ABSTRACT
A rapid method for quantitative determination of fat and moisture in a variety of mayonnaise products (regular, reduced- and low-fat) was developed using Fourier Transform Infrared (FTIR) spectroscopy. Both the fat and moisture contents could be effectively solvated out of the soluble solids using 1-propanol without sonication. A load cell assembly with a 100 mm Teflon spacer gave sufficient separation under 2 cm−1 resolution using 100 scans. Two distinct peaks were obtained on the ratioed sample spectra of regular mayonnaise: 1748 cm−1 and 1650 cm−1 for C˭O stretching of oil and H-O-H bending of water, respectively. Data was then normalized using the Window-based software that acquired spectra information seamlessly from the instrument. The experimental measurements of the fat and moisture contents were in excellent agreement with those using the modified Mojonnier and the dehydration method, respectively. The method was accurate over all varieties of mayonnaise studied (p < 0.05).
INTRODUCTION
In order to meet the demands of health-conscious consumers, commercial mayonnaise products are now available in different varieties, including regular, reduced- and low-fat formula. Classified as a permanent emulsion, mayonnaise is created when a shear force is applied to disperse the oil phase into tiny droplets that remain throughout the water phase. The bipolar affinity of emulsifiers (e.g., lecithin from egg yolk) creates a desirable stability at the interface by forming an aligned monolayer around the oil droplets.Citation[1] Inherent from its original formulation, the quality of the reduced- and low-fat products is affected by the emulsion stability, which is highly dependent on appropriate formulation of fat and moisture contents under specific processing (shearing) conditions.Citation[2] Therefore, development of a reliable method for rapid, quantitative determination of fat and moisture in mayonnaise is crucial to the quality control and, if necessary, manufacturing adjustments.
On the other hand, the very stable emulsion created by the combination of shearing with emulsification results in significant difficulties in analyzing the water and oil contents in mayonnaise.Citation[3] Conventionally, lengthy sample preparations are required to separate the emulsion, still giving results that are not as accurate as they could be, as seen in the Mojonnier method (AOAC 989.05)Citation[4] and the Soxhlet method (AOAC 963.15) for fat determination, or the vacuum-oven method (AOAC 926.12) for moisture analysis. Other methods involving imidazole, a chemical destabilizer capable of separating emulsified phases, have been found hazardous to the environment and should be eliminated in a food processing facility.Citation[5] Practically, calcium-induced destabilization is commonly used in the food industry prior to solvent extraction.Citation[6] Nevertheless, the use of extraction for fat analysis inevitably receives considerations such as structure or chemical composition alteration, solvent toxicity, flammability and cost, simplicity and speed of the procedure, as well as reproducibility.Citation[7] Although a new ultracentrifuge separation method involving freezing and a precentrifugation treatment has been reported,Citation[8] a portion of emulsion phase (5% w/w) remained inseparable.
Recent development of near-infrared (NIR) spectroscopy offers an alternative to the aforementioned techniques and has triggered extensive research efforts using similar technologies.Citation9-10 It has been found that functional groups such as isolated or conjugated double bonds, hydroxyl, epoxy, or ester functions in fatty acids, FAMEs, or triacylglycerols give rise to unique absorption bands in the mid-infrared (MIR) spectral region (between 4000 and 600 cm−1) in comparison with NIR spectroscopy.Citation[11] Fourier Transform Infrared (FTIR), a technique taking advantage of these unique MIR absorption bands, has been recognized to provide excellent rapidity and reliability for structure conformation.Citation[12] By using FTIR, the sensitivity increases, the size of the sample required for analysis has decreased, and the number of new applications has exploded.Citation[13] van de Voort and coworkers developed FTIR spectroscopic methods for analysis of edible fats and oils, including iodine value and saponification number, free fatty acids, peroxide value, and cis and trans content.Citation14-16 However, published data are limited to high-fat mayonnaise using a DOS-based program, leaving considerable discrepancy for its application to reduced- and low-fat formulas, besides lack of user-friendliness provided by many Windows-based programs. The time required for sample preparation and analysis was reported to be 5–7 min per sample, excluding 5 min per sample sonication for extracting fat and moisture.
The objectives of the work were (1) to establish FTIR for the determination of fat and moisture in various mayonnaise products; and (2) to verify the analytical performance of FTIR on fat and moisture, respectively, using the modified Mojonnier method and the dehydration method.
MATERIALS AND METHODS
Sample Preparation
Commercial mayonnaise products, including regular, reduced- and low-fat varieties, were purchased from local supermarkets and stored at 4°C prior to sample preparation. Mayonnaise samples (approximately 5 g each) were transferred to respective screw cap test tubes containing 9 mL of anhydrous 1-propanol (Sigma-Aldrich, St. Louis, MO, USA). The mixture was vortexed for 5 min prior to centrifugation at 800×g (IEC Centra-4B Centrifuge) for 1 min at room temperature to separate the solids. The supernatant was collected for FTIR analysis. All samples were prepared in duplicate.
FTIR Operation and Calibration
A Perkin Elmer 1720X FTIR Spectrometer (Perkin Elmer, Norwalk, CT, USA) with Spectrum for Windows CD version 2.0 software was used. The FTIR was equipped with a CaCl2 demountable transmission-flow cell and a Teflon spacer was used for this study. A Whatman Purge Gas Generator Model 75-62 (Whatman Inc., Haverhill, MA, USA) was connected to the cell to improve baseline stability. The CaCl2 cell was glove-handled to avoid scratches while assembled and rinsed with alcohol or chloroform and allowed to dry by evaporation. The scanning sequence was initialized after the FTIR was turned on and the system booted up. To scan the background, “Spectrum for Windows” was first chosen from the computer desktop. Once the program was loaded with a “new” file, “instrument” was chosen from the tool bar, then “background.” The scanning configurations were set as follows: (1) 100 scans of the background; (2) “Range” from 3000 cm−1 to 1500 cm−1; and (3) “Resolution” 2 cm−1. Once the file was named, click “ok” to initiate the scanning process. As soon as the scans were completed the cell assembly was removed and flushed with 1-propanol several times.
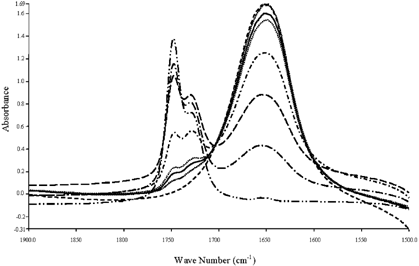
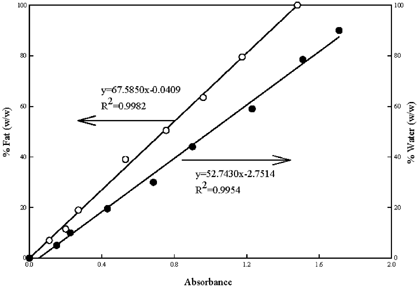
Calibration curves correlating % water and fat with respective absorbance were prepared using standard solutions containing mixtures of known amounts of water and vegetable oil (Crisco) in place of mayonnaise samples following the same procedures described in sample preparation. The spectra of various standard solutions are shown in Fig. . Linear relationships between % fat and % water and the corresponding absorbance at 1748 and −1650 cm−1, respectively, were obtained (both R2>0.99) (Fig. ). The calibration curve for oil content has been corrected to account for the displacement of 1-propanol by the insoluble solids in mayonnaise and the efficiency of extraction per se, as discussed and suggested by van der Voort et al.Citation[16]
Sample Analysis and Data Processing
The cell assembly was flushed with the sample and wipe-cleaned before each scan to ensure the best representation of the sample. Spectra were then compared against calibration standards and the solvent background spectrum using the “normalization” function in the software to eliminate artifacts and enhance the signal-to-noise ratio (SNR) of the peak in question. To obtain quantitative information, the peak heights of the normalized absorption bands at the 1748 cm−1 (C˭O stretching of oil) and 1650 cm−1 (H-O-H bending) frequencies were correlated to the amounts of fat and moisture, respectively, using the calibration curve shown in Fig. .
Modified Mojonnier Method and Dehydration Method
A modified Mojonnier methodCitation[4] using ether extraction was employed to verify the fat content in mayonnaise with the following modifications. For each sample, 2.5 g of mayonnaise was weighed into test tubes and then diluted with 10 mL distilled water at room temperature. Five mL of 95% ethyl alcohol was added after the addition of phenolphthalein. Ten mL each of ethyl ether and petroleum ether were used for the first extraction. Five mL each of ethyl ether and petroleum ether were used for the second extraction. The moisture content of mayonnaise was verified by measuring the weight loss of samples after dried in a commercial food dehydrator at 60°C for 48 hrs (AOAC 925.09).Citation[4]
RESULTS AND DISCUSSION
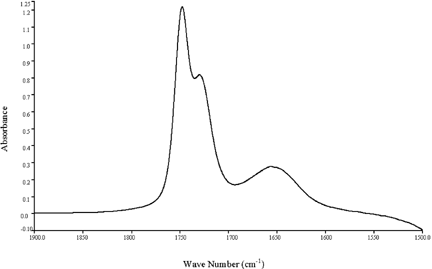
Figure shows a typical spectrum of regular mayonnaise samples. With a 100-µm Teflon spacer and 100 scans under 2 cm−1 resolution, two distinct peaks were obtained on the sample spectra of regular mayonnaise: 1748 cm−1 and 1650 cm−1 for C˭O stretching of oil and H-O-H bending, respectively. It is obvious that the peak representing the oil content is dominant in a regular mayonnaise, in agreement with most mayonnaise formula that contains vegetable oil (75–80%), vinegar (9–12%), and a pre-mix composed of water, egg yolk, starch, spices, and stabilizers (10–11%).Citation[16]
Figure 3. A typical spectrum of a regular mayonnaise sample showing a dominant oil peak at 1748 cm−1.
Interestingly, a secondary peak adjacent to the C˭O peak was observed at around 1730 cm−1, similar to that reported by van der Voort and co-workers.Citation[16] Although no specific functional group has been assigned to this particular peak, in order to give rise to linear calibration plots, it was proposed by their group to develop secondary coefficients in the calibration equations of oil and water standards to account for the interaction of fat and water, as indicated by the overlap of their absorbances. An excellent discussion on taking into account the displacement effects caused by the insoluble solids and the relative extraction efficiency of the solvent system can be found in their work.Citation[16] However, the authors admitted that, the biases for both fat and water are substantially higher than can be accounted for by displacement effects only, even if one assumes high extraction efficiency. As also seen in Figs. and , the height of the overlapped peak at 1730 cm−1 was signified at both reduced- and low-fat formula due to the overlapping effect due to the increase of the water peak in those formula. Therefore, to improve its signal-to-noise ratio (SNR), further processing of the spectrum is required to eliminate possible artifacts.
Figure 4. A typical spectrum of a reduced-fat mayonnaise sample showing a water peak (1650 cm−1) higher than the oil peak (1748 cm−1).
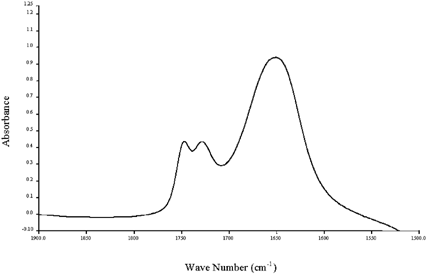
Figure 5. A typical spectrum of a low-fat mayonnaise sample showing a dominant water peak at 1650 cm−1.
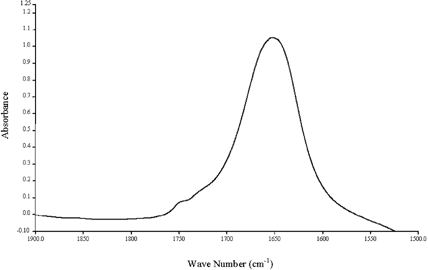
Figure shows an example ‘normalization’ process for obtaining the corrected peak height at 1748 cm−1 of a reduced-fat mayonnaise sample. A corrected peak was obtained by matching the height of the water peak with a superimposed, water-only spectrum at 1650 cm−1. This new peak is more distinct than the one in the original spectrum, resulting in a high SNR. The rationale for such an operation is simple. Since the strength and number of chemical interactions with nearest neighbor molecules varies from place to place within a chemical sample, it is generally recognized that there are many chemical environments within a sample. Therefore, molecules in each of these chemical environments absorb infrared radiation at slightly different wave numbers, contributing to the overall width of a molecule's infrared band. Functional groups, chemical structural fragments within molecules, tend to absorb infrared radiation of the same wavenumber range regardless of the structure of the rest of the molecule. In many cases, a sample with many chemical environments has wide bands, and a sample with few chemical environments has narrow bands. Also keeping in mind is that because the strength and number of hydrogen bonds within a sample of water is variable, it usually causes wide infrared bands, independent of the resolution.
Figure 6. An example ‘normalization’ process for obtaining the corrected peak height at 1748 cm−1 of a reduced-fat mayonnaise sample. (a) Matching the height of the water peak with a superimposed, water-only spectrum at 1650 cm−1 (— the original spectrum; ---- the water-only peak); (b) The corrected peak after subtracting the water-only peak from the original spectrum.
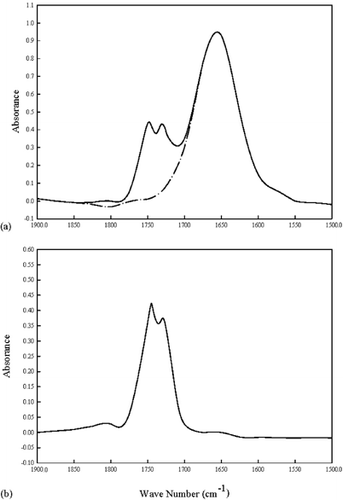
Therefore, subtracting such a peak out of the spectrum will allow us to see discrete peaks of interest, in this case the C˭O peak. Similar operations could be easily performed to obtain discrete H-O-H peaks for all mayonnaise samples by subtracting out of the spectrum a superimposed, oil-only spectrum that matches the height of the corresponding oil peak. This is particularly valuable since the performance of any infrared spectrometer is determined by measuring its SNR, which is calculated by measuring the peak height and dividing it with a baseline value in the spectrum. In addition, FTIR is known for its throughput (or Jacquinot) and multiplex (or Fellgett) advantages, both lead to a higher SNR than in dispersive spectrometers.Citation[13] Further increase in SNR has been identified to be more accurate and more sensitive for a wider range of products than an instrument with a lower SNR.Citation[17]
To fully evaluate the representability of the corrected peaks, each sample was analyzed in triplet using the modified Mojonnior method and the dehydration method for their fat and moisture contents, respectively. As seen in Table , the results obtained from FTIR after the normalization operations were in excellent agreement with the experimental measurements using the chemical methods (p<0.05), indicating a valid approach to quantitatively determine the fat and moisture contents in all varieties of mayonnaise samples studied. In addition, the average time of sample preparation and analysis was only 5 min per sample; significantly reduced the time required using other methods. Therefore, seamless processing of FTIR spectra using the ‘normalization’ function in the Windows-based software provides a powerful tool in obtaining valuable information about fat and moisture in mayonnaise without sophisticate experimental procedures. It is likely that other emulsion products, such as margarine and salad dressing, could be analyzed by this technique.
Table 1. Verification of the FTIR Results with the Modified Mojonnier Method and the Dehydration Method
ACKNOWLEDGMENTS
The authors would like to acknowledge the funding support provided by John Hall and his associates as well as the technical assistance provided by Tanya Zhong.
REFERENCES
- van Nieuwenhuyzen , W. and Szuhaj , B.F. 1998 . Effects of Lecithins and Proteins on the Stability of Emulsions . Fett/Lipid , 100 ( 7 ) : 282 – 291 .
- Curt , C. 1994 . Review – Evaluation of Emulsion Stability – Principle, Applications, Advantages and Drawbacks . Sci. Des Alimenta , 14 ( 6 ) : 699 – 724 .
- Aluko , R.E. and Mine , Y. 1998 . Characterization of Oil-in-water Emulsions Stabilized by Hen's Egg Yolk Granule . Food Hydrocolloid , 12 ( 2 ) : 203 – 210 .
- 1995 . “ AOAC. ” . In Official Methods of Analysis , 16 Washington, DC : Association of Official Analytical Chemists . Methods No. 925.09, 926.12, 963.15, 989.05
- Breider , M.A. , Ulloa , H.M. , Pegg , D.G. and Gough , A.W. 1998 . Nitro-imidazole Radiosensitizer-induced Toxicity in Cynomolgus Monkeys . Toxicol. Pathol. , 26 ( 5 ) : 651 – 656 .
- Agboola , S.O. and Dalgleish , D.G. 1995 . Calcium-induced Destabilization of Oil-in-water Emulsions Stabilized by Caseinate or by β-lactoglobulin . J. Food Sci. , 60 : 399 – 404 .
- Mossoba , M.M. and Firestone , D. 1996 . New Methods for Fat Analysis in Foods . Food Test. Anal. , : 24 – 33 .
- Jacobsen , C. , Meyer , A.S. and Adler-Nissen , J. 1998 . Oxidation Mechanisms in Real Food Emulsions: Method for Separation of Mayonnaise by Ultracentrifugation . J. Food Lipids , 5 ( 2 ) : 87 – 101 .
- Jordan , J.R. 1992 . Near-infrared – Exciting, New Technology . Am. Lab. , 24 ( 18 ) : P20 – Q20 .
- Davies , A.M.C. and Brocklehurst , T.F. 1986 . Near-infrared Reflectance Analysis of Oil Concentration in an Emulsion – a Study of Oil and Water Migrations in a Mayonnaise-based Salad . J. Sci. Food Agr. , 37 ( 3 ) : 310 – 316 .
- Hashimoto , A. and Kameoka , T. 2000 . Mid-infrared Spectroscopic Determination of Sugar Contents in Plant-cell Culture Media Using an ATR Method . Appl. Spectrosc. , 54 ( 7 ) : 1005 – 1011 .
- Kameoka , T. , Okuda , T. , Hashimoto , A. , Noro , A. , Shiinoki , Y. and Ito , K. 1998 . FTIR Analysis of Sugars in Aqueous Solution Using ATR Method . J. Jpn. Soc. Food Sci. Technol. , 45 ( 3 ) : 192 – 198 .
- Smith , B.C. 1996 . Fundamentals of Fourier Transform Infrared Spectroscopy 202 pp New York : CRC Press .
- van de Voort , F. , Memon , K. , Sedman , J. and Ismail , A. 1996 . Determination of Solid Fat Index by Fourier Transform Infrared Spectroscopy . J. Am. Oil Chem. Soc. , 73 ( 4 ) : 411 – 416 .
- van de Voort , F.R. 1992 . Fourier-transform Infrared Spectroscopy Applied to Food Analysis . Food Res. Int. , 25 ( 5 ) : 397 – 403 .
- van de Voort , F.R. , Sedman , J. and Ismail , A.A. 1993 . A Rapid FTIR Quality-control Method for Determining Fat and Moisture in High-fat Products . Food Chem. , 48 ( 2 ) : 213 – 221 .
- Yang , H. and Irudayaraj , J. 2000 . Characterization of Semisolid Fats and Edible Oils by Fourier Transform Infrared Photoacoustic Spectroscopy . J. Am. Oil Chem. Soc. , 77 ( 3 ) : 291 – 295 .