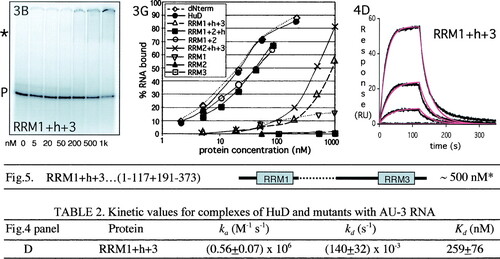
Volume 20, no. 13, p. 4765-4722, 2000. Recently, upon reinitiating studies focused on the role of HuD RRM2 in RNA binding, we obtained results that differed from the ones published. Extensive analysis of plasmids, and a variety of old and new protein preparations, revealed that although the construct was correct, previously the wrong plasmid had been used for a series of protein preparations. Tryptic digestion indicates that what was thought to be an RRM1+h+3 mutant HuD protein was in fact an RRM3 deletion mutant instead, yielding a protein of almost identical size but different domain composition. We have repeated all the analyses reported in the original paper with the correct protein (see corrected figure panels below). The key conclusion of the paper remains unaltered. (All three RRM domains play distinct roles in RNA binding. While RRM1 is the most important domain, RRM3 is required to form a stable HuD/RNA complex. The role of RRM3 in binding is masked in steady-state analyses of a domain 3 deletion mutant because its removal increases both the on and off rates of the complex.) However, our conclusion regarding the role of RRM2 needs to be revised. While we originally thought its role was distinct but similar to that of RRM3, it is clear from the data below that RRM2 plays a unique role in RNA binding that is more similar to that of RRM1. Removal of this domain strongly affects RNA binding ability, as seen by gel shift analysis (Fig. 3B and G and 5). The more accurate kinetic analysis (Fig. 4D; Table 2) shows that it weakens association by ∼7-fold and increases the dissociation rate by almost 50-fold, resulting in a loss of affinity of over 2 orders of magnitude. (By comparison, as described in the original paper, RRM1 deletion decreases association ∼20-fold and increases dissociation 100-fold, resulting in a 2,000-fold loss in affinity.) We sincerely apologize for any inconvenience our error may have caused.
Free access
HuD RNA Recognition Motifs Play Distinct Roles in the Formation of a Stable Complex with AU-Rich RNA
Reprints and Corporate Permissions
Please note: Selecting permissions does not provide access to the full text of the article, please see our help page How do I view content?
To request a reprint or corporate permissions for this article, please click on the relevant link below:
Academic Permissions
Please note: Selecting permissions does not provide access to the full text of the article, please see our help page How do I view content?
Obtain permissions instantly via Rightslink by clicking on the button below:
If you are unable to obtain permissions via Rightslink, please complete and submit this Permissions form. For more information, please visit our Permissions help page.
Related research
People also read lists articles that other readers of this article have read.
Recommended articles lists articles that we recommend and is powered by our AI driven recommendation engine.
Cited by lists all citing articles based on Crossref citations.
Articles with the Crossref icon will open in a new tab.
Your download is now in progress and you may close this window
- Choose new content alerts to be informed about new research of interest to you
- Easy remote access to your institution's subscriptions on any device, from any location
- Save your searches and schedule alerts to send you new results
- Export your search results into a .csv file to support your research
Login now Don't have an account?
Register for free
Login or register to access this feature
Login now Don't have an account?
Register for free
- Choose new content alerts to be informed about new research of interest to you
- Easy remote access to your institution's subscriptions on any device, from any location
- Save your searches and schedule alerts to send you new results
- Export your search results into a .csv file to support your research
Register now or learn more