Abstract
The gelation profile of yoghurts from conventionally treated (85°C/30 min) and UHT treated (143°C/6s) milks at 16, 18, and 20% total solids was analyzed during fermentation for 4 hrs using the invasive Rapid Visco Analyzer (RVA) and the non-invasive ultrasonic spectroscope. The viscosity measured by the RVA and the ultrasonic velocity measured by the ultrasonic spectroscope exhibited similar sigmoid trends with respect to fermentation time. The ultrasonic spectroscope detected the onset of gelation of yoghurt milk earlier (by an average of 52 min) than did the RVA, indicating a higher sensitivity of ultrasonic spectroscopy. The delay of gelation time of UHT-treated yoghurt milk as compared to conventionally treated yoghurt milk was detected by both techniques. A non-significant (P > 0.05) effect of solids content in the yoghurt milks on their gelation time was also observed by both instruments.
INTRODUCTION
According to the FAO/WHO Standards, yoghurt is “the coagulated milk product obtained by lactic acid fermentation through the action of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus.” The yoghurt gel is classified as an acid-induced gel.[Citation1] On acidification, the micelles become unstable, and in an unstirred solution, coagulation occurs with the formation of a gel network in which casein is the main component.[Citation2] Casein micelles aggregate as a result of charge neutralisation, leading to the formation of chains and clusters that are somehow linked together to give a three-dimensional network.[Citation3]
In the past, gelation of yoghurt has been studied using viscosity measurement.[Citation4–8] Parnell-Clunies et al.[Citation5] reported that the gel formation is a multistage process comprising an induction stage (no change in viscosity), flocculation stage (rapid change in viscosity), metastable equilibrium stage (constant viscosity), and syneresis stage (decrease in viscosity). On the other hand, other researchers have used the ultrasonic spectroscopy method to analyse the pre-gelation and gelation processes in acidified milk (colloidal calcium phosphate solubilization, casein micelle aggregation, gel formation).[Citation9–15]
Although, the gelation of yogurt during fermentation has been studied using viscosity measurement and ultrasonic spectroscopy, a comparison of these two methods has not been reported. This research compared the measurement of gelation of yoghurts by Rapid Visco Analyzer (RVA) and ultrasonic spectroscopy.
MATERIALS AND METHODS
Preparation of Yoghurt Premix
Raw milk was obtained from the University of Queensland herd at the Gatton Campus. The cream was removed with a centrifugal separator. The skim milk was thermized at 63°C for 15 s and stored at 1°C. The thermized milk was used as a stock of raw milk throughout this experiment to avoid variation in the composition of milk used in yoghurt production.
Thermized milk was mixed with sugar in the ratio of 100 parts of milk to 2 parts of sugar. The total solids level was then adjusted to 16, 18 and 20% by addition of low-heat skim milk powder (Murray Goulburn Co-operative Co., Ltd., Australia). The milk of each level of total solids was divided into 2 parts. The first part was heated at 143°C for 6 s by steam infusion UHT heating (APV, Copenhagen, Denmark), homogenized at 20/5 MPa (2-stage) at 70–75°C, and packaged aseptically into laminated plastic bags using an Intersept® aseptic filler. The other part was conventionally heated at 85°C for 30 min in glass bottles (control). Both UHT and control milks were kept at 4°C until used for yoghurt production the following day.
Production of Yoghurt and Rapid Visco Analyzer Analysis
The yoghurt cultures, Lactobacillus delbrueckii ssp. bulgaricus (LB) and Streptococcus thermophilus (ST), were propagated in skim milk media (10% w/v skim milk sterilized at 115°C for 5 min) at 37°C for 24 h before use. The UHT and control milks fortified with low-heat skim milk powder to 16, 18 and 20% (w/w) total solids were heated to 45°C and 1.5% (w/v) of each yoghurt bacterium were added. Twenty-five milliliters of yoghurt premixes were distributed into 16 RVA containers. The containers were then placed into the incubator at 45°C. During fermentation at 45°C for 4 h, samples were collected every 15 min and manually stirred for 30 s before measuring viscosity using a Rapid Visco Analyzer (RVA-4, Newport Scientific, Warriewood, Australia). Since the yoghurt is thixotropic and viscosity might be affected by agitation, the viscosities of the samples were read at 1 minute at 150 rpm stirrer speed. After the viscosity measurement, the sample was discarded and another fresh sample undergoing fermentation was used at the next analysis. The viscosity was measured in Rapid Visco Units (RVU). According to the RVA manufacturer, one RVU is approximately equivalent to 12 mPas.[Citation16] The test results from this viscometer are highly empirical; therefore, the exact shear rates are unknown. According to Lai et al.,[Citation17] the average shear rate present in RVA is around 20.1/revolution. This means that the shear rate applied in this research at 150 rpm was on average 3015/s.
Ultrasonic Spectroscopy Analysis
A high resolution ultrasonic spectrometer (HR-US101, Heath Scientific Company Ltd., Bletchley, UK) was used in this research. All measurements were performed differentially with two identical resonator cells of 2 ml volumes at a frequency of about 7.8 MHz. The ultrasonic velocity and attenuation in the references and samples were automatically measured by a PC-controlled HRUS 101 spectrometer. Buckin and Smyth [Citation11] provide more details on the ultrasonic spectroscopic technique.
Two 20-ml portions of yoghurt premix were degassed in a vacuum chamber for 10-15 min under stirring. The degassing was necessary to avoid perturbation in measurements due to the presence of air in the ultrasonic path. One portion contained cultures, and the other was used as a reference (without cultures). The sample and reference were quickly loaded into the cells of the ultrasonic spectroscope. From the time the cultures were added to the milk, the gelation time was recorded. The velocity of ultrasound was measured continuously for 4 h at 45°C. This was a measurement of the ultrasound propagation in the sample relative to the propagation in water at 20°C.
Statistical Analysis
A completely randomised 2×3 factorial design with 3 replications was used in this experiment. The means were compared by the Duncan's multiple range tests.
RESULTS AND DISCUSSION
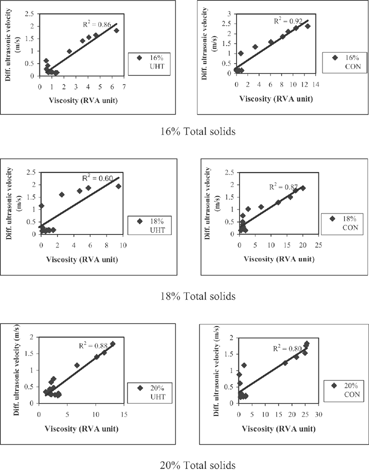
The viscosity measured by RVA and the differential ultrasonic velocity measured by the ultrasonic spectroscopy are presented in . The patterns of viscosity measured by RVA and differential ultrasonic velocity changes measured by ultrasonic spectroscopy during 4 hours of fermentation were similar, both showing a sigmoid shape with 3 phases characterised by a small change, rapid increase, and slow increase in measured values in subsequent phases. The ultrasonic method picked up the changes in differential ultrasonic velocity (point A, ) before the RVA could detect the changes in viscosity. When the RVA picked up the changes in viscosity (point B, ), the differential ultrasonic velocity was already in phase 2, in which the differential ultrasonic velocity was increasing. This was observed in yoghurts from both UHT- and conventionally treated milk at all levels of total solids.
Figure 1 Change in differential ultrasonic velocity (measured by ultrasonic spectroscopy) and viscosity (measured by RVA) during fermentation of yoghurts from conventionally treated milk at 16% total solids (points A and B show the time at which the measured parameters start rising).
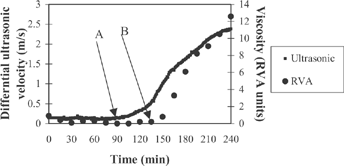
The times of the onset of gelation of yoghurts from UHT- and conventionally treated milk measured by RVA and ultrasonic spectroscopy are shown in . The onset-of-gelation time measured by RVA is the approximate time when the viscosity of the product begins to rise (point B, ). For ultrasonic spectroscopy, the onset-of-gelation time is the time of the first upward change in the differential ultrasonic velocity curve (point A, ). Note that the ultrasonic spectroscopy allowed the measurements in real time, whereas the RVA measurements were performed at 15 minutes intervals.
There was a significant difference (p<0.05) between the onset-of-gelation times measured by RVA and ultrasonic spectroscopy. Gelation times detected by ultrasonic spectroscopy were approximately 52 min earlier than those detected by RVA for all treatments. The gelation times detected by RVA were approximately 165 and 150 min, whereas for ultrasonic spectroscopy they were approximately 124 and 97 min for yoghurt from UHT- and conventionally treated milk, respectively. The higher sensitivity of ultrasonic spectroscopy may be attributed to its ability to detect changes in the chemical composition (lactic acid formation) and changes in the nature of the casein micelle due to the acidification. The RVA can only detect when there is a sufficient change in the physical structure, such as gel network formation and marked hydration of protein molecules. In addition, clearly shows that the gelation time of yoghurt from UHT-treated milk at all levels of total solids was delayed according to measurements by both RVA and ultrasonic spectroscopic methods.
Table 1 Comparison of onset-of-gelation time observed from destructive (RVA) and non-destructive (ultrasonic spectroscopy) methods for yoghurts made from UHT-treated milk (UHT) and conventionally treated milk (CON)
The viscosity of yoghurt from conventionally treated milk was positively correlated with the ultrasonic velocity, with correlation coefficients between 0.6 and 0.92 (). This correlation occurs despite the fact that at earlier stages of fermentation there were changes in ultrasonic velocity before there was any change in viscosity (). However, the variation in ultrasonic velocity at lower viscosity values (early stages of fermentation) did not show any clear trend. The variation in the preparation of the samples (manually stirring and difference in temperature distribution in the incubator) for RVA analysis may influence the viscosity values, while in the ultrasonic spectroscopic analyses, the samples were undisturbed. Therefore, the readings by ultrasonic spectroscopy may be more reliable and accurate.
CONCLUSION
This work suggests that ultrasonic spectrometry is superior to viscosity measurement by RVA for the detection of gelation during yogurt fermentation. Its noninvasive nature and sensitivity make it an attractive technique for investigating texture changes during yogurt fermentation.
ACKNOWLEDGEMENTS
The financial support from Assumption University, Bangkok (Scholarship to WK) and Dairy Australia are gratefully acknowledged.
Notes
2. Roefs, S.P.F.M. Structure of Acid Casein Gels. A study of gels formed after acidification in the cold, Wageningen Agricultural University: the Netherlands, 1986.
6. Guinee, T.P.; Mullins, C.G.; Reville, W.J. 1994 Rheological and synergetic properties of yoghurt stabilized with different dairy ingredients. Second Food Ingredients Symposium, Moorepark, Ireland, National Dairy Products Research Centre. 13–14 April 1994.
16. Anonymous (998). Applications manual for the Rapid Visco-Analyser. Newport Scientific Pty. Ltd., Warriewood, NSW, Australia.
REFERENCES
- 2000 . “ FAO/WHO Standard Codex Alimentarius: milk and milk products: Codex general standard for the use of dairy terms ” . In Food and Agriculture Organisation of the United Nation (FAO) and World Health Organisation (WHO) Standard , 2nd , Rome : FAO . 12 S.1
- 2. Roefs, S.P.F.M. Structure of Acid Casein Gels. A study of gels formed after acidification in the cold, Wageningen Agricultural University: the Netherlands, 1986.
- Skriver, A. Characterization of stirred yogurt by rheology, microscopy and sensory analysis, 1995 http://www.mli.kvl.dk/dairy/phd/as.htm. (http://www.mli.kvl.dk/dairy/phd/as.htm.)
- Guirguis , N , Broome , M.C. and Hickey , M.W. 1984 . The effect of partial replacement of skim milk powder with whey protein concentrate on the viscosity and syneresis of yoghurt . Australian Journal of Dairy Technology , 39 ( 1 ) : 33 – 35 .
- Parnell-Clunies , E. , Kakuda , Y. , deMan , J.M. and Cazzola , F. 1988 . Gelation profiles of yoghurt as affected by heat treatment of milk . J. of Dairy Sci. , 71 ( 3 ) : 582 – 588 .
- 6. Guinee, T.P.; Mullins, C.G.; Reville, W.J. 1994 Rheological and synergetic properties of yoghurt stabilized with different dairy ingredients. Second Food Ingredients Symposium, Moorepark, Ireland, National Dairy Products Research Centre. 13–14 April 1994.
- Morris , H.A. , Ghaleb , H.M. , Smith , D.E. and Bastian , E.D. 1995 . A comparison of yogurt fortified with nonfat dry milks and whey protein concentrates . Cultured Dairy Products J. , 30 ( 1 ) : 2 – 4 . 31
- Fenelon , M.A. , Guinee , T.P. , Kelly , P.M. , O'Kennedy , B.T. and Wilkinson , M.G. 2000 . The effect of total protein, casein: whey protein ratio and fat content on the rheological and synergetic properties of yogurts . Irish J. Agri. Food Research , 39 : 171
- Povey , M.J.W. 1997a . “ Rapid determination of food material properties ” . In Ultrasound in Food Processing , Edited by: Povey , M.J.W. and Mason , T.J. 30 – 65 . London : Blackie Academic & Professional .
- Povey , M.J.W. 1997b . “ Measuring aggregation in colloids using ultrasound velocity and attenuation ” . In Food Colloids. Proteins, Lipids and Polysaccharides , Edited by: Dickenson , E. and Bergenstahl , B. 150 – 167 . London, , United Kingdom : Royal Society Chemistry .
- Buckin , V. and Smyth , C. 1999 . High-resolution ultrasonic resonator measurements for analysis of liquids . Seminars in Food Analysis , 4 : 113 – 130 .
- Smyth , C. , Dawson , K. and Buckin , V.A. 1999 . Ultrasonic analysis of heat-induced coagulation in calcium fortified milk . Progressive Colloid Polymer Sci. , 112 : 221 – 226 .
- Kudryashov , E. , Smyth , C. , Duffy , G. and Buckin , V. 2000 . Ultrasonic high-resolution longitudinal and shear wave measurements in food colloids: monitoring of gelation processes and detection of pathogens . Progressive Colloid Polymer Sci. , 115 : 287 – 294 .
- Smyth , C. , Kudryashov , E.D. and Buckin , V. 2001 . High-frequency shear and volume viscoelastic moduli of casein particle gel . Colloids and Surfaces A: Physiochemical and Engineering Aspects , 183–185 : 517 – 526 . [CROSSREF]
- Buckin, V.; Kuryashov, E.; Morrissey, S.; Smith, C.; O'Driscoll, B.High-resolution ultrasonic spectroscopy for analysis of biocolloids. International Labmate 2002, 27(2 April) http:// www.productsearch.co.uk / internationallabmate.com/ features/ April2002 (http:// www.productsearch.co.uk / internationallabmate.com/ features/ April2002)
- 16. Anonymous (998). Applications manual for the Rapid Visco-Analyser. Newport Scientific Pty. Ltd., Warriewood, NSW, Australia.
- Lai , K.P. , Steffe , J.F. and Ng , P.K.W. 2000 . Average shear rates in the Rapid Visco-Analyser (RVA) mixing system . Cereal Chem. , 77 ( 6 ) : 714 – 716 .