Abstract
The kinetics of chlorophylls (a and b) degradation were studied in yerba maté leaves at different temperatures (50 to 808C) and water activities (0.1 to 0.8). Chlorophyll changes followed a first order-kinetic reaction in all cases. Rate constant values were fitted to different models to describe temperature and water activity dependence. When an Arrhenius model was used, the activation energy was found to be dependant on water activity for chlorophyll a (80.4–99.7 kJ/mol), while for chlorophyll b activation energy showed only a little variation (88.1–89.5 kJ/mol). Color parameter a* underwent a great decrease while parameters L* and b* had only a little variation.
INTRODUCTION
Color, an important parameter in food quality, is associated with the presence of pigments in tissues. Chlorophyll is an important pigment that provides green color to many food products. However, thermal processing (i.e., blanching, drying) used to preserve some foods usually produces an important reduction of the chlorophyll concentration, thus, causing color changes. During processing, chlorophyll degradation follows two distinctive pathways[Citation1]: a) loss of magnesium to form pheophytin followed by the cleavage of the phytol chain to form pheophorbide; and b) cleavage of the phytol chain to form chlorophyllide with the subsequent formation of pheophorbide after lost of magnesium. The removal of the magnesium is the result of heat or acidic substitution. Cleavage of the phytol chain is promoted by either chemical hydrolysis or enzymatic action.
Green color is an important quality attribute in yerba maté, a plant that grows in the central region of South America. The twigs and leaves of the plant, which are used to make a widely consumed tea, are annually cut and rapidly transported for processing. Industrial processing of yerba maté consists of four stages: blanching with hot burning gases (300-350°C), drying on a conveyor belt (where temperature material in bed is about 50-80°C), grinding, and seasoning.[Citation2] It has a similar composition to the traditional tea (thea sinensis): protein, 10.12%; sugar, 4.45%, fiber, 28.88%; ether extract, 3.15%; caffeine, 1.02%; water extract, 36.65%; starch, 8.58%; and ash, 6.76%. A tea prepared with 2g of yerba maté and 100 mL of water has a pH= 4.5.[Citation3] There is an extensive body of research about chlorophyll degradation in many products, including temperature, water activity (aw), and pH—some factors that affect chlorophyll and color degradation. Temperature influence was widely studied by many researchers on different products.[Citation4,Citation5,Citation6,Citation7,Citation8,Citation9,Citation10] In all cases, a first order degradation kinetic was used with temperatures according to an Arrhenius model and activation energies varying between 63 and 92 kJ/mol.
The effect of pH in fresh vegetables is related to chlorophyllase activity. Its optimum pH activity depends on the type of vegetable, and it is generally lower than 7. In processed vegetables (like our test material), due to the fact that magnesium is replaced by hydrogen ions, losses of chlorophylls diminish with an increase of pH. These losses were studied by Ryan-Stonehaun[Citation11] in peas, who found a decrease in the rate constant when the pH was increased from 5.5 to 7.5. Fuke and others[Citation12] found a similar behavior in kiwi fruit paste (they have worked at pH= 3.6, 6.7, and 9.5) and Min and others[Citation13] in cabbage, lettuce, and spinach (they have worked with a pH= 5.7 and 9).
The influence of water activity was widely studied on spinach by Lajollo and others.[Citation14] They followed the chlorophyll degradation at two temperatures and five water activity levels and found that for aw > 0.52, the degradation rate increased with water activity, while below this value the influence of water activity on chlorophyll degradation was minor. For example, at 37°C and aw = 0.11, chlorophyll a degradation was about 20% in 120 days; while with aw = 0.75, this degradation took place in 1.5 days. Morawicki and others,[Citation15] studying the influence of water activity on chlorophyll degradation in yerba maté, found that for aw levels less than 0.33, the water activity has no influence on the degradation rate. Color changes and chlorophyll degradation during the industrial processing of yerba maté was studied by Schmalko and Alzamora.[Citation15] They found that 80% of chlorophyll loss occurs during the blanching step (with hot burning gases), while only 10% takes place during the drying. The objective of this research was to study the kinetics of degradation of chlorophylls a and b and color changes in yerba maté leaves at typical industrial temperatures (50-80°C) and water activities (0.1-0.8) used at the drying step of yerba maté.
MATERIAL AND METHODS
Material
Yerba maté (Ilex paraguariensis Saint Hilaire), used as test material, was obtained from local producers. Processing, as was described earlier, consists of four stages. For the experiments, samples were taken after the blanching step. At this point, the leaves of yerba maté have mean moisture content of 16 ± 5% (wet basis).[Citation16]
Sample Preparation
Leaves were separated from branches and partially air dried at 60°C until the approximate moisture content was reached. This moisture content level was obtained from the aw and temperature values given by the experimental design and desorption isotherms of yerba maté leaves.[Citation17] Then leaves were ground with a cutter mill to a size of 40 mesh. In order to assure uniformity in the moisture content and to reach the desire aw, samples were maintained in a tightly closed container, which contains the corresponding saturated salt solution at refrigeration temperature (5-8°C) during 10 days.
Experimental Method
Different levels of water activity were generated using saturated salt solutions that were placed at the bottom of closed containers. The containers were maintained in an oven at the experimental temperatures. In each container, 12 flasks with the samples (approximately 5g) were placed at the beginning of the experiment; they then were removed periodically to determine chlorophyll, moisture content, and color. The first sample was taken after 30 minutes, corresponding to t = 0 (time theoretically calculated to reach the required temperature). The others samples were removed at different times according to the levels of temperature and water activity used; they varied between 0.5 and 408 hours. Experiments were carried out in triplicate. Different levels of water activities were generated with the following saturated salt solutions: KCl (0.789-0.812); NaNO3 (0.652-0.690); NaBr (0.497-0.514); MgCl2 (0.260-0.305); and LiCl (0.105-0.111).[Citation18] Temperature levels used in this research were 50, 60, 70, and 80°C.
Chlorophyll Content
Chlorophyll was quantified using an HPLC method with ultrasonic extraction of the analytes ([CitationMorawicki et al., 1999; Ryan-Stoneham and Tong, 2000). Ten ml of an acetone-water solution (85:15 in volume) was added to 1g of sample, and then sonicated for 5 min in an ultrasonic bath (Crest Ultrasonic Corporation 690 D). An aliquot was then taken and filtered though a 0.22 µm syringe filter. The solution (10 uL) was then injected in a Shimadzu LC6A chromatograph, with an integrator CR3A, UVis 200 linear detector, and a C18 Altima column, 5 Micron 250 × 4.6 mm. Operation conditions were: mobile phase: ethyl acetate: methanol: water (55:35:10 in volume); flow: 2 ml/min and detector UV-Vis at 435 nm; 0.01 AUFS. Calibration curves were generated by injecting chlorophylls a and b, from algae nidulans, at concentrations varying between 0.0074 and 0.666 mg/mL for chlorophyll a; and 0.0037 and 0.333 mg/mL for chlorophyll b. Standards were dissolved in an acetone:water solution (85:15 in volume).[Citation15]
Color Determination and Water Content
Color was measured with a Color Touch Colorimeter, Model ISO (Technidyne Corporation). Response parameters were in the CIELAB scale; where: L*= lightness; a*=green-red axis and b*= blue-yellow axis. Measures were carried out by triplicate with an observer angle of 10°. Water content was determined by measuring the loss in mass at 103 ± 2°C during 6 h.[Citation19]
RESULTS AND DISCUSSION
The concentrations of chlorophylls a and b at time zero were 1.850 and 0.853 mg/g(db), respectively, while their initial concentration ratios (a/b) varied between 1.75 and 2.52. It should be mentioned that leaves of yerba maté suffered a heat and drying treatment in order to condition the sample for this experiment. Consequently, the ratios in this experiment are different from those existing in green leaves (3:1).[Citation20] Mean values (of 3 replicates) of chlorophylls a and b concentrations were fit to first order equation:
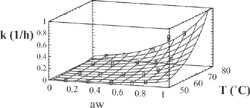
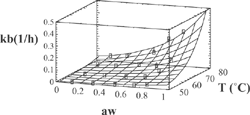
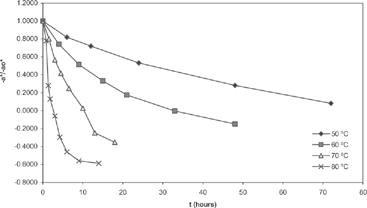
where C and C0 are the chlorophyll concentrations at time t and time zero, respectively; and k is the rate constant. shows experimental and fitted lines at 60°C at different water activities for chlorophyll a and b., and presents experimental and fitted data for a particular saturated salt solution (NaNO3) at different temperatures. and show the rate constant for chlorophyll degradation and correlation coefficients of the fitted line for chlorophylls a and b, respectively. Rate constants varied between 0.0046-0.5433 h−1 for chlorophyll a and 0.0042-0.2968 h−1 for chlorophyll b. Correlation coefficients varied between 0.980-0.998. In all cases, chlorophyll a degradation rate constant was greater than b, and their relation varied between 1.10 and 2.54. Many researchers determined chlorophylls’ degradation rate constants (k). They have worked with fresh products at high aw. Van Loey et al.,[Citation8] and Weemaes et al.[Citation9] working at 80°C with broccoli found rate contents values between 0.61-1.51 h−1 for chlorophyll a and 0.33-0.95 h−1 for chlorophyll b while Ryan-Stoneham and Tong[Citation11] found in pureed peas constant values of 2.8 h−1 and 1.3 h−1 for chlorophyll a and b, respectively, at 80°C and pH 5.5. In all cases, rate constant values were higher than those found in this research. This fact is probably due to the fact that they used higher aw. For chlorophyll a, the constants of the first order kinetic equation increased with the temperature for each level of aw (). The rate constant had also an increasing trend with rising levels of aw. However, there was no difference, or the differences were very small, in the rate constant values at low aw (for MgCl2 and LiCl saturated solutions). To study the dependence of the rate constant with the temperature and water activity, we fitted different models, without considering interactions between aw and temperature:
Figure 1 Experimental and theoretical values for chlorophylls a and b at 60°C and different water activities.
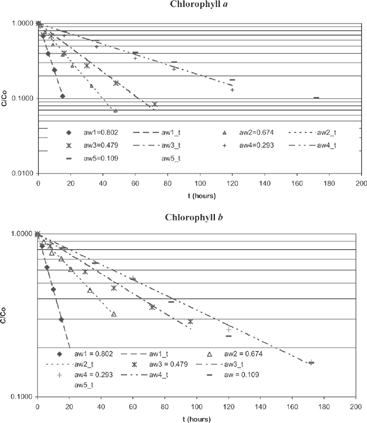
Figure 2 Experimental and theoretical values for chlorophylls a and b at aw2 (0.652-0.690) and different temperatures.
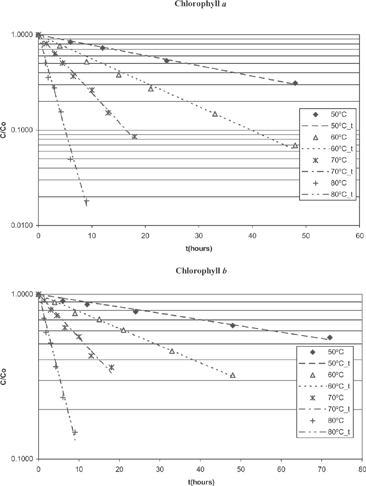
Table 1 Constants of the first order kinetic “k” (Eq. 1) and correlation coefficient for chlorophyll a in hours−1.
Table 2 Constants of the first order kinetic “k” (Eq. 1) and correlation coefficient for chlorophyll b in hours−1.
where T is the absolute temperature, ko is a constant, f(aw) is a function of aw (linear, logarithmic, negative reciprocal), R is the gas constant, and Ea is the activation energy. When these models were fitted to rate constant values, a high mean percentage error (> 30%) and a dependence of Ea on aw were found. Others equations that considered the dependence of ko and Ea with water activity were evaluated. The best fit was obtained with Eq. (3). Mean percentage errors were lower than that obtained with Eq. (2) (). Uddin and others,[Citation21] studying ascorbic acid degradation, found a similar dependence of activation energy with water content.
Table 3 Constant values of Eq. (3) (a, b, c, d) for chlorophylls a and b and color and mean percentage error (MPE).
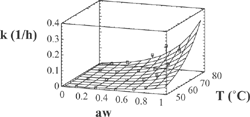
The agreement between experimental and predicted values, according to Eq. (3), of “k” vs. temperature and water activity is shown in and . Dependence of the activation energy with water activity, given by constant d (), is high for chlorophyll a and varied between 80.4-99.7 kJ/mol. For chlorophyll b, this dependence is small, and the activation energy varied between 88.1-89.5 kJ/mol. Values of activation energy were higher than those found for others vegetables.[Citation6,Citation8,Citation11] Degradation of chlorophylls, like others pigments, produce color changes. Particularly for chlorophyll, the degradation affects the coordinate “a*” of color, that represents the greenness.[Citation6,Citation9,Citation16,Citation22] For the present research, a high variation of this parameter (between –6.89 and 3.24) was observed in all the experiments (). However, a small variation of the parameters L* and b* was found. L* varied between 39 and 46 with a ± 2 error, while b* values varied between 27 and 31 with a deviation lower than ± 3. In many instances, it is useful to assimilate physical properties to concentration units and fit them to a first order equation. In this case, we fitted color parameter “a*” to the following equation:
where, a*, , and
are the actual, initial, and final values of “a*”, respectively. shows rate constants obtained with Eq. (4) and the correlation coefficients. These values were fitted to Eq. 3, and the values of constants and mean percentage error were found to be similar for chlorophylls a and b (). There was a good agreement between experimental and predicted values ().
Table 4 Constants of the first order kinetic “k” (Eq. 4) and correlation coefficient for color parameter “a*” in hours−1.
CONCLUSIONS
Temperature and water activity affected the degradation of chlorophylls in yerba maté. This degradation followed a first order kinetics with a good agreement between the experimental results and the kinetic equation. The rate constants (at different temperatures) were fitted to a model that considered their dependence with temperature and water content. Furthermore, the ratio of chlorophyll a to b did not remain constant during the experiment. This ratio fluctuated between 1.10 and 2.54. Values of rate constant were fitted to a model that considered the dependence on temperature and water content. A dependence of energy activation of chlorophyll a with water activity was found. When color variations (coordinates L*, a*, b*) were measured, a great variation of a* and low variations of L* and b* were found.
ACKNOWLEDGMENT
The authors would like to thank Mr. Fernando Felicia for his help in color measurements.
Notes
19. IRAM 20503 (Instituto de Racionalización de Materiales). Yerba Mate: Determinación de la pérdida de Masa a 103 °C, 1995
REFERENCES
- Heaton , W.H. , Rickey , Y.Y. and Marangoni , A.G. 1996 . Discoloration of coleslaw is caused by chlorophyll degradation . Journal of Agricultural and Food Chemistry , 44 : 395 – 398 .
- Ramallo , L.A. , Pokolenko , J.J. , Balmaceda , Z.G. and Schmalko , M.E. 2001 . Moisture diffusivity, shrinkage, and apparent density variation during drying of leaves at high temperatures . International Journal of Food Properties , 4 : 163 – 170 . [CROSSREF]
- Ramallo , L.A. , Smorczewski , M. , Valdez , E.C. , Paredes , A.M. and Schmalko , M.E. 1998 . Contenido nutricional de la Yerba mate en tres deferentes formas de consumo . La Alimentación Latinoamericana , 225 : 48 – 52 .
- Canjura , F. L. , Schwartz , S.J. and Nunes , R.V. 1991 . Degradation kinetics of chlorophylls and chlorophyllides . Journal of Food Science , 56 : 1639 – 1643 .
- Maharaj , V. and Sankat , C.K. 1996 . Quality changes in dehydrated dashen leaves: effects of blanching pre-treatments and drying conditions . Food Research International , 29 : 563 – 568 . [CROSSREF]
- Steet , J.A. and Tong , C.H. 1996 . Degradation kinetics of green color and chlorophylls in peas by colorimetry and HPLC . Journal of Food Science , 61 : 924 – 927 .
- Quintero-Ramos , A. , Bourne , J.B. and Anzaldúa-Morales , A. 1998 . Optimization of low temperature blanching of frozen jalapeño pepper (Capsicum annuum) using response surface methodology . Journal of Food Science , 63 : 519 – 522 .
- Van Loey , A. , Ooms , V. , Weemaes , C. , Van den Broeck , I. , Ludikhuyze , L. , Indrawati , D. S. and Hendrickx , M. 1998 . Thermal and pressure-temperature degradation of chlorophyll in broccoli (Brassica oleracea L. Italica) juice: A kinetic study . Journal of Agriculture and Food Chemistry , 46 : 5289 – 5294 . [CROSSREF]
- Weemaes , C.A. , Ooms , V. , Van Loey , A.M. and Hendrickx , M.E. 1999 . Kinetics of chlorophyll degradation and color loss in heated Broccoli juice . Journal of Agriculture and Food Chemistry , 47 : 2404 – 2409 . [CROSSREF]
- Shivhare , U.S. , Gupta , A. , Bawa , A.S. and Gupta , P. 2000 . Drying characteristics and product quality of okra . Drying Technology , 18 : 409 – 419 .
- Ryan-Stoneham , T. and Tong , C.H. 2000 . Degradation kinetics of chlorophyll in peas as a function of pH . Journal of Food Science , 65 : 1296 – 1302 .
- Fuke , Y. , Sasago , K. and Matsuoko , H. 1995 . Determination of chlorophylls in kiwi fruit and their changes during ripening . Journal of Food Science , 50 : 1220 – 1223 .
- Zhang , M. , Li , C. and Cao , P. 2004 . Effects of processing conditions of the green-leafy vegetable juice enriched with selenium on its quality stability . Journal of Food Engineering , 62 : 393 – 398 . [CROSSREF]
- Lajollo , F. L. , Tannenbaum , S.R. and Labuza , TP . 1971 . Reaction at limited water concentration 2. Chlorophyll degradation . Journal of Food Science , 36 : 850 – 853 .
- Morawicki , R.O. , Schmalko , M.E. and Känzig , RG . 1999 . Chlorophyll stability in yerba maté leaves in controlled atmospheres . Brazilian Archives of Biology and Technology , 42 : 85 – 90 .
- Schmalko , M.E. and Alzamora , S.M. 2001 . Color, chlorophyll, caffeine, and water content variation during yerba maté processing . Drying Technology , 19 : 599 – 610 . [CROSSREF]
- Känzig , R.G. , Schmalko , M.E. and Novo , M.A. 1987 . Comparación estadística de las isotermas de sorción de la yerba mate . Revista de la SGCyT-UNaM , 1 : 25 – 29 .
- Greenspan , L. 1997 . Humidity fixed points of binary saturated aqueous solutions . Journal of Research of the National Bureau of Standards-A. Physics and Chemistry , 81-a 1 : 89 – 96 .
- 19. IRAM 20503 (Instituto de Racionalización de Materiales). Yerba Mate: Determinación de la pérdida de Masa a 103 °C, 1995
- Francis , F.J. 1996 . “ Pigments and other colorants ” . In Food Chemistry , 3rd , 659 New York : Marcel Dekker Inc. .
- Uddin , M.S. , Hawlader , N.A. and Zhou , L. 2001 . Kinetics of ascorbic acid degradation in dried kiwifruits during storage . Drying Technology , 19 : 437 – 446 . [CROSSREF]
- Barth , M.M. , Perry , A.K. , Schmidt , S.J. and Kein , B.P. 1992 . Misting affects market quality and enzyme activity of broccoli during retail storage . Journal of Food Science , 47 : 954 – 957 .