Abstract
The thermal properties of plums (Prunus domestica) and prunes were investigated in the moisture content of 14.2-80.4% (wet basis) near room temperature (approximately 28°C). The apparent density of the fruits increased from 1042.9 to 1460.0 kg/m3, and the bulk density increased from 706.6 to 897.5 kg/m3 as the plums were dried, following classical empirical models as a function of moisture content. It was found that specific heat, effective thermal diffusivity, and effective thermal conductivity of the prunes increased with the moisture content of the samples, which can be represented by using different empirical models.
INTRODUCTION
In recent years, much attention has been given to the quality of processing products. Prunes are generally produced by drying fresh plums (Prunus domestica) with a stream of hot air. They can be consumed as ingredient of many commodities, such as bakery products (fruit bread, fruit loaf, cookies, coffee cakes, and pies), dairy foods (ice cream, yogurt, and desserts), cereals and cereal-based products, confectionery foods, condiments, and delicatessen products.[Citation1]
Specific heat, thermal diffusivity, and thermal conductivity are the most commonly used thermal properties that affect many food processing operations, such as simulation process of drying, rehydration, and packaging. These operations can be analyzed by mathematical modeling, which usually consists of solving the system of coupled differential equations for heat and mass transfer. This approach requires knowledge of these properties––which are scarce for foodstuffs in the literature––mainly as a function of moisture content. During unit operations, such as drying of fruits, heat transfer occurs through a mass of samples, so the effective (bulk) thermal conductivity and thermal diffusivity are more important than the properties of a single fruit. A similar consideration can be done for density of the samples and the bulk density of the drying process.[Citation2,Citation3] The objective of this work was to obtain data on the thermophysical properties of prunes: namely density, specific heat, effective thermal conductivity, and thermal diffusivity; and presenting equations to predict these properties as a function of moisture content.
MATERIAL AND METHODS
Sample Preparation
Samples of plums cv. Angeleno were acquired in a local market and stored at 7°C prior to use in experiments. The fruits were pretreated in a solution of 1.5% ethyloleate for 60 seconds at 50°C and then rinsed with tap water. Samples of plums and prunes were prepared by air drying at 65°C for the time required to achieve the moisture content range of 0.14–0.84 kg water/kg sample. The moisture contents of partial dried plums were equilibrated by using a packing plastic film for 24 hours at room temperature. The moisture content was analyzed by the vacuum oven method.[Citation4] The composition of the plum was determined, and the main results are shown as follow:[Citation4] total sugars: 10g/100g; fiber: 1.4g/100g; ash: 0.4g/100g; carbohydrate: 11g/100g; and vitamin C (total ascorbic acid): 6.5 mg/100g.
Experimental Apparatus and Measurement Procedure
Bulk and apparent density
The bulk density of plum and prunes at different moisture contents was determined by measuring the weight of the sample occupying a container of known volume. Preliminary measurements of the bulk density of sucrose powder were necessary to find a suitable container and to determine the reproducibility of experimental data. Tests showed no significant differences between the results obtained from a cylindrical container with a height to diameter ratio of 1.333 (0.40 m height × 0.30 m diameter) and a container of similar shape but having a height to diameter ratio of 2.25 (0.90 m × 0.40 m). The format of container did not affect the precision of measurements. Further, there were no significant differences in bulk density values between three and six determinations; hence bulk density was determined in three replicates. In the experiment, the container was filled with samples through a funnel that was fixed in such a way that its discharge outlet was at a level of 0.3 m above the lip of the container. The sample was carefully leveled to the lip of the container using a plastic ruler, and the material remaining in the container weighed on an electronic balance with a precision of 1mg.
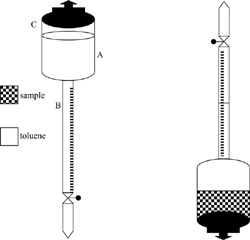
The apparent density of plum and prunes at different moisture contents was determined by the liquid displacement method; toluene was used as the liquid medium. Toluene, which is less dense than the samples, was used to ensure complete submergence of the material into the liquid. Samples weighing from 10 to 15 g were coated with a thin film of liquid paraffin by dipping to block off entry of the toluene into the open pores. The coated samples were then immersed in toluene according to Fig. .[Citation5]
The amount of liquid paraffin coating was determined by weighing, and a corresponding correction in volume was incorporated in the calculation of apparent density. Three replicates were made for each moisture content level. In order to establish the accuracy of the method used for measuring volume displacement, the experiment was repeated using sucrose powder of a known volume. The standard deviation of volume displacement reading was ±0.11 ml. Hence, the liquid displacement method used in the study was considered sufficiently accurate. An electronic balance measured the weight with an accuracy of 1 mg. The experimental apparatus (Fig. ) consists of a compartment (A), in which the sample is placed, and of a measuring burette. The lid C can close compartment A hermetically. The apparatus is filled with a suitable portion of a liquid, and the volume displacement is measured by turning it upside down twice, once without and once with the sample immersed.
Porosity
The bulk porosity of the sample, , was evaluated from the densities data by adopting the following relation:
Effective bulk thermal conductivity
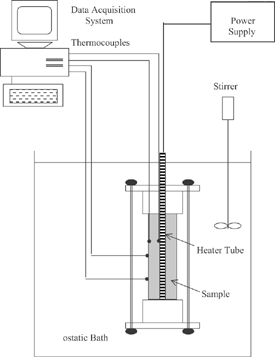
The effective thermal conductivity of prunes was obtained by using the experimental apparatus shown in Fig. . This method consisted of a cylindrical cell made of chromium-plated brass, similar to the apparatus used by Gabas et al.[Citation6] The methodology was based on maintaining a known heat flux from the center to external surface of the cell cylinder, and the temperatures were measured at two points in the radial direction. Both ends of the cell were fitted with nylon stoppers to prevent axial heat transfer. The power input to the heater resistance was made by means of a laboratory DC power supply (model MPS-3006D, Minipa, São Paulo, Brazil), which permitted an adjustment of the current with a stability of 0.05%. Temperatures were monitored with an accuracy of 0.6°C by a HP data logger model 75.000-B, an interface HP-IB, and a HP PC running a data acquisition program written in IBASIC. In order to measure temperature, respectively, one and three copper-constantan thermocouples were embedded at the surfaces of the inner and outer cylinders.
In the steady state, conduction inside the cell is described by the Fourier equation in cylindrical coordinates, with boundary conditions corresponding to heat transfer between the cylindrical surfaces kept at constant temperatures. An integrated form is given by the aquation
where is the heat flux in the thermal resistance (W), r the radius (m), R1 and R2 the external and internal radius of the cylinder (m), respectively, S the surface area of a cylinder of radius r (m2), T the temperature (°C), T1 the steady state temperature at the internal cylinder (°C), T2 the steady state temperature in the thermostatic bath where cell was immersed (°C), and λ the thermal conductivity of the sample at the average temperature (T1+T2)/2 (W/m °C). Eq. (2) can be applied to calculate the sample thermal conductivity, λ, from experimental measurements of T1 and T2 under steady state conditions.[Citation7]
Effective bulk thermal diffusivity
The thermal diffusivity of prunes was obtained by using the method of Dickerson,[Citation8] which is based on transient heat transfer, and can be expressed as
Specific heat
The specific heat was estimated from the effective thermal conductivity, bulk density, and effective thermal diffusivity data given by Eq. (4)
Empirical models
Empirical models are widely popular for the prediction of thermal conductivity, specific heat, thermal diffusivity, and densities due to the complexity of theoretical models and the scarce information on the chemical composition of these products in the literature. In this work, five typical models were applied in the prediction of these properties. The models are described as
where A, B, and C are the empirical constants and X is the fraction of moisture content in wet basis.
RESULTS AND DISCUSSION
Apparent and Bulk Density
The apparatus used for measuring bulk density and apparent density was calibrated by using sucrose (food grade) which has a well-known ρb and ρap. Eleven replicated experiments were carried, out and the average result is presented in Table , as well as a reference value reported by Rahman.[Citation9] A good agreement was observed between the experimental and reference values. The reproducibility of results was also satisfactory, as shown by standard deviations included on this Table. Bulk and apparent densities as a function of moisture content are shown on Table . The bulk porosity was calculated by using Eq. (1).
Table 1 Data obtained during calibration of the experimental apparatus for bulk density and apparent density.
Table 2 Bulk density (ρb), apparent density (ρap), and bulk porosity (ϵb) of plums and prunes at various moisture contents.
It was observed that the bulk density increased linearly while the moisture content decreased. This can be explained by the collapsing of the fruit cells, which resulted in a decreasing of air pores between the fruits at the same time a decrease in the volume occupied for the samples occurred. The results were best fit by the model 5 that is presented with respective coefficient determination (r2=0.961) of the following form:
The change of apparent density with moisture content was also fitted to Eqs. (5) to (9), and the best fit was obtained with a quadratic equation (Model 4) as follows (r2= 0.964):
Similar quadratic equation adjusted the apparent density of yerba mate twigs as a function of moisture content in duplicate.[Citation3]
Thermophysical Properties
The apparatus used for measuring effective thermal conductivity and thermal diffusivity was calibrated by using powdered magnesia and sawdust for effective thermal conductivity and lactose and glucose for effective thermal diffusivity, which has a well-known λeff and α. Eleven replicated experiments were carried out, and the average results are presented in and , as well as a reference value reported by Perry and Chilton[Citation10] and Rahman.[Citation9] A good agreement was observed between the experimental and reference values. The reproducibility of results was also satisfactory, as shown by the standard deviations included on these Tables. The results on the effective thermal conductivity, thermal diffusivity, and specific heat at different moisture contents are presented in Table . It can be seen that the increase on moisture content takes to an increase of these properties. The effective thermal conductivity of the fruits has been found to vary with moisture content by Model 1 (Eq. 5) leading to the following result (r2 = 0.995):
Table 3 Data obtained during calibration of the experimental apparatus for effective thermal conductivity and the reference values in similar bulk densities.
Table 4 Data obtained during calibration of the experimental apparatus for effective thermal diffusivity, α
Table 5 Effective thermal conductivity, effective thermal diffusivity, and specific heat of plums and prunes at various moisture contents.
According to Niesteruk,[Citation11] thermal properties of fruit and vegetables vary linearly with the moisture content, but not for all the range of humidity (0–100%). This can be due to different properties of water bounded in the product during drying. The effect of moisture content on effective thermal diffusivity (Table ) exhibited a good fit by Model 1, as follows (r2 = 0.981):
The specific heat was calculated from Eq. (4), and its dependence with the moisture content was well fitted by using Model 2 (Eq. 6). Results can be expressed by Eq. (14) with coefficient determination (r2) of 0.963:
Specific heat and thermal conductivity of mushrooms for a wide range of moisture contents with other two input variables (temperature and bulk density) was investigated by Shrivastava and Datta.[Citation2] The results of this investigation showed that changes in the moisture content range 10.24–89.68% (wet basis) had a strong linear correlation on these properties. According to Madamba et al.,[Citation12] the moisture content had a highly significant effect on the specific heat and thermal conductivity of garlic. These thermal properties were also investigated by Vagenas et al.[Citation13] for sultana grapes and raisins and varied linearly with the moisture content (14–80% wet basis).
CONCLUSIONS
Simple apparatus could have been used in the accurate measurement of bulk and apparent densities as well as thermal properties of plums and prunes as a function of moisture content. The results of bulk density and apparent density showed an increase with a decrease in moisture content, and they were well adjusted by using linear and quadratic models, respectively. The effective bulk thermal conductivity increased from 0.154 to 0.4 W/m K according to an increase of moisture content range of 0.142–0.804 (wet basis). At the same range of increasing moisture content, the thermal diffusivity presented a value from 0.958 to 1.6 × 10−7 m2/s. On the other hand, the specific heat showed an increase, varying from 1796 to 3536 J/kg K. It was obtained by different empirical equations in order to represent these properties as a function of the moisture content of the plums.
NOMENCLATURE
|
| = |
Heat flux in the thermal resistance (W) |
| Ω | = |
Constant rate of temperature rise (°C/s) |
| ρ | = |
Density (kg/m3) |
| α | = |
Effective thermal diffusivity (m2/s) |
| ϵ | = |
Porosity |
| λ | = |
Thermal conductivity (W/m °C) |
| Cp | = |
Specific heat (J/kg K) |
| L | = |
Length (m) |
| R | = |
Radius (m) |
| S | = |
Surface area (m2) |
| T | = |
Temperature (°C) |
| Subscript | = | |
| ap | = |
apparent |
| b | = |
bulk |
| c | = |
continuous phase |
| s | = |
surface |
| sa | = |
sample |
| eff | = |
effective |
REFERENCES
- Gabas , A.L. , Menegalli , F.C. and Telis-Romero , J. 2002 . Influence of drying conditions on the rheological properties of prunes . Drying Technology , 20 ( 7 ) : 1485 – 1502 .
- Shrivastava , M. and Datta , A.K. 1999 . Determination of specific heat and thermal conductivity of mushrooms (Pleurotus florida) . Journal of Food Engineering , 39 : 255 – 260 . [CROSSREF]
- Schmalko , M.E. , Morawicki , R.O. and Ramallo , L.A. 1997 . Simultaneous determination of specific heat and thermal conductivity using the finite-difference method . Journal of Food Engineering , 31 : 531 – 540 . [CROSSREF]
- AOAC . 1990 . Official Methods of Analysis , 15th , 1298 Washington, DC : Association of Official Analytical Chemists .
- Zogzas , N.P. , Maroulis , Z.B. and Marinos-Kouris , D. Densities, shrinkage and porosity of some vegetables during air drying . Drying Technology , 12 ( 7 ) 1653 – 1666 .
- Gabas , A.L. , Telis-Romero , J. and Telis , V.R.N. 2003 . Influence of fluid concentration on freezing point depression and thermal conductivity of frozen orange juice . International Journal of Food Properties , 6 ( 3 ) : 543 – 556 . [CROSSREF]
- Bellet , D. , Sengelin , M. and Thirriot , C. 1975 . Determination des proprietes thermophysiques de liquids non-newtoniens a l’áide d'une cellule a cylinders coaxiaux . International Journal of Heat and Mass Transfer , 18 ( 10 ) : 1177 – 1187 . [CROSSREF]
- Dickerson , R.W. 1965 . An apparatus for measurement of thermal diffusivity of foods . Food Technology , 19 : 198 – 204 .
- Rahman , S. 1995 . Food Properties Handbook , 1st , 500 pp Boca Raton, Florida : CRC Press, Inc. .
- Perry , R. and Chilton , C. 1986 . Manual de Engenharia Química , 5th , Rio de Janeiro : Guanabara Dois .
- Niesteruk , R. 1996 . Changes on thermal properties of fruits and vegetables during drying . Drying Technology , 14 ( 2 ) : 415 – 422 .
- Madamba , P.S. , Driscoll , R.H. and Buckle , K.A. 1995 . Models for the specific heat and thermal conductivity of garlic . Drying Technology , 13 ( 1&2 ) : 295 – 317 .
- Vagenas , G.K. , Marinos-Kouris , D. and Saravacos , G.D. 1990 . Thermal properties of raisins . Journal of Food Engineering , 11 : 147 – 158 . [CROSSREF]