Abstract
Objective: To evaluate the pharmacokinetic (PK) characteristics of a modified fentanyl iontophoretic transdermal system (ITS).
Research design and methods:This was a prospective, open-label, single-center, randomized, 3-period, 5-treatment, 6-sequence study. Each subject was randomly assigned to receive three treatments in a sequence consisting of intravenous fentanyl citrate, fentanyl ITS at 170 μA, and then one of three other fentanyl ITS treatments at 140, 200 or 230 μA.
Main outcome measures: The following PK parameters were determined: Cmax, tmax, t1/2, AUC23 – 25 and amount of fentanyl absorbed into systemic circulation (i.e., Dose Absorbed).
Results: Fifty-two subjects received at least one fentanyl treatment. Serum exposure (Cmax and AUC23 – 25) and Dose Absorbed increased with increasing current. The median tmax ranged from 23.0 to 23.2 h across the 4 ITS groups. Mean t1/2 values ranged from 11.0 to 13.0 h. The Dose Absorbed from the fentanyl ITS at 170 μA met bioequivalence criteria when compared to data from an earlier version of the fentanyl ITS.
Conclusions: Exposure of fentanyl and the amount of fentanyl absorbed increased with the magnitude of applied current with the ITS. The fentanyl ITS at 170 μA is bioequivalent to an earlier version of the system.
1. Introduction
Fentanyl [propanamide, N-phenyl-N-[1-(2-phenylethyl)-4-piperidyl] is a synthetic opioid that has been administered parenterally as an anesthetic and analgesic since the 1960s. In addition to the intravenous (i.v.) route of fentanyl administration, oral transmucosal, oral buccal, sublingual and transdermal preparations are available for analgesic indications. An iontophoretic, transdermal system that provides patient-controlled systemic delivery of fentanyl to patients for the management of moderate to severe pain in the hospital setting is being studied. The fentanyl iontophoretic transdermal system (ITS) is a prefilled, adhesive, needle-free system. Iontophoresis (electrotransport or electrically-assisted transport) technology provides controlled systemic delivery of drugs across the skin, with the application of a small electrical current. This method of drug delivery can provide patient-controlled analgesia (PCA) in a noninvasive manner.
A fentanyl ITS (Trade name IONSYS®) was developed to provide PCA in adult post-operative patients in the hospital setting. A fentanyl dose of 40 μg administered over 10 min was determined to be an safe and efficacious dose for treatment of post-operative pain with i.v. PCA in a 24 h Phase II study in patients (n = 150) following major surgery Citation[1]. In this study, doses of 20, 40 and 60 μg (expressed as the base) were infused over 10 min upon patient activation of the infusion pump containing a solution of fentanyl citrate. The 40 μg dose provided better efficacy than the 20 μg dose, with fewer opioid-related adverse events than the 60 μg dose.
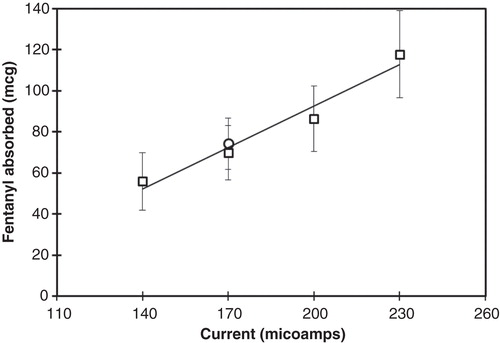
Two ITS dose-ranging pharmacokinetic (PK) studies were performed to determine the current level required to deliver 40 μg of fentanyl across the skin in 10 min Citation[2]. In the first PK study, a linear relationship between the magnitude of the applied current over a range from 100 to 230 μA and the amount of fentanyl absorbed in 10 min was observed, with a current of 170 μA providing a nominal dose of 40 μg. In the second study, two consecutive 10 min doses were administered with the fentanyl ITS at current levels of 100 and 170 μA. As shown in , the amount of fentanyl absorbed at 170 μA was approximately twice the amount observed in the first study, consistent with the delivery of two consecutive doses versus one.
Table 1. Fentanyl dose absorbed from an iontophoretic transdermal system Citation[2].
Other PK studies Citation[3-7] were performed with prototype versions of the ITS to characterize absorption and elimination kinetics as a function of current magnitude, dosing duration, site of application and demographic variables. Fentanyl absorption was found to be independent of dose duration for treatments where the total charge delivered by the ITS was held constant Citation[3]. Compared with intravenously infused fentanyl, the serum concentrations resulting from ITS application had a slightly dampened rate of increase and decrease in serum concentrations attributed the barrier properties of the stratum corneum, and similar intersubject variability in fentanyl PK parameter values Citation[4]. In addition, the effect of the frequency of fentanyl administration over 24 h was compared to intermittent i.v. administration Citation[5,6]. Fentanyl absorption was found to increase from about 16 μg per dose initially to a steady state value of about 40 μg per dose over the first 10 h, regardless of the frequency of dose administration. In another study, application of the system to the upper outer arm or chest resulted in similar maximum serum concentrations (Cmax) and AUC Citation[6]. However, both Cmax and AUC values were about one-fourth lower when the system was applied to the lower inner arm. Subject age, bodyweight, gender and ethnicity had no significant effect on PK parameters Citation[6]. In addition, the same investigators concluded that there was no significant difference in AUC values for single- and multiple-day treatments. Passive diffusion of fentanyl from the ITS across the skin over 24 h was found to be about 2 μg/h and therefore have no predicted clinical effect on patients Citation[7].
The integrated design and key components of the original fentanyl ITS have been discussed by Phipps et al. Citation[8]. Phase III trials were performed with the original fentanyl ITS for treatment of acute postoperative pain in adult patients during hospitalization Citation[9-14]. Patients were treated with the fentanyl ITS for up to 3 days after their operation. Dose administration was started when the patient pressed a recessed button on the top of the system twice within 3 sec. A maximum of six doses could be administered per hour with each system operating for up to 24 h or until 80 doses were administered. The results of these trials demonstrated that treatment of postoperative pain with the ITS was superior to a placebo ITS, and provided similar efficacy to administration of morphine by i.v. PCA.
The original fentanyl ITS received European marketing authorization in January of 2006 and FDA approval in May of 2006 for the management of acute post-operative pain in adult patients requiring opioid analgesia in a hospital setting. The original system was never launched in the US due to the detection of corrosion in a small number of samples during storage caused by moisture from the hydrogels. To eliminate corrosion, the electronics were separated from the hydrogels to completely isolate the electronics from the humidity produced by the hydrogels during storage.
The modified fentanyl ITS evaluated in this study consisted of two parts: a Drug Unit containing the fentanyl hydrogel and a Controller containing the electronics. The two parts were assembled by the healthcare professional immediately prior to application to the subject. The hydrogel formulation and dosing parameters of the modified ITS were the same as the original ITS.
Four ITS units set at different currents (140, 170, 200 and 230 μA) were used in this study. The primary objective of this study was to measure five key PK parameters as a function of applied current: AUC, maximum fentanyl concentration (Cmax), time to Cmax (tmax), terminal half-life (t1/2) and the amount of fentanyl absorbed (Dose Absorbed).
A key second objective of this study was to evaluate the bioequivalence of the modified fentanyl ITS 170 μA (test) to the original ITS 170 μA (reference) Citation[2] using Dose Absorbed values. The original version of the ITS was unavailable, so a direct comparison of PK performance in a crossover bioequivalence study was not possible. Therefore, to bridge the different subject groups used in the two studies, the i.v. infusion treatment used in the original study was replicated in this study (i.e., i.v. infusion of 80 μg of fentanyl each hour) Citation[2]. This strategy allowed the AUC data from the two studies to be converted to ‘Dose Absorbed’ using i.v. data for each individual from the respective study.
2. Subjects and methods
This was a single-center, randomized, open-label, 3-period, 5-treatment, 6-sequence PK study in healthy adult subjects. Written informed consent was obtained from all potential subjects prior to any study procedure being performed. The study was conducted between 26 November 2012 and 22 February 2013 with PRACS Institute, Ltd. Institutional Review Board approval prior to screening subjects. The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and clinical research guidelines established by the Code of Federal Regulations (Title 21, CFR Parts 50, 56 and 312) and ICH Guidelines.
2.1 Subjects
Healthy male or female volunteers, 18 – 45 years of age, inclusive, with no clinically relevant abnormalities as assessed by the investigator and determined by medical history, physical examination and screening tests were eligible for inclusion. In addition, a naloxone challenge by subcutaneous injection was performed within 24 h prior to the fentanyl dosing to assess opioid tolerance, with a negative finding required for the volunteer to continue in the study.
2.2 Materials
Commercially available fentanyl citrate solution (i.v.), naloxone (i.v. and/or s.c.) and naltrexone (PO) were provided by the clinical investigator.
Fentanyl was delivered transdermally from the modified ITS which was preprogrammed to apply currents of 140, 170, 200, or 230 μA.
2.3 Dosing
Subjects received 3 fentanyl treatments, one administered via i.v. infusion and 2 administered via the ITS. Each subject was randomly assigned to receive a treatment sequence consisting of i.v. fentanyl citrate, fentanyl ITS 170 μA, and then 1 of 3 other fentanyl ITS treatments at 140, 200, or 230 μA. The six different treatment sequences are summarized in . Fentanyl citrate, equivalent to 80 μg fentanyl base, was administered by an i.v. infusion pump (Medfusion 2001 Syringe Infusion Pump) over 20 min every hour through 23.33 h. The fentanyl ITS treatments were applied to the upper outer arm and provided two consecutive fentanyl doses every hour, administered over 10 min each, through 23.33 h (48 total doses). Fentanyl-induced opioid effects were blocked or antagonized by coadministration of oral naltrexone 50 mg every 12 h, which began 14 h before the start of fentanyl treatment and ended ∼ 11 h after each treatment. At least 6 days separated each of the treatment periods. Subjects remained at the study site during fentanyl dosing and for 24 h following completion of each treatment.
Table 2. Treatment sequences.
2.4 Sampling schedule
Blood samples for fentanyl serum PK analysis were obtained at baseline (just prior to the start of fentanyl administration on Day 1), and 23, 23.17, 23.25, 23.33, 23.42, 23.50, 23.67, 23.83, 24, 24.25, 24.50, 24.75, 25, 26, 28, 32, 36, 42, and 48 h after the start of fentanyl administration. Blood samples were collected into Vacutainer® tubes with no anticoagulant, from the arm without a system applied, and were processed within 2 h. After clotting was complete (∼ 30 min), the samples were spun at 3000 rpm at 4°C for 10 min. Serum was evenly divided between two aliquots and stored at -20 (± 10) °C until shipment to the bioanalytical laboratory.
2.5 Drug concentration measurements
Samples were shipped to the central bioanalytical laboratory and assayed for fentanyl concentration using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. The lower limit of quantitation was 0.020 ng/ml and the upper limit of quantitation was 10.00 ng/ml. All samples were assayed within the established stability period of 5 months.
Each 200 μl aliquot of standard, QC sample, and study sample was mixed with 100 μl of internal standard working solution (2.000 ng/ml), 200 μl of 10% NH4OH and 7.0 ml of methyl tert-butyl ether. After vortexing and centrifuging, the aqueous layer was frozen in a dry-ice/IPA solution and the organic layer was decanted and dried at 40°C under air. The residue was reconstituted with 300 μl of reconstitution solution (20% acetonitrile-water containing 0.1% formic acid solution) and an aliquot was then injected onto an LC-MS/MS system for analysis.
2.6 PK parameters
The primary endpoint was to determine 5 standard PK parameters (AUC, Cmax, tmax, t½, and Dose Absorbed). All PK parameters were derived from a noncompartmental analysis of the fentanyl concentration-versus-time data using Phoenix™ WinNonlin® Version 6.3 (Pharsight Corp., Mountain View, CA). Actual sampling times were used to calculate the PK parameters except for the initial pre-dose samples, which were always reported as zero, regardless of time deviations. Any concentration below the limit of quantitation (BLQ) was reported as BLQ. The mean concentrations and associated statistics were calculated using quantifiable levels. Concentration data not reported in the bioanalytical dataset were considered missing, and therefore were not interpolated in the PK analyses.
The following PK parameters were calculated for this analysis: AUC23 – 25 was calculated using the linear trapezoidal rule for the period from 23 through 25 h; Cmax, the maximum fentanyl concentration, was determined by inspection; tmax, the time to maximum fentanyl concentration, was determined by inspection; t½, the terminal half-life, was calculated from the elimination rate constant λz. The amount of fentanyl absorbed (Dose Absorbed) for each treatment by each subject was calculated from the ratio of the AUC23 – 25 for the ITS treatments to the AUC23 – 25 for the i.v. infusion treatment and then multiplied by the i.v. fentanyl dose of 80 μg.
2.7 Statistical methods
PK endpoints were analyzed descriptively for the PK analysis population. The PK analysis population consisted of all subjects who completed at least one treatment period (n = 43). Descriptive statistics were reported for each treatment/period and included the number of observations, mean, median, geometric mean, standard deviation, coefficient of variation (CV), and the minimum and maximum values. Descriptive statistics for tmax only included the number of observations, median, and the minimum and maximum values. Area-under-the-curve (AUC23 – 25) values were determined with the linear trapezoid method for serum fentanyl concentrations from 23.0 through 25.0 h. Individuals whose PK profiles did not meet the minimum criteria for t½ determination, or for which a t½ could not be calculated, were not included in the descriptive statistics for t½.
The bioequivalence of the modified fentanyl ITS (test) to the original system (reference) was evaluated using Dose Absorbed values per FDA guidance Citation[15]. The 90% CIs for the difference of the Dose Absorbed means of the two ITSs on a log scale were computed using the total between-subject variance. The difference of the two means of log-transformed Dose Absorbed data, and the corresponding 90% CIs, were back-transformed (taking anti-logs) to provide the 90% CIs for the ratio of the population geometric means (test/reference) on the original scale. Bioequivalence was concluded if the 90% CIs for the ratio of the population geometric means were fully contained within the bounds of 80 and 125%.
2.8 Sample size calculation
In this study, enrollment of 54 subjects with an estimated dropout rate of 20% was expected to result in 43 evaluable subjects. An bioequivalence test of means using two one-sided t-tests on data from a parallel group design, with sample sizes of 31 in the historical reference group Citation[2] and 43 in the current treatment group, achieved 90% power at a 5% significance level when the ratio of the means is 0.95, the CV on the original unlogged scale is 25%, and the bioequivalence limits of the mean ratio are 0.80 and 1.25.
3. Results
3.1 Subjects
Fifty-two subjects received fentanyl and 43 subjects completed at least one treatment and had reportable PK data (). Thirty-six of the 52 subjects completed a 3-treatment sequence. Sixteen subjects were discontinued from the study. Subjects had a mean age of 28.3 years and mean body mass index of 24.2 kg/m2. Thirty-six of the 52 subjects were male and 39 subjects were Caucasian.
Table 3. Summary of subject enrollment and disposition.
3.2 PK parameters
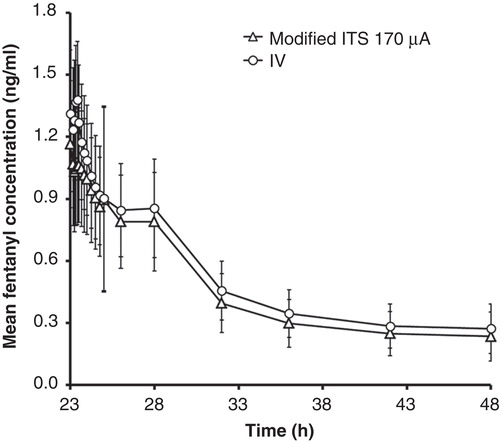
Serum fentanyl concentration profiles exhibited a similar shape for each of the fentanyl ITS treatments and the i.v. treatment (). The mean concentration-versus-time profiles demonstrate increasing concentrations of fentanyl with increased current for the fentanyl ITS treatments (). In addition, the mean Cmax occurred at or slightly after the completion of the final dose delivery at 23.33 h, indicating rapid transdermal absorption of fentanyl into systemic circulation. The variability in fentanyl serum concentrations for the i.v. treatment were similar to the iontophoretic transdermal treatment groups as illustrated with overlapping profiles in for the i.v. and 170 μA treatments, with coefficients of variation at each time point ranging from 20 to 40%.
Figure 1. Mean serum fentanyl concentration-time profiles after i.v. infusion or iontophoretic transdermal system treatments. (A) 23 – 48 h. (B) 23 – 25 h.
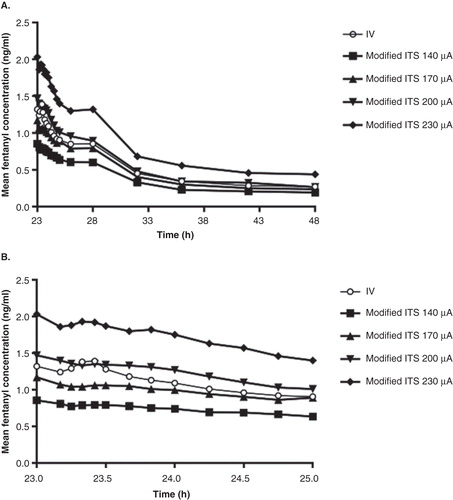
Figure 2. Comparison of mean (± standard deviation) fentanyl serum concentrations for the modified iontophoretic transdermal system at 170 μA (n = 41) and the i.v. infusion treatment (n = 43).
Independent of the route of administration or the current, the decline in fentanyl concentrations after completing treatments appeared to plateau from 25.5 through 28 h for all dose groups and demonstrated a similar elimination phase. The mean apparent t1/2 and λZ were similar across the four fentanyl ITS treatment groups, and mean t1/2 was slightly shorter than the observed t1/2 for the i.v. treatment ().
Table 4. Fentanyl pharmacokinetic parameters after intravenous infusion or modified iontophoretic transdermal system.
Mean fentanyl exposure (Cmax and AUC23 – 25) increased with increasing current (). Box plots illustrating increasing AUC23 – 25 with increasing current are shown in . In addition, the Dose Absorbed increased in proportion to the magnitude of the applied current as shown in .
Figure 3. Box plot comparisons of AUC 23 – 24 after i.v. infusion or modified iontophoretic transdermal system administration of fentanyl by treatment group. Standard box plot is defined by the interquartile range (IQR) with upper (75%) and lower (25%) quartiles (the box). Whiskers indicate the 1.5 IQR (50% of the difference between the third and fourth quartiles). Outliers (outside of the 1.5 IQR) are designated by markers. The median is represented by a solid line.
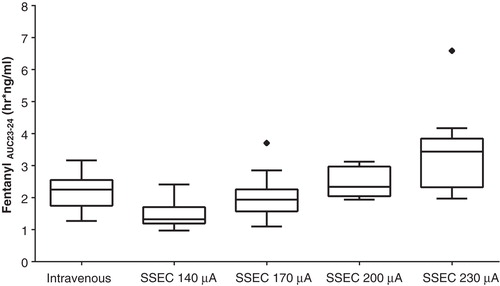
Figure 4. Mean dose absorbed (with standard deviations) from two consecutive doses using the modified iontophoretic transdermal system at various applied currents (line is a linear regression fit with r2 = 0.96), compared to dose absorbed from the original iontophoretic transdermal system at 170 μA (circle).
Variability in exposures were similar for the i.v. and transdermal iontophoretic treatments, with CVs of 22.5 and 26.7% for AUC23 – 25 and Cmax for the i.v. treatment, respectively; compared to the transdermal iontophoretic treatments with ranges of 18.6 – 36.6% for AUC23 – 25 and 18.8 – 34.8% for Cmax.
3.3 Comparison of the dose absorbed from the modified ITS and the original ITS
Statistical comparison and bioequivalence results for the modified ITS at 170 μA relative to original ITS are summarized in . The ratio of the geometric means of the Dose Absorbed for the modified ITS (test) and the original ITS products (reference; 93.18%) as well as the 90% CIs (87.13, 99.65%) were fully contained within the pre-established bioequivalence bounds of 80 – 125%, indicative of bioequivalence.
Table 5. Statistical comparison of fentanyl dose absorbed from the modified ITS (test, n = 40) and the original ITS (reference, n = 31) Citation[2].
3.4 Safety
The effects of fentanyl were blocked by use of oral antagonist naltrexone. Treatment-emergent adverse events were primarily local reactions at the site of system application or the infusion site. Each of the AEs was assessed by the investigator as mild or moderate in severity and had resolved by the end of the study. There were no SAEs, deaths or withdrawals due to an AE. There were no individual clinically significant laboratory abnormalities or any clinically meaningful changes from baseline to end of study in any hematology, serum chemistry or urinalysis parameters. There were no clinically meaningful changes observed from baseline to end of study in vital signs or ECG data.
4. Discussion
The active ingredient in the ITS is fentanyl as the hydrochloride salt which is dissolved in the anode hydrogel formulation. The cationic form of fentanyl has very low passive transport across human skin Citation[7] but passes readily across skin when an electrical potential gradient is applied (i.e., a voltage drop) Citation[2,3].
Because the passive flux of fentanyl ion is negligible, the rate of transdermal delivery of fentanyl ion, Rf, from the anode hydrogel formulation is explained by the following expression derived from Faraday’s Law of Electrolysis:
where, tf is the fentanyl transport number (fraction of charge that is carried by the fentanyl ion), I is the current, Mf is molecular weight of fentanyl ion, Zf is the valence of the fentanyl ion, and F is Faraday’s constant. Therefore, the rate of fentanyl delivery is directly proportional to the magnitude of the applied current. The amount of fentanyl delivered to the patient for a specific current is determined by the duration of the applied current. For fixed dose duration, the amount of fentanyl absorbed will be proportional to current, as demonstrated in .
The transport number, tf, is an important parameter determined by the concentration and mobility of fentanyl ions within the skin relative to the concentration and mobility of all other mobile ions in the skin. The transport number can be calculated from rearrangement of EquationEquation 11 :
The linear regression of the data presented in yields a slope of 0.67 grams fentanyl per ampere resulting from delivery of two consecutive 10 min doses from the ITS. Therefore, the rate of fentanyl delivery per ampere (i.e., Rf/I) is 5.6 × 10-4 g/sec/ampere. Substitution of this value into EquationEquation 22 with the fentanyl charge (+1), Faraday’s constant (96487 C/mol) and the molecular weight of fentanyl ion (337 g/mol) yields a transport number of 0.16. This value indicates that 16% of all ions passing across the skin were fentanyl ions. The majority of ions crossing the skin during treatment (i.e., the other 84%) can be attributed to the electromigration of chloride ions from the body through the skin and into the anode hydrogel. The chloride ion carries the majority of the current because of its greater mobility relative to the larger fentanyl ion and presumed larger concentration within the skin.
The fentanyl ITS evaluated in this study is a modified version of a previous ITS. The modifications altered the design of the ITS to accommodate a digital display and a two-part configuration. Since the composition of the anode hydrogel and all delivery parameters were identical to the original ITS, the amount of fentanyl absorbed was expected to be unaffected by the design modifications. This prediction was confirmed by the data shown in .
Statistical comparison of the Dose Absorbed in 170 μA group of the modified fentanyl ITS (test) relative to the original fentanyl ITS (reference) indicated that the ratio of the geometric means of test versus reference as well as the 90% CI were fully contained within the bounds of the CI limit interval of 80 – 125% (). Therefore, these data demonstrate that the modifications made to the original fentanyl ITS did not significantly affect the amount of fentanyl absorbed and that standard bioequivalence criteria Citation[15] were achieved. The original fentanyl ITS was utilized in the Phase III studies that demonstrated that fentanyl ITS was superior efficacy to placebo Citation[9,14] and has therapeutically equivalent efficacy to morphine administered i.v. as patient-controlled analgesia in adult patients with acute post-operative pain Citation[10-13]. Therefore, the clinical results of the Phase III trials are relevant to the modified ITS because of the bioequivalence of the original fentanyl ITS and modified fentanyl ITS.
One limitation of the study was that it was conducted in healthy volunteers and not in patients with acute post-operative pain. In addition, the effects of fentanyl were negated by using oral naltrexone, and therefore, no efficacy data could be obtained from this study. Another limitation is that the original version of the ITS was unavailable, so a direct comparison of PK performance in a crossover bioequivalence study was not possible. Therefore, to bridge between the different subject groups used in the two studies, the i.v, infusion treatment used in the original study was replicated in this study (i.e., i.v. infusion of 80 μg of fentanyl each hour) Citation[2]. This strategy allowed the AUC data from the two studies to be converted to ‘Dose Absorbed’ using i.v. data from the respective study.
5. Conclusion
In this study, PK exposure (Cmax and AUC23 – 25) and the Dose Absorbed from the final two consecutive doses administered increased proportionally with increasing current, as predicted from a derivation of Faraday’s Law of Electrolysis. Bioequivalent fentanyl absorption was established for the modified fentanyl ITS relative to the original (historical) fentanyl ITS.
Acknowledgements
The authors gratefully acknowledge Kirsten Reimen (InClin) for assistance with planning and execution of this study and Starr Grundy (SD Scientific) for editorial assistance. Results of this study were presented in part at the Annual Meeting of the International Anesthesia Research Society (May 17-20, 2014; Montréal, Canada).
Declaration of interest
Study funding was provided by Incline Therapeutics, Inc., a wholly owned subsidiary of The Medicines Company (Parsippany, NJ). The analyses and writing of this manuscript were supported financially by The Medicines Company. Brad Phipps and Nitin Joshi are employees of The Medicines Company. Raymond S Sinatra is Acting Director of Pain Medicine at the West Haven VA Medical Center, West Haven CT, and serves as a consultant to The Medicines Company. Kelly Regal and Jinfang Li are employees of ProPharma Services and provided PK and statistical analysis services to Incline Therapeutics.
Notes
Bibliography
- Camu F, Van Aken H, Bovill JG. Postoperative analgesic effects of three demand-dose sizes of fentanyl administered by patient-controlled analgesia. Anesth Analg 1998;4:890–5
- Sathyan G, Jaskowiak J, Evashenk M, Gupta S. Characterisation of the pharmacokinetics of the fentanyl HCl patient-controlled transdermal system (PCTS): effect of current magnitude and multiple-day dosing and comparison with IV fentanyl administration. Clin Pharmacokinet 2005;44(Suppl 1):7–15
- Gupta SK, Bernstein KJ, Noorduin H, et al. Fentanyl delivery from an electrotransport system: delivery is a function of total current, not duration of current. J Clin Pharmacol 1998;38:951–8
- Gupta SK, Southam M, Sathyan G, Klausner M. Effect of current density on pharmacokinetics following continuous or intermittent input from a fentanyl electrotransport system. J Pharm Sci 1998;8:976–81
- Gupta SK, Sathyan G, Phipps B, et al. Reproducible fentanyl doses delivered intermittently at different time intervals from an electrotransport system. J Pharm Sci 1999;8:835–41
- Gupta SK, Hwang S, Southam M, Sathyan G. Effects of application site and subject demographics on the pharmacokinetics of fentanyl HCl patient-controlled transdermal system (PCTS). Clin Pharmacokinet 2005;44(Suppl 1):25–32
- Sathyan G, Phipps B, Gupta SK. Passive absorption of fentanyl from the fentanyl HCl iontophoretic transdermal system. Curr Med Res Opin 2009;2:363–6
- Phipps JB, Padmanabhan RV, Young W, et al. E-TRANS technology. In: Rathbone MJ, Hadgraft MS, Roberts MS, editors. Modified-release drug delivery technology. Marcel Dekker, Inc; New York: 2002. p. 499–511
- Chelly JE, Grass J, Houseman TW, et al. The safety and efficacy of a fentanyl patient-controlled transdermal system for acute postoperative analgesia: a multicenter, placebo-controlled trial. Anesth Analg 2004;2:427–33; table of contents
- Grond S, Hall J, Spacek A, et al. Iontophoretic transdermal system using fentanyl compared with patient-controlled intravenous analgesia using morphine for postoperative pain management. Br J Anaesth 2007;6:806–15
- Hartrick CT, Bourne MH, Gargiulo K, et al. Fentanyl iontophoretic transdermal system for acute-pain management after orthopedic surgery: a comparative study with morphine intravenous patient-controlled analgesia. Reg Anesth Pain Med 2006;6:546–54
- Minkowitz HS, Rathmell JP, Vallow S, et al. Efficacy and safety of the fentanyl iontophoretic transdermal system (ITS) and intravenous patient-controlled analgesia (IV PCA) with morphine for pain management following abdominal or pelvic surgery. Pain Med 2007;8:657–68
- Viscusi ER, Reynolds L, Chung F, et al. Patient-controlled transdermal fentanyl hydrochloride vs intravenous morphine pump for postoperative pain: a randomized controlled trial. JAMA 2004;11:1333–41
- Viscusi ER, Reynolds L, Tait S, et al. An iontophoretic fentanyl patient-activated analgesic delivery system for postoperative pain: a double-blind, placebo-controlled trial. Anesth Analg 2006;1:188–94
- US Department of Health and Human Services. Guidance for industry: statistical approaches to establishing bioequivalence. Available from: http://www.fda.gov/downloads/Drugs/Guidances/ucm070244.pdf [Last accessed 30 July 2014]
