ABSTRACT
A wide variation of the reported experimental data of thermal conductivity of solid food materials makes difficult their utilization in food processing applications. The published values of thermal conductivity of various foods were classified and analyzed statistically to reveal the influence of material moisture content and temperature. Empirical models, relating thermal conductivity to material moisture content and temperature, are fitted to all literature data for each material. The data are screened carefully using residual analysis techniques. A potential model is proposed and parameters are estimated for different foods.
INTRODUCTION
Thermal conductivity depends strongly on moisture, temperature and structure of the material. In porous materials the void fraction and the pore structure and distribution affect thermal conductivity significantly. Generally, in multiphase systems (solids, water and air), the effect of geometric distribution of the phases is taken into account by using structural models Citation[4], Citation[6].
A large number of structural models were summarized and analyzed in detail by Marinos-Kouris and Maroulis Citation[3], and Rahman Citation[5]. According to the most popular consideration, an Arrhenius model is adopted for the effect of temperature, and the effect of moisture is introduced into the Arrhenius parameters. Literature data for thermal conductivity of food materials were selected and presented in a recent review by Krokida et al. Citation[2].
The purpose of this paper is (a) to propose a mathematical model in estimating the thermal conductivity of some food materials as a function of temperature and moisture, and (b) to fit the model simultaneously to the available literature data and to estimate the model parameters for different foods.
MATHEMATICAL MODEL
A material with intermediate moisture is assumed to consist of a uniform mixture of two different components (a) a dried material and (b) a wet material with infinite moisture. The thermal conductivity of the mixture can be estimated using the two phase parallel model:
where λ the effective thermal conductivity (W/mK), λX o the thermal conductivity of the dried material (phase a) (W/mK), λX i the thermal conductivity of the wet material (phase b) (W/mK), X the material moisture content (kg/kg db), and T(°C) the material temperature. The conductivities of both phases depend on temperature by an Arrhenius-type model:
where T r =60°C a reference temperature, R=0.0083143 kJ/mol K the ideal gas constant, and λ o , λ i , E o , E i =adjustable parameters of the proposed model. The reference temperature of 60°C was chosen as a typical temperature of air drying of foods. Thus, the thermal conductivity of a material is characterized and described by four parameters with physical meaning:
-
λ o (W/mK): thermal conductivity at moisture X=0 and temperature T=T r
-
λ i (W/mK): thermal conductivity at moisture X=∞ and temperature T= T r
-
E o (kJ/mol): activation Energy for heat conduction in dry material at X=0
-
E i (kJ/mol): activation Energy for heat conduction in wet material at X=∞
The resulting model is summarized in Table .
Table. 1. Mathematical Model for Calculating Thermal Conductivity as a function of Moisture and Temperature
PROCEDURE OF REGRESSION ANALYSIS
The proposed model is fitted the data using a non linear regression analysis method. It is fitted to all literature data for different material and the estimated model parameters are obtained. Then the residuals are examined and the data with large residuals are removed. This procedure is repeated until an accepted standard deviation between experimental and calculated values is obtained Citation[1].
RESULTS AND DISCUSSION
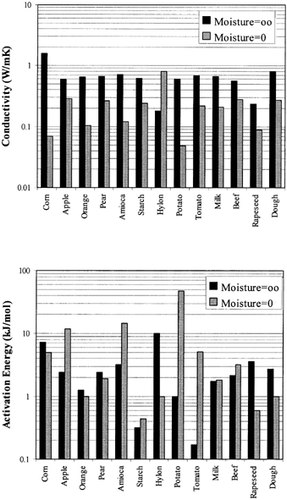
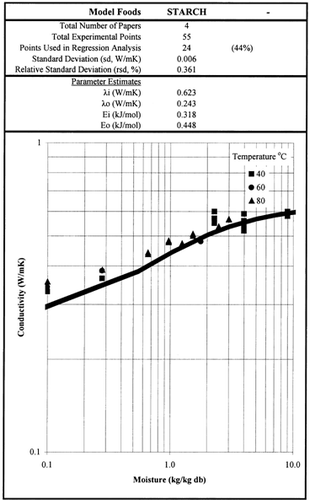
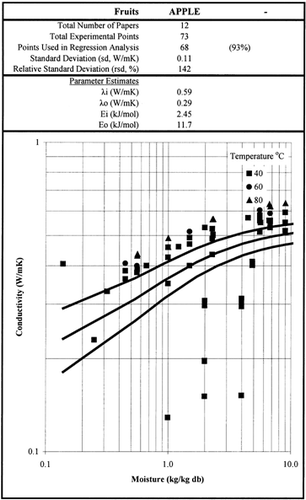
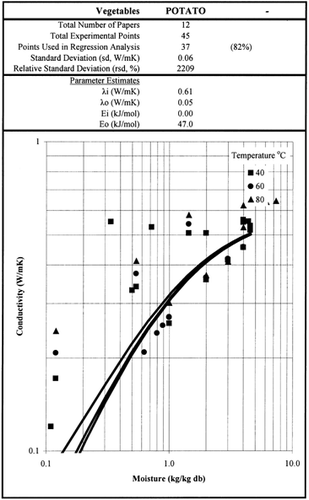
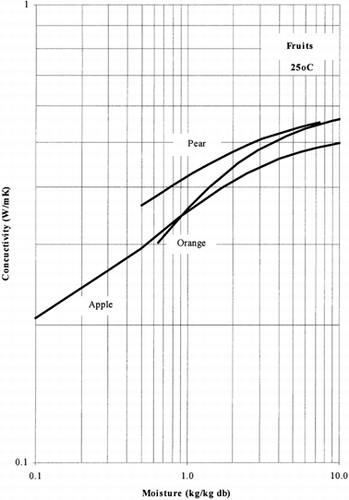
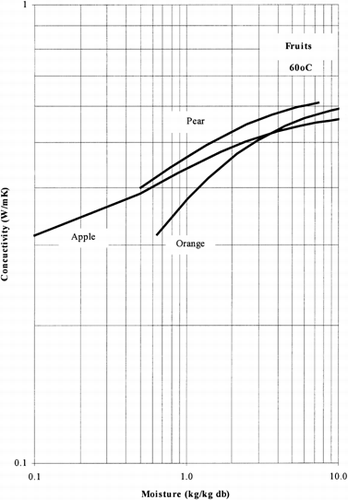
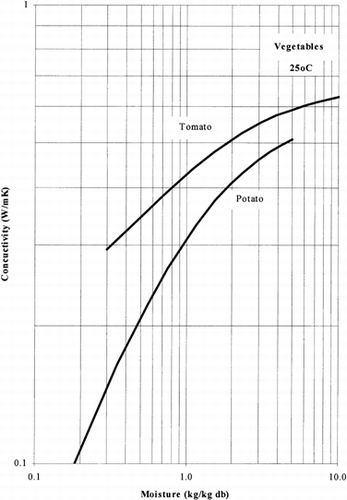
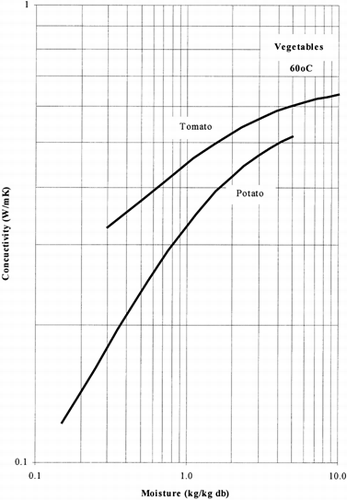
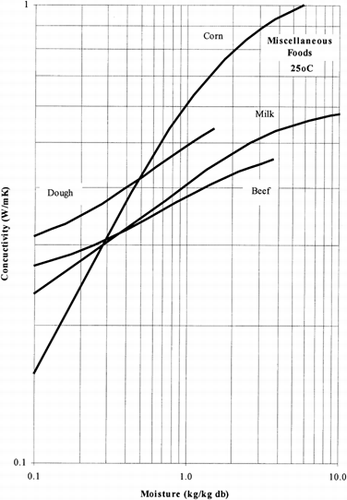
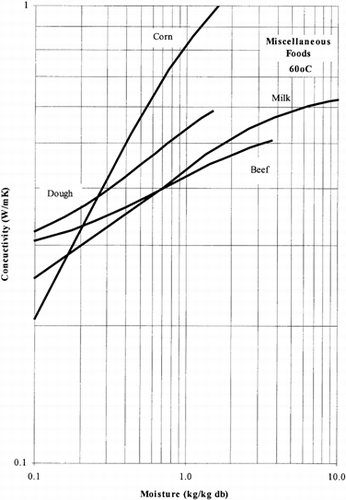
Among the available data only 13 materials have more than 10 data points, which come from more than 3 publications. The procedure is applied to these data and the results of the estimated parameters is presented in Table and in Fig. . It is clear that thermal conductivity is larger in wet materials. The activation energy is, in general, different for wet and dried material but no specific trend was observed. Figs. present the retrieved data from the literature and the calculated model values for several materials. Figs. summarize the predicted model values for various fruits and vegetables at ambient and 60°C temperature. It must be noted that the regression procedure was applied simultaneously to all the data of each material, regardless the data sources. Thus, the results are not based only on the data of one author, and consequently they average out experimental errors to some extent.
Table 2. Parameter Estimates of the Model Presented in Table 1
The thermal conductivity parameters (λ o , λ i ) as shown in Fig. , vary in the range of 0.05 to 1.0 W/mK. It should be noted that the thermal conductivity of air is about 0.026 W/mK, while that of water is 0.60 W/mK. Values of thermal conductivity of foods higher than 0.60 W/mK are normally found in frozen food materials (λice=2 W/mK). Thus it is important to include the effect of air in the case of dried foods and ice in the case of frozen foods.
CONCLUSION
The well known parallel model was fitted adequately to thermal conductivity literature data for some foods. However in many cases limited data are available to carry out a successful regression.
Acknowledgments
REFERENCES
- Draper , N. and Smith , H. 1981 . Applied Regression Analysis , Second Edition New York, , USA : Wiley .
- Krokida , M.K. , Panagiotou , N.M. , Maroulis , Z.B. and Saravacos , G.D. 2001 . Thermal Conductivity: Leterature Data compilation for Foodstuffs . International Journal of Food Properties , 4 ( 1 ) : 111 – 140 .
- Marinos-Kouris , D. and Maroulis , Z.B. 1995 . “ Thermophysical Properties for the Drying of Solids ” . In In Handbook of Industrial Drying , Ed. Edited by: Mujumdar , A.S. New York : Marcel Dekker .
- Rahman , M.S. 1991 . Thermal Conductivity of Four Food Materials as a Single function of Porosity and Water Content . Journal of Food Engineering , 15 ( 4 ) : 261 – 268 .
- Rahman , M.S. 1995 . Food Properties Handbook New York : CRC Press .
- Rahman , M.S. , Chen , X.D. and Perera , C.O. 1997 . An Imporved Thermal Conductivity Prediction Model of Fruits and Vegetables as a function of Temperature, Water Content and Porosity . Journal of Food Engineering , 31 ( 2 ) : 163 – 170 .