Abstract
Objectives: To compare, from a biological and clinical perspective, a significant group of patients with AML with inv(3)(q21q26.2) or t(3;3)(q21;q26.2) with another group of AML carrying different abnormalities of 3q at q21 or q26, the latter named as the AML abn(3q) group. Methods: We developed a national survey with the participation of 13 Spanish hospitals, and retrospectively reviewed (from 1990 to 2010) these subtypes of AML. Fifty-five patients were collected: 35 with AML inv(3)/t(3;3) and 20 with AML abn(3q). A data collecting page that included main features at diagnosis, therapeutic approach and response, and survival variables, was distributed and completed. Results: We did not find significant differences in sex, age, history of myelodysplastic syndrome or chemo-/radiotherapy, clinical presentation, WBC and platelet counts, hemoglobin level, blasts immunophenotype, serum lactatedehydrogenase, peripheral blood and bone marrow cellular dysplasia, and bone marrow biopsy findings. Although the association with monosomy 7 was significantly more frequent in AML inv(3)/t(3;3), this did not seem to influence outcome. The lack of response to the different modalities of treatment and the aggressive course of the disease were the standard in both cohorts of patients. Discussion: Although not yet recognized by the World Health Organization classification, our results are in agreement with the findings of other authors, who include both subsets of AML together in the same group of adverse prognosis. Conclusion: In an attempt to simplify and bound entities with similar genetic background and clinical behavior, it would be desirable to bring together both subgroups of AML in a single section.
Introduction
Since the early 1990s, the so-called 3q21q26 syndrome was defined as a myelodysplastic syndrome (MDS), an acute myeloid leukemia (AML) or the accelerated phase or blast crisis of a chronic myeloproliferative disease, associated with chromosomal defects of 3q in bands q21 and q26.Citation1–Citation3 In 2008, the World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues recognizes AML with inv(3)(q21q26.2) or t(3;3)(q21;q26.2) as a separate entity, characterized by a specially aggressive clinical behavior.Citation4 These abnormalities occurring at the long arm of chromosome 3 involve the oncogene EVI1 at 3q26.2 (or its longer form MDS1-EVI1), and RPN1 at 3q21, leading to the RPN1–EVI1 fusion transcript.Citation5–Citation7 RPN1 may act as an enhancer of EVI1 expression resulting in increased cell proliferation and impaired cell differentiation and inducing haematopoietic cell transformation.Citation8
Typically, the 3q21q26 syndrome is characterized by abnormalities of megakaryocytopoiesis and lack of response to conventional chemotherapy, but these features are not unique to inv(3)/t(3;3), and they can be observed in other AML with interchromosomal rearrangements between 3q21 or 3q26 and other chromosomes. In fact, controversy exists concerning if all AML with any 3q abnormality (at least those in which 3q21 or 3q26 are committed) could be reunited in the same group. Our purpose was to compare, from a biological and clinical perspective, a group of patients with AML inv(3)(q21q26.2) or t(3;3)(q21;q26.2) with another group carrying different abnormalities of chromosome 3 at q21 or q26.
Patients and methods
Sample collection
We retrospectively studied the register of patients with the diagnosis of AML with inv(3)(q21q26.2) or t(3;3)(q21;q26.2) from 1990 to 2010, obtained from a national survey with the participation of 13 Spanish hospitals. Additionally, those cases of AML with other cytogenetic aberrations involving 3q21 or 3q26, different from inv(3) and t(3;3), were also registered and named as the AML abn(3q) group. Patients carrying t(9;22) BCR-ABL1 were excluded. Given that AML with inv(3)/t(3;3) is not considered as a subgroup of AML irrespective of blast percentage [as it is, for example, those with t(15;17)(q22;q12)], the patients with a diagnosis of MDS associated with inv(3) or t(3;3), were also excluded.
A data collecting page, previously designed, was distributed to the participating hospitals. Analyzed data at diagnosis included age, sex, motive for initial consultation, previous history of MDS or administration of chemotherapy/radiotherapy, presence or absence of hepatosplenomegaly or lymphadenopathy, hemogram (with special attention to white blood cell count, WBC; hemoglobin level; platelet count; and mean corpuscular volume, MCV), serum lactatedehydrogenase (LDH), bone marrow aspiration findings, morphologic changes in the peripheral blood and bone marrow, subtype of AML according to ancient FAB classification, phenotype of blastic population (flow cytometry), karyotypic study, overexpression of EVI1, and bone marrow biopsy features. Data concerning the therapeutic approach (achieving or not complete remission, number and type of chemotherapy schemes, and performing or not of hematopoietic stem cell transplantation, HSCT) and those related to survival [mortality rate, disease-free survival (DFS), and overall survival (OS)] were also recorded.
Morphology features of blood and bone marrow specimens, and blast cell immunophenotype
A review of morphological findings in the peripheral blood and bone marrow (both aspirate and biopsy) smears at diagnosis was performed and registered. In the peripheral blood, we studied the presence of dyshemopoietic features in each lineage and the percentage of blast cells. In bone marrow aspirate specimens, we evaluated the following morphological parameters: marrow cellularity, presence and type of erythroid, myeloid, and megakaryocytic dysplasia, and percentage of blast cells. Bone marrow biopsy examination included cellularity, quantity and morphology of megakaryocytes, and reticulin fibrosis degree.
The phenotypic analysis was performed on the peripheral blood or bone marrow aspirate specimens, using different flow cytometers with 2-, 3- or 4-color flow cytometric analysis, and according to local panels of monoclonal antibodies. Procedures concerning incubation of the cells with monoclonal antibodies, lysing of the erythrocytes, washing steps, and cytometric analysis were standard. Cases were considered positive if 20% or more of the cells expressed the specific antigen in the adequate gate.
Complete hematological remission was defined as less than 5% bone marrow blasts in cytomorphology plus normal hematopoiesis of all cell lines and a restitution of a normal peripheral blood with at least 1500/ml neutrophils and at least 100 000/ml platelets.
Statistical analysis
All statistical analyses were performed using the SPSS 17.0 software package (SPSS, Chicago, IL, USA). Survival times were calculated from the day of diagnosis until death. The median OS was determined by Kaplan–Meyer estimates and compared by the logrank test. Groups were compared with the Pearson Chi-Square, Mann–Whitney U test, or Fisher's exact tests, as appropriate. All P-values are two-sided and only P-values of <0.05 were considered statistically significant.
Results
We collected 55 patients: 35 with AML inv(3)/t(3;3) and 20 with AML associated with other 3q aberrations. Considering the genetic findings, the distribution of cases that make up the group of AML abn(3q) is shown in . The age (mean ± standard deviation) was 50 ± 20 years (range 14–84) in the first group and 53 ± 19 years (range 2–79) in the second one. There was a slight predominance of males (54%) in patients with AML inv(3)/t(3;3) and women (55%) in AML abn(3q).
Table 1. Specific cytogenetic abnormalities in patients who constitute the group of AML abn(3q)
The main characteristics at the diagnosis of the two groups are shown in . When comparing both groups, we did not find differences in sex, age, history of previous MDS or chemo-/radiotherapy, clinical presentation, WBC and platelet counts, hemoglobin level, serum LDH, degree of cellular dysplasia (significant in both groups in peripheral blood and aspirate smears), and bone marrow biopsy findings. History of previous chemotherapy or radiotherapy treatments was 23.5% in AML with inv(3)/t(3;3) and 30% in AML abn(3q). The presence of hepatomegaly, splenomegaly or lymphadenopathies at the diagnosis is not frequent in any group, and all morphological FAB diagnoses from M0 to M7 (except M3) are recorded, without a predominant subtype over the rest. Patients presented with anemia [94% in AML inv(3)/t(3;3) and 100% in AML abn(3q)], leukopenia (26% and 30%, respectively) or leukocytosis (46 and 30%), and either thrombocytosis (9% and 10%), a normal platelet count (26 and 5%) or, more frequently, thrombocytopenia (65 and 85%). An increased serum LDH and dysplastic features in the three hematopoietic series were fairly frequent in both groups. Pseudo-Pelger–Hüet neutrophils and giant hypogranular platelets in the peripheral blood, and particularly atypical small hypolobed megakaryocytes in the bone marrow, were the more prominent dysplastic findings in the two series ( and ).
Figure 1. AML with t(3;3)(q21;q26.2). Presence of blasts and dysplastic megakaryocytes in aspirate smear (Wright, x400).
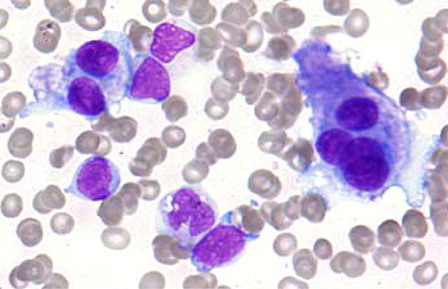
Figure 2. AML with inv(3)(q21q26.2). Bone marrow biopsy showing a marked increase of atypical small mono- and bilobated megakaryocytes (PAS, x400).
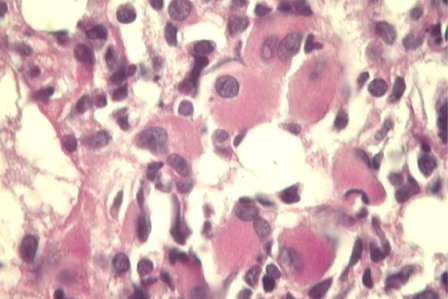
Table 2. Main characteristics at diagnosis of the patients in the group of AML with inv(3)(q21q26) or t(3;3)(q21;q26) vs. those with AML carrying other 3q21 or 3q26 abnormality
The immunophenotype of blasts () was similar in the two cohorts: an immature myeloid pattern, CD34 + CD33 + CD13 + CD117 + HLA-DR + , also most commonly CD38 positive, and with an aberrant CD7 expression, was the more frequent observed phenotypic repertoire. When compared both groups of patients, only the frequency of CD34 positive cases was significantly higher in the inv(3)/t(3;3) group (96 vs. 73%, P = 0.04).
Table 3. Immunophenotypic features of blasts in AML with inv(3)(q21q26.2) or t(3;3)(q21;q26.2), and those from patients with AML carrying other cytogenetic aberrations involving 3q21 or 3q26
Of note, the association with monosomy 7 was significantly more prevalent in the inv(3)/t(3;3) group (43 vs. 10%, P = 0.011), while the presence of del(5q) or a complex karyotype was found in similar proportions (9 vs. 10%, and 26 vs. 30%, respectively). Owing to the retrospective nature of our study, in which many of the samples were not available for analysis, oncogene EVI1 was investigated by RT–PCR only in eight patients with AML inv(3)/t(3;3) and one with AML abn(3q): in the first group, EVI1 was overexpressed in five cases (62.5%) and also in the only patient analyzed in the second group.
When intensive chemotherapy was used (80 and 78% of the cases, respectively), complete remission after first cycle of induction was achieved only in 6.2% of the patients with inv(3)/t(3;3) and 21.4% in the other group. In those cases failing to reach complete remission, a second cycle of induction was successful in 28.6% in the first group and 25% in the second one. Allogeneic HSCT was performed in 29% of patients with inv(3)/t(3;3) compared to none in the group of patients with other 3q aberration (P = 0.05). Mortality rate and median OS and DFS were similar in both groups, extremely unfavorable. In the group of AML inv(3)/t(3;3) the DFS was 2.7 ± 1.6 months for 2.2 ± 1.1 months in the AML abn(3q) group (P = 0.872), while OS was 8.8 ± 1.8 months in the first group and 5.9 ± 1.5 months in the second one (P = 0.083). Closing the data collection of this study, only two patients were alive in the group of AML inv(3)/t(3;3) and one in AML abn(3q).
Discussion
Various categories of 3q abnormalities in AML can be distinguished according to their genetic features: inv(3)(q21q26.2)/t(3;3)(q21;q26.2), balanced t(3q26), balanced t(3q21), and other 3q abnormalities. But the revised 2008 WHO classification of hematopoietic tumors only recognizes AML with inv(3)(q21q26.2) or t(3;3)(q21;q26.2) as an independent clinicopathological entity, with an extremely poor prognosis. Less is known about the risk assignment and clinical and genetic characterization of AML with chromosome 3q abnormalities other than inv(3)/t(3;3). In this multicenter retrospective study, we wanted to compare a significant group of patients with AML inv(3)(q21q26.2)/t(3;3)(q21;q26.2) and other with the diagnosis of AML associated with abnormalities in 3q21 or 3q26. From a methodological point of view, although a recent study shows that MDS with inv(3)(q21q26.2)/t(3;3)(q21;q26.2) may be considered as an AML with recurrent genetic abnormalities, irrespective of blast percentage,Citation8 and it is generally accepted that MDS or CMML with inv(3)/t(3;3) is aggressive diseases with a high risk of progression to AML,Citation9 we did not include MDS patients in the study.
AML with inv(3)/t(3;3) is around 0.8–2.5% of all AMLCitation8,Citation10–Citation14 and its incidence according to the sex is not clear. At the time of the diagnosis the median age is in general younger than AML and MDS without inv(3)/t(3;3), as previously reported.Citation8 This seems to be also valid for patients with MDS/AML and other abnormality at 3q. In the review reported by Jotterand et al.,Citation3 the median age of 13 of 16 patients (81.2%) with MDS and 3q defect was younger than 55 years, just opposite to those without 3q defect (88.5% over 55 years). In our series, 69% of cases of AML inv(3)/t(3;3) were under 60 years at diagnosis, and 60% of those carrying other abn(3q). AML with inv(3)/t(3;3) has been also described in children,Citation15 and our study included a 2-year-old boy with AML associated with 48,XX,t(1;3)(q11;q26), + 8, + 21c[40].
Anemia was a constant in the two cohorts of patients that we studied, and a normal platelet count or thrombocytosis (reported as relatively frequent in AML with 3q abnormalities) was found in 35% of patients in the inv(3);t(3;3) group and 15% of the abn(3q) group. Specifically, considering thrombocytosis when platelet count > 450 × 109/l, it was seen in 5.7% of cases in the cohort of inv(3)/t(3;3) (very similar to the 6.7% reported by Sun et al.Citation16), and 5% in the other cohort. We find thrombocytopenia at diagnosis in 65% of cases of AML inv(3)/t(3;3), when other authors describe only 7–22%.Citation8 It has been widely observed and reported that abnormality involving 3q21 and 3q26 bands is strongly associated with trilineage myelodysplasia and a prominent presence of small mono- or bilobed megakaryocytes and micromegakaryocytes in the bone marrow.Citation2–Citation4,Citation10 Although somewhat more pronounced in AML inv(3);t(3;3) than AML abn(3q), significant dysplastic changes were observed in the three hematopoietic series in the majority of our patients, both in peripheral blood and bone marrow smears. As already indicated, all morphological possibilities according to FAB classification were possible in both groups, except for M3.Citation2,Citation3,Citation13 Although usually not performed, the bone marrow biopsy in cases of AML with some 3q abnormality showed a variable degree of cellularity (not exceptionally hypocellular), blastosis and fibrosis, and the typical picture of increased small, hypolobated (monolobed or bilobed) megacaryocytes. Of note, the association of AML inv(3);t(3;3) and central diabetes insipidus, which has been previously reported,Citation17 was found in two patients.
In the inmunophenotypic studies, there is a high degree of similarity between the groups, and more than 80% of the blast cells express CD34, CD13, CD33, CD117, CD38 and HLA-DR, i.e. pan-myeloid and immature markers. In about 60% of cases, aberrant expression of CD7 is present, and MPO is positive in about one-third of cases. In the literature, there is no remarkable series analyzing the phenotype of blasts in AML abn(3q). For the group of AML inv(3)/t(3;3), our results are broadly consistent with those reported by Medeiros et al.,Citation11 but we find a higher frequency in the expression of CD38 (82 vs. 28%) and CD4 (39 vs. 19%), and a lower expression of CD56 (20 vs. 40%). Megakaryocytic markers are expressed, in both studies, in fewer than 20% of cases. Regarding the results for CD38, our findings are more in line with those published by the WHO classification in 2008.Citation4
Associated with inv(3)/t(3;3), secondary karyotypic abnormalities are common and may precede the development of the 3q26.2 abnormality. The most frequent anomaly reported in the literature is monosomy 7, occurring in approximately half of cases, followed by complex karyotypes and 5q deletions.Citation3,Citation4,Citation8,Citation11,Citation16 The frequency of monosomy 7 in our series was 42.9%, which is very similar to that reported by other authors. In contrast, the coexistence of this monosomy was significantly rarer in patients with AML abn(3q), only 10% (P = 0.011), and the most frequent additional cytogenetic anomaly in this second cohort was 5q deletion (30%).
AML with inv(3)(q21q26.2) or t(3;3)(q21;q26.2) is an aggressive disease associated with a poor response and high rates of resistance to conventional chemotherapy, short survival and poor prognosis in the studies reported. Weisser et al.Citation11 showed an OS of 336 days in a cohort of 35 patients, slightly higher than the 8.8 months in our series, but equally onerous. They found a slightly better percentage of complete remissions with intensive chemotherapy (46%) that we did (overall, 35%). In their study, a higher initial WBC and age above 60 years were associated with shorter OS. As in our cohort and other relevant studies,Citation14 survival length did not seem to be significantly different in patients with or without monosomy 7 as additional cytogenetic aberration, although this fact was not confirmed in other related works.Citation13 Rogers et al.Citation8 also found a short OS (7.9 months) in 63 cases of AML inv(3)/t(3;3).
In our study, regarding the HSCT as a potentially curative therapy, the fact that 29% of patients with inv(3)/t(3;3) were treated with this intensification approach against none in the group of other 3q abnormalities (P = 0.05), gives an idea of the different clinical perception of both types of leukemia. The follow-up of the patients demonstrated that the HSCT in AML with inv(3)/t(3;3) did not influence the outcome in this type of leukemia, as previously reported.Citation18 Recently, some authors have reported that patients with MDS/AML inv(3)/t(3;3) receiving allogeneic HSCT had a relatively better outcome compared to patients receiving chemotherapy alone or supportive therapy. However, chemotherapy with allogeneic HSCT in their cohort lost prognostic significance in multivariable analysis. Although OS by the Kaplan–Meier survival curve showed a relatively better OS (15 months) than chemotherapy alone (9 months) or supportive therapy (3.2 months), outcome was dismal and there was no significant improvement of OS in this cohort.Citation8 In contrast, other authors have found a benefit with allogeneic HSCT when compared with standard chemotherapy in this set of patients.Citation11,Citation16
In the literature, there are few studies that specifically examine the cases of AML with 3q21 or 3q26 abnormalities other than inv(3)/t(3;3),Citation19–Citation24 and even fewer studies comparing somehow the biological features and clinical behavior of both types of acute leukemia.Citation13,Citation14,Citation25 Most papers that collect AML abn(3q) do on single cases or very small series. Li et al.Citation25 compared 17 cases of MDS/AML with t(3;21)(q26.2;q22) and 17 cases with MDS associated with inv(3) (q21q26.2)/t(3;3)(q21;q26.2), because these entities share 3q26 locus abnormalities. Multilineage dysplasia and frequent association with –7/7q were similar in both groups, but MDS/AML cases associated with t(3;21) have a higher frequency of therapy-related disease (16/17 vs. 6/17) and even shorter survival times than MDS with inv(3)/t(3;3) (median, 4.7 vs. 14 months, P = 0.03). In our series, history of previous chemotherapy or radiotherapy treatments was similar: 23.5% in AML with inv(3)/t(3;3) and 30% in AML abn(3q). For AML inv(3)/t(3;3) this is in line with data from larger series of patients (17.4% in the work of Rogers et al.Citation8).
Lugthart et al.Citation13 found that median survival for patients with AML inv(3)/t(3;3) was 10.3 months, 13.7 for AML (t3q26) and 20.9 for AML (t3q21). This apparent advantage in terms of survival for the AML group in which 3q21 is involved was no confirmed in the work of Grimwade et al.Citation14 These latter authors, analyzing the prognostic significance of many recurring cytogenetic abnormalities in a large number of patients with AML (n = 5876) found that, in multivariable analyses, the presence of inv(3)(q21q26)/t(3;3)(q21;q26) or abn(3q) [excluding t(3;5)(q25;q34)] equally predicted a significantly poorer outcome. Estimated 10-year OS was only 3% for AML inv(3)/t(3;3) and 11% for AML abn(3q). Interestingly, AML with t(3;5)(q25;q34) is not involved 3q21 or 3q26.
In conclusion, in our experience clinical feautures of AML with inv(3)/t(3;3) and other AML abn(3q) are similar. Although the association with monosomy 7 was more frequent in the first group, this does not seem to influence outcome. Notably, the therapeutic approach differed between both groups: it seems that allogeneic HSCT is in mind of oncohematologists only in AML with inv(3)/t(3;3), while it is not considered in AML abn(3q). However, although the rarity of this type of leukemias does not allow to analyze large series of patients (specially those AML carrying 3q21 or 3q26 aberrations independently), there is growing evidence that support the possibility that they actually can be grouped into a single subtype of AML. Our results are in agreement with the findings of other authors, who include both subsets of AML together in the same group of adverse prognosis. In an attempt to simplify and bound entities with similar genetic background and clinical behavior, it would be desirable to bring together in one section those AML which carry alterations in 3q21 and/or 3q26. As a result, collecting these leukemias with similar features into a single subtype could help to unify therapeutic approaches aimed at providing better clinical outcomes.
Disclaimer statements
Contributors JMR, TMS, EL, FS, LF: Conceived and designed the study. JMR, TMS: Analysed and interpreted the data. Wrote the article in whole or in part. CS, MLPS, ES, JTN, FM, EA, AD, MR, MDB, AB, SGV, ET, TV, MO, AB, MMR, VP: Collected the data.
Funding None.
Conflicts of interest None.
Ethics approval None.
References
- Jenkins RB, Tefferi A, Solberg LA, Dewald GW. Acute leukemia with abnormal thrombopoiesis and inversions of chromosome 3. Cancer Genet Cytogenet. 1989;39:167–79.
- Jotterand M, Parlier V, Mühlematter D, Grob JP, Beris Ph. Three new cases of chromosome 3 rearrangement in bands q21 and q26 with abnormal thrombopoiesis bring further evidence to the existence of a 3q21q26 syndrome. Cancer Genet Cytogenet. 1992;59:138–60.
- Fonatsch C, Gudat H, Lengfelder E, Wandt H, Silling-Engelhardt G, Ludwig WD, et al. Correlation of cytogenetic findings with clinical features in 18 patients with inv(3)(q21q26) or t(3;3)(q21;q26). Leukemia 1994;8:1318–26.
- Swerdlow SH, Campo E, Lee Harris N, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC press; 2008.
- Levy ER, Parganas E, Morishita K, Fichelson S, James L, Oscier D, Gisselbrecht S, Ihle JN, Bucle VJ. DNA rearrangements proximal to the EVI 1 locus associated with the 3q21q26 syndrome. Blood 1994;83:1348–54.
- Martinelli G, Ottaviani E, Buonamici S, Isidori A, Borsaru G, Visani G, et al. Association of 3q21q26 syndrome with different RPN1/EVI1 fusion transcripts. Haematologica 2003;88:1221–8.
- Barjesteh van Waalwijk van Doorn-Khosrovani S, Erpelinck C, van Putten WL, Valk PJ, van der Poel-van de Luytgaarde S, Hack R, et al. High EVI1 expression predicts poor survival in acute myeloid leukemia: a study of 319 de novo AML patients. Blood 2003;101:837–45.
- Rogers HJ, Vardiman JW, Anastasi J, Raca G, Savage NM, Cherry AM, et al. Complex or monosomal karyotype and not blast percentage is associated with poor survival in acute myeloid leukemia and myelodysplastic syndrome patients with inv(3)(q21q26.2)/t(3;3)(q21;q26.2): a Bone Marrow Pathology Group study. Haematologica 2014;99:821–9.
- Cui W, Sun J, Cotta CV, Medeiros J, Lin P. Myelodysplastic syndrome with inv(3)(q21q26.2) or t(3;3)(q21;q26.2) has a high risk for progression to acute myeloid leukemia. Am J Clin Pathol. 2011;136:282–8.
- Testoni N, Borsaru G, Martinelli G, Carboni C, Ruggeri D, Ottaviani E, et al. 3q21 and 3q26 cytogenetic abnormalities in acute myeloblastic leukemia: biological and clinical features. Haematologica 1999;84:690–4.
- Weisser M, Haferlach C, Haferlach T, Schnittger S. Advances age and high initial WBC influence the outcome of inv(3)(q21q26)/t(3;3)(q21;q26) positive AML. Leuk Lymphoma 2007;48:2145–51.
- Medeiros BC, Kohrt HE, Arber DA, Bangs Cd, Cherry AM, Majeti R, Kogel KE, Azar CA, Patel S, Alizadeh AA. Inmunophenotypic features of acute myeloid leukaemia with inv(3)(q21q26.2)/t(3;3)(q21q26.2). Leuk Res. 2010;34:594–7.
- Lugthart S, Gröschel S, Beverloo HB, Kayser S, Valk P, van Zelderen-Bhola SL, et al. Clinical, molecular, and prognostic significance of WHO type inv(3)(q21q26.2)/t(3;3)(q21;q26.2) and various other 3q abnormalities in acute myeloid leukemia. J Clin Oncol. 2010;28:3890–98.
- Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010;116:354–65.
- Haimi M, Elhasid R, Moustafa N, Gershoni-Baruch R. Aberration of 3q and monosomy 7 in a child with acute myelogenous leukemia. Cancer Genet Cytogenet. 2007;174:78–81.
- Sun J, Konoplev SN, Wang X, Cui W, Chen SS, Medeiros LJ, et al. De novo acute myeloid leukemia with inv(3)(q21q26.2) or t(3;3)(q21;q26.2): a clinicopathologic and cytogenetic study of an entity recently added to the WHO classification. Mod Pathol. 2011;24:384–9.
- Chung HJ, Seo EJ, Kim KH, Jang S, Park CJ, Lee HJ, et al. Hematologic and clinical features of 3q21q26 syndrome: extremely poor prognosis and association with central diabetes insipidus. Korean J Lab Med. 2007;27:133–8.
- Reiter E, Greinix H, Rabitsch W, Keil F, Schwarzinger I, Jaeger U, et al. Low curative potential of bone marrow transplantation for highly aggressive acute myelogenous leukemia with inversion inv (3)(q21q26) or homologous translocation t(3;3)(q21q26). Ann Hematol. 2000;79:374–7.
- Horsman DE, Gascoyne RD, Barnett MJ. Acute leukaemia with structural rearrangements of chromosome 3. Leuk Lymphoma 1995;16:369–77.
- Lim G, Kim MJ, Oh SH, Cho SY, Lee HJ, Suh JT, et al. Acute myeloid leukemia associated with t(1;3)(p36;q21) and extreme thrombocytosis: a clinical study with literature review. Cancer Genet Cytogenet. 2010;203:187–92.
- Granada I, Luño E, Ribera JM, Sanzo C, Sancho JM, Muñiz SG, et al. Translocation (3;10)(q26;q22): a new nonrandom abnormality in three patients with 3q26 Involvement. Cancer Genet Cytogenet. 2000;121:99–100.
- Yamamoto K, Nagata K, Tsurukubo Y, Morishita K, Hamaguchi H. A novel translocation t(3;22)(q21;q11) involving 3q21 in myelodysplastic syndrome-derived overt leukemia with thrombocytosis. Leuk Res. 2000;24:435–57.
- Chang VT, Aviv H, Howard LM, Padberg F. Acute myelogenous leukemia associated with extreme symptomatic thrombocytosis and chromosome 3q translocation: case report and review of literature. Am J Hematol. 2003;72:20–26.
- Yamagata N, Shimazaki C, Kikuta T, Hirai H, Sumikuma T, Sudo Y, et al. A translocation between 3q21 and 12q24 in a patient with minimally differentiated acute myeloidleukemia (AML-M0). Cancer Genet Cytogenet. 1997;97:90–93.
- Li S, Yin C, Medeiros J, Bueso-Ramos C, Lu G, Lin P. Myelodysplastic syndrome/acute myeloid leukemia with t(3;21)(q26.2;q22) is commonly a therapy-related disease associated with poor outcome. Am J Clin Pathol. 2012;138:146–52.
