Abstract
Objectives: Lithium may inhibit lipid peroxidation (LP) and protein oxidation, stimulate cell proliferation, increase neurogenesis, and delay cell death. Oxidative stress (OxS) is a state of imbalance between oxidative processes and antioxidant defenses, which may play an important role in the pathophysiology and disease course of bipolar disorder (BD). The aim of this study was to estimate the influence of lithium, administered alone or in combination with haloperidol, on selected OxS parameters in human plasma in vitro.
Methods: The OxS parameters evaluated were thiobarbituric acid reactive substances (TBARS) and total antioxidant capacity (TAC). Plasma samples from healthy volunteers were incubated with drug concentrations used in psychiatry.
Results: Incubation of plasma with lithium or haloperidol alone did not produce statistically significant changes of TBARS levels in comparison with control samples. However, significantly higher TBARS levels were observed in samples incubated with haloperidol plus lithium compared to control, haloperidol, or lithium samples. The TAC value did not differ between samples.
Conclusions: Lithium does not influence OxS parameters in human plasma in vitro during short-term observation when applied at concentrations used in psychiatry. However, lithium increased the TBARS level in the samples when given in combination with haloperidol, which may be one of the mechanisms behind the neurotoxicity associated with combined lithium and haloperidol administration.
Introduction
Lithium was introduced to modern psychiatry as long ago as the 1940s. Nowadays, it remains a key element in the treatment of bipolar disorder (BD): a first-line treatment for acute mania and maintenance therapy and a second-line option for acute bipolar depression.Citation1 The fact that long-term lithium treatment prevents suicide lends it additional advantages over other mood stabilizers.Citation2 Previous studies have proposed many sites of action of lithium in the central nervous system (CNS). In the last two decades, a growing body of evidence has shown that lithium has several neuroprotective effects.Citation3
Oxidative stress (OxS) is a state of imbalance between oxidative processes and antioxidant defence.Citation4 Increased OxS induces lipid peroxidation (LP) in membranes, proteins, and DNA.Citation5 The antioxidant system involves the coordinated effects of antioxidant enzymes, macromolecules and small molecules, with the cumulative effect being referred to as total antioxidant capacity (TAC).Citation6 OxS may well play an important role in the pathophysiology, severity of symptoms and disease course of BD.Citation7
In the treatment of acute mania, lithium is commonly combined with haloperidol or another first-generation antipsychotic. Importantly, chronic treatment with typical antipsychotics, especially haloperidol, appears to increase the OxS parameters detected in plasma.Citation8 However, a systematic review by Lepping et al. reveals no clear conclusion in this regard.Citation9 Mood stabilizers, especially lithium, have been demonstrated to influence OxS in cell cultures and animal models but the results differ profoundly.Citation10–Citation12 Single human studies indicate that lithium possesses antioxidant properties and influences OxS parameters in blood plasma.Citation13–Citation15 However, these effects demand further investigation, particularly with regard to identifying the specific targets that mediate these unique properties.
The present study investigates the effects of lithium administered alone and in combination with haloperidol on selected OxS parameters in human plasma in vitro. Two different doses of lithium were administered, which resulted in appropriate concentrations within the therapeutic range of the drug as administered to patients: 0.7 and 1.0 mmol/l. The aim of this study was to confirm whether even short-term treatment with lithium can decrease the level of OxS markers in human plasma in vitro, when given either alone or in combination with haloperidol, as a means of preventing the oxidation caused by treatment with haloperidol alone.
Materials and methods
Subjects
Blood samples were taken from 58 healthy subjects ranging in age from 20 to 40 years. The inclusion criteria were a lack of psychiatric or somatic disorders, no history of head injuries, no allergies, no lipid or carbohydrate metabolism disorders, a normal body mass index and abdominal circumference, no pharmacological treatment, no use of any addictive substances or antioxidant supplementation, consumption of a balanced diet, and similar socio-economic conditions to the rest of the group.
All subjects signed an informed consent form for participation in the study, in accordance with the protocol accepted by the Medical University of Lodz Committee for Research on Human Subjects (number RNN/13/08/KE). Mental status was assessed with the M.I.N.I. (Mini International Neuropsychiatric Interview), Polish version 5.0.0. Fasting blood (20 ml) was collected into EDTA tubes between 7:30 and 8:30 a.m. Blood samples were centrifuged twice for 15 min at 4 °C at 1500 g to obtain platelet-poor plasma.
Incubation with drugs
All the reagents and chemicals used in these experiments were of the highest analytical grade of purity. Lithium carbonicum and haloperidol (4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-1-(4-fluorophenyl)butan-1-one) were obtained from Sigma–Aldrich in the form of active substances. Lithium salt was dissolved in distilled water and haloperidol in dimethylsulfoxide (DMSO) to form stock solutions (100 mmol/l for lithium carbonicum and 1 mg/ml for haloperidol). Drug solutions were added to samples containing 0.5 ml of plasma to obtain final concentrations corresponding with those used in psychiatry: 0.67 and 1.00 mmol/l for lithium (prophylactic and therapeutic concentrations used to manage BD) and 10 ng/ml for haloperidol.Citation16 For each experiment, a control sample without the drug was prepared with plasma and DMSO. The final concentration of DMSO in all samples was 0.01%. The plasma samples from each of the volunteers were labeled as follows: control, lithium prophylactic (Li0.67), lithium therapeutic (Li1.00), haloperidol (hpl), lithium prophylactic + haloperidol (Li0.67 + hpl), and lithium therapeutic + haloperidol (Li1.00 + hpl). The plasma samples were incubated for 24 hours at 37°C. The OxS parameters evaluated were LP and TAC content.
Selected oxidative stress parameters
Thiobarbituric acid reactive substances (TBARS) level is a direct index of cell LP. The concentration of TBARS was measured according to the Rice-Evans method.Citation4 To assess TAC, an improved ABTS radical cation decolorization assay was used.Citation5 All the experiments were performed in triplicate.
Statistical analysis
All calculations were performed using Statistica v.10.0 software (Statsoft, Inc.). As the normal values of TBARS and TAC seen in humans vary considerably, all measurements were converted to percentages of controls. Mean values, standard deviation (SD) and standard error of the mean were calculated. The data was analyzed with the one-way analysis of variance. Statistical significance was set at 5% (alpha = 0.05). Post hoc comparisons were carried out with the Tukey test. The results are shown in bar graphs.
Results
The levels of TBARS in plasma incubated with prophylactic and therapeutic concentrations of lithium were very similar to those observed in controls. Similarly, no differences were observed between the samples from subjects administered 0.67 and 1.00 mmol/l lithium (Fig. ). Nonetheless, the post hoc analyses showed that the TBARS levels in the Li0.67 + hpl samples were significantly higher than those seen in controls (P < 0.05). TBARS levels in the Li0.67 + hpl sample were also significantly higher than in the Li1.00 (P < 0.01) or hpl (P < 0.05) samples, and significantly higher in the Li1.00 + hpl samples than the hpl samples (P < 0.01) (Fig. ).
Figure 1 The comparison of influence of lithium (prophylactic and therapeutic concentration), haloperidol and their combination on lipid peroxidation, expressed as TBARs levels, in human plasma (in vitro). The results are expressed in percentages of control and shown as mean ± SD.
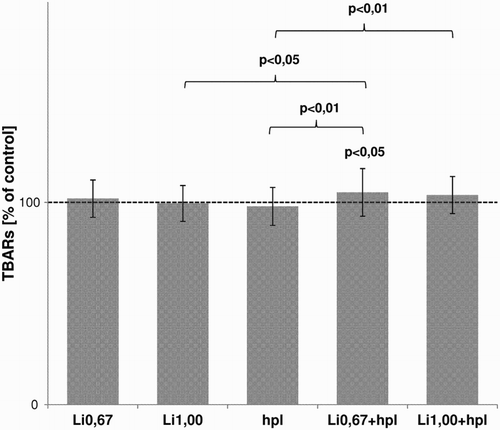
The level of TAC did not differ between samples.
Discussion
The present findings showed that lithium did not affect the level of LP markers (TBARS) or TAC in human plasma in vitro compared with controls and haloperidol samples.
Similarly, Abdalla et al. did not report any influence of short-term (7 days) or long-term (1 month) treatment with lithium on the concentration of LP or antioxidative defense parameters in the erythrocytes and brain tissue of examined healthy rats.Citation11 Similarly, chronic therapeutic concentrations of lithium have also not been shown to have any impact on TBARS levels in the liver and kidneys of mice.Citation17 The fact that the present study and the two discussed above all use tissue from healthy subjects may account for the similar results; lithium was also shown not to prevent OxS in ALS (amyotrophic lateral sclerosis) mice, despite blocking apoptosis.Citation18
Contrary to the results of the first part of our study, many previous studies have already demonstrated that lithium treatment may attenuate OxS. Chronic treatment with therapeutic doses of lithium significantly inhibited the increase of peroxidation markers induced by glutamate, H2O2 and high lithium concentrations, and were associated with reduced cytotoxicity in primary cultured neuronal cells.Citation12,Citation19,Citation20 The main difference between the cited studies and the present one seems to be the pretreatment OxS level. Similarly, lithium was seen to exert a protective influence against increased oxidative status in the brain tissue of the tested animals, but not the blood, liver, or kidneys.Citation21–Citation23 It may be assumed that the protective effects of lithium influence neuronal cells rather than other tissues. Interestingly, chronic lithium treatment reversed some of the OxS parameter levels to normal values in animal models of depression,Citation24 mania,Citation25,Citation26 or sepsisCitation27 which again, might indicate that lithium acts under pathological rather than neutral conditions. On the other hand, Vasconcellos et al. note that lithium treatment in rats induced an antioxidant effect in the hippocampus, but it was not able to prevent a stress-induced increase in damage to macromolecules such as LP.Citation28
The lack of agreement between the results of the first part of our study and those of studies demonstrating the antioxidative properties of lithium may be explained by short time of incubation, low level of primary OxS (using blood only from healthy volunteers and not bipolar patients), and type of peripheral tissue examined (blood). Moreover, some of the peripheral antioxidant effects may be secondary to CNS effects and thus might be seen only in in vivo experiments.
Moreover, in contrast to the present results, some studies associate lithium with pro-oxidative activity. Long-term treatment with therapeutic levels of lithium caused a significant increase in the levels of LP and an imbalance of SOD, reduced glutathione, catalese (CAT) and glutathione-S-transferase in rats. Changes in OxS parameters correlated with functional damage, and were seen in the liver, kidneys, lung tissue, and erythrocytes.Citation11,Citation29–Citation31
In addition, individual in vivo studies also contradict the present findings with regard to the influence of lithium on OxS parameters in humans. It was demonstrated that acute treatment with lithium significantly reduced the increased levels of TBARS seen in unmedicated manic patients, normalized the antioxidant enzyme activity ratio (SOD/CAT) and increased TAC levels.Citation13,Citation14,Citation32 Lithium was also shown to have antioxidative activity in non-pathological conditions (in the plasma of healthy volunteers).Citation15
To summarize, a number of different factors may explain the heterogeneity of the results observed in the literature regarding the influence of lithium on OxS parameters. The key factors may include lithium concentration, duration of treatment, tissue type (CNS vs. peripheral tissue) and the primary state of the oxidative balance. In addition, single antioxidant enzyme measurements and their ratios may seem to be more relevant than TAC level in assessing the prevention of OxS by neuropsychiatric medications. However, the present study had been designed before this knowledge emerged.
Our hypothesis that lithium carries antioxidant properties demands supplementary information. It may be concluded that chronic treatment with therapeutically effective lithium concentrations can decrease the vulnerability of neuronal cells to induced OxS.
The results of the second part of the study show that OxS parameters in the lithium + haloperidol samples were significantly higher than those in other samples. Despite the fact that both medications were used in therapeutic concentrations and none of them alone caused increased LP, the combination should be used with particular caution. These findings suggest that, contrary to our primary hypothesis, lithium may not prevent but rather potentiate haloperidol-induced oxidation.
Lithium has been administered concomitantly with neuroleptic drugs, particularly haloperidol, for the treatment of some mental disorders for hundreds of years. Despite the very high therapeutic potential of this combination, especially in manic episodes, physicians and patients have noted its severe adverse effects. Neurotoxicity associated with the combination of lithium and high-potency neuroleptics was first reported by Cohen and Cohen in 1974.Citation33 Subsequently, multiple authors have tried to clarify the incidence, risk factors and symptomatology of this adverse effect. The resultant neurotoxicity is characterized by variable combinations of delirium, extrapyramidal symptoms, and cerebellar symptoms.Citation34 In 1996, a review of spectrum cases of lithium + neuroleptic neurotoxicity recorded in the Spontaneous Reporting System Database of the U.S. FDA, together with other extant literature, indicted that the plasma level of lithium and combination therapy with haloperidol were significant factors in the development of sequelae.Citation35
The precise mechanism by which the lithium + haloperidol combination induces toxicity is, however, poorly understood, and various mechanisms have been proposed to account for this. Nemes et al. report that patients treated with a lithium + haloperidol combination had significantly higher brain and plasma levels of the neuroleptic than a group treated with haloperidol alone.Citation36 Another study showed that the haloperidol + lithium combination was associated with significantly more frequent photic and other EEG changes than the combination of lithium with other neuroleptics.Citation37 As long ago as the 1970s and 1980s, it was proposed that OxS was responsible for combined haloperidol + lithium toxicity. In 1979, Guynn and Faillace studied the brain metabolism of rats treated with lithium, haloperidol or the combination of both medications. The only significant differences found concerned brain redox parameters: a reduction in both the cytoplasmic free [NAD+]/[NADH][H+] and [NADP+]/[NADPH] ratio in the lithium + haloperidol group compared with the control group.Citation38 A few years later, Sawas and Gilbert demonstrated that the neurotoxic and nephrotoxic effects of lithium carbonate and haloperidol they observed in rats might be a consequence of increased LP in brain and kidney tissues.Citation39 This observation is in accordance with the results of our study.
A review of extant literature indicates that this is the first study since 1985, and only the third one to examine possible mechanism of lithium + haloperidol toxicity in terms of OxS. Consequently, the increased LP level in lithium + haloperidol plasma samples seems to be the most interesting and valuable result of our study, and may suggest that the extracellular matrix plays an independent role in mediating neuronal toxicity of the medications.
Limitations
The results of the present study have some limitations. A significant weakness of our experimental design is the difficulty in extrapolation of these findings to in vivo conditions. An important limitation of the study is using only blood from healthy volunteers and not from patients with BP (depressive, manic, or euthymic states). Another apparent limitation of the present study is the short incubation time with the active substances and the low level of primary OxS. In spite of this, the results may be important for future in vivo studies.
Conclusions
In conclusion, the primary finding of our study was the increased LP level in human samples treated with a lithium + haloperidol combination, likely to be one of the causes of the toxicity of the drug combination. Lithium was not found to have any influence on OxS parameters in human plasma in vitro when administered alone.
Disclaimer statements
Contributors None.
Funding Supported by grant 502-11-756 from the Medical University of Lodz, Poland, and by grant N402 456138 from the Polish Ministry of Science and Higher Education.
Conflict of interest There is no conflict of interest.
Ethics approval All subjects examined in the study signed an informed consent form for participation in the study in accordance with the protocol accepted by the Committee for Research on Human Subjects of the Medical University of Lodz (number RNN/13/08/KE).
Acknowledgments
The authors are thankful to the Head of Department of Pharmaceutical Biochemistry, Medical University of Lodz, Prof. Marek Mirowski for providing the facilities required to carry out the research work.
References
- Connolly KR, Thase ME. The clinical management of bipolar disorder: a review of evidence-based guidelines. Prim Care Companion CNS Disord 2011;13(4): PCC.10r01097.
- Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ 2013;346:f3646. doi: 10.1136/bmj.f3646.
- Machado-Vieira R, Manji HK, Zarate Jr CA. The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord 2009;11(Suppl. 2):92–109. doi: 10.1111/j.1399-5618.2009.00714.x.
- Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol 1997;82:291–5. doi: 10.1113/expphysiol.1997.sp004024
- Rice-Evans CA, Diplock AT, Symons MCR. Techniques in free radical research. Amsterdam, London, New York, Tokyo: Elsevier; 1991.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free Radic Biol Med 1999;26(9–10):1231–7. doi: 10.1016/S0891-5849(98)00315-3
- Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev 2011;35:804–17. doi: 10.1016/j.neubiorev.2010.10.001
- Singh OP, Chakraborty I, Dasgupta A, Datta S. A comparative study of oxidative stress and interrelationship of important antioxidants in haloperidol and olanzapine treated patients suffering from schizophrenia. Indian J Psychiatry 2008;50:171–6. doi: 10.4103/0019-5545.43627
- Lepping P, Delieu J, Mellor R, Williams JHH, Hudson PR, Hunter-Lavin CJ. Antipsychotic medication and oxidative cell stress: a systematic review. J Clin Psychiatry 2011;72(3):273–85. doi: 10.4088/JCP.09r05268yel
- Abdalla DSP, Bechara EJH. The effect of chlorpromazine and Li2CO3 on the superoxide dismutase and glutathione peroxidase activities of rat brain, liver and erythrocytes. Biochem Mol Biol Int 1994;34:1085–90.
- Malhotra A, Dhawan DK. Zinc improves antioxidative enzymes in red blood cells and hematology in lithium-treated rats. Nutr Res 2008;28(1):43–50. doi: 10.1016/j.nutres.2007.11.002
- Nciri R, Desmoulin F, Allagui MS, Murat JC, Feki AE, Vincent C, et al. Neuroprotective effects of chronic exposure of SH-SY5Y to low lithium concentration involve glycolysis stimulation, extracellular pyruvate accumulation and resistance to oxidative stress. Int J Neuropsychopharmacol 2013;16(2):365–76. doi: 10.1017/S1461145712000132
- Machado-Vieira R, Andreazza AC, Viale CI, Zanatto V, Cereser Jr V, da Silva Vargas R, et al. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: a possible role for lithium antioxidant effects. Neurosci Lett 2007;421:33–6. doi: 10.1016/j.neulet.2007.05.016
- Aliyazicioglu R, Kural B, Colak M, Karahan SC, Ayvaz S, Deger O. Treatment with lithium, alone or in combination with olanzapine, relieves oxidative stress but increases aterogenic lipids in bipolar disorder. Tohoku J Exp Med 2007;213:79–87. doi: 10.1620/tjem.213.79
- Khairova R, Pawar R, Salvadore G, Juruena MF, de Sousa RT, Soeiro-de-Souza MG, et al. Effects of lithium on oxidative stress parameters in healthy subjects. Mol Med Rep 2012;5(3):680–2. doi:10.3892/mmr.2011.732.
- Ulrich S, Neuhof S, Braun V, Meyer FP. Therapeutic window of serum haloperidol concentration in acute schizophrenia and schizoaffective disorder. Pharmacopsychiatry 1998;31(5):163–9. doi: 10.1055/s-2007-979322
- Nciri R, Allagui MS, Vincent C, Murat JC, Croute F, El Feki A. Chronic lithium administration triggers an over-expression of GRP94 stress protein isoforms in mouse liver. Food Chem Toxicol 2010;48:1638–43. doi: 10.1016/j.fct.2010.03.038
- Shin JH, Cho SI, Lim HR, Lee JK, Lee YA, Noh JS, et al. Concurrent administration of Neu2000 and lithium produces marked improvement of motor neuron survival, motor function, and mortality in a mouse model of ALS. Mol Pharmacol 2007;71:965–75. doi: 10.1124/mol.106.030676
- Shao L, Young LT, Wang JF. Chronic treatment with mood stabilizers lithium and valproate prevents excitotoxicity by inhibiting oxidative stress in rat cerebral cortical cells. Biol Psychiatry 2005;58:879–84. doi: 10.1016/j.biopsych.2005.04.052
- LaiJ S, Zhao Z, Warsh JJ, Li PP. Cytoprotection by lithium and valproate varies between cell types and cellular stresses. Eur J Pharmacol 2006;539:18–26. doi: 10.1016/j.ejphar.2006.03.076
- Kiełczykowska M, Pasternak K, Musik I, Wrońska-Tyra J, Hordyjewska A. The influence of different doses of lithium administered in drinking water on lipid peroxidation and the activity of antioxidant enzymes in rats. Polish J Environ Stud 2006;15:747–51.
- Bhalla P, Dhawan DK. Protective role of lithium in ameliorating the aluminium-induced oxidative stress and histological changes in rat brain. Cell Mol Neurobiol 2009;29(4):513–21. doi: 10.1007/s10571-008-9343-5
- Castro AA, Casagrande TS, Moretti M, Constantino L, Petronilho F, Guerra GCB, et al. Lithium attenuates behavioral and biochemical effects of neuropeptide S in mice. Peptides 2009;30:1914–20. doi: 10.1016/j.peptides.2009.07.004
- Song C, Killeen AA, Leonard BE. Catalase, superoxide dismutase and glutathione peroxidase activity in neutrophils of sham-operated and olfactory-bulbectomised rats following chronic treatment with desipramine and lithium chloride. Neuropsychobiology 1994;30:24. doi: 10.1159/000119131
- Frey BN, Valvassori SS, Réus GZ, Martins MR, Petronilho FC, Bardini K, et al. Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J Psychiatry Neurosci 2006;31:326–32.
- JornadaL K, Valvassori SS, Steckert AV, Moretti M, Mina F, Ferreira CL, et al. Lithium and valproate modulate antioxidant enzymes and prezentouabain-induced oxidative damage in an animal model of mania. J Psychiatr Res 2011;45:162–8. doi: 10.1016/j.jpsychires.2010.05.011
- Albayrak A, Halici Z, Polat B, Karakus E, Cadirci E, Bayir Y, et al. Protective effects of lithium: a new look at an old drug with potential antioxidative and anti-inflammatory effects in an animal model of sepsis. Int Immunopharmacol 2013;16:35–40. doi: 10.1016/j.intimp.2013.03.018
- Vasconcellos APS, Nieto FB, Crema LM, Diehl LA, Almeida LM, Prediger ME, et al. Chronic lithium treatment has antioxidant properties but does not prevent oxidative damage induced by chronic variate stress. Neurochem Res 2006;31:1141–51. doi: 10.1007/s11064-006-9139-2
- Chadha VD, Bhalla P, Dhawan DK. Zinc modulates lithium-induced hepatotoxicity in rats. Liver Int 2008;28(4):558–65. doi: 10.1111/j.1478-3231.2008.01674.x
- Oktem F, Ozguner F, Sulak O, Olgar S, Akturk O, Yilmaz HR. Lithium-induced renal toxicity in rats: protection by a novel antioxidant caffeic acid phenethyl ester. Mol Cell Biochem 2005;277:109–115. doi: 10.1007/s11010-005-5426-5
- Sahin O, Sulak O, Yavuz Y, Uz E, Eren I, Yilmaz HR, et al. Lithium-induced lung toxicity in rats: the effect of caffeic acid phenethyl ester (CAPE). Pathology 2006;38(1):58–62. doi: 10.1080/00313020500464904
- Banerjee U, Dasgupta A, Rout JK, Singh OP. Effects of lithium therapy on Na(+)-K(+)-ATPase activity and lipid peroxidation in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 2012;37(1):56–61. doi: 10.1016/j.pnpbp.2011.12.006.
- Cohen WJ, Cohen NH. Lithium carbonate, haloperidol, and irreversible brain damage. JAMA 1974;230(9):1283–7. doi: 10.1001/jama.1974.03240090023018
- Colvard MD, Gentry JD, Mullis DM. Neurotoxicity with combined use of lithium and haloperidol decanoate. Prim Care Companion CNS Disord 2013;15(6). pii:PCC.13l01563. doi:10.4088/PCC.13l01563.
- Goldman SA. Lithium and neuroleptics in combination: is there enhancement of neurotoxicity leading to permanent sequelae? J Clin Pharmacol 1996;36(10):951–62. doi: 10.1002/j.1552-4604.1996.tb04763.x
- Nemes ZC, Volavka J, Lajtha A, Cooper TB, Sershen H. Concurrent lithium administration results in higher haloperidol levels in brain and plasma of guinea pigs. Psychiatry Res 1987;20(4):313–6. doi: 10.1016/0165-1781(87)90092-8
- Saran A, Addy O, Foliart RH, Schubert DS, Halaris A. Electroencephalographic changes and other indices of neurotoxicity with haloperidol-lithium therapy. Neuropsychobiology 1989;20(3):152–7. doi: 10.1159/000118490
- Guynn RW, Faillace LA. The effect of the combination of lithium and haloperidol on brain intermediary metabolism in vivo. Psychopharmacology 1979;61:155–9. doi: 10.1007/BF00426730
- Sawas AH, Gilbert JC. Lipid peroxidation as a possible mechanism for the neurotoxic and nephrotoxic effects of a combination of lithium carbonate and haloperidol. Arch Int Pharmacodyn Ther 1985;276(2):301–12.
