Since the favorable results reported in the United Kingdom Prospective Study (UKPDS) in 1998, metformin became the first line antidiabetic medication, combined with diet and exercise, for the management of hyperglycemia of type 2 diabetes (T2D).[Citation1] Metformin results in similar or even better glucose control compared to other oral antidiabetic agents while not exposing the diabetic patient to weight gain or hypoglycemia, with a good safety profile considering the long clinical experience and, last but not least, at a minimal cost.[Citation1]
Figure 1. Characteristics of the different formulations of metformin: delayed release compared with extended release and immediate release. GI: gastrointestinal; GLP-1: glucagon-like peptide-1; BID: twice a day; TID: three times a day.
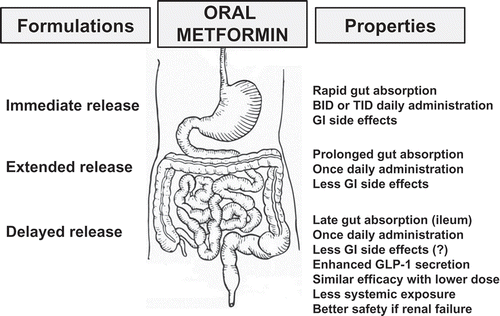
The development of extended-release (XR) metformin offers some advantages over classical immediate-release (IR) metformin formulation, especially one daily dosing option and better gastrointestinal tolerance, thus improving patient adherence.[Citation2,Citation3] Both formulations of metformin are contraindicated in patients with moderate to severe renal impairment because of an increased risk of accumulation and lactic acidosis.[Citation4] This remains a clinically relevant concern even if contraindications have been dampened during recent years even in subgroups of T2D patients initially considered at high risk of lactic acidosis.[Citation5]
When metformin is administered orally, approximately 40% of the dose are absorbed in the upper small intestine (duodenum and proximal jejunum) and only around 10% are absorbed in the ileum and colon.[Citation6,Citation7] Thus, bioavailability is approximately 50% with the current metformin IR or XR formulations. Absorbed metformin circulates in the plasma unbound and is eliminated unchanged by the kidneys.[Citation6,Citation7] Metformin is supplied to the liver directly from the gut via the portal vein, enters hepatocytes through the organic cation transporter-1 (OCT-1), and accumulates in the liver at concentrations approximately 10 times higher than those in plasma.[Citation7] This is in line with the current concept that the antihyperglycemic action of metformin is primarily exerted in the liver, via its ability to suppress gluconeogenesis and hepatic glucose output.[Citation8] Unabsorbed metformin accumulates in the mucosa of the bowel (at concentrations 300 times greater than in plasma),[Citation9] and is ultimately eliminated in the feces.
There is growing evidence suggesting that the gastrointestinal tract may play an important role in the action of metformin.[Citation8,Citation10] Recent human data showed that the pharmacology of metformin includes alteration of bile acid recirculation and gut microbiota resulting in enhanced enteroendocrine hormone secretion.[Citation11] The effect seems predominant on glucagon-like peptide-1 (GLP-1), a well-known incretin hormone secreted by L cells of the ileum.[Citation11]
A delayed release (DR) preparation of metformin was already clinically evaluated more than 40 years ago.[Citation12] However, afterwards advances in metformin therapy focused on XR rather than on DR preparations () (see comment in Expert opinion section below).[Citation2,Citation3] Maximum plasma metformin concentrations are reached more slowly with the XR formulation compared with conventional IR metformin, although both provide similar systemic exposure at a given total daily dose.[Citation2] Recently, a DR formulation of metformin has been designed by Elcelyx Therapeutics (NewMet®) to release metformin primarily in the distal small intestine and restrict metformin to the bowel, in contrast to currently available IR and XR metformin products that both target high systemic exposure. Metformin DR development is based on the recent findings that metformin may exert its glucose-lowering activity by action at the intestinal L cells of the ileum, and not only via systemic exposure.[Citation11] This is consistent with the L cells being the primary source of several glucose-regulating and satiety-regulating hormones, especially GLP-1. Metformin DR uses an enteric-coating technology to accomplish once-daily, targeted delivery to the region of the gut where the density of L cells is high.
A recent human study tested such a metformin DR formulation. Results were intriguing and provided further argument for a primary glucose-lowering effect of metformin present in the gut, not the systemic circulation.[Citation13] In a Phase 1, randomized, four-period crossover pharmacokinetic study in 20 healthy volunteers, the bioavailability of 1,000 mg metformin DR twice daily was approximately 50% that of metformin IR and XR.[Citation13] In a 12-week, Phase 2, multicenter, placebo-controlled, dose-ranging study, 240 subjects with T2D were randomized to receive metformin DR 600, 800, or 1,000 mg administered once daily; blinded placebo; or unblinded metformin XR 1,000 or 2,000 mg once daily used as reference. All doses of metformin DR produced statistically significant, clinically relevant, and sustained reductions in fasting plasma glucose levels over 12 weeks compared with placebo, with an approximately 40% increase in potency compared with metformin XR (). The placebo-subtracted changes from baseline in glycated haemoglobin (HbA1c) level at 12 weeks were consistent with changes in fasting plasma glucose (FPG) levels. The dissociation of the glycemic effect from plasma exposure with gut-restricted metformin DR in patients with T2D provides evidence for a predominantly lower bowel-mediated mechanism of metformin action at therapeutic doses.[Citation13] Delaying release of metformin to the distal intestine reduces bioavailability while still achieving similar glucose-lowering efficacy. However, some results deserve further discussion. For instance, the 95% CI for the reduction in FPG with the three doses of metformin DR have substantial overlap with each other (and with metformin XR 1000 mg), such that a dose response is not so clear. Furthermore, changes in HbA1c in the three metformin DR groups do not show a dose response, and the reduction for the higher dose is less than that for the mid-range dose, in contrast to the pattern seen for FPG. Finally, and most importantly, Buse and colleagues did not compare the different DR formulations to the highest dose of metformin XR, which had greater reduction in HbA1c. Consequently, it is not possible to determine whether all of the glucose-lowering effect of metformin can be explained by lower-bowel gut-based mechanisms.
Table 1. Main results (changes from baseline) of a 12-week, phase 2, placebo-controlled, dose-ranging study comparing metformin DR 600, 800, or 1,000 mg administered once daily; blinded placebo; or unblinded metformin XR 1,000 or 2,000 mg once-daily (reference) in patients with T2D (drug-naïve or after a 14–17 day wash-out) and an estimated glomerular filtration rate 60 ml/min/1.73 m2. Adapted from data reported in reference.[Citation13]
A crucial question now arises: will metformin DR provide better management of T2D ? If yes, better management may concern either efficacy or safety or both. First of all, will the use of metformin DR improve glucose control ? Currently we don’t have a precise answer to this question because available data showed almost equipoise glucose-lowering efficacy with metformin DR and metformin XR, even if the dose of metformin DR may be significantly reduced: 1,000 mg metformin DR administration resulted in a slightly lower reduction in FPG than 2,000 mg metformin XR but a slightly greater reduction than 1,000 mg metformin XR ().[Citation13] Thus, metformin DR once-daily resulted in almost comparable glucose lowering to metformin XR, with a 40% lower dose and substantially lower systemic exposure. In patients who are not allowed to sufficiently increase the daily dose of metformin XR (because of tolerance or safety concern), a better glucose control may be expected with a lower dose of metformin DR. However, in patients who could titrate classical metformin formulation to a maximal dose, the potential advantage of metformin DR remains unclear.
Second, in absence of better efficacy, will the use of metformin DR improve tolerance and safety? Using lower doses of metformin and bypassing the upper gastrointestinal tract may lead to improved digestive tolerability, without the need for dose titration upon initiation of therapy. However, in a recent comparison trial, gastrointestinal adverse events were almost similar between metformin DR and metformin XR,[Citation13] a prolonged-release formulation that is associated with better gastrointestinal tolerance compared with IR formulation.[Citation2,Citation3] The mechanism behind the gastrointestinal adverse effects of metformin remains unclear. OCT1, which is critical for the transport of metformin from the gut lumen into cells, may play a prominent role in the tolerability of metformin and taking into account its polymorphism has been proposed for personalizing metformin therapy.[Citation14]
Interestingly, changes in plasma lactate levels were negligible and less marked with metformin DR than those measured with metformin XR in a head-to-head comparative study (),[Citation13] perhaps due to lower metformin exposure. Alternatively, the human intestine might be an important source of metformin-induced lactate production [Citation9] and reducing the oral dose (and thus potential metformin gut accumulation) by specifically targeting the ileum may also contribute to smaller lactate increase. Consequently, a potential advance provided by metformin DR may be the use of the biguanide in patients with chronic kidney disease and at higher risk of lactic acidosis.[Citation4] The relationship between metformin exposure and lactate production was examined across the spectrum of renal function ranging from normal to severely impaired in patients with T2D.[Citation15] Although plasma metformin concentrations increased with decreasing renal function, the area under-the-curve of metformin plasma concentrations was significantly reduced by 25–50% after oral administration of a single 1,000 mg dose of metformin DR compared to a 1,000 mg dose of metformin XR. In contrast to what was observed with metformin XR, no relationship was detected between plasma metformin and plasma lactate levels with metformin DR, most likely because metformin concentrations in the systemic circulation were not high enough. Thus metformin DR could potentially provide a treatment option for patients with renal impairment pending the results future studies.[Citation4]
1. Expert opinion section
Modified-release or controlled-release formulations are able to control ‘rate’ as well as ‘site’ of drug release. They are designed to maintain drug concentration within the therapeutic window for maximum or desirable period of time. Thereby, an XR form allows at least a twofold reduction in dosage frequency as compared to the same drug presented as an immediate-release (conventional) dosage form, generally thereby allowing once-daily administration. In contrast, DR formulations are designed to control only site of drug release. In the case of metformin, DR product is formulated to avoid early gastrointestinal absorption and to deliver the active drug to the lower bowel: the main objective is to potentiate the gut-based GLP-1-dependent mechanisms of metformin action with lower systemic plasma exposure ().
The assumption that metformin acts substantially through activation of the L cells in the lower bowel is supported by several rodent data and early clinical data.[Citation8–Citation11] The observation that metformin DR formulation, which delivers the drug predominantly to the lower bowel (ileum where L cells are predominant), acts with a similar glucose-lowering efficacy to that of metformin XR, but with significantly lower systemic exposure may be of clinical significance. It is too early to speculate whether metformin DR will replace metformin XR in all patients with T2D in the future. However, if preliminary data are confirmed with this investigational new drug in development (NewMet®), such a gut-restricted metformin DR may be administered in T2D patients with renal impairment without the risk of metformin-associated lactic acidosis.[Citation4] However, the precise efficacy and safety profile of metformin DR relative to existing IR or XR formulations remain to be confirmed. In this regard, large-scale phase 3 clinical trials are mandatory as well studies in patients with renal impairment who cannot benefit from metformin therapy owing to the contraindications currently in place.[Citation1,Citation4,Citation5]
The use of metformin DR instead of metformin XR may be also a way of personalizing metformin therapy targeting differently postprandial versus fasting hyperglycemia. Indeed, the mechanism of glucose lowering may actually be different with DR vs XR formulations. Metformin DR may work predominantly by increasing GLP-1 and have a greater impact on postprandial glucose than metformin XR. In contrast, metformin XR that increases systemic/liver exposure reduces hepatic glucose production and thereby could have its greatest impact on fasting glucose. However, this hypothesis requires further confirmation.
Finally, the considerable progress in our understanding of the complex mechanisms underlying the glucose-lowering action of metformin points toward potential new molecular targets for the development of novel antidiabetic therapies.
Declaration of interest
A.J. Scheen has received lecture/advisor fees from AstraZeneca/BMS, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Novartis, NovoNordisk, Sanofi and Takeda. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care. 2015;38:140–149.
- Ali S, Fonseca V. Overview of metformin: special focus on metformin extended release. Expert Opin Pharmacother. 2012;13:1797–1805.
- Chacra AR. Evolving metformin treatment strategies in type-2 diabetes: from immediate-release metformin monotherapy to extended-release combination therapy. Am J Ther. 2014;21:198–210.
- DeFronzo R, Fleming GA, Chen K, et al. Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism. 2016;65:20–29.
- Scheen AJ, Paquot N. Metformin revisited: a critical review of the benefit-risk balance in at-risk patients with type 2 diabetes. Diabetes Metab. 2013;39:179–190.
- Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996;30:359–371.
- Graham GG, Punt J, Arora M, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98.
- Gruszka A. New insight into the mechanisms of the anti-hyperglycemic action of metformin. Br J Med Med Res. 2015;13(1):1–9. doi:10.9734/BJMMR/2016/23354
- Bailey CJ, Wilcock C, Scarpello JH. Metformin and the intestine. Diabetologia. 2008;51:1552–1553.
- Hur KY, Lee M-S. New mechanisms of metformin action: focusing on mitochondria and the gut. J Diabetes Investig. 2015;6:600–609.
- Napolitano A, Miller S, Nicholls AW, et al. Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. PLoS One. 2014;9:e100778.
- Campbell IW, Clarke BF, Duncan LJP. A clinical evaluation of a delayed release preparation of metformin. J Int Med Res. 1973;1:551–556.
- Buse JB, DeFronzo RA, Rosenstock J, et al. The primary glucose-lowering effect of metformin resides in the gut, not the circulation. Results from short-term pharmacokinetic and 12-week dose-ranging studies. Diabetes Care. 2016;39:198–205.
- Scheen AJ. Personalising metformin therapy: a clinician’s perspective. Lancet Diabetes Endocrinol. 2014;2:442–444.
- Bakris GL, Mudaliar S, Kim T, et al. Effects of new metformin formulation in stage 3 and 4 CKD: a pilot study. J Am Soc Nephrol. 2014;25:549A.
