Abstract
The effects of enzymatic treatment of corn gluten on the extraction of lutein and zeaxanthin (non-provitamin A carotenoids), which have multiple bio-functional properties in human beings, were studied. They were extracted from corn gluten meal (CGM) after pretreatment by protease (neutrase 0.5 L) under the following conditions: enzyme content 214 (U/g protein), solid content 15%, and hydrolysis time of 2 h. The yields of crude lutein, crude zeaxanthin, and total carotenoids were increased from 74.1, 77.1, and 393.6 to 113.5, 140.1, and 599.1 (µg/g, CGM on moisture-free basis), respectively.
Introduction
Lutein and zeaxanthin are xanthophylls commonly occurring in yellow corn, fruits, dark-green leafy vegetables, and many flower petals. They are frequently used in the poultry industry as natural colorants to enhance pigmentation of the skin and the egg yolk of chicken. Numerous epidemiological studies have suggested that lutein and zeaxanthin have specific biological functions in controlling cancer development, enhancing immune function, decreasing the risk of heart disease, and protecting against age-related macular degeneration (AMD).Citation[1] Lutein and zeaxanthin are the only carotenoids in the macular of the retina that are responsible for sharp and detailed vision.Citation[2] Recent studies on AMD, one of the most important of the irreversible blinding diseases, showed that the course of AMD is decisively influenced by the concentration of lutein and zeaxanthin in the retina. Compelling evidence suggests that the consumption of lutein and zeaxanthin from diet or supplements might significantly reduce the risk of developing AMD.Citation[3] Corn gluten meal (CGM) is one of the major by-products in the wet milling process of corn starch production with high concentration of carotenoids (200–400 µg/g), consisting of about 92% xanthophylls.Citation[4] Some researchers have reported processes for recovering xanthophylls from CGM.Citation5–8 However, according to these processes, the carotenoids are not effectively extracted from CGM as they probably bind to zein. It is, therefore, with this background that we decided to investigate the effects of protease treatment on carotenoids, especially during the extraction processes of lutein and zeaxanthin.
Materials and Methods
Materials and Reagents
Wet corn gluten meal (before dryness, protein content 75.76%) was kindly provided by Zhumadian Medicine Company (Zhumadian, Henan, China). All-trans-lutein (90% purity) was purchased from Fluka (Fluka Chemie Gmbh CH-9471 Buchs, 081/7552511 packed in Sigma-Aldrich, pf, D-89552 Steinheim, 073291970 Switzerland). HPLC-grade solvents were purchased from Merk KgaA (Darmstadt, Germany). Analytical-grade reagents were purchased from Zhenxing Chemical Company (Shanghai, China). The commercial enzyme Protease (neutrase 0.5 L, 53500 U/mL, measured according to the Folin-phenol methodCitation[9]) was provided by Novo Laboratories, Inc. (Wilton, CT).
Enzymatic Treatment of Corn Gluten Meal
Corn gluten meal was mixed with protease to form a slurry in a brown jar. In order to evaluate the effect of solid-to-water ratio of the mixture on the yield of carotenoids, crude lutein and crude zeaxanthin slurries were prepared containing 5%, 10%, and 15% solid. The solid content was determined by taking into account the moisture content of the corn gluten meal. To determine the effects of enzyme content on the yield of carotenoids (crude lutein and crude zeaxanthin), a series of slurries containing no enzyme (control), enzyme (protease) 53.5, 107, 160.5, and 214 (U/g protein) were prepared. The pH of all the slurries was adjusted to 6.5, as recommended by the enzyme manufacturers, with dilute sodium hydroxide solution (2 mol/L). The pH was measured with a PHS-3C pH meter (Shanghai Leizi Instrument Company, Shanghai, China). Each slurry was thoroughly mixed and stirred at 37°C for about 2–6 h. After an enzymatic treatment and pH (5.0) adjustment with dilute hydrochloric acid (2 mol/L) to precipitate protein, the hydrolyzed corn gluten meal was centrifuged at 2500 rpm for 15 min. The supernatant was discarded, while the precipitate was dried under 40°C to a moisture content lower than 10%, put into plastic bags, and stored in the dark.
Carotenoid Extraction
Two point five grams of enzyme treated corn gluten meal was mixed with 37.5 mL 95% ethanol in a brown bottle, sealed and stirred for about 2–4 h. The mixture was filtered, and the cake was re-extracted for several times until the filtrate was colorless. All the filtrates were combined and concentrated to dryness by rotary evaporation at 40°C. The residues were dissolved in acetone (CH3COCH3) to precipitate zein, and the solution was filtered in a 25 mL brown-colored volumetric flask. The cake was repeatedly washed with acetone until the filtrate was colorless. Then the precipitate was discarded, while the filtrate was diluted to 25 mL and stored at −20°C until analysis. Next, 0.25 mL of the solution was diluted with 4.75 mL of acetone to form a 5 mL solution. The UV-visible spectrum was measured by a UV-visible spectrometer (UV1100, Beijing Ruili Analytical Instrument Company, Beijing, China) and the absorbance was determined at its maximum absorbing wavelength. All the experiments were performed under dim light or in dark room.
Thin Layer Chromatography (TLC)
The lutein standard and the CGM extract were subjected to TLC (20 × 20 cm; 0.2 mm layer thickness), with magnesium oxide (MgO) as support (Shanghai Dunhuang Chemical Company, Shanghai, China) and a solvent mixture of petroleum ether (30–60°C)/acetone (65/35,v/v) as developing solvent.Citation[10] After the development of the chromatogram, the plate was removed and dried in a nitrogen stream. Then the plate was soaked or sprayed with saturated methanolic silver nitrate (AgNO3) solution for the identification of the end structure of carotenoids, as described by Isaksen, M. and Francis, G. W.Citation[11] Comparing the R f value to the standard and the color change after the reaction with silver ion identified the strip of lutein and zeaxanthin. The strip of lutein and zeaxanthin was scraped off from the plate and dissolved in 1 mL acetone and 1 mL absolute ethanol, respectively. The resulting slurries were filtered, washed with acetone and absolute ethanol, respectively, until the filtrates were colorless. These solutions were subjected to LC/MS analysis. The crude lutein and crude zeaxanthin content were determined on an UV-visible spectrometer (UV1100, Beijing Ruili Instrument Company, Beijing, China) at the maximum wavelength of lutein and zeaxanthin. All the experiments described above were performed in a dark room.
Identification of Lutein and Zeaxanthin by LC/MS
Positive-ion mass spectra were obtained using a Waters Platform ZMD 4000 MS equipped with an LC/APCI-MS interface (Waters Corporation, Milford, MA U.S.A.). The mass spectrometer was interfaced to a Waters 2690 HPLC system equipped with an auto injector and photodiode-array UV/visible absorbance detector. The parameters were ApcI Pin: 3.69 kV; Cone: 27 V; Source Heater: 120°C; ApcI Probe Heater: 300°C; Multiplier: 650 V; Gas flow: 3.8 L/h; the range of m/z: 350–800 M. The HPLC separation was carried out using a reversed-phase column (Symmetry column C-8 150 × 2.1 mm i.d.). The solvent system consisted of a 30 min linear gradient methanol/water (85/15) to 100% methanol at a flow rate of 0.3 mL/min. Absorbance spectra were recorded from 350–600 nm using a photodiode-array detector (Waters PDA996 Detector) located in-line between the HPLC column and the APCI interface.
Quantification of Lutein, Zeaxanthin, and Total Carotenoids
Crude lutein was quantified using the calibration curve prepared by measuring the O.D λmax of the lutein standard at concentrations ranging from 0.592 to 2.368 µg/mL. The linearity of the calibration curve was R 2 = 0.9971. Crude zeaxanthin was quantified according to its extinction coefficient in ethanol (2348). Total carotenoids were quantified according to the method described by Lichtenthaler and WellburnCitation[12] Wang, Y. and Li.Citation[13]
Orthogonal Experiments
The method employed was a three-variable, with three levels of each variable orthogonal design. The three independent variables for enzyme treatment were enzyme content, solid content of slurry, and hydrolysis time. The experimental design in the levels of variables is shown in Table . CGM was treated with enzyme and dried before carotenoids were extracted, separated, and quantified according to the methods described above. The yields of crude lutein, crude zeaxanthin, and total carotenoids were determined.
Table 1 Orthogonal experimental design in levels of variables of enzymatic treatment of CGM
Results and Discussion
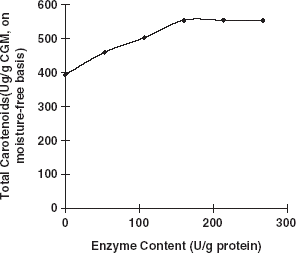
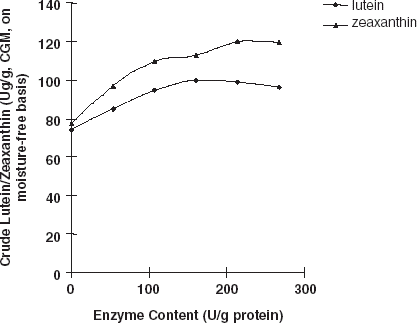
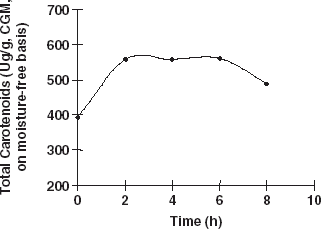
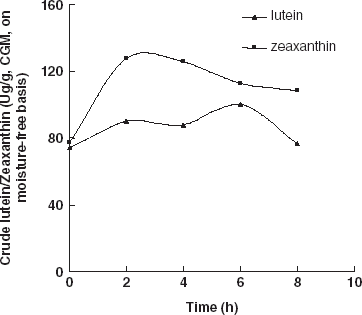
Carotenoids do not only exist in the liposome systems of animal or plant materials, but they are also known to generally bind to some proteins, carbohydrates, and liposome to form compounds.Citation[5] Citation[13] If the bonds of the compounds are broken and the entrapped carotenoids released, then these entrapped carotenoids could be extracted easily and thoroughly. When CGM was hydrolyzed with protease, results indicated that protein hydrolysis resulted with an increase in the extraction efficiency of total carotenoids, lutein, and zeaxanthin. Lutein, zeaxanthin, and total carotenoids extracted from enzymes treated CGM were significantly higher (averagely increased 32.9, 52.2, and 40.8%, respectively) when enzyme content was above 160.5 (U/g protein) compared with those obtained from the control group (Fig. and ). When the enzyme content increased to 160.5 (U/g protein) and higher, the total carotenoid yield did not increase but was maintained at a relatively steady level. Therefore, the enzyme content (160.5 U/g protein) was enough for the carotenoid-protein to break. More enzyme and further hydrolysis of the protein were not necessary and did not facilitate the release of more carotenoids from the complex. Similar results could be seen in Fig. . Currently, we do not have an exact explanation for this result, but it seems logical to attribute it to the destruction of the protein network during hydrolysis. Some reports have shown that carotenoids (including lutein and zeaxanthin) bind to some proteins to form complexes.Citation[14] Citation[15] When CGM was treated with protease, the protein-network and the bounded carotenoid-protein complex were disintegrated, and carotenoids were released in a free type. Further study is still needed to explain fully the effects of enzymatic treatment of CGM on carotenoid extraction.
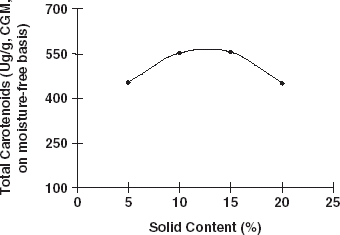
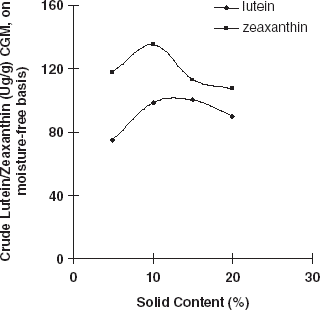
The effects of solid content and hydrolysis time on lutein, zeaxanthin, and the total carotenoid extraction were investigated. If the solid content in the slurry was higher than 12%, the yield of crude zeaxanthin decreased. And if the solid content was higher than 15%, the yield of crude lutein and total carotenoids decrease, (Fig. and ). This result occured because the enzyme (protease) needs a suitable solid content to hydrolyze protein. When the hydrolysis time was prolonged, the yield of crude lutein, crude zeaxanthin, and total carotenoids decreased (Fig. and ). This is probably due to the fact that carotenoids are sensitive to heat, O2, and light. Therefore, some of the carotenoids would have been damaged during long time hydrolysis. Hence, the hydrolysis time should be limited to about 6 h.
In order to optimize conditions for enzymatic treatment of CGM, orthogonal experiments were employed. The results are shown on Tables and . The optimum enzyme treatment conditions for total carotenoids, lutein, and zeaxanthin extraction were A3 (214(U/g protein)), B2 (hydrolysis time 2 h), and C3 (solid content 15%), A3 B2 C2 or A3 B2 C3, and A3 C3 B2, respectively. The enzyme treatment conditions for the carotenoids extraction, studied in this paper, were almost identical. We obtained the optimum conditions: enzyme contents 214(U/g protein), solid content 15%, and hydrolysis time 2 h. After the enzyme pretreatment of wet CGM under these optimum conditions, the yield of crude lutein, crude zeaxanthin, and total carotenoids were 113.5, 140.1, and 599.1 (µg/g, CGM, on moisture-free basis), respectively. These yields were higher than any yields obtained in the orthogonal experiments and proved the optimum enzymatic treatment condition.
Table 2 The results of orthogonal experiments for enzymatic treatment of CGM (Yield of carotenoids: µg/g, CGM, on moisture-free basis)
Table 3 The statistically analyzed results of orthogonal experiments for enzymatic treatment of CGM
Conclusion
Enzyme (protease) treatment can increase the extraction efficiency of lutein, zeaxanthin, and total carotenoids from corn gluten meal. The optimum conditions of enzymatic treatment of CGM were obtained as: enzyme contents 214 (U/g protein), solid content 15%, and hydrolysis time 2 h. The yields of crude lutein, crude zeaxanthin, and total carotenoids were increased from 74.1, 77.1, and 393.6 (µg/g, CGM, on moisture-free basis) to 113.5, 140.1, and 599.1 (µg/g, CGM, on moisture-free basis), respectively. The results suggest that the enzyme treatment destroyed the protein network and/or bonds of the caroteniod-protein complex and the entrapped carotenoids could be extracted easily. Further studies are needed to obtain an exact explanation.
References
- Park , J.S. , Chew , B.P. and Wong , T.S. 1998 . Dietary lutein absorption from marigold extract is rapid in BALB/c mice . J. Nutr. , 128 : 1802 – 1806 .
- Johnson , E.J. , Hammond , B.R. , Kyung-Jin , Y. , Jian , Q. , Xiang , D.W. , Castaneda , C.D. , Snodderly , M. and Russell , R.M. 2000 . Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density . Am. J. Clin. Nutr. , 71 : 1555 – 1562 .
- Dachtler , M. , Kohler , K. and Klaus , A. 1998 . Reversed-phase high-performance liquid chromatographic identification of lutein and zeaxanthin stereoisomers in bovine retina using a C30 bonded phase . Journal of Chromatography B. , 720 : 211 – 216 .
- Blessin , C.W. 1963 . Carotenoids of corn and sorghum I . Analytical procedure. Cereal Chem. , 39 : 236 – 242 .
- Muralidhara , H.S. Process for Recovering Xanthophylls from Corn Gluten . Patent US005602286A . Feb. 11, 1997 .
- Muralidhara , H.S. and Cornuelle , T.L. Processes for Recovering Xanthophylls from Corn Gluten Meal . Patent US005847238A . Dec. 8, 1998 .
- Muralidhara , H.S. Improved Process for Recovering Xanthophylls from Corn Gluten . Patent WO 96/40607 . Dec. 1996 .
- Cheryan , M. Method for Extracting Xanthophylls from Corn . Patent US006169217B1 . Jan. 2, 2001 .
- Zeng , T. and Gong , Y. 1996 . Guide to Cereal and Food Biochemistry Experiments 77 pp Henan, , China : Henan Medical University Press .
- Lu , Y. , Chen , Z. and Yao , H. 2003 . Isolation and identification of lutein and zeaxanthin from corn gluten by thin-layer chromatography . Journal of Wuxi University of Light Industry , 22 ( 5 ) : 11
- Isaksen , M. and Francis , G.W. 1990 . Silver ion spray reagent for the discrimination of β- and ε-end groups in carotenoids on thin-layer chromatograms . Chromatographia , 29 ( 7/8 )
- Lichtenthaler and Wellburn. 1983 . Determination of the total carotenoids and chlorophylls a and b of leaf extracts in different solvents . Biochemical Society Transactions , 603 : 591 – 592 .
- Wang , Y. and Li , Q. 1997 . Advancement, Production, and Application of Nature Carotenoids 158 pp Beijing, , China : Medical Science and Technology Publishing House .
- Takagi , S. , Shiroishi , M. and Takagi , T. 1980 . Interaction of lutein with ovalbumin and other proteins: association and acquisition of novel optical activity . Agric. Biol. Chem. , 44 ( 9 ) : 2111 – 2117 .
- Huei-mei , C. and Meyers , S.P. 1982 . Extraction of astraxanthin pigment from crawfish waste using a soy oil process . Journal of Food Science , 47 : 892 – 900 .