Abstract
Thirty-six commercially and traditionally produced samples including seven fruit concentrates based on different fruit sources, seventeen boiled juices, and twelve tomato and paprika pastes were analysed for the determination of the hydroxymethyl furfural (HMF) and soluble solids content. The HMF concentrations of analysed food products showed a wide variability and were in a range between 0.4 and 3500 ppm. The HMF concentrations were found to be in a range of 0.4-4.5 ppm for fruit concentrates, 12.8-3500 ppm for boiled juices, and 0.4-18 ppm for tomato and paprika pastes. The soluble solids contents of food products ranged within the values of 13-66 0Brix for fruit concentrates, 64-79 0Brix for boiled juices, 29-54 0Brix for tomato pastes, and 55-61 0Brix for paprika paste. The HMF contents of the analysed concentrates showed significant differences (p<0.05). The highest HMF concentration was found for the boiled juice samples.
INTRODUCTION
Heat processing such as pasteurization, frying, roasting, or drying is the most common way of preserving food. Under adequate conditions, foods retain their expected organoleptic and nutritional properties; however, overprocessing may cause damage of constituents and decrease in the nutritional value.[Citation1] Nonenzymic browning reactions are one of the significant deteriorative reactions limiting the storage life of processed foods. They contribute color, flavor, and nutritional quality changes in foodstuffs during processing and storage. Additionally, many mutagenic and carcinogenic products, including melanoidins, furans, carbolines, and a variety of other hetorocyclic amines, are formed by several mechanisms of nonenzymic browning reactions.[Citation2] Thus, the safety of the food products that are exposed to nonenzymic browning is important for the health effects. There are many qualitative and quantitative methods for evaluating the extent of browning in food products; such as measurement of intensity of brown color and determination of intermediate and final products of nonenzymic browning reactions.[Citation3]
Hydroxymethyl furfural (HMF) is a recognized indicator of nonenzymic browning, and it is often used as an index of deteriorative changes which take place during excessive heating and/or storage of foods. In fresh foods, the HMF level is close to zero.[Citation4] However, it is found to be at a significant level in processed foods, and it is often used as a quality indicator.[Citation1,Citation5,Citation10] HMF has also been reported to be mutagenic.[Citation3] Thermal treatments during the manufacturing processes and unsuitable storage temperatures affect the quality of fruit-based products through nonenzymic browning reactions. Fruit juices and/or purees undergo flavor, taste, color, and nutritional changes when stored at warm temperatures and/or for prolonged periods of time.[Citation5, Citation6,Citation11,Citation13] HMF is one of the most widely used indices in nonenzymic browning studies on juices and fruit derivatives. The HMF content is important since it indicates the degree of heating of the treated products during processing and quantify this compound is considered as a quality parameter for concentrated food products. A large body of work has evaluated HMF as an indicator of nonenzymic browning in different fruit juices and concentrates,[Citation6,Citation9,Citation11,Citation14,Citation15] in jams and fruit-based infant foods,[Citation1] in boiled juice,[Citation8,Citation16,Citation18] and in tomato paste.[Citation7] The HMF formation in food products generally changes according to composition, processing type, and storage condition. The detection and quantitative determination of this component become important for the producer because HMF content gives some idea about the effects of processing type and processing and storage conditions on the quality of the food products. Since the food constituents participating in nonenzymic browning reactions in foods represent major dietary constituents, it is necessary to observe the safety and quality of food products that are consumed.
Numerous analytical methods have been developed for the determination of HMF in various food products.[Citation7,Citation12,Citation19 Citation22] The classical methods for the quantitative determination of HMF in food products are based on spectrophotometric measurements. In general, these methods lack specifity, are time consuming, use toxic or hazardous chemicals, and require a strick control of both reaction time and temperature.[Citation7,Citation12,Citation19] Chromatographic techniques used include ion exchange chromatography, gas chromatography, and, more recently, high performance liquid chromatography using UV detection at 280-285 nm.[Citation12,Citation19,Citation23] The high performance liquid chromatography (HPLC) method offers improved accuracy, sensitivity, and specifity compared to other methods, especially colorimetric methods.[Citation19] The objectives of this study are 1) to determine the HMF contents of various food products including fruit juice concentrates, boiled juice, and tomato and paprika pastes, and 2) to coordinate food products according to applied processes, especially heat treatment, and determine the effect of processing conditions on HMF formation in these products.
MATERIALS AND METHODS
Materials
The total number of samples was 36 and were collected under three main groups: 7 samples of fruit concentrates, 17 samples of boiled juices, and 12 samples of tomato and paprika pastes. Seven samples of fruit concentrates from various types of fruits including cherry, strawberry, apple, orange, peach, briar rose, and apricot were analysed. Analysed boiled juice samples included three commercially and fourteen traditionally produced boiled juices, which were obtained from local producers and markets. Also, they were grouped according to the fruit sources: four of them were mulberry, nine of them were grape, and four of them were boiled pomegranate juices. Twelve samples of pastes, including six samples of tomato pastes, and six samples of paprika pastes, were collected from local producers and markets. Clarifying agents included potassium ferrocyanide (Carrez I, K4Fe(CN)63H2O) and zinc acetate (Carrez II, Zn(CH3CO2)2H2O), which were purchased from Riedel De-Haen (Riedel De-Haen, Germany). Pure hydroxymethyl furfural standard was purchased from Sigma (Steinhein, Switzerland). HPLC grade methanol was purchased from Merck (Darmstad, Germany).
Sample Preparation
Each sample was homogenized by stirring with a spatula. A 10 g portion of sample was placed in 100 ml flask and 2 ml of each of Carrez I and Carrez II reagents was added. Then, the volume was taken up to mark with triple distilled water, and the mixture was homogenized. After standing 5 min, the mixture was filtered through filter paper and centrifuged for 10 min at 2500g. Next, 2 ml of homogenized supernatant was injected through HPLC after filtering through a disk filter (Minisart, Sartorius) having 0.2 μm pore size. Analyses were carried out in duplicate, and the results are presented as the mean ± standard deviation.
Methods
The analysis of HMF was carried out by HPLC. The chromatographic system used in this study consisted of a Schimadzu high performance liquid chromatography equipped with a LC-10 AD VP model quartet pump (Schimadzu, Japan), Hewlett Packard 1100 series UV detector (Hewlett Packard, Japan), Waters-Novapak C-18 column (150 mm*3.9 id), Waters-Novapak C-18 guard column, 20 μl injection port (Rheodyne, Japan), and software (Borwin, France). The mobile phase of HPLC consisted of a solution of methanol:water (10:90 v/v) mixture. The elution was isocratic, and the flow rate of mobile phase was 1 ml/min. The UV detector was set at 280 nm. The peak area obtained from the final chromatogram was calculated by Borwin (version 1.21) package programme. The HMF concentration of samples was obtained from the calibration curve of standard solution. Soluble solids content was determined by refractometry following the AOAC method.[Citation24]
Statistical Analysis
Statistical analysis of the obtained data was carried out by using SPSS (version 10.0) package programme at 95% confidence interval.[Citation25] One-way ANOVA with Duncan's multiple range test was used to compare experimental data.
RESULTS AND DISCUSSION
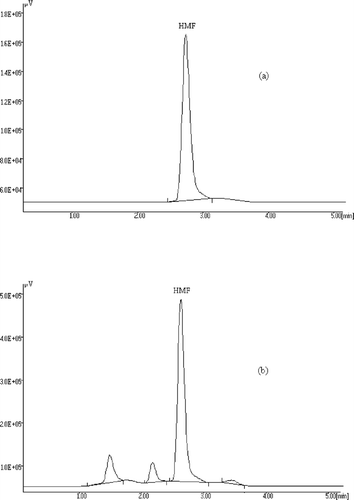
In this study, HMF contents of various concentrated food products, including fruit juice concentrates, boiled fruit juices, and tomato and paprika pastes, were determined. The same method and chromatographic conditions were applied to all of the samples with no need for modification. Under the chromatographic conditions of HPLC, the characteristic chromatographic peak of HMF appeared after 2.6 min. The typical chromatograms of a standard pure HMF and a boiled grape juice sample are shown in . The lowest HMF level that can be detected by HPLC was found as 0.1 ppm. The recovery of HMF from samples was determined by standard HMF addition method and the results were corrected according to 100% recovery. The recoveries were found as 97, 98, and 99.3% for pastes, fruit juice concentrates, and boiled juices, respectively.
Nonenzymic browning reactions more readily take place in concentrated fruit juice than in fresh juice, because the rate of the reaction depends on the amount of total soluble solids present in the juice or concentrate.[Citation14] Seven different samples of fruit juice concentrates were examined for the determination of HMF content. HMF concentration and soluble solids content of fruit juice concentrate, boiled juice, and paste samples are given in at . The soluble solids content of samples varied within the range of 13-66 0Brix. As shown in , the HMF was detected in all analysed samples, indicating the presence of this compound as the degradation product of nonenzymic browning reactions. HMF concentration of fruit juice concentrates varied from 0.4 to 4.5 ppm, regardless of the soluble solids content. Higher HMF concentrations of apple, cherry, and orange juice concentrates were significantly different (p<0.05) than those of others.
Table 1 Soluble solids content and HMF concentration of commercial fruit juice concentrates, boiled juices, and tomato and paprika pastes
In fruit juice concentrates, high temperature manufacturing processes such as concentration of fruit juices, dehydration of fruits, or storage at improper temperature were probably the cause of the formation of HMF. Garza et al.[Citation9] observed significant increase in HMF content during thermal treatment of peach puree at several high temperatures including 85, 90, and 98˚C. The HMF concentrations of all analysed fruit juice concentrates were appropriate to the standard HMF limit that should be max 25 ppm.[Citation26] Therefore, the possible high content of HMF was not observed in these products. This is a clear indication of a proper process of the fruit juice concentrates examined. The further risk of an increase in the browning products will be lower for concentrates produced under similar proper conditions (low temperature, vacuum application, etc.). If these concentrates are stored properly, they will reach the final consumer in good condition from the standpoint of the browning.
Boiled juice is produced by concentration of fruit juice up to 70-80% soluble solids content. The soluble solids content of boiled juice samples varied within the range of 64-79 0Brix. Highly variable HMF concentrations, changing from 12.8 to 3500 ppm, were obtained for the boiled juices (). These were 12.8-152 ppm for boiled mulberry juice, 31-200 ppm for boiled grape juice, and 514-3500 ppm for boiled pomegranate juice. The results were in agreement with the findings of Velioglu and Artik.[Citation27] Their findings showed that the HMF concentration was in a range of 13.4-236 ppm for several types including grape, fig, and mulberry boiled juices. It was observed that the HMF concentrations of analysed samples were not very dependent on the changing soluble solids content, whereas there was a significant difference (p<0.05) between the HMF concentration of different boiled juice types. It can be seen in that a higher range of HMF concentrations (514-3500 ppm) was obtained in boiled pomegranate juices. Pomegranate juices are highly acidic, with a pH of 3.5,[Citation28] and, thus, formation of HMF in those juices might be higher with respect to boiled grape and mulberry juices with a pH of 5.5.[Citation29] It was observed that the formation of HMF in boiled juice is highly dependent on pH.[Citation30]
When the HMF concentrations of boiled juice samples were compared with the standards,[Citation31] only 24% of the studied samples were in the limits of HMF concentration of the referred standard. This is a clear indication of excessive nonenzymic browning (caramelization and/or Maillard) in a significant number of boiled juice samples. HMF results of boiled juices were also significantly different (p<0.05) with respect to type of manufacturing processes. Traditionally produced samples had higher HMF values with respect to commercially produced ones. This may be due to the higher temperature and longer evaporation time of traditional method.[Citation16] Bozkurt et al.[Citation8] showed that the effect of increasing temperature on both HMF accumulation and brown pigment formation was significant in boiled juice. In the traditional method of boiled juice production, a longer time of evaporation process is necessary to meet desired soluble solids content (about 70 0Brix). During this time, the HMF content of boiled juice was probably increased, since the formation of HMF in boiled juice is significantly dependent on the time of process.[Citation16] Batu[Citation17] found that the HMF concentration of vacuum processed boiled grape juice was 32.2 ppm. On the other hand, it has been reported that the HMF concentration was 681.4 ppm in boiled grape juice produced by traditional method.[Citation17] From these finding, the results obtained in this study were in agreement with those reported in literature.
In this study, twelve samples of tomato and paprika pastes were analysed for the determination of HMF content. HMF concentration in analysed samples fell within the range of 0.4-18 ppm (). The soluble solids contents of analysed pastes changed from 29 to 61 0Brix, and paprika pastes had higher soluble solids contents with respect to tomato pastes. There was a significant difference (p<0.05) between the HMF concentrations of the two different types of pastes. HMF concentrations ranged within 3.6-18 ppm for tomato pastes and 0.4-6 ppm for paprika pastes. According to Duncan's multiple range test at 95% confidence interval, tomato pastes had higher HMF content with respect to paprika pastes. In tomato paste, 50-65% of the total solids are the reducing sugars, mainly glucose and fructose in almost equal proportions.[Citation32] Because of the high sugar content and low pH of the system, tomato pastes may give suitable conditions for occurrence of browning reactions and, subsequently, formation of accumulation product, hydroxymethyl furfural. When sugars are heated in the presence of acids, particularly organic acids, they yield furan compounds such that hexoses yielding HMF as the main furan derivative.[Citation33] The effect of processing type on HMF content is significant (p<0.05) for tomato pastes and non-significant (p<0.05) for paprika pastes. Various heat treatments are applied in commercial production of tomato and paprika pastes in which color has major importance. During hot breaking of tomatoes, high break temperatures may darken the color of the final product because of the caramelization of sugars. Furthermore, during concentration of tomato and/or paprika puree, nonenzymic browning reactions may excessively take place due to high evaporation temperature. Evaporation under partial vacuum pressure takes place at a lower temperature compared to open tank evaporation; consequently, the resulting paste retains most of the color and flavor of fresh fruit. In traditional processing, fruit juices or purees are concentrated by draining of the serum part of juice through a filter, such as a cheese-cloth (only for tomato paste). After draining, the resulting paste is evaporated under hot sunshine by spreading of the pastes on slabs.[Citation32] Thus, there might be lower contribution of nonenzymic browning on the overall browning in traditionally produced paste due to lower temperature.
CONCLUSION
In general, the HMF concentration in most of analysed concentrates has been found to be low, with the exception of boiled juice samples. When average HMF concentrations of each type of concentrates (fruit concentrates, boiled juices, tomato and paprika pastes) were statistically compared, it was seen that nonenzymic browning is significantly different (p<0.05) in the samples. HMF concentrations in boiled juice samples were the highest of all, with an average value of 727 ppm. The effect of processing type on HMF content was significant for boiled juices and tomato and paprika pastes. A higher HMF concentration was found in open type processed boiled juices with respect to vacuum type. Commercially produced tomato pastes had higher HMF concentrations than traditionally produced ones. Thus, it is necessary to improve processing conditions to prevent excessive browning in concentrates.
Notes
25. SPSS 1999. SPSS 10.0 for windows, statistical software. SPSS Inc., USA.
31. Anonymous. Pekmez, TSE No: 3791; Turkish Standard Institute: Ankara, 1983.
REFERENCES
- Rada-Mendoza , M. , Olano , A. and Villamiel , M. 2002 . Determination of hydroxymethylfurfural in commercial jams and in fruit-based infant foods . Food Chemistry , 79 : 513 – 516 .
- Friedman , M. 1996 . Food browning and its prevention: an overview . Journal of Agricultural and Food Chemistry , 44 : 631 – 653 . [CROSSREF]
- O'Brien , J. and Morrisey , P.A. 1989 . Nutritional and toxicological aspects of the Maillard browning reaction in foods . Critical Reviews in Food Science and Nutrition , 28 : 211 – 235 .
- Babsky , N.E. , Toribio , J.L. and Lozano , J.E. 1986 . Influence of storage on the composition of clarified apple juice concentrate . Journal of Food Science , 51 : 564 – 567 .
- Marcy , E.J. and Rouseff , R.L. 1984 . High performance liquid chromatographic determination of furfural in orange juice . Journal of Agriculture and Food Chemistry , 32 : 979 – 981 . [CROSSREF]
- Lee , H.S. and Nagy , S. 1988b . Relationship of sugar degradation to detrimental changes in citrus juice quality . Food Technology , 42 : 91–94 – 97 .
- Poretta , S. and Sandei , L. 1991 . Determination of 5-(hydroxymethyl)-2-furfural (HMF) in tomato products: proposal of a rapid HPLC method and its comparison with the colorimetric method . Food Chemistry , 39 : 51 – 57 . [CROSSREF]
- Bozkurt , H. , Gögüs , F. and Eren , S. 1998 . Nonenzymic browning reactions in boiled grape juice and its models during storage . Food Chemistry , 64 : 89 – 93 . [CROSSREF]
- Garza , S. , Ibarz , A. , Pagan , J. and Giner , J. 1999 . Nonenzymatic browning in peach puree during heating . Food Research International , 32 : 335 – 343 . [CROSSREF]
- Ramirez-Jimenez , A. , Guerra-Hernandez , E. and Garcia-Villanova , B. 2000 . Browning indicators in bread . Journal of Agriculture and Food Chemistry , 48 : 4176 – 4181 . [CROSSREF]
- Lee , H.S. and Nagy , S. 1988a . Quality changes and nonenzymic browning intermediates in grapefruit juice during storage . Journal of Food Science , 53 : 168 – 172 .
- Lo Coco , F. , Novelli , V. , Valentini , C. and Ceccon , L. 1997 . High performance liquid chromatographic determination of 2-furaldehyde and 5-hydroxymethyl-2-furaldehyde in fruit juices . Journal of Chromatographic Science , 35 : 578 – 583 . [PUBMED] [INFOTRIEVE]
- Ahmed , J. , Shivhare , U.S. and Kaur , M. 2002 . Thermal colour degradation kinetics of mango puree . International Journal of Food Properties , 5 : 359 – 366 . [CROSSREF]
- Toribio , J.L. and Lozano , J.E. 1986 . Heat induced browning of clarified apple juice at high temperatures . Journal of Food Science , 51 : 172 – 175 .
- Gögüs , F. , Düzdemir , C. and Eren , S. 2000 . Effects of some hydrocolloids and water activity on nonenzymic browning of concentrated orange juice . Nahrung , 44 : 438 – 442 . [CROSSREF]
- Gögüs , F. and Eren , S. 1997 . Pekmez imalati esnasinda esmerlesme reaksiyonlarinin hizinin belirlenmesi . Gida Teknolojisi , 2 : 34 – 39 .
- Batu , A. 1991 . Farkli iki yönteme göre elde edilen kuru üzüm pekmezinin kimyasal bileşiminde oluşan değişmeler üzerinde bir araştirma . Cumhuriyet Üniversitesi Tokat Ziraat Fakültesi Dergisi , 7 : 179 – 190 .
- Tosun , I. and Üstün , N.S. 2003 . Nonenzymic browning during storage of white hard grape pekmez (zile pekmezi) . Food Chemistry , 80 : 441 – 443 . [CROSSREF]
- Poretta , S. 1992 . Chromatographic analysis of Maillard reaction products: review . Journal of Chromatography , 624 : 211 – 219 . [CROSSREF]
- Kim , H.J. and Richardson , M. 1992 . Determination of 5-hydroxymethylfurfural by ion-exclusion chromatography with UV detection . Journal of Chromotography , 593 : 153 – 156 . [CROSSREF]
- Corradini , D. and Corradini , C. 1992 . Separation and determination of 5-hydroxymethyl-2-furaldehyde and 2-furaldehyde in fruit juices by micellar electrokinetic capillary chromatography with direct sample injection . Journal of Chromatography , 624 : 503 – 509 . [CROSSREF]
- Ames , J.M. , Arnoldi , A. , Bates , L. and Negroni , M. 1997 . Analysis of methanol-extractable nonvolatile Maillard reaction products of a model extrusion-cooked cereal product . Journal of Agriculture and Food Chemistry , 45 : 1256 – 1263 . [CROSSREF]
- Lee , H.S. , Rouseff , R.L. and Nagy , S. 1986 . HPLC determination of furfural and 5-hydroxymethyl furfural in citrus juices . Journal of Food Science , 51 : 1075 – 1076 .
- Association of Official Analytical Chemists . 1990 . Official Methods of Analysis , 15th , Arlington : AOAC .
- 25. SPSS 1999. SPSS 10.0 for windows, statistical software. SPSS Inc., USA.
- International Federation of Fruit Juice Producers . 1964 . Analysen-analyses , Switzerland : Fruit Union Suisse .
- Velioğlu , S. and Artik , N. 1993 . Bazi pekmez örneklerinin standarda (TS 3792) uygunluğunun belirlenmesi üzerine araştirma . Standard , 4 : 51 – 54 .
- Velioğlu , S. , Ünal , C. and Cemeroğlu , B. 1997 . Chemical characterization of pomegranate juice . Quality Control , 8 : 307 – 309 .
- Üstün , N.S. and Tosun , I. 1997 . Pekmezlerin Bileşimi . Gida , 22 ( 6 ) : 417 – 423 .
- Bozkurt , H. A M.S. thesis . 1996 . University of Gaziantep. Gaziantep
- 31. Anonymous. Pekmez, TSE No: 3791; Turkish Standard Institute: Ankara, 1983.
- Peter , G.G. 1973 . Tomato Paste and Other Tomato Products , 17 – 30 . London : Food Trade Press. Ltd. .
- Resnik , S. and Chirife , J. 1979 . Effect of moisture content and temperature on some aspects of nonenzymatic browning in dehydrated apple . Journal of Food Science , 44 : 601 – 605 .