Abstract
To analyze the impact of chloropicrin and 1,3-dichloropropene on fungal community structure in bulk soil and spinach rhizosphere soil in a field, developed a new nested polymerase chain reaction (PCR) method to facilitate the detection of major fungal taxa and we used the method to monitor 18S rDNA PCR-denaturing gradient gel electrophoresis (DGGE) profiles for 3 years. The cropping system consisted of soil fumigation in September and two subsequent consecutive spinach cultivations each year. The soil was treated with fumigants (chloropicrin at 20 mL m−2 or 1,3-dichloropropene at 32 mL m−2) and covered with polyethylene film for approximately 2 weeks. The nested PCR method with primer pairs AU2/AU4 and GC-FR1/FF390 successfully amplified sequences from all major fungal taxa, but not from plants or oomycetes, which were amplified from rhizosphere soil samples using direct PCR with the primer pair GC-FR1/FF390. The DGGE analysis of the nested PCR products indicated that the chloropicrin treatment had a greater impact on the fungal community than 1,3-dichloropropene, both in terms of the magnitude and duration of the effect. Chloropicrin treatment changed DGGE profiles drastically and reduced the diversity index H′ in both bulk soil and rhizosphere soil 2 months after fumigation. Profiles and diversity indices did not recover completely after 1 year. Bands with high sequence similarity to ascomycetous fungi decreased in intensity and, conversely, bands inferred to represent chytridiomycota became dominant. In contrast, 1,3-dichloropropene did not reduce the diversity index significantly after 2 months. The DGGE profiles of 1,3-dichloropropene plots revealed a smaller change 2 months after fumigation, but became indistinguishable from those of control plots after 6 months. Spinach cultivation also affected the soil fungal community structure because the differences in DGGE profiles between control and chloropicrin plots were smaller in rhizosphere soil than in bulk soil 2 months after fumigation. These results suggest that the rhizosphere effect may contribute to minimizing the effect of chemical fumigation.
INTRODUCTION
In the past, pre-planting soil fumigation with methyl bromide (MeBr) has been widely used around the world to control insects, nematodes, weeds and pathogens in high-value crops (CitationKobara 1998). However, the use of MeBr in soil fumigation was banned after 2005 because of its environmental risk and, therefore, the use of alternatives, such as chloropicrin (CP) and 1,3-dichloropropene (1,3-D), has increased (CitationDungan et al. 2003; CitationTateya 2005). In Japan, especially in areas that produce vegetables such as spinach, lettuce and tomato, continuous monoculture is widely adopted to increase profit, a practice that leads to occasional outbreaks of pests (CitationIwasaki 1999). Many areas are dependent on chemical fumigation of the soil for consistent production. Most of these fumigants are known to have a broad biocidal activity (CitationAnderson 1993) and can potentially harm beneficial organisms in addition to the targeted agricultural pests.
Fungi play important and diverse roles in agricultural ecosystems; they are plant pathogens, mycorrhizal symbionts and, most importantly, principal decomposers of organic material. However, there are relatively few studies on the effects of chemical fumigants on the non-target soil fungal community compared with the number of studies reporting the effects on specific target plant pathogens (CitationBrowning et al. 2006; CitationHamm 2003; CitationTakehara et al. 2003) and on the bacterial community (CitationDungan et al. 2003; CitationIbekwe et al. 2001). CitationItoh et al. (2000) and CitationTanaka et al. (2003) reported that the count of viable fungi decreased after CP fumigation. CitationDe Cal et al. (2005) used a culture-dependent method with selective media to show that chemical fumigants reduced members of the soil fungal population, such as Fusarium spp., Pythium spp. and Verticillium spp. However, cultivation techniques have limitations in their ability to describe total fungal community structure and dynamics in soil (CitationVandenkoornhuyse et al. 2002). Culture-independent methods using respiratory quinone (CitationKatayama et al. 2001) and phospholipid fatty acid analysis (PLFA) (CitationIbekwe et al. 2001; CitationKlose et al. 2006) have been used, but only provide an estimate of total fungal biomass in the soil.
Molecular techniques of direct DNA extraction followed by cloning or electrophoresis are increasingly being used. These methods have been useful in revealing the community structure of microbes, including unculturable microbes. Sequence analysis of the recovered DNA, usually of ribosomal RNA genes, allows researchers to infer the taxonomic position of microbes that exist in the sampled environment. One such technique, polymerase chain reaction–denaturing gradient gel electrophoresis (PCR-DGGE), has been used to analyze the genetic diversity of the rhizosphere fungal community in terms of the effects of plant growth (CitationGomes et al. 2003) and fertilizer and pesticide application (CitationGirvan et al. 2004; CitationSigler and Turco 2002; CitationYao et al. 2006). This technique provides novel insights and significant advances into research on soil fungal communities.
Various PCR-DGGE primer sets targeted for 18S rDNA are available for assessing fungal diversity in using soil DNA samples. A number of these primers have been noted for their limitations in primer bias and specificity (CitationAnderson and Cairney 2004). Primers are often designed to amplify a taxonomic range of fungi that is as broad as possible. Consequently, some primers also amplify other eukaryotic DNAs from mixed DNA samples because of high sequence similarity between 18S rDNA of fungi and that of other closely related eukaryotes belonging to the chromista and protozoa (CitationAnderson et al. 2003). In addition, the primer specificity sometimes varies among DNA samples (CitationAnderson and Cairney 2004). Although primer pair GC-FR1/FF390 was reported to be specific for fungi, we found that it could also amplify plant DNA in rhizosphere soil samples. Consequently, we wished to improve the PCR-DGGE method for the specific detection of fungal DNA.
The aim of the present study was to analyze the effect of two chemical fumigants (CP and 1,3-D) and spinach growth on fungal community structure in a field. We began by developing a nested PCR-DGGE method with a new combination of primer pairs, AU2/AU4 (CitationVandenkoornhuyse et al. 2002) and GC-FR1/FF390 (CitationVainio and Hantula 2000). Using this method, we monitored the 18S rDNA PCR-DGGE profiles of bulk soil and rhizosphere soil samples in a spinach field for 3 years.
MATERIALS AND METHODS
Field design and sampling
Experiments were carried out in a field at the National Institute for Agro-Environmental Sciences (NIAES), Japan. The soil was a Dystric-Silic Andisol, light clay (pH 5.5), containing 8.34% organic matter and 41.8% clay and fumigated with 99.5% CP (Mitsui Chemicals, Tokyo, Japan) or 92% 1,3-D (Asahi D-D 92; Kashima Chemicals, Ibaraki, Japan) in three replicated plots (each 5 m × 6 m) arranged according to the randomized Latin square method every September from 2001 to 2003. Each treatment consisted of three replicated plots: plots 2, 4 and 9 for the untreated control; plots 3, 5 and 7 for CP-treated; and plots 1, 6 and 8 for 1,3-D treated. The fumigants were injected at a depth of 17 cm with 30-cm spacing in a hound's-tooth pattern at a rate of 2 mL per spot (20 mL m−2) for CP and 2.4 mL per spot (32 mL m−2) for 1,3-D. After injection, the soil was covered with 0.05-mm-thick polyethylene film for approximately 2 weeks and then tilled to disperse the fumigants 1 week before the sowing of spinach (Spinacia oleracea L.), according to conventional fumigation practice in Japan. The field was sown with spinach in October (cv. Solomon, Sakata Seed Company, Yokohama, Japan) and in May (cv. Active, Sakata Seed Company) with an interval of 70 cm between rows. Whole spinach plants were plowed into the soil in April and July as green manure, except for one portion in each plot that was harvested for yield survey.
Bulk soil samples were taken periodically from September 2001 to April 2004 during the three fumigation trials (). Three core samples (15 cm of topsoil) were taken from the space between the rows in each plot and mixed after sieving to make one composite bulk soil sample. Rhizosphere soil samples were taken from November 2002 to April 2004 during the last two fumigation trials (). One composite rhizosphere soil sample representing each plot consisted of rhizosphere soil from 10 randomly selected spinach plants. Each plant was shaken carefully to remove loose soil. The soil adhering to the roots was defined as rhizosphere soil. Roots with adhering soil were weighed and added to 50-mL polypropylene tubes containing twice the soil volume of sterile sodium phosphate buffer (200 mmol L−1; pH 8). Samples in tubes were mixed by vortex for 3 min and sonicated for 5 min, and then the
Table 1 Sampling scheme of bulk soil and rhizosphere soil
DNA extraction from soil
Tubes containing 500 mg of bulk soil or 1 mL of rhizosphere soil slurry were stored at −20°C until use. DNA was extracted using the FastDNA Spin kit for soil (Bio101, Vista, CA, USA) according to the manufacturer's recommendations, except that skim milk was added at 40 mg g−1 bulk soil and 20 mg mL−1 rhizosphere soil slurry. It was necessary to use skim milk as a competitor during DNA extraction from some types of Andisol because of the problem of high DNA adsorption to clay particles (CitationHoshino and Matsumoto 2004).
Five or 10 µL of extract was subjected to electrophoresis on a 1% agarose gel (NipponGene, Toyama, Japan) in TAE buffer (20 mmol L−1 Tris, 10 mmol L−1 acetate, 0.5 mmol L−1 ethylenediaminetetraacetic acid [pH 7.4]) at 100 V for 25 min and visualized using ethidium bromide staining (0.5 µg mL−1). Gels were photographed and scanned with the ImageMaster VDS (Amersham Biosciences, Uppsala, Sweden). Genomic DNA in extracts was quantified by comparing their fluorescence intensity on agarose gels with that of DNA standard samples following the method of CitationZhou et al. (1996) using the ImageMaster software. DNA standard curves were obtained for each agarose gel containing three replicated lanes with 100–250 ng HindIII-digested λ DNA fragments (Invitrogen, Carlsbad, CA, USA).
Polymerase chain reaction amplification
Fungal 18S rRNA genes were amplified from soil DNA by PCR for DGGE. Primers for direct PCR were fungal universal primers GC-FR1 and FF390 (CitationVainio and Hantula 2000). The PCR mixtures (50 µL) contained 0.3 µmol L−1 of each primer, 200 µmol L−1 of each dNTP, 1.4 mmol L−1 MgSO4, 5 µL 10× PCR buffer, 1 U KOD-plus (TOYOBO, Osaka, Japan), 25 ng template DNA and sterile water. The PCR program was initial denaturation at 94°C for 2 min, then 40 cycles of denaturation at 94°C for 15 sec, annealing at 50°C for 30 sec, and extension at 68°C for 30 sec. For nested PCR, the first PCR was with primers AU2 and AU4 (CitationVandenkoornhuyse et al. 2002) and the second PCR was with primers GC-FR1 and FF390. In the first PCR, the PCR mixture (50 µL) contained the same constituents as for direct PCR, except that the primers were replaced by AU2 and AU4 and MgSO4 was 1.2 mmol L−1. The first PCR program was as follows: initial denaturation at 94°C for 2 min, followed by 30 cycles of denaturation at 94°C for 15 sec, annealing at 45°C for 30 sec, and extension at 68°C for 1 min 30 sec. In the second PCR, 1 µL of the first PCR product was transferred to the second reaction mixture containing the same constituents as for direct PCR. The second PCR program was the same as the first one, except that the number of cycles was 30.
The PCR was conducted using a TaKaRa PCR Thermal Cycler SP (Takara, Shiga, Japan). The products were quantified on a 1.5% agarose gel using HindIII-digested λ DNA as the standard.
Denaturing gradient gel electrophoresis of fungal 18S rDNA fragments
The DGGE based on the method of CitationMuyzer et al. (1993) was carried out using the D Code System (Bio-Rad, Hercules, CA, USA). The PCR products were loaded at 200 ng per lane on 8% (wt/vol) polyacrylamide gels (37.5:1, acrylamide : bisacrylamide) in TAE buffer. Gels had a denaturing gradient ranging from 30% to 60%, where 100% denaturant contained 7 mol L−1 urea and 40% formamide. Electrophoresis was at 60°C and 75 V for 22 h. Gels were stained with SYBR Green I (1:10,000 dilution; FMC BioProducts, Rockland, ME, USA) for 30 min, photographed, scanned and analyzed with the Molecular Imager FX system (Bio-Rad).
Quantitative analysis of DGGE profiles
Banding patterns of DGGE profiles were analyzed using the Discovery Series Quantity One software (version 4.2, Bio-Rad). After background subtraction of single lanes, banding patterns were digitized. The intensity and position of individual bands were transferred into spreadsheets. Band intensity was expressed as the percentage of total band intensity in one lane. For these calculations, we excluded bands inferred not to be fungi, but instead inferred to be other eukaryotes by the sequencing analysis described below. The patterns were analyzed in two ways. First, the Shannon–Weaver index of general diversity, H′ (CitationShannon and Weaver 1949), was used as a parameter for the structural diversity of the microbial community and was calculated as H′ = –∑Pi ln Pi . Pi is the importance probability of the bands in a gel lane and is calculated as Pi = ni /N, where ni is the intensity of a band and N is the sum of intensities of all bands in the densitometric profile. Second, similarities of all possible pairs of gel tracks were expressed as squared distances. A multidimensional scaling (MDS) map was generated using the program SPSS 9.0 J for Windows (SPSS, Chicago, IL, USA). The MDS map shows every band pattern on one plot, where relative changes in community structure can be visualized and interpreted as the distances between the points (CitationAraya et al. 2003). The closer the points are to each other, the more similar the DGGE banding patterns represented by the points are. Dendrograms were generated based on the unweighted pair group with the arithmetic mean (UPGMA) clustering method using the Black box program (CitationAoki 1996).
Statistical analysis
Analyses were carried out on 18S rDNA PCR-DGGE data for each treatment with triplicate cultivation plots. The statistical significance of any differences was determined using Student's t-tests.
Sequencing of DGGE bands
Dominant bands in DGGE gels were excised from the gels and re-amplified with the GC-FR1/FF390 primer pair. The PCR products were again subjected to DGGE to ensure that products contained single bands and showed electrophoretic mobility identical to that of the original bands. When the products were not homogeneous, target bands were excised and amplified again. Then, they were used as templates for direct sequencing after purification using SUPEC-02 (Takara). Sequencing reactions were carried out using the BigDye Terminator v3.0 DNA sequencing kit (Applied Biosystems, Foster City, CA, USA) and the FR1 (without GC clamp) or FF390 primers. Reaction products were analyzed with the ABI PRISM 3100 Genetic Analyzer (Applied Biosystems).
Phylogenetic analysis
Sequences of DGGE bands were identified with the FASTA search from the database of the DNA Data Bank of Japan (DDBJ). The 18S rDNA partial sequences obtained in the present study are available in the DDBJ database under the accession numbers of AB262807 to AB262846. Bands from direct PCR were labeled with lower-case letters indicating their sampling site (b, bulk soil; r, rhizosphere soil), followed by arbitrarily assigned band numbers. Bands from nested PCR were labeled with upper-case letters indicating their nature (C, bands detected consistently in control plots; S, bands detected in specific seasons in control plots; L, bands detected in CP plots; D, bands detected in 1,3-D plots) and sampling site (B, bulk soil; R, rhizosphere soil), followed by band numbers.
RESULTS
Comparison between DGGE profiles of direct and nested PCR products
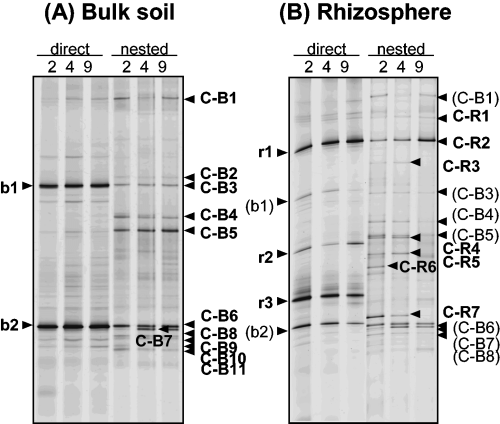
Comparisons were made between DGGE profiles of direct and nested PCR products from bulk soil and rhizosphere soil samples from untreated control plots (2, 4 and 9) collected 2 months after fumigation in the second trial (2 M(2)) (). The DGGE profiles of direct PCR products showed two and five major bands in bulk and rhizosphere soil, respectively, whereas
Figure 1 18S rDNA denaturing gradient gel electrophoresis profiles of the fungal community using direct and nested polymerase chain reaction (PCR) in untreated control plots (2, 4 and 9) at 2 months in the second trial (December 2002). (A) Bulk soil and (B) rhizosphere soil. Bands from direct PCR were labeled with lower-case letters indicating their sampling site (b, bulk soil; r, rhizosphere soil), followed by arbitrarily assigned band numbers. Bands from nested PCR are labeled with upper-case letters indicating their nature (C, bands detected consistently in control plots) and sampling site (B, bulk soil; R, rhizosphere soil), followed by band numbers.
Effect of fumigation on the fungal community
Sequence analysis of DGGE bands
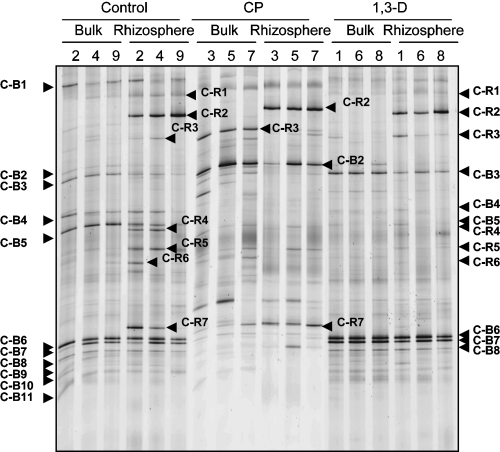
The structure of the fungal community of the control, CP and 1,3-D plots at 2 M(2) were compared using 18S rDNA PCR-DGGE. In bulk soil samples of all replicated control plots, bands C-B3, C-B4, C-B5, C-B6 and C-B7 were intense (). C-B3, C-B6 and C-B7 displayed high sequence similarity to known isolated fungi (C-B3, Mortierella alpina and Mortierella chlamydospora, 99.7%; C-B6, Myrothecium cinctum, 100%; C-B7, Bionectria ochroleuca and Bionectria pityrodes, 99.7%). Band C-B4 showed 83.7% similarity to Basidiobolus microsporus and C-B5 showed 88.9% similarity to Gaertneriomyces semiglobiferus and 88.9% similarity to Bensingtonia ciliate (). In all replicated CP plots, all of these five bands became faint or disappeared, but C-B2 became prominent and C-R3 appeared. C-R3 was also present in rhizosphere soil samples of control plots. C-B2 showed 92.5% similarity to Entophlyctis sp. and C-R3 showed 97.6% similarity to Macrobiotophthora vermicola (). C-B4 and C-B5 disappeared after 1,3-D treatment and other bands became more intense ().
Rhizosphere soil samples had specific bands (C-R1 to C-R7) in addition to those also detected in bulk soil (C-B1, C-B3, C-B4, C-B5, C-B6, C-B7 and C-B8) (). Band C-R2 showed the highest intensity and was detected in all rhizosphere soil samples from control and fumigant-treated plots (). The DNA sequence of C-R2 showed 96.8% similarity to Chytridium polysiphoniae (). In CP plots, bands C-B6, C-B7 and C-B8 became faint or disappeared, as did C-B4, C-B5, C-R4, C-R5 and C-R6, whereas C-R7 remained detectable. In 1,3-D plots, C-R4, C-R5, C-R6 and C-R7 became faint or disappeared, as did C-B4 and C-B5.
Comparison of DGGE profiles among treatments
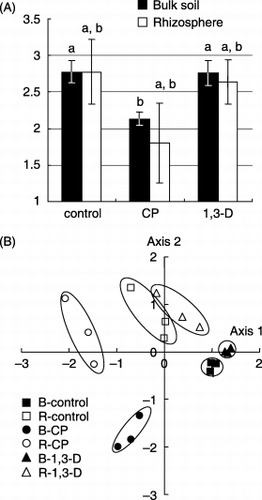
The Shannon diversity index H′ () was calculated from the DGGE profiles (). The index for bulk soil in the CP plots was significantly lower than that in the control plots (P < 0.05). The index for the rhizosphere soil in the CP plots also tended to be lower than that of the control plots, but the difference was not significant. The diversity indices of bulk soil and rhizosphere soil, in the 1, 3-D plots were almost equivalent to those of the control plots.
The DGGE profiles shown in were also analyzed using MDS (). In the MDS map, each treatment consisting of three replicated samples was grouped together. Samples from the bulk soil and the rhizosphere soil were positioned separately within the same treatments. The squared distances between the CP plot and the control were 1592 ± 317 in bulk soil and 945 ± 287 in rhizosphere soil, and those between the 1,3-D plot and the control were 595 ± 108 in the bulk soil and 478 ± 287 in the rhizosphere soil. The squared distances between the CP plot and the control were significantly larger than those between 1,3-D and the control in both bulk soil and rhizosphere soil (P < 0.001 and
Table 2 Sequence analysis of bands from 18S rDNA denaturing gradient gel electrophoresis using direct and nested polymerase chain reaction (PCR) from bulk soil and rhizosphere soil samples in untreated control plots (Fig. 1)
Figure 2 18S rDNA denaturing gradient gel electrophoresis profiles of the fungal community using nested polymerase chain reaction (PCR) from bulk soil and rhizosphere soil in untreated control plots (2, 4 and 9), chloropicrin (CP) plots (3, 5 and 7) and 1,3-dichloropropene (1,3-D) plots (1, 6 and 8) 2 months after fumigation in the second trial (December 2002). Bands from nested PCR are labeled with upper-case letters indicating their nature (C, bands detected consistently in control plots) and sampling site (B, bulk soil; R, rhizosphere soil), followed by band numbers.
Temporal changes after fumigation
The structure of the fungal community in the untreated control, CP-treated and 1,3-D-treated plots was monitored by DGGE for three consecutive years ().
Control plots
The DGGE profiles of bulk soil from the control plots did not show any appreciable change with time (). All the bands detected at 2 M(2) (C-B1 to C-B11 in ) were present throughout the trials. Some bands, such as S-B1, S-B2, S-B5, S-B6 and S-B7, appeared only on specific sampling dates, and the intensity of bands S-B3, S-B4 and C-B5 changed with time. Most of these bands (S-B1 to S-B6) were also detected in rhizosphere soil (, ). For example, band S-B1 detected at 6 M(2) in bulk soil corresponds to band C-R2 in rhizosphere soil. Band C-R2 showed high intensity at the beginning of spinach cultivation. S-B2 and S-B6 were detected in bulk soil just after the plowing of spinach plants at 7 M(1) and 7 M(2), respectively. S-B2 and S-B6 correspond to C-R3 and C-R7, respectively, which were detected in rhizosphere soil at all sampling dates. In addition, S-B4 was detected at 6 M(1) and after 3 M(2), and peaked at 6 M(1) and 6 M(2). C-R5 in rhizosphere soil, corresponding to S-B4 in bulk soil, increased in intensity with cultivation time from 1 M(2) to 4 M(2) and remained detectable from 1 M(3) to 6 M(3). These bands detected in bulk soil of the control plots were also detected in bulk soil of the fumigated plots and showed the same trends ().
The DGGE profiles in spinach rhizosphere soil of the control plots changed drastically with time (). During both trials, some bands became prominent and DGGE profiles became simplified. For example, in the second trial the intensity of C-R5 increased over time, while intensity of C-R2, C-R3, C-R4 and C-B5 decreased.
Table 3 Sequence analysis of bands from 18S rDNA denaturing gradient gel electrophoresis using nested polymerase chain reaction (PCR) from bulk soil and rhizosphere soil samples in control, chloropicrin (CP) or 1,3-dichloropropene (1,3-D) plots (Fig. 4)
Figure 3 Quantitative analysis of the 18S rDNA denaturing gradient gel electrophoresis profiles shown in Fig. 2. (A) Shannon's diversity index and (B) multi-dimensional scaling (MDS) map based on the squared distance of similarity. Each treatment consisted of three plot replicates. B, bulk soil; R, rhizosphere soil; CP, chloropicrin; 1,3-D, 1,3-dichloropropene.
Chloropicrin-treated plots
DGGE profiles and DNA sequencing
The DGGE profiles of bulk soil changed drastically over time after CP fumigation (). Bands C-B6 to C-B11 disappeared, whereas band C-B2 appeared. Then, the total number of bands increased, with the appearance of new bands rather than the reappearance of bands that had disappeared. In particular, bands C-B6 to C-B11, each inferred to be ascomycota or basidiomycota, disappeared early in the first trial, never to reappear. New and CP-plot-specific bands, L-B1 to L-B3, were detected from 2 M to 6 M in the first and/or second trials. Their sequences identified closest relatives in the chytridiomycota Rhizophlyctis harderi, Rhizophydium sp. and Powellomyces sp., respectively (). Other CP-plot-specific bands appeared just after plowing spinach plants into the soil. Bands L-B7 and L-B10 appeared at 7 M(1) and showed high similarity to Plectosphaerella cucumerina (anamorph: Fusarium tabacinum) and Taphrina sp., respectively (). The closest relatives identified by bands L-B8, which appeared at 9M-B(2), and L-B9, which appeared from 6 M(2) to 6 M(3), were not fungal, but were instead metazoa (82.0%) and cercozoa (85.7%), respectively ().
In rhizosphere soil DGGE profiles of CP-treated plots, one or two bands were dominant at 1 M(2) and 1 M(3), and then the number of bands increased with cultivation time (). In contrast, the rhizosphere soil DGGE profiles of control plots simplified with increasing cultivation time (). The prominent band C-B2 in bulk soil samples after fumigation was also prominent in rhizosphere soil samples at 1 M(2) and 1 M(3). After 1 M cultivation, C-B2 gradually decreased, while bands C-R4, C-R5, C-R6 and C-R7, which were also detected in control plots, became apparent. Bands C-B6, C-B7 and C-B8, present in rhizosphere soil of the control plots, were faint or could not be detected in the rhizosphere soil of CP-treated plots. The profile for C-R2 was similar for the control and CP plots, being readily detected at 1 M and 2 M and decreasing thereafter.
As DGGE profiles of bulk soil revealed that drastic change occurred at 1 M, but not just after (JA) fumigation in the first trial, more detailed observations were made in the second and third trials, introducing sampling times at 3 days (3D) and 1 week (1W) and 2 weeks (2W) after fumigation (). The DGGE profiles did not show any differences from JA(2) to 2W(2) and from JA(3) to 1W(3), but markedly changed thereafter.
Numerical analysis of DGGE profile data
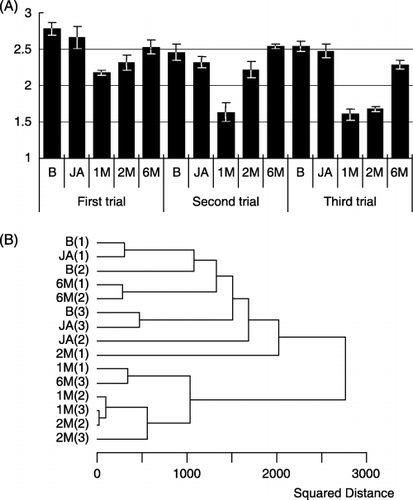
The Shannon diversity index H′ was calculated from DGGE profiles of bulk soil samples before fumigation (B), just after fumigation (JA) and 1 (1 M), 2 (2 M) and 6 (6 M) months after fumigation for the three trials. The change in diversity with time showed the same tendency in each trial (). The indices did not show a significant decrease in JA samples compared to B samples. From the JA time point forward in each trial, the index decreased and was lowest in 1 M samples, and then subsequently increased from 2 M to 6 M. The index at 1 M in the second and third trials was lower than that at 1 M in the first trial.
We next created a dendrogram using the UPGMA clustering method (). The dendrogram defined two separate, major clusters, demonstrating a drastic change in DGGE profiles between the JA samples and the 1 M samples. B and JA samples from all trials, together with 2 M(1), 6 M(1) and 6 M(2), defined one major cluster. The other major cluster consisted of 1 M from all trials, plus 2 M(2), 2 M(3) and 6 M(3). Samples of 1 M and 2 M in the second and third trials were grouped in a tight sub-cluster, indicating that DGGE
Figure 4 Temporal change after fumigation in fungal 18S rDNA denaturing gradient gel electrophoresis profiles from bulk soil and rhizosphere soil samples in (A) untreated control plot 4, (B) chloropicrin (CP) plot 5 and (C) 1,3-dichloropropene (1,3-D) plot 6. Bands from nested polymerase chain reaction (PCR) are labeled with upper-case letters indicating their nature (C, bands detected consistently in control plots; S, bands detected in specific seasons in control plots; L, bands detected in CP plots; D, bands detected in 1,3-D plots) and sampling site (B, bulk soil; R, rhizosphere soil), followed by band numbers. Arrows show the duration of spinach cultivation. After growth, spinach plants were plowed into the soil. Asterisks show the timing of the fumigation treatment.
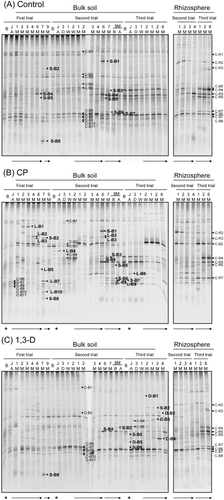
Figure 5 Quantitative analysis of 18S rDNA denaturing gradient gel electrophoresis profiles of bulk soil samples from chloropicrin (CP) plots using (A) Shannon's diversity index and (B) dendrogram cluster analysis. Samples were taken before (B) and just after (JA) fumigation, and then 1, 2 and 6 months (1 M, 2 M and 6 M, respectively) after fumigation for the three trials (September 2001 to April 2004). Numbers in parentheses indicate the trial number. The dendrogram was calculated on the basis of squared distance of similarity using the clustering algorithm of the unweighted pair group method with the arithmetic mean.
1,3-dichloropropene plots
The DGGE profiles of bulk soil samples did not show any drastic changes after 1,3-D fumigation, but were slightly different from profiles of control plots (). Bands C-B4, C-B5, S-B4 and S-B5 disappeared from 1 M(1). S-B4 appeared again at 4 M(2), and C-B4, C-B5 and S-B5 gradually appeared after 9 M-A(2). All of these four bands decreased in intensity or disappeared again after the third fumigation. In contrast, four bands specific to the 1,3-D plots (D-B1 to D-B4) appeared after the third fumigation. D-B1 and D-B2 showed high similarity to zygomycetous fungi, Conidiobolus lamprauges (100%) and Umbelopsis isabellina (99.7%), respectively (). The closest relative of D-B3 was Olpidium brassicae (96.1%), and that of D-B4 was Podoscypha petalodes (86.8%). In contrast to the changes observed in DGGE profiles of bulk soil, the DGGE profiles of the rhizosphere fungal community in 1,3-D-treated plots were very similar to those in the control plots (compare ).
Temporal change in DGGE profile similarity between treatments
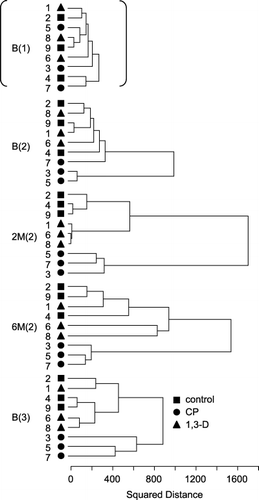
The UPGMA dendrograms of control and fumigated plots were compared in terms of time after fumigation. The time points we chose for this comparison were: before fumigation in the first trial (B(1)); before fumigation and 2 and 6 months after fumigation in the second trial (B(2), 2 M(2), 6 M(2)); and before fumigation in the third trial (B(3)) (). We included all cultivation plot replicates in this analysis (denoted by numbers 1–9 in ). Although samples collected at B(1) did not show any clear separation from each other, the dendrogram was clearly separated into two major clusters for B(2) samples. Samples of control and 1,3-D plots were part of the same cluster, which included a CP plot sample (plot 7) in a distant position. The two other CP plot samples (plots 3 and 5) constituted the other cluster. At 2 M(2), samples of each cultivation condition fell into unique clusters. From 6 M(2) forward, samples of control and 1,3-D plots merged with each other again. All samples of CP plots remained separated on the dendrogram from other samples at 6 M(2) and at B(3) (approximately 1 year after the second fumigation). Cluster length in the UPGMA dendrograms expresses dissimilarity as the squared distance between samples within a cluster. After fumigation, dissimilarity between treatments was greatest at 2 M(2) and then decreased gradually (). In the first and third trials, the temporal change in similarity between treatments showed a similar tendency to that in the second trial, but the distance among treatments in the first trial was smaller than that in the second trial (data not shown).
DISCUSSION
Polymerase chain reaction amplification of fungal 18S rDNA for DGGE
Microbial community profiling using PCR-DGGE analysis of ribosomal small subunit genes is more problematic for fungi than it is for bacteria (CitationAnderson and Cairney 2004). This bias is due to several factors that include the DNA extraction methods used, PCR primer specificity and variation in 18S rDNA copy number among fungal species. Moreover, taxonomic identification of fungi based on 18S rDNA sequences is usually limited to the genus or family level because of the relative lack of variation among 18S rRNA genes between closely related fungal species and the lack of an exhaustive
Figure 6 Dendrogram analysis of 18S rDNA denaturing gradient gel electrophoresis profiles of bulk soil samples taken in the first trial before fumigation (B(1)), in the second trial before, 2 and 6 months after fumigation (B(2), 2 M(2) and 6 M(2), respectively), and in the third trial before fumigation (B(3)). Untreated control plots (2, 4 and 9; ▪), and 1,3-dichloropropene (1,3-D) plots (1, 6 and 8; ▴) chloropicrin (CP) plots (3, 5 and 7; •). The dendrogram was calculated on the basis of squared distance of similarity using the clustering algorithm of the unweighted pair group method with the arithmetic mean.
The primer pair GC-FR1 and FF390 was reported to not produce a detectable amount of PCR amplification products from bacterial, plant and some soil animal DNA (CitationPennanen et al. 2001; CitationVainio and Hantula 2000). In our experiments, however, plant and oomycete DNA was amplified from rhizosphere soil DNA samples by direct PCR using these primers (, ). Similar results have been reported for other fungal primer pairs. For example, primers nu-SSU-817 and nu-SSU1196 were initially reported to amplify only fungal DNA from mixed community DNA (CitationBorneman and Hartin 2000), but they were later shown to also amplify invertebrate 18S rDNA sequences from genomic DNA extracted from agricultural soil (CitationAnderson et al. 2003). Primer specificity varies depending on the origin of the sample and the diversity of eukaryote DNA contained within the extracted DNA (CitationAnderson and Cairney 2004). In our experiments, plant DNA was not amplified from bulk soil samples by direct PCR using primers GC-FR1 and FF390. We assume that plant DNA was present in bulk soil DNA samples, but the amount must have been less than that in rhizosphere soil samples. These primers might only amplify plant and other eukaryote DNA if the fraction of fungal DNA is low in a mixed DNA sample.
The primer pair AU2 and AU4 was reported to amplify DNA of all fungal phyla from plant roots without amplification of plant and oomycete DNA (CitationVandenkoornhuyse et al. 2002). We examined a nested PCR method, with the first PCR primer pair AU2/AU4 for fungal-DNA specific amplification followed by the second PCR primer pair GC-FR1/FF390. Use of the nested PCR method avoided amplification of plant and oomycete DNA, even from rhizosphere soil samples (, ). Thus, the nested PCR method using AU2/AU4 and GC-FR1/FF390 is useful for the detection of fungi associated with plants. In some cases, direct PCR with GC-FR1/FF390 would be advantageous because oomycetes sometimes detected by these primers include important plant pathogens, such as Phytophthora spp. and Pythium spp. Thus, primer selection and PCR methods should be considered according to the aim of the particular research.
Some sequences of DNA bands amplified by the nested PCR method, however, showed higher similarity to cercozoa and metazoa than to fungi (,). In addition, the relative intensity of some bands in the DGGE profiles differed between samples amplified using direct and nested PCR, even if the bands corresponding to plants and oomycetes were excluded. These results indicate that each PCR method is biased, and underscore the specificity and bias problems that remain to be solved for fungal DNA amplification from various environmental samples. Limitations of these methods must always be taken into consideration when interpreting data. In the present report, we excluded the non-fungal bands from our numerical fungal community analysis, although these bands showed interesting behavior.
Effect of spinach plants on the soil fungal community
Fungal community structure in spinach rhizosphere soil was different from that in bulk soil and changed with cultivation time. CitationGomes et al. (2003) obtained similar results when examining the maize rhizosphere. One factor contributing to this difference is considered to be root exudates, such as amino acids, sugars and organic acids, which are important nutritional sources for microorganisms. The variability of DGGE profiles within replicates was larger for rhizosphere soil samples than for bulk soil (), which might reflect the greater environmental diversity in the rhizosphere. The rhizosphere effect may also contribute to minimizing the effect of chemical fumigation: 2 months after fumigation, the differences in DGGE profiles between control and CP plots were smaller in rhizosphere soil samples than in bulk soil samples (), indicating that the fungal community recovered from fumigation faster in the rhizosphere. The viable population of fungi as measured by standard dilution plate counts with rose bengal medium also showed faster recovery of the fungal community in rhizosphere in 1 M(1) samples (data not shown).
C-B3, C-B6 and C-B7, which were detected as major bands in DGGE profiles of bulk soil, had high sequence similarity to Mortierella alpina, Myrothecium cinctum and Bionectria ochroleuca (anamorph: Clonostachys rosea), respectively (). These organisms and their relatives are well-known soil fungi and are often detected in agricultural soils by culture-dependent (CitationGirlanda et al. 2001) and culture-independent methods (CitationGomes et al. 2003; CitationSmit et al. 1999). In contrast, band C-R2, detected in rhizosphere soil samples, appears to be a chytridiomycete. The fungi of phylum chytridiomycota reproduce by means of posteriorly uniflagellate zoospores and are widespread in both terrestrial and aquatic ecosystems. Chytridiomycetes are important plant parasites and biodegraders of material containing cellulose, chitin and keratin (CitationPowell 1993).
In bulk soil DGGE profiles, season-specific bands appeared or increased in intensity at a later stage of spinach growth or just after plowing spinach plants into the soil. Most of these bands corresponded to major bands detected in rhizosphere soil. Bands S-B7 (100% similarity to Cryptococcus luteolus and Bullera sinensis) and S-B8 (100% similarity to Taphrina sp. and 99.0% to Cryptococcus carnescens) were not detected as bands in rhizosphere soil during autumn cultivation. CitationGomes et al. (2003) showed that basidiomycetes Bullera spp. and Cryptococcus spp. constituted the dominant fungal populations in the rhizosphere of senescent maize plants. Thus, these fungal species, which might exist in rhizosphere soil of spinach based on our analysis of bands S-B7 and S-B8, may have high affinity to the rhizosphere of plants in general. Some species of fungi are suggested to develop in the rhizosphere and to proliferate by degrading plant debris in bulk soil when spinach plants have been plowed under.
Impact of fumigation on the soil fungal community
The impact of fumigation on the soil fungal community was greater in the CP treatment than in the 1,3-D treatment in terms of the magnitude of the effect after 2 months and the extent of recovery after 1 year. In 2 M(2) samples, the difference in DGGE profiles was greater between CP and control plots than between 1,3-D and control plots, both in bulk soil and rhizosphere soil (). The diversity index H′ in CP plots decreased significantly relative to the control, whereas H′ in 1,3-D plots did not (). Although the similarity in DGGE profiles between CP and control plots tended to increase with time, the soil fungal community of CP plots still differed from the control, constituting an independent cluster 1 year after fumigation (). In contrast, the DGGE profiles of 1,3-D plots became indistinguishable from those of the control plots, as measured by cluster analysis (). The fumigant 1,3-D is an effective nematicide and is sometimes combined with CP to improve efficacy against soil-borne fungal pathogens. CitationDe Cal et al. (2005) reported that the soil fungal population isolated on Potato dextrose ager (PDA) plates decreased after application of 1,3-D : CP (61:35) at 35 mL m−2 or CP alone at 40 g m−2. However, a mixture with a lower CP content (1,3-D : CP [83:17] at 35 mL m−2) occasionally failed to reduce the soil fungal population significantly. CitationYao et al. (2006) reported that DGGE profiles of the fungal community changed with the application of 1,3-D : CP (83:17) at 300 L ha−1 (30 mL m−2) at an apple replant site, but that the profiles converged and became indistinguishable from those of pre-plant soil samples 10 months after replanting. The reason for the difference in recovery from fumigation is attributed mainly to the application dosage of CP; other authors used CP at 5.1 mL m−2, whereas we used CP at sixfold this level (32 mL m−2). Our CP-treated plots may require more than 1 year to recover.
In the DGGE profiles of the CP plots, band C-B2 was most prominent, while bands considered to be ascomycota disappeared and did not reappear even 1 year after fumigation. C-B2 was inferred to be chytridiomycota, and half of the bands that newly appeared in CP plots were also inferred to be chytridiomycota (L-B1 to L-B5, , ). Soil disturbance, such as fumigation and crop cultivation, often increases the concentration of biologically available nutrients. Populations of chytrids increase in response to disturbance (CitationLozupone and Klein 2002). Chytrids can rapidly reproduce, releasing highly chemotactic zoospores when conditions are favorable (CitationLozupone and Klein 2002). These characteristics could allow them to quickly exploit nutrients released after soil disturbance, increasing their overall population. Starting with the second trial, we made three additional small plots adjacent to the field to analyze the impact of MeBr fumigation. The DGGE profiles in MeBr plots were similar to those in CP plots 2 months after fumigation in the second and third trials (data not shown). Band C-B2 was also prominent in the MeBr plots. Band C-B2 was inferred to be derived from a fast-growing fungus after fumigation.
Samples of CP-treated plots at JA did not show any changes in DGGE profile compared with those at B, but colony counts of fungi on rose bengal plates decreased from 105 at B to 102 at JA (data not shown). These results highlight an important caveat of DNA-based DGGE analysis. Frequent sampling in the third trial revealed that the DGGE profiles did not change until the colony count increased to 103 at 2 W (data not shown). We also found that DNA-based DGGE profiles detected changes in the bacterial community more slowly than RNA-based profiles (CitationHoshino and Matsumoto 2006). These findings suggest that viable fungi diminished just after CP treatment, but that their DNA remained in the soil for at least a few weeks.
In the 1,3-D plots, a few DGGE bands disappeared and others increased in relative intensity. The effect of 1,3-D on the nematode population was larger than that of CP in the same fields (CitationAraki, 2002). In addition to its direct effect on fungi, fumigation could also indirectly affect the fungal population by damaging the nematode population.
Spinach yield in CP plots was more than twice that in control plots, whereas the yield in 1,3-D plots was slightly higher than that in the control plots (Y Kobara, unpubl. data, 2006). Spinach yield might be related to changes in the structure of the fungal community, although disease symptoms or specific fungal pathogens were not detected in this field. In addition, other factors, such as the community structure of other organisms (including bacteria, nematodes and soil insects) and nutritional exudates from dead biomass, were likely to be related to spinach production.
In this report we revealed the impact of two chemical fumigants on the structure of the fungal community using culture-independent, 18S rDNA PCR-DGGE with nested PCR using a new combination of primer pairs. We found that DGGE bands whose sequences showed high similarity to chytridiomycota dominated in samples after CP fumigation and in rhizosphere soil samples. Chytridiomycota cannot be detected by conventional dilution-plate counting, and are usually studied using culture and microscopic protocols based on baiting techniques, using a “bait” substrate known to attract chytrids under flooded conditions (CitationLozupone and Klein 2002). Our results indicate that PCR-DGGE can be used to detect an array of fungal community members, including chytridiomycota, in a single experiment. If we were to study a broad fungal community using culture-dependent methods, we would need to use various culture conditions and techniques specific to each fungal group.
In conclusion, the nested PCR-DGGE method described here is useful for assessing the impact of fumigation on the structure of the soil fungal community, including fastidious fungal group members, and in particular for a comparison among treatments and for monitoring community change after treatment.
ACKNOWLEDGMENTS
We thank Yuzo Kobara, National Institute for Agro-Environmental Sciences (NIAES), for help with field experiments, Kazuki Togami, NIAES, for soil analysis and Seiya Tsushima, NIAES, for suggestions on an early draft of this manuscript. We also thank Kazuko Taniguchi for preparing the soil samples.
REFERENCES
- Kobara , Y . 1998 . Depletion of stratospheric ozone by methyl bromide (in Japanese) . JAgricSci , 53 : 289 – 294 .
- Dungan , RS , Ibekwe , AM and Yates , SR . 2003 . Effect of propargyl bromide and 1,3-dichloropropene on microbial communities in an organically amended soil . FEMS MicrobiolEcol , 43 : 75 – 87 .
- Tateya , A . 2005 . Montreal giteisyo ni okeru syuukamechiru hukaketsuyouto no genjyo . Kongetsu No Nougyo , 4 : 19 – 24 .
- Iwasaki , Y . 1999 . Dojyo kunjyo-zai no tokusei to syori-jyoken ni yoru yakuzai no kouka . Kongetsu No Nougyo , 11 : 25 – 28 .
- Anderson , JPE . 17–21 August 1993 . “ Side-effects of pesticides on carbon and nitrogen transformations in soils ” . In Procof the International Symposium on Environmental Aspects of Pesticide Microbiology , 17–21 August , 61 – 67 . Sigtuna, , Sweden : Swedish University of Agricultural Science Uppsala . 1992
- Browning , M , Wallace , DB , Dawson , C , Alm , SR and Amador , JA . 2006 . Potential of butyric acid for control of soil-borne fungal pathogens and nematodes affecting strawberries . Soil BiolBiochem , 38 : 401 – 404 .
- Hamm , PB , Ingham , RE , Jaeger , JR , Swanson , WH and Volker , KC . 2003 . Soil fumigant effects on three genera of potential soilborne pathogenic fungi and their effect on potato yield in the Columbia Basin of Oregon . Plant Dis , 87 : 1449 – 1456 .
- Takehara , T , Kuniyasu , K , Mori , M and Hagiwara , H . 2003 . Use of a nitrate-nonutilizing mutant and selective media to examine population dynamics of Fusarium oxysporumf. sp. spinaciae in soil . Phytopathology , 93 : 1173 – 1181 .
- Ibekwe , AM , Papiernik , SK , Gan , J , Yates , SR , Yang , C-H and Crowley , DE . 2001 . Impact of fumigants on soil microbial communities . ApplEnvironMicrobiol , 67 : 3245 – 3257 .
- Itoh , K , Takahashi , M , Tanaka , R , Suyama , K and Yamamoto , H . 2000 . Effect of fumigants on soil microbial population and proliferation of Fusarium oxysporuminoculated into fumigated soil . JPesticSci , 25 : 147 – 149 .
- Tanaka , S , Kobayashi , T , Iwasaki , K , Yamane , S , Maeda , K and Sakurai , K . 2003 . Properties and metabolic diversity of microbial communities in soils treated with steam sterilization compared with methyl bromide and chloropicrin fumigations . Soil SciPlant Nutr , 49 : 603 – 610 .
- De Cal , A , Martinez-Treceno , A , Salto , T , Lopez-Aranda , JM and Melgarejo , P . 2005 . Effect of chemical fumigation on soil fungal communities in Spanish strawberry nurseries . ApplSoil Ecol , 28 : 47 – 56 .
- Vandenkoornhuyse , P , Baldauf , SL , Leyval , C , Straczek , J and Young , JP . 2002 . Extensive fungal diversity in plant roots . Science , 295 : 2051
- Katayama , A , Funasaka , K and Fujie , K . 2001 . Changes in the respiratory quinone profile of a soil treated with pesticides . BiolFertilSoils , 33 : 454 – 459 .
- Klose , S , Acosta-Martinez , V and Ajwa , HA . 2006 . Microbial community composition and enzyme activities in a sandy loam soil after fumigation with methyl bromide or alternative biocides . Soil BiolBiochem , 38 : 1243 – 1254 .
- Gomes , NCM , Fagbola , O Costa , R . 2003 . Dynamics of fungal communities in bulk and maize rhizosphere soil in the tropics . ApplEnvironMicrobiol , 69 : 3758 – 3766 .
- Girvan , MS , Bullimore , J , Ball , AS , Pretty , JN and Osborn , AM . 2004 . Responses of active bacterial and fungal communities in soils under winter wheat to different fertilizer and pesticide regimens . ApplEnvironMicrobiol , 70 : 2692 – 2701 .
- Sigler , WV and Turco , RF . 2002 . The impact of chlorothalonil application on soil bacterial and fungal populations as assessed by denaturing gradient gel electrophoresis . ApplSoil Ecol , 21 : 107 – 118 .
- Yao , S , Merwin , IA , Abawi , GS and Thies , JE . 2006 . Soil fumigation and compost amendment alter soil microbial community composition but do not improve tree growth or yield in an apple replant site . Soil BiolBiochem , 38 : 587 – 599 .
- Anderson , IC and Cairney , JWG . 2004 . Diversity and ecology of soil fungal communities: increased understanding through the application of molecular techniques . EnvironMicrobiol , 6 : 769 – 779 .
- Anderson , IC , Campbell , CD and Prosser , JI . 2003 . Potential bias of fungal 18S rDNA and internal transcribed spacer polymerase chain reaction primers for estimating fungal biodiversity in soil . EnvironMicrobiol , 5 : 36 – 47 .
- Vainio , EJ and Hantula , J . 2000 . Direct analysis of wood-inhabiting fungi using denaturing gradient gel electrophoresis of amplified ribosomal DNA . MycolRes , 104 : 927 – 936 .
- Hoshino , YT and Matsumoto , N . 2004 . An improved DNA extraction method using skim milk from soils that strongly adsorb DNA . Microbes Environ , 19 : 13 – 19 .
- Zhou , J , Bruns , MA and Tiedje , JM . 1996 . DNA recovery from soils of diverse composition . ApplEnvironMicrobiol , 62 : 316 – 322 .
- Muyzer , G , de Waal , EC and Uitterlinden , AG . 1993 . Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA . ApplEnvironMicrobiol , 59 : 695 – 700 .
- Shannon , CE and Weaver , W . 1949 . The Mathematical Theory of Communication , Urbana : University of Illinois Press .
- Araya , R , Tani , K , Takagi , T , Yamaguchi , N and Nasu , M . 2003 . Bacterial activity and community composition in stream water and biofilm from an urban river determined by fluorescent in situ hybridization and DGGE analysis . FEMS MicrobiolEcol , 43 : 111 – 119 .
- AokiS1996 http://aoki2.si.gunma-u.ac.jp/BlackBox/BlackBox.html (http://aoki2.si.gunma-u.ac.jp/BlackBox/BlackBox.html)
- Pennanen , T , Paavolainen , L and Hantula , J . 2001 . Rapid PCR-based method for direct analysis of fungal communities in complex environmental samples . Soil BiolBiochem , 33 : 697 – 699 .
- Borneman , J and Hartin , RJ . 2000 . PCR primers that amplify fungal rRNA genes from environmental samples . ApplEnvironMicrobiol , 66 : 4356 – 4360 .
- Girlanda , M , Perotto , S Moenne-Loccoz , Y . 2001 . Impact of biocontrol Pseudomonas FluorescensCHA0 and a genetically modified derivative on the diversity of culturable fungi in the cucumber rhizosphere . ApplEnvironMicrobiol , 67 : 1851 – 1864 .
- Smit , E , Leeflang , P , Glandorf , B , Dirk van Elsas , J and Wernars , K . 1999 . Analysis of fungal diversity in the wheat rhizosphere by sequencing of cloned PCR-amplified genes encoding 18S rRNA and temperature gradient gel electrophoresis . ApplEnvironMicrobiol , 65 : 2614 – 2621 .
- Powell , MJ . 1993 . Looking at mycology with a janus face: A glimpse at Chytridiomycetes active in the environment . Mycologia , 85 : 1 – 20 .
- Lozupone , CA and Klein , DA . 2002 . Molecular and cultural assessment of chytrid and Spizellomycespopulations in grassland soils . Mycologia , 94 : 411 – 420 .
- HoshinoYT MatsumotoN2006DNA-versus-RNA-based denaturing gradient gel electrophoresis profiles of a bacterial community during replenishment after soil fumigation Soil BiolBiochem doi:10.1016/j.soilbio. 2006.08.013
- Araki , M . 2002 . Restoration process of soil nematode population and species diversity of nematodes after soil fumigation . Jap. J. Nematol , 32 : 93