred method of obtaining Tg values. This study shows that for both synthetic porous specimens and biological porous specimens, the level of porosity affects the measurement of the Tg. Most likely the effect of porosity on measuring the Tg is an artifact of the DMTA test. Therefore, the effect of porosity should be acknowledged when discussing Tg measurements and future work should be performed to elucidate the actual cause of the observed effect of porosity.
Acknowledgments
Notes
Purdue Research Paper #16590
REFERENCES
- Brent , J.L. , Mulvaney , S.J. , Cohen , C. and Bartsch , J.A. 1997 . Thermomechanical Glass Transition of Extruded Cereal Melts . J. Cereal Sci. , 26 : 301 – 312 .
- Kawas , M.L. and Moreira , R.G. 2001 . Characterization of Product Quality Attributes of Tortilla Chips During the Frying Process . J. Food Engin. , 47 : 97 – 107 .
- Kalichevsky , M.T. , Jaroszkiewski , S. , Ablett , S. , Blanshard , J.M.V. and Lilliford , P.J. 1992 . The Glass Transition of Amylopectin Measured by DSC, DMTA and NMR . Carbohydr. Polym. , 9 : 77 – 88 .
- Brent , J.L. , Mulvaney , S.J. , Cohen , C. and Bartsch , J.A. 1997 . Viscoelastic Properties of Extruded Cereal Melts . J. Cereal Sci. , 26 : 313 – 328 .
- Nicholls , R.J. , Appelqvist , I.A.M. , Davies , A.P. , Ingman , S.J. and Lilliford , P.J. 1995 . Glass Transitions and the Fracture Behaviour of Gluten and Starches within the Glassy State . J. Cereal Sci. , 21 : 25 – 36 .
- Clayton , J.T. and Huang , C.T. 1984 . “ Porosity of Extruded Foods ” . In Engineering and Food Edited by: McKenna , B.M. Vol. 2 , 611 – 620 . London : Elsevier Applied Science Publishing .
- Stanley , D.W. and deMan , J.M. 1978 . Structural and Mechanical Properties of Textured Proteins . J. Texture Studies , 9 : 59 – 76 .
- Bourne , M.C. 1982 . Food Texture and Viscosity New York : Academic Press .
- Stanley , D.W. 1986 . Chemical and Structural Determinants of Texture in Fabricated Foods . Food Technol. , 40 ( 3 ) : 65 – 68 .
- Hayter , A.L. and Smith , A.C. 1988 . The Mechanical Properties of Extruded Food Foams . J. Mater. Sci. , 23 : 736 – 741 .
- Guraya , H.S. and Toledo , R.T. 1996 . Microstructural Characteristics and Compression Resistance as Indices of Sensory Texture in a Crunchy Snack Product . J. Texture Studies , 27 : 687 – 701 .
- Kirby , A.R. and Smith , A.C. 1988 . Impact Studies on Extruded Food Foams . J. Mater. Sci. , 23 : 2251 – 2254 .
- Moore , D. , Sanei , A. , Van Hecke , E. and Bouvier , J.M. 1990 . Effect of Physical/Structural Properties of Extrudates . J. Food Sci. , 55 ( 5 ) : 1383 – 1387 . 1402
- Gogoi , B.K. , Alavai , S.H. and Rizvi , S.S.H. 2000 . Mechanical Properties of Protein Stabilized Starch-based Supercritical Fluid Extrudates . Int. J. Food Properties , 3 ( 1 ) : 37 – 58 .
- Attenburrow , G.E. , Goodbrand , R.M. , Taylor , L.J. and Lilliford , P.J. 1989 . Structure, Mechanics and Texture of a Food Sponge . J. Cereal Science , 9 : 61 – 70 .
- Gibson , L.J. , Ashby , M.F. and Schajer , G.S. 1982 . The Mechanics of Two-dimensional Cellular Materials . P. Roy. Soc. Lond. A. Mat. , 382 ( 1782 ) : 25 – 42 .
- Hayter , A.L. , Smith , A.C. and Richmond , J. 1986 . The Physical Properties of Extruded Food Foams . J. Mater. Sci. , 21 : 3729 – 3736 .
- Barrett , A.H. and Peleg , M. 1992 . Extrudate Cell Structure-texture Relationships . J. Food Sci. , 57 ( 5 ) : 1253 – 1257 .
- Duizer , L.M. , Campanella , O.H. and Barnes , G.R.G. 1998 . Sensory, Instrumental, and Acoustic Characteristics of Extruded Snack Food Products . J. Texture Studies , 29 : 397 – 411 .
- Bradley , R.L. Moisture and Total Solids Analysis. 1998 . Food Analysis Edited by: Nielsen , S.S. 122 – 127 . Gaithersburg, MD : Aspen Publishers Inc. .
- Sterling , M. , Okos , M.R. and Campanella , O.H. Determination of Transition Temperature of Semolina Using Plate-plate Viscometer . Proceedings of the AIChE Annual Meeting . pp. 398 – 402 .
- Gibson , L.J. , Easterling , K.E. and Ashby , M.F. 1981 . The Structure and Mechanics of Cork . Proc. R. Soc. Lond. , A377 : 99 – 117 .
- Vincent , J.F.V. 1982 . The Mechanical Design of Grass . J. Mater. Sci. , 17 : 856 – 860 .
- LeMeste , M. , Huang , V.T. , Panama , J. , Anderson , G. and Lentz , R. 1992 . Glass Transition of Bread . Cereal Foods World , 37 ( 3 ) : 264 – 267 .
- Peleg , M. 1995 . A Note on the tan δ (T) peak as a Glass Transition Indicator in Biosolids . Rheol. Acta , 34 : 215 – 220 .
- Peleg , M. 1994 . Mathematical Characterization and Graphical Presentation of the Stiffness-temperature-moisture Relationship of Gliadin . Biotechnol. Prog. , 10 : 652 – 654 .
- Krokida , M.K. , Karanthos , V.T. and Maroulis , Z.B. 1998 . Effect of Freeze-drying Conditions on Shrinkage and Porosity of Dehydrated Agricultural Products . J. Food Engin. , 35 : 369 – 380 .
- Fan , J. , Mitchell , J.R. and Blanshard , J.M.V. 1996 . The Effect of Sugars on the Extrusion of Maize Grits: I The Role of the Glass Transition in Determining Product Density and Shape . Int. J. Food Sci. Technol. , 31 : 55 – 65 .
- Rahman , M.S. 2001 . Toward Prediction of Porosity in Foods During Drying: A Brief Review . Drying Technol. , 19 ( 1 ) : 1 – 13 .
- Hoseney , R.C. 1992 . “ Glass Transition and its Role in Cereals ” . In Principles of Cereal Science and Technology , 2 307 – 320 . St. Paul, MN : American Association of Cereal Chemists .
- Marousis , S.N. and Saravacos , G.D. 1990 . Density and Porosity in Drying Starch Materials . J. Food Sci. , 55 ( 5 ) : 1367 – 1370 . 1372
- Tanaka , Y. and Tanaka , K. 1991 . Effect of Preparation and Filler Surface on Dynamic Mechanical Properties of Epoxy Resin Composites Filled with Silica Microballoons . Kobunshi Ronbunshu , 48 ( 6 ) : 373 – 379 .
- Weipert , D. 1997 . Determining Rheological Properties of Cereal Products using Dymanic Mechanical Analysis in Compression Mode . Cereal Foods World , 42 ( 3 ) : 132 – 137 .
- Mansare , T. , Gors , C. and More , M. 1998 . Phase Transitions of MBBA Confined in Porous Solids . J. Thermal Analysis , 51 : 823 – 830 .
- Jackson , C.L and McKenna , G.B. 1991 . The Melting Behavior of Organic Materials Confined in Porous Solids . J. Chem. Phys. , 93 ( 12 ) : 9002 – 9011 .
- Jackson , C.L and McKenna , G.B. 1991 . The Glass Transition of Organic Liquids Confined to Small Pores . J. Non-crystalline Solids. , : 131 – 133 . 221–224
- Purdue Research Paper #16590