ABSTRACT
Inactivated trivalent influenza vaccines (IIV3s) are designed to protect against illness caused by two influenza A virus subtypes and one influenza B virus lineage. They may provide inadequate protection due to the co-circulation of viruses from two antigenically distinct influenza B lineages. Incorporating strains from both B lineages as in inactivated quadrivalent influenza vaccines (IIV4s) reduces this risk. We summarize the evidence supporting two IIV4s manufactured by GSK Vaccines. Compared to IIV3s, these two IIV4s demonstrated noninferior immunogenicity against the shared influenza strains and superior immunogenicity for the strain of the additional B lineage, particularly in subjects who were seronegative for that B strain. One IIV4’s efficacy in children aged 3–8 years was 55.4% against influenza of any severity and 73.1% against moderate-to-severe influenza. Both IIV4s were well-tolerated with a similar safety profile to IIV3s. These IIV4s are more likely than IIV3s to protect against the added influenza B strain.
Seasonal influenza is a serious public health burden due to its associated morbidity, mortality and healthcare resource utilization worldwide.[Citation1] Annual seasonal influenza epidemics are estimated to result in about 3–5 million cases of severe illness and about 250,000 to 500,000 deaths.[Citation1] Besides being a direct burden on healthcare resources, influenza illnesses and complications are attributed with significant productivity losses to the society.[Citation2–Citation5] The causative pathogen, influenza virus, belongs to the RNA virus family Orthomyxoviridae and can be classified into A, B, and C types [Citation6]. Influenza attack rates can be 5–10% in adults and as high as 20%–30% in children.[Citation6] Children serve as a major source of influenza transmission within communities and are at high risk of influenza-related clinic and emergency department visits during community outbreaks.[Citation7] Hospitalization rates are highest in elderly individuals aged ≥65 years and children <1 year of age.[Citation8] Most deaths caused by influenza occur in elderly individuals aged ≥65 years.
Influenza A virus is considered as the most prevalent pathogen in typical seasonal epidemics and the cause of all past pandemics.[Citation9] Influenza B virus is known to cause a significant proportion of influenza-related illnesses and complications. The influenza B virus is less diverse than influenza A, as it undergoes slower antigenic drift. These attributes of influenza B may have contributed to the perception that influenza B causes mild disease not warranting vigorous prophylaxis. Yet the clinical presentation and severity of symptoms of influenza A and influenza B illness were shown to be similar.[Citation10] Moreover, influenza B infections were more frequently observed among subjects with a high-risk medical condition than those caused by influenza A.[Citation10] As with influenza A, groups at particular risk of influenza B disease include pregnant women, children aged <5 years, elderly individuals aged ≥65 years, and individuals with underlying non-communicable health conditions such as heart disease, asthma, and diabetes.
Influenza B virus lineages
Four antigenically distinct human influenza virus groups are reported to circulate on a regular basis.[Citation11] There are two A subtypes (A/H1N1 and A/H3N2) and two B lineages (B/Victoria and B/Yamagata).[Citation11,Citation12] The B lineages were first identified in the 1988–1989 influenza season.[Citation12] In a typical season, influenza A circulation predominates; however, often there is co-circulation of influenza A and B viruses, with waxing and waning levels of influenza B from year to year. The contribution of influenza B to overall influenza disease burden is unpredictable. This trend can be discerned from recent surveillance data from different worldwide regions. Surveillance data from 1999 to 2013 showed that during these annual influenza seasons, the prevalence of influenza B ranged from 1% to 46% (United States [US][Citation13,Citation14]) and 2–65% (United Kingdom [UK][Citation15]) with 4/14 influenza seasons with influenza B circulation levels of >30%. During 2000−2013, influenza B circulation accounted for 0–70% (Germany [Citation16,Citation17]) and 1–74% (Brazil [Citation18]), with 5/13 seasons with >30% influenza B circulation levels. Epidemiological data (1995–2010) from the Asia-Pacific region described in a recent review report that frequencies of influenza B were higher in Taiwan (6.4–62.9%) than Korea (0.0–16.4%), but whether these differences truly reflect higher incidence, timing, or characteristics of study populations or method of surveillance remains unclear.[Citation19]
Burden of influenza B in the US
In the US during the 2014–2015 influenza season, despite the drifted A/H3N2 strain predominating the season, influenza B accounted for 15.3% of influenza cases, with the majority of influenza B cases reported during the latter part of the influenza season.[Citation20] Another study based on regression modeling, over 12 winter seasons, estimated that influenza B was responsible for 29% (1–95%) of all influenza-attributable mortality regardless of age between October 1997 and March 2009.[Citation21] From yet another study, it was shown that during nine influenza seasons (2001 to 2011), influenza B was responsible for the significant disease burden compared to influenza A in terms of general practitioner (GP) visits in 5–17 years old (43% influenza-related GP visits). Comparable effects were observed in adults <65 years old (50% influenza-related GP visits) and adults ≥65 years old (26% influenza-related GP visits).[Citation22]
Burden of influenza B in Europe
In England and Wales, during 12 consecutive influenza seasons (1996 to 2008), influenza B accounted for at least 40% of all influenza detections in 5/12 different influenza seasons. Over these five seasons, the average number of respiratory deaths attributed to influenza B was 2,456 per year. By comparison, 12,500 deaths per year were attributed to influenza A for the seven remaining seasons.[Citation23] During the same period (1996 to 2008), the relative proportion of GP visits due to influenza B was 24%, and the remainder was due to influenza A.[Citation24] Data from the UK (October 2007 to May 2008) showed that the overall proportions of influenza cases across all age groups were 69.2% and 30.8% for Influenza A and Influenza B, respectively.[Citation15] In contrast, influenza B was the prevalent type in children aged ≤14 years (94.7%), and the remaining cases were due to influenza A/H3N2.[Citation25] Similar trends for influenza B-associated burden were observed for the same period in other European countries such as Italy (all age-groups: 48%; children aged ≤14 years: 74%) and Spain (all age-groups: 29%; children aged ≤14 years: 60%).[Citation25]
Influenza B co-circulation and mismatch
The strains for inclusion in the annual seasonal influenza vaccine are recommended annually by the World Health Organization (WHO) based on the predominant virus types that are expected to circulate during the forthcoming influenza season for the northern and the southern hemisphere.[Citation1,Citation26] The commonly used seasonal influenza vaccines are trivalent formulations including two influenza A subtypes (A/H1N1 and A/H3N2) and only one influenza B lineage (B/Victoria or B/Yamagata).
Since the last decade, circulation of both influenza B lineages in one influenza season has been largely documented.[Citation27–Citation41] Although influenza viruses evolve relatively slowly, population immunity can unpredictably prompt a rapid change in the dominant B lineage, including during the time between vaccine virus selection and the onset of influenza season. If this occurs, the lineage of the influenza B strain recommended for inclusion in inactivated trivalent influenza vaccines (IIV3s) may no longer match the predominant circulating B lineage, resulting in a vaccine B lineage mismatch. Vaccine B lineage mismatch occurs frequently. For example, in the US, at least 50% mismatch of the predominant B lineage with the vaccine-recommended B lineage was reported in six of 13 influenza seasons between 2000 and 2013.[Citation42,Citation43] Similar occurrences of at least 50% mismatch of the predominant B lineage with the vaccine-recommended B lineage was observed between 2001 and 2013 in Germany (6 of 13 influenza seasons [Citation28–Citation39]), the UK (5 of 13 influenza seasons [Citation40]) and Brazil (5 of 13 influenza seasons [Citation41]). The public health consequences resulting from a vaccine mismatch depends on several factors such as influenza viral strain virulence and transmission dynamics, previous experience in the population to the drift strain, and cross-reactivity responses induced by vaccination. Mismatch between formulated IIV3s and the prevalent strains may result in severe illness and economic burden.[Citation2,Citation44–Citation46] The cross-protection against the circulating B lineage not included in the IIV3s is limited. Thus, these vaccines provide inadequate protection in the event of significant co-circulation of both influenza B lineages or during mismatched seasons.[Citation42,Citation47,Citation48]
Prevention of seasonal influenza
According to the WHO, vaccine effectiveness (VE) of seasonal influenza vaccines, estimated in observational studies, is a measure of how well the seasonal influenza vaccines prevents influenza illness in the general population during a given influenza season. Influenza B lineage mismatch could result in partial/incomplete protection of individuals when IIV3s are deployed; the effect of B lineage mismatch on VE is illustrated by the examples summarized in .[Citation48–Citation50]
Table 1. Examples of impact of B-lineage mismatch on vaccine effectiveness.
In contrast to VE studies as explained above, randomized and blinded controlled trials provide well-controlled relatively unbiased estimates of the effect of IIVs on the risk of acquiring influenza (i.e., vaccine efficacy). A recent meta-analysis of 34 randomized controlled trials evaluated the impact of lineage mismatch on vaccine efficacy against laboratory-confirmed influenza.[Citation51] This meta-analysis provides confirmation that IIV3s efficacy against influenza B (77% [95% confidence interval [CI]: 18–94]) is reduced when there is a mismatch for the B-lineage (52% [95% CI: 19–72]). The suboptimal protection afforded by IIV3s when there is unpredicted B lineage mismatch can be resolved by the use of inactivated quadrivalent influenza vaccines (IIV4s). The clinical development required to support licensure of the two GSK Vaccines’ IIV4s are described below.
Influenza vaccines
Currently, multiple influenza vaccines are licensed for use in Europe and the US (Supplementary File 1).[Citation52,Citation53]
GSK’s quadrivalent influenza vaccines
GSK Vaccines manufactures two IIV4s: Fluarix™ Quadrivalent, produced in Dresden, Germany (D-IIV4) and FluLaval™ Quadrivalent, produced in Quebec, Canada (Q-IIV4). GSK Vaccines acquired these 2 separate manufacturing sites in Dresden and Quebec which have slightly differing manufacturing processes, which is the rationale why GSK Vaccines has two IIV4 vaccines with minor differences in excipients. The hemagglutinin antigen content in both GSK Vaccines’ IIV4s is identical (15 µg/strain). Fluarix™ Quadrivalent is approved in several countries for active immunization of individuals aged 3 years and onwards to prevent disease caused by the influenza A subtype viruses and B type viruses contained in the vaccine. FluLaval™ Quadrivalent is currently licensed in the US (for individuals aged 3 years and onwards) and in Canada and Mexico for individuals from ages of 6 months onwards.
Overview of clinical evidence for GSK’s influenza vaccines
Background on the clinical development of IIV4s
Clinical development of D-IIV4 began in 2008 and was planned to extend to Q-IIV4 in 2009. However, the emergence of influenza A/H1N1pdm09 and the WHO’s subsequent pandemic declaration forced postponement of this plan until the 2010−2011 influenza season. Both D-IIV4 and Q-IIV4 have been evaluated in large clinical development programs that have enrolled more than 39,000 subjects to support their licensure in numerous jurisdictions around the world. The majority of clinical trials in the programs were aimed to assess IIV4s’ immunogenicity and safety, and vaccine efficacy trials in specific populations are still planned as described below.
The efficacy of GSK Vaccines’ IIV3 (manufactured in Dresden; D-IIV3) for the prevention of influenza due to strains matched to the vaccine in healthy adults aged 18−64 was shown in 2006 − 2007 to be 66.9% (95% CI 51.9–77.4).[Citation54] This study was considered predictive of the efficacy of D-IIV4 because the manufacturing process for individual strains was unchanged; therefore, no clinical endpoint trial of D-IIV4 was considered necessary to support its benefit for adults and older children. On the other hand, the clinical benefit of D-IIV4 for children 6 to 35 months of age was unknown [Citation55]; therefore, a phase III trial to evaluate the efficacy of D-IIV4 for the prevention of influenza (moderate-to-severe illness, and any severity) in this age group was conducted over multiple seasons. Data from this study will become available in 2016 (NCT01439360).
The efficacy of GSK Vaccines’ IIV3 (manufactured in Quebec; Q-IIV3) for the prevention of influenza due to strains matched to the vaccine in healthy adults aged 18−49 years was estimated over two seasons in 2005 − 2007 to be 46.3% (lower limit [LL] 97.5% CI 9.8%).[Citation56] As this result did not satisfy the pre-specified success criterion, an evaluation of the efficacy of Q-IIV4 was considered necessary for licensure. Therefore, a phase III trial to evaluate the efficacy of Q-IIV4 for the prevention of influenza in healthy children 3 to 8 years of age was conducted (NCT01218308; 2010–2011).[Citation57] The data from this trial was considered to also be predictive of benefit for older children and adults.
Endpoints and immunogenicity threshold criteria in influenza vaccine trials
Antibody responses to the influenza virus hemagglutinin surface glycoprotein that are measured for each virus strain included in an IIV by the hemagglutination inhibition (HI) test are widely accepted indicators of active immunization that are predictive of clinical benefit. Consequently, all IIV4 clinical trials conducted to support licensure included HI antibody assessments before and after vaccination. Antibody titers ≥1:40 have been associated with protection from influenza illness in up to 50% of subjects,[Citation58,Citation59] and increasing titers of HI antibody may confer greater protection. Geometric mean titers (GMT) were obtained by computing the anti-log of the arithmetic mean of the log-transformed inverse titers. Subjects with HI antibody titers ≥1:10 were considered to be seropositive. HI antibody responses were described as seroconversion rate (SCR), seroprotection rate (SPR), and the seroconversion factor (SCF). Immunogenicity criteria for licensure are defined according to Center for Biologics Evaluation and Research (CBER [Citation60]) and Committee for Medicinal Products for Human Use[Citation61] ().
Table 2. Vaccine protection and immunogenicity criteria for licensure.
For both D-IIV4 and Q-IIV4 studies, noninferiority was demonstrated when the upper limit (UL) of the 2-sided 95% CI for the adjusted GMT ratio of IIV3/IIV4 was ≤ 1.5 and the UL of the 2-sided 95% CI for the SCR difference (IIV3 minus IIV4) was ≤10.0%. On the other hand, superiority for D-IIV4 (for the strain present in the quadrivalent formulation but not in the trivalent formulation) was demonstrated when the LL of the 2-sided 95% CI on the adjusted GMT ratio (D-IIV4/IIV3-Vic and D-IIV4/IIV3-Yam) was >1.0 and the LL of the 2-sided 95% CI for the SCR difference (D-IIV4 minus IIV3-Vic and D-IIV4 minus IIV3-Yam) was >0.0%. For Q-IIV4, the criteria were LL of the 2-sided 95% CI on the adjusted GMT ratio (Q-IIV4/IIV3-Vic and Q-IIV4/IIV3-Yam) was >1.5, and the LL of the 2-sided 95% CI for the SCR difference (Q-IIV4 minus IIV3-Vic and Q-IIV4 minus IIV3-Yam) was >10.0%. Additionally, manufacturing lot-to-lot consistency was demonstrated if the 2-sided 95% CI limit for the GMT ratio was between 0.67 and 1.5 for all four strains.
D-IIV4
Immunogenicity
Immunogenicity data for D-IIV4 are available from phase I–III studies. Clinical studies were conducted in adults aged 18–60 years,[Citation62] adults aged ≥18 years,[Citation63] children aged 18–47 months,[Citation64] and children aged 3–17 years and 6–35 months [Citation65] (see Supplementary File 2).
In a phase I/II study (NCT00714285; 2008–2009 [Citation62]) conducted in healthy adults aged 18–60 years (N = 209), D-IIV4 had noninferior immunogenicity relative to the D-IIV3 control (formulated with a B/Victoria strain) for the shared strains (per strain, UL 95% CI for adjusted GMT ratio [D-IIV3/D-IIV4 < 1.5]) and superior immunogenicity for the additional B strain (B/Yamagata) in the D-IIV4 ([LL 95% CI for HI GMT ratio [D-IIV4/D-IIV3] >1.0).[Citation62] These early results supported the likely acceptability of an IIV4 in primed adults and set the stage for a full phase III development.[Citation62]
In a phase III trial (NCT01204671; 2010–2011 [Citation63]) conducted in adults in stable health aged ≥18 years (N = 4,659), D-IIV4 had noninferior immunogenicity relative to two formulations of D-IIV3 (each formulated with either a B/Victoria or B/Yamagata strain, each) for the shared vaccine strains (per strain, LL 95% CI for adjusted GMT ratio [D-IIV3/D-IIV4] ≤1.5 and for SCR difference [D-IIV3 minus D-IIV4] ≤10%) and superior immunogenicity for the respective additional B lineage strain (LL 95% CI for adjusted GMT ratio [D-IIV4/D-IIV3] >1.0 and LL 95% CI SCR difference [D-IIV4 minus D-IIV3] >0.0%). In a sub-group analysis by age, though immune responses were generally lower for subjects aged ≥65 years than those aged 18–65 years, the D-IIV4 candidate was immunogenic for all four vaccine strains, and HI antibody responses against all strains fulfilled CBER immunogenicity acceptance criteria in both age strata (18–65 years, and ≥65 years).[Citation63] Importantly, D-IIV4 manufacturing consistency was confirmed by demonstrating equivalent immunogenicity per strain for three consecutive vaccine lots.[Citation63]
A phase II study (NCT00985790; 2009–2010 [Citation64]) was conducted in children aged 18–47 months who had received D-IIV3, formulated with a B/Yamagata strain) the previous year (vaccine primed, N = 192) and an independent cohort of children who had not (vaccine unprimed, N = 407). The objective was to assess the immunogenicity and safety of D-IIV4 relative to D-IIV3, formulated with a B/Victoria strain. Using similar noninferiority (UL 95% CI for adjusted GMT ratio [D-IIV3/D-IIV4] ≤1.5 and for SCR difference [D-IIV3 minus D-IIV4] ≤10.0%) and superiority (LL 95% CI for adjusted GMT ratio [D-IIV4/D-IIV3] >1.0 and for SCR difference [D-IIV4 minus D-IIV3] >0.0%) immunological thresholds defined earlier, GMTs were noninferior for D-IIV4 versus D-IIV3 formulated with a B/Victoria strain for shared vaccine strains in a pooled analysis of primed and unprimed children (adjusted GMT ratio: A/H1N1: 1.05; 95% CI: 0.84–1.33, A/H3N2: 1.02; 95% CI: 0.85–1.23, B/Yamagata: 1.05; 95% CI: 0.81–1.37). GMTs were superior for the alternate B-lineage (B/Yamagata: 4.25; 95% CI: 3.47–5.21). One dose of D-IIV4 in primed children and two D-IIV4 doses in unprimed children was immunogenic against all four vaccine strains – 87.0%, 88.6%, 69.8%, and 97.9% of children had post-vaccination titers of ≥1:40 against A/H1N1, A/H3N2, B/Victoria, and B/Yamagata, respectively.[Citation64]
In a phase III trial (NCT01196988; 2010–2011 [Citation65]) of children in stable health aged 3–17 years (N = 2,738), D-IIV4 had noninferior immunogenicity relative to D-IIV3, formulated with a B/Victoria or a B/Yamagata strain, against shared vaccine strains (per strain, UL 95% CI for adjusted GMT ratio [D-IIV3/D-IIV4] ≤1.5 and for SCR difference [D-IIV3 minus D-IIV4] ≤10.0%). D-IIV4 had superior immunogenicity relative to D-IIV3 for the alternate-lineage B lineage (LL 95% CI for adjusted GMT ratio [D-IIV4/D-IIV3] >1.0 and for SCR difference [D-IIV4 minus D-IIV3] >0.0%). D-IIV4 was immunogenic in children aged 3–17 years, with SCRs of 91.4%, 72.3%, 70.0%, and 72.5% against A/H1N1, A/H3N2, B/Victoria, and B/Yamagata, respectively. In the open-label arm of the same study, in children aged 6–35 months (N = 277), D-IIV4 was also immunogenic and elicited immune responses against all four vaccine strains with SCRs of 78.0%, 68.5%, 68.1%, and 82.3% against influenza A/H1N1, A/H3N2, B/Victoria, and B/Yamagata, respectively, thus encouraging further development of D-IIV4 in young children from the age of 6 months.[Citation65]
To confirm the benefit of D-IIV4 in children 6–35 months, a clinical endpoint study (NCT01439360) in 12,037 children aged 6–35 months [Citation66] is ongoing with the objective of showing efficacy of the D-IIV4 vaccine, with results expected in 2016.[Citation66]
Reactogenicity and safety
IIV4s contain 25% more inactivated split virus antigen quantified as hemagglutinin than do IIV3s; therefore, increased reactogenicity change in its safety profile were risks that had to be assessed in the clinical trial program. Overall, D-IIV4 had an acceptable safety profile in all four studies (Supplementary File 2). Most injection site and general adverse events (AEs) were transient in nature, mild to moderate in severity, and similar in frequency between D-IIV4 and IIV3 groups. In all studies,[Citation62–Citation65] the rates of unsolicited AEs over 21–28 days after vaccination and of medically attended and serious AE (SAE) during the entire clinical trials were comparable between D-IIV4 and IIV3 groups. These outcomes provided assurance that D-IIV4 posed no incremental safety risk relative to IIV3s.
Q-IIV4
Immunogenicity
Clinical development of Q-IIV4 was supported by early data generated with D-IIV4. Therefore, the initial trials of Q-IIV4 were in phase III for subjects aged 3 years and older. The initial phase III study of Q-IIV4 in healthy children also included a small uncontrolled evaluation in children 6−35 months of age. There have been two additional controlled trials of Q-IIV4 in children aged 6−35 months to support its eventual licensure in this group beyond Canada and Mexico. Overall, immunogenicity data are available for adults aged ≥18 years,[Citation67,Citation68] children aged 3–17 years,[Citation69] and children aged 6–35 months [Citation69–Citation71] (Supplementary File 3).
In a phase III study (NCT01196975; 2010–2011[Citation68]) conducted in adults in stable health aged ≥18 years (N = 1,703), Q-IIV4 had noninferior immunogenicity relative to Q-IIV3, formulated with either a B/Victoria or B/Yamagata strain, for the shared vaccine strains (per strain UL 95% CI for adjusted GMT [Q-IIV3/Q-IIV4] ≤1.5 and for SCR difference [Q-IIV3 minus Q-IIV4] ≤10%) and superior immunogenicity for the additional B lineage strain (LL 95% CI for adjusted GMT ratio [D-IIV4/D-IIV3] >1.5 and LL 95% CI SCR difference [Q-IIV4 minus Q-IIV3] >10%). Though immune responses were generally lower for subjects aged ≥65 years than those aged 18–65 years, the Q-IIV4 candidate was immunogenic for all four vaccine strains, and HI antibody responses against all strains fulfilled CBER immunogenicity acceptance criteria in both age strata (18–65 years and ≥65 years). Importantly, Q-IIV4 manufacturing consistency was confirmed by demonstrating equivalent immunogenicity per strain for three consecutive vaccine lots (NCT01440387; NCT01153685).[Citation67]
In a phase III trial (NCT01198756; 2010–2011 [Citation69]) conducted in children in stable health aged 3–17 years (N = 2,793), Q-IIV4 had noninferior immunogenicity relative to D-IIV3 for the shared strains (per strain, UL 95% CI for adjusted GMT [D-IIV3/Q-IIV4] ≤1.5 and for SCR difference [D-IIV3 minus Q-IIV4] ≤10%) and superior immunogenicity (LL 95% CI for adjusted GMT [Q-IIV4/D-IIV3] >1.5 and for SCR difference >10%) for the additional B lineage strain, suggesting broader protection. In an open-label arm of the study conducted in children aged 6–35 months (N = 301), Q-IIV4 was less immunogenic than in children aged 3–17 years, but nevertheless met CBER immunogenicity acceptance criteria.[Citation69]
A phase II randomized controlled study (NCT01974895; 2013–2014 [Citation71]) of Q-IIV4 was conducted in US children aged 6–35 months (N = 314) using the only US-licensed IIV3 for this age group as control vaccine.[Citation71] Q-IIV4 was shown to be immunogenic with SCRs of 80.4%, 72.0%, 86.0%, and 66.4% against the A/H1N1, A/H3N2, B/Yamagata, and B/Victoria strains, respectively; these results met CBER SCR licensure criteria. Notably, Q-IIV4 had similar immunogenicity for the shared vaccine strains relative to the IIV3, formulated with a B/Yamagata strain, and superior immunogenicity for the additional B/Victoria strain. Based on these results, a phase III randomized controlled study (NCT01711736; 2012–2013 [Citation70]) was conducted in children aged 6–35 months (N = 601) using D-IIV3 as a control vaccine in this age group. That study demonstrated that Q-IIV4 had noninferior immunogenicity relative to D-IIV3 for the shared strains (per strain, UL 95% CI for adjusted GMT [D-IIV3/Q-IIV4] ≤1.5) and superior immunogenicity (LL 95% CI for adjusted GMT [Q-IIV4/D-IIV3] >1.5 and for SCR difference [D-IIV3 minus Q-IIV4]>10%) for the additional B lineage.[Citation70] This phase III study has set the stage for a pivotal phase III trial (NCT02242643, 2014–2015) conducted in healthy children aged 6 − 35 months in the US and Mexico to evaluate the immunogenic noninferiority of Q-IIV4 versus the only US-licensed IIV4 for this age group. Results from this trial will become available in 2016.
The above clinical trials were performed with a preservative-free formulation of Q-IIV4 presented in pre-filled syringes. In order to offer a multidose vial presentation of Q-IIV4, a formulation in which thimerosal as a preservative was added as a terminal step, had to be tested for acceptable immunogenicity. This was done in a phase III study (NCT01440387; 2011–2012 [Citation67]) conducted in adults in stable health aged ≥18 years (N = 112) where enrollment was equally divided into two age strata (18–60 years and >60 years).[Citation67] A study of the same design conducted in a similar age-stratified population (N = 120) one year earlier using the previously licensed formulation of Q-IIV3 (NCT01153685; 2010–2011), in which thimerosal was added as a preservative in a very early process step, served as a control.[Citation67] Q-IIV3 and Q-IIV4 were immunogenic in both age strata (18–60 years and >60 years). Q-IIV4 produced by the new manufacturing process elicited HI antibody responses in adults aged >60 years that met or exceeded all three immunogenicity acceptance criteria for the four vaccine strains (SCR >30%, SPR >60%, SCF >2.0); the immune responses of adults aged 18–60 years met or exceeded all three immunogenicity acceptance criteria for the two influenza A vaccine strains (SCR >40%, SPR >70%, SCF >2.5) and two immunogenicity acceptance criteria (SPR >70%, SCF >2.5) for the two influenza B vaccine strains. SCRs against both influenza B lineages were below the immunogenicity acceptance criteria, which is observed in the past, given the particularly high baseline GMTs against the influenza B lineages.[Citation67]
Efficacy
Demonstration of Q-IIV4 efficacy was necessary to attain licensure of Q-IIV4. This requirement was met in a phase III study (NCT01218308; 2010–2011 [Citation57]) conducted in healthy children aged 3 − 8 years (N = 5,220) who were randomized to receive Q-IIV4 or inactivated hepatitis A vaccine as control (Supplementary File 3). The clinical endpoints were real-time polymerase chain reaction-confirmed influenza A or B of any severity (body temperature of 37.8 °C or higher, with at least one of the following: cough, sore throat, runny nose, or nasal congestion) or of moderate to severe intensity (body temperature higher than 39 °C, physician-confirmed acute otitis media, lower respiratory tract illness, or serious extrapulmonary complications such as myositis, encephalitis, seizure, or myocarditis). The moderate-to-severe secondary end-point captures clinically significant outcomes leading to healthcare consultations or hospitalizations. Q-IIV4 efficacy was 55.4% (97.5% CI: 39.1–67.3) against influenza of any severity and 73.1% (97.5% CI: 47.1–86.3) against moderate-to-severe influenza. Most breakthrough cases in the Q-IIV4 group were of mild intensity. Among children with moderate-to-severe disease, the Q-IIV4, as compared with the control vaccine, was associated with 69% fewer medical visits, 75% fewer hospitalizations, 77% fewer absences from school, and 61% fewer parental absences from work.
Reactogenicity and safety
The reactogenicity of the Q-IIV4 in all age groups was comparable to IIV3s (Supplementary file 3) and to that of D-IIV4 (Supplementary file 2), with one exception. Injection site pain was modestly more common with Q-IIV4 (>30%) than D-IIV3 control (>20%) in children aged 3–17-years after the first dose. In the two randomized controlled phase II studies of Q-IIV4 in children aged 6–35 months,[Citation69,Citation70] the frequencies of both injection site and systemic AEs including fever, notable for its link with febrile seizures, following vaccination were comparable in the Q-IIV4 and IIV3 recipients, after dose 1 or 2, and across the age strata (6−17 months and 18−35 months of age).[Citation71] In all studies,[Citation57,Citation67–Citation71] regardless of age group, the rates of unsolicited AEs over 21−28 days after vaccination and of medically attended and SAE during the entire clinical trials were comparable between Q-IIV4 and control groups. These outcomes provided assurance that Q-IIV4 posed no incremental safety risk relative to IIV3s.
Cross-reactivity profiles of D-IIV4 and Q-IIV4
Background
The efficacy or effectiveness of an IIV depends on factors such as age, health status, vaccine coverage, and importantly, also antigenic match between the vaccine strains and those circulating. As shown above, data illustrating the detrimental effect of B lineage mismatch on protection were summarized. Nevertheless, even with vaccine B lineage mismatch, IIV3s may afford some cross-protection attributable to cross-reactive humoral immunity that is boosted after vaccination. Nonetheless, this cross-protection has been suggested to be low.[Citation42,Citation45,Citation48,Citation72]
Cross-reactivity between influenza B lineages
We performed a systematic post-hoc analysis in the pivotal phase III trials (NCT01196988 [Citation65], NCT01204671 [Citation63], NCT01198756 [Citation69], NCT01196975 [Citation68]) to evaluate the levels of HI antibody B-lineage cross-reactivity observed in IIV3 controls. This was expressed as the post-vaccination GMT ratio (IIV4/IIV3) for the B lineage not covered in the trivalent vaccine, and the results were stratified according to whether subjects were seronegative (HI antibody <1:10) or seropositive to the IIV3-omitted B lineage at baseline. We hypothesized that individuals who were seronegative for at least one B lineage vaccine strain would elicit lower cross-reactive antibody responses, potentially placing them at higher risk of breakthrough infection on exposure. For these individuals, the administration of an IIV4 rather than an IIV3 would be expected to provide enhanced benefit.
As shown in and , B lineage cross-reactive HI antibody responses were consistently observed in the IIV3 groups, but always at a lower level than in the IIV4 groups. The contrast in response was greater for those who were seronegative at baseline. Persons seropositive to the B lineage absent in IIV3s on average showed improvements in their HI antibody response to that B lineage by 2-fold when they received IIV4s instead of IIV3s. Persons seronegative to the B lineage omitted in IIV3s on average improved their HI antibody response to that lineage by 4-fold, if they receive IIV4s instead of IIV3s. As hypothesized, IIV4s afforded the greatest improvement in HI response (the magnitude of which is associated with reduced risk of influenza illness) to seronegative individuals, but IIV4s also afforded benefit to seropositive individuals. Based on data from clinical trials conducted in North America and Europe in 2010−2011, the risk of being seronegative for HI antibodies to at least one of the B lineages decreases with age, but is 5−10% even for individuals aged 65 years or more. In summary, some degree of cross-reactivity to the non-vaccine influenza B lineage was observed in the IIV3 groups. However, when there is a mismatch between the vaccine-recommended B lineage and the circulating lineage, use of IIV3s could potentially leave a proportion of the vaccinated population more vulnerable to influenza B and its complications, whereas use of IIV4s maximizes prevention of influenza B.
Figure 1. Adjusted GMT data from D-IIV4 trials (by baseline status). (A) Children Aged 3–17 years. (B) Adults Aged ≥18 years.
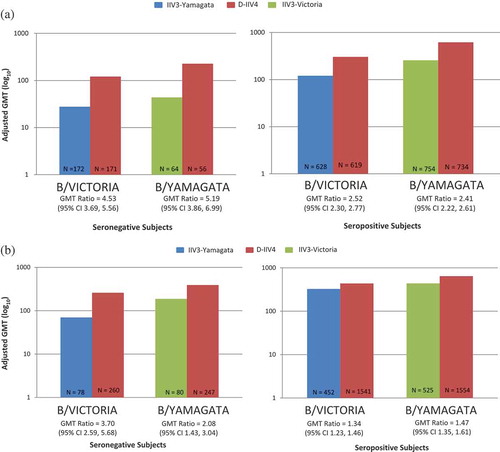
Figure 2. Adjusted GMT data from Q-IIV4 trials (by baseline status). (A) Children Aged 3–17 years. (B) Adults Aged ≥18 years.
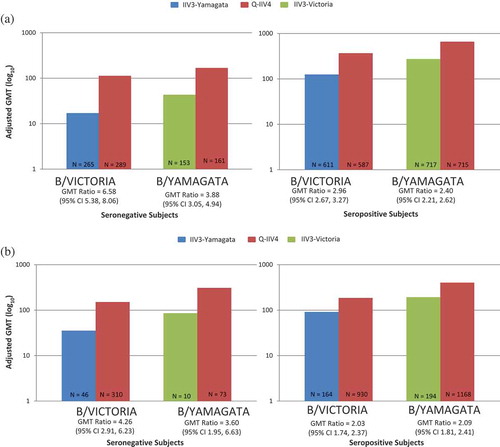
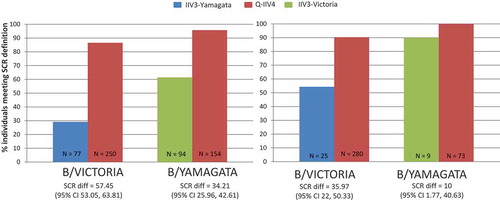
Additionally, it is observed that both IIV4 s are better positioned than the IIV3s to seroconvert individuals seronegative at baseline. Notably, in the baseline seropositive population, this benefit of IIV4s is also evident ( and ).
Figure 3. SCR data in baseline seronegative population from D-IIV4 trials. (A) Children Aged 3–17 years. (B) Adults Aged ≥18 years.
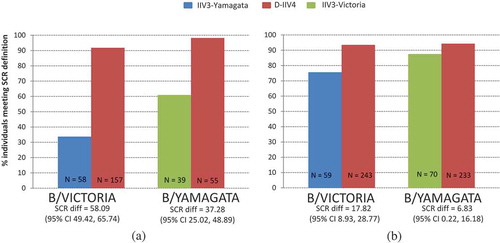
Figure 4. SCR data in baseline seronegative population from Q-IIV4 trials. (A) Children Aged 3–17 years. (B) Adults Aged ≥18 years.
Expert commentary
Global epidemiological data have demonstrated that influenza disease burden caused by influenza B virus could be high. In addition to this, epidemiological data showed that in approximately 50% of each influenza seasons over the last decade, there was a mismatch between the circulating B lineage and the included B lineage in trivalent vaccines. The consequence of this B mismatch may be lower vaccine efficacy and effectiveness, which results in a greater number of cases and burden on the healthcare system. Several influenza vaccine manufacturers have responded to the issue of frequent B lineage mismatch by developing quadrivalent vaccines that contains the additional B-lineage not previously included in the trivalent vaccines. GSK Vaccines has embarked on two large clinical development programs to develop two seasonal IIV4s, Fluarix™ Quadrivalent and FluLaval™ Quadrivalent. Both of these quadrivalent vaccines have demonstrated noninferior immunogenicity for the shared strains included in the IIV3s, as well as superior immunogenicity for the additional B-lineage included in the IIV4s across adult and pediatric age groups included in the trials. Antibody titers ≥1:40 have been associated with protection from influenza illness in up to 50% of subjects [Citation58,Citation59]; increasing titers of HI antibody may confer greater protection. One potential limitation is the limited data available for the very young infants (i.e., 6–35 mo) and future immunogenicity and efficacy data in this age group is anticipated to fill this data gap. The addition of the fourth strain did not impair the safety profile of the IIV4s, and the safety profile is identical to that documented with IIV3s use. FluLaval™ Quadrivalent has also demonstrated efficacy in children against any influenza as well as moderate-severe influenza, which provides information on clinically relevant outcomes.
With the development of these two IIV4s from GSK and other manufacturers, the WHO, since 2012, has recommended the inclusion of an additional B-lineage in quadrivalent vaccines manufacturing for both northern and southern hemispheres.[Citation73] Additionally, national authorities in Canada and the US have started acknowledging the value of IIV4s and thus recommending it in their respective national guidelines.[Citation26,Citation74] Some recent reviews suggest that this trend will continue and it can be anticipated that future standard of influenza prevention will move towards influenza vaccination with quadrivalent vaccines. The wider utilization of quadrivalent vaccines is expected to further reduce the burden of influenza.[Citation27,Citation75]
Five-year view
Globally, there are four licensed quadrivalent vaccines being marketed as of 2015.[Citation26] We expect that in the next five years, more quadrivalent vaccines will become available. We expect the standard of influenza protection to evolve from currently used trivalent formulations to quadrivalents. This evolution of standard of care may also include quadrivalent vaccines containing adjuvants as well as vaccines manufactured from egg-free processes (i.e., cell-culture, plant-based, recombinant technology etc.).[Citation76,Citation77] It seems clear that quadrivalent vaccines will be the standard of care until they are displaced by a vaccine offering more universal or multi-season protection.
Supplementary material
Supplemental data for this article can be accessed here.
Influenza B is responsible for significant overall influenza-related morbidity and mortality worldwide.
Influenza B-lineage co-circulation and varying degrees of mismatch contribute to additional public health burden.
Quadrivalent influenza vaccines aim to address this problem by providing broader protection against both influenza B lineages, as compared to trivalent influenza vaccines.
GSK Vaccines manufactures two inactivated quadrivalent influenza vaccines (IIV4) with comparable profiles: D-IIV4 (Fluarix™ Tetra, manufactured in Dresden, Germany) and Q-IIV4 (FluLaval™ Tetra, manufactured in Quebec, Canada). Both these vaccines are also licensed in some countries under the names Fluarix™ Quadrivalent and FluLaval™ Quadrivalent.
Both D-IIV4 and Q-IIV4 have demonstrated noninferior immunogenicity to the influenza strains shared with the inactivated trivalent influenza vaccines (IIV3s) and superior immunogenicity to the additional B-lineage not present in the counterpart IIV3s.
Both D-IIV4 and Q-IIV4 are licensed as of 3 years of age, except for the Q-IIV4 which is licensed from 6 months of age in Canada and Mexico.
Both D-IIV4 and Q-IIV4 have demonstrated similar reactogenicity and safety profiles compared to the IIV3s, despite their increase in antigen content (60 µg vs 45 µg).
Q-IIV4 has also demonstrated 55% efficacy against influenza of any severity and 73% efficacy against moderate-to-severe influenza.
Future trend will be towards greater adoption of quadrivalent influenza vaccines globally with multiple manufacturers gaining marketing authorization for quadrivalent influenza vaccines currently in clinical development.
Trademarks
InfluvacTM and ImuvacTM are trademarks of Abbot Healthcare. FluenzTM, FlumistTM, FluenzTM Tetra, and FlumistTM Quadrivalent are trademarks of AstraZeneca. FluarixTM, AlpharixTM, InflusplitTM, FluviralTM, FluarixTM Tetra, AlpharixTM Tetra, InflusplitTM Tetra, FluarixTM Quadrivalent, and FlulavalTM Quadrivalent are trademarks of the GSK group of companies. AgrippalTM, FluvirinTM, OptafluTM, FluadTM, AgrifluTM, and FlucelvaxTM are trademarks of Novartis. Fluval ABTM is a trademark of Omnivest. EnziraTM is a trademark of Pfizer/CSL Australia. VaxigripTM, IntanzaTM, FluzoneTM, FluzoneTM High-Dose, FluzoneTM Quadrivalent, and FluzoneTM Intradermal Quadrivalent are trademarks of Sanofi Pasteur. FluBlokTM is a trademark of Protein Sciences. AfluriaTM is a trademark of Pfizer/CSL Australia/BioCSL.
Financial & competing interests disclosure
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the studies conducted and analysis. GlaxoSmithKline Biologicals SA also funded all costs associated with the development and the publishing of the present manuscript. All authors had full access to the data and agreed with the submission of the publication. All authors are employees of the GSK group of companies. R Bekkat-Berkani, R Ray, VK Jain, and BL Innis report ownership of stock options and/or restricted shares. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. R Bekkat-Berkani, R Ray, VK Jain, V Chandrasekaran, and BL Innis were involved in the conception and/or the design of the project. R Bekkat-Berkani, R Ray, and BL Innis participated in the collection or generation of the project data. R Ray, VK Jain, V Chandrasekaran, and BL Innis performed the project. R Bekkat-Berkani, R Ray, VK Jain, and V Chandrasekaran contributed materials and/or analysis tools. R Ray, VK Jain, V Chandrasekaran, and BL Innis were involved in the analyses and/or interpretation of the data. All authors reviewed the manuscript and approved the final version prior submission.
FLU_Review_Clinical_supp_files_revised_for_cats.docx
Download MS Word (51.8 KB)Acknowledgement
The authors thank Gael Dos Santos (Business & Decision Life Sciences on behalf of GSK Vaccines) for his review of the paper, Amrita Ostawal for medical writing services (consultant publications writer to GSK Vaccines), and Bruno Dumont (Business & Decision Life Sciences on behalf of GSK Vaccines) for editorial assistance and publication coordination.
References
- Reference annotations
- • Of interest
- •• Of considerable interest
- World Health Organization. [ cited 2015 Jul]. Available from: http://www.who.int/mediacentre/factsheets/fs211/en/
- Karve S, Meier G, Davis KL, et al. Influenza-related health care utilization and productivity losses during seasons with and without a match between the seasonal and vaccine virus B lineage. Vaccine. 2013;31(33):3370–3388.
- Keech M, Beardsworth P. The impact of influenza on working days lost: a review of the literature. Pharmacoeconomics. 2008;26(11):911–924.
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–5096.
- Xue Y, Kristiansen IS, de Blasio BF. Modeling the cost of influenza: the impact of missing costs of unreported complications and sick leave. BMC Public Health. 2010;10:724.
- Centers for Disease Control and Prevention. The Pink Book: Influenza 2012. [cited 2015 Apr]. Available from http://www.cdc.gov/vaccines/pubs/pinkbook/flu.html
- Glezen WP. Emerging infections: pandemic influenza. Epidemiol Rev. 1996;18(1):64–76.
- Zhou H, Thompson WW, Viboud CG, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis. 2012;54(10):1427–1436.
- Centers for Disease Control and Prevention. How the flu virus can change: “drift” and “shift” [ cited 2015 Apr]. Available from: http://www.cdc.gov/flu/about/viruses/change.htm
- Irving SA, Patel DC, Kieke BA, et al. Comparison of clinical features and outcomes of medically attended influenza A and influenza B in a defined population over four seasons: 2004-2005 through 2007-2008. Influenza Other Respir Viruses. 2012;6(1):37–43.
• The findings from this paper demonstrate that symptoms of influenza A and influenza B are identical.
- McCullers JA, Huber VC. Correlates of vaccine protection from influenza and its complications. Hum Vaccin Immunother. 2012;8(1):34–44.
- Rota PA, Wallis TR, Harmon MW, et al. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology. 1990;175(1):59–68.
• The paper provides a comprehensive overview on the entire evolution of the influenza B lineages.
- Centers for Disease Control and Prevention. Influenza season summaries [ cited 2015 Apr]. Available from: http://www.cdc.gov/flu/weekly/pastreports.htm
- Centers for Disease Control and Prevention. Influenza season summaries [ cited 2015 Apr]. Available from: http://www.cdc.gov/flu/weekly/pastreports.htm
- World Health Organization. FluNet (UK) [ cited 2015 May]. Available from http://www.who.int/influenza/gisrs_laboratory/flunet/en/
- Robert Koch Institut. Influenza-assoziierte Mortalität in Deutschland 1985–2006 [ cited 2015 May]. Available at: http://edoc.rki.de/documents/rki_fv/re3BNEVpkzVE/PDF/26k5qbYqF4loFg.pdf
- Robert Koch Institut. Bericht zur Epidemiologie der Influenza in Deutschland Saison 2012/13 [ cited 2015 May]. Available from: https://influenza.rki.de/Saisonberichte/2012.pdf
- World Health Organization. FluNet (Brazil) [ cited 2015 May]. Available from: http://www.who.int/influenza/gisrs_laboratory/flunet/en/
- Paul Glezen W, Schmier JK, Kuehn CM, et al. The burden of influenza B: a structured literature review. Am J Public Health. 2013;103(3):e43–51.
- Centers for Disease Control and Prevention. FluView. Week 16: April 19, 2015 [ cited 2015 May]. Available from: http://www.cdc.gov/flu/weekly/pdf/External_F1513.pdf
- Matias G, Taylor R, Haguinet F, et al. Estimates of age-specific influenza-related mortality and hospitalizations in the United States from 1997 to 2009. Poster presented at Options for the Control of Influenza VIII. Vol. P1–200. 2013 Sep 5–10; Cape Town. p. 146.
- Matias G, Haguinet F, Schuck-Paim C, et al. Model estimates of age-specific influenza-attributable physician office visits in the United States in recent years. Poster presented at The 19th WONCA Europe Conference; 2014 Jul 2–5; Lisbon, Portugal. [cited 2015 May]. Available from: http://www.woncaeurope2014.org/en
- Matias G, Lustig R, Schuck-Paim C, et al. Model estimates of age specific influenza related mortality in the UK in recent years. Poster presented at Influenza Vaccines for the World (IVW 2012); 2012 Oct 9–12; Valencia. [cited 2015 May]. Available from: http://www.meetingsmanagement.co.uk/index.php?option=com_content&view=article&id=6&Itemid=7
- Matias G, Taylor R, Haguinet F, et al. Model estimates of age-specific influenza-related outpatient visits in the UK in recent years. Poster presented at Options for the Control of Influenza VIII. Vol. P1–203. 2013 Sep 5–10; Cape Town. p. 148–149.
- Paget WJ, Balderston C, Casas I, et al. Assessing the burden of paediatric influenza in Europe: the European Paediatric Influenza Analysis (EPIA) project. Eur J Pediatr. 2010;169(8):997–1008.
- Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices--United States, 2013-2014. MMWR Recomm Rep. 2013;62(RR–07):1–43.
•• This is an example of a national authority (US-CDC) acknowledging the clinical value offered by a quadrivalent vaccine.
- Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother. 2012;8(1):81–88.
- Robert Koch Institut, influenza season reports. [ cited 2015 July]. Available from: https://influenza.rki.de/Saisonberichte/2012.pdf
- Robert Koch Institut, influenza season reports. [ cited 2015 Jul]. Available from: https://influenza.rki.de/Saisonberichte/2011.pdf
- Robert Koch Institut, influenza season reports. [ cited 2015 Jul]. Available from: https://influenza.rki.de/Saisonberichte/2010.pdf
- Robert Koch Institut, influenza season reports. [ cited 2015 Jul]. Available from: https://influenza.rki.de/Saisonberichte/2009.pdf
- Robert Koch Institut, influenza season reports. [ cited 2015 Jul]. Available from: https://influenza.rki.de/Saisonberichte/2008.pdf
- Robert Koch Institut, influenza season reports. [ cited 2015 Jul]. Available from: https://influenza.rki.de/Saisonberichte/2007.pdf
- Robert Koch Institut, influenza season reports. [ cited 2015 Jul]. Available from: https://influenza.rki.de/Saisonberichte/2006.pdf
- Robert Koch Institut, influenza season reports. [ cited 2015 Jul]. Available from: https://influenza.rki.de/Saisonberichte/2005.pdf
- Robert Koch Institut, influenza season reports. [ cited 2015 Jul]. Available from: https://influenza.rki.de/Saisonberichte/2004.pdf
- Robert Koch Institut, influenza season reports. [ cited 2015 Jul]. Available from: https://influenza.rki.de/Saisonberichte/2003.pdf
- Robert Koch Institut, influenza season reports. [ cited 2015 Jul]. Available from: https://influenza.rki.de/Saisonberichte/2002.pdf
- Robert Koch Institut, influenza season reports. [ cited 2015 Jul]. Available from: https://influenza.rki.de/Saisonberichte/2001.pdf
- Health Protection Agency. Annual reports [ cited 2015 Jul]. Available from: https://www.gov.uk/government/statistics/annual-flu-reports
- Bricks L, Paiva T, Carvalhanas T, et al. Influenza B circulation in Brazil and characterization of 75 B strains isolated from patients from São Paulo state, Brazil (2002-2013). Abstract 026, presented at ESPID 2014; 2014. [cited 2015 May]. Available from: http://cmoffice.kenes.com/cddemo/data/HtmlApp/main.html#open-authors
- Belshe RB, Coelingh K, Ambrose CS, et al. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine. 2010;28(9):2149–2156.
- Centers fo Disease Control and Prevention. FluView Surveillance Reports Past Seasons (2010–2011 to 2013–2014) [ cited 2015 Apr]. Available from: http://www.cdc.gov/flu/pastseasons/index.htm
- Eiros-Bouza JM, Perez-Rubio A. Burden of influenza virus type B and mismatch with the flu vaccine in Spain. Rev Esp Quimioter. 2015;28(1):39–46.
- Heikkinen T, Ikonen N, Ziegler T. Impact of Influenza B Lineage-Level Mismatch Between Trivalent Seasonal Influenza Vaccines and Circulating Viruses, 1999-2012. Clin Infect Dis. 2014;59(11):1519–1524.
•• A retrospective population-level study of lineage-level mismatch between the vaccine and circulating strains of influenza B viruses showing substantial adverse impact, especially among children and adolescents.
- Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007;25(39–40):6852–6862.
- Skowronski DM, De Serres G, Dickinson J, et al. Component-specific effectiveness of trivalent influenza vaccine as monitored through a sentinel surveillance network in Canada, 2006-2007. J Infect Dis. 2009;199(2):168–179.
- Skowronski DM, Janjua NZ, Sabaiduc S, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011-2012 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis. 2014;210(1):126–137.
- Menniti-Ippolito F, Da Cas R, Traversa G, et al. Vaccine effectiveness against severe laboratory-confirmed influenza in children: results of two consecutive seasons in Italy. Vaccine. 2014;32(35):4466–4470.
- Heinonen S, Silvennoinen H, Lehtinen P, et al. Effectiveness of inactivated influenza vaccine in children aged 9 months to 3 years: an observational cohort study. Lancet Infect Dis. 2011;11(1):23–29.
- Tricco AC, Chit A, Soobiah C, et al. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. 2013;11:153.
•• A large meta-analyses demonstrating drop in vaccine efficacy due to B-strain mismatch.
- European Centre for Disease Prevention and Control. Influenza Vaccination [ cited 2015 Aug]. Available from: http://ecdc.europa.eu/en/healthtopics/seasonal_influenza/vaccines/Pages/influenza_vaccination.aspx
- Centers for Disease Control and Prevention. Influenza (Flu) [ cited 2015 Aug]. Available from: http://www.cdc.gov/flu/protect/vaccine/vaccines.htm
- Beran J, Vesikari T, Wertzova V, et al. Efficacy of inactivated split-virus influenza vaccine against culture-confirmed influenza in healthy adults: a prospective, randomized, placebo-controlled trial. J Infect Dis. 2009;200(12):1861–1869.
- Pavia-Ruz N, Angel Rodriguez Weber M, Lau YL, et al. A randomized controlled study to evaluate the immunogenicity of a trivalent inactivated seasonal influenza vaccine at two dosages in children 6 to 35 months of age. Hum Vaccin Immunother. 2013;9(9):1978–1988.
- Jackson LA, Gaglani MJ, Keyserling HL, et al. Safety, efficacy, and immunogenicity of an inactivated influenza vaccine in healthy adults: a randomized, placebo-controlled trial over two influenza seasons. BMC Infect Dis. 2010;10:71.
- Jain VK, Rivera L, Zaman K, et al. Vaccine for prevention of mild and moderate-to-severe influenza in children. N Engl J Med. 2013;369(26):2481–2491.
- Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004;103(1–2):133–138.
- Hobson D, Curry RL, Beare AS, et al. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) . 1972;70(4):767–777.
- Center for Biologics Evaluation and Research (CBER). Guidance for Industry: Clinical data needed to support the licensure of seasonal inactivated influenza vaccines. Food and Drug Administration (FDA) [ cited 2015 Jul]. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091990.pdf
- Committee for Proprietary Medicinal Products (CPMP): Note for guidance on harmonisation of requirements for influenza vaccines. The European Agency for the Evaluation of Medicinal Products [ cited 2015 Jul]. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf
- Beran J, Peeters M, Dewe W, et al. Immunogenicity and safety of quadrivalent versus trivalent inactivated influenza vaccine: a randomized, controlled trial in adults. BMC Infect Dis. 2013;13:224.
- Kieninger D, Sheldon E, Lin WY, et al. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged >/=18 years. BMC Infect Dis. 2013;13:343.
- Rodriguez Weber MA, Claeys C, Aranza Doniz C, et al. Immunogenicity and safety of inactivated quadrivalent and trivalent influenza vaccines in children 18-47 months of age. Pediatr Infect Dis J. 2014;33(12):1262–1269.
- Domachowske JB, Pankow-Culot H, Bautista M, et al. A randomized trial of candidate inactivated quadrivalent influenza vaccine versus trivalent influenza vaccines in children aged 3-17 years. J Infect Dis. 2013;207(12):1878–1887.
- 115345. GSK Clinical Study Register. [ cited 2015 Aug]. Available from: http://www.gsk-clinicalstudyregister.com/study/115345?study_ids=115345#ps
- Jain VK, Chandrasekaran V, Wang L, et al. A historically-controlled Phase III study in adults to characterize the acceptability of a process change for manufacturing inactivated quadrivalent influenza vaccine. BMC Infect Dis. 2014;14:133.
- Tinoco JC, Pavia-Ruz N, Cruz-Valdez A, et al. Immunogenicity, reactogenicity, and safety of inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine in healthy adults aged >/=18 years: a phase III, randomized trial. Vaccine. 2014;32(13):1480–1487.
- Langley JM, Carmona Martinez A, Chatterjee A, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine candidate: a phase III randomized controlled trial in children. J Infect Dis. 2013;208(4):544–553.
- Langley JM, Wang L, Aggarwal N, et al. Immunogenicity and reactogenicity of an inactivated quadrivalent influenza vaccine administered intramuscularly to children 6 to 35 months of age in 2012–2013: a randomized, double-blind, controlled, multicenter, multicountry, clinical trial. J Pediatric Infect Dis Soc. 2014;4(3):242–251.
- Wang L, Chandrasekaran V, Domachowske JB, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine in US children 6–35 months of age during 2013–2014: results from a phase II randomized trial. J Pediatric Infect Dis Soc. 2015. pii: piv0419. [Epub ahead of print].
- Pebody RG, Andrews N, Fleming DM, et al. Age-specific vaccine effectiveness of seasonal 2010/2011 and pandemic influenza A(H1N1) 2009 vaccines in preventing influenza in the United Kingdom. Epidemiol Infect. 2013;141(3):620–630.
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2012-2013 northern hemisphere influenza season 2012. [cited 2015 Aug]. Available from: http://www.who.int/influenza/vaccines/virus/recommendations/201202_recommendation.pdf?ua=1
- Canada Advisory Committee Statement on Seasonal Influenza Vaccine 2014-2015. [ cited 2015 Aug]. Available from: http://publications.gc.ca/collections/collection_2014/aspc-phac/HP40-114-2014-eng.pdf
- Zhang H, Wang L, Compans RW, et al. Universal influenza vaccines, a dream to be realized soon. Viruses. 2014;6(5):1974–1991.
- Flucelvax. [ cited 2015 Jul]. Available from: http://www.cdc.gov/flu/protect/vaccine/cell-based.htm
- Medicago. Seasonal flu vaccine [ cited 2015 Jul]. Available from: http://www.medicago.com/English/Products/Flu-vaccine/default.aspx
