Abstract
The thermophysical properties (initial freezing point, unfreezable water, enthalpy of freezing, and specific heat) of alginate-restructured sweet potato (SP) puree at freezing and refrigeration temperatures were determined using differential scanning calorimetry. Restructuring of SP puree increased the amount of unfreezable (bound) water in the puree from 0.44 g H2O/g solids to about 0.56 g H2O/g solids and reduced the freezing point from −2.5 to −3.2°C. During freezing (or melting), the specific heat increased from about 1.9 to 90 kJ/kg. After freezing, the specific heats of restructured and nonrestructured SP puree were respectively 3.695 and 3.404 kJ/kg. Between 358 and 403 kJ/kg of heat have to be removed when SP puree (at 20°C) is to be frozen to −40°C.
Introduction
Despite the high nutritional value of sweetpotato—SP (beta carotene, ascorbic acid, and minerals),Citation[1] Citation[2] the per capita consumption of SP in the United States has declined from about 12 kg in the 1930s to only 2.25 kg in 1988.Citation[3] This is because of texture control problems of SP, which have limited its use in the food-processing industry. About 60% of the harvested roots are sold fresh. Preparation of the fresh roots at home is inconveniently long. For example, baking of the roots takes 80–90 min at 240°C.
Restructuring of SP puree with alginate has been shown to be an effective method to control SP product textural characteristics and to utilize virtually all harvested roots.Citation[4] Texturization of SP puree was achieved by the addition of alginate, tetrasodium phyrophosphate, and calcium sulfate. The authors used this technology to develop model products such as a simulated baked SP and a French-fry type SP product. Freezing is one of the unit operations essential to the production of both restructured (RSP) and nonrestructured (NRSP) SP puree.
Thermophysical properties at freezing temperatures are crucial to the design and selection of freezing and refrigeration systems and in estimating the time required to freeze and thaw food products. Some important thermophysical properties include initial freezing point, unfreezable water content, unfrozen water fraction, latent heat of fusion, specific heat, and enthalpy.Citation5–7 Initial freezing point (T i ) is the temperature at which crystallization begins.Citation[8] It is one of the basic parameters in thermal property models that are used in the estimation of freezing times. This property is often a function of product composition. Unfreezable water content (w u ) is the amount of water remaining unfrozen in a product at the reference temperature of −40°C.Citation[9] It is a measure of the amount of unfrozen water in the system. The reference temperature of −40°C has been used by many investigators because negligible amounts of water are frozen below this temperature for the majority of food productsCitation[10] Citation[11] and because this temperature covers the typical range of industrial processes. Unfrozen water fraction (n w ) is the amount of water remaining unfrozen at a certain temperature. Both unfreezable water and unfrozen water weight fractions are needed in the theoretical calculation of the enthalpy of a food system.Citation[7]
Enthalpy (H) is used for calculating the total heat to be removed and to determine the rate of heat removal during refrigeration and freezing of food products.Citation[12] Enthalpy is a relative value above a reference temperature. Enthalpy of freezing, or latent heat of fusion (H L ), is the heat of phase change. Enthalpy data and latent heat of fusion are essential to the design of freezers and frozen storage processes and to model mathematically the freezing process using the enthalpy formula method.Citation[13] Apparent specific heat is the rate of enthalpy change with respect to temperature. It is the summation of the sensible heat and the latent heat of fusion in the frozen temperature range. Apparent specific heat is one of the most important thermal properties used in modeling nonlinear heat conduction problems during freezing and frozen storage processes.Citation[11]
Various researchers have used differential scanning calorimetry to determine the thermophysical properties of food materials such as carrots, reindeer meat, white bread,Citation[14] apples,Citation[12] and meats.Citation[8] Citation[15] To our knowledge, the information on the thermophysical properties of sweet potato puree at freezing temperatures is not available in literature.
The aim of this study was to determine the thermophysical properties of restructured (RSP) and nonrestructured (NRSP) sweet potato puree between −40 and 20°C using differential scanning calorimetry (DSC).
Materials and Methods
Restructured Puree Preparation
Raw SP roots (Beauregard cultivar) that have been cured and stored at 13–16°C and 80–90% relative humidity for 4 months were used in this study. The roots were made into puree using the method described by Walter and HooverCitation[16] except that the roots were hand-peeled instead of lye-peeled. The peeled roots were cut into slices (0.95 cm thick) and steam-cooked for 20 min. The cooked slices were then pureed using a hammer mill (model D, Fitzpatrick Co., Chicago, IL) fitted with a 0.15 cm screen. Pureed samples were packaged into polyethylene bags and stored in the refrigerator for experimental measurements. Moisture content of the puree was 73.3% and was determined by placing 10 g samples in an air-convection oven set at 103°C for 24 h. Alcohol insoluble solids,Citation[16] starch content,Citation[17] and sugar contentCitation[18] of the puree were also determined.
Cooked puree was restructured using the alginate-calcium system by mixing 3000 g of the treated puree with a mixture containing 6.28 g of tetrasodium phyrophosphate and 70 g of sucrose. After thoroughly mixing, a mixture containing 12.2 g of alginate and 70 g of sucrose was added and mixing was continued. Finally, 18 g of CaCl22H2O suspended in 70 mL of water was added and the mixing completed. The restructured SP puree was cured overnight at 4°C and held at −40°C in a freezer until needed.Citation[19]
Thermophysical Properties Determination
Thermophysical properties determination was carried out on samples of NRSP and RSP. The differential scanning calorimeter (DSC) used in this study was a Perkin-Elmer DSC 7 equipped with intracooler II refrigeration unit and dry box (Perkin Elmer Corp., Norwalk, CT). The instrument was calibrated with indium (melting point of 429.8K) and dodecane (melting point of 263.5K with enthalpy of 214.35 J/g) before use. Samples were weighed (60 mg) into the manufacturer's stainless steel pans. The sample was cooled to −40°C in the insulated chamber of the DSC by the application of liquid nitrogen. The samples were then subject to controlled heating to 20°C at the rate of 2°C/min (this low heating rate minimizes the temperature lags likely to occur in the event of a poor thermal contact of the sample-capsule-base systemCitation[20] Thermal lags were corrected by the method suggested by Wang and Kolbe.Citation[8]
Apparent specific heat (C p ), enthalpy (H, from −40 to 20°C) and enthalpy of freezing (H L ) were calculated by the software provided by the DSC manufacturer. H L was used to calculate the amount of unfreezable water present in the samples according to the procedure described by Ross.Citation[21] The unfreezable water content (w u ) is the difference between the total water content of the product and the amount of water detected by the DSC fusion endotherm. Unfrozen water fraction at any temperature (n w ) was determined by the summation of unfreezable water and melted water divided by the total mass of the sample. Testing of all samples was done in duplicates.
To ascertain the accuracy of the measurements, the specific heat and latent heat of fusion of HPLC grade water were measured. The specific heat of the water was found to be within 2% of published values. Based on three measurements, an average H L value of 335.5 ± 0.4 kJ/kg was obtained for water.
Statistical Testing
Analysis of varianceCitation[22] was used to statistically test the effect of process variables on experimental data. Statistical testing was performed at the 95% confidence interval.
Results and Discussion
The total moisture content, initial freezing points (T i ), enthalpy of fusion (H L ), and the amount of unfreezable water (w u ) for the SP puree samples are given in Table . Restructuring significantly affected (P < 0.05) all the thermophysical properties of SP puree listed in Table . T i was determined from the peak temperature of the DSC thermograms. As expected, Ti of the samples (−2.5 and −3.2°C for NRSP and RSP respectively) were below that of water (0°C) and within the range expected for processed food materials.Citation[23] The amount of unfreezable water in RSP was higher than that of NRSP because some of the water in the puree is used during the formation of the alginate/calcium binding complex and hence unavailable (i.e., bound) for freezing. Enthalpy of RSP is lower than NRSP primarily due to the higher amount of water unavailable to freeze RSP. Per 100 g weight of sample, the amount of alcohol insoluble solids starch and sugar in the puree was found to be 31.7, 2.13, and 12.03% respectively.
Table 1 Water mass fraction, initial freezing point (T i ), enthalpy of freezing (H L ), and unfreezable water fraction (w u ) SP puree
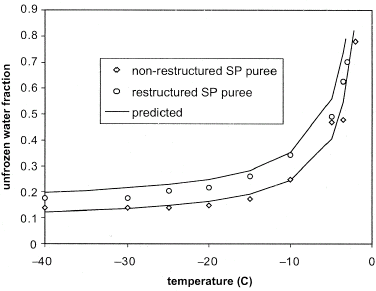
Experimental data (in symbols) for the effect of temperature on unfrozen water fraction (n w ) for both samples are given in Fig. . Results indicate that the unfrozen water fraction reduced rapidly at the initial freezing point of the two samples and then decreased gradually to the unfreezable water content (w u ). The formation of ice at the initial freezing temperature resulted in a three-phase system, with the product solids being concentrated within the unfrozen water phase.Citation[7] This caused a higher mass fraction of soluble solids in the unfrozen product fraction and a corresponding depression of the freezing temperature, hence the relationship depicted in Fig. .
Figure 1 Effect of temperature on the unfrozen water fraction in nonrestructured and restructured SP puree.
At any temperature T (below the initial freezing point) and assuming that a frozen food is an equilibrium mixture of water, ice, and solutes,Citation[24] the combination of Raoult's law and Classius-Clapeyron relation after adjusting for presence of bound water was utilized in this study to predict unfrozen water fraction. The mole fraction of water (X w ) in the puree at any temperature T below the freezing point of water (T w ) is given byCitation[7]:
The mole fraction of water (X w ) in the samples (0.986 for RSP and 0.985 for NRSP) at their freezing points was determined by substituting T i for T in Eq. (Equation1). The effective molecular weight of the solids (M s ) in the samples (459.1 for RSP and 336.93 for NRSP) was then estimated from the relation below:
The amount of unfrozen water (w) in each sample at any temperature T was then obtained by combining Eqs. (Equation1) and (Equation2) to obtain:
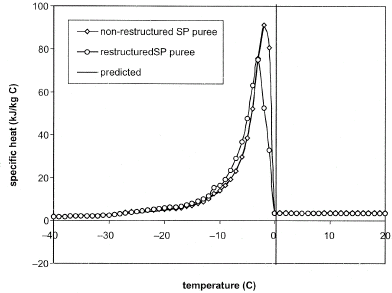
The apparent specific heat of NRSP and RSP is given in Fig. . In the temperature range (∼−20 to 0°C) where there is phase change, the specific heat of the samples dramatically increased from about 1.9 kJ/kg°C to more than 70 kJ/kg°C. Similar increases in specific heat were obtained for fruits, vegetables, and meat products.Citation[8] Citation[12] Citation[14] Citation[15] The peak specific heats of the NRSP and RSP were respectively 72.5 and 91.3 kJ/kg °C. Below the freezing point (T i ), Eqs. (Equation6) and (Equation7) were used to relate the specific heats of NRSP and RSP to temperature. Similar equations have been used to relate the specific heats of meats to temperature above and below their freezing points.Citation[15]
Nonrestructured SP puree (NRSP)
Restructured SP puree (RSP)
Above freezing point, the specific heat of NRSP was consistently higher than that of RSP samples. We attribute this to the presence of higher moisture in the NRSP puree. For each sample type, statistical testing at 95% confidence interval showed that the specific heats were not significantly affected by temperature within a range of T i to 20°C. The specific heat values were, therefore, averaged within this temperature range to obtain values of 3.70 kJ/kg (standard deviation of 0.01558) for NRSP puree and 3.40 kJ/kg (standard deviation of 0.0173) for RSP puree.
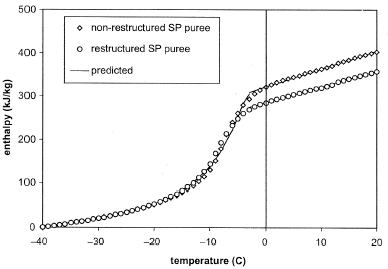
Figure shows that the total heat required to freeze RSP puree (358 kJ/kg) was lower than that required to freeze the NRSP (403 kJ/kg). This is because some of the water in the restructured SP puree was used in the formation of the alginate/calcium binding complex and, hence, unavailable (i.e., bound) for freezing. Enthalpy increased in a linear fashion from −40 to about −20°C. Beyond −20°C, the increase in enthalpy with temperature was exponential in nature. The enthalpy curves obtained in this study are similar to the curves that have been documented for other food materials subjected to freezing.Citation[15] Citation[25] The temperature dependence of enthalpy exhibited only weak discontinuity at the initial freezing point compared to the temperature dependence of apparent specific heat (Fig. ), which had a strong discontinuity. This feature of temperature dependence of enthalpy has been shown to minimize the undesirable phenomena of “peak jumping” and “oscillations” in numerical schemes when the freezing process is modeled by the enthalpy formulation method,Citation[24] hence, the importance of estimating the enthalpy of food materials at temperatures above and below their freezing point.
Figure also shows that most of the differences in the total heat of both samples occurred at temperatures greater than their freezing points. Statistical analysis using analysis of variance at 95% confidence intervalCitation[22] showed that between temperatures of −40°C and T i , the enthalpy of the RSP sample was not significantly different from the enthalpy of NRSP. Consequently, the data from both samples were averaged and the equation below was used to relate the enthalpy of SP to temperature (at temperatures from −40°C to the initial freezing point). The closeness of the prediction from Eq. (Equation8) to experimental data is shown in Fig. .
At temperatures greater than the freezing points of the samples, the enthalpy was a linear function of temperature (Eqs. (Equation9) and (Equation10)).
Restructured SP puree:
T > T i ; R 2 = 0.997, s.e. = 2.10
Nonrestructured SP puree:
T > T i ; R 2 = 0.998, s.e. = 1.98
Conclusions
It was shown in this study that the thermophysical properties of SP puree can be obtained from DSC thermograms. The addition of alginate/calcium complex bound some of the water in the puree. This in turn increased the amount of water in the puree that was unavailable for freezing and reduced the amount of energy required to freeze the texturized SP puree. Analysis of experimental data showed that up to 403 kJ/kg of heat need to be removed to freeze SP puree. The modified Clausius-Clapyeron equation gave a good prediction (R 2 of 0.98) of the amount of water that is unfrozen in the puree at any temperature.
Nomenclature
| C p | = |
Specific heat (J/kg K) |
| H | = |
Enthalpy (J/kg) |
| H L | = |
Latent heat of fusion (J/kg) |
| m | = |
Mass fraction |
| M | = |
Molecular weight |
| n w | = |
Unfrozen water fraction |
| R u | = |
Universal gas constant (8.314 J/mol K) |
| T | = |
Temperature (°C) |
| T i | = |
Initial freezing point (°C) |
| TK | = |
Temperature (K) |
| w u | = |
Unfreezable water content (g H2O/g of dry solids) |
| X | = |
Mole fraction of water |
Subscripts
| s | = |
Solids |
| w | = |
Water |
References
- Woolfe , J.A. 1992 . Sweet Potato: An Untapped Food Resource 643 pp Cambridge : Cambridge University Press .
- USDA. 1984 . “ Potatoes and sweetpotatoes ” . In Final estimate 1978–1982 , Statistical Bulletin No 709 37 pp Washington, DC : U.S. Department of Agriculture .
- Lucier , G. 1993 . Sweetpotato consumption beyond the Holiday Niche . Agric. Outlook , 201 : 20 – 23 .
- Truong , V.D. and Walter , W.B. Jr. 1994 . Physical and sensory properties of sweetpotato puree texturized with cellulose derivatives . J. Food Sci. , 59 : 1175 – 1180 .
- Gabas , A.L. , Telis-Romero , J.T. and Telis , V.R.N. 2003 . Influence of fluid concentration on freezing point depression and thermal conductivity of frozen orange juice . Int. J. Food Prop. , 6 : 543 – 556 .
- Succar , J. 1985 . Estimation of thermophysical properties of food at freezing temperatures . ASHRAE Trans. , 91 : 312 – 332 .
- Heldman , D.R. 1974 . Predicting the relationship between unfrozen water fraction and temperature during food freezing using freezing point depression . Trans. ASAE , 17 : 63 – 66 .
- Wang , D.Q. and Kolbe , E. 1991 . Thermal properties of surimi analyzed using DSC . J. Food Sci. , 56 : 302 – 308 .
- Fennema , O.R. 1996 . Food Chemistry, , 2nd Ed. 1069 pp New York : Marcel Dekker, Inc. .
- Heldman , D.R. and Singh , R.P. 1981 . Food Process Engineering, , 2nd Ed. 415 pp Westport, CT : AVI Publishing Co. Inc. .
- Succar , J. and Hayakawa , K. 1983 . Empirical formulae for predicting thermal physical properties of food at freezing or defrosting temperatures . Lebens. Wiss. Tech. , 16 : 326 – 331 .
- Ramaswamy , H.S. and Tung , M.A. 1981 . Thermophysical properties of apple in relation to freezing . J. Food Sci. , 46 : 724 – 728 .
- Mannapperuma , J.D. and Singh , R.P. 1988 . Prediction of freezing and thawing times of foods using a numerical method based on enthalpy formulation . J. Food Sci. , 53 : 626 – 630 .
- Roos , Y.H. 1986 . Phase transitions and unfreezable water content of carrots, reindeer meat and white bread studied using differential scanning calorimetry . J. Food Sci. , 51 : 684 – 686 .
- Tocci , A.M. and Mascheroni , R.H. 1998 . Characteristics of differential scanning calorimetry determination of thermophysical properties of meats . Lebens. Wiss. Tech. , 31 : 418 – 426 .
- Walter , W.M. Jr. and Hoover , M.W. 1984 . Effect of pre-processing storage conditions on the composition, microstructure, and acceptance of sweetpotato patties . J. Food Sci. , 49 : 1258 – 1261 .
- Holm , J. , Bjorck , I. , Drews , A. and Asp , N.G. 1986 . A rapid method for the analysis of starch . Starch/Starke , 38 : 224 – 226 .
- Walter , W.M. Jr. 1992 . Use of refractive index to monitor changes in sugar content of stored sweetpotatoes . HortSci. , 27 : 333 – 335 .
- Truong , V.D. , Walter , W.M. Jr. and Giesbrecht , F.G. 1995 . Texturization of sweet potato puree with alginate effects of tetrasodium pyrophosphate and calcium sulfate . J. Food Sci. , 60 : 1054 – 1059, 1074 .
- Tocci , A.M. , Flores , E.S. and Mascheroni , R.H. 1997 . Enthalpy, heat capacity and thermal conductivity of boneless mutton between −40 and + 40°C . Lebens. Wiss. Tech. , 30 : 184 – 191 .
- Ross , K.D. 1978 . Differential scanning calorimetry of nonfreezable water in solute-macromolecule-water systems . J. Food Sci. , 43 : 1812 – 1815 .
- SAS. 1996 . SAS Users’ Guide Cary, NC : Statistical Analysis Systems Inc. .
- ASHRAE. 2002 . ASHRAE Handbook (Refrigeration)—Thermal Properties of Foods 8.1 – 8.30 pp . Atlanta, GA : American Society of Heating and Refrigerating Airconditioning Engineers .
- Singh , R.P. and Mannapperuma , J.D. 1990 . “ Developments in food freezing ” . In Biotechnology and Food Process Engineering Edited by: Schwartzberg , H.G. and Rao , M.A. 309 – 358 . New York : Marcel Dekker Inc. .
- Heldman , D.R. 1982 . Food properties during freezing . Food Tech. , 36 : 92 – 96 .
- Chen , C.S. 1985 . Thermodynamic analysis of the freezing and thawing of foods: ice content and Mollier diagram . J. Food Sci. , 50 : 1163 – 1166 .