Abstract
To screen the highly efficient and specific B-cell chronic lymphocytic leukemia/lymphoma 11B (BCL11B) small interfering RNA (siRNA) which are able to downregulate the BCL11B gene expression in human T-cell acute lymphoblastic leukemia, thereby inhibiting the leukemic T-cell proliferation and inducing apoptosis, four BCL11B-siRNAs and the scrambled non-silencing siRNA control (sc) were designed and obtained by chemosynthesis. After nucleofection, BCL11B expression in the mRNA and the protein levels were measured by qRT–PCR and immunoblotting, respectively. The biological consequences based on the highly efficient and specific BCL11B-siRNA were demonstrated by CCK-8 kit, morphological changes (Hoechst 33258 staining), high-resolution imaging, and flow cytometry. Reduction in the BCL11B mRNA level was observed at 24 or 48 hours in molt-4 T cells with BCL11B-935-siRNA, BCL11B-434-siRNA, or BCL11B-748-siRNA, respectively. BCL11B protein expression levels were reduced by 34·77% and 41·73% in the BCL11B-935-siRNA- and BCL11B-434-siRNA-treated cells, compared with the control level at 72 hours. In comparison with BCL11B-434-siRNA treatment group, the Molt-4 cells transfected with the BCL11B-935-siRNA showed significantly inhibited proliferation and effectively induced apoptosis (P<0·05). When highly efficient and specific BCL11B-935-siRNA was used to analyze the inhibition of BCL11B mRNA level in primary T-cell acute lymphoblastic leukemia (T-ALL) cells, similar result was obtained. In conclusion, siRNAs targeting the different exon domains resulted in different silencing effects and biological consequences. Suppression of BCL11B by RNA interference could inhibit the proliferation and induce the apoptosis effectively in leukemic T cells, which might be considered as a new target therapeutic strategy in T-cell malignancies.
Introduction
T-cell malignancies, including T-cell acute lymphoblastic leukemia (T-ALL), T- prolymphocytic leukemia (T-PLL), and T-cell non-Hodgkin’s lymphoma (T-NHL), represent a heterogeneous group of lymphoid neoplasms, which occur mainly via the proliferation of malignant T-cell clones. T-ALL accounts for 10–15% of newly diagnosed ALL cases in children and 20–25% of ALL cases in adults.Citation1Citation1,2 One-third of NHL cases are T-NHL. Overall, these are aggressive malignancies that do not respond well to chemotherapy and have a poorer prognosis than their B-cell counterparts.Citation3
The development of targeted therapies, including monoclonal antibodies and gene treatments, continues. Some studies have focused on monoclonal antibodiesCitation4–Citation9 and gene inhibitors targeting T-cell malignancies;Citation10–Citation12 however, most therapies have targeted B-cell malignancy and myeloid leukemia. Few clinical trials have been performed. Further advances in the treatment of T-cell malignancies require the development of novel agents that target specific malignancies, without significant toxicity.
In 2001, synthetic small interfering RNAs (siRNA) were shown to be able to induce RNAi in mammalian cells by Elshabir et al.Citation13 This discovery led to a surge in interest in harnessing RNAi for biomedical research and drug development. siRNA is a promising gene-targeted agent that has shown great potential, particularly in the field of cancer treatment.Citation14–Citation16 A combination of c-raf siRNA and bcl-2 siRNA induced apoptosis in HL-60, U937, and THP cell lines, and increased chemosensitivity to etoposide and daunorubicin.Citation17 The B-cell chronic lymphocytic leukemia/lymphoma 11B (BCL11B) gene plays a crucial role in T-cell development, differentiation, and proliferation,Citation18–Citation21 and the altered expression, mutation, disruption, or rearrangement of BCL11B has been associated with T-cell malignancies.Citation22–Citation24 Based on the finding that BCL11B overexpression is detected in most T-cell malignancies,Citation21Citation25Citation21,25,26 our previous study showed that the inhibition of BCL11B expression by BCL11B-674-siRNA led to apoptosis in Jurkat, and huT78 cell lines.Citation27 To confirm the potential of BCL11B siRNA as a therapeutic agent, in the present study, we screened the highly efficient and specific BCL11B-siRNAs which target different exon sequences in a human T-ALL cell line Molt-4, the higher efficient BCL11B-935-siRNA was used further to evaluate its BCL11B downregulation, proliferation inhibition, and apoptosis induction of leukemic T cells.
Methods
Samples
Three newly diagnosed T-ALL patients and one case with T-cell lymphoma/leukemia were collected with informed consent. The diagnosis of T-ALL was based on cytomorphology, immunohistochemistry, and cytoimmunological analysis. Peripheral blood was collected by heparin anticoagulation and peripheral mononuclear cells (PBMCs) (contained more than 70% leukemic T cells) were separated using the Ficoll–Hypaque gradient centrifugation method. All procedures were conducted in accordance with the guidelines of the Medical Ethics Committees of the Health Bureau of Guangdong Province, China.
Cell culture
Molt-4 human T-cell leukemia cells (Institutes for Biological Sciences, Cell Resource Center, Chinese Academy of Sciences, Shanghai, China) and PBMCs of three T-ALL patients and one case with T-cell lymphoma/leukemia were grown in Rosewell Park Memorial Institute 1640 medium (Gibco-BRL, Grand Island, NY, USA) with 10% fetal calf serum (Sijiqing Co., Hangzhou, China), which were maintained in a humidified incubator at 37°C and 5% CO2. All experiments were performed using cells in the exponential growth phase.
siRNA design and synthesis
siRNAs named BCL11B-434-siRNA (Chinese patent application no. 200910193249·8), BCL11B-674-siRNA, BCL11B-748-siRNA, and BCL11B-935-siRNA (Chinese patent application no. 200910193248·3), which respectively target the second, second, third and fourth exon domains of BCL11B gene (ACCESSION NM_138576), and the scrambled non-silencing siRNA control (BCL11B-sc), were designed with online software (http://www.invitrogen.com) and synthesized by Invitrogen (Carlsbad, CA, USA). Alexa Red Oligo (Invitrogen) was used to measure transfection efficiency.
Nucleofection
Malignant T cells were collected by centrifugation and resuspended at 2·5×106 of Molt-4 cells or 1×107 of PBMCs per 100 μl of the appropriate NucleofectorTM kit solution (Amaxa Biosystems, Cologne, Germany). Malignant T cells were nucleofected with 3 μg of BCL11B-siRNA or control non-silencing scrambled (sc) RNA using the C-005 (Molt-4 cells) or U-014 (PBMCs) program of a Nucleofection Device II (Amaxa Biosystems); Mock-transfected cells nucleofected without siRNA were used as a negative control. After nucleofection, the cells were immediately mixed with 500 μl of pre-warmed culture medium and transferred to culture plates. Molt-4 cells were incubated at 37°C for 3 days. Three independent experiments on Molt-4 cells were preformed every 24 hours. At 4 hours post-transfection, interleukin-2 (300 U/ml; Gibco-BRL) was added in the PBMCs, and cells were incubated at 37°C for 1 day.
RNA isolation, reverse transcription, and real-time qRT–PCR
Total RNA was isolated from different samples (Molt-4 cells and PBMCs) using Trizol (Invitrogen). The cDNA for qRT-PCR was synthesized with a Superscript II RNaseH reverse transcriptase kit (Invitrogen).
Primers and probes for BCL11B and the reference gene beta-2-microglobulin (beta-2-MG) were synthesized by Tib Molbiol (Berlin, Germany). Quantification of BCL11B mRNA was performed as previously described.Citation22Citation22,26
Immunoblotting
Molt-4 cells (1·5×106) of each group were collected at 72 hours after nucleofection, and proteins were extracted using a RIPA total protein lysate kit (Shennengbocai, Shanghai, China). Protein quantification was performed according to conventional methods. Protein samples (30 μg) were added to SDS loading buffer, heated at 100°C for 5 minutes, and then electrophoresed in 10% SDS-polyacrylamide gels at a constant voltage of 60 V for 30 minutes, followed by 80 V for 45 minutes (Bio-Rad, Hercules CA, USA). The separated proteins were transferred onto nitrocellulose membranes (Invitrogen) using a tank system (Bio-Rad). The membranes were blocked with 3% blocking reagent for 1 hour and then incubated with polyclonal rabbit anti-human BCL11B antibody (1∶5000; Bethyl Laboratories, Inc., Montgomery, TX, USA) or mouse anti-actin antibody (1∶500; Boshide, Wuhan, China), followed by incubation with goat anti-rabbit or goat anti-mouse IgG antibody, respectively (eBioscience, San Diego, CA, USA). Immunoreactive proteins were visualized by chemiluminescence (Beyo-ECL; Beyotime, Shanghai, China), and images were obtained on X-ray film (Kodak, Rochester, NY, USA). The expression level of BCL11B was calculated by Image quantitation analysis software, using beta-actin as a reference.
Cell proliferation assays
The proliferation of Molt-4 cells was indirectly assayed using a CCK-8 kit (Dojindo, Kumamoto, Japan), which stains living cells. After transfection, approximately 5×104 Molt-4 cells (100 μl) and control cells were incubated in triplicate in 96-well plates. At 24, 48, and 72 hours later, CCK-8 reagent (10 μl) was added to each well and incubated at 37°C for 2 hours. The optical density at 450 nm was measured using an automatic microplate reader (Synergy4; Bio-Tek, Winooski, VT, USA).
Cell apoptosis analysis
At 72 h post-transfection, 5×104 of Molt-4 cells were fixed, washed twice with PBS, and stained with Hoechst 33258 staining solution according to the manufacturer’s instructions (Beyotime, Haimen, China). Changes in the nuclei of cells after Hoechst 33258 staining were observed under a confocal laser scanning microscope (LSM 510 META DuoScan; Carl Zeiss, Jena, Germany). Molt-4 cells transfected with both BCL11B-935-siRNA and Alexa Red Oligo were monitored from 48 to 72 hours post-transfection using a Delta Vision high-resolution imaging system (Applied Precision, Inc., Issaquah, WA, USA). Molt-4 cells (5×105) of each group were collected at 72 hours after transfection, then prepared by FITC-labeled anti-Annexin-V (BD Pharmingen, San Diego, CA, USA) and propidium iodide (Kaiji, Nanjing, China) according to the manufacturers’ protocols, and were measured by flow cytometry (Beckman Coulter, Fullerton, CA, USA). The results were analyzed using Win MDI 2·9 software.
Statistical analysis
Statistical analyses were performed with paired t-tests and one-way ANOVE using SPSS 13·0 statistical software. Kruskal–Wallis analysis was used for the BCL11B gene mRNA levels in different samples. Differences were considered statistically significant at P<0·05.
Results
BCL11B-specific siRNAs suppresses BCL11B expression in Molt-4 cells and primary leukemic T cells
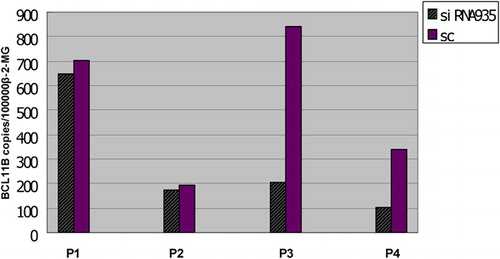
To determine the suppression of BCL11B expression in the Molt-4 cells after siRNA treatment, BCL11B mRNA expression was analyzed by qRT–PCR at 24, 48, and 72 hours after nucleofection, and BCL11B protein expression was analyzed by immunoblotting at 72 hours after nucleofection. Efficiencies of Alexa Red Oligo transfected Molt-4 cells were 39·98±5·47% (). BCL11B-935-siRNA group took silencing results from 24 to 72 hours post-transfection. BCL11B mRNA level in the Molt-4 cells were 63·37±13·90 copies/105 beta-2-MG and 81·23±29·42 copies/105 beta-2-MG at 24 hours with BCL11B-935-siRNA and BCL11B-674-siRNA, while the sc control level was 181·08±59·75 copies/105 beta-2-MG. Reduction in the BCL11B mRNA level were also observed at 48 hours with BCL11B-434-siRNA and BCL11B-748-siRNA (). BCL11B protein expression levels were reduced by 34·77% and 41·73% in the BCL11B-935-siRNA- and BCL11B-434-siRNA-treated cells, compared with the control level at 72 hours, while the reductions with BCL11B-674-siRNA- and BCL11B-748-siRNA-treated cells were 15·56% and 11·66% (). So, the highly efficient and specific BCL11B-935-siRNA was used to treat leukemic T cells from four patients. After BCL11B-935-siRNA treatment, BCL11B mRNA level in primary T-ALL cells of three T-ALL were 282·77±247·57 copies/105 beta-2-MG and 519·48±303·41 copies/105 beta-2-MG at 24 hours with BCL11B-935-siRNA and sc. The BCL11B expression levels from different T-ALL samples were variant, the inhibition rates of BCL11B expression level were very different, and the reduced BCL11B expression rates in leukemic T cells from four patients were 7·66%, 9·02%, 75·59% and 70·16%, respectively ().
Figure 1. Inhibition of BCL11B expression in Molt-4 cells by RNA interference. Alexa Red Oligo transfected Molt-4 cells (A) and MOCK transfected Molt-4 cells used as control (B) at 10 hours after transfection measured by fluorescence microscope (magnification, ×200) and FCM (positive cells are shown as the P3 domain). (C) Suppression of BCL11B mRNA expression measured by qRT-PCR after nucleofection with BCL11B siRNAs (3 μg), compared with expression in cells treated with control non-silencing RNA. Beta-2-MG: beta-2-microglobulin (reference gene). (D) BCL11B protein level in Molt-4 cells at 72 hours after nucleofection with BCL11B siRNAs (3 μg). Non-treated cells (nc), mock-transfected (mock), and scrambled non-silencing RNA (sc)-treated cells were used as controls.
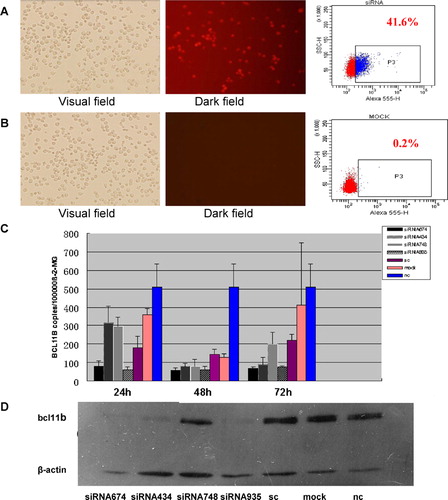
BCL11B suppression inhibits proliferation and induces apoptosis in Molt-4 cells
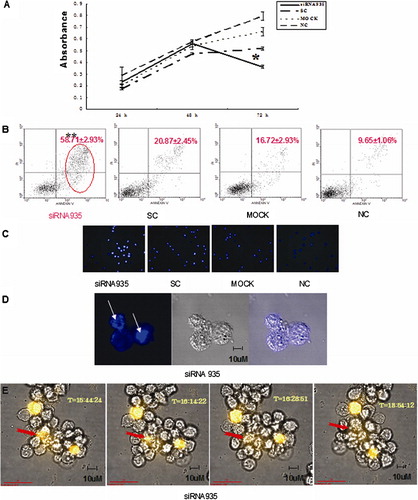
Based on the interference effects of BCL11B-siRNAs, BCL11B-935-siRNA was used to assess the biological consequences. The proliferation rate of Molt-4 cells transfected with BCL11B-935-siRNA was significantly decreased at 72 hours, compared with controls (P<0·05) (), and the transfected Molt-4 cells showed a significant increase in Annexin V/PI-positive cells, reaching 58·71±2·93% (P<0·05) (). Furthermore, morphological changes consistent with apoptosis were observed by Hoechst staining () and by high-resolution imaging ().
Figure 3. Biological consequences of BCL11B silencing. (A) Absorbances of BCL11B siRNA935-treated and control cells at different time points, as measured by the CCK-8 method. Results represent mean values of three independent experiments. *P<0·05, compared with scrambled non-silencing RNA-treated cells. (B) Induction of apoptosis by BCL11B suppression in Molt-4 cells 72 hours after nucleofection with BCL11B-935-siRNA (3 μg). **P<0·05, compared with control non-silencing RNA-treated cells. (C) Hoechst 33258-stained Molt-4 nuclei at 72 hours after transfected in siRNA935 group were mostly dense stain, showing white color, while the normal nuclei in control groups showed light blue with fluorescence microscope. Magnification, ×100. (D) Laser scanning confocal microscopy further revealed morphological changes of apoptosis with white arrows in siRNA935 group. Magnification, ×630. (E) High-resolution live-cell imaging of Molt-4 cells transfected with BCL11B-935-siRNA and Alexa Red Oligo showed changes over time from 48 to 72 hours. The red arrow denoted Molt-4 cell revealed the process of apoptosis as irregular shape changes, shrinking of volume, and then condensation of apoptosis with fluorescence quenching. Magnification, ×630.
Discussion
The functions of the BCL11B gene as a transcriptional factor and a possible tumor suppressor have been described,Citation19–Citation21 but the course of events in vivo remains controversial. To confirm the potential of BCL11B-siRNA as a therapeutic agent, we compared different BCL11B-siRNAs which target different exon sequences and screened the highly efficient and specific BCL11B-siRNA in leukemic T cells. Moreover, in order to identify an efficient siRNA, it may be necessary to test three to four different siRNAs for their ability to inhibit the expression of the intended gene.Citation28
Exogenous siRNA delivery results always in a transient RNAi effect.Citation28 In principle, the observation of the RNAi effect was detected between 24 and 72 hours after siRNA transfection. Experimentally proven, siRNAs took post-transcriptional gene silencing, and control groups did not have obvious influence on the protein levels at 72 hours after nucleofection. However, siRNAs targeting different exon domains showed different levels of effectiveness in BCL11B gene silencing and biological consequences followed. Molt-4 cells responded well to BCL11B-935-siRNA and showed a robust knockdown, whereas BCL11B-748-siRNA showed limited effect on the protein level. BCL11B-748-siRNA targeted on BCL11B gene exon 3; however, BCL11B gene has two transcripts of the selected mRNA alternative splicing on exon 3, the wild-type transcripts within exon 3, and the isoform without exon 3. Although Molt-4 cells express both two transcripts, the BCL11B-748-siRNA only induced silencing in wild-type transcripts. Thus, BCL11B-748-siRNA might not be suggested to be application in transcripts deleted in exon 3. Furthermore, if BCL11B-748-siRNA might take a stronger effect on the simple exon 3 expressing transcript, remains to be detected.
BCL11B gene encodes a C2H2 zinc finger protein, and is krüppel like transcription factor. BCL11B Krüppel protein contains six zinc finger-like domains and a proline-rich region, the major protein encoded 894 amino acids, of which five zinc finger-like domain corresponding to exon 4 coding region, is also the most important functional structure domain.Citation29 BCL11B-935-siRNA targeting on exon 4, obtained efficient and stable silencing effects and biological effects. BCL11B-434-siRNA targeting on exon 2 containing a zinc finger-like domain, also achieved expected effect. Thus, selection and optimization of effective siRNA sequences is required based on the functional encoding protein sequence.
Suppression of BCL11B by siRNA-935 effectively inhibited proliferation and induced apoptosis in Molt-4 cells. This result is similar to the suppression of BCL11B by siRNA-674, which targets the second exon of the BCL11B gene, in the Jurkat cell line.Citation27 The dynamic process of cell apoptosis was confirmed by high-resolution imaging in the present study. The different results of the silencing effects in the mentioned siRNAs above were due to the different dosages. Moreover, our results provide further evidence for a major role of BCL11B in negatively regulating apoptosis in T-cell malignant cell lines.
Overexpression of BCL11B was detected in most primary T-cell malignancies;Citation26 to test the effect of BCL11B-935-siRNA which showed the best inhibition efficiency in Molt-4 cells, in primary leukemic T cells, we used the BCL11B-935-siRNA to treat leukemic T cells from four patients. Significant effect could be found only in two case, in which the reduction of expression level of BCL11B gene was more than 70%, whereas the reduction of BCL11B expression only 7·66% and 9·02% in the remains two cases, respectively. It may due to the heterogeneity of T-cell malignancies,Citation30 although overexpression of BCL11B gene was identified in all samples. Further work is needed to analyze more samples and to characterize the feature of leukemic T cells with sensitivity or resistance for BCL11B-siRNA. Moreover, we will focus on the molecular mechanisms of BCL11B siRNA-mediated cell death and to further evaluate its influence on proliferation and differentiation of hematopoietic stem/progenitor cells.
In conclusion, our findings provided further evidence for an anti-apoptotic function of BCL11B in T-cell malignancies. Downregulated BCL11B expression results in apoptosis in Molt-4 cells, suggesting that BCL11B siRNA may be considered a new targeted therapeutic strategy for T-cell malignancies.
Xin Huang, Si Chen and Qi Shen contributed equally to the study. This work was supported by grants from the National Natural Science Foundation of China (no. 30771980), the Fundamental Research Funds for the Central Universities (no. 21610604), and the Guangdong Science and Technology Project (nos. 2007B030703008 and 2009B050700029).
References
- Rivera GK, Crist WM. Acute lymphoblastic leukemia. In: , Handin R I, Stossel T P, Lux S E, ed, editors. Blood. Principles and practice of hematology. Philadelphia, PA: J. B. Lippincott Co.; 1995. p.743–59.
- Uckun FM, Sensel MG, Sun L, Steinherz PG, Trigg ME, Heerema NA. Biology and treatment of childhood T-lineage acute lymphoblastic leukemia. Blood 1998;91:735–46.
- Morris JC, Waldmann TA, Janik JE. Receptor-directed therapy of T-cell leukemias and lymphomas. J Immunotoxicol 2008;5:235–48.
- Dearden CE, Matutes E, Cazin B, Tjønnfjord GE, Parreira A, Nomdedeu B, et al.. High remission rate in T-cell prolymphocytic leukemia with CAMPATH-1H. Blood 2001;98:1721–6.
- Rodig SJ, Abramson JS, Pinkus GS, Treon SP, Dorfman DM, Dong HY, et al.. Heterogeneous CD52 expression among hematologic neoplasms: implications for the use of alemtuzumab (CAMPATH-1H). Clin Cancer Res 2006;12:7174–9.
- Ravandi F, O’Brien S. Alemtuzumab in CLL and other lymphoid neoplasms. Cancer Invest 2006, 24:718–25.
- Ishida T, Ueda R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci 2006;97:1139–46.
- Peipp M, Küpers H, Saul D, Schlierf B, Greil J, Zunino SJ, et al.. A recombinant CD7-specific single-chain immunotoxin is a potent inducer of apoptosis in acute leukemic T cells. Cancer Res 2002;62:2848–55.
- Sato T, Yamochi T. CD26 regulates p38 mitogen-activated protein kinase-dependent phosphorylation of integrin beta, adhesion to extracellular matrix, and tumorigenicity of T-anaplastic large cell lymphoma Karpas 299. Cancer Res 2005;65:6950–6.
- Steinbach D, Wittig S, Cario G, Viehmann S, Mueller A, Gruhn B, et al.. The multidrug resistance-associated protein 3 (MRP3) is associated with a poor outcome in childhood ALL and may account for the worse prognosis in male patients and T-cell immunophenotype. Blood 2003;102:4493–8.
- McKallip RJ, Lombard C, Fisher M, Martin BR, Ryu S, Grant S, et al.. Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood 2002;100:627–34.
- Villalba M, Altman A. Protein kinase C-theta (PKCtheta), a potential drug target for therapeutic intervention with human T cell leukemias. Curr Cancer Drug Targets 2002;2:125–37.
- Elbashir S, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001;411:494–8.
- Oh YK, Park TG. siRNA delivery systems for cancer treatment. Adv Drug Deliv Rev 2009;61:850–62.
- Devi RS. siRNA-based approaches in cancer therapy. Cancer Gene Ther 2006;13:819–29.
- Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov 2009;8:129–38.
- Cioca DP, Aoki Y, Kijosawa K. RNA interference is a functional pathway with therapeutic potential in human myeloid leukemia cell lines. Cancer Gene Ther 2003;10:125–33.
- Li L, Leid M, Rothenberg EV. An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science 2010;329:89–93.
- Wakabayashi Y, Watanabe H, Inoue J, Takeda N, Sakata J, Takeda N, et al.. Bcl11bis required for differentiation and survival of αβ T lymphocytes. Nat Immunol 2003;4:533–9.
- Cismasiu VB, Ghanta S, Duque J, Albu D, Chen HM, Kasturi R, et al.. BCL11Bparticipates in the activation of interleukin-2 gene expression in CD4+ T lymphocytes. Blood 2006;108:2695–702.
- Liu P, Li P, Burke S. Critical roles of Bcl11b in T-cell development and maintenance of T-cell identity. Immunol Rev 2010;238:138–49.
- Przybylski GK, Dik WA, Wanzeck J, Grabarczyk P, Majunke S, Martin-Subero JI, et al.. Disruption of the BCL11B gene through inv(14)(q11·2q32·31) results in the expression of BCL11B-TRDC fusion transcripts and is associated with the absence of wild-type BCL11B transcripts in T-ALL. Leukemia 2005;19:201–8.
- Karlsson A, Nordigården A, Jönsson JI, Söderkvist P.Bcl11bmutations identified in murine lymphomas increase the proliferation rate of hematopoietic progenitor cells. BMC Cancer 2007;7:195.
- Su XY, Della-Valle V, Andre-Schmutz I, Lemercier C, Radford-Weiss I, Ballerini P, et al.. HOX11L2/TLX3 is transcriptionally activated through T-cell regulatory elements downstream of BCL11B as a result of the t(5;14)(q35;q32). Blood 2006;108:4198–201.
- Oshiro A, Tagawa H, Ohshima K, Karube K, Uike N, Tashiro Y, et al.. Identification of subtype-specific genomic alterations in aggressive adult T-cell leukemia/lymphoma. Blood 2006;107:4500–7.
- Huang X, Chen S, Shen Q, Yang LJ, Li B, Zhong LY, et al.. Analysis of the expression pattern of the BCL11B gene and its relatives in patients with T-cell acute lymphoblastic leukemia. J Hematol Oncol 2010;3:44.
- Grabarczyk P, Przybylski GK, Depke M, Völker U, Bahr J, Assmus K, et al.. Inhibition of BCL11B expression leads to apoptosis of malignant but not normal mature T cells. Oncogene 2007;26:3797–810.
- Heidenreich O. Oncogene suppression by small interfering RNAs. Curr Pharm Biotechnol 2004;5:349–54.
- Avram D, Fields A, Pretty On Top K, Nevrivy DJ, Ishmael JE, Leid M. Isolation of a novel family of C2H2 zinc finger proteins implicated in transcriptional repression mediated by chicken ovalbumin upstream promoter transcription factor (COUP-TF) orphan nuclear receptors. J Biol Chem 2000;275:10315–22.
- Onciu M, Lai R, Vega F, Bueso-Ramos C, Medeiros LJ. Precursor T-cell acute lymphoblastic leukemia in adults: age-related immunophenotypic, cytogenetic, and molecular subsets. Am J Clin Pathol 2002;117:252–8.