Abstract
Cardiovascular magnetic resonance (CMR) imaging can precisely quantify cardiac size and function, but moreover depict tissue changes that are associated with various forms of myocardial inflammation. Thereby, CMR can often detect myocardial inflammation before contractility is obviously impaired. Various CMR techniques to assess aspects of inflammation including T2-weighted imaging, and early- and late-contrast enhanced T1-weighted imaging, are reviewed regarding technical challenges and clinical usefulness. In this article we discuss the available evidence regarding clinical application of CMR in different forms of myocardial inflammation.
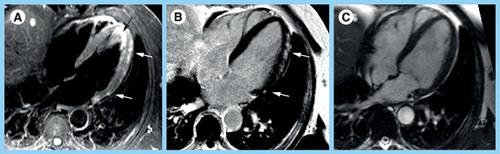
(A) Triple-inverted T2-weighted fast spin echo (short tau inversion recovery) reveals epicardial hyperintense edema in the apical-lateral and basal-lateral wall (white arrows). The bright signal in the apical lumen represents slow flow, but not edema (black arrow). (B) The fast low-angle shot gradient echo late gadolinium enhancement (LGE) image with phase-sensitive reconstruction delineates focal fibrosis in the same location (white arrows). (C) The diastolic steady state free precession cine frame illustrates the extent of the lateral wall and confirms the epicardial location of the myocarditis lesions. There is a small rim of pericardial fluid, hypointense in (A) and (B), hyperintense in (C). There is a focal hepatic lesion: (A) bright in T2 and (B) hypointense in LGE and (C) cine, most likely representing a liver cyst.
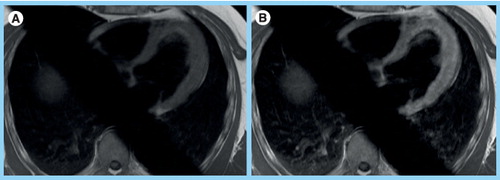
Both images are from an axial stack of images that are obtained in an interleaved fashion over minutes before and after contrast to reflect accumulation in the interstitial space. (B) Note the increased myocardial signal intensity in the postcontrast image reflecting global left ventricular inflammation. Global relative enhancement was 4. Skeletal muscle in the right and left lower corner of the images is available for comparison. The body coil was used for image acquisition. The oblique saturation bars were applied to reduce aortic and atrial flow artifacts.
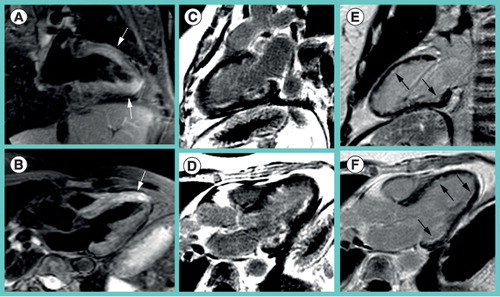
As it turned out afterwards, she had pulmonary infiltrates, eosinophilia, a history of sinusitis and asthma. Myocardial biopsy confirmed Churg–Strauss vasculitis. (A) T2-weighted images depict edema in the anterior and apical-inferior wall (arrow) as well as (B) anteroseptal wall (arrow). (C & E) Late gadolinium enhancementimages in the two-chamber-view and (D & F) three-chamber-view delineate subendocardial fibrotic lesions in the anterior, posterolateral and inferior wall. (E & F) Follow-up after 1 year revealed fibrotic lesions that persisted but had shrinked in size (arrows).
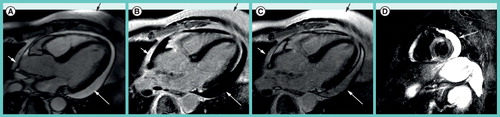
(A) On steady state free precession cine images, pericardial fluid (white arrows) appears slightly brighter than fat (black arrow). (B) On the late gadolinium enhancement (LGE) phase-sensitive inversion-recovery image the effusion (white arrow) appears hypointense, while pericardial and subcutaneous fat (black arrow) appear bright. (C) On magnitude LGE images the effusion (white arrow) has intermediate signal intensity less bright than fat (black arrow). (D) The triple-inverted fast spin echo (short tau inversion recovery) image highlights bright pericardial fluid (white arrow), but also the fluid-filled stomach, the spleen and the kidney.
Cardiovascular magnetic resonance imaging
For more than 15 years cardiovascular magnetic resonance (CMR) imaging has proven its clinical usefulness to depict cardiac anatomy, function and tissue composition noninvasively for various indications without any radiation involved Citation[1]. CMR is considered the gold standard to assess left and right ventricular mass and systolic function as well as to depict myocardial infarction scars. The particular strength of CMR is its ability to characterize tissue properties beyond mere depiction of anatomy or function. We will review the potential and the limitations of CMR to depict myocardial inflammation.
Cardiovascular magnetic resonance images are most often acquired during a breathhold of 8–20 s triggered by an ECG signal. A CMR study protocol is not uniform, but may include various elements depending on local availability and expertise. CMR offers several techniques to depict different aspects of inflammation that we will briefly describe later. Currently a combination of these techniques is recommended for CMR imaging of myocarditis Citation[2].
T2-weighted imaging
After the transverse magnetic pulse the protons no longer rotate in a coherent way, but interact with each other and their surroundings. This spin–spin interaction contributes to the decay of transverse magnetization. The time constant of transverse magnetization decay is called T2. Protons from small mobile water molecules behave differently compared with protons within large molecules such as fatty acids Citation[3]. Images with contrast based on these mechanisms are called T2-weighted images Citation[4]. Water and fluid typically appear bright on T2-weighted images Citation[5]. Image contrast does not only depend on changes in absolute water content, but also on shifts between free and bound water. The feasibility of T2-weighted edema imaging has been demonstrated nicely in animal models of acute infarction Citation[6,7].
Any acute inflammatory or ischemic tissue injury will result in edema. Edema can be intracellular or interstitial. It may result in capillary compression, impaired diastolic and systolic cardiac function Citation[4,8,9].
Edematous lesions within the myocardium might be focal or diffuse. If no focal lesions are anticipated, the global signal has to be quantified. However, the myocardial signal directly depends on heart rate, as the repetition time as one of the main determinants of contrast is fixed to be two times the cardiac cycle length. To overcome this problem and to enable comparisons between studies and patients, skeletal muscle as a reference structure has been suggested Citation[10]. Myocardial signal is indexed to skeletal muscle signal intensity assuming first, that the heart rate affects the signal intensity of skeletal muscle in the same way as it does affect the myocardial signal and second, that the skeletal muscle is free of inflammation. If a signal comparison with reference structures elsewhere is desired, one should employ either the body coil or coil sensitivity correction algorithms to ensure equal distribution of signal across the field of view.
Despite successful clinical application in various centers Citation[11,12] and a wide range of settings including acute infarction Citation[11,13,14] and acute myocarditis Citation[10,15,16], cardiac T2-weighted spin echo imaging suffers from several drawbacks Citation[4,17,18]: first, the pulse sequence confines several magnetic pulses, which are supposed to hit the heart at the exact same position and time during the cardiac cycle to yield in the desired image contrast. In case of arrhythmia, higher heart rates or pronounced diastolic wall motion in younger individuals signal loss in the posterolateral wall might result, as these magnetic pulses cancel each other out. Second, the black blood image requires a certain speed of blood flow to achieve blood suppression by new blood flowing into the slice of interest that has not been affected by magnetization. In case of severe wall motion abnormalities the slow flowing blood picks up magnetization and appears bright. This bright signal within the left ventricular (LV) lumen might be hard to differentiate from myocardial edema within the wall. Increasing the slice thickness for the blood suppression pulse and reducing the imaging slice thickness might alleviate the problem. Thinner slices, however, will result in even lower signal to noise ratio. Finally, the contrast between hyperintense edematous regions and hypointense remote areas requires a homogenous signal across the chest due to even coil sensitivity. Increased signal intensities in myocardial areas closer to the coil might mock false-positive findings.
The triple-inversion short tau inversion recovery (STIR) sequence suffers from the disadvantages mentioned earlier, but results in an excellent contrast owing to its additional fat suppression, T1 weighting Citation[19,20] and proton density weighting effects Citation[21]. As a net result, the third 180-inversion pulse additionally enhances water signal . As an alternative to spin-echo imaging, Kellman et al. proposed a T2-prepared steady state free precession (SSFP) sequence Citation[22,23], whereas Aletras et al. suggested a hybrid spin echo SSFP sequence (ACUTE), which sustains contrast but suffers less from suboptimal blood suppression Citation[24]. In ischemic edema the ACUTE sequence resulted in less interobserver variability than the STIR sequence Citation[25]. However, there is no consensus yet regarding the optimal sequence for myocardial T2-weighted imaging and careful assessment of these images is pivotal.
Recently, attempts have been made to quantify myocardial edema more easily with T2 maps Citation[26,27]. The map is calculated based on a set of images obtained with identical parameters, but various echo times. On the map the signal intensities in relation to echo time should directly reflect the T2-time. Normal myocardium has a T2-time of 55 ms, edema in acute infarction has a T2-time of approximately 70 ms. Whether this difference of 15 ms is large enough to separate normal from abnormal areas remains to be determined. There is no published experience yet for T2 mapping in inflammatory edema.
Contrast-enhanced T1-weighted imaging
After injection, gadolinium chelate contrast agents quickly leave the vascular compartment and enter the interstitial space. The gadolinium chelate complex cannot enter the intact cell owing to its large molecular size. Unlike conventional x-ray, contrast dye gadolinium agents rarely cause adverse effects. To avoid the rare development of nephrogenic systemic fibrosis in patients with advanced renal failure, clinicians should follow the guidelines and restrict the use of less stable contrast agents in patients with a glomerular filtration rate lower than 30 ml/min. Since awareness for this potential adverse effect has risen no further cases of nephrogenic systemic fibrosis have been reported Citation[28].
Early contrast enhancement
Friedrich et al. found increased early contrast uptake within 4 min after contrast administration in patients with acute myocarditis compared with controls Citation[29]. Hyperemia, increased distribution volume in edematous interstitial space and capillary leakage are believed to contribute to this phenomenon. Therefore this marker is unspecific, but it characterizes a temporary phenomenon typically present for approximately 4–6 weeks after onset of inflammation. In contrast to persistent late gadolinium lesions, early enhancement therefore has the potential to monitor disease activity in chronic inflammatory disease Citation[30,31]. Technically early enhancement is based on a T1-weighted fast spin echo image acquired during shallow breathing over 4 min with signal averaging before and after contrast administration . Alternative sequences for early enhancement are not yet available, but clinically required to overcome motion artifacts and other drawbacks of spin echo imaging Citation[2].
Using perfusion sequences it has been demonstrated that even familial dilated cardiomyopathy features an increased distribution volume of gadolinium Citation[32], highlighting the limited specificity of early contrast enhancement, when applied alone.
Late gadolinium enhancement
Late gadolinium enhancement (LGE) has become very popular for imaging myocardial infarction. Technically it is well standardized and widely available Citation[33]. Images are taken 15–25 min after contrast administration when contrast is already washed out from blood and normal myocardium, but is still present in scar or fibrous tissue. Interestingly, not only can classical infarction scars be visualized, but so can a variety of nonischemic lesions including those of acute myocarditis. A recent review is given by Mewton et al.Citation[34]. The technique to obtain the images and the signal intensity patterns do not differ between ischemic and nonischemic lesions, but the spatial distribution does. Nonischemic lesions are located intramural in the middle layer of the myocardial wall or within the epicardial portion of the wall Citation[35–37]. They are often multiple and not confined to a single coronary territory . To a certain extent the distribution pattern may be suggestive for certain nonischemic myocardial diseases Citation[35,36]. Over time the lesions do persist but may shrink to a certain extent .
Clinical aspects of acute myocarditis
Acute myocarditis comprises a wide clinical spectrum from subclinical disease to severe heart failure Citation[38]. Generally, viral etiology is presumed, but other infectious agents may also be important depending on geographical region Citation[38]. Currently, in central Europe, biopsy often reveals parvovirus B19-targeting myocardial endothelial cells but not the myocytes. However, biopsy is prone to sample error and clinically indicated only in a few scenarios Citation[39]. A scientific statement by the AHA/ACC/ESC suggested in 2007 that among 14 different clinical scenarios myocardial biopsy does appear appropriate in just two of them; that is. first “unexplained new-onset heart failure with hemodynamic compromise within 2 weeks” and second “unexplained new-onset heart failure for 2 weeks to 3 months with dilated left ventricle and new ventricular arrhythmias or second or third-degree atrioventricular block or failure to respond to usual care within 1 to 2 weeks” Citation[39]. Clinically, biopsy does not appear to be helpful in cases when CMR has confirmed a ‘typical’ pattern of myocarditis or excluded any myocardial abnormality. In that sense, CMR may offer therapeutic guidance for those patients with an acute cardiovascular event, but normal coronary arteries Citation[40–42]. Still, some authors (using late gadolium enhancement CMR only) advocate a complementary role of CMR and biopsy Citation[43]. Serum antibody titers do not correlate with myocardial virus load Citation[44]. Therefore, it does not appear clinically helpful to determine them. Some investigators even question the importance of bioptic evidence of parvovirus being present Citation[45]. All this explains the interest in sophisticated imaging to support or exclude the clinical suspicion for myocarditis.
CMR in myocarditis
Owing to its excellent spatial resolution, CMR is able to depict the subtle temporary increase in LV mass during myocardial edema Citation[46,47]. The CMR markers for inflammation are unspecific on their own. The combination of CMR techniques may allow a more accurate diagnosis Citation[10].
Ideally, patients with acute myocarditis should be imaged within the first 2 weeks as the sensitivity of CMR sharply decreases thereafter. The most impressive images are seen in young males with an infarction-type myocarditis, whereas patients with heart failure-type myocarditis might not even present with LGE lesions, but may feature more subtle and diffuse contrast uptake.
Myocardial edema in acute myocarditis is often regional similar to acute myocardial infarction and most often found in a posterolateral region. There is extensive interest in imaging ischemic edema for assessment of area at risk in acute myocardial infarction Citation[6,7]. The edema is mostly larger than the necrotic infarction itself, the difference corresponds to the salvaged area at risk and can be quantified by CMR Citation[11,23,48]. In contrast to myocardial infarction, multiple small edematous foci may be found in myocarditis corresponding to multiple patches of fibrosis in late enhancement . Multiple small fibrotic lesions are most often located in the mid or epicardial portion of the lateral and posterolateral wall Citation[16,49,50]. Therefore, it is important for imaging to cover as much myocardial territory as possible and not to limit imaging to an exemplary single midventricular slice. These LGE lesions can be detected early at the time of the initial clinical presentation and do not represent chronic sequelae during the late phase of the disease. Even if these patchy fibrotic lesions are the most striking, CMR finding and LGE images are obtained with the most standardized technique, clinicians should be aware that true myocarditis exists without LGE lesions Citation[10,51]. The diagnosis of myocarditis should therefore not be based on LGE lesions alone. In addition, early myocardial enhancement is often increased over the first weeks after myocarditis even in the absence of LGE lesions. Whether localizing lesions using CMR helps to increase the number of positive biopsy results, is still under debate Citation[49,52].
Among other clinical pictures there is a distinct pattern of infarction-like myocarditis predominantly affecting young men Citation[53–56]. They typically suffer from diarrhea for 2–3 days and then experience an episode of severe chest pain prompting hospital admission. These young males present with signs similar to mild inferior infarction, including mild impairment of systolic LV function often pronounced in the inferolateral segments, global or even inferior ECG changes and a slightly elevated troponin. The most impressive CMR findings can be expected in this patient category. Moreover, this is a nice example for the ability of CMR to detect tissue changes even in the presence of normal LV function Citation[15,46].
In an acute inpatient setting of acute coronary syndrome without obstructive coronary disease, the most important contribution of CMR is the differentiation of myocarditis from myocardial infarction Citation[40,43,57,58] or takotsubo cardiomyopathy (TTC) Citation[59].
Thus far there are not enough data yet to clarify a prognostic role of CMR in acute myocarditis. Preliminary experience suggests that young men do have a favorable prognosis even in the presence of LGE lesions Citation[31,55]. In a broader and older patient spectrum mortality might be higher Citation[54]. Septal LGE and enlarged LV size at initial presentation were found to be markers of unfavorable remodeling at follow-up in a single-center study Citation[50]. Echo data suggest that some patients may develop diastolic dysfunction after myocarditis Citation[60].
An expert consensus paper regarding CMR imaging in myocarditis has been published in 2009 Citation[2]. Multicenter trials to assess the reproducibility of CMR findings are underway.
CMR in pericarditis
Three recent papers have reviewed the potential diagnostic contributions of CMR for pericardial disease in comparison to other imaging modalities Citation[61–63]. Both, SSFP cine CMR and T2-weighted images have a high sensitivity for even small amounts of hyperintense serous pericardial fluid that are hard to detect on echo images . Owing to different relaxation behavior, hemorrhagic or exudative effusions appear less bright on CMR. In acute pericarditis the pericardium may appear bright on LGE images Citation[64]. If the pericardium is 4 mm or thicker, it is most often directly visible on CMR. However, constrictive physiology can be present even in the absence of thickened pericardium. CMR can visualize restricted relaxation of the cardiac chambers and the typical septal bounce Citation[63]. The clinician might still be interested in echo flow patterns across the atrioventricular valves to confirm a constrictive situation Citation[62]. Even small amounts of calcification are more easily delineated by computed tomography Citation[61]. In selected cases CMR tagging might reveal adhesions by persisting rigid tagging patterns across the myocardial and the pericardial layers Citation[65].
Systemic inflammation
They are systemic inflammatory diseases affecting a variety of organ systems, including the heart. If cardiac involvement is present, this often adversely affects the prognosis and therefore requires adjustments in clinical management. In a typical clinical situation the underlying disease in a particular patient has been known for a long time, but unclear symptoms or ECG changes prompt cardiac imaging. Often the inflammatory disease is chronic with active and less active phases. In this situation CMR might occasionally help to guide clinical decisions. The inflammatory markers described earlier are most often unspecific, but might be helpful to detect temporary or permanent myocardial affection in this context. However, the skeletal muscle might no longer be an ideal reference structure as it might be involved in the disease as well.
Churg–Strauss syndrome
Churg–Strauss syndrome (CSS) is a small vessel vasculitis that can affect the heart Citation[66]. Myocarditis, pericarditis, impaired LV function and mitral regurgitation can be observed. Using CMR, we encountered all aspects of myocardial inflammation including increased myocardial signal intensity on T2-weighted images, increased early enhancement and pathological late gadolinium enhancement Citation[67]. Of note, in CSS subendocardial LGE lesions can be found atypical for other forms of nonischemic myocardial disease Citation[68,69]. These lesions often affect more than one coronary territory and most likely develop owing to inflammatory destruction of coronary capillaries. Again, CMR detects tissue changes even in the presence of normal LV function Citation[67].
Sarcoidosis
Sarcoidosis, a multisystemic granulomatous inflammation of unknown origin, can induce a variety of cardiac affections including arrhythmias and heart failure Citation[70]. Cardiac involvement is clinically obvious in only 5% of cases, whereas autopsy series detected cardiac involvement in 20–50% of patients. Importantly, myocardial biopsy has a very low sensitivity. Therefore, negative myocardial biopsies do not rule out cardiac sarcoidosis Citation[70]. In the early phase of the disease reversible myocardial changes such as myocardial edema and increased early contrast uptake can be found in the presence of normal wall motion Citation[71], which often normalize after steroid therapy Citation[72,73]. At a later stage cardiac dilatation and fibrotic lesions develop that can be detected as areas of LGE. Notwithstanding the uncertainties of referral and publication bias, a fourth to a fifth of patients with cardiac sarcoidosis present with LGE lesions Citation[73–75]. Large series have demonstrated a variety of LGE patterns Citation[74]. From our experience the lesions generally appear very bright and almost transmural, but are located more on the epicardial side. This nicely matches historic descriptions from pathology Citation[76,77].
Lupus
Lupus erythematosus is a multisystem connective tissue disease, mainly affecting women, that involves the heart in 60–70% of individuals. Beside a premature atherosclerosis with early myocardial infarctions in women, myocarditis and pericardial effusions can frequently be detected Citation[78]. CMR has been used to detect myocardial scars in those patients Citation[79]. Recent work by Ishimori et al. even revealed abnormal myocardial CMR stress perfusion despite normal coronary angiography, indicating microvascular impairment Citation[80]. Increased myocardial T2-times were able to differentiate Lupus patients in active inflammation from those in inactive phases or controls Citation[81]. Abdel-Aty et al. applied a comprehensive approach including T2-weighting, and early- and late-contrast enhancement to depict all aspects of inflammation in Lupus patients Citation[82].
Rheumatoid arthritis
Cardiovascular magnetic resonance data are sparse in patients with rheumatoid arthritis (RA) and cardiac involvement. Puntmann et al. found increased LV cavity and slightly increased signal in T2, but no scars in LGE imaging in 24 patients with rheumatoid arthritis Citation[47]. In a series of 75 RA patients Giles et al. found decreased LV mass as the most striking CMR finding compared with controls Citation[83].
Transplant rejection
Only few reports with small numbers of patients have been published regarding clinical CMR imaging in transplant cardiomyopathy. A recent review by Butler et al. describes the value of T2-quantification Citation[84], first employed by Marie et al.Citation[85]. Taylor et al. found increased early-contrast enhancement and hyperintense myocardium on T2-weighted images in patients with bioptic evidence of transplant rejection Citation[86]. A small CMR pilot series described subtle LGE lesions in asymptomatic transplant patients that await confirmation in larger studies Citation[85,87]. Korosoglou et al. found stress perfusion and diastolic strain to be sensitive CMR markers for the detection of allograft vasculopathy in a study of 69 transplant patients Citation[88].
Takotsubo cardiomyopathy
In TTC or broken heart syndrome, postmenopausal women are typically admitted to the hospital with a clinical picture similar to acute myocardial infarction Citation[89]. The coronaries are normal, but a severe LV apical ballooning is obvious, resembling a Japanese fishing tool. Carefully assessing the history reveals a stressful trigger event in 77% of the patients Citation[90]. CMR nicely depicts apical ballooning as does echo. The wall motion abnormality can even occur in the inferior or mid portion of the left ventricle. It is of great importance to follow the wall motion abnormality closely over the next 10 days, when most of the ballooning should disappear. Only, if imaging confirms the improving LV function, the diagnosis of TTC is justified. A follow-up after 3 or 6 months only might not distinguish TTC from transient impairment of LV function in myocarditis or tachycardiomyopathy. In clinical routine CMR is important to definitely exclude (embolic) myocardial infarction or myocarditis Citation[41]. The absence of any LGE lesion in combination with the typical ballooning constitutes the diagnosis Citation[91]. In some cases edema in the apical region has been observed Citation[92,93]. However, this might be technically challenging in the presence of insufficient blood suppression owing to slow apical flow.
Limitations
Only part of human CMR studies in myocarditis have histopathological confirmation owing to the limitations of myocardial biopsy Citation[94]. There are only very few data regarding imaging inflammation in animals Citation[95]. This has to do with difficulties in generating stable animals models of myocardial inflammation and technical challenges in quantification of edema ex vivo. Some of the evidence is extrapolated from animal models of ischemic edema that is easier to reproduce, but may not completely be identical to inflammatory edema.
There is currently no clinical role for CMR in the detection of valvular endocarditis. Transesophageal echocardiography supersedes CMR in temporal and spatial resolution, and is therefore still a better choice to depict small mobile valvular lesions.
Conclusion
Cardiovascular magnetic resonance can depict ventricular size and function with great precision but without radiation. Unlike any other imaging modality, it can moreover visualize tissue changes that are associated with inflammation and monitor them longitudinally. Thereby, CMR can often detect inflammatory disease before obvious impairments in systolic function are detectable by other imaging methods. In the acute phase of inflammation CMR can visualize myocardial edema on T2-weighted images. In certain settings, early contrast enhancement is increased and a subgroup of patients shows lesions on late enhancement images that can clearly be differentiated from coronary infarction scars. Thereby, noninvasive and serial assessment of myocardial inflammation is feasible.
Expert commentary & five-year view
The combined use of T2-weighted imaging and contrast enhanced T1-weighted imaging will gain more widespread acceptance. More stable sequences for edema imaging will pave this way. Automatic quantification of hyperintense areas will reduce investigator bias Citation[96]. We expect better ways to quantify early-contrast enhancement. Parametric imaging for T1 and T2 may play a role in future CMR imaging of inflammation. Myocardial edema not only changes T2 contrast but also T1 contrast Citation[19,97]. Therefore, more effort will be invested to explore the potential of T1 mapping for the assessment of myocardial inflammation. Multicenter trials are underway to assess reproducibility in different centers and on different hardware platforms. More animal data are needed for validation and technical improvement. CMR will deliver therapy-guiding outcome data from long-term studies in various forms of myocardial inflammation, thereby assessing the prognostic impact of various CMR markers. CMR might be useful as a surrogate end point in clinical studies of immunomodulating therapy for myocarditis.
Key issues
• Cardiovascular magnetic resonance (CMR) imaging can assess cardiac size and function noninvasively without radiation, but can moreover depict tissue changes that are associated with inflammation.
• These CMR markers are unspecific on their own. Their combination however, allows assessing myocardial inflammation and enables longitudinal monitoring.
• CMR is clinically helpful as these inflammatory markers are detectable even in the presence of normal ventricular size and function.
• Multicenter studies and more animal data are needed to further refine the available evidence from single center human data.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Hundley WG, Bluemke DA, Finn JP et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation121, 2462–2508 (2010).
- Friedrich MG, Sechtem U, Schulz-Menger J et al. Cardiovascular magnetic resonance in myocarditis: a JACC White paper. J. Am. Coll. Cardiol.53, 1475–1487 (2009).
- Friedrich MG. Myocardial edema – a new clinical entity? Nat. Rev. Cardiol.7, 292–296 (2010).
- Edwards NC, Routledge H, Steeds RP. T2-weighted magnetic resonance imaging to assess myocardial oedema in ischaemic heart disease. Heart95, 1357–1361 (2009).
- Abdel-Aty H, Simonetti O, Friedrich MG. T2-weighted cardiovascular magnetic resonance imaging. J. Magn. Reson. Imaging26, 452–459 (2007).
- Aletras AH, Tilak GS, Natanzon A et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation113, 1865–1870 (2006).
- Abdel-Aty H, Cocker M, Meek C, Tyberg JV, Friedrich MG. Edema as a very early marker for acute myocardial ischemia: a cardiovascular magnetic resonance study. J. Am. Coll. Cardiol.53, 1194–1201 (2009).
- Desai KV, Laine GA, Stewart RH et al. Mechanics of the left ventricular myocardial interstitium: effects of acute and chronic myocardial edema. Am. J. Physiol. Heart Circ. Physiol.294, H2428–H2434 (2008).
- Bijnens B, Sutherland GR. Myocardial oedema: a forgotten entity essential to the understanding of regional function after ischaemia or reperfusion injury. Heart94, 1117–1119 (2008).
- Abdel-Aty H, Boye P, Zagrosek A et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J. Am. Coll. Cardiol.45, 1815–1822 (2005).
- Eitel I, Desch S, Fuernau G et al. Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J. Am. Coll. Cardiol.55, 2470–2479 (2010).
- Wright J, Adriaenssens T, Dymarkowski S, Desmet W, Bogaert J. Quantification of myocardial area at risk with T2-weighted CMR: comparison with contrast-enhanced CMR and coronary angiography. JACC Cardiovasc. Imaging2, 825–831 (2009).
- Cury RC, Shash K, Nagurney JT et al. Cardiac magnetic resonance with T2-weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation118, 837–844 (2008).
- Carlsson M, Ubachs JF, Hedstrom E, Heiberg E, Jovinge S, Arheden H. Myocardium at risk after acute infarction in humans on cardiac magnetic resonance: quantitative assessment during follow-up and validation with single-photon emission computed tomography. JACC Cardiovasc. Imaging2, 569–576 (2009).
- Jeserich M, Olschewski M, Bley T et al. Cardiac involvement after respiratory tract viral infection – detection by cardiac magnetic resonance. J. Comput. Assist. Tomogr.33, 15–19 (2009).
- Yelgec NS, Dymarkowski S, Ganame J, Bogaert J. Value of MRI in patients with a clinical suspicion of acute myocarditis. Eur. Radiol.17, 2211–2217 (2007).
- Wince WB, Kim RJ. Molecular imaging: T2-weighted CMR of the area at risk – a risky business? Nat. Rev. Cardiol.7, 547–549 (2010).
- Eitel I, Friedrich MG. T2-weighted cardiovascular magnetic resonance in acute cardiac disease. J. Cardiovasc. Magn. Reson.13, 13 (2011).
- Dwyer AJ, Frank JA, Sank VJ, Reinig JW, Hickey AM, Doppman JL. Short-Ti inversion-recovery pulse sequence: analysis and initial experience in cancer imaging. Radiology168, 827–836 (1988).
- Francone M, Carbone I, Agati L et al. Utility of T2-weighted short-tau inversion recovery (STIR) sequences in cardiac MRI: an overview of clinical applications in ischaemic and non-ischaemic heart disease. Radiol. Med.116, 32–46 (2011).
- Zhou X, Rundell V, Liu Y et al. T(2)-weighted STIR imaging of myocardial edema associated with ischemia-reperfusion injury: the influence of proton density effect on image contrast. J. Magn. Reson. Imaging33, 962–967 (2011).
- Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn. Reson. Med.57, 891–897 (2007).
- Berry C, Kellman P, Mancini C et al. Magnetic resonance imaging delineates the ischemic area at risk and myocardial salvage in patients with acute myocardial infarction. Circ. Cardiovasc. Imaging3, 527–535 (2010).
- Aletras AH, Kellman P, Derbyshire JA, Arai AE. ACUT2E TSE-SSFP: a hybrid method for T2-weighted imaging of edema in the heart. Magn. Reson. Med.59, 229–235 (2008).
- Payne AR, Casey M, McClure J et al. Bright-blood T2-weighted MRI has higher diagnostic accuracy than dark-blood short tau inversion recovery MRI for detection of acute myocardial infarction and for assessment of the ischemic area at risk and myocardial salvage. Circ. Cardiovasc. Imaging4, 210–219 (2011).
- Giri S, Chung YC, Merchant A et al. T2 quantification for improved detection of myocardial edema. J. Cardiovasc. Magn. Reson.11, 56 (2009).
- Verhaert D, Thavendiranathan P, Giri S et al. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovasc. Imaging4, 269–278 (2011).
- Wang Y, Alkasab TK, Narin O et al. Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium-based contrast agent guidelines. Radiology260(1), 105–111 (2011).
- Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation97, 1802–1809 (1998).
- Gutberlet M, Spors B, Thoma T et al. Suspected chronic myocarditis at cardiac MR: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology246, 401–409 (2008).
- Zagrosek A, Abdel-Aty H, Boye P et al. Cardiac magnetic resonance monitors reversible and irreversible myocardial injury in myocarditis. JACC Cardiovasc. Imaging2, 131–138 (2009).
- Jerosch-Herold M, Sheridan DC, Kushner JD et al. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol.295, H1234–H1242 (2008).
- Kim RJ, Shah DJ, Judd RM. How we perform delayed enhancement imaging. J. Cardiovasc. Magn. Reson.5, 505–514 (2003).
- Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J. Am. Coll. Cardiol.57, 891–903 (2011).
- Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur. Heart J.26, 1461–1474 (2005).
- Bohl S, Wassmuth R, Abdel-Aty H et al. Delayed enhancement cardiac magnetic resonance imaging reveals typical patterns of myocardial injury in patients with various forms of non-ischemic heart disease. Int. J. Cardiovasc. Imaging24, 597–607 (2008).
- Hunold P, Schlosser T, Vogt FM et al. Myocardial late enhancement in contrast-enhanced cardiac MRI: distinction between infarction scar and non-infarction-related disease. Am. J. Roentgenol.184, 1420–1426 (2005).
- Cooper LT Jr. Myocarditis. N. Engl. J. Med.360, 1526–1538 (2009).
- Cooper LT, Baughman KL, Feldman AM et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation116, 2216–2233 (2007).
- Assomull RG, Lyne JC, Keenan N et al. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. Eur. Heart J.28, 1242–1249 (2007).
- Eitel I, Behrendt F, Schindler K et al. Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. Eur. Heart J.29, 2651–2659 (2008).
- Mather AN, Fairbairn TA, Artis NJ, Greenwood JP, Plein S. Diagnostic value of CMR in patients with biomarker-positive acute chest pain and unobstructed coronary arteries. JACC Cardiovasc. Imaging3, 661–664 (2010).
- Baccouche H, Mahrholdt H, Meinhardt G et al. Diagnostic synergy of non-invasive cardiovascular magnetic resonance and invasive endomyocardial biopsy in troponin-positive patients without coronary artery disease. Eur. Heart J.30, 2869–2879 (2009).
- Mahfoud F, Gartner B, Kindermann M et al. Virus serology in patients with suspected myocarditis: utility or futility? Eur. Heart J.32, 897–903 (2011).
- Stewart GC, Lopez-Molina J, Gottumukkala RV et al. Myocardial parvovirus B19 persistence: lack of association with clinicopathologic phenotype in adults with heart failure. Circ. Heart Fail.4, 71–78 (2011).
- Zagrosek A, Wassmuth R, Abdel-Aty H, Rudolph A, Dietz R, Schulz-Menger J. Relation between myocardial edema and myocardial mass during the acute and convalescent phase of myocarditis – a CMR study. J. Cardiovasc. Magn. Reson.10, 19 (2008).
- Puntmann VO, Taylor PC, Barr A, Schnackenburg B, Jahnke C, Paetsch I. Towards understanding the phenotypes of myocardial involvement in the presence of self-limiting and sustained systemic inflammation: a magnetic resonance imaging study. Rheumatology49, 528–535 (2010).
- Dall’armellina E, Karia N, Lindsay AC et al. Dynamic changes of edema and late gadolinium enhancement after acute myocardial infarction and their relationship to functional recovery and salvage index. Circ. Cardiovasc. Imaging4, 228–236 (2011).
- Mahrholdt H, Goedecke C, Wagner A et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation109, 1250–1258 (2004).
- Mahrholdt H, Wagner A, Deluigi CC et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation114, 1581–1590 (2006).
- Rottgen R, Christiani R, Freyhardt P et al. Magnetic resonance imaging findings in acute myocarditis and correlation with immunohistological parameters. Eur. Radiol.21, 1259–1266 (2011).
- Yilmaz A, Kindermann I, Kindermann M et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation122, 900–909 (2010).
- Angelini A, Calzolari V, Calabrese F et al. Myocarditis mimicking acute myocardial infarction: role of endomyocardial biopsy in the differential diagnosis. Heart84, 245–250 (2000).
- Caforio AL, Calabrese F, Angelini A et al. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur. Heart J.28, 1326–1333 (2007).
- Costantini M, Oreto G, Albanese A et al. Presumptive myocarditis with ST-elevation myocardial infarction presentation in young males as a new syndrome. Clinical significance and long term follow up. Cardiovasc. Ultrasound9, 1 (2011).
- Cocker MS, Abdel-Aty H, Strohm O, Friedrich MG. Age and gender effects on the extent of myocardial involvement in acute myocarditis: a cardiovascular magnetic resonance study. Heart95, 1925–1930 (2009).
- Monney PA, Sekhri N, Burchell T et al. Acute myocarditis presenting as acute coronary syndrome: role of early cardiac magnetic resonance in its diagnosis. Heart97(16), 1312–1318 (2010).
- Leurent G, Langella B, Fougerou C et al. Diagnostic contributions of cardiac magnetic resonance imaging in patients presenting with elevated troponin, acute chest pain syndrome and unobstructed coronary arteries. Arch. Cardiovasc. Dis.104, 161–170 (2011).
- Eitel I, Behrendt F, Schindler K, Gutberlet M, Schuler G, Thiele H. Takotsubo cardiomyopathy or myocardial infarction? Answers from delayed enhancement magnetic resonance imaging. Int. J. Cardiol.135, e9–e12 (2009).
- Escher F, Westermann D, Gaub R et al. Development of diastolic heart failure in a 6-year follow-up study in patients after acute myocarditis. Heart97, 709–714 (2011).
- Yared K, Baggish AL, Picard MH, Hoffmann U, Hung J. Multimodality imaging of pericardial diseases. JACC Cardiovasc. Imaging3, 650–660 (2010).
- Verhaert D, Gabriel RS, Johnston D, Lytle BW, Desai MY, Klein AL. The role of multimodality imaging in the management of pericardial disease. Circ. Cardiovasc. Imaging3, 333–343 (2010).
- Bogaert J, Francone M. Cardiovascular magnetic resonance in pericardial diseases. J. Cardiovasc. Magn. Reson.11, 14 (2009).
- Taylor AM, Dymarkowski S, Verbeken EK, Bogaert J. Detection of pericardial inflammation with late-enhancement cardiac magnetic resonance imaging: initial results. Eur. Radiol.16, 569–574 (2006).
- Kojima S, Yamada N, Goto Y. Diagnosis of constrictive pericarditis by tagged cine magnetic resonance imaging. N. Engl. J. Med.341, 373–374 (1999).
- Noth I, Strek ME, Leff AR. Churg–Strauss syndrome. Lancet361, 587–594 (2003).
- Wassmuth R, Gobel U, Natusch A et al. Cardiovascular magnetic resonance imaging detects cardiac involvement in Churg–Strauss syndrome. J. Card. Fail.14, 856–860 (2008).
- Chun W, Grist TM, Kamp TJ, Warner TF, Christian TF. Infiltrative eosinophilic myocarditis diagnosed and localized by cardiac resonance imaging. Circulation110, e19 (2004).
- Debl K, Djavidani B, Buchner S et al. Time course of eosinophilic myocarditis visualized by CMR. J. Cardiovasc. Magn. Reson.10, 21 (2008).
- Kim JS, Judson MA, Donnino R et al. Cardiac sarcoidosis. Am. Heart J.157, 9–21 (2009).
- Vignaux O, Dhote R, Duboc D et al. Clinical significance of myocardial magnetic resonance abnormalities in patients with sarcoidosis: a 1-year follow-up study. Chest122, 1895–1901 (2002).
- Shimada T, Shimada K, Sakane T et al. Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium–DTPA-enhanced magnetic resonance imaging. Am. J. Med.110, 520–527 (2001).
- Schulz-Menger J, Wassmuth R, Abdel-Aty H et al. Patterns of myocardial inflammation and scarring in sarcoidosis as assessed by cardiovascular magnetic resonance. Heart92, 399–400 (2006).
- Patel MR, Cawley PJ, Heitner JF et al. Detection of myocardial damage in patients with sarcoidosis. Circulation120, 1969–1977 (2009).
- Cheong BY, Muthupillai R, Nemeth M et al. The utility of delayed-enhancement magnetic resonance imaging for identifying nonischemic myocardial fibrosis in asymptomatic patients with biopsy-proven systemic sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis.26, 39–46 (2009).
- Shirani J, Roberts WC. Subepicardial myocardial lesions. Am. Heart J.125, 1346–1352 (1993).
- Bagwan IN, Hooper LV, Sheppard MN. Cardiac sarcoidosis and sudden death. The heart may look normal or mimic other cardiomyopathies. Virchows Arch.458(6), 671–678 (2011).
- Roman MJ, Salmon JE. Cardiovascular manifestations of rheumatologic diseases. Circulation116, 2346–2355 (2007).
- O’Neill SG, Woldman S, Bailliard F et al. Cardiac magnetic resonance imaging in patients with systemic lupus erythematosus. Ann. Rheum. Dis.68, 1478–1481 (2009).
- Ishimori ML, Martin R, Berman DS et al. Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovasc. Imaging4, 27–33 (2011).
- Singh JA, Woodard PK, Davila-Roman VG et al. Cardiac magnetic resonance imaging abnormalities in systemic lupus erythematosus: a preliminary report. Lupus14, 137–144 (2005).
- Abdel-Aty H, Siegle N, Natusch A et al. Myocardial tissue characterization in systemic lupus erythematosus: value of a comprehensive cardiovascular magnetic resonance approach. Lupus17, 561–567 (2008).
- Giles JT, Malayeri AA, Fernandes V et al. Left ventricular structure and function by cardiac magnetic resonance imaging in rheumatoid arthritis. Arthritis Rheum.62, 940–951 (2010).
- Butler CR, Thompson R, Haykowsky M, Toma M, Paterson I. Cardiovascular magnetic resonance in the diagnosis of acute heart transplant rejection: a review. J. Cardiovasc. Magn. Reson.11, 7 (2009).
- Marie PY, Angioi M, Carteaux JP et al. Detection and prediction of acute heart transplant rejection with the myocardial T2 determination provided by a black-blood magnetic resonance imaging sequence. J. Am. Coll. Cardiol.37, 825–831 (2001).
- Taylor AJ, Vaddadi G, Pfluger H et al. Diagnostic performance of multisequential cardiac magnetic resonance imaging in acute cardiac allograft rejection. Eur. J. Heart Fail.12, 45–51 (2010).
- Usta E, Burgstahler C, Aebert H et al. The challenge to detect heart transplant rejection and transplant vasculopathy non-invasively – a pilot study. J. Cardiothorac. Surg.4, 43 (2009).
- Korosoglou G, Osman NF, Dengler TJ et al. Strain-encoded cardiac magnetic resonance for the evaluation of chronic allograft vasculopathy in transplant recipients. Am. J. Transplant.9, 2587–2596 (2009).
- Akashi YJ, Goldstein DS, Barbaro G, Ueyama T. Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation118, 2754–2762 (2008).
- Schneider B, Athanasiadis A, Schwab J et al. [Clinical spectrum of tako-tsubo cardiomyopathy in Germany: results of the tako-tsubo registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte (ALKK)]. Dtsch Med. Wochenschr.135, 1908–1913 (2010).
- Fernandez-Perez GC, Aguilar-Arjona JA, de la Fuente GT et al. Takotsubo cardiomyopathy: assessment with cardiac MRI. AJR Am. J. Roentgenol.195, W139–W145 (2010).
- Eitel I, Lucke C, Grothoff M et al. Inflammation in takotsubo cardiomyopathy: insights from cardiovascular magnetic resonance imaging. Eur. Radiol.20, 422–431 (2010).
- Joshi SB, Chao T, Herzka DA et al. Cardiovascular magnetic resonance T2 signal abnormalities in left ventricular ballooning syndrome. Int. J. Cardiovasc. Imaging26, 227–232 (2010).
- Elliott P, Arbustini E. The role of endomyocardial biopsy in the management of cardiovascular disease: a commentary on joint AHA/ACC/ESC guidelines. Heart95, 759–760 (2009).
- Korkusuz H, Esters P, Huebner F, Bug R, Ackermann H, Vogl TJ. Accuracy of cardiovascular magnetic resonance in myocarditis: comparison of MR and histological findings in an animal model. J. Cardiovasc. Magn. Reson.12, 49 (2010).
- Johnstone RI, Greenwood JP, Biglands JD, Plein S, Ridgway JP, Radjenovic A. Assessment of tissue edema in patients with acute myocardial infarction by computer-assisted quantification of triple inversion recovery prepared MRI of the myocardium. Magn. Reson. Med.66(2), 565–574 (2011).
- Higgins CB, Herfkens R, Lipton MJ et al. Nuclear magnetic resonance imaging of acute myocardial infarction in dogs: alterations in magnetic relaxation times. Am. J. Cardiol.52, 184–188 (1983).