Abstract
A derivatization procedure for the production of a cold gelling whey protein isolate (WPI) has been identified. The cold gelling derivatized whey protein isolate (dWPI) imparted greater viscosity and water holding ability when rehydrated at room temperature than unmodified whey powders. The objective of this study was to further characterize the foaming and emulsifying functionality of the derivatized ingredient. Samples were prepared by hydrating dWPI and WPI in deionized water and, when needed, adjusting the sample pH from 3.4 to 6.8, with 6M NaOH. Yield stress, drainage, and overrun were measured for 6.5% WPI and dWPI foams. Emulsifying capacity and creaming stability were determined for various WPI and dWPI emulsions. The overrun of dWPI foams was approximately 50% lower than WPI foams at pH 3.4 and 6.8. Foams of the derivatized ingredient were significantly more stable than WPI foams. The derivatized ingredient displayed a similar emulsifying capacity to WPI at pH 3.4 and pH 7.0, and differences were not observed in creaming of dWPI and WPI emulsions. Information on foaming and emulsifying ability of derivatized protein ingredients will expedite the development of applications with the novel dairy ingredient, particularly in those foods desiring an all-natural, or all dairy, food label.
INTRODUCTION
Whey proteins are “label-friendly” ingredients widely used for the formation and stabilization of food emulsions and foams.[Citation1–3] The ability of whey proteins to serve as emulsifying or foaming agents depends on their ability to adsorb at water-oil or water-air interfaces and prevent droplet coalescence.[Citation4] Surface-active functional performance of whey proteins is directly linked to protein structure, which varies based on several environmental factors (pH, ionic strength, and protein concentration).
Emulsifying properties are important to many food applications, and several authors have characterized the emulsifying properties of whey proteins.[Citation5–10] An emulsion is a suspension of two immiscible liquids. Emulsifying properties are commonly discussed in terms of emulsifying capacity (EC) and emulsion stability (ES) (Citation11), and various methods have been applied to measure both properties. Emulsifying capacity is a qualitative term used to describe the amount of oil a protein can adsorb to stabilize a 2-phase system. Swift et al.[Citation12] developed an approach for evaluating EC in model food systems. The basic method consisted of blending a protein slurry with oil to form an oil-in-water emulsion. Additional oil was added to the system until the emulsion collapsed or inverted to a water-in-oil emulsion. EC was reported as the amount of oil added per 100 mg protein. Adopting this approach, Chobert et al.[Citation13] compared the emulsifying capacity of whey protein concentrate (WPC) hydrolyzed at various levels by trypsin. The emulsifying capacity of unhydrolyzed WPC was almost constant from pH 1.0-9.0, with a slight decrease in EC near the isoelectric pH (∼5.2). The emulsifying capacity improved after enzymatic treatment of the proteins.
Food emulsions are thermodynamically unstable and, given enough time, will collapse as the two phases attempt to minimize contact area.[Citation14] Emulsion stability has been empirically characterized by a number of authors[Citation13,Citation15,Citation16] to quantify the extent of creaming (or settling) in an emulsion. Creaming occurs as a result of the differences in density between the two phases under the influence of gravity, leading to phase separation.[Citation14] One method described by Demetriades and McClements[Citation15] requires measurement of cream and serum layer height, and relating the two values through calculation of a creaming index. The creaming index was correlated with emulsion stability to provide indirect information about the extent of droplet aggregation in an emulsion.[Citation15] Kulmyrzaev et al.[Citation17] used this method to study the influence of pH on droplet creaming of whey protein emulsions. This study found that oil-in-water emulsions were stable to creaming at low and high pH, but were less stable at a pH close to the isoelectric pH of whey protein.
Protein foams are dependent on protein surface activity and film forming properties,[Citation18] which are governed by protein structure. The characterization of liquid foams is difficult because the structure is complex, delicate, and often unstable.[Citation19] Prud'homme and Khan[Citation20] suggested the use of a vane device for measuring the yield stress of foams. A vane is a bladed, rheological device intended to minimize slip and sample disturbance. Numerous researchers have applied this method to describe yield properties of foods.[Citation21–23] Pernell et al.[Citation19] successfully applied the vane method to relate egg white and whey protein foam yield stress to “stiffness.”
Emulsifying and foaming properties of whey proteins are improved by partial protein unfolding leading to additional exposed hydrophobic regions.[Citation1] Processing-induced structure modifications affect protein functionality. For example, Resch and Daubert[Citation24] described a whey protein modification process involving a thermal treatment at low pH to form a weak gel, then spray drying the gel to a powder. The spray–dried derivatized whey powders imparted greater viscosity and water holding ability when rehydrated at room temperature than unmodified whey powders—a functionality improvement. The goal of this research was to determine the emulsifying and foaming ability of derivatized whey protein isolates (dWPI), thus further characterizing functionality of the protein ingredient. Understanding foaming and emulsifying properties of dWPI will provide insight for potential, functional roles in food formulations.
MATERIAL AND METHODS
A commercial whey protein isolate (WPI) powder, containing approximately 93.1% (w/w) protein, was used for all experiments (Bipro, Davisco International Inc., Le Sueur, MN). The elemental content of WPI was analyzed by the Analytical Services Laboratory (Raleigh, NC), using a Perkin-Elmer PE 2400 CHN Elemental Analyzer (Perkin Elmer Corp., Norwalk, CT). The protein content was calculated on a nitrogen basis (N × 6.38). All chemicals, NaCl, NaOH, and HCl, were purchased from Fisher Scientific Company (Norcross, GA), and corn oil was purchased from a local supermarket.
Derivatized Powder Production
Whey protein isolate powder was dispersed in deionized (DI) water to form an 8% (w/w) protein solution and allowed to stir for approximately 1hr. The pH was slowly adjusted to 3.4 with 6M HCl. The solution was transferred for thermal treatment to a double-jacketed steam kettle equipped with a scraped surface agitator. The solution temperature was increased to 80°C at approximately 5°C/min and held at 80°C with constant agitation for 1hr. Following heat treatment, the solution had formed a semisolid gel. The gel was transferred at a flow rate of 2 liter/hr by a peristaltic pump (Model 7553-80, Cole-Parmer Instrument Company, Vernon Hills, IL) to a pilot scale spray dryer (Anhydro, Attlesboro Falls, MA) operating at inlet temperature of 88°C, outlet temperature of 32°C, and 15 psi at the nozzle. Approximately 20 replications of this procedure were required to produce enough powder for experimentation. All lots were combined into one stock dWPI powder, and the stock was stored in an airtight container prior to analyses.
Emulsifying Capacity
Emulsifying capacity (EC) measurements were made using the modified method described by Webb et al.[Citation25] Whey protein isolate (WPI) and dWPI solutions (0.001, 0.003, 0.005, 0.010, 0.015% protein, w/w) were prepared by dispersing the powders in DI water. Minor pH adjustments were made with 1N NaOH or HCl prior to final dilution to ensure a solution pH of 3.4. A 150 ml aliquot of each sample was added to a commercial Waring blender (Model 5011, Waring Products, Inc., New Hartford, CT) equipped with two electrodes placed such that each was in continuous contact with the solution during the blending operation (). The blender was always operated on HI speed (22,000 RPM with no load). During blending, corn oil was added continuously at a rate of 1ml per second (Peristaltic Pump Model 7553-80, Cole-Parmer Instrument Company, Vernon Hills, IL) through a Tygon tube (7 mm diameter) positioned such that the dispensing end was in close proximity to the blender blades. This location prevented the accumulation of sizable quantities of oil on top of the developing emulsion. The oil temperature was maintained at approximately 23°C throughout the experiment. Emulsion formation and inversion was monitored by measuring resistance with a multimeter (Omega HHM26, Omega Engineering, Stamford, CT) equipped with an RS232 output to a computer. Inversion of the emulsion was clearly defined as the point at which resistance abruptly spiked. Each sample was run in triplicate, and the amount of oil added was calculated by time and flow rate measurement. The average ml oil emulsified per 100 mg protein was reported as emulsifying capacity. A blank containing only water was also tested to allow for correction of EC values.
Emulsion Stability
Stock solutions containing DI water and 5 mg/ml protein (WPI or dWPI) were prepared. Sodium azide (0.04% w/w) was added to each stock solution to prevent microbial growth, and then the pH of each solution was adjusted to 3.4 or 6.8 prior to final volume adjustment. Equal amounts of corn oil and protein solution (25 ml each) were homogenized in an Omni Mixer Homogenizer (Model 17105, Ivan Sorvall, Inc., Newtown, CT) at speed 3.5 (approx. 5,000 rpm) for 2 min.
Emulsion stability was measured according to the method of Keowmaneechai and McClements.[Citation26] Ten grams of each emulsion was placed in a glass test tube (internal diameter 16 mm and height 100 mm) and then stored at an ambient temperature for 14 days. After storage, all of the emulsions separated into three layers, with a creamed layer at the top, a transparent serum layer at the bottom, and an emulsion layer in the middle. The total height of the emulsions (He) and the height of the serum (Hs) were measured in triplicate. The creaming index was reported as:
Foam Preparation
Stock solutions containing 6.5% (w/w) protein were prepared using unmodified WPI or the dWPI ingredient. Prior to final weight adjustment with DI water, the pH of each solution was adjusted to either 3.4 or 6.8 with 1M NaOH and 1M HCl. Solutions were stored overnight at 4°C and allowed to equilibrate at room temperature under mild agitation for at least 1 hr prior to foaming experiments. The apparent viscosity of each solution was measured prior to foam formation. To minimize sedimentation, a Haake VT550 viscometer (Karlsruhe, Germany) equipped with a Haake pitched-paddle impeller (blade diameter = 0.04143 m, blade height = 0.02692 m) was adopted. Applying the matching viscosity method,[Citation27] an apparent viscosity was calculated for each solution. Apparent viscosity readings were obtained over a 2 min interval while shearing the sample at 50 s−1 (100 rpm). Foams were formed using a Kitchen Aid Ultra Power Mixer (Kitchen Aid, St. Joseph's, MI) with a 4.5 qt (4.3 L) stationary bowl and rotating beaters. Solutions (200 ml) were whipped at speed setting 8 (planetary rpm of 225 and beater rpm of 737) for 20 min. At least three replications were made for each treatment.
Foam Characterization
Drainage
Foam drainage was measured to better understand the stability of foams over time. Foams were formed as previously outlined with slight modification to the bowl. A 0.25” hole was drilled into the underside of the bowl and sealed during whipping. After foam formation, the bowl was positioned on a ring stand with the hole directly over a scale, tared with a weigh boat. The bowl was marked so that the position of the hole on the ring stand was consistent throughout each replication. The weight of liquid drained from the foam was measured every 5-10 min for 1 hr. Percent drainage was calculated as:
Overrun
Overrun is a measurement of how much air is whipped into a system. For this study, a standard weigh boat of known volume was filled with protein solution prior to whipping. The weight of this volume of solution was recorded, and the solution was whipped as outlined above. Following foam formation, the foam was gently scooped from the bowl in a circular pattern with a rubber spatula, filling the same weigh boat. Four replications were made for each foam, and the mean was used to calculate overrun according to Campbell and Mougeot:[Citation28]
Yield stress
Yield stress of each foam was measured in triplicate, using vane rheometry according to the method of Pernell et al.[Citation19] The appropriately sized vane was used with a Brookfield YR-1 (Brookfield Engineering Laboratories, Inc. Middleboro, Massachusetts) viscometer, set to rotate at a speed of 0.3 rpm. Upon completion of whipping, the beaters were carefully removed, and the vane was lowered to the proper measurement position. Yield stress was measured in triplicate at three different positions within the foam.
RESULTS AND DISCUSSION
Emulsifying Capacity
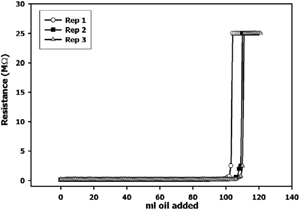
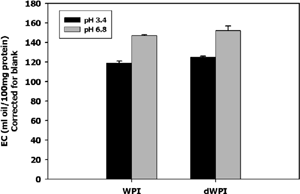
shows three replications of a 1 mg/ml WPI solution. Continuous resistance measurement allowed a precise detection of the inversion point from an oil-in-water (o/w) emulsion to a water-in-oil (w/o) emulsion. This inversion point is seen as a spike in resistance, since water, the continuous phase in an o/w emulsion, conducts electricity better than oil. There was not a significant difference (p< 0.05) between EC of dWPI and WPI (). Emulsifying capacity was dependent on pH, showing greater emulsifying capacity (p<0.05) at pH 6.8 than at pH 3.4.
Emulsion Stability
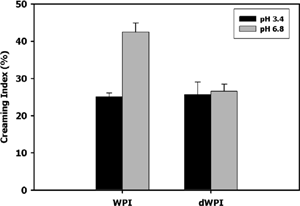
presents apparent viscosity of 6.5% (w/w) protein WPI and dWPI solutions. At 6.5% (w/w) protein, the derivatized whey ingredient displayed apparent viscosities more than twice those of unmodified WPI. Prior to the emulsion stability measurement, the derivatized whey ingredient was hypothesized to improve emulsion stability by increasing the apparent viscosity of the continuous phase, thereby limiting oil droplet flocculation. Visual inspection of emulsified samples after seven days storage at 25°C showed very little phase separation or creaming. Kulmyrzaev et al.[Citation17] reported similar results for WPI at pH 3.4 and 6.8. The samples were allowed to settle for an additional seven days to see if differences occurred over longer storage periods. On day 14, the height of the emulsion (He) and serum (Hs) layers was measured, and the creaming index was calculated (). The creaming index of WPI and dWPI was similar (approx. 25%) at pH 3.4. However, at pH 6.8, the WPI sample showed nearly twice as much creaming as dWPI. Most likely, the elevated apparent viscosity of the dWPI solution reduced the capacity for oil droplet flocculation, thereby increasing the overall stability to creaming.[Citation29]
Table 1 Apparent viscosity of 6.5% (w/w) protein WPI and dWPI solutions or disperations at 25°C.
Foam Characterization
Drainage
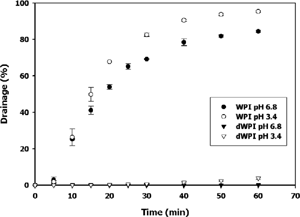
The drainage of a foam over a fixed time is used to define stability [Citation30]. At pH 3.4 and 6.8, foams produced from 6.5% protein (w/w) dWPI were more stable (p<0.05) than unmodified WPI foams of the same concentration (). In the case of unmodified WPI, at least 80% of the original foam volume drained after 60 min, whereas dWPI foams showed less than 5% drainage over the same period. The pH of the foaming solution also affected stability. Unmodified WPI at pH 3.4 was less stable (p<0.05) than unmodified WPI at pH 6.8. Derivatized whey protein samples showed similar, though less pronounced trends. Halling [Citation30] suggested that foam expansion is often higher for more rapidly draining foams. Increased foam stability may be due to differences in foam expansion, or overrun. Therefore, our next step measured the density, or overrun, of dWPI and WPI foams.
Overrun
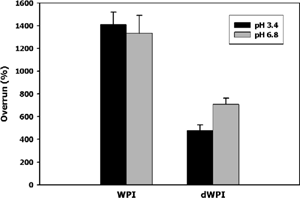
Derivatized whey protein foams demonstrated lower overrun than WPI foams independent of pH (). This result supported the earlier hypothesis that high foam expansion (or low density) leads to low foam stability. However, an exception to this hypothesis was seen in the dWPI sample at pH 6.8. Overrun of the dWPI sample at pH 6.8 was significantly higher (p<0.05) than dWPI at pH 3.4. In other words, the sample at pH 3.4 was more dense than the sample at pH 6.8. According to the hypothesis, the dWPI sample at pH 6.8 should be less stable and drain faster. However, according to , the dWPI sample at pH 6.8 was more stable to drainage than the dWPI sample at 3.4. A visually noticeable difference between stiffness of dWPI foams at pH 3.4 and pH 6.8 was noted during this experiment. The dWPI samples at pH 6.8 produced very stiff peaks after the 20 min of whipping required for this test. Further tests were therefore required to quantitatively characterize physical foam characteristics.
Yield stress
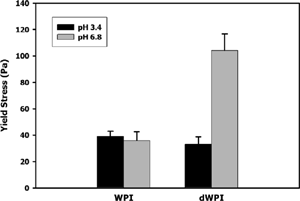
The yield stress of the WPI foams was hypothesized lower than dWPI foams due to overall differences in density or overrun. However, this hypothesis did not hold entirely true. Yield stresses of WPI foams at pH 3.4 and 6.8 were approximately 40 Pa (), and derivatized whey foams at pH 3.4 also had yield stress values of approximately 40 Pa. The dWPI foams at pH 6.8 had significantly higher (p<0.001) yield stresses than all other samples. Possibly, the heat denaturation of the dWPI ingredient, along with increased disulfide-sulfide interchange at pH 6.8, improved protein-protein interactions at the air/water interface. Increased protein-protein interactions would improve the elasticity of the continuous liquid phase of the foams, therefore increasing yield stress.
CONCLUSIONS
Derivatized whey protein isolates are capable of stabilizing foams and emulsions at an equivalent or surpassed level of native whey protein isolates. Emulsifying capacity of dWPI and WPI were similar at pH 3.4 and pH 6.8. However, dWPI creates a more stable emulsion than WPI at pH 6.8. This study showed that derivatized whey protein isolates create foams that are more stable and have lower overrun than unmodified WPI foams at pH 3.4 and 6.8. Unlike WPI foams, dWPI foam stiffness can be improved through pH adjustment. Results from this study should facilitate future formulations using the derivatized whey ingredient.
ABBREVIATIONS
| WPI | = |
whey protein isolate |
| WPC | = |
whey protein concentrate |
| dWPI | = |
derivatized whey protein isolate |
| DI | = |
deionized |
| EC | = |
emulsifying capacity |
| ES | = |
emulsion stability. |
ACKNOWLEDGEMENTS
The authors would like to thank Davisco Foods International, Inc. for providing whey protein isolate and the Southeast Dairy Food Research Center for supporting this study.
REFERENCES
- Kinsella , J.E. and Whitehead , D.M. 1989 . Properties in whey: chemical, physical, and functional properties . Advances in Food and Nutrition Research , 33 : 343 – 438 .
- Bryant , C.M. and McClements , D.J. 1998 . Molecular basis of protein functionality with special consideration of cold-set gels derived from heat-denatured whey . Trends in Food Science and Technology , 9 : 143 – 151 . [CROSSREF]
- Dickinson , E. 1999 . Adsorbed protein layers at fluid interfaces: interactions, structure and surface rheology . Colloids and Surfaces B-Biointerfaces , 15 : 161 – 176 . [CROSSREF]
- Dalgleish , D.G. 1996 . “ Food Emulsions ” . In Emulsions and Emulsion Stability , Edited by: Sjoblom , J. 287 – 325 . New York, NY : Marcel Dekker, Inc. .
- McClements , D.J. , Monahan , F.J. and Kinsella , J.E. 1993 . Disulfide bond formation affects stability of whey protein isolate emulsions . J. Food Sci. , 58 : 1036 – 1039 .
- Hunt , J.E. and Dalgleish , D.G. 1994 . Effect of pH on the stability and surface composition of emulsions made with whey protein isolate . J. Agric. Food Chem. , 42 : 2131 – 2135 . [CROSSREF]
- Dickinson , E. , Rolfe , S.E. and Dalgleish , D.G. 1989 . Competitive adsorption in oil-in-water emulsions containing α-lactalbumin . Food Hydrocolloids , 3 : 193
- Dickinson , E. 1997 . Properties of emulsions stabilized with milk proteins: over-view of some recent developments . J. Dairy Sci. , 80 : 2607 – 2619 .
- Demetriades , K. and McClements , D.J. 1998 . Influence of pH and heating on physicochemical properties of whey protein-stabilized emulsions containing a nonionic surfactant . J. Agric. Food Chem. , 46 : 3936 – 3942 . [CROSSREF]
- Das , K.P. and Kinsella , J.E. 1989 . pH dependent emulsifying properties of β-lactoglobulin . J. Dispos. Sci. Technol. , 10 ( 1 ) : 77 – 102 .
- Pearce , K.N. and Kinsella , J.E. 1978 . Emulsifying properties of proteins: evaluation of a turbidimetric technique . J. Agric. Food Chem. , 26 ( 3 ) : 716 – 723 . [CROSSREF]
- Swift , C.E. , Lockett , C. and Fryar , A.J. 1961 . Comminuted meat emulsions: the capacity of meats for emulsifying fats . Food Technology , 15 : 468
- Chobert , J.-M. , Bertrand-Harb , C. and Nicolas , M.-G. 1988 . Solubility and emulsifying properties of caseins and whey proteins modified enzymatically by trypsin . J. Agric. Food Chem. , 36 : 883 – 892 . [CROSSREF]
- Rousseau , D. 2000 . Fat crystals and emulsion stability—a review . Food Research International , 33 : 3 – 14 . [CROSSREF]
- Demetriades , K. , Coupland , J.N. and McClements , D.J. 1997 . Physical properties of whey protein stabilized emulsions as related to pH and ionic strength . J. Food Sci. , 62 ( 2 ) : 342 – 347 .
- Imm , J.Y. and Regenstein , J.M. 1997 . Interaction of commercial dairy proteins with chicken breast myosin in an emulsion system . J. Food Sci. , 62 : 967–971 – 975 .
- Kulmyrzaev , A. , Chanamai , R. and McClements , D.J. 2000 . Influence of pH and CaCl2 on the stability of dilute whey protein stabilized emulsions . Food Research International , 33 : 15 – 20 . [CROSSREF]
- Phillips , L.G. , German , J.B. , O’Neil , T.E. , Foegeding , E.A. , Harwalkar , V.R. , Kilara , A. , Lewis , B.A. , Mangino , M.E. , Morr , C.V. , Regenstein , J.M. , Smith , D.M. and Kinsella , J.E. 1990 . Standardized procedure for measuring foaming properties of three proteins, a collaborative study . Journal of Food Science , 55 ( 5 ) : 1441 – 1453 .
- Pernell , C.W. , Foegeding , E.A. and Daubert , C.R. 2000 . Measurement of the yield stress of protein foams by vane rheometry . Journal of Food Science , 65 : 110 – 114 .
- Prud'homme , R.K. and Khan , S.A. 1996 . “ Experimental results on foam rheology ” . In Food Colloids , Edited by: Prud'homme , R.K. and Khan , S.A. 217 – 241 . New York, NY : Marcel Dekker, Inc. .
- Yoo , B. , Rao , M.A. and Steffe , J.F. 1995 . Yield stress of food dispersions with the vane method at controlled shear rate and shear stress . Journal of Texture Studies , 26 : 1 – 10 .
- Daubert , C.R. , Tkachuck , J.A. and Truong , V.D. 1998 . Quantitative measurement of food spreadability using the vane method . Journal of Texture Studies , 29 : 427 – 435 .
- Tung , M.A. , Speers , R.A. , Britt , I.J. , Owen , S.R. and Wilson , L.L. 1990 . “ Yield stress characterization of structured foods. ” . In Engineering and Food , Edited by: Spiess , W.E.L. and Schubert , H. Vol. I , 79 – 88 . London : Elsevier Applied Science . Physical Properties and Process Control
- Resch , J.J. , Daubert , C.R. and Foegeding , E.A. 2004 . A comparison of drying operations on the rheological properties of whey protein thickening ingredients . International Journal of Food Science and Tecnology , 39 : 1 – 9 . [CROSSREF]
- Webb , N.B. , Ivey , F.J. , Craig , H.B. , Jones , V.A. and Monroe , R.J. 1979 . The measurement of emulsifying capacity by electrical resistance . Journal of Food Science , 35 : 501 – 504 .
- Keowmaneechai , E. and McClements , D.J. 2002 . Influence of EDTA and citrate on physicochemical properties of whey protein-stabilized oil-in-water emulsions containing CaCl2 . J. Agric. Food Chem. , 50 : 7145 – 7153 . [CROSSREF]
- Steffe , J.F. 1996 . “ Introduction to Rheology ” . In Rheological Methods in Food Process Engineering , 2nd , 1 – 93 . East Lansing, MI : Freeman Press .
- Campbell , G.M. and Mougeot , E. 1999 . Creation and characterization of aerated food products . Trends in Food Science and Technology , 10 : 283 – 296 . [CROSSREF]
- Keowmaneechai , E. and McClements , D.J. 2002(b) . Effect of CaCl2 and KCl on physiochemical properties of model nutritional beverages based on whey protein stabilized oil-in-water emulsions . Journal of Food Science , 67 ( 2 ) : 665 – 671 .
- Halling , P.J. 1981 . Protein-stabilized foams and emulsions . CRC Critical Reviews in Food Science and Nutrition , 15 ( 2 ) : 155 – 203 .