Abstract
This study aimed to determine the effect of induced mild hypothermia (34°C) on the production of two cytokines (interleukin (IL-6) and tumor necrosis factor (TNF)alpha) and reactive nitrogen and oxygen species in plasma and the heart of acutely septic rats. After anesthesia and in conditions of normothermia (38°C) or mild hypothermia (34°C), acute sepsis was induced by cecal ligation and perforation. For each temperature three groups were formed: (1) baseline (blood sample collected at T0 hour), (2) sham (blood sample at T4 hours) and (3) septic (blood sample at T4 hours). At either temperature sepsis induced a significant increase in plasma IL-6, TNF-alpha and HO• concentration, compared with the sham groups (P ≤ 0.016). Compared with the normothermic septic group, septic rats exposed to mild hypothermia showed a mild decrease in TNF-alpha concentration (104 ± 50 pg/ml vs. 215 ± 114 pg/ml; P > 0.05) and a significant decrease in IL-6 (1131 ± 402 pg/ml vs. 2494 ± 691 pg/ml, P = 0.038). At either temperature sepsis induced no enhancement within the heart of lipoperoxidation (malondialdehyde content) or antioxidant activities (superoxide dismutase and catalase). In conclusion, during acute sepsis, induced mild hypothermia appears to reduce some pro-inflammatory and oxidative responses. This may, in part, explain the beneficial effect of hypothermia on survival duration of septic rats.
Introduction
Sepsis, a major cause of death within intensive care units, is defined as a pathological state following infection associated with systemic inflammatory response.Citation1–Citation3 During sepsis secretion of excessive pro-inflammatory mediators, such as cytokines, has been established.Citation4 Among these cytokines, tumor necrosis factor (TNF)-alpha and interleukin (IL)-6 (which respectively activate and regulate the inflammatory system in response to infectious stimuli) are released in large quantities by various immune cell types in the early stage of sepsis.Citation5 This phenomenon may be amplified by an increase in reactive oxygen species (ROS) and reactive nitrogen species (RNS) such as nitric oxide (NO).Citation6 These reactive species represent a key element in the deleterious cascade process when endogenous antioxidant defences (for example, superoxide dismutase: SOD and catalase: CAT)Citation7 are overcome and/or when a redox imbalance occurs.Citation8,Citation9 In sepsis several potential ROS sources, including mitochondrial electron transport and NADPH oxydase through macrophages and neutrophils activity,Citation7 are thought to be responsible for such an event leading to severe dysfunction and/or damage across multiple organs.Citation10 For example, NO generated within macrophages and other immune system cells, is implicated in pathologic vasodilatation resulting in hypotension.Citation11
In the laboratory, the rat model employed has two main objectives, firstly, to investigate acute sepsis and its early physiological mechanisms and secondly, to explore all treatments likely to promote an increase in survival duration of patient, thereby extending the available time for therapeutic intervention. Recently, we reliably demonstrated that the survival duration of septic rats increased by 42% when they were maintained at a core temperature of 34°C (mild hypothermia), compared with those at 38°C (normothermia).Citation12,Citation13 The mean survival durations were 442 minutes ± 12 and 311 minutes ± 36 in mild hypothermia and normothermia, respectively.Citation13 The protective effect of mild hypothermia has also been found in human tissue injuries such as cerebral or myocardial ischemia.Citation14,Citation15 Multiple mechanisms involving protective responses for induced hypothermia have been identified, including a reduced metabolic rate, slower energy depletion and decreases in both inflammatory response and ROS production.Citation16 A temperature of 34°C is used in our experiments because deep hypothermia (32°C) may give rise to more side effects and harmful impacts, as has been demonstrated in the clinical context.Citation17
The aim of this experimental study was to examine the influence of mild hypothermia on the plasma level of two pro-inflammatory cytokines, IL-6 and TNF-alpha, and two free radicals, NO and HO•, from induced normothermic (38°C) and hypothermic (34°C) septic rats. Our hypothesis was that the increase in survival duration in septic rats exposed to mild hypothermia, as observed in our previous studies,Citation12,Citation13 might be linked to a decrease in inflammatory responses and metabolic rate resulting in decreased RNS and ROS production. Heart tissue levels of two antioxidant enzymes activities (SOD and CAT) and a lipoperoxidation index (malondialdehyde (MDA) content) were also quantified. This organ was preferentially chosen because of the frequently reported protective effects of hypothermia on the heart in relation to other pathologic conditions.
Materials and methods
Animals
This study was performed in accordance with the European Council revised guidelines on animal care for experimental and other scientific purposes. The protocol was approved by the local ethics committee for animal experimentation (Authorization number: R-2011-KL-01). All efforts were made to minimize the number of animals used and their suffering. Experiments were performed on 36 male Sprague-Dawley rats. Rats of median weight 296 g (range 236–349 g) were obtained from Janvier SAS (Le Genest St Isle, France). Prior to the experiment the rats were housed for 1 week in the University vivarium in standard conditions (mean temperature 21°C, relative humidity 27%, 12-hour light:dark cycle) during which they had access to rat food and water ab libitum.
Anesthesia and temperature control
Anesthesia and analgesia were induced by intraperitoneal injection of ketamine (10 mg/100 g body mass) and xylazine (1 mg/100 g body mass) mixture. An intraperitoneal catheter was then inserted to allow subsequent continuous anesthetic and analgesic infusion (0.4 mg xylazine, 12 mg ketamine in 1 ml saline solution) until the end of the experiment. The infusion rate was 0.5 ml/hour per 100 g body mass. Resuscitation was also completed with subcutaneous saline solution injection (3 ml/100 g body mass). The experiments were conducted with animals breathing room air.
The procedure to maintain core temperature was adapted from L'Her et al.Citation12 and was used to counteract core temperature changes induced by sepsis and anesthesia. In summary, during the entire experiment, the animals were placed on a thermoregulated mattress (Huber Polystat CC1, Peter Huber Kältemaschinenbau GmbH, Germany) and core temperature was maintained constant at 38°C or 34°C (±0.5°C) corresponding to induced normothermia and induced mild hypothermia, respectively. Temperature was continuously monitored using a telethermometer, connected to a rectal probe (YSI telethermometer; Yellow Spring Instruments, Yellow Spring, OH, USA). Surgical procedures were performed after animals had reached the target temperature.
Sepsis induction
Sepsis induction was performed using a cecal ligation and puncture model adapted from L'Her et al.Citation12 and Rittirsch et al.Citation18 In sum, a 2-cm midline incision was made in the abdominal wall (laparotomy). The cecum was carefully extruded and ligated on the middle to avoid bowel obstruction. Afterwards, the cecum was punctured once from side to side using a pulmonary trocar (8 Gauge) and replaced in the abdominal cavity. For sham rats, anesthesia, laparotomy, and cecum extrusion were performed without any cecal puncture.
Experimental procedure
The 36 rats were randomly assigned to one of three experimental sub-groups for each temperature (38°C or 34°C; n = 6 rats for each; ). The three experimental sub-groups were considered depending on blood sample procedure. In the baseline group for each temperature blood samples were obtained immediately after surgery. In the two other groups for each temperature (sham group and septic group) a blood sample was obtained 4 hours later.
In all groups, 1 hour before obtaining a blood sample, a catheter was inserted into the right jugular vein to administer salicylic acid (2.5 mg/kg of body mass) in order to trap blood HO•. A catheter was also inserted, 5 minutes before sepsis (or laparotomy) induction, into the carotid artery to collect a blood sample (8 ml) on ethylenediamminetetraacetate. Finally, after collecting the arterial blood sample, each heart was frozen in liquid nitrogen and preserved at −80°C until biochemical analysis.
Biochemical analysis
In plasma
Blood samples were centrifuged at 1600 × g for 10 minutes at 4°C. A part of the collected supernatant (1800 µl) was recovered to quantify HO• production and the remainder (1400 µl) frozen at −80°C until an assay of plasma cytokines (IL-6, TNF-alpha) and NO could be conducted.
HO• quantification
In vivo HO• production was determined using an indirect method adapted from Coudray and FavierCitation19 Hydroxylation of salicylic acid gives two stable metabolites (2,3 and 2,5 dihydroxybenzoic acid, DHBA) which were further separated and quantified by high-performance liquid chromatography (HPLC) coupled with electrochemical detection at a potential of 700 mV. For this experiment, only 2,3 DHBA quantification was performed (2,5 DHBA is a metabolite which can be produced by enzymatic pathways through the cytochrome P-450 system of the liverCitation20). The 2,3 DHBA extraction was performed on plasma with HCl and ethyl acetate. The mixture was centrifuged at 3000 × g for 10 minutes at 4°C and the supernatant evaporated under nitrogen gas until a dry residue was obtained. This residue was re-suspended in ultrapure water, homogenized and filtered at 0.45 µm. The sample was then placed on a plate cooled to 4°C and injected into the HPLC column (chromatographic conditions, see Amerand et al.Citation21). HO• production was expressed in 2,3 DHBA ng/ml of plasma/hour. The detection limit and the intra-assay variation coefficient of the 2,3 DHBA quantification were respectively 20 nM and 9%.
Cytokine content
TNF-alpha and IL-6 were measured with a solid phase sandwich Elisa, using a commercial immunoassay kit (RTA00 and R600B, respectively; R&D Systems Europe, Abingdon, UK). Optical density was read at 450 nm and cytokines were expressed in pg/ml of plasma. The sensitivity of the assay, the analytical range and the variation coefficient were, respectively, 21 pg/ml, 0–4000 pg/ml, and 8% for IL-6 and 5 pg/ml, 0–800 pg/ml, and 5% for TNF-alpha.
NO determination
Plasma NO concentration was determined by a colorimetric assay based on the Griess reaction after enzymatic conversion of nitrate to nitrite by nitrate reductase. The nitrite was then made to react with sulfanilamide and N-(1-naphthyl)-ethylenediamine dihydrochloride to give a violet diazo dye. Quantification was performed in accordance with the manufacturer's instructions (11746081001, Roche Diagnostics, Germany). Results were expressed in μmol/l of plasma. The detection limit was 0.02 mg/l for NO.
In heart
Antioxidant enzyme activities and protein content
A sample of each frozen heart (approximately 200 mg) was weighed and pounded with a polytron at 4°C in a buffer solution (see Mortelette et al.Citation22). After an initial centrifugation at 5000 × g for 10 minutes, the supernatant was again centrifuged at 12 000 × g for 15 minutes before analysis. Enzyme activity and protein content were determined by ultraviolet spectrophotometry at 25°C. SOD activity was measured at 480 nm by a method involving inhibition of the adrenaline-adenochrome reaction. One unit (U) of SOD is the amount needed to observe 50% inhibition. SOD activity was expressed in U/mg protein. CAT activity was determined at 240 nm through its ability to transform H2O2 into H2O and O2−. CAT activity was expressed in mmol H2O2/min/mg of protein. Protein content was measured by the indirect Lowry technique and expressed in mg protein/mg of tissue.
Malondialdehyde content
The method for determining MDA content in each heart was adapted from Mortelette et al.Citation22 by the thiobarbituric acid (TBA)/MDA complex quantification. A sample of each frozen heart was pounded with a polytron at 4°C in a solution containing 1% of butylhydroxytoluene and 1% of phosphoric acid. The mixture was then maintained at 100°C for 30 minutes during which, in the presence of phosphoric acid, a complex was formed between MDA and thiobarbituric acid (TBA, 7.4 mM). Then, after butanolic extraction under nitrogen gas, the TBA/MDA complex was re-suspended in the mobile phase and separated by HPLC (chromatographic conditions, see Sébert et al.Citation23) detected by UV spectrophotometry at 532 nm. Results were expressed in nmol/g tissue. The detection limit was 100 nM for MDA.
Statistical analysis
Results were expressed as median ± interquartile range. Differences in median values between groups were assessed using Wilcoxon rank sum tests. Differences where P < 0.05 were considered significant.
Results
There was no significant difference in any of the variables measured between the baseline and sham groups at either 34 or 38°C. For this reason, we chose to compare the septic group to the sham group only.
In plasma: inflammatory and oxidative parameters
Cytokines level
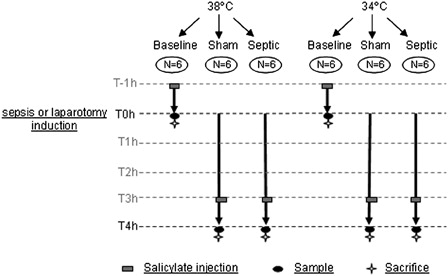
As shown in A and B, sepsis induced a significant increase in IL-6 and TNF-alpha plasma concentrations compared with the sham groups, at both temperatures (P ≤ 0.01). Compared with the normothermic septic group, septic rats exposed to mild hypothermia showed a mild decrease in TNF-alpha concentration (104 ± 50 pg/ml vs. 215 ± 114 pg/ml, P > 0.05) and a significant decrease in IL-6 (1131 ± 402 pg/ml vs. 2494 ± 691 pg/ml, P = 0.038).
Figure 2. Cytokines values (A, IL-6 and B, TNF-α) in plasma for each experimental condition (n = 6 for each group except for septic group in normothermia where n = 4). Data are expressed as median ± interquartile range. *Significant difference between septic and sham normothermia groups. #Significant difference between septic and sham hypothermia groups. +Significant difference between septic normothermia and septic hypothermia groups (P < 0.05).
NO and HO• quantification
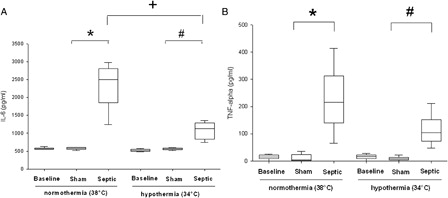
In normothermia, plasma NO concentration significantly increased in the septic group, compared with the sham group (28.5 ± 4.7 µmol/l vs. 20.6 ± 6 µmol/l, P = 0.01) (). The sham group exposed to mild hypothermia showed a significant decrease in NO concentration compared with the normothermic sham group (10.4 ± 5.5 µmol/l vs. 20.6 ± 6 µmol/l, P ≤ 0.03). In septic rats no significant decrease in plasma NO concentration was observed during mild hypothermia compared with normothermia. Regardless of the temperature, sepsis induced a significant increase in plasma HO• concentration, compared with the sham group (P ≤ 0.016) (). In septic rats, a non-significant decrease in the HO• production was observed in hypothermia, compared to normothermia.
Figure 3. Nitric oxide (NO) level in plasma for each experimental condition (n = 6 for each group except for septic group in normothermia where n = 4 and for sham and septic groups in induced mild hypothermia where n = 5). *Significant difference between septic and sham normothermia groups (P < 0.05). xSignificant difference between sham normothermia and sham hypothermia groups (P < 0.05).
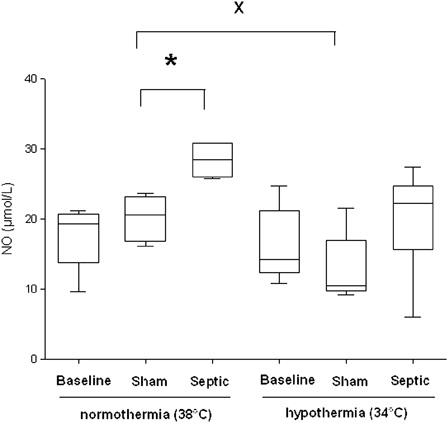
Figure 4. Hydroxyl radical (HO•) production in plasma per hour for each experimental condition (n = 6 for each group except for sham and septic groups in normothermia where n = 5 and n = 4, respectively). Data are expressed as median ± interquartile range *Significant difference between septic and sham normothermia groups. #Significant difference between septic and sham hypothermia groups (P < 0.05).
In heart: antioxidant enzyme activities, protein and malondialdehyde contents
At either temperature, no significant effect was observed for the above-mentioned parameters during sepsis except for MDA in hypothermia where a significant decrease was observed in the septic rats compared to the sham rats ().
Table 1. SOD and CAT activity and MDA concentrations in the heart for each experimental condition
At either temperature there was no significant difference between septic groups and control groups in either SOD or CAT activity (). Under mild hypothermia, however, the septic group showed significantly lower MDA compared with the control group (36 ± 9 nmol/g vs. 42 ± 5 nmol/g, P ≤ 0.05)
Discussion
In this study acute sepsis was observed to induce an inflammatory response (increased pro-inflammatory cytokines) associated with increasing NO and HO• concentrations at a systemic level, and yet without corresponding oxidative changes to the heart. Mild hypothermia may globally decrease the overall plasma levels of these systemic insult markers.
As preliminary comment, two precisions were important to mention. First, the experimental conditions of this work and the model used (ligature position, perforation size, anesthesia throughout the experiment) explain the short survival duration. Second, induced mild hypothermia, with its beneficial effects in terms of survival duration, likely differs significantly to hypothermia developed by septic patients. In clinical practice the prognosis is usually deleterious when septic patients exhibit spontaneous hypothermia.Citation24
In our acute sepsis model two pro-inflammatory cytokines, TNF-alpha and IL-6, were determined in plasma. TNF-alpha is an activator of the cytokine cascade and IL-6 appears to be a good marker of sepsis severity.Citation4 Anesthesia itself did not produce changes in plasma concentrations of IL-6 or TNF while in the sepsis groups there was a significant increase. Thus, in our experiment the variation of plasmatic cytokine levels confirmed the validity of the sepsis model, consistent with previous research.Citation25 With mild hypothermia during sepsis, our experimental results indicate a significant decrease in plasmatic level of IL-6, as in the severe septic shock model of Rim et al.Citation26 A decrease was also observed for TNF-alpha but to a lesser, non-significant degree. Unfortunately, the plasma HO• determination requires a relatively large volume of blood (3.5 ml). Yet, monitoring IL-6 and TNF-alpha kinetics would have been interesting because it is know that cytokine level are time dependant.Citation5 Among the possible mechanisms involved in the cytokine decrease, nuclear factor κ B (NF-κB), an important and highly inducible transcriptional factor with a pivotal role in the induction of genes responsible for stress and inflammatory responses, could be implicated. Indeed, a study lead by Webster et al.Citation27 on a cerebral ischemia model demonstrated that mild hypothermia reduces NF-kB pathway activation, an inflammatory response, by decreasing IκB (inhibitor of NF-κB) protein phosphorylation.
The use of hypothermia in the treatment of sepsis retains considerable potential especially as to date, human trials of anti-inflammatory therapies have been relatively disappointing in improving outcomes during sepsis.Citation4,Citation28 Reasons for this lack of efficacy are unclear. Timing and dosage of these interventions may be critical, since these treatments affect proximal steps in the cytokine cascade. Blocking pro-inflammatory cytokine production may inhibit host defence functions that are critical for recovery from sepsis. There may also be a balance between excessive inflammation (which causes tissue injury) and inadequate inflammatory response (which compromises host defence functions).Citation4 In this context, mild hypothermia may hold significant potential for regulating parts of the inflammatory process.
During sepsis, many studies have shown that ROS and RNS may play a central pathogenic role.Citation8,Citation9 Among ROS, HO• is a powerful oxidant that reacts immediately with neighbouring biological substrates such as nucleic acids, polyunsaturated fatty acids, and proteins.Citation29 The choice of HO• measurement within our study was made because the accumulation of hydrogen peroxide and superoxide anion, in the presence of free iron, largely released from myoglobin and haemoglobin in sepsis, could lead to the formation of HO• via the metal catalyzed Fenton reaction. Moreover, endogenous nitrous oxide (NO) can react with superoxide and produce peroxynitrite, which may in turn decompose to form the hydroxyl radical.Citation30 Owing to the extremely short half-life of HO•, salicylic acid has been used as an HO• trapper. The concentration of injected salicylic acid in this study (2.5 mg/kg bm [body mass]) was considered sufficient to trap HO• and yet not sufficiently high as to induce anti-inflammatory and/or antioxidant effects.Citation19 In this study the plasma HO• level observed in the sepsis groups confirmed a significant increase in ROS production, as previously reported.Citation8 With mild hypothermia HO• concentrations followed the tendency of plasma cytokines levels to decrease. Previous studies have shown that hypothermia generally attenuates oxidative stress generated, for example, by ischemia-reperfusion insult.Citation31,Citation32 thus allowing endogenous anti-oxidative mechanisms to prevent or attenuate oxidative damage to cells.Citation15 To explain, at least in part, the beneficial effects of hypothermia, previous studies have acknowledged that the kinetic properties of most enzyme systems are temperature-dependent.Citation33 Hypothermia, by reducing the metabolic rate and in turn, oxygen consumption at the mitochondrial level, could also reduce ROS production and its consequential deleterious effects. No oxidative damage was observed in the heart, suggesting this organ was protected. Indeed, in the normothermic group, sepsis did not have a significant effect on heart antioxidant activities (SOD and CAT) or MDA content (lipoperoxidation index). Only a significant MDA decrease in the mild hypothermic septic group, compared with the hypothermic sham group, was observed but this remains difficult to explain. We can hypothesize that the design of our severe experimental sepsis and thus the short sampling time (at 4 hours) may not have been sufficient to observe changes in the pro-oxidant/anti-oxidant balance within the heart. Demirbilek et al.Citation34 found that MDA concentration increased significantly within the heart during sepsis, but these measurements were performed 24 hours post sepsis inducement. In our study both the absence of oxidative stress within the heart and the increase in HO• production at the systemic level suggest that other tissues or organs suffered oxidative injuries whereas the heart, as a vital organ, may have remained relatively preserved during the onset of sepsis. Indeed, Wu et al.Citation35 have shown that lipoperoxidation during hemorrhagic shock in rats was not a major mechanism responsible for myocardial damage following mild hypothermia while it may be significant in other tissues.
In this study, a significant increase in plasma NO concentration was observed in septic rats during normothermia, in keeping with previous research.Citation36 Mediators of inflammation such as cytokines have been shown to promote enzyme inducible NO synthetase, thereby increasing NO production.Citation34 With mild hypothermia, plasma NO concentration tends to decrease. This may be explained, at least in part, both by the reduced amount of IL-6 and TNF-alpha pro-inflammatories observed in this study and also by an inhibition of NOS among other enzymes, as shown by Scumpia et al.Citation37 in endotoxemic rats. By this mechanism hypothermia may, therefore, attenuate vasodilation and thus increase systemic vascular resistance, this effect being synergic with increasing hypothermia-induced vascular resistance.Citation38 In the same manner, Schortgen et al.Citation39 have recently demonstrated the importance of temperature control. They have shown that the control of fever by external cooling permitted a reduction in the use of vasopressors and reduced early deaths in septic patients, probably by accelerating hemadynamic stabilization.
In conclusion, in this study acute sepsis was observed to induce a major inflammatory response, (increase of two pro-inflammatory cytokines), associated with increasing NO and HO• concentrations at a systemic level, and yet without corresponding changes to the heart. Mild hypothermia may globally decrease the overall plasma levels of these systemic insult markers. These results could explain, at least in part, the beneficial effects of mild hypothermia on survival duration.
Acknowledgments
This research was supported by a grant from the Brittany Regional council. The authors thank Peter Buzzacott and Joanne Gardner Le Gars for their helpful suggestions and English revisions.
References
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250–6.
- Gerlach H, Toussaint S. Organ failure in sepsis. Curr Infect Dis Rep 2007;9:374–81.
- Schmittinger CA, Wurzinger B, Deutinger M, Wohlmuth C, Knotzer H, Torgersen C, et al. How to protect the heart in septic shock: a hypothesis on the pathophysiology and treatment of septic heart failure. Med Hypotheses 2010;74:460–5.
- Blackwell TS, Christman JW. Sepsis and cytokines: current status. Br J Anaesth 1996;77:110–7.
- Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Dupont E. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest 1993;103:565–75.
- Taylor DE, Piantadosi CA. Oxidative metabolism in sepsis and sepsis syndrome. J Crit Care 1995;10:122–35.
- Macdonald J, Galley HF, Webster NR. Oxidative stress and gene expression in sepsis. Br J Anaesth 2003;90:221–32.
- Biswal S, Remick DG. Sepsis: redox mechanisms and therapeutic opportunities. Antioxid Redox Signal 2007;9:1959–61.
- Victor VM, Rocha M, Esplugues JV, De la Fuente M. Role of free radicals in sepsis: antioxidant therapy. Curr Pharm Des 2005;11:3141–58.
- Goode HF, Cowley HC, Walker BE, Howdle PD, Webster NR. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Crit Care Med 1995;23:646–51.
- Price S, Anning PB, Mitchell JA, Evans TW. Myocardial dysfunction in sepsis: mechanisms and therapeutic implications. Eur Heart J 1999;20:715–24.
- L'Her E, Amerand A, Vettier A, Sebert P. Effects of mild induced hypothermia during experimental sepsis. Crit Care Med 2006;34:2621–3.
- Léon K, Pichavant-Rafini K, Quemener E, Sébert P, Egreteau PY, Ollivier H, et al. Oxygen blood transport during experimental sepsis: Effect of hypothermia. Crit Care Med 2012;40:912–8.
- Vigue B, Geeraerts T, Le Guen M, Engrand N, Ract C. Therapeutic hypothermia. Ann Fr Anesth Reanim 2006;25:838–44.
- Moore EM, Nichol AD, Bernard SA, Bellomo R. Therapeutic hypothermia: benefits, mechanisms and potential clinical applications in neurological, cardiac and kidney injury. Injury 2011;42:843–54.
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med 2009;37:S186–202.
- Weinrauch V, Safar P, Tisherman S, Kuboyama K, Radovsky A. Beneficial effect of mild hypothermia and detrimental effect of deep hypothermia after cardiac arrest in dogs. Stroke 1992;23:1454–62.
- Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 2009;4:31–6.
- Coudray C, Favier A. Determination of salicylate hydroxylation products as an in vivo oxidative stress marker. Free Radic Biol Med 2000;29:1064–70.
- Halliwell B, Kaur H, Ingelman-Sundberg M. Hydroxylation of salicylate as an assay for hydroxyl radicals: a cautionary note. Free Radic Biol Med 1991;10:439–41.
- Amerand A, Vettier A, Sebert P, Cann-Moisan C. In vitro effect of hydrostatic pressure exposure on hydroxyl radical production in fish red muscle. Redox Rep 2005;10:25–8.
- Mortelette H, Amerand A, Sebert P, Belhomme M, Calves P, Moisan C. Effect of exercise training on respiration and reactive oxygen species metabolism in eel red muscle. Respir Physiol Neurobiol 2010;172:201–5.
- Sebert P, Menez JF, Simon B, Barthelemy L. Effects of hydrostatic pressure on malondialdehyde (MDA) determination in brain from yellow freshwater eels. C R Acad Sci III 1995;318:757–60.
- Peres Bota D, Lopes Ferreira F, Melot C, Vincent JL. Body temperature alterations in the critically ill. Intensive Care Med 2004;30:811–6.
- Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 2000;181:176–80.
- Rim KP, Kim K, Jo YH, Lee JH, Rhee JE, Kang KW, et al. Effect of therapeutic hypothermia according to severity of sepsis in a septic rat model. Cytokine 2012;60:755–61.
- Webster CM, Kelly S, Koike MA, Chock VY, Giffard RG, Yenari MA. Inflammation and NFkappaB activation is decreased by hypothermia following global cerebral ischemia. Neurobiol Dis 2009;33:301–12.
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138–50.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39:44–84.
- Ritter C, Andrades M, Moreira JC, Dal-Pizzol F. Antioxidants and sepsis: can we find the ideal approach? Crit Care Med 2004;32:1445–6.
- Bayir H, Adelson PD, Wisniewski SR, Shore P, Lai Y, Brown D, et al. Therapeutic hypothermia preserves antioxidant defenses after severe traumatic brain injury in infants and children. Crit Care Med 2009;37:689–95.
- Huet O, Kinirons B, Dupic L, Lajeunie E, Mazoit JX, Benhamou D, et al. Induced mild hypothermia reduces mortality during acute inflammation in rats. Acta Anaesthesiol Scand 2007;51:1211–6.
- Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med 2009;37:1101–20.
- Demirbilek S, Ersoy MO, Demirbilek S, Karaman A, Akin M, Bayraktar M, et al. Effects of polyenylphosphatidylcholine on cytokines, nitrite/nitrate levels, antioxidant activity and lipid peroxidation in rats with sepsis. Intensive Care Med 2004;30:1974–8.
- Wu X, Stezoski J, Safar P, Bauer A, Tuerler A, Schwarz N, et al. Mild hypothermia during hemorrhagic shock in rats improves survival without significant effects on inflammatory responses. Crit Care Med 2003;31:195–202.
- Giusti-Paiva A, Martinez MR, Felix JV, da Rocha MJ, Carnio EC, Elias LL, et al. Simvastatin decreases nitric oxide overproduction and reverts the impaired vascular responsiveness induced by endotoxic shock in rats. Shock 2004;21:271–5.
- Scumpia PO, Sarcia PJ, DeMarco VG, Stevens BR, Skimming JW. Hypothermia attenuates iNOS, CAT-1, CAT-2, and nitric oxide expression in lungs of endotoxemic rats. Am J Physiol Lung Cell Mol Physiol 2002;283:L1231–8.
- Kuhn LA, Turner JK. Alterations in pulmonary and peripheral vascular resistance in immersion hypothermia. Circ Res 1959;7:366–74.
- Schortgen F, Clabault K, Katsahian S, Devaquet J, Mercat A, Deye N, et al. Fever control using external cooling in septic shock: a randomized controlled trial. Am J Respir Crit Care Med 2012;185:1088–95.