Abstract
Introduction: Although significant scientific advances have been made over the past six decades in developing safe, nontoxic and effective radiation/medical countermeasures (MCMs) for acute radiation syndrome (ARS), no drug has been approved by the US FDA. The availability of adequate animal models is a prime requisite under the criteria established by the FDA ‘animal rule’ for the development of novel MCMs for ARS and the discovery of biomarkers for radiation exposure.
Areas covered: This article reviews the developments of MCMs to combat ARS, with particular reference to the various animal models (rodents: mouse and rat; canine: beagle; minipigs and nonhuman primates [NHPs]) utilized for the in-depth evaluation. The objective, pathways and challenges of the FDA Animal Efficacy Rule are also discussed.
Expert opinion: There are a number of well-defined animal models, the mouse, canine and NHP, that are being used for the development of MCMs. Additional animal models, such as the minipig, are under development to further assist in the identification, efficacy testing and approval of MCMs under the FDA Animal Efficacy Rule.
1. Introduction
Proliferation of radioactive material, nuclear weapons and nuclear power facilities has increased the likelihood of military forces and civilians encountering a local or widespread radiological hazard Citation[1,2]. Nuclear or radiological exposure can result from detonation of improvised nuclear devices by terrorists or hostile forces, the deliberate spread of radioactive material from industrial or medical sources, accidental or intentional destruction of nuclear facilities or accidents involving radiation sources Citation[2-5].
Radiation accidents can result in different types of radiation exposure which alters the diagnostic and therapeutic measures, as well as the outcomes. The clinical course of acute radiation syndrome (ARS) depends on the absorbed radiation dose and its distribution. Multiorgan involvement and multiorgan failure need be taken into account Citation[6]. Radiation-induced damage to the hematopoietic tissue, skin, lung and gastrointestinal (GI) systems plays an important role in the diagnosis and the treatment of radiation accident victims since reliable information about the radiation exposure from physical dosimetry and results from biodosimetric methods are usually not available in accident situations; the estimation of radiation effects can be performed on the basis of clinical signs and symptoms as described in the medical treatment protocols for radiation accident victims system Citation[7,8]. Since the hematopoietic system is most vulnerable to ionizing radiation, diagnostic and therapeutic measures dealing with the hematopoietic syndrome are most important. The main specific therapeutic principles are replacement with blood products, the administration of cytokines like G-CSF and GM-CSF and the transplantation of hematopoietic stem cells. There is a strong need for internationally recognized guidelines for the treatment of severely radiation-exposed patients Citation[9].
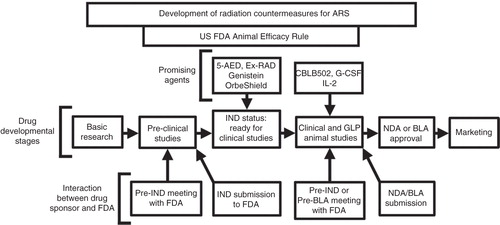
Although the search for medical countermeasures (MCMs) was initiated more than six decades ago, no safe and effective MCM for ARS has been approved by the US FDA Citation[10-12]. There are several promising MCMs at advanced stages of development under the Animal Efficacy Rule (); some of these also have US FDA investigation new drug (IND) status and may receive FDA approval Citation[13]. Two agents G-CSF and GM-CSF have been procured for the strategic national stockpile (SNS) Citation[14,15]. The Center for Disease Control currently holds both IND and emergency use authorization (EUA) applications with the FDA for the use of G-CSF in the event of a nuclear or radiological incident Citation[16]. The EUA application for CBLB502, a Toll-like receptor (TLR) ligand and stimulator of NF-B may be filed soon with the FDA.
Figure 1. Stages of MCM development under the FDA Animal Efficacy Rule are shown. Important steps for developing MCM and FDA approvals are depicted in the above figure.
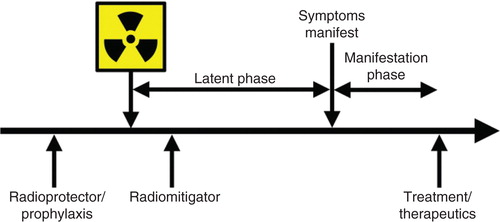
A good MCM for radiation exposure should be easy to handle, stable under extreme conditions, thermoresistant, cheap, deployable, valid (quality assurance) and must meet medical needs for rapid diagnosis and therapeutic support. MCMs fall into three broad classes based on their administration in relation to radiation exposure () Citation[17]. Radioprotectors, also called prophylactic agents, are administered before radiation exposure to prevent radiation injury by acting during the initial radiochemical events; these include the free radical scavengers, amifostine and vitamin E isomers (tocols) Citation[18,19], which must be present at the time of radiation exposure. Radiation mitigators are administered after irradiation but before the appearance of overt evidence of injury, to accelerate recovery. Radiomitigators include ACE inhibitors and immunomodulators (captopril, 5-androstenediol [5-AED], IL-12 and CBLB502 [truncated flagellin]) Citation[12,13,20-22]. Radiation therapeutics or treatments are the agents given after symptoms manifest to stimulate and facilitate repair or regeneration of organ and tissue functions Citation[15,23-29]. G-CSF and GM-CSF fall under this category Citation[15].The efficacy of a MCM to mitigate lethality of irradiation may depend on the interval between irradiation and MCM administration. This article focuses on animal models useful in developing MCMs for radiation.
Figure 2. Terminology of therapeutic approaches in relation to radiation exposure is shown. Radioprotectors are administered before exposure and radiation mitigators are administered after radiation exposure during the prodromal/latent phase. Radiation therapeutics or treatments are given after symptoms manifest.
2. FDA Animal Efficacy Rule for the development of radiation countermeasures
In 2002, the US FDA issued what has become known as the ‘Animal Efficacy Rule’ (21 Code of Federal Regulations [CFR] Parts 314.600 – 314.650 for drugs and 21 CFR 601.90 for biological products). It was intended to expedite the development of new drugs and biological products as MCMs against chemical, biological, radiological and nuclear threats; the Animal Efficacy Rule applies only to new drugs or biologics for which definitive human efficacy studies cannot be conducted because it would be unethical to knowingly expose humans to lethal or debilitating stimuli, or for which field trials have not been feasible Citation[11,30,31].
The FDA may grant marketing approval for new products for which safety has been established and for those which meet the requirements of CFR Parts 314.600 – 314.650 or 601.90, based on adequate and well-controlled animal studies to establish that the drug is likely to produce clinical benefit in humans. The criteria of the FDA’s Animal Efficacy Rule relevant to development using animal models are stated below:
There is a reasonably well-understood pathophysiological mechanism of the toxicity of the agent (radiation) and of its prevention or substantial reduction by the drug.
The effect of drug/biologic is demonstrated in more than one animal species expected to react with a response predictive for humans, unless the effect is demonstrated in a single animal species that represents a sufficiently well-characterized animal model for predicting the response in humans.
The animal study’s end point is related to the desired benefit in humans, generally the enhancement of survival or prevention of major morbidity.
Pharmacokinetics and pharmacodynamics or other relevant data or information of the product, in animals and humans, allows selection of an effective human dose.
The mechanisms of radiation injury remain poorly understood (point 1). Good animal models with appropriate end points are needed to satisfy points 2 and 3. There are several approaches for the treatment of radiation injuries Citation[32]; as a result, it is highly unlikely that any single model system will be adequate for assessing all potential classes of agents. It is also unlikely that any one animal model will be appropriate for studying all sub-syndromes of ARS. Several animal models discussed below have been used in the development of the MCMs for ARS (, ) Citation[13,17,23,33,34]. Some investigators have used additional models, ferrets Citation[35-37], guinea pigs Citation[38,39] and rabbits Citation[40-43] to study radiation injury and evaluate MCMs. The availability of suitable, well-characterized animal models of ARS is critical for the development of novel MCMs.
Figure 3. Evaluation of MCM for ARS in various animal models is shown. Those drugs that have been evaluated in multiple species are only mentioned under the highest animal model used for evaluation to date. Some drugs have been evaluated using additional models that are not mentioned here.
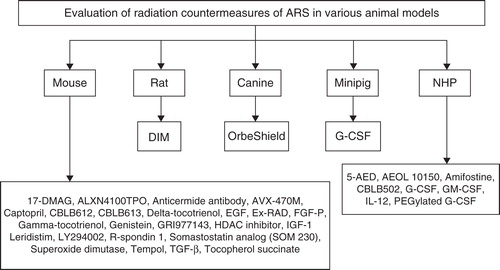
Table 1. Animal models of ARS for studying radiation injuries and development of medical countermeasures.
Animal models rarely reflect the human disease precisely; therefore, animal efficacy data will never be as convincing as the human efficacy data used for other drugs or vaccines. Without human efficacy data, it is more critical to understand how and why a MCM works to provide confidence that the MCM will be effective in humans. Substituting animals for humans in efficacy tests for novel MCM was not intended to make it easier to obtain FDA approval; in fact, more information is required about the animal model, the mechanism of injury and course of disease and the mechanism of action of the MCM than when efficacy studies can be performed in humans. The Animal Efficacy Rule does not completely eliminate the requirement of testing the drug in humans. Clinical trials are still required to evaluate the safety of the MCM and to help determine the appropriate dose of the drug for efficacy in humans. A different mindset and a consistent strategy are required for the approval of MCM that will be needed during a chemical, biological or radiation/nuclear defense emergency. The FDA also requires post-marketing efficacy studies if the MCM is ever administered in response to a radiation event. Therefore, as part of the FDA license application, pharmaceutical companies need to develop plans to execute such post-marketing clinical trials for drug efficacy – a process that will, undoubtedly, be extremely difficult in the midst of a national public health crisis.
The challenges of fulfilling the Animal Efficacy Rule requirements, compared with the traditional licensure pathway, may be one reason it has rarely been used to approve new drugs. Since 2001 only five drugs (not to combat ARS) have been approved, despite significant investment by the federal government, to promote development of MCMs against potential threats Citation[10,44-47].
3. Radiation injury
In humans, ARS manifests following exposure to total-body or partial-body (PB) irradiation, at doses > 1 Gy, delivered at a relatively high-dose rate. Clinical components of the ARS include hematopoietic (H-ARS) (2 – 6 Gy), gastrointestinal (GI-ARS) (> 6 Gy) and cerebrovascular (> 10 Gy) sub-syndromes Citation[48]. Although a broad range of radiation doses can cause both hematopoietic and GI injuries, their impact on radiation-induced mortality is dose- and time-dependent. The cerebrovascular syndrome is considered incurable; in contrast, individuals receiving lower radiation doses that result in hematopoietic or GI sub-syndromes are more likely to be amenable to MCMs. Therefore, these two sub-syndromes are specific targets for the development of novel MCMs. H-ARS is characterized by a severe loss of hematopoietic stem cells followed by cytopenia; GI-ARS is characterized by massive apoptotic cell death in the intestinal epithelium followed by disintegration of the mucosal epithelial barrier or intestinal wall and death from fluid and electrolyte imbalance, intestinal bleeding and sepsis. The lowest lethal doses cause severe bone marrow damage but only modest GI damage. These ‘sub-syndromes’ are somewhat oversimplified because multiorgan dysfunction is also involved Citation[49-51].
3.1 Effects of ionizing radiation
Ionizing radiation includes α- and β-particles, γ- and X-rays, neutrons, high-speed electrons, high-speed protons and other particles capable of producing ions. Radiosensitivity is the relative susceptibility of cells, tissues and organ systems to the injurious action of radiation. In general, it has been found that cell radiosensitivity is directly proportional to the rate of cell division and inversely proportional to the degree of cell differentiation Citation[32]. In brief, the most radiosensitive cells are those which have a high division rate, have a high metabolic rate and are of a non-specialized type. Cells in the S phase of the cell cycle are least sensitive, followed by the G1 phase, then the G2 phase, with the most sensitive being in the M phase.
High doses of ionizing radiation delivered to the whole body of healthy adults within short periods of time can produce acute effects. These effects will develop within hours, days or weeks, depending on the dose received; the larger the dose, the sooner a given symptom manifests. The acute effects apply when the whole body is relatively uniformly irradiated. The effects can be significantly different when only portions of the body or an individual organ system are irradiated. A dose of 5 Gy delivered uniformly to the whole body of a human may cause death, whereas a dose of 5 Gy delivered only to the skin will cause hair loss and skin reddening. The hematopoietic, GI, pulmonary, cutaneous and CNS are more susceptible to radiation injury than other organ systems Citation[34].
Of the five types of ionizing radiation mentioned above, only exposure to neutrons can turn nonradioactive objects radioactive. Several MCMs have been evaluated for efficacy against neutrons. G-CSF has been evaluated as a MCM against mixed field radiation (neutron and γ-photon) in the mouse model Citation[52]. Although G-CSF was effective as a mitigator, the thrombopoietin mimetic, ALXN4100TPO (effective against γ-irradiation), was ineffective against mixed field, thus suggesting that MCMs should be tested against a variety of radiation qualities using appropriate animal models Citation[53]. Glycosylated IL-6 was tested in baboons exposed to mixed field (5 Gy, neutron:γ ratio of 5.5) and findings provided the rationale for the use of IL-6 as a therapeutic agent for the treatment of thrombocytopenia in nonhuman primates (NHPs) at a clinically well-tolerated dose Citation[54]. Additional MCMs have been tested for efficacy against mixed field using combined injury (irradiation plus skin wounding) murine model Citation[55].
3.2 Differences in radiosensitivity based on animal strains
Mouse strain differences in radiosensitivity and survival have long been observed Citation[56] and represent innate immune response and enteric flora differences. Reports indicate that changes in the bacterial population can impact radiosensitivity and survival Citation[57,58]. Recently, significant strain-related differences in radiation-induced pulmonary damage among the C57L/J, CBA/J and C57BL/6J strains have been reported Citation[59-61]. The lungs of C57L/J mice are quite analogous to the lungs of humans and NHPs in respect to the radiation dose response and time course of pathological damage following thoracic irradiation. CBA/J mice remain an excellent model for studying radiation-induced pneumonitis without contracted fibrosis. C57L/J and CBA/J strains may elucidate pneumonitis and fibrosis mechanisms. Strain differences can be exploited to better understand the molecular mechanisms of radiation-induced injury and evaluation of MCM Citation[61].
3.3 Dose and dose rate of irradiation
The dose rate will determines the biological consequences of a given absorbed dose; as the dose rate is increased and exposure time decreases, the biological effect of a given dose (X-rays or γ-rays) usually increases and the sooner the cell injury response will appear Citation[32]. Repair times range from 1 to 24 h; therefore, irradiations that take place over the course of hours or days are likely to have less effect per unit dose than irradiations that take place in seconds or minutes.
3.4 Effect of age at the time of radiation exposure
Age, at the time of exposure, is an important effect modifier for acute irradiation, but there is a paucity of data on age-dependence for early health effects. The young and old experience the most definitive symptoms at somewhat lower doses than adults. Lower radiation doses are required to induce the GI-ARS in younger mice compared with older mice. This is related to the difference in the radiosensitivity of the crypt clonogenic population in young animals Citation[62]. The effects of MCMs under evaluation on pediatric, juvenile and aged populations are highly relevant and must be studied.
Developmental abnormalities are significant only for embryos, fetuses and newborns Citation[63]. In Japan, the studies of children who were exposed in utero at the time of the nuclear detonations in 1945, have shown that the highest risk of severe mental retardation caused by irradiation exposure lies between weeks 8 and 15 of gestation, with lower risks occurring at weeks 16 through 25; the lowest risks occur before and after these periods Citation[63]. Age is a key variable for the late effect of carcinogenesis, with the young suffering higher risks than those irradiated as adults.
3.5 Influence of sex on the effects of irradiation
Sex does not emerge as a key effect modifier for early health effects but does make a difference for late effects. In particular, female breast tissue is highly radiosensitive Citation[64], and the female’s thyroid is twice as sensitive to radiation carcinogenesis as the male’s thyroid Citation[64,65]. Several studies have used only male animals to eliminate the impact of the estrus cycle in response to radiation Citation[62]. The differential effects of MCMs on outbred animals of either gender are relevant.
3.6 Uniformity of irradiation (whole-body and PB, unilateral versus homogeneous irradiation)
Individuals may receive fairly uniform whole-body irradiation, as was the case for many of the Japanese survivors of the nuclear detonation, but non-uniform irradiation has been observed in many accidents, medical procedures or following the intake of radionuclides Citation[66,67]. Accidental exposures to external sources usually results in heterogeneous rather than homogenous dose distribution, particularly when only parts of the body are much closer to a source than others Citation[68,69]. Inhalation of insoluble α- and β-emitters may lead primarily to lung irradiation, whereas dermal contact with β-emitting radioactive materials may result in irradiation of the skin.
The hematological effects of PB irradiation (unilateral or bilateral) of various regions of the body have been studied extensively using various species such as mice Citation[70], rats Citation[71], rabbits Citation[72], canines Citation[73-75] and NHPs Citation[76]. At doses of irradiation, capable of inducing the GI-ARS, survival increases significantly if an estimated 40% of the bone marrow is shielded (head, thorax and forelimbs – PB BM40) from irradiation Citation[62]. This strategy of PB irradiation has been extensively studied. There are several reports of mice and NHPs where 5% bone marrow has been spared (PB BM5) which contributed to rescue animals from H-ARS Citation[62,77]. GM-CSF efficacy has been evaluated in NHPs using non-uniform total-body irradiation (TBI), demonstrating early recovery of granulocyte-macrophage colony-forming unit activity Citation[76].
3.7 Radiation syndromes
Since most of the MCM are being developed for specific sub-syndromes, it is logical to discuss these syndromes.
3.7.1 Hematopoietic ARS
H-ARS, defined as the development of neutropenia, thrombocytopenia and anemia, appears at the lowest end of the dose range resulting in injury; it occurs due to the radiosensitivity of committed progenitor cells in these lineages Citation[3,78-80]. Doses of ≥ 2 Gy result in decreased lymphocyte counts and immune suppression, making victims susceptible to secondary infections. Exposure may result in bone marrow failure and lethal hemorrhage or infections. Without recovery or treatment, death may occur within 2 – 4 weeks; therefore, protection or reconstitution of the hematopoietic systems is a major concern in the development of MCMs Citation[81,82].
The time it takes for each symptom to manifest is dependent on the rates of stem cell maturation and differentiation. The time until symptom manifestation, the extent of the cell count nadir and the recovery time (return to baseline) for each cell lineage have all been used as secondary end points to assess the extent of hematopoietic injury. Pluripotent hematopoietic stem cells located in the bone marrow are capable of surviving doses of radiation that typically result in H-ARS; however, as the dose increases, the fraction of surviving stem cells is reduced and the recovery of the multiple blood cell lineages is increasingly delayed. MCMs for H-ARS treatment expedite the recovery of the multiple blood cell lineages in various irradiated animals ( and ).
Figure 4. The effect of GT3 administration on sternal cellularity and megakaryocytes in irradiated mice is shown. GT3 was administered 24 h before irradiation. Mice were harvested 24 h post-irradiation for sterna. After fixation and processing, sternal bone marrow was stained (hematoxylin and eosin staining) to assess cellularity and megakaryocytes. Representative areas are shown above (100×, and an enlarged section of each photomicrograph at 400× magnification). GT3-treated sample has better cellularity compared with vehicle control.
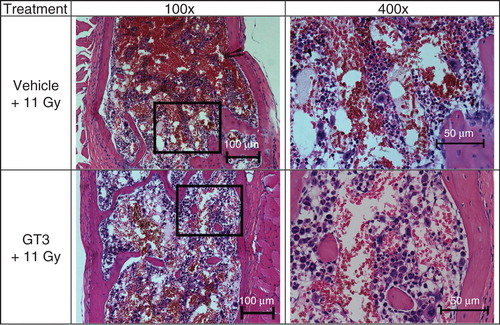
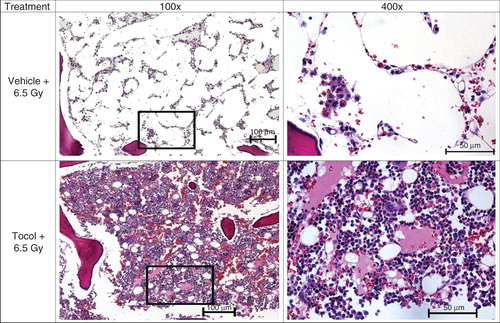
Figure 5. Effects of tocol treatment on bone marrow cellularity in irradiated nonhuman primates (NHPs) are shown. NHPs were treated with tocol or vehicle 24 h before 6.5 Gy whole-body 60Co γ-radiation. Sample of vehicle-treated NHP was collected on day 17 as a result of moribundity and tocol-treated NHP sample was collected on day 60 post-irradiation at the time of termination of the experiment. Sternum samples were collected, and after fixation were processed for hematoxylin and eosin stained sections to assess the cellularity. Representative areas are shown above (100×, and an enlarged section of each photomicrograph at 400× magnification). Tocol-treated NHP sample has better cellularity compared to vehicle control.
The damaging effects of radiation on hematopoiesis have been well established and animal models have been highly valuable in characterizing them Citation[3,17,33,34,78]. Multiple animal models of H-ARS have been developed to study MCMs, although the three that have been used in the majority of H-ARS studies are mouse, canine and NHPs Citation[17,21,33]. The most widely used and well-characterized end point for H-ARS assessment after TBI is the LD50, defined as the lethal dose that results in the death of 50% of the affected population. In mice, development of bone marrow syndrome is significantly faster, with half the population dying within 30 days of irradiation (LD50/30); for larger animal models, a 45- to 60-day period is considered (LD50/60) Citation[83]. The peak incidence of human death occurs around 30 days post-exposure, although it may continue up to 60 days.
3.7.2 Gastrointestinal ARS
The GI tract is considered to be particularly sensitive to radiation exposure. Intestinal radiation injury, consisting of enterocyte depletion, mucosal barrier breakdown and mucositis with secretory diarrhea, occurs as a consequence of many concurrent and sequential pathophysiological events. Other elements of the intestine also contribute to system dysfunction: the enteric muscularis, immune system, microvasculature and nervous system and the resident bacteria and fungi, although classical GI injury is attributed to death of clonogenic crypt epithelial stem cells Citation[84]. The pathological aspect of GI injury emphasizes protein modifications, changes in redox status, secondary effects due to inflammation and release of cytokines and the functional consequences of cell loss Citation[85]. Cellular compartments may contribute to and modulate organ dysfunction, but the key event in the pathophysiology of GI injury is enterocyte depletion, with vascular damage possibly contributing at higher radiation doses Citation[86]. The physiological approach emphasizes early vomiting and diarrhea as common GI-related symptoms of exposure which greatly exacerbate the problem of fluid and electrolyte loss, which may result in death Citation[32]. Even at radiation doses below the threshold for GI syndrome, mucosal barrier breakdown allows bacteria to translocate into circulation, which can cause sepsis and death in the setting of concomitant immune suppression Citation[87]. Long term tissue remodeling after radiation damage alters the structure, motility and absorption of the gut; fibrosis renders it more rigid and susceptible to adhesions, stenosis and perforation Citation[88].
Lethality as a result of intestinal failure is the primary end point for GI syndrome studies. The current end point for studying acute GI syndrome is animal lethality within 10 days after radiation exposure (LD50/10). The classical histological end point in mice is the number of regenerating crypts measured in the intestine at defined times (small intestine: 3.5 days for mouse) ( and ); intestinal crypt cell survival does not necessarily correlate with animal survival due to the contribution of possible concurrent damage to the immunohematopoietic system Citation[89]. Functional assays of GI injury include GI motility and permeability, bacterial translocation into the blood stream and plasma levels of citrulline Citation[90-92]. Radiation-induced vomiting as an end point has been studied extensively in canines; however, the mechanisms underlying this symptom may vary between models because they do not directly correlate among canines, NHPs or humans Citation[93-96]. Abdominal irradiation has been shown to triple the frequency of colonic giant migrating contractions, with more than half originating in the small intestine resulting in diarrhea Citation[97,98].
Figure 6. Effect of progenitor cells administered after irradiation on jejuna tissue area, crypt number, villus height and number, mitotic figures and length of basal lamina is shown. Photomicrographs of jejunal cross-sections are shown demonstrating the effect of progenitor cells administered 2 h after irradiation on jejuna tissue area, crypt number, villus height and number, mitotic figures per high power field and length of basal lamina containing five enterocytes 4 and 8 days after 13 Gy irradiation. CD2F1 male mice were irradiated with a dose of 13 Gy 60Co γ-radiation and transfused with 6 million progenitors or vehicle 2 h post-irradiation through para-orbital sinus injection. Tissue samples were harvested on days 4 and 8 post-irradiation, fixed and processed. Cross-sections of jejunum (4 µm) were stained (hematoxylin and eosin). Representative areas are shown (circumference at ×40, villus height at ×100, mitotic figure at ×1000 and basal lamina at ×600 magnifications) in photomicrographs: (A) circumference, (B) villus height, (C) mitotic figures and (D) basal lamina.
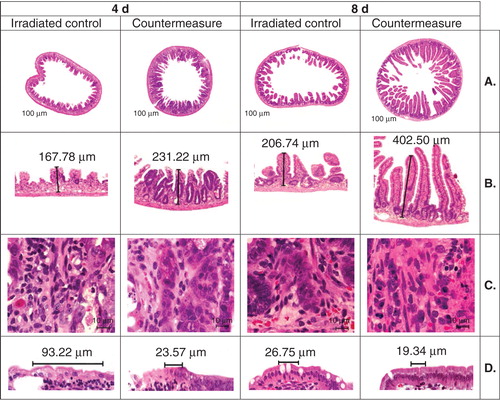
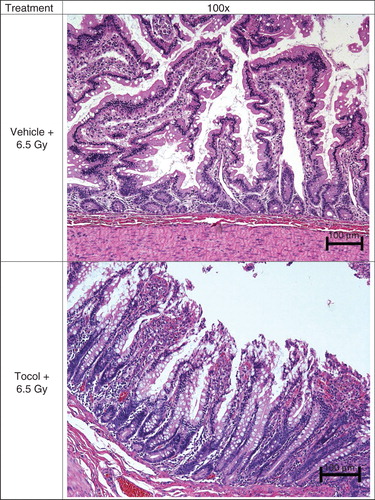
Figure 7. Effect of tocol treatment on jejunal tissue in irradiated nonhuman primates (NHPs) is shown. NHPs were treated with tocol or vehicle 24 h before 6.5 Gy whole-body 60Co γ-radiation. Jejunal samples of vehicle-treated NHP were collected on day 17 as a result of moribundity and tocol-treated NHP samples were collected on day 60 post-irradiation at the time of termination of the experiment. Tissue samples were fixed and processed for hematoxylin and eosin stained sections for assessment. Tocol-treated NHP jejunum has better structural organization cellularity compared with vehicle control.
3.7.3 Pulmonary syndrome
The lung is a very sensitive organ which, when exposed to radiation, may result in acute and chronic inflammation that can lead to fatal lung fibrosis Citation[99]. The majority of animal models display a higher tolerance to pulmonary radiation than humans. Lung injury manifests within few months after irradiation; inflammatory pneumonitis develops within 2 – 4 months after irradiation and fibrosis is observed after 4 – 6 months Citation[100-103]. Histologically, pneumonitis is characterized by interstitial and airspace edema, inflammatory infiltrate of predominantly macrophages and loss of epithelial cells Citation[99]. Although clinical symptoms may not develop for weeks to months, radiation immediately triggers a cascade of molecular and cellular events that proceed during a clinically latent period; this process involves endothelial cells, macrophages, epithelial cells, fibroblasts, proinflammatory and profibrotic cytokines and stimulation of various gene expression and transcription factors Citation[104,105].
There are several models under development for studying pulmonary effects Citation[106-108]. The mouse is one of the most characterized species in terms of pulmonary injury pathogenesis; however, within the species, there is a great deal of variability in histopathological sequelae and severity of lung injury between strains in response to thoracic irradiation Citation[59-61,109]. Rodent lung pathology differs from that of human in respect of lobularity, relative thickness of the septa and pleura and blood supply Citation[103]. The three large animal models, swine, canines and NHPs, have their advantages and disadvantages for studying pulmonary injury. The swine lung has a similar physiology to human but there are limited data available for this model using either PB- or whole-body irradiation Citation[110,111]. There is a wealth of information on the radiation-induced pulmonary response of canines Citation[112], although the physiologies differ from that of humans. Despite the close similarities in the physiology of the NHPs to humans, limited information is available in regard to radiation-induced pulmonary injury Citation[34]. There is a recent report of delayed lung injury using rhesus NHP; the thorax was exposed to photon radiation and medical management was administered according to a standardized treatment protocol; primary end point was mortality at 180 days post-irradiation. A comparative multiparameter analysis was provided, focusing on the lethal dose–response relationship characterized by a LD50/180 of 10.27 Gy (9.88, 10.66) and slope of 1.112 probits per linear dose Citation[113].
Since this pulmonary sub-syndrome may be associated with high morbidity and mortality, treatment following high-dose irradiation will ideally include treatments that will also delay pulmonary syndrome Citation[114-116]. Still, existing models need to be validated for radiation damage to the lungs to better understand the mechanisms responsible for the radiation damage as well as cellular and organ responses to the injury Citation[117]. The NHP is the model of choice for studying pulmonary syndrome, although the canine model also satisfies the large animal model requirements.
3.7.4 Cutaneous radiation syndrome
Accidental exposure of the human skin to ionizing radiation > 3 Gy results in a distinct clinical representation, which is characterized by a transient and faint erythema after a few hours, followed by severe erythema, blistering and necrosis. Depending on severity of damage, the latter generally occurs 10 – 30 days after exposure but, in severe cases, it may appear within 48 h. The therapeutic approaches should include topical and systemic anti-inflammatory measures at the earliest conceivable point and should be maintained throughout the acute and subacute stages, as this reduces the need for surgical intervention, once necrosis has occurred. Antifibrotic measures in the chronic stage addresses the chronic inflammatory nature, during which overexpression of TGF-β may be a target for therapeutic intervention Citation[118]. The recent clinical strategy relies on excision based on dose reconstruction aimed at preventing deleterious recurrent necrosis and then autologous keratinocyte therapy combined with allogeneic stem cell injections Citation[119,120]. There has been an explosion of interest in use of mesenchymal stem/stromal cells, progenitors and adipose stromal cells as tools for cell and gene therapy of the H-ARS and cutaneous radiation syndrome Citation[119-127]. The swine is widely used as a large animal model to study the skin effects of radiation exposure. The cutaneous data for NHPs are limited Citation[34].
3.7.5 Cerebrovascular syndrome
Cerebrovascular symptoms only occur at whole body doses in excess of 10 Gy. Also known as neurovascular sub-syndrome, it results from localized changes in the CNS, including impaired capillary circulation, damage to the blood–brain barrier, interstitial edema, acute inflammation, petechial hemorrhages, inflammation of the meninges and hypertrophy of perivascular astrocytes Citation[17,33,34]. Signs and symptoms include persistent and severe nausea, vomiting, accompanied by headache, neurological deficits, disorientation, confusion, loss of balance and seizures. Physical examination may show papilledema, ataxia and reduced or absent deep tendon and corneal reflexes Citation[128]. As previously stated, because damage resulting from such extremely high radiation exposure has been deemed untreatable, the scientific community has focused its efforts on finding preventative and mitigating treatments for only hematopoietic, pulmonary and GI syndromes of ARS.
3.8 Animal models suitable for the various components of ARS
The choice of animal model depends on the specific pathophysiological process being studied. Rodent models lack prodromal manifestations, but they are outstanding models for studying enterocyte depletion, changes in the absorption and the secretion of the bowel and bacterial translocation from gut to circulation. Another major advantage using murine model is the availability of large number of strains. For studies of MCMs to combat GI syndrome, the mouse is the preferred small animal model. Irrespective of radiation dose or mouse strain, diarrhea begins 3.5 – 5 days post-irradiation Citation[62]. The severity of diarrhea rather than the timing of the onset is radiation dose-dependent. There is a direct correlation with the timing of the diarrhea within that time range and evidence of sustained loss of mucosal integrity. The mouse is also the preferred small animal model for studying pulmonary and cutaneous syndromes Citation[34]. Murine models provide greater opportunity for mechanistic studies due to the availability of genetically modified animals. For example, Smad3 knockout mice are protected against radiation-induced cutaneous injury, suggesting involvement of TGF-β pathway Citation[129].
The minipig model is potentially more useful than the rodent model, but not the canine or NHP, for evaluating radiation-induced clinical symptoms of GI syndrome such as vomiting or diarrhea, as rodents do not manifest these symptoms. The swine GI tract has similar physiological parameters as humans, including transit time and pH value, and swine are less prone to emesis when compared to canines Citation[130]. The small size, the defined microbiologic and genetic backgrounds and docility of the Göttingen minipig are additional advantages for H-ARS studies. The pig is also widely used as a large animal model to study the skin effects of radiation exposure Citation[131]. Pig skin is generally considered to be the most similar to human skin Citation[132].
Prodromal syndrome with vomiting is best studied in the canine or ferret. Ferrets exhibit a radiosensitivity similar to that of humans and display the same prodromal symptoms of retching, vomiting and diarrhea Citation[133]. Canine model is suitable for GI injury studies where vomiting is used as an end point; however, the mechanisms of radiation-induced vomiting and delayed gastric emptying do not correlate with NHPs or humans Citation[134-139].
NHPs and canines are the most widely used large animal models to study GI injury Citation[77,136]. Both NHPs and canines are capable of vomiting and experiencing diarrhea; their associated GI tract contractions can be used for monitoring response to drugs that are used to mitigate radiation sickness. Vomiting and diarrhea are important considerations in the development of mitigating agents since they exacerbate fluid losses and limit the use of oral mitigating agents. There is a recent report suggesting that the minipig may be used as a model for the human GI-ARS Citation[130]. However, there are no definitive reports that describe the human GI-ARS in response to acute radiation exposure. NHP is the preferred large animal model for pulmonary syndrome Citation[34].
4. MCMs development for the ARS using various animal models
There is a large number of MCMs for ARS at various stages of development. In this article, we have focused on the development of promising agents using various animal models (). The objective of this article is to update the readers with the pathway used for developing MCMs () and to present the status of selected MCMs at advanced stages of development. Rodent, canine and NHP models are used most frequently for the development of MCMs. Some studies have used additional animal models, such as minipigs and ferrets, to evaluate the effects of radiation Citation[36,130,140-142].
4.1 Rodents
The mouse is the most frequently used animal model for medical research. Rats have been used in relatively limited number of studies for MCM development. Among the many advantages of using the mouse as an animal model, the most important is their anatomical, physiological and genetic similarity to humans; over 95% of the mouse genome matches the human genome. The mouse body size, short generation time and an accelerated lifespan (one mouse year equals about 30 human years), provides advantages relative to cost, space and time required to perform respective studies. Additionally, more research reagents are available for mice compared to any other animal model. The main disadvantage of the rodent models is the small body thickness, which does not account for the intrinsic heterogeneity of radiation dose distribution inherent to human exposure. The majority of investigators have employed inbred mouse strains, although several have used hybrid strains Citation[17,33,34]. The most common mouse strains used are BALB/c, C3H/HeN, B6D2F1/J, CD2F1 and C57BL/6. These strains show considerable variation in their response to irradiation, as demonstrated by their range of LD50/30 values (6.5 – 9.0 Gy), with the BALB/c being most sensitive and the C57BL/6 being the most resistant (‘drift’ in the dose–response relationship and LD50/30 may occur in any laboratory). The BALB/c strain has been reported to have a double-stranded DNA repair defect that may account for its enhanced radiation sensitivity Citation[143]. Thymocytes, splenocytes and crypt cells of C57BL/6 mice are more sensitive to radiation-induced apoptosis than those of the C3H/HeN Citation[34]. The mouse is deemed the most appropriate species for testing MCMs in a small animal due to the breath of the current literature. A large number of MCMs for ARS have been evaluated in murine models () Citation[13,18,144]. The strain differences outlined above suggest that MCMs should be tested in more than one mouse strain, preferably the C57BL/6 and C3H/HeN.
4.2 Canine
The beagle has been favored as the canine model for radiation studies; the canine hematopoietic and immune systems are similar to that of humans. There is a wealth of information on the pulmonary response in the canine model, of which a significant proportion was performed using inhaled radionuclides, although there are no published models of acute radiation-induced lung injury in the canine Citation[34]. However, as with rodents, the physiology of the canines differs from that of the humans; some investigators group the canine and rodent models in respect to pulmonary anatomy and function. There is a large database and well-characterized model of H-ARS using different radiation quality (cobalt-60, X-ray, mixed field neutron:γ), dose rate and PB irradiation, with and without the use of medical management in the beagle Citation[17,33,34].
OrbeShield (beclomethasone dipropionate) is being developed by Soligenix, Inc. (Princeton, NJ, USA) for the treatment of GI-ARS. OrbeShield has been tested in the canine model of GI-ARS and demonstrated a survival benefit Citation[145]. Canines received TBI (12 Gy, 0.7 Gy/min, Clinac600 linear accelerator), followed by autologous bone marrow infusion and supportive care. This study demonstrated that OrbeShield when administered 2 or 24 h after exposure to lethal dose of TBI provided significant survival benefits Citation[146]. This study also suggests that OrbeShield has the potential to rescue inflamed tissues in the radiation-damaged GI mucosa and improve survival when therapy is initiated as late as 24 h after high-dose irradiation. This is a promising study demonstrating significant efficacy in the canine model, although these results have not been published in a peer-reviewed journal. Amifostine and G-CSF have also been evaluated in the canine model and demonstrated beneficial effects against radiation injury Citation[134-136,147,148].
4.3 Göttingen minipig
Swine (specifically Göttingen minipig) have become the more frequently used large animal models in medical research procedures and are now regarded as an appropriate animal model for drug validation studies Citation[124,125,130]. The body thickness of a minipig is comparable to a young human, resulting in radiation absorption patterns that are quite similar. The pathophysiology of H-ARS in the minipig is similar to that observed in humans, NHPs and canines, although the animal is very sensitive to TBI with an LD50/30 less than the canine and that estimated for the humans Citation[149,150]. The LD50/30 of minipig is 1.73 Gy Citation[149], which is due to its abnormally high radiosensitivity of its vascular/endothelial cells. This raises questions concerning the possibility of genetic defects in DNA repair and may limit its utility as a model for human ARS.
The attributes described above make the minipig an additional large animal model to NHPs and canines for studying the radiation-induced H-ARS as well as potential MCM efficacy. The minipig model has recently been used to validate the efficacy of G-CSF as a mitigator/MCM for the radiation-induced H-ARS. It was determined that G-CSF enhanced survival, stimulated recovery from neutropenia and induced mobilization of hematopoietic progenitor cells Citation[151]. There is a report studying high-dose radiation-induced GI syndrome in minipig model using a small number of animals Citation[130]. This study used 10 and 15 Gy abdominal irradiation (60Co γ-radiation, 1.4317 Gy/min) to suggest that the minipig mimics human GI syndrome. Plasma citrulline levels were also evaluated as a biomarker for radiation-induced intestinal damage. Another report used 5 – 12 Gy TBI (60Co γ-radiation, 0.6 Gy/min) in Göttingen minipig to study GI-ARS Citation[152] and demonstrated a dose-dependent occurrence of parameters associated with GI-ARS (plasma citrulline, diarrhea, vomiting, bacterial translocation and intestinal crypt loss). This exploratory study used only two to six minipigs per radiation dose, and therefore has its limitations. Most minipigs died within 10 days post-irradiation, suggesting supportive care is a necessity to extend survival time to allow sufficient time to assess crypt epithelial cell transit time and evaluate countermeasure efficacy. There is still no comprehensive study defining the GI-ARS dose–response relationship and survival/probit curve for acute TBI in the minipig.
4.4 Nonhuman primates
The NHP model is considered the gold standard of animal models; it has ≥ 95% DNA sequence identity with humans, and a high degree of similarity in terms of receptors and pathways of physiological responses. This model most closely reproduces the clinical, histopathological and pathophysiological aspects of radiation injury in humans. Due to the longer lifespan and similar supportive care requirements, it is possible to link the dose–effect relationships between the NHPs and humans. In addition to rhesus macaque, several studies have been conducted using baboons to evaluate radiation injuries as a result of X-rays Citation[153], 60Co γ-photons Citation[154], and mixed field exposure Citation[54]. Promising MCMs under development for H-ARS have been tested in NHPs for efficacy and pharmacokinetics/pharmacodynamics. A few important agents at advanced stages of development are discussed below.
4.4.1 Growth factors: G-CSF, GM-CSF and PEGylated G-CSF
G-CSF (filgrastim, Neupogen) is an agent which has been evaluated for efficacy against radiation injuries in animal models across four species Citation[13,15,23,135,155]. G-CSF has also completed a good laboratory practice (GLP) compliant study in the NHP model to show that G-CSF increases survival after exposure to 7.5 Gy (LD50/60) of linear accelerator (LINAC)-derived photon radiation Citation[24]. In this study, G-CSF (10 μg/kg/day) was administered beginning 1 day after irradiation and continued daily until the absolute neutrophil count was > 1000/μl for 3 consecutive days. All NHPs received supportive care including blood products. The primary end point was overall survival over the 60-day in-life study. Secondary end points included mean survival time of decedents and all hematologic-related parameters. G-CSF significantly reduced overall mortality at 60 days compared to the controls. G-CSF also decreased the duration of neutropenia but did not affect the absolute neutrophil count nadir. G-CSF, administered at this dose and schedule, effectively mitigated the lethality of the H-ARS. G-CSF administration initiated 48 h after irradiation did not improve NHP survival Citation[156]. During a recently conducted FDA meeting, members overwhelmingly voted (17:1) to support the concept that G-CSF will produce clinical benefits to humans who have been exposed to radiation with doses capable of inducing myelosuppression Citation[157].
PEGylated G-CSF (Pegfilgrastim/Neulasta) can be administered in a reduced schedule and still retain the therapeutic benefits comparable to the more extensive dosing regimens required for G-CSF. Two weekly injections of PEGylated G-CSF are equivalent or significantly better in virtually all measured parameters (survival, granulopoiesis) compared to 17 – 21 days of daily filgrastim injections Citation[158,159].
Unlike G-CSF, GM-CSF (sargramostim, Leukine) is more species-specific. GM-CSF enhances the recovery of blood leukocyte levels in NHPs when administered alone or in combination with other cytokines Citation[76,160,161]. Similar to G-CSF, GM-CSF administration decreased the severity and duration of neutropenia, enhanced neutrophil recovery and overall blood leukocyte counts, and increased granulocyte-macrophage colony-forming units in the bone marrow of NHPs.
4.4.2 CBLB502
CBLB502/Entolimod is a recombinant protein (truncated flagellin) that acts as an agonist of TLR 5, an innate immunity receptor. Studies conducted with CBLB502 by Cleveland BioLabs, Inc. (Buffalo, NY, USA) using the NHP model suggested that it may be a promising treatment for lethally irradiated humans, due to an extended therapeutic time window for drug administration after radiation exposure Citation[162,163]. Cleveland BioLabs has also conducted CBLB502 studies in NHPs under GLP compliance. A single administration of CBLB502 administered either before or after lethal TBI leads to a significant improvement in animal survival and neutropenia. CBLB502 reduces radiation damage of both hematopoietic and GI tissues and improves tissue regeneration Citation[162]. Using rodents, canines and NHPs, two cytokines (G-CSF and IL-6) have been reported as candidate biomarkers of CBLB502’s radioprotective/mitigative efficacy Citation[163].
4.4.3 IL-12
Currently, recombinant human IL-12 is being developed as a radiomitigator by Neumedicines, Inc. (Pasadena, CA, USA) under the name HemaMax. Allometrically equivalent doses of mouse and human HemaMax had similar pharmacokinetics and significantly increased mouse and NHP survival when administered 24 h post-irradiation when no supportive care (including fluids and blood products) was provided Citation[164]. A Phase II equivalent study (randomized, double blinded, GLP) demonstrated that single administration of IL-12 to NHPs significantly increased survival and reduced radiation-induced hematopoietic injury when administered 24 h post-irradiation. Administration of IL-12 promoted multilineage hematopoietic recovery and immune function in NHPs. In another study by the same group, G-CSF failed to demonstrate efficacy in the NHP model. Further, administration of G-CSF failed to improve the survival benefit already provided by IL-12 in NHPs Citation[165]. However, this report conflicts with the substantial and consistent clinical database and preclinical data in four species relative to the efficacy of G-CSF in mitigating radiation-induced myelosuppression and mortality. The cause for the disparity in results remains to be assessed. In both studies of IL-12 in NHPs, a radiation dose of 7.0 Gy was used. Neumedicines is developing IL-12 for the treatment of H-ARS for a Biologic License Application submission to the FDA under the Animal Efficacy Rule Citation[166].
4.4.4 AEOL 10150
AEOL 10150 (mesoporphyrin mimetic) is a novel, well-tolerated (as evidenced in initial clinical trials) antioxidant with significant protective and mitigative potential relative to ARS, particularly acute pulmonary injury Citation[167-171]. Results of a pilot efficacy study conducted in NHPs suggested that daily administration of AEOL 10150 (5 mg/kg for 4 weeks), initiated 24 h after irradiation (11.5 Gy whole thorax lung exposure, 0.8 Gy/min), improved survival and effectively mitigated potentially fatal radiation-induced lung injury Citation[172]. Exposure of the whole thorax to 11.5 Gy resulted in radiation-induced lung injury in all animals through the 180-day study duration. AEOL 10150 treatment resulted in 28.6% survival following radiation exposure that proved to be 100% fatal in the control cohort. Plasma analysis suggested that AEOL 10150 treatment led to lower TGF-β1 levels. All animals received supportive care, including dexamethasone, based on clinical signs during the planned 180-day in-life phase of the study. The results of this study demonstrate that treatment with AEOL 10150 results in reduced clinical, radiographical, anatomical and molecular evidence of radiation-induced lung injury and merits further investigation as a MCM against radiation-induced pulmonary syndrome.
4.4.5 5-AED
5-AED has been investigated as a radioprotector and radiomitigator (Hollis-Eden Pharmaceuticals, San Diego, CA, USA) Citation[173,174]. The radiomitigative efficacy of 5-AED was confirmed in NHPs exposed to either 4 or 6 Gy of TBI; both of these radiation doses are capable of inducing only H-ARS. 5-AED treatments improved overall blood profiles, including blood platelet levels Citation[173,174]. There is no report for efficacy of this agent against GI syndrome in NHPs.
4.4.6 Amifostine
In addition to mice and canines, amifostine (WR2721, Ethyol) has been found to be effective in NHPs following intravenous and intraperitoneal administration Citation[175,176]. However, oral administration of amifostine before irradiation failed to protect NHPs or canines. This is the only effective radioprotective agent that has been fully approved for human use by the US FDA Citation[177-179], although it has only been authorized for use for a very narrowly defined medical indication, namely the reduction of xerostomia (dry mouth) that results from salivary gland injury in patients undergoing radiotherapy for the treatment of head and neck cancer patients Citation[180].
5. Conclusion
There are several promising MCMs under advanced stages of development such as CBLB502, G-CSF and HemaMax (IL-12). There are seven agents which have received US FDA IND status, which permits clinical trial initiation Citation[23]. The following drugs, 5-AED, AEOL 10150, CBLB502, G-CSF, GM-CSF, PEGylated G-CSF and IL-12 have been evaluated in the NHP model Citation[13,76,158,159]. As stated above, the Center for Disease Control currently holds both IND and EUA applications with the FDA for the use of G-CSF in the event of a nuclear or radiological incident Citation[16], and the US federal government has procured G-CSF and GM-CSF to be available in the SNS under the Pandemic and All-Hazards Preparedness Reauthorization Act of 2013 Citation[181].
There are large numbers of promising MCMs that have been evaluated in the murine model; several of them are moving forward with an objective to get FDA approval Citation[13,21,23]. Several strains of mice have been used for investigating the efficacy and mechanisms of action of these MCMs. The rat model has also been used for the evaluation of few MCMs such as 3,3′-diindolylmethane Citation[144].
The Animal Efficacy Rule imposes the additional burden on investigators to establish a candidate drug’s mode of action in at least one animal model representing an accurate human pathology of ARS. Because this pathway is uncommonly used, the FDA currently provides only general guidelines for navigating this regulatory mechanism to drug sponsors and other investigators. Availability of suitable animal models is one of the limiting factors in developing MCMs utilizing the Animal Efficacy Rule. Development of adequate large animal models will facilitate and also expedite the development and approval of agents currently being investigated and newly discovered.
For studying radiation injury, only two large animal models (NHP and canine) have been well characterized Citation[33,34]. Canines have been recommended for radiation-induced GI injury studies and NHPs are preferred for pulmonary studies; renal dysfunction has been demonstrated in both models Citation[34]. Additional long-lived large animal models for ARS need to be developed and validated, to facilitate advanced development of MCM. Swine have been suggested as a promising species for drug evaluation Citation[33]. Recent studies with a minipig model of H-ARS have started to characterize this model Citation[130,151], however, additional studies are required to characterize this model for acute GI-ARS. It may prove to be an additional model to assess MCM efficacy for the H-ARS.
6. Expert opinion
There are several standardized animal models available to compare the relative efficacy of radioprotector, mitigator or therapeutic MCMs that are consistent with FDA Animal Efficacy Rule requirements Citation[157]. Although the Animal Efficacy Rule allows approval based on a single sufficiently well-characterized animal species, the usual expectation is that efficacy will be demonstrated in more than one species. All studies intended to support approval under the Animal Efficacy Rule must be carried out under the procedures and controls outlined in FDA’s GLP for nonclinical laboratory study regulations (21 CFR Part 58) Citation[157].
Animal models are required to show efficacy, mechanisms, natural history, pharmacokinetics and pharmacodynamics, and identify and validate organ-specific biomarkers. Rodent models are useful for identifying potential MCMs and can be used to study their mechanisms of action and potential biomarkers, whereas NHPs, canine and porcine models are useful for validation of the efficacy of the MCM and biomarkers. Therefore, multiple animal models are utilized to comprehensively investigate any one MCM before final approval. While developing new animal models, consideration should be given to the assessment of radiation damage and treatments for special populations based on age, gender, immune suppression and other underlying clinical conditions. Animal models are needed to provide a better understanding of the basic mechanisms of radiation injury which will be helpful in designing a novel class of MCM. The NHP model, preferred by the FDA, provides an avenue for both acute and long-term studies of multiple organ syndromes or late/delayed effects and is possibly the most relevant model to the human exposure response. NHPs are necessary for preclinical development because of the similarities of drug metabolism and physiology between NHPs and humans. Previous experience has shown that canine pharmacokinetics are not always relevant to primate efficacy Citation[182]; consequently, the NHP is generally the large animal model of choice for toxicity, pharmacokinetics, biomarker, radiation injury and MCM investigations.
Early discussions with the FDA are important to identify the appropriate, validated animal models and study end points that would yield acceptable, convincing data to approve the MCMs by the FDA. Before conducting Phase I clinical trials of new therapeutic agents, an application for an IND must be submitted to FDA. The FDA reviews the application within 30 days with a focus on safety; the IND is either found ‘safe to proceed’ or ‘placed on clinical hold’. The latter situation is usually caused by insufficient preclinical toxicology and pharmacokinetic data, which are crucial components of the package, particularly for new molecule entities. After obtaining IND status for the agent, clinical studies for pharmacokinetics and toxicity can proceed; after accumulating sufficient clinical and efficacy data in animal models under GLP conditions, an application for new drug or biologic can be filed.
No radiation medical countermeasure (MCM) for acute radiation syndrome (ARS) is currently approved by the US FDA for use in humans.
MCM for ARS are being developed following the US FDA Animal Efficacy Rule since efficacy evaluation for such agents is not feasible in human volunteers due to ethical considerations.
FDA emphasizes the use of well-characterized animal models in which the pathophysiology of radiation injury is well understood and predictive of the human response.
For studying radiation injury, only two large animal models (nonhuman primate and canine) have been well characterized. Several groups are currently working to develop and characterize the minipig model of radiation injury and evaluation of MCM.
The available research sites must characterize current animal models and include the capability to study both the ARS and the delayed effects of acute radiation exposure.
Acknowledgments
The authors are thankful to Col. L Andrew Huff and Captain David Lesser for helpful discussions. The opinions or assertions contained herein are the professional views of the authors and are not necessarily those of the Department of Defense, USA. Mention of specific therapeutic agents does not constitute endorsement by the US Department of Defense, and trade names are used only for the purpose of clarification. The authors express appreciation to Stephen Y Wise and Oluseyi O Fatanmi for outstanding photomicrography support. The authors apologize to those having contributed substantially to the topics discussed herein that they were unable to cite because of space constraints.
Declaration of interest
VK Singh is employee of the US Department of the Defense. Furthermore, VL Newman and AN Berg are affiliated with the Armed Forces Radiobiology Research Institute, a US Department of Defense research laboratory, as research project staff. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
This box summarizes key points contained in the article
Bibliography
- Bushberg JT, Kroger LA, Hartman MB, et al. Nuclear/radiological terrorism: emergency department management of radiation casualties. J Emerg Med 2007;32:71-85
- Flynn DF, Goans RE. Nuclear terrorism: triage and medical management of radiation and combined-injury casualties. Surg Clin North Am 2006;86:601-36
- Waselenko JK, MacVittie TJ, Blakely WF, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med 2004;140:1037-51
- DiCarlo AL, Maher C, Hick JL, et al. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep 2011;5(Suppl 1):S32-44
- Goans RE. Clinical care of the radiation-accident patient: patient presentation, assessment and initial diagnosis. In: Ricks RC, Berger ME, O’Hara FM, editors. The medical basis for victims. Partheon Publishing Group; Boca Raton, FL: 2002. p. 11
- Dorr H, Meineke V. Acute radiation syndrome caused by accidental radiation exposure - therapeutic principles. BMC Med 2011;9:126
- Gourmelon P, Benderitter M, Bertho JM, et al. European consensus on the medical management of acute radiation syndrome and analysis of the radiation accidents in Belgium and Senegal. Health Phys 2010;98:825-32
- Weinstock DM, Case CJr, Bader JL, et al. Radiologic and nuclear events: contingency planning for hematologists/oncologists. Blood 2008;111:5440-5
- Dainiak N, Gent RN, Carr Z, et al. Literature review and global consensus on management of acute radiation syndrome affecting nonhematopoietic organ systems. Disaster Med Public Health Prep 2011;5:183-201
- Aebersold P. FDA experience with medical countermeasures under the animal rule. Adv Prev Med 2012;2012:507571
- Gronvall GK, Trent D, Borio L, et al. The FDA animal efficacy rule and biodefense. Nat Biotechnol 2007;25:1084-7
- Singh VK, Ducey EJ, Brown DS, Whitnall MH. A review of radiation countermeasure work ongoing at the Armed Forces Radiobiology Research Institute. Int J Radiat Biol 2012;88:296-310
- Singh VK, Newman VL, Romaine PL, et al. Radiation countermeasure agents: an update (2011 - 2014). Expert Opin Ther Pat 2014;24:1229-55
- U.S. Department of Health and Human Services. HHS boosts stockpile of products to treat acute radiation syndrome. 2013. Available from: http://www.hhs.gov/news/press/2013pres/09/20130926a.html [Last accessed 12 February 2014]
- Singh VK, Newman VL, Seed TM. Colony-stimulating factors for the treatment of the hematopoietic component of the acute radiation syndrome (H-ARS): a review. Cytokine 2015;71:22-37
- Centers for Disease Control and Prevention. Population monitoring in radiation emergencies: a guide for state and local public health planners. 2007. Available from: http://www.bt.cdc.gov/radiation/pdf/population-monitoring-guide1sted.pdf [Last accessed 20 October 2014]
- Stone HB, Moulder JE, Coleman CN, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3-4, 2003. Radiat Res 2004;162:711-28
- Singh VK, Beattie LA, Seed TM. Vitamin E: tocopherols and tocotrienols as potential radiation countermeasures. J Radiat Res 2013;54:973-88
- Weiss JF, Landauer MR. History and development of radiation-protective agents. Int J Radiat Biol 2009;85:539-73
- Seed TM. Radiation protectants: current status and future prospects. Health Phys 2005;89:531-45
- Dumont F, Le Roux A, Bischoff P. Radiation countermeasure agents: an update. Expert Opin Ther Pat 2010;20:73-101
- Moulder JE. 2013; Dade W. Moeller lecture: medical countermeasures against radiological terrorism. Health Phys 2014;107:164-71
- Singh VK, Romaine PL, Newman VL. Biologics as countermeasures for acute radiation syndrome: where are we now? Expert Opin Biol Ther 2014; In press
- Farese AM, Cohen MV, Katz BP, et al. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res 2013;179:89-100
- Herodin F, Roy L, Grenier N, et al. Antiapoptotic cytokines in combination with pegfilgrastim soon after irradiation mitigates myelosuppression in nonhuman primates exposed to high irradiation dose. Exp Hematol 2007;35:1172-81
- Drouet M, Mourcin F, Grenier N, et al. Single administration of stem cell factor, FLT-3 ligand, megakaryocyte growth and development factor, and interleukin-3 in combination soon after irradiation prevents nonhuman primates from myelosuppression: long-term follow-up of hematopoiesis. Blood 2004;103:878-85
- Farese AM, Casey DB, Smith WG, et al. Leridistim, a chimeric dual G-CSF and IL-3 receptor agonist, enhances multilineage hematopoietic recovery in a nonhuman primate model of radiation-induced myelosuppression: effect of schedule, dose, and route of administration. Stem Cells 2001;19:522-33
- Wagemaker G, Neelis KJ, Hartong SCC, et al. The efficacy of recombinant TPO in murine And nonhuman primate models for myelosuppression and stem cell transplantation. Stem Cells 1998;16(Suppl 2):127-41
- MacVittie TJ, Farese AM, Patchen ML, Myers LA. Therapeutic efficacy of recombinant interleukin-6 (IL-6) alone and combined with recombinant human IL-3 in a nonhuman primate model of high-dose, sublethal radiation-induced marrow aplasia. Blood 1994;84:2515-22
- Nightengale SL, Prasher JM, Simonson S. Emergency use authorization (EUA) to enable use of needed products in civilian and military emergencies, United States. Emerging Infect Dis 2002;7:1046-55
- U.S. Food and Drug Administration. Guidance for Industry: product Developoment Under the Animal Rule. 2014. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM399217.pdf [Last accessed 18 July 2014]
- Hall EJ, Giaccia AJ. Radiobiology for the radiobiologist. 7th edition. Lippincott Williams and Wilkins; Philadelphia, PA: 2012
- Augustine AD, Gondre-Lewis T, McBride W, et al. Animal models for radiation injury, protection and therapy. Radiat Res 2005;164:100-9
- Williams JP, Brown SL, Georges GE, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res 2010;173:557-78
- Sanzari JK, Wan XS, Krigsfeld GS, et al. The effects of gamma and proton radiation exposure on hematopoietic cell counts in the ferret model. Gravit Space Res 2013;1:79-94
- Krigsfeld GS, Savage AR, Billings PC, et al. Evidence for radiation-induced disseminated intravascular coagulation as a major cause of radiation-induced death in ferrets. Int J Radiat Oncol Biol Phys 2014;88:940-6
- King GL, Landauer MR. Effects of zacopride and BMY25801 (batanopride) on radiation-induced emesis and locomotor behavior in the ferret. J Pharmacol Exp Ther 1990;253:1026-33
- Kerekes J, Novak J, Koteles GJ. Micronucleus frequency in peripheral lymphocytes for the differential diagnosis of radiation injuries combined with thermal burns. J Burn Care Rehabil 1988;9:275-8
- Maleki S, Kamrava SK, Sharifi D, et al. Effect of local irradiation with 630 and 860 nm low-level lasers on tympanic membrane perforation repair in guinea pigs. J Laryngol Otol 2013;127:260-4
- Su YX, Benedek GA, Sieg P, et al. Radioprotective effect of lidocaine on neurotransmitter agonist-induced secretion in irradiated salivary glands. PLoS ONE 2013;8:e60256
- Gabka CJ, Benhaim P, Mathes SJ, et al. An experimental model to determine the effect of irradiated tissue on neutrophil function. Plast Reconstr Surg 1995;96:1676-88
- Gratwohl A, John L, Baldomero H, et al. FLT-3 ligand provides hematopoietic protection from total body irradiation in rabbits. Blood 1998;92:765-9
- Georgieva S, Popov B, Bonev G. Radioprotective effect of Haberlea rhodopensis (Friv.) leaf extract on gamma-radiation-induced DNA damage, lipid peroxidation and antioxidant levels in rabbit blood. Indian J Exp Biol 2013;51:29-36
- Albuquerque EX, Pereira EF, Aracava Y, et al. Effective countermeasure against poisoning by organophosphorus insecticides and nerve agents. Proc Natl Acad Sci USA 2006;103:13220-5
- U.S. Food and Drug Administration. FDA approves new antibacterial treatment for plague. 2012. Available from: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm302220.htm [Last accessed 10 February 2014]
- U.S. Food and Drug Administration. FDA approves raxibacumab to treat inhalational anthrax. 2012. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm332341.htm [Last accessed 10 February 2014]
- U.S. Food and Drug Administration. FDA approves first Botulism Antitoxin for use in neutralizing all seven known botulinum nerve toxin serotypes. 2013. Available from: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm345128.htm [Last accessed 13 February 2014]
- McCann DGC. Radiation poisoning: current concepts in the Acute Radiation Syndrome. Am J Clin Med 2006;3:13-21
- Fliedner TM, Dorr DH, Meineke V. Multi-organ involvement as a pathogenetic principle of the radiation syndromes: a study involving 110 case histories documented in SEARCH and classified as the bases of haematopoietic indicators of effect. Br J Radiol Suppl 2005;27:1-8
- Hill RP. Radiation effects on the respiratory system. Br J Radiol Suppl 2005;27:75-81
- Moulder JE, Cohen EP. Radiation-induced multi-organ involvement and failure: the contribution of radiation effects on the renal system. Br J Radiol Suppl 2005;27:82-8
- Cary LH, Ngudiankama BF, Salber RE, et al. Efficacy of radiation countermeasures depends on radiation quality. Radiat Res 2012;177:663-75
- Satyamitra M, Lombardini E, Graves JIII, et al. A TPO receptor agonist, ALXN4100TPO, mitigates radiation-induced lethality and stimulates hematopoiesis in CD2F1 mice. Radiat Res 2011;175:746-58
- Herodin F, Mestries JC, Janodet D, et al. Recombinant glycosylated human interleukin-6 accelerates peripheral blood platelet count recovery in radiation-induced bone marrow depression in baboons. Blood 1992;80:688-95
- Ledney GD, Elliott TB. Combined injury: factors with potential to impact radiation dose assessments. Health Phys 2010;98:145-52
- Hanson WR, Fry RJ, Sallese AR, et al. Comparison of intestine and bone marrow radiosensitivity of the BALB/c and the C57BL/6 mouse strains and their B6CF1 offspring. Radiat Res 1987;110:340-52
- Ciorba MA, Riehl TE, Rao MS, et al. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut 2012;61:829-38
- Duran-Struuck R, Hartigan A, Clouthier SG, et al. Differential susceptibility of C57BL/6NCr and B6.Cg-Ptprca mice to commensal bacteria after whole body irradiation in translational bone marrow transplant studies. J Transl Med 2008;6:10
- Jackson IL, Vujaskovic Z, Down JD. Revisiting strain-related differences in radiation sensitivity of the mouse lung: recognizing and avoiding the confounding effects of pleural effusions. Radiat Res 2010;173:10-20
- Jackson IL, Vujaskovic Z, Down JD. A further comparison of pathologies after thoracic irradiation among different mouse strains: finding the best preclinical model for evaluating therapies directed against radiation-induced lung damage. Radiat Res 2011;175:510-18
- Jackson IL, Xu P, Hadley C, et al. A preclinical rodent model of radiation-induced lung injury for medical countermeasure screening in accordance with the FDA animal rule. Health Phys 2012;103:463-73
- Booth C, Tudor G, Tudor J, et al. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys 2012;103:383-99
- Strom JS. Health impacts from acute radiation exposure. Office of Security Affairs US Department of Energy under Contract DE-AC06-76RLO 1830. Pacific Northwest National Laboratory; Richland, Washington: 2003
- International Commission on Radiological Protection. 1990 Recommendations of the International Commission on Radiological Protection. ICRP Publication No. 60 Pergamon Press; New York, NY: 1991
- National Council on Radiation Protection and Measurements. Induction of Thyroid Cancer by Ionizing Radiation, NCRP Report No. 80. NCRP Publications; Bethesda, MD: 1985
- Herodin F, Valente M, Abend M. Useful radiation dose biomarkers for early identification of partial-body exposures. Health Phys 2014;106:750-4
- Reeves G. Overview of use of G-CSF and GM-CSF in the treatment of acute radiation injury. Health Phys 2014;106:699-703
- Miyamoto K, Watanabe Y, Yukawa M, et al. Reconstruction of two victims’ posturing based on the induced radioactivities in their bones in the criticality accident in Tokai-Mura, Japan. Health Phys 2002;83:19-25
- Da Silva FC, Hunt JG, Ramalho AT, Crispim VR. Dose reconstruction of a Brazilian industrial gamma radiography partial-body overexposure case. J Radiol Prot 2005;25:289-98
- Croizat H, Frindel E, Tubiana M. Abscopal effect of irradiation on haemopoietic stem cells of shielded bone marrow–role of migration. Int J Radiat Biol Relat Stud Phys Chem Med 1976;30:347-58
- Carsten AL, Noonan TR. Hematological effects of partial-body and whole-body X-irradiation in the rat. Radiat Res 1964;22:136-43
- Scarantino CW, Rubin P, Constine LSIII. The paradoxes in patterns and mechanism of bone marrow regeneration after irradiation. 1. Different volumes and doses. Radiother Oncol 1984;2:215-25
- Baltschukat K, Nothdurft W. Hematological effects of unilateral and bilateral exposures of dogs to 300-kVp X rays. Radiat Res 1990;123:7-16
- Baltschukat K, Fliedner TM, Nothdurft W. Hematological effects in dogs after irradiation of the lower part of the body with a single myeloablative dose. Radiother Oncol 1989;14:239-46
- Nothdurft W, Calvo W, Klinnert V, et al. Acute and long-term alterations in the granulocyte/macrophage progenitor cell (GM-CFC) compartment of dogs after partial-body irradiation: irradiation of the upper body with a single myeloablative dose. Int J Radiat Oncol Biol Phys 1986;12:949-57
- Monroy RL, Skelly RR, Taylor P, et al. Recovery from severe hematopoietic suppression using recombinant human granulocyte-macrophage colony-stimulating factor. Exp Hematol 1988;16:344-8
- MacVittie TJ, Farese AM, Bennett A, et al. The acute gastrointestinal subsyndrome of the acute radiation syndrome: a rhesus macaque model. Health Phys 2012;103:411-26
- Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol 2002;30:513-28
- Goans RE, Holloway EC, Berger ME, Ricks RC. Early dose assessment following severe radiation accidents. Health Phys 1997;72:513-18
- Inoue T, Hirabayashi Y, Mitsui H, et al. Survival of spleen colony-forming units (CFU-S) of irradiated bone marrow cells in mice: evidence for the existence of a radioresistant subfraction. Exp Hematol 1995;23:1296-300
- Gianni AM, Bregni M, Siena S, et al. Rapid and complete hemopoietic reconstitution following combined transplantation of autologous blood and bone marrow cells. A changing role for high dose chemo-radiotherapy? Hematol Oncol 1989;7:139-48
- Laterveer L, Zijlmans JM, Liehl E, et al. Accelerated platelet reconstitution following transplantation of bone marrow cells derived from IL-6-treated donor mice. Ann Hematol 1996;73:239-45
- Farese AM, Cohen MV, Katz BP, et al. A nonhuman primate model of the hematopoietic acute radiation syndrome plus medical management. Health Phys 2012;103:367-82
- Withers HR, Elkind MM. Dose-survival characteristics of epithelial cells of mouse intestinal mucosa. Radiology 1968;91:998-1000
- Denham JW, Hauer-Jensen M, Peters LJ. Is it time for a new formalism to categorize normal tissue radiation injury? Int J Radiat Oncol Biol Phys 2001;50:1105-6
- Ch’ang HJ, Maj JG, Paris F, et al. ATM regulates target switching to escalating doses of radiation in the intestines. Nat Med 2005;11:484-90
- Geraci JP, Jackson KL, Mariano MS. The intestinal radiation syndrome: sepsis and endotoxin. Radiat Res 1985;101:442-50
- Carr KE. Effects of radiation damage on intestinal morphology. Int Rev Cytol 2001;208:1-119
- Mason KA, Withers HR, McBride WH, et al. Comparison of the gastrointestinal syndrome after total-body or total-abdominal irradiation. Radiat Res 1989;117:480-8
- Krimsky M, Dagan A, Aptekar L, et al. Assessment of intestinal permeability in rats by permeation of inulin-fluorescein. J Basic Clin Physiol Pharmacol 2000;11:143-53
- Kobayashi T, Ohmori T, Yanai M, et al. The analysis of the defense mechanism against indigenous bacterial translocation in X-irradiated mice. Microbiol Immunol 1991;35:315-24
- Lutgens LC, Blijlevens NM, Deutz NE, et al. Monitoring myeloablative therapy-induced small bowel toxicity by serum citrulline concentration: a comparison with sugar permeability tests. Cancer 2005;103:191-9
- Lang IM, Sarna SK, Condon RE. Gastrointestinal motor correlates of vomiting in the dog: quantification and characterization as an independent phenomenon. Gastroenterology 1986;90:40-7
- Dubois A, Jacobus JP, Grissom MP, et al. Altered gastric emptying and prevention of radiation-induced vomiting in dogs. Gastroenterology 1984;86:444-8
- Danquechin Dorval E, Mueller GP, Eng RR, et al. Effect of ionizing radiation on gastric secretion and gastric motility in monkeys. Gastroenterology 1985;89:374-80
- Makrauer FL, Oates E, Becker J, et al. Does local irradiation affect gastric emptying in humans? Am J Med Sci 1999;317:33-7
- Otterson MF, Sarna SK, Moulder JE. Effects of fractionated doses of ionizing radiation on small intestinal motor activity. Gastroenterology 1988;95:1249-57
- Otterson MF, Sarna SK, Leming SC, et al. Effects of fractionated doses of ionizing radiation on colonic motor activity. Am J Physiol 1992;263:G518-26
- Travis EL. Organizational response of normal tissues to irradiation. Semin Radiat Oncol 2001;11:184-96
- Marks LB, Yu X, Vujaskovic Z, et al. Radiation-induced lung injury. Semin Radiat Oncol 2003;13:333-45
- Sharplin J, Franko AJ. A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the intermediate and late phases. Radiat Res 1989;119:15-31
- Sharplin J, Franko AJ. A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the early phase. Radiat Res 1989;119:1-14
- McLaughlin RFJr, Tyler WS, Canada RO. Subgross pulmonary anatomy of the rabbit, rat, and guinea pig, with additional notes on the human lung. Am Rev Respir Dis 1966;94:380-7
- Vujaskovic Z, Marks LB, Anscher MS. The physical parameters and molecular events associated with radiation-induced lung toxicity. Semin Radiat Oncol 2000;10:296-307
- Haston CK, Zhou X, Gumbiner-Russo L, et al. Universal and radiation-specific loci influence murine susceptibility to radiation-induced pulmonary fibrosis. Cancer Res 2002;62:3782-8
- Epperly MW, Carpenter M, Agarwal A, et al. Intraoral manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) radioprotective gene therapy decreases ionizing irradiation-induced murine mucosal cell cycling and apoptosis. In Vivo 2004;18:401-10
- Carpenter M, Epperly MW, Agarwal A, et al. Inhalation delivery of manganese superoxide dismutase-plasmid/liposomes protects the murine lung from irradiation damage. Gene Ther 2005;12:685-93
- Chen L, Brizel DM, Rabbani ZN, et al. The protective effect of recombinant human keratinocyte growth factor on radiation-induced pulmonary toxicity in rats. Int J Radiat Oncol Biol Phys 2004;60:1520-9
- Travis EL, Tucker SL. The relationship between functional assays of radiation response in the lung and target cell depletion. Br J Cancer Suppl 1986;7:304-19
- Hopewell JW, Rezvani M, Moustafa HF. The pig as a model for the study of radiation effects on the lung. Int J Radiat Biol 2000;76:447-52
- Mandel L, Travnicek J, Talafantova M, Zahradnickova M. The LD50/30 and the survival time in whole-body gamma-irradiated conventional and germfree Minnesota miniature piglets. Z Versuchstierkd 1980;22:96-100
- Slauson DO, Hahn FF, Benjamin SA, et al. Inflammatory sequences in acute pulmonary radiation injury. Am J Pathol 1976;82:549-72
- Garofalo M, Bennett A, Farese AM, et al. The delayed pulmonary syndrome following acute high-dose irradiation: a rhesus macaque model. Health Phys 2014;106:56-72
- Adawi A, Zhang Y, Baggs R, et al. Disruption of the CD40-CD40 ligand system prevents an oxygen-induced respiratory distress syndrome. Am J Pathol 1998;152:651-7
- Adawi A, Zhang Y, Baggs R, et al. Blockade of CD40-CD40 ligand interactions protects against radiation-induced pulmonary inflammation and fibrosis. Clin Immunol Immunopathol 1998;89:222-30
- Kaufman J, Sime PJ, Phipps RP. Expression of CD154 (CD40 ligand) by human lung fibroblasts: differential regulation by IFN-gamma and IL-13, and implications for fibrosis. J Immunol 2004;172:1862-71
- DiCarlo AL, Jackson IL, Shah JR, et al. Development and licensure of medical countermeasures to treat lung damage resulting from a radiological or nuclear incident. Radiat Res 2012;177:717-21
- Peter RU. [Cutaneous radiation syndrome after accidental skin exposure to ionizing radiation]. Hautarzt 2013;64:894-903
- Lataillade JJ, Doucet C, Bey E, et al. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Med 2007;2:785-94
- Bey E, Prat M, Duhamel P, et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen 2010;18:50-8
- Semont A, Francois S, Mouiseddine M, et al. Mesenchymal stem cells increase self-renewal of small intestinal epithelium and accelerate structural recovery after radiation injury. Adv Exp Med Biol 2006;585:19-30
- Abdel-Mageed AS, Senagore AJ, Pietryga DW, et al. Intravenous administration of mesenchymal stem cells genetically modified with extracellular superoxide dismutase improves survival in irradiated mice. Blood 2009;113:1201-3
- Saha S, Bhanja P, Kabarriti R, et al. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS ONE 2011;6:e24072
- Agay D, Scherthan H, Forcheron F, et al. Multipotent mesenchymal stem cell grafting to treat cutaneous radiation syndrome: development of a new minipig model. Exp Hematol 2010;38:945-56
- Forcheron F, Agay D, Scherthan H, et al. Autologous adipocyte derived stem cells favour healing in a minipig model of cutaneous radiation syndrome. PLoS ONE 2012;7:e31694
- Singh VK, Wise SY, Fatanmi OO, et al. Preclinical development of a bridging therapy for radiation casualties: appropriate for high risk personnel. Health Phys 2014;106:689-98
- Singh VK, Wise SY, Fatanmi OO, et al. Progenitors mobilized by gamma-tocotrienol as an effective radiation countermeasure. PLoS ONE 2014;9:e114078
- Lopez M, Martin M. Medical management of the acute radiation syndrome. Rep Pract Oncol Radiother 2011;16:138-46
- Flanders KC, Sullivan CD, Fujii M, et al. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol 2002;160:1057-68
- Shim S, Jang WS, Lee SJ, et al. Development of a new minipig model to study radiation-induced gastrointestinal syndrome and its application in clinical research. Radiat Res 2014;181:387-95
- Lippincott SW, Wilson JD, Montour JL. Radiation effects on pig skin. Exposure to different densities of ionization. Arch Pathol 1975;99:105-10
- Hopewell JW. The skin: its structure and response to ionizing radiation. Int J Radiat Biol 1990;57:751-73
- King GL. Characterization of radiation-induced emesis in the ferret. Radiat Res 1988;114:599-612
- Yu ZY, Li M, Han AR, et al. RhG-CSF improves radiation-induced myelosuppression and survival in the canine exposed to fission neutron irradiation. J Radiat Res 2011;52:472-80
- MacVittie TJ, Monroy RL, Patchen ML, Souza LM. Therapeutic use of recombinant human G-CSF (rhG-CSF) in a canine model of sublethal and lethal whole-body irradiation. Int J Radiat Biol 1990;57:723-36
- Herrera JL, Vigneulle RM, Gage T, et al. Effect of radiation and radioprotection on small intestinal function in canines. Dig Dis Sci 1995;40:211-18
- MacVittie TJ, Farese AM, Jackson WIII. Defining the full therapeutic potential of recombinant growth factors in the post radiation-accident environment: the effect of supportive care plus administration of G-CSF. Health Phys 2005;89:546-55
- Alpen EL. The historical background for large-animal studies with neutrons of various energies. Radiat Res 1991;128:S37-41
- Summers RW, Flatt AJ, Prihoda MJ, Mitros FA. Effect of irradiation on morphology and motility of canine small intestine. Dig Dis Sci 1987;32:1402-10
- Sanzari JK, Wan XS, Krigsfeld GS, et al. Effects of solar particle event proton radiation on parameters related to ferret emesis. Radiat Res 2013;180:166-76
- King GL, Rabin BM, Weatherspoon JK. 5-HT3 receptor antagonists ameliorate emesis in the ferret evoked by neutron or proton radiation. Aviat Space Environ Med 1999;70:485-92
- Martin C, Roman V, Agay D, Fatome M. Anti-emetic effect of ondansetron and granisetron after exposure to mixed neutron and gamma irradiation. Radiat Res 1998;149:631-6
- Okayasu R, Suetomi K, Yu Y, et al. A deficiency in DNA repair and DNA-PKcs expression in the radiosensitive BALB/c mouse. Cancer Res 2000;60:4342-5
- Fan S, Meng Q, Xu J, et al. DIM (3,3’-diindolylmethane) confers protection against ionizing radiation by a unique mechanism. Proc Natl Acad Sci USA 2013;110:18650-5
- Soligenix. 2014. Available from: http://www.soligenix.com/prod_def_sgx202.shtml [Last accessed 25 March 2014]
- Georges GE, Kuver RP, Jordan R, et al. Post-exposure oral 17,21-beclomethasone dipropionate (BDP) improves survival in a canine gastrointestinal acute radiation syndrome (GI-ARS) model. 58th Annual Meeting of the Radiation Research Society. San Juan, PR; September 30 - October 3, 2012
- Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist 2007;12:738-47
- Li M, Yu ZY, Xing S, et al. High dose granulocyte colony-stimulating factor enhances survival and hematopoietic reconstruction in canines irradiated by 2.3 Gy mixed fission neutron and gamma ray. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2011;19:991-8
- Moroni M, Lombardini E, Salber R, et al. Hematological changes as prognostic indicators of survival: similarities between Gottingen minipigs, humans, and other large animal models. PLoS ONE 2011;6:e25210
- Moroni M, Coolbaugh TV, Lombardini E, et al. Hematopoietic radiation syndrome in the Gottingen minipig. Radiat Res 2011;176:89-101
- Moroni M, Ngudiankama BF, Christensen C, et al. The Gottingen minipig is a model of the hematopoietic acute radiation syndrome: G-colony stimulating factor stimulates hematopoiesis and enhances survival from lethal total-body gamma-irradiation. Int J Radiat Oncol Biol Phys 2013;86:986-92
- Elliott TB, Deutz NE, Gulani J, et al. Gastrointestinal acute radiation syndrome in gottingen minipigs (Sus scrofa domestica). Comp Med 2014;64:456-63
- Mahmud N, Pang W, Cobbs C, et al. Studies of the route of administration and role of conditioning with radiation on unrelated allogeneic mismatched mesenchymal stem cell engraftment in a nonhuman primate model. Exp Hematol 2004;32:494-501
- Norol F, Drouet M, Mathieu J, et al. Ex vivo expanded mobilized peripheral blood CD34+ cells accelerate haematological recovery in a baboon model of autologous transplantation. Br J Haematol 2000;109:162-72
- Patchen ML, MacVittie TJ, Solberg BD, Souza LM. Therapeutic administration of recombinant human granulocyte colony-stimulating factor accelerates hemopoietic regeneration and enhances survival in a murine model of radiation-induced myelosuppression. Int J Cell Cloning 1990;8:107-22
- Farese AM, Brown CR, Smith CP, et al. The ability of filgrastim to mitigate mortality following LD50/60 total-body irradiation is administration time-dependent. Health Phys 2014;106:39-47
- U.S. Food and Drug Administration. FDA Advisory Committee Briefing Document: a Joint Meeting of the Medical Imaging Drugs Advisory Committee and the Oncologic Drugs Advisory Committee. 2013. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/MedicalImagingDrugsAdvisoryCommittee/UCM350151.pdf [Last accessed 5 February 2014]
- Farese AM, Cohen MV, Stead RB, et al. Pegfilgrastim administered in an abbreviated schedule, significantly improved neutrophil recovery after high-dose radiation-induced myelosuppression in rhesus macaques. Radiat Res 2012;178:403-13
- Hankey KG, Farese AM, Gibbs AM, et al. Pegfilgrastim administration significantly improves survival in an LD50/60 model of totalbody irradiation (TBI) in nonhuman primates (NHP). 59th Annual Meeting of the Radiation Research Society; New Orleans, LA; September 14-18, 2013. p. 318
- Farese AM, Williams DE, Seiler FR, MacVittie TJ. Combination protocols of cytokine therapy with interleukin-3 and granulocyte-macrophage colony-stimulating factor in a primate model of radiation-induced marrow aplasia. Blood 1993;82:3012-18
- Neelis KJ, Hartong SC, Egeland T, et al. The efficacy of single-dose administration of thrombopoietin with coadministration of either granulocyte/macrophage or granulocyte colony-stimulating factor in myelosuppressed rhesus monkeys. Blood 1997;90:2565-73
- Cleveland BioLabs, Inc. 2014. Available from: http://www.cbiolabs.com/ [Last accessed 10 September 2014]
- Krivokrysenko VI, Shakhov AN, Singh VK, et al. Identification of granulocyte colony-stimulating factor and interleukin-6 as candidate biomarkers of CBLB502 efficacy as a medical radiation countermeasure. J Pharmacol Exp Ther 2012;343:497-508
- Gluzman-Poltorak Z, Mendonca SR, Vainstein V, et al. Randomized comparison of single dose of recombinant human IL-12 versus placebo for restoration of hematopoiesis and improved survival in rhesus monkeys exposed to lethal radiation. J Hematol Oncol 2014;7:31
- Gluzman-Poltorak Z, Vainstein V, Basile LA. Recombinant interleukin-12, but not granulocyte-colony stimulating factor, improves survival in lethally irradiated nonhuman primates in the absence of supportive care: evidence for the development of a frontline radiation medical countermeasure. Am J Hematol 2014;89:868-73
- Neumedicines. 2014. Available from: http://www.neumedicines.com/ [Last accessed 27 October 2014]
- Pearlstein RD, Higuchi Y, Moldovan M, et al. Metalloporphyrin antioxidants ameliorate normal tissue radiation damage in rat brain. Int J Radiat Biol 2010;86:145-63
- Zhang Y, Zhang X, Rabbani ZN, et al. Oxidative stress mediates radiation lung injury by inducing apoptosis. Int J Radiat Oncol Biol Phys 2012;83:740-8
- Aeolus Pharmaceuticals. 2013. Available from: http://www.aeoluspharma.com/ [Last accessed 30 September 2013]
- Batinic-Haberle I, Tovmasyan A, Roberts E, et al. SOD therapeutics: latest insights into their structure-activity relationships and impact upon the cellular redox-based pathways. Antioxid Redox Signal 2014;20:2372-415
- Orrell RW. AEOL-10150 (Aeolus). Curr Opin Investig Drugs 2006;7:70-80
- Garofalo MC, Ward AA, Farese AM, et al. A pilot study in rhesus macaques to assess the treatment efficacy of a small molecular weight catalytic metalloporphyrin antioxidant (AEOL 10150) in mitigating radiation-induced lung damage. Health Phys 2014;106:73-83
- Stickney DR, Dowding C, Garsd A, et al. 5-androstenediol stimulates multilineage hematopoiesis in rhesus monkeys with radiation-induced myelosuppression. Int Immunopharmacol 2006;6:1706-13
- Stickney DR, Dowding C, Authier S, et al. 5-androstenediol improves survival in clinically unsupported rhesus monkeys with radiation-induced myelosuppression. Int Immunopharmacol 2007;7:500-5
- Geary RS, Swynnerton NF, Miller MA, et al. Intraduodenal administration of ethiofos (WR-2721): dose proportionality study in the rhesus monkey. Res Commun Chem Pathol Pharmacol 1989;65:147-59
- Davidson DE, Grenan MM, Sweeney TR. Biological Characteristics of some improved radioprotectors. In: Brady LW, editor. Radiation sensitizers, their use in the clinical management of cancer. Masson; New York: 1980. p. 309-20
- Wasserman TH, Brizel DM. The role of amifostine as a radioprotector. Oncology (Williston Park) 2001;15:1349-54. discussion 57-60
- MedImmune. 2013. Available from: http://www.medimmune.com/docs/default-source/pdfs/prescribing-information-for-amifostine.pdf [Last accessed 30 September 2013]
- Seed TM, Inal CE, Singh VK. Radioprotection of hematopoietic progenitors by low dose amifostine prophylaxis. Int J Radiat Biol 2014;90:594-604
- Culy CR, Spencer CM. Amifostine: an update on its clinical status as a cytoprotectant in patients with cancer receiving chemotherapy or radiotherapy and its potential therapeutic application in myelodysplastic syndrome. Drugs 2001;61:641-84
- U.S. Food and Drug Administration. About the pandemic and all-hazards preparedness reauthorization act of 2013 (PAHPRA), emergency preparedness and response. 2014. Available from: http://www.fda.gov/emergencypreparedness/medicalcountermeasures/ucm346195.htm [Last accessed 15 February 2014]
- Ahlem CN, White SK, Page TM, Frincke JM. Differential metabolism of androst-5-ene-3beta,17beta-diol between rats, canines, monkeys and humans. Steroids 2011;76:669-74
- Yan Y, Ran X, Wei S. Changes of immune functions after radiation, burns and combined radiation-burn injury in rats. Chin Med Sci J 1995;10:85-9
