Abstract
Ocular fibrosis leads to significant visual impairment and blindness in millions of people worldwide, and is one of the largest areas of unmet need in clinical ophthalmology. The antimetabolites, mitomycin C and 5-fluorouracil, are the current gold standards used primarily to prevent fibrosis after glaucoma surgery, but have potentially blinding complications like tissue damage, breakdown and infection. This review thus focuses on the development of new classes of small molecule therapeutics to prevent post-surgical fibrosis in the eye, especially in the context of glaucoma filtration surgery. We discuss recent advances and innovations in ophthalmic wound healing research, including antibodies, RNAi, gene therapy, nanoparticles, liposomes, dendrimers, proteoglycans and small molecule inhibitors. We also review the challenges involved in terms of drug delivery, duration of action and potential toxicity of new anti-fibrotic agents in the eye.
Fibrosis & wound healing
Fibrosis and scarring are involved in the pathogenesis or failure of treatment of virtually all the major blinding diseases. Glaucoma is the second leading cause of blindness in the world. By 2020, its prevalence is estimated to reach around 79.6 million people worldwide, with more than 11 million individuals suffering from bilateral blindness Citation[1]. Glaucoma filtration surgery is the mainstay of surgical treatment for medically uncontrolled glaucoma. Even with new surgical techniques, postoperative scarring remains a critical determinant of the long-term surgical outcome and intraocular pressure after drainage surgery Citation[2]. The antimetabolites, mitomycin C (MMC) and 5-fluorouracil (5-FU), have improved the surgical outcome of glaucoma filtration surgery, but lead to non-specific cytotoxicity and the risk of sight-threatening complications like tissue damage, breakdown and infection. In addition, some patients still scar and fail surgery despite antimetabolite therapy. There is thus a large unmet need to develop new anti-fibrotic therapeutics to prevent scarring and post-surgical fibrosis in the eye.
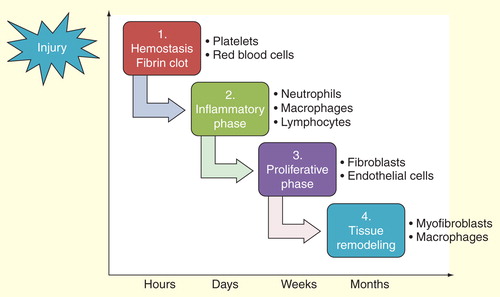
Wound healing is a complex multifactorial process consisting of a cascade of overlapping events, including hemostasis, inflammation, cell proliferation and tissue remodeling . After injury, hemostasis takes place, and blood and fibrin clots are formed to reduce blood loss. This leads to an inflammatory phase where neutrophils, macrophages and lymphocytes are attracted to the wound site as part of the immune response. This is then followed by a proliferative phase where fibroblasts are activated to lay down extracellular matrix, leading to re-epithelialization, angiogenesis and granulation tissue formation. The final step is tissue remodeling and scar formation, and matrix metalloproteinases (MMPs) play an important role in the process.
In this review, we focus on the development of novel small molecule therapeutics to modulate ophthalmic wound healing, especially in the context of post-surgical scarring after glaucoma filtration surgery. We discuss the recent advances and also the pitfalls of different types of therapeutic molecules, including antibodies, RNAi, gene therapy, nanoparticles, liposomes, dendrimers, proteoglycans and small molecule inhibitors, to prevent scarring and promote healthy cell regeneration in the eye.
Antibodies
Monoclonal antibodies are only a few nanometers in size, but currently represent one of the largest classes of therapeutic molecules. Unlike antimetabolites, one of the key advantages of an antibody is its target specificity in ocular therapeutics . TGF-β plays a pivotal role in wound healing as it increases fibroblast proliferation Citation[3], controls extracellular matrix synthesis Citation[4] and accelerates myofibroblast differentiation Citation[5]. Our group has previously shown that subconjunctival monoclonal antibody to TGF-β2 decreased conjunctival scarring in an experimental model of glaucoma surgery Citation[6], but did not significantly prolong bleb survival after glaucoma filtration surgery in a randomized clinical trial (RCT) Citation[7]. However, it is likely that the doses used were not high enough for a sufficiently long period of tissue exposure, and that a prolonged or slow release dosing regimen might have been required Citation[8]. Furthermore, it is likely that a broader coverage of the different isoforms might also have been necessary.
Table 1. Summary of properties of different therapeutic molecules in ocular fibrosis.
Connective tissue growth factor (CTGF) is a key downstream mediator of TGF-β-induced fibrosis, and can accelerate fibroblast proliferation and secretion of extracellular matrix Citation[9]. Wang et al. have shown that subconjunctival injection of a CTGF antibody can maintain larger bleb areas and lower intraocular pressures in a rabbit model of trabeculectomy Citation[10]. Yuan et al. have also found that CTGF is overexpressed in filtration blebs, suggesting that CTGF might play an important role in the process of wound healing after trabeculectomy Citation[11].
VEGF is a crucial mediator of angiogenesis, and stimulates both fibroblasts and endothelial cells in wound healing Citation[12,13]. Bevacizumab is a recombinant humanized monoclonal antibody against VEGF, and several studies have shown that it decreases scar formation and prolongs bleb survival after experimental glaucoma filtration surgery Citation[12,14,15]. However, Rodríguez-Agirretxe et al. reported that combined MMC and bevacizumab implants decreased intraocular pressure to a lesser extent than MMC alone, and that bevacizumab could interact with MMC Citation[16]. In the first RCT, Nilforushan et al. found that the MMC group had better intraocular pressure control but similar bleb morphology to the subconjunctival bevacizumab group Citation[17], and other studies have also suggested that the antibody alone is currently less effective than MMC Citation[18]. Vandewalle et al. recently reported that perioperative administration of intracameral bevacizumab significantly reduced the need for needling interventions, and led to a higher success rate after trabeculectomy Citation[19]. Further RCTs are needed to investigate the best way of using anti-VEGF therapies in glaucoma surgery, and the optimal doses and combinations with other anti-scarring agents.
PlGF is a VEGF-homolog that binds to VEGF-R1 and acts on pathological angiogenesis and inflammation Citation[20]. Bergen et al. showed that intracameral injection of a monoclonal PlGF antibody increased bleb area and survival in a mouse model of glaucoma surgery Citation[21]. Furthermore, anti-PlGF treatment seemed to be more effective than anti-VEGF-R2 treatment in improving the surgical outcome, possibly due to its additional effect on inflammation.
Lysyl oxidase (LOX) is another important class of enzymes that catalyses the covalent crosslinking of collagens and elastin in the extracellular matrix Citation[22]. Bergen et al. have found that LOX and LOX-like 2 (LOXL2) are both upregulated in Tenon’s capsule Citation[23]. The authors also showed that targeting LOXL2 with an inhibitory monoclonal antibody, GS-607601, reduced pathological angiogenesis, inflammation, fibrosis and prolonged bleb survival in a rabbit model of trabeculectomy Citation[23].
Integrins are protein heterodimers consisting of non-covalently associated α- and β-subunits, and the adhesive interactions mediated by integrins are necessary for cell proliferation Citation[24]. Paikal et al. reported that different integrin antibodies inhibited the attachment and proliferation of human Tenon’s fibroblasts in vitro Citation[25]. Further in vivo studies are needed to determine whether integrin antibodies can significantly limit scar formation after glaucoma surgery without significant toxicity.
siRNA & shRNA therapy
RNAi is another promising therapeutic approach as it can be used to silence the expression of unwanted genes in fibrosis . RNAi is mediated by siRNAs, shRNAs and miRNAs. siRNAs are small double-stranded exogeneous RNA molecules (20–25 base pairs) Citation[26], and shRNAs are short sequences of RNA with a tight hairpin turn that can be used in post-transcriptional gene silencing Citation[27].
Table 2. Tested anti-fibrotic gene therapeutic targets in the eye.
Transcription factors are key molecules that regulate gene expression by controlling the transcription of DNA into messenger RNA. NF-κB is a transcription factor that activates fibroblasts and is regulated by IκB kinase subunit b. Duan et al. showed that siRNAs targeting IκB kinase subunit b effectively downregulated NF-κB in human Tenon’s fibroblasts and suppressed fibroblast proliferation Citation[28].
Growth factors, especially TGF-β, also play a pivotal role in ocular fibrosis. Nakamura et al. reported that siRNAs efficiently knocked down TGF-βRII expression in human corneal fibroblasts, and that direct ocular application of TGF-βRII siRNAs could be used to decrease scarring in the subconjunctival space in the mouse Citation[29].
Cell cycle regulators also represent potential anti-fibrotic gene therapeutic targets. S phase kinase-interacting protein 2 is a key cell cycle regulator that targets p27. Wang et al. found that siRNA silencing of S phase kinase-interacting protein 2 decreased proliferation and cell viability in rabbit Tenon’s fibroblasts, and had potential as an anti-scarring therapy in glaucoma filtration surgery Citation[30].
Proteins are also important components of the extracellular matrix in the eye. Seet et al. showed that knocking down secreted protein, acidic, rich in cysteine (SPARC) impaired contraction in human Tenon’s fibroblasts and reduced the expression of profibrotic genes, namely collagen I, MMP-2, MMP-9, MMP-14, IL-8 and TGF-β Citation[31]. Yuan et al. found that shRNAs effectively suppressed the expression of keratoepithelin and myocilin in trabecular meshwork cells Citation[32]. Comes and Borrás also reported that siRNAs effectively silenced Matrix GLA protein expression in trabecular meshwork cells, and that intracameral delivery of siRNAs could become a future drug delivery technique in glaucoma Citation[33].
The success of siRNA therapeutics will depend largely on the efficient ocular delivery of siRNAs. In a Phase I study, Kaiser et al. showed that intravitreal injections of siRNA-027 were well tolerated in the eye, and led to an improvement in visual acuity and foveal thickness in patients with wet age-related macular degeneration Citation[34]. Ocular delivery of naked siRNAs is, however, unlikely to be sufficient in ocular fibrosis as siRNAs are not permeable across cell membranes and are not resistant against enzymatic degradation. Viral delivery systems, including adenoviral vectors Citation[35] and lentiviral vectors Citation[36], have shown good transfection efficacy. Non-viral delivery systems have also been used, including cationic liposomes Citation[37] and cationic copolymers like CS-g-(PEI-b-mPEG) Citation[28].
miRNA therapy
There is now increasing evidence of the key role of miRNAs in fibrosis . miRNAs are small single-stranded endogenous RNA transcripts (21–25 nucleotides) that are involved in post-transcriptional gene modulation Citation[38]. One of the most studied miRNAs in fibrosis is miR-29, and downregulation of miR-29 has been linked to tissue scarring in various body systems, namely cardiac fibrosis Citation[39], pulmonary fibrosis Citation[40], liver fibrosis Citation[41], renal fibrosis Citation[42], skin fibrosis Citation[43] and fibrosis in cancer Citation[44,45].
Li et al. showed that miR-29b suppressed collagen type I gene expression and was significantly downregulated in TGF-β1-activated human Tenon’s fibroblasts Citation[46]. The authors also reported that increasing intracellular miR-29b expression, by exogenous miR-29b delivery, effectively decreased fibroblast activity in vitro Citation[46].
In addition, Luna et al. showed that miR-29b downregulated the expression of multiple genes involved in the synthesis of extracellular matrix in human trabecular meshwork cells, and decreased cell cytotoxicity in the presence of chronic oxidative stress Citation[47]. Increasing miR-29b expression might thus represent a therapeutic approach to limit extracellular matrix deposition, prevent trabecular meshwork cell loss and facilitate aqueous humor outflow in glaucoma Citation[47].
miR-29 is one of the well-described TGF-β-associated miRNAs involved in fibrosis Citation[39,40]. miR-29 regulates multiple genes encoding for extracellular matrix proteins, including COL1A1, COL1A2, COL3A1, FBN1, ELN and SPARC Citation[47,48]. Some level of cross-talk has also been reported between miR-29 and TGF-β in trabecular meshwork cells, suggesting that miR-29 could represent an important modulator of TGF-β in aqueous outflow in glaucoma Citation[49].
The tumor suppressor gene p53 enhances the rate of transcription of several genes that regulate cell-cycle arrest and apoptosis Citation[50]. Park et al. have shown that miR-29 positively regulates p53, and might thus inhibit fibroblast proliferation in conjunctival scarring Citation[51]. The authors have also found that miR-29 directly suppresses CDC42 (a Rho family GTPase) and p85α (the regulatory subunit of PI3K) Citation[51]. As the Rho/MRTF/SRF (Myocardin related transcription factor/Serum response factor) pathway is increasingly being associated with fibrosis Citation[52,53], upregulating miR-29 could also decrease ocular scarring by suppressing the Rho-actin pathway. Although specificity has been thought to be an advantage in many clinical situations, this overlap of activity may be advantageous in scarring inhibition due to the overlap of multiple pathways.
Other miRNAs that have been identified as key triggers of fibrosis driven by TGF-β include miR-21 Citation[54,55], miR-192 Citation[56], miR-216a and miR-217 Citation[57]. miR-21 is expressed in the lungs of patients with idiopathic pulmonary fibrosis. Mice treated with miR-21 antisense probes were also protected from bleomycin-induced pulmonary fibrosis Citation[54], and cardiac fibrosis induced by pressure overload Citation[55]. miR-21 is thought to promote fibrosis by regulating TGF-β1 and MAPK signaling in activated myofibroblasts.
Future research will focus on the development and effective ocular delivery of miRNA-based therapies to prevent scarring in the eye. miR-29 mimics to increase miR-29 expression, and pharmacological inhibitors to prevent the downregulation of miR-29 expression, both have therapeutic potential. Locked nucleic acid-modified oligonucleotides are single-stranded bicyclic RNA analogs that irreversibly bind to miRNAs Citation[58], and could potentially be used for the ocular delivery of miRNA therapies. Miravirsen is a locked nucleic acid that sequesters miR-122, and provides proof-of-concept of the efficacy of miRNA therapy in human disease Citation[59].
Viral gene transfer & gene therapy
Instead of blocking unwanted mRNA expression like siRNAs, shRNAs and miRNAs, other methods of gene therapy involve increasing the expression of inhibitory genes on the cell cycle . Human p53 is a tumor suppressor gene that plays a key role in arresting cell cycle progression to allow DNA repair, or to induce apoptosis if the damage is too extensive Citation[50]. Using a recombinant adenovirus (rAd) for p53, Johnson et al. induced overexpression of p53 in human Tenon’s fibroblasts, and significantly inhibited fibroblast proliferation and DNA synthesis Citation[60].
Perkins et al. also showed that a rAd containing the p21 gene, which is a crucial downstream effector of p53, inhibited the proliferation of rabbit Tenon’s fibroblasts and performed similarly to MMC in terms of decrease in intraocular pressure Citation[61]. In a primate model of glaucoma surgery, Heatley et al. found that rAd.p21 gene therapy prevented conjunctival scarring and achieved even better intraocular pressure control than the MMC-treated group Citation[62]. Both rAd.p21 gene therapy studies did not report any of the tissue complications seen with MMC treatment.
In addition, Akimoto et al. used a rAd containing the cytosine deaminase gene to convert the pro-drug 5-fluorocytosine into 5-FU in glaucoma surgery Citation[63]. In an earlier study, the same authors reported that blocking the transcription factor E2F using the hemagglutinating virus of Japan on cationic liposomes inhibited fibroblast proliferation in glaucoma surgery Citation[64].
Furthermore, Yamanaka et al. reported that a rAd expressing Smad7 blocked the expression of Smad2/3 and suppressed collagen type I, α-smooth muscle actin and VEGF in human conjunctival fibroblasts Citation[65]. The authors have thus suggested that Smad7 gene transfer could become an effective strategy to prevent excessive conjunctival scarring in glaucoma filtration surgery.
Yamanaka et al. also showed that adenoviral gene transfer of a dominant negative p38MAPK decreased conjunctival fibrosis both in vitro and in vivo Citation[66]. They found that p38MAPK gene transfer blocked the TGF-β increase in collagen type I expression, CTGF and the monocyte/macrophage chemoattractant protein-1. The authors have thus suggested that p38MAPK inhibition could become a future anti-scarring therapy in glaucoma filtration surgery.
A difficult challenge with gene therapy remains the delivery of the gene into the target cell. The main disadvantage of non-viral methods, including ballistic DNA injections Citation[67] and liposomes Citation[68], is that the gene expression is short-lived and lasts only a few days. Viral methods achieve longer gene expression, and adenovirus is a double-strain DNA virus shown to be an efficient vector for gene transfer in ocular cells Citation[69]. There is, however, a rare but inherent risk of mutagenesis with the use of viral vectors, and at least two cases of lymphoproliferative disorders related to gene therapy have been described Citation[70].
Nanoparticles
Nanotechnology and nanoparticle research currently represent an area of great research interest due to its translational potential in a wide variety of scientific fields. Nanoparticles typically range in size between 1 and 100 nm , and several research groups worldwide are developing nanoparticles that could be used to modulate wound healing in the eye. Nanoparticles have several key advantages: a large surface area, for example, a standard teaspoon of 10-nm diameter silica nanoparticles has more surface area than a dozen double-sized tennis courts, good penetration and improved bioavailability by enhancing aqueous solubility and targeted drug delivery to a specific location in the eye.
Using the rabbit model of glaucoma filtration surgery, Butler et al. showed that topical silver nanoparticles (Ag-NPs) achieved an improved and sustained reduction in intraocular pressure, and led to blebs with decreased fibrosis and ischemia compared with MMC Citation[71]. Ag-NPs have also been shown to accelerate wound healing after skin burns, with decreased scar tissue formation and anti-inflammatory effects Citation[72]. Ag-NPs have a good safety profile in the eye Citation[73], but large particle diameters and higher concentrations have been associated with increased cytotoxicity and genotoxicity Citation[74].
SPARC silencing has been shown to decrease collagen gel contraction in vitro Citation[31], and to prolong bleb survival in a mouse model of glaucoma filtration surgery Citation[75]. Tan et al. have shown that layer-by-layer nanoparticles could be used as an efficient delivery vehicle for SPARC silencing and siRNA therapeutics Citation[76]. No toxic side effects were observed with the layer-by-layer nanoparticles.
Shao et al. have also reported that low-density lipoprotein (LDL)-MMC-chitosan nanoparticles could be used as a target drug delivery system to specifically bind to LDL receptors on activated human Tenon’s fibroblasts Citation[77]. The LDL-MMC-chitosan nanoparticles are associated with high selective targeting, good biocompatibility, low immunogenicity, decreased toxicity to normal cells and increased effectiveness of the anti-scarring agent MMC in glaucoma filtration surgery.
In addition, Santos et al. showed that subconjunctival administration of nanosized complexes of antisense TGF-β2 phosphorothioate oligonucleotides with polyethylenimine (PEI) increased intracellular penetration of TGF-β2 antisense oligonucleotides in conjunctival cells Citation[78]. The authors also found that the sustained release of these nanosized complexes significantly improved bleb survival in a rabbit model of trabeculectomy Citation[78].
Duan et al. also reported that cationic nano-copolymers combined with IKKβ targeting siRNA [CS-g-(PEI-b-mPEG)/IKKβ-siRNA] inhibited fibroblast proliferation in vitro Citation[28]. Ye et al. showed that subconjunctival injection of these nano-copolymers decreased subconjunctival scarring and increased bleb survival in a monkey model of glaucoma filtration surgery Citation[79]. The authors also found that the cationic nano-copolymers were well tolerated and non-toxic in the eye.
Liposomes
There is now increasing research in using liposomes as a drug delivery system for anti-scarring agents in the eye. Liposomes are small composite structures composed of a lamellar phase lipid bilayer, and vary in size between low to tens of micrometers . Peng et al. showed that subconjunctival injections of liposomes containing homoharringtonine, an alkaloid and protein synthesis inhibitor, reduced the intraocular pressure and inhibited scarring in a rabbit model of trabeculectomy Citation[80].
Simmons et al. also developed a liposomal delivery system for 5-FU to prolong the ocular levels of the drug in glaucoma filtration surgery Citation[81]. They found that liposomal 5-FU prolonged the scleral and conjunctival concentrations of the drug in rabbits, while reducing the peak ocular concentrations. Liposomal 5-FU could thus help improve the surgical outcome of glaucoma surgery, as well as decrease the risk of ocular side effects.
Daunorubicin is an anthracycline anti-cancer drug that is used to block DNA synthesis. Varma et al. showed that daunorubicin was safe and effective at lowering intraocular pressure in glaucoma filtration surgery Citation[82]. Shinohara et al. also showed that daunorubicin encapsulated in empty liposomes was effective in preventing proliferative vitreoretinopathy in a rabbit model Citation[83]. The authors did not report any adverse side effects with liposomal daunorubicin.
Tilleul et al. also showed that a liposome formulation of the anti-mitotic mitoxantrone reduced intraocular pressure and improved the surgical outcome of glaucoma surgery, when injected subconjunctivally at the end of surgery in rabbits Citation[84]. The effect of mitoxantrone in liposome formulation was similar to MMC application, and decreased the occurrence of corneal opacity compared with those treated with mitoxantrone in solution.
Dendrimers & proteoglycans
Other classes of therapeutic molecules that have been developed to modulate ophthalmic wound healing are dendrimers and proteoglycans . Dendrimers are hyperbranched nanomolecules that can be chemically synthesized to have precise structural characteristics. Our group has shown that the dendrimer glucosamine [d(+)-glucosamine] and dendrimer glucosamine 6-sulfate [d(+)-glucosamine 6-sulfate] have immunomodulatory and anti-angiogenic properties respectively, and that their combined use significantly increased the long-term surgical success in a rabbit model of glaucoma filtration surgery Citation[85]. We did not observe any local or systemic side effects with the dendrimer drugs.
Decorin is a small proteoglycan that binds to TGF-β and can inhibit its activity in wound healing Citation[86]. Grisanti et al. reported that decorin significantly decreased intraocular pressure and post-surgical fibrosis in a rabbit model of trabeculectomy Citation[87]. Decorin was also well tolerated in the experimental model of glaucoma filtration surgery, and no adverse effects, such as inflammation or blurring of the optical media, were observed.
Small molecule inhibitors & drugs
Small molecule inhibitors and drugs are showing a lot of potential as effective anti-fibrotic agents for the eye . Honjo et al. showed that the Rho-associated protein kinase (ROCK) inhibitor, Y-27632, prevented fibroblast-mediated matrix contraction in vitro, and increased bleb survival in a rabbit model of glaucoma filtration surgery Citation[88]. There were no adverse side effects observed with the Y-27632 inhibitor. ROCK inhibitors may block TGF-β-induced scarring by downregulating pathways generating mechanical tension, and thus improve the long-term success of glaucoma surgery. Inoue and Tanihara have also shown that a ROCK inhibitor significantly decreased intraocular pressure in vivo by directly affecting the trabecular meshwork and Schlemm’s canal Citation[89].
Rac1 is a small Rho GTPase involved in regulating cytoskeletal organization, and plays an important role in protrusive activity and wound healing Citation[90]. Xu et al. have shown that Rac1 inhibition reverses the phenotype of fibrotic fibroblasts in the skin Citation[91]. Our group has previously shown that the Rac1 inhibitor, NSC23766, efficiently inhibited conjunctival tissue and fibroblast-mediated matrix contraction Citation[92]. NSC23766 did not cause any significant toxicity, and was associated with reduced MMP1 expression during matrix contraction.
In a high-throughput screen, Evelyn et al. have also identified the small molecule inhibitor CCG-1423 as being more effective in reducing MRTF/SRF-regulated gene transcriptional signaling than ROCK inhibitors Citation[93]. Bell et al. have more recently optimized a second-generation inhibitor, CCG-203971, that is more potent and less cytotoxic than CCG-1423 Citation[94]. CCG inhibitors have shown efficacy in decreasing fibrosis in the skin Citation[95] and colon Citation[96], and it will be interesting to see their effects on wound healing in the eye.
MMPs are a large family of calcium-dependent zinc-containing endopeptidases that play a crucial role in tissue remodeling and degradation of collagens and extracellular matrix Citation[97–99]. Our group has previously shown that MMP inhibition decreased matrix contraction and collagen production in vitro Citation[98], as well as scarring in a rabbit model of glaucoma filtration surgery Citation[97,99].
Furthermore, inhibitors for activin receptor-like kinase 5 (ALK5), also known as TGF-β receptor type I, have been studied in ocular fibrosis. Sapitro et al. reported that the ALK5 inhibitor, SB-505124, suppressed TGF-β activity and promoted bleb survival in a rabbit model of trabeculectomy Citation[100]. Xiao et al. also showed that the ALK5 inhibitor, SB-431542, decreased post-surgical scarring and fibrosis after experimental glaucoma filtration surgery Citation[101].
Pirfenidone (PFD) is an interesting small molecule drug that downregulates a series of key profibrotic cytokines and growth factors like TGF-β Citation[102]. Zhong et al. showed that postoperative topical PFD was safe and improved bleb survival in a rabbit model of glaucoma filtration surgery Citation[103]. Other authors have also found that PFD decreased scarring and improved wound healing after corneal burns Citation[104], strabismus surgery Citation[105] and proliferative vitreoretinopathy Citation[106].
Expert commentary
To date, there has been significant progress made in the development of small molecule therapeutics to modulate ophthalmic wound healing using different approaches . Given that fibrosis-related events play a part in most of the blinding diseases in the world and also account for over 40% of all deaths, this represents one of the largest areas of unmet need in clinical ophthalmology and clinical medicine.
Figure 2. Schematic diagram summarizing the different therapeutic molecules in ocular fibrosis and their modes of action.
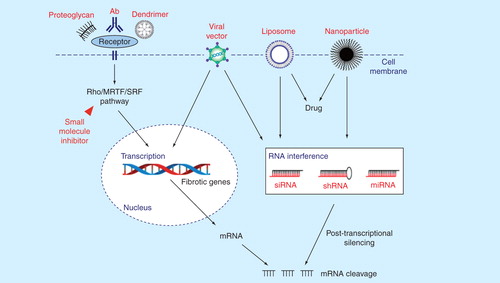
Box 1 describes the properties of the ideal anti-scarring agent or combination of agent(s). Antibody therapeutics is currently a fast growing area of research, and there have also been major advances in the field of gene modulating therapies, nanoparticles, liposomes, dendrimers and small molecule inhibitors in ocular fibrosis. Clinical trials using antisense oligonucleotides and ribozymes have so far proved to be disappointing, but RNAi is more potent than antisense oligonucleotides Citation[107].
The eye has the distinct advantage of being a closed compartment where small molecule therapeutics can be injected close to the target tissues, thus minimizing the risks of side effects to the rest of the body. This includes both the intraocular and subconjunctival space after glaucoma surgery that is easily accessible in the outpatient clinical setting. Drug delivery and duration of action remain major hurdles in the development of novel anti-scarring therapies. New slow release drug delivery systems, like ocular tablets and in situ gels, are being developed to decrease the need for multiple injections in glaucoma surgery Citation[108]. RNAi formulations (RNAi + delivery system) are also being designed to increase the cellular uptake, to protect against enzymatic degradation and to permit the long-term delivery of RNAi therapies Citation[26].
Another key challenge with new anti-fibrotic agents is their potential toxicity in humans. Several small molecule inhibitors have shown promising results as anti-fibrotic agents, but their ocular toxicity have yet to be fully investigated. Viral vector delivery systems have also shown high transfection efficacy, but is limited by the risks of mutagenesis and host immunity Citation[109]. The unselective silencing of additional genes represents another potential side effect with RNAi therapeutics, and a possible solution might be to chemically modify one or both of the siRNA strands to minimize the risk of off-target effects Citation[110].
Five-year view
It is well established that some group of patients scar much more than others. However, there is a current lack of reliable biomarkers to stratify the risk of scarring and post-surgical fibrosis in the eye. In the next 5 years, the hope is that advances in genotyping and phenotyping, using modern tissue biomarkers and high-resolution real-time in vivo imaging techniques, will help to identify the groups of patients that would scar more aggressively, and thus help to develop a more personalized and stratified approach in anti-fibrotic ocular therapeutics.
Although fibroblasts underlie the scarring process after glaucoma filtration surgery, there are also other cell types, like inflammatory cells, which play an important role in the wound healing process. The aim of future research will thus be to develop combination therapies targeting several pathways that would be more efficacious than monotherapies, especially in the high-risk groups. The ability to predict the risk of scarring and to tailor the anti-fibrotic treatment regimen to each individual patient will be an extremely useful tool clinically to prevent undertreating, or exposing patients to unnecessary treatments with potential side effects. Furthermore, development of new therapies in combination with surgical devices, where scarring is currently still a significant long-term problem, further increases both the challenges and the gains to be made in clinical outcomes in many pathological situations affecting the eye and other body organs.
Finally, apart from a few studies on anti-TGF-β and anti-VEGF therapies, there is a current lack of well-designed randomized controlled trials to compare the efficacy and safety of different therapeutic molecules in ocular fibrosis. Similar to cancer therapeutics, more refined human studies with long-term follow-ups are needed, alongside carefully chosen comparative control groups. In addition, a combination of agents may afford us much better control of the scarring process, as has been the case in cancer therapy. The ultimate goal in the future will be to modulate the wound healing process in each patient toward a more regenerative repair process with no scarring, possibly combined with new bioengineered devices. This will allow us to achieve a new generation of outcome goals not previously possible. This includes audacious goals such as our 10, 10, 10 target for glaucoma surgery: a postoperative intraocular pressure of 10 mmHg, carried out in 10 minutes and lasting for at least 10 years. New therapies to prevent long-term scarring, together with new bioengineering technologies, will help to make this possible in the future.
Effective anti-fibrotic effects, but allows some healing (e.g., maintains tissue flow ∼10 mmHg after glaucoma surgery).
High specificity for target(s), but able to adequately suppress multiple pathways that constitute complex fibrosis.
Safe to handle.
Short initial delivery method, ideally at the time of surgery.
Long duration of action, ideally >10 years (or easily delivered at intermittent intervals, e.g. yearly).
Inexpensive and easily mass produced.
Minimal toxicity.
Applicable in many different situations in the eye and body.
Fibrosis and scarring are responsible for the pathogenesis or failure of treatment of all the major blinding diseases, with postoperative wound healing responses posing a major problem for most ocular surgery on a worldwide scale.
Fibrosis prevention is one of the largest areas of unmet need in clinical medicine.
Antibodies are a fast growing class of therapeutics and hold a lot of potential in the development of new anti-fibrotic treatments.
Gene modulation and gene therapy represent an exciting and innovative approach to prevent scarring and post-surgical fibrosis in the eye.
There have also been significant advances in small molecule therapeutics, including nanoparticles, liposomes, proteoglycans, dendrimers and inhibitors, to modulate ophthalmic wound healing.
Optimal drug delivery and potential ocular toxicity remain major challenges in the development of effective anti-fibrotic therapies.
Future research will focus on developing a more personalized and stratified approach in anti-fibrotic ocular therapeutics, combinations of therapies and the need for well-designed randomized controlled human trials.
New therapies to prevent scarring together with new bioengineering technologies will also allow far better long-term clinical outcomes in the future.
Financial & competing interests disclosure
C Yu-Wai-Man is funded by an NIHR BRC Francis Crick Institute Clinical Research Training Fellowship. Our research is supported by the National Institute for Health Research Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology (NIHR BRC), the Medical Research Council, Moorfields Trustees, Moorfields Eye Charity, the Freemasons Grand Charity, the Michael and Ilse Katz Foundation, the Helen Hamlyn Trust and Fight for Sight. The authors have no other relevant affiliations or financial involvement with any organization or entity, with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Notes
References
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90:262-7
- Addicks EM, Quigley HA, Green WR, Robin AL. Histologic characteristics of filtering blebs in glaucomatous eyes. Arch Ophthalmol 1983;101(5):795-8
- Battegay EJ, Raines EW, Seifert RA, et al. Transforming growth factor beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine platelet-derived growth factor loop. Cell 1990;63:515-24
- Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type B: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in-vitro. Proc Natl Acad Sci USA 1986;83:4167-71
- Tomasek JJ, McRae J, Owens GK, Haaksma CJ. Regulation of alpha-smooth muscle actin expression in granulation tissue myofibroblasts is dependent on the intronic CArG element and the transforming growth factor-beta1 control element. Am J Pathol 2005;166(5):1343-51
- Cordeiro MF, Gay J, Khaw PT. Human anti-transforming growth factor-beta2 antibody: a new glaucoma anti-scarring agent. Invest Ophthalmol Vis Sci 1999;40:2225-34
- Khaw PT, Grehn F, Hollo G, et al. A phase III study of subconjunctival human anti-transforming growth factor beta(2) monoclonal antibody (CAT-152) to prevent scarring after first-time trabeculectomy. Ophthalmology 2007;114:1822-30
- Mead AL, Wong TT, Cordeiro MF, et al. Evaluation of anti-TGF-beta2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest Ophthalmol Vis Sci 2003;44:3394-401
- Ruiz-Ortega M, Rodríguez-Vita J, Sanchez-Lopez E, et al. TGF-beta signaling in vascular fibrosis. Cardiovasc Res 2007;74(2):196-206
- Wang JM, Hui N, Fan YZ, et al. Filtering bleb area and intraocular pressure following subconjunctival injection of CTGF antibody after glaucoma filtration surgery in rabbits. Int J Ophthalmol 2011;4(5):480-3
- Yuan HP, Li XH, Yang BB, et al. Expression of connective tissue growth factor after trabeculectomy in rabbits. Zhonghua Yan Ke Za Zhi 2009;45(2):168-74
- Li Z, Bergen T, Veire S, et al. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtration surgery. Invest Ophthalmol Vis Sci 2009;50(11):5217-25
- Wilgus TA, Ferreira AM, Oberyszyn TM, et al. Regulation of scar formation by vascular endothelial growth factor. Lab Invest 2008;88(6):579-90
- Memarzadeh F, Varma R, Lin L, et al. Postoperative use of bevacizumab as an antifibrotic agent in glaucoma filtration surgery in the rabbit. Invest Ophthalmol Vis Sci 2009;50:3233-7
- How A, Chua JLL, Charlton A, et al. Combined treatment with bevacizumab and 5-fluorouracil attenuates the postoperative scarring response after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci 2010;51:928-32
- Rodríguez-Agirretxe I, Vega SC, Rezola R, et al. The PLGA implant as an antimitotic delivery system after experimental trabeculectomy. Invest Ophthalmol Vis Sci 2013;54(8):5227-35
- Nilforushan N, Yadgari M, Kish S, Nassiri N. Subconjunctival bevacizumab versus mitomycin C adjunctive to trabeculectomy. Am J Ophthalmol 2012;153:352-7
- Akkan JU, Cilsim S. Role of Subconjunctival Bevacizumab as an Adjuvant to Primary Trabeculectomy: a Prospective Randomized Comparative 1-Year Follow-up Study. J Glaucoma 2013. [Epub ahead of print]
- Vandewalle E, Pinto L, Bergen T, et al. Intracameral bevacizumab as an adjunct to trabeculectomy: a 1-year prospective, randomised study. Br J Ophthalmol 2014;98(1):73-8
- Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 2001;7(5):575-83
- Bergen T, Jonckx B, Hollanders K, et al. Inhibition of placental growth factor improves surgical outcome of glaucoma surgery. J Cell Mol Med 2013;17(12):1632-43
- Rodríguez C, Rodríguez-Sinovas A, Martínez-González J. Lysyl oxidase as a potential therapeutic target. Drug News Perspect 2008;21(4):218-24
- Bergen T, Marshall D, Veire S, et al. The role of LOX and LOXL2 in scar formation after glaucoma surgery. Invest Ophthalmol Vis Sci 2013;54(8):5788-96
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol 1995;11:549-99
- Paikal D, Zhang G, Cheng Q, Lee DA. The effect of integrin antibodies on the attachment and proliferation of human Tenon’s capsule fibroblasts. Exp Eye Res 2000;70(4):393-400
- Gallas A, Alexander C, Davies M, et al. Chemistry and formulations for siRNA therapeutics. Chem Soc Rev 2013;42(20):7983-97
- Paddison PJ, Caudy AA, Bernstein E, et al. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev 2002;16:948-58
- Duan Y, Guan X, Ge J, et al. Cationic nano-copolymers mediated IKKb targeting siRNA inhibit the proliferation of human Tenon’s capsule fibroblasts in vitro. Mol Vis 2008;14:2616-28
- Nakamura H, Siddiqui SS, Shen X, et al. RNA interference targeting transforming growth factor-beta type II receptor suppresses ocular inflammation and fibrosis. Mol Vis 2004;10:703-11
- Wang F, Qi LX, Su Y, et al. Inhibition of cell proliferation of Tenon’s capsule fibroblast by S-Phase kinase-interacting protein 2 targeting SiRNA through increasing p27 protein level. Invest Ophthalmol Vis Sci 2010;51(3):1475-82
- Seet LF, Su R, Toh LZ, Wong TT. In vitro analyses of the anti-fibrotic effect of SPARC silencing in human Tenon’s fibroblasts: comparisons with mitomycin C. J Cell Mol Med 2012;16(6):1245-59
- Yuan C, Zins EJ, Clark AF, Huang AJ. Suppression of keratoepithelin and myocilin by small interfering RNAs (siRNA) in vitro. Mol Vis 2007;13:2083-95
- Comes N, Borrás T. Functional delivery of synthetic naked siRNA to the human trabecular meshwork in perfused organ cultures. Mol Vis 2007;13:1363-74
- Kaiser PK, Symons RC, Shah SM, et al. RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am J Ophthalmol 2010;150(1):33-9
- Shen C, Buck AK, Liu X, et al. Gene silencing by adenovirus-delivered siRNA. FEBS Lett 2003;539:111-14
- Rubinson DA, Dillon CP, Kwiatkowski AV, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet 2003;33:401-6
- Templeton NS. Cationic liposomes as on vivo delivery vehicles. Curr Med Chem 2003;10:1279-87
- Bartel D. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281-97
- Rooij E, Sutherland LB, Thatcher JE, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. PNAS 2008;105(35):13027-32
- Xiao J, Meng XM, Huang XR, et al. miR-29 inhibits bleomycin-induced pulmonary fibrosis in mice. Mol Ther 2012;20(6):1251-60
- Ogawa T, Lizuka M, Sekiya Y, et al. Suppression of type I collagen production by microRNA-29b in cultured human stellate cells. Biochem Biophys Res Commun 2010;391:316-21
- Qin W, Chung ACK, Huang XR, et al. TGF-b/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol 2011;22:1462-74
- Cheng J, Wang Y, Wang D, Wu Y. Identification of collagen 1 as a post-transcriptional target of miR-29b in skin fibroblasts: therapeutic implication for scar reduction. Am J Med Sci 2013;346(2):98-103
- Sengupta S, Boon JA, Chen I, et al. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci USA 2008;105:5874-8
- Fabbri M, Garzon R, Cimmino A, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA 2007;104(40):15805-10
- Li N, Cui J, Duan X, et al. Suppression of type I collagen expression by miR-29b via PI3K, Akt, and Sp1 pathway in human Tenon’s fibroblasts. Invest Ophthalmol Vis Sci 2012;53(3):1670-8
- Luna C, Li G, Qiu J, et al. Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol Vis 2009;15:2488-97
- Kapinas K, Kessler CB, Delany AM. miR-29 suppression of osteonectin in osteoblasts: regulation during differentiation and by canonical Wnt signaling. J Cell Biochem 2009;108(1):216-24
- Luna C, Li G, Qiu J, et al. Cross-talk between miR-29 and transforming growth factor-betas in trabecular meshwork cells. Invest Ophthalmol Vis Sci 2011;52(6):3567-72
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell 1997;88:323-31
- Park SY, Lee JH, Ha M, et al. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol 2009;16:23-9
- Small EM, Thatcher JE, Sutherland LB, et al. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res 2010;107(2):294-304
- Luchsinger LL, Patenaude CA, Smith BD, Layne MD. Myocardin-related transcription factor-A complexes activate type I collagen expression in lung fibroblasts. J Biol Chem 2011;286(51):44116-25
- Liu G, Friggeri A, Yang Y, et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med 2010;207(8):1589-97
- Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008;456(7224):980-4
- Chung A, Huang X, Meng X, Lan H. miR-192 Mediates TGF-β/Smad3-Driven Renal Fibrosis. J Am Soc Nephrol 2010;21:1317-25
- Kato M, Putta S, Wang M, et al. TGF-β activates Akt kinase via a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol 2009;11(7):881-9
- Kurreck J, Wyszko E, Gillen C, Erdmann VA. Design of antisense oligonucleotides stabilized by locked nucleic acids. Nucleic Acids Res 2002;30:1911-18
- Janssen H, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med 2013;368:1685-94
- Johnson KTM, Rodicker F, Heise K, et al. Adenoviral p53 gene transfer inhibits human Tenon’s capsule fibroblast proliferation. Br J Ophthalmol 2005;89:508-12
- Perkins TW, Faha B, Ni M, et al. Adenovirus-mediated gene therapy using human p21WAF-1/Cip-1 to prevent wound healing in a rabbit model of glaucoma filtration surgery. Arch Ophthalmol 2002;120(7):941-9
- Heatley G, Kiland J, Faha B, et al. Gene therapy using p21WAF-1/Cip-1 to modulate wound healing after glaucoma trabeculectomy surgery in a primate model of ocular hypertension. Gene Ther 2004;11:949-55
- Akimoto M, Miyahara T, Arai J, et al. A new delivery system for 5-fluorouracil using prodrug and converting enzyme. Br J Ophthalmol 2002;86:581-6
- Akimoto M, Hangai M, Okazaki K, et al. Growth inhibition of cultured human Tenon’s fibroblastic cells by targeting the E2F transcription factor. Exp Eye Res 1998;67:395-401
- Yamanaka O, Ikeda K, Saika S, et al. Gene transfer of Smad7 modulates injury-induced conjunctival wound healing in mice. Mol Vis 2006;12:841-51
- Yamanaka O, Saika S, Ohnishi Y, et al. Inhibition of p38MAP kinase suppresses fibrogenic reaction in conjunctiva in mice. Mol Vis 2007;13:1730-9
- Tsai J, Whitsitt J, Davidson J. Transfection of luciferase gene by gold particle bombardment in rabbit organ cultured corneas. Invest Ophthalmol Vis Sci 1996;37(S683):3123
- Abraham N, DaSilva J, Dunn M. Retinal pigment epithelial cell-based gene therapy against hemoglobin toxicity. Int J Mol Med 1998;1:657-63
- Larkin DF, Oral HB, Ring CJ, et al. Adenovirus-mediated gene delivery to the corneal endothelium. Transplantation 1996;61(3):363-70
- Kang EM, Tisdale JF. The leukemogenic risk of integrating retroviral vectors in hematopoietic stem cell gene therapy applications. Curr Hematol Rep 2004;3:274-81
- Butler MR, Ponce CM, Weinstock YE, et al. Topical silver nanoparticles result in improved bleb function by increasing filtration and reducing fibrosis in a rabbit model of filtration surgery. Invest Ophthalmol Vis Sci 2013;54(7):4982-90
- Tian J, Wong KK, Ho CM, et al. Topical delivery of silver nanoparticles promotes wound healing. Chem Med Chem 2007;2(1):129-36
- Kim JS, Song KS, Sung JH, et al. Genotoxicity, acute oral and dermal toxicity, eye and dermal irritation and corrosion and skin sensitisation evaluation of silver nanoparticles. Nanotoxicology 2013;7(5):953-60
- Lee KJ, Browning LM, Nallathamby PD, et al. In vivo quantitative study of sized-dependent transport and toxicity of single silver nanoparticles using zebrafish embryos. Chem Res Toxicol 2012;25(5):1029-46
- Seet LF, Su R, Barathi VA, et al. SPARC deficiency results in improved surgical survival in a novel mouse model of glaucoma filtration surgery. PLoS One 2010;5(2):e9415
- Tan YF, Mundargi RC, Chen MH, et al. Layer-by-layer nanoparticles as an efficient siRNA delivery vehicle for SPARC silencing. Small 2014;10(9):1790-8
- Shao T, Li X, Ge J. Target drug delivery system as a new scarring modulation after glaucoma filtration surgery. Diagn Pathol 2011;6:64
- Santos AL, Bochot A, Doyle A, et al. Sustained release of nanosized complexes of polyethylenimine and anti-TGF-β2 oligonucleotide improves the outcome of glaucoma surgery. J Control Release 2006;112:369-81
- Ye H, Qian Y, Lin M, et al. Cationic nano-copolymers mediated IKKβ targeting siRNA to modulate wound healing in a monkey model of glaucoma filtration surgery. Mol Vis 2010;16:2502-10
- Peng D, Yu K, Zeng S, et al. An experimental study on homoharringtonine liposome and glaucoma filtration surgery. Yan Ke Xue Bao 1999;15(1):51-4
- Simmons ST, Sherwood MB, Nichols DA, et al. Pharmacokinetics of a 5-fluorouracil liposomal delivery system. Br J Ophthalmol 1988;72(9):688-91
- Varma D, Sihota R, Agarwal HC. Evaluation of efficacy and safety of daunorubicin in glaucoma filtering surgery. Eye (Lond) 2007;21(6):784-8
- Shinohara K, Tanaka M, Sakuma T, Kobayashi Y. Efficacy of daunorubicin encapsulated in liposome for the treatment of proliferative vitreoretinopathy. Ophthalmic Surg Lasers Imaging 2003;34(4):299-305
- Tilleul P, Denis P, Maignen F, et al. Effects of different formulations of mitoxantrone (solutions, nanospheres, liposomes) on glaucoma surgery in rabbits. Ophthalmic Res 1997;29(4):218-26
- Shaunak S, Thomas S, Gianasi E, et al. Polyvalent dendrimer glucosamine conjugates prevent scar tissue formation. Nat Biotechnol 2004;22(8):977-84
- Hildebrand A, Romarís M, Rasmussen LM, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J 1994;302(Pt 2):527-34
- Grisanti S, Szurman P, Warga M, et al. Decorin modulates wound healing in experimental glaucoma filtration surgery: a pilot study. Invest Ophthalmol Vis Sci 2005;46(1):191-6
- Honjo M, Tanihara H, Kameda T, et al. Potential role of rhoassociated protein kinase inhibitor Y-27632 in glaucoma filtration surgery. Invest Ophthalmol Vis Sci 2007;48(12):5549-57
- Inoue T, Tanihara H. Rho-associated kinase inhibitors: a novel glaucoma therapy. Prog Retin Eye Res 2013;37:1-12
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol 1999;144:1235-44
- Xu SW, Liu S, Eastwood M, et al. Rac inhibition reverses the phenotype of fibrotic fibroblasts. PLoS One 2009;4(10):e7438
- Tovell VE, Chau CY, Khaw PT, Bailly M. Rac1 inhibition prevents tissue contraction and MMP mediated matrix remodeling in the conjunctiva. Invest Ophthalmol Vis Sci 2012;53:4682-91
- Evelyn CR, Wade SM, Wang Q, et al. CCG-1423: a small-molecule inhibitor of RhoA transcriptional signaling. Mol Cancer Ther 2007;6:2249-60
- Bell JL, Haak AJ, Wade SM, et al. Optimization of novel nipecotic bis(amide) inhibitors of the Rho/MKL1/SRF transcriptional pathway as potential anti-metastasis agents. Bioorganic Med Chem Letters 2013;23:3826-32
- Haak AJ, Tsou PS, Amin MA, et al. Targeting the myofibroblast genetic switch: inhibitors of MRTF/SRF-regulated gene transcription prevent fibrosis in a murine model of skin injury. J Pharmacol Exp Ther 2014. [ Epub ahead of print]
- Johnson LA, Rodansky ES, Haak AJ, et al. Novel Rho/MRTF/SRF inhibitors block matrix-stiffness and TGF-β-induced fibrogenesis in human colonic myofibroblasts. Inflamm Bowel Dis 2014;20(1):154-65
- Wong TTL, Mead AL, Khaw PT. Matrix metalloproteinase inhibition modulates postoperative scarring after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci 2003;44(3):1097-103
- Daniels JT, Cambrey AD, Occleston NL, et al. Matrix metalloproteinase inhibition modulates fibroblast-mediated matrix contraction and collagen production in vitro. Invest Ophthalmol Vis Sci 2003;44(3):1104-10
- Wong TT, Mead AL, Khaw PT. Prolonged antiscarring effects of ilomastat and MMC after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci 2005;46(6):2018-22
- Sapitro J, Dunmire JJ, Scott SE, et al. Suppression of transforming growth factor-β effects in rabbit subconjunctival fibroblasts by activin receptor-like kinase 5 inhibitor. Mol Vis 2010;16:1880-92
- Xiao YQ, Liu K, Shen JF, et al. SB-431542 inhibition of scar formation after filtration surgery and its potential mechanism. Invest Ophthalmol Vis Sci 2009;50(4):1698-706
- Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther 1999;291:367-73
- Zhong H, Sun G, Lin X, et al. Evaluation of pirfenidone as a new postoperative antiscarring agent in experimental glaucoma surgery. Invest Ophthalmol Vis Sci 2011;52(6):3136-42
- Chowdhury S, Guha R, Trivedi R. Pirfenidone nanoparticles improve corneal wound healing and prevent scarring following alkali burn. PLoS One 2013;8(8):e70528
- Jung KI, Choi JS, Kim HK, Shin SY. Effects of an anti-transforming growth factor-β agent (pirfenidone) on strabismus surgery in rabbits. Curr Eye Res 2012;37(9):770-6
- Choi K, Lee K, Ryu SW, et al. Pirfenidone inhibits transforming growth factor-β1-induced fibrogenesis by blocking nuclear translocation of Smads in human retinal pigment epithelial cell line ARPE-19. Mol Vis 2012;18:1010-20
- Brantl S. Antisense-RNA regulation and RNA interference. Biochmical Biophysica Acta 2002;1575:15-25
- Parkinson G, Gaisford S, Ru Q, et al. Characterisation of ilomastat for prolonged ocular drug release. AAPS Pharm Sci Tech 2012;13(4):1063-72
- Sun JY, Anand-Jawa V, Chatterjee S, Wong KK. Immune responses to adeno-associated virus and its recombinant vectors. Gene Ther 2003;10:964-76
- Bharadwaj AS, Appukuttan B, Wilmarth PA, et al. Role of the retinal vascular endothelial cell in ocular disease. Prog Retin Eye Res 2013;32:102-80