Abstract
A method to develop a model for latent heat of vaporization of moisture in red chillies was developed from moisture isotherm data. The latent heat of vaporization of moisture in red chillies was high at low moisture contents and low at high moisture contents. The moisture inside the chillies behaved almost like free water when the moisture content of chillies was above 150% (dry basis). The developed model is useful in determining the total latent heat of vaporization of moisture in chillies within the moisture limits of 400-5% d.b.
Keywords:
INTRODUCTION
Red chillies are the dried ripe fruits of the species of genus Capsicum. Chillies, which contain a high moisture content (300-400% d.b.) after harvest, are highly perishable and hence processing and storage of chillies are of considerable importance both to farmers as well as to processors and consumers. Reducing the moisture content and providing aeration to the chillies after harvesting is essential to avoid development of microflora and subsequent loss of quality or even total spoilage.[Citation1] Therefore, chillies need to be dried quickly without impairing colour and pungency.
Information about the amount of heat energy required to vaporize moisture from chillies during the drying process is important for the proper design of a dryer. The latent heat of vaporization of moisture in fruits or vegetables is often determined by considering the evaporation of free water as given in steam tables. The use of such data, especially at low moisture contents, leads to considerable error in drying energy calculations. At high moisture contents, the moisture inside the fruits or vegetables behaves almost like free water, which can be removed with relative ease. However, at lower moistures, it is strongly bound by physical and chemical forces.[Citation2]
The binding of moisture inside food material is due to adsorption, a surface phenomenon, though other factors such as capillary, solution, and chemical binding are also possible.[Citation3] Because the food material is a hygroscopic, it exhibits a vapor pressure less than that of free water at the same temperature and, therefore, the heat energy required to vaporize moisture from food material would be higher than that of free water.[Citation4] The energy required to evaporate moisture from grain especially at low moisture content is higher than that of free water and depends on the type of crop.[Citation5] The latent heat of vaporization of the product can be determined from the equilibrium moisture content data.[Citation6,Citation7]
In simulation studies of a dryer, the total latent heat of vaporization of moisture at a given chilli bed temperature and moisture range is frequently needed. Since at each layer of chillies bed the temperature and moisture content vary with time, a model for total latent heat of vaporization of moisture in chillies in terms of moisture range and temperature will be useful. In the present study, a systematic approach to develop such a model for total latent heat of vaporization of moisture in chillies was adopted which can be extended to any food material. Regression equations were developed for latent heat of vaporization of moisture from brown parboiled rice,[Citation2] wheat,[Citation6] malt,[Citation7] shelled corn, [Citation8] soybean,[Citation9] and alfalfa pellets.[Citation10]
MATERIALS AND METHODS
A method was proposed for determining the latent heat of vaporization of moisture of any liquid using vapor pressure data.[Citation11] The thermodynamic function Clausius–Clapeyron equation, which expresses the temperature dependence of vapor pressure for phase transition from an adsorbed liquid to the vapor phase, was used. The equation was given as:
At normal temperature and pressure, the specific volume of liquid can be considered to be negligible compared to the specific volume of vapor. By assuming the vapor behaves like an ideal gas, the following equation is derived after following the known procedure:[Citation11]
The vapor pressure of moisture in chillies at each moisture content was calculated as:
To determine the latent heat of vaporization of moisture present in the chillies, an equilibrium relative humidity data was generated within a moisture content of 400-5% d.b. using a modified Oswin formula, which was proved to be the best for describing the desorption of chillies:[Citation12]
The calculated values of pv and pvs at the same equilibrium moisture contents were then plotted on logarithmic scales. The slopes of the resulting straight lines were determined to give the latent heat of vaporization ratio (Lc/L). An equation was developed to describe the latent heat of vaporization ratio as a function of moisture content. A model was also developed to determine the total latent heat of vaporization of moisture in chillies within the moisture limits of 400-5% d.b.
RESULTS AND DISCUSSION
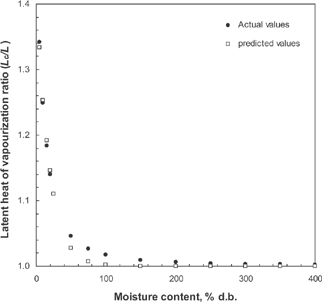
The equilibrium relative humidity data generated by using the modified Oswin formula and the calculated equilibrium vapor pressure in chillies at different temperatures and moisture contents are presented in . shows that the latent heat required for vaporizing moisture from chillies decreased with increasing moisture content. This phenomenon confirms the fact that at higher moisture levels, the strength of water binding decreases. This result in confirms with the result quoted in the case of alfalfa pellets.[Citation10] Lower values of latent heat of vaporization of moisture from chillies compared to those of wheat[Citation13] and brown parboiled rice[Citation2] below 12% d.b. moisture content indicate that the chillies desorb moisture easier than wheat and brown parboiled rice. The quantity of latent heat required for vaporizing moisture from chillies increased considerably as the moisture content of chillies decreased below 100% d.b. (). Similar results were noticed in the case of brown parboiled rice,[Citation2] wheat,[Citation6] malt,[Citation7] shelled corn,[Citation8] soybean,[Citation9] and alfalfa pellets.[Citation10] The latent heat of vaporization of moisture from chillies at a moisture content of 5% d.b. was more by 34.21% than that of free water (). Furthermore, the latent heat of vaporization of free water is not significantly different than the latent heat of vaporization of moisture from red chillies for moisture contents above 150% d.b. (). Beyond this moisture content, the moisture existing in the chillies may be considered as free water. A relationship between the latent heat of vaporization of moisture ratio and the equilibrium moisture content can be expressed by the following expression:
Table 1 Equilibrium relative humidity (ERH) and actual pressure of water vapor (pv ) in chillies at different moisture contents and temperatures
Table 2 Latent heat of vapourization ratio (Lc/L) at different moisture contents
with a R2 value of 0.99. The total latent heat of vaporization of moisture from chillies, Lc (M 1 to M 2) within a moisture range of M 1 and M 2 can be determined by using the following formula:
Substituting Lc value in equation 7,
Considering ‘dM’ as an infinitesimal increment in moisture content and integrating the above equation within the limits M1 and M2 ,
Substituting the values of M1 (more value) and M2 (less value) resulted in a negative value for Lc (M 1 to M 2), which implies the heat requirement to vaporize the moisture from chillies. Hence, it is multiplied with –1 to get a positive quantity of Lc (M 1 to M 2).
where M1 ≤ 400% d.b. and M2 ≥ 5% d.b.
However, the latent heat of vaporization of free moisture, L (kJ/kg) can be expressed as a function of temperature, t (°C) as follows:[Citation14]
Equation (10) is useful in determining the total latent heat of vaporization of moisture from chillies within the moisture limits of 400-5% d.b.
cONCLUSIONS
The latent heat of vaporization of moisture from red chillies was high at low moisture contents and low at high moisture contents. The moisture inside the chillies behaved almost like free water when the moisture content of chillies was above 150% d.b. The developed model is useful in determining the total latent heat of vaporization of moisture in chillies within the moisture limits of 400-5% d.b.
NOMENCLATURE
| a,b,c | = |
dimensionless coefficients |
| C | = |
constant of integration |
| ERH | = |
equilibrium relative humidity, decimal |
| L | = |
latent heat of vaporization of free moisture, kJ/kg |
| Lc | = |
latent heat of vaporization of moisture from chillies, kJ/kg |
| Lc (M 1 to M 2) | = |
total latent heat of vaporization of moisture from chillies within a moisture range of M1 and M2 , kJ |
| M | = |
equilibrium moisture content, % d.b. |
| M1 | = |
moisture content of fresh chillies, % d.b. |
| M2 | = |
moisture content of dried chillies, % d.b. |
| pv | = |
actual pressure of water vapor (kPa) |
| pvs | = |
saturation pressure of water vapor (kPa) |
| t | = |
temperature, °C |
| T | = |
absolute temperature, K |
| vl | = |
specific volume of liquid, m3/kg |
| vv | = |
specific volume of vapor, m3/kg |
| Wb | = |
weight of bone dry material of chillies, kg |
REFERENCES
- Singh , H. and Alam , A. 1982 . Techno-economic study on chilli drying . J. Agr. Eng. , 19 ( 1 ) : 27 – 32 .
- Palanimuthu , V. and Chattopadhyay , P.K. 1993 . Prediction of heat of vaporization of moisture from cereal grains – A modelling approach . Drying Technol. , 11 : 1855 – 1862 .
- Henderson , S.M. 1952 . A basic concept of equilibrium moisture . Agr. Eng. , 33 : 29 – 32 .
- Lal , R. and Agarwal , K.K. 1968 . “ Principles and Practices of Rice Drying ” . In Dept. Agrl. Eng , 54 Kharagpur, India : Indian Institute of Tecnology .
- Bala , B.K. 1997 . Drying and Storage of Cereal Grains , 63 New Delhi, India : Oxford & IBH Publishing Co. .
- Gallaher , G.L. 1951 . A method of determining the latent heat of agricultural crops . Agr. Eng. , 32 : 34 – 38 .
- Bala , B.K. and Woods , J.L. 1984 . Simulation of deep bed malt drying . J. Agr. Eng. Res. , 30 : 235 – 244 .
- Strohman , R.D. and Yoerger , R.R. 1967 . A new equilibrium moisture content equation . Trans. ASAE , 10 ( 5 ) : 675 – 677 .
- Alam , A. and Shove , G.C. 1973 . Hygroscopicity and thermal properties of soybeans . Trans. ASAE , 16 ( 4 ) : 707 – 709 .
- Fasina , O.O. and Sokhansanj , S. 1993 . Equilibrium moisture relations and heat of sorption of alfalfa pellets . J. Agr. Eng. Res. , 56 : 51 – 63 . [CROSSREF]
- Othmer , D.F. 1940 . Correlating vapor pressure and latent heat data . J. Industrial Eng. Chem. , 32 : 841 – 856 . [CROSSREF]
- Kaleemullah , S. 2004 . Moisture sorption isotherms of red chillies . Biosystems Eng. , 88 ( 1 ) : 95 – 104 . [CROSSREF]
- Cenkowski , S. , Jayas , D.S. and Hao , D. 1992 . Latent heat of vaporization for selected foods and crops . Canadian Agr. Eng. , 34 : 281 – 286 .
- ASAE . 1992 . “ Psychrometric Data ” . In Standards , 4 – 11 . St. Joseph, Michigan, USA : ASAE .