Abstract
Lung cancer is the most common cause of cancer death worldwide. Surgical resection has played a major role in the treatment of non-small-cell lung cancer (NSCLC); however, the disease is often detected in a progressive and inoperable form. Surgical resection may also be impossible for early-stage NSCLC due to medical conditions, such as pulmonary or cardiovascular disease and old age. Radiotherapy plays an important role for these patients. Proton-beam therapy is a particle radiotherapy with an excellent dose localization that permits treatment of lung cancer by administering a high dose to the tumor while minimizing damage to the surrounding normal tissues. Thus, proton beams are increasingly being used for lung cancer. In this context, the authors review the current knowledge on proton-beam therapy for the treatment of NSCLC.
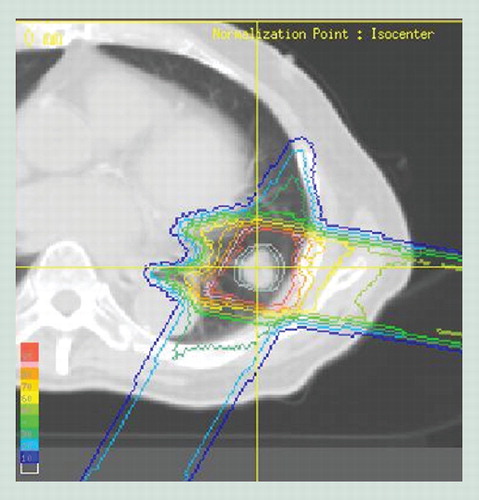
Because of the low lung density, the doses do not fall off sharply at the target depth. Also, the edge at the end of the beam range is irregular, because the scapula and ribs are in the pathway. The beam range of the part that passes through bones is smaller than that of the part of the beam that avoids bone.
History of proton beam therapy
A proton (H+) is a positively charged particle that is obtained by stripping a hydrogen atom of its electron. Protons were first identified in decay of uranium by Ernest Rutherford in 1899. Wilson et al. first proposed utilizing high energy protons for radiotherapy in 1946 based on a description of the favorable dose distribution of accelerated protons inside the body Citation[1]; this phenomenon is now referred to as the Bragg peak. Four years later, the first clinical application of proton beam therapy (PBT) was conducted on the pituitary gland, with the goal of suppressing the function of this gland in order to reduce metastases from breast cancer. The Uppsala Group in Sweden pioneered proton radiotherapy for cancer patients in the 1950s. In the early 1960s, the Harvard Cyclotron Group developed most of the techniques that are in current use. At present, there are more than 30 PBT facilities and more than 67,000 people have been treated worldwide. Eleven new facilities began operation especially in the last 5 years from 2007 to 2011, and more are scheduled to open in 2012 Citation[101].
Physical characteristics
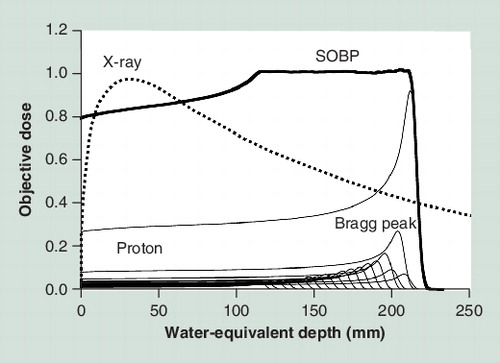
PBT has excellent dose localization because of the Bragg peak, with a relatively low plateau dose at entry, followed by a sharp peak, and then a rapid fall off, both laterally and distally to zero. This peak is spread out in conformity to the tumor (spread out of Bragg peak [SOBP]) because it is too narrow and sharp to give doses to a tumor with an inhomogeneous shape . Proton penetration depth is finite and controllable, and 4D image-guided treatment and intensity-modulated techniques are possible in PBT, as well as in photon radiotherapy, because of the flexibility in choosing beam number, orientation and scanning Citation[2]. The proton range and sharpness of the Bragg peak is strongly affected by the energy and density of the tissues through which the beam passes in the body. The particle range is inversely proportional to tissue density Citation[3]. Thus, because the density of the lung is very low, the beam range tends to become extended. Inhomogeneity in the beam’s path, that is, the higher density of bone and relatively lower density of the lung, also affects on shape of distal fall off and curvature of the isodose lines Citation[2,3]. Tissue density is also affected by organ motion due to respiration, which is a very important factor in PBT for lung tumors. In 1992, respiratory gating PBT at end expiration using a laser range finder that monitors the movement of the body surface was started at Tsukuba University (Ibaraki, Japan) Citation[4,5]. More recently, 4D computer tomography has been developed. This method provides improved calculation of actual organ motion and better optimization of the dose during treatment, which allows more precise respiratory gating to be possible Citation[6–8].
Thus, the margin decision in PBT differs from that in photon radiotherapy, with distal coverage, changes of tissue density along the pathway and respiratory organ motion all taken into consideration. A range compensator for distal margins and a lateral smearing technique to null the beam misalignment are generally used Citation[9,10]. The margins should be field specific, and a different planning target volume is needed for each beam direction Citation[11,12].
Biological characteristics
A proton beam is categorized as low linear energy transfer (LET) radiation, similar to photon radiotherapy. The LET is a measure of energy transfer to matter from an ionizing particle travelling through the matter. It is closely related to energy per unit distance and provides an indication of ion-induced damages. Higher LET radiation is proposed to be more effective for destroying cells. Differences in biological effects due to radiation quality are quantified as the relative biological effective (RBE) dose. The RBE is defined as the ratio of the photon dose to the proton dose required to give the same biological effect under identical irradiation conditions. Proton beams used in clinical practice are normally considered to have an RBE of 1.1 Citation[2,13]. That is, the biological effects of protons are similar to those of photons, with no apparent clinical advantage; therefore, proton therapy is generally thought to be applicable for most uses of photon radiotherapy. However, this concept is uncertain because the actual effect depends on several factors, including the measured end point, tissue, dose level and depth of the SOBP Citation[13]. The RBE increases by 5–10% in the very distal part of the SOBP relative to the mid-SOBP Citation[2]. Therefore, proton beams are usually designed to avoid critical organs in the distal fall off of the beam.
Most radiation-induced lethal cell death is caused by mechanisms linked to mitosis. Apoptosis contributes to tumor growth kinetics and response to radiotherapy, and some radiosensitive tumors have a tendency to undergo apoptosis Citation[14]. There may be differences in the kinetics and extent of apoptosis induction between protons and photon Citation[15,16]. Gerelchuluun et al. showed greater induction of in situ DNA double-strand breaks and apoptosis by proton beams in the early phase after irradiation, compared with photon beams. At 10% survival, the RBE of protons to x-rays was around 1.1 but the average ratios of early apoptosis induced by protons to that induced by x-rays at 1.8 Gy were 2.13 ± 0.79 at 12 h and 1.04 ± 0.36 at 20 h Citation[16]. This indicates that the biological effect may differ with different end points, and that clinical effects may potentially vary depending on the type of disease.
Clinical results of PBT for early stage non-small-cell lung cancer
Surgical resection is the first treatment choice for early stage non-small-cell lung cancer (NSCLC) and has an associated 5-year survival rate of 60–80%. However, in some cases, standard surgical resection may not be possible because of comorbidities, old age and patient refusal. Stereotactic body radiotherapy (SBRT) has been increasingly applied for these patients as an emerging modality for the treatment of early stage NSCLC, since it permits safe treatment of a limited disease with escalated radiation doses. Timmerman et al. reported the results of photon SBRT for 55 patients (80% with stage IA tumor and 20% with stage IB tumor) with 60 Gy in three fractions within 14 days, prescribed to the edge of planning target margin. The 3-year local control rate was 97.6% and the 3-year disease-free and overall survival rate were 48.3 and 55.8%, respectively Citation[17].
PBT has also been used for stage IA/IB NSCLC, but the clinical evidence for efficacy is limited Citation[4,18–22]. Nihei et al. reported the results of PBT for 37 patients with stage I NSCLC. Most patients received a RBE-weighted dose of 80–88 Gy (RBE), in fractions of 3.5–4.9 Gy (RBE). The 2-year local progression-free and overall survival rates were 80 and 84% for stage IA and IB patients, respectively. Pulmonary toxicity of grades 2 and 3 was observed in six patients, including five with stage IB disease Citation[18]. Hata et al. reported the results of treatment of 21 patients with stage I NSCLC with a proton dose of 50–60 Gy (RBE) in ten fractions. The 2-year local control rate was 95% and only one patient experienced local recurrence. The 2-year overall survival rate was 74% and there was no treatment toxicity of grade 3 or severer Citation[19]. However, these studies were small and most were retrospective. Therefore, a meta-analysis was performed to compare the efficacy of chemoradiotherapy, photon SBRT, PBT and carbon ion therapy for stage I inoperable NSCLC Citation[23]. Carbon ion therapy is one of the particle radiotherapies, as well as PBT, but carbon ion beams are categorized as high LET radiation. In this analysis, survival and severe adverse events were compared among radiotherapy with conventional radiotherapy (CRT), SBRT, concurrent chemoradiotherapy, PBT and carbon ion therapy. Toxicities were infrequent for all treatment modalities, and the 2- and 5-year survival rate were estimated at 53 and 19% for CRT, 73 and 42% for SBRT, 61 and 40% for PBT and 74 and 42% for carbon ion therapy, respectively. Overall survival with CRT was statistically significantly lower than those with SBRT, PBT and carbon ion therapy, with no significant differences among SBRT, PBT and carbon ion therapy.
The dosimetric advantages of protons have been widely discussed Citation[24–28]. Hoppe et al. performed a dosimetric comparison between SBRT (including intensity modulated radiotherapy [IMRT]) and PBT for eight patients with stage I NSCLC, and found that normal tissue doses were significantly lower in all patients who received PBT Citation[25]. However, the dosimetry of proton beams, especially for the distal range, is very sensitive to the tissue density in the proton beam pathway . Therefore, the beam range and target coverage may be changed if the pathway is misaligned due to respiratory motion, variation in set up and tumor shrinkage. Seco et al. found that these uncertainties result in larger required planning margins for protons compared with photons, with a subsequent increase in the dose to the surrounding organs Citation[28]. Register et al. found that clinical target volume coverage was comparable between the two methods, but that coverage of the planning target volume was marginally worse with proton beams Citation[26].
The difference in dose conformity between SBRT and PBT is due to a difference in the number of ports. Two or three ports are usually used in PBT, whereas 7–12 ports are used in SBRT. However, there seems to be little clinical impact of the dosimetric differences, as mentioned above. Thus, proton beams can provide a sufficient dose distribution using a smaller number of ports. This has a major advantage for patients with pulmonary morbidities such as chronic obstructive pulmonary disease and interstitial pneumonitis, because a larger low dose area is correlated with a higher risk of development of radiation-induced lung injury in these patients Citation[29–31]. A history of radiation of the lung is also associated with a higher incidence of radiation-induced lung injury, and sometimes limits further radiotherapy Citation[32]. Westover et al. analyzed the results of PBT for 15 patients with inoperable stage I NSCLC who could not receive photon SBRT because of pulmonary morbidities, difficult locations, prior chest radiation and other reasons. The treatment dose was 42–50 Gy (RBE) in three fractions. The 2-year survival rate and local control rates were 64 and 100%, respectively, and there were no severe toxicities. The results suggested that PBT is potentially advantageous in these cases, and permits for dose escalation that cannot be safely achieved with photons for larger tumors Citation[27].
Clinical results of PBT for advanced NSCLC
Dose escalation in radiation therapy may be promising for improvement of local control in patients with unresectable advanced NSCLC, but is limited by the risk of toxicity in neighboring organs. Concurrent chemoradiotherapy is a particular risk for increased toxicity. Historically, a significant benefit of dose escalation in radiotherapy for advanced NSCLC has been suggested. In the 1980s and 1990s, various irradiation schedules were tried to deliver high doses with minimal toxicity for unresectable NSCLC Citation[33–35]. One such approach was the dose escalation study (RTOG 8311) performed by Cox et al. as a Phase I/II trial of hyperfractionated radiotherapy [83]. Patients received doses increased in five 4.8 Gy steps from 60 to 79.2 Gy using 1.2 Gy fractions delivered twice a day Citation[33]. There was no significant difference in toxicity and among the doses, 69.6 Gy was recommended because patients who received this dose had significantly better survival compared with those who received lower doses, while patients who received more than 69.6 Gy had no additional survival benefit.
Subsequently, chemoradiotherapy was established and a radiation dose of about 60 Gy was agreed upon for concurrent chemoradiotherapy to prevent increased toxicity. In the last decade, modern precise radiotherapy and diagnostic imaging have allowed target volume definition which permits higher doses to be delivered to a smaller volume Citation[36]. Socinski et al. conducted a Phase I/II study of induction and concurrent carboplatin and paclitaxel for stage III NSCLC patients with dose escalation from 60 to 74 Gy. At 74 Gy, only 8% of the patients experienced grade 3/4 esophagitis and the median survival time of 26 months suggested a survival benefit Citation[37]. The results of this study encouraged performance of further Phase II studies of concurrent chemoradiotherapy at a dose of 74 Gy. The results of these studies were also favorable with the median survival of 18–37 months and >grade 3 esophagitis and pulmonary toxicity in only 11–17% and 0–30% of the patients, respectively Citation[38–43].
Other recent studies have also suggested a benefit of dose escalation Citation[44–46]. Kong et al. analyzed the dose-response relationship in patients who are treated by standalone radiotherapy in a dose escalation study (63–103 Gy), and found that a higher dose was the only significant factor associated with improved outcome Citation[46]. Wang et al. found that higher radiation doses had a significant and independent impact on survival, as well as utilization of chemotherapy based on the evaluation of 237 patients with stage III NSCLC Citation[45]. Machtay et al. investigated the biological effective dose for patients treated in RTOG trials, and found that a 1-Gy biological effective dose increase in radiation dose intensity was significantly associated with approximately 4% relative improvement in survival and local-regional control Citation[44]. However, a Phase III dose escalation study (RTOG 0617) was closed early because a higher dose of 74 Gy did not produce a survival benefit compared with a standard dose of 60 Gy. There was no significant difference in treatment-related toxicity between the higher and lower doses Citation[47]. These were surprising results that occurred for unknown reasons. Cox warned that these results were counterintuitive and that they were contrary to considerable evidence that higher radiation doses lead to better tumor control Citation[48].
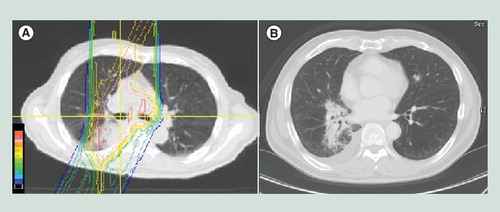
PBT has also been used for stage III NSCLC with the goal of reduced toxicity by focusing higher doses on the tumor. Few studies have compared the dose distribution between proton and photon beams for stage III NSCLC compared with those for early stage NSCLC, however, the advantage of PBT for stage III NSCLC seems to be more significant than that for early NSCLC Citation[49,50]. Roelofs et al. investigated if PBT reduces the dose and irradiated volume of normal tissue with dose escalation in 25 patients with stage IA–IIIB disease, including 20 cases of stage IIB–IIIB disease Citation[49]. PBT gave the lowest dose to organs at risk (lung, esophagus, cord, heart) compared with 3D conformal radiotherapy and IMRT, while maintaining doses of 70 Gy to the target. The lung volume receiving 20 Gy (V20) with PBT (16.8%) was similar to that with IMRT (16.3%), but the mean lung dose with PBT (13.5 Gy) was significantly lower than that for IMRT (16.4 Gy) and 3D conformal radiotherapy (18.9 Gy), and V5 for protons was less than half the values for both photon modalities. Nichols et al. evaluated dose distribution in eight patients with stage III disease, and found that PBT gave about a 30% reduction of normal lung V20 in all patients Citation[50]. Salama et al. investigated pulmonary toxicities in a photon dose escalation study, and found a strong correlation with patients with N3 or V20 ≥38% Citation[51]. Thus, using proton beams, reduction of V20 seems to be possible owing to excellent dose localization. The dose distribution of proton beams for stage III NSCLC and CT images after PBT was shown in . The doses for the anterior lung and esophagus were decreased, and fibrosis was limited in the posterior lung. Over a long treatment period for advanced NSCLC, tumor shape and volume may change and replanning is useful. Koay et al. showed improvement of target coverage and sparing of the esophagus and spinal cord after adaptive planning especially for large tumors Citation[52].
The clinical value of PBT has not been fully established, but high-dose PBT has the potential to be conducted safely for patients with stage III NSCLC. Oshiro et al. evaluated clinical results of standalone PBT for 57 patients with stage III NSCLC using a median dose of 74 Gy (RBE). The median survival was 21.3 months, and grade 3 or severer lung toxicities occurred in only three patients. Two out of these three had grade 4 or 5 lung toxicities, but they had severe interstitial pneumonitis before diagnosis of NSCLC, and grade 3 or severer esophageal toxicity was not observed Citation[53]. Recent trials of high-dose PBT with concurrent chemotherapy have also shown good safety and efficacy Citation[8,54]. Chang et al. reported the results of concurrent chemoproton therapy at a dose of 74 Gy (RBE) and weekly paclitaxel (50 mg/m2) and carboplatin (area under the blood concentration–time curve [AUC] 2) Citation[8]. The median survival was 29.4 months and total local failure was only 20.5%. Toxicity was modest with only one and five patients developing grade 3 pneumonitis and esophagitis, respectively. No grade 4 or 5 radiation-related toxicity occurred. It was concluded that high-dose PBT with concurrent chemotherapy is well tolerated and may be useful for inoperable stage III NSCLC.
Expert commentary
Many studies have compared the dose distribution between protons and photons, but there has been no clinical comparison of treatment outcomes. This might suggest a possible need for a Phase III study to compare PBT and photon therapy. However, there is the opposite perspective that the clearly superior dose distributions of proton beams compared with photon beams provides enough evidence to conduct PBT Citation[55,56]. As far as the biological effect is similar, the superior dose distribution should achieve equaling or better clinical results. However, proton beams may have different kinetics from photon beams, and it will be very interesting to see if this has an influence on clinical effects. Recently, less invasive surgery has been aggressively conducted for very early adenocarcinoma including minimally invasive adenocarcinoma and adenocarcinoma in situ. It will also be of interest to determine whether PBT or SBRT can be applied for these diseases when surgical resection is judged to be impossible. Besides, some studies have shown favorable results for operable early stage NSCLC treated with photon SBRT Citation[57,58], and these results indicate that SBRT or PBT could be used for operable NSCLC. However, lobectomy or pneumonectomy is the standard treatment for invasive NSCLC, therefore, an additional assessment of indication of SBRT or PBT for operable NSCLC may be required. The efficacy of concurrent chemoproton therapy for advanced NSCLC also requires careful evaluation. Thus, although there may be ‘enough’ evidence to use proton beams clinically, many clinical issues still need to be resolved and this may require further prospective studies of PBT.
Five-year view
The results of ongoing studies of PBT for advanced NSCLC with or without chemotherapy may establish the efficacy of PBT in the near future. PBT with pencil beam scanning is also likely to become more common. The common proton beam delivery system used today permits passive scattering PBT, in which proton beams are spread out to cover the field and extent in depth of the planning target volume in a beam line that starts just before entry into the patient. By contrast, the trend is moving toward use of pencil beam scanning, which is already used in some facilities. Pencil beam scanning delivers the dose inside the patient through the sequential superposition of many elementary pencil beams. Using this approach, intensities and energies of all pencil beams can be simultaneously optimized and the SOBP can be changed to fit the length of the target along the beam axis for each beam. Therefore, proton doses can be delivered to a complex target shape more precisely and with reduction of doses outside the target. Pencil beam scanning has been used for static targets in some facilities, and expected to be beneficial for NSCLC Citation[59]; however, it is not currently in practical use for NSCLC. This scanning technique is very sensitive to organ motion, and margin decisions for this technique are more complicated than those with use of a scattering beam Citation[12,60,61]. A beam-specific margin is needed as well as a scattering beam, and furthermore, each scanned beam can overlap and separate due to respiratory displacement, which produces hot spots and cold spots. Rescanning and fast scanning techniques are now under development to solve these problems Citation[62,63]. Scanning PBT for NSCLC is likely to be put into practice in the near future, and this will facilitate intensity-modulated PBT for NSCLC, which may give more favorable results than current PBT. There is also likely to be progression in other modalities such as photon radiotherapy, carbon-ion therapy and less invasive surgery for early stage NSCLC, and selection of an appropriate morality in each case will require careful consideration.
Key issues
• Proton beam therapy (PBT) can give an excellent dose distribution using a small number of ports due to the Bragg peak.
• PBT is appropriate for patients with early stage non-small-cell lung cancer (NSCLC) in addition to photon stereotactic body radiotherapy.
• PBT is potentially advantageous for patients with inoperable early stage NSCLC who cannot receive photon stereotactic body radiotherapy because of pulmonary comorbidities and difficult tumor locations.
• PBT for advanced NSCLC may be more advantageous than that for early stage NSCLC in terms of reduction of the dose to normal tissue.
• The significance of dose-escalation concurrent chemoradiotherapy with dose escalation for stage III NSCLC remains uncertain. However, dose-escalated PBT with concurrent chemotherapy can be conducted safely and effectively whereas this is difficult using photon radiotherapy because of toxicities. Further studies of this issue are required.
Acknowledgements
The authors thank Professor Takeji Sakae, Proton Medial Research Center of Tsukuba University, for advice on the proton physics in the manuscript.
Financial & competing interests disclosure
This article was partly supported by a Grant-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science, Tokyo, Japan (No. 23791390), and the ‘Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program)’, initiated by the Council for Science and Technology Policy (CSTP). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Wilson RR. Radiological use of fast protons. Radiology 47(5), 487–491 (1946).
- International Commission on Radiation Units and Measurements. Prescribing, recording, and reporting proton-beam therapy (ICRU Report 78). J. ICRU 7(2), (2007).
- Breuer H, Smit BJ. Proton Therapy and Radio-Surgery (1st Edition). Springer, Berlin, Germany, 40 (2000).
- Shioyama Y, Tokuuye K, Okumura T et al. Clinical evaluation of proton radiotherapy for non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 56(1), 7–13 (2003).
- Inada T, Tsuji H, Hayakawa Y, Maruhashi A, Tsujii H. Proton irradiation synchronized with respiratory cycle. Nihon Igaku Hoshasen. Gakkai Zasshi. 52(8), 1161–1167 (1992).
- Kang Y, Zhang X, Chang JY et al. 4D Proton treatment planning strategy for mobile lung tumors. Int. J. Radiat. Oncol. Biol. Phys. 67(3), 906–914 (2007).
- Rietzel E, Chen GT, Choi NC, Willet CG. Four-dimensional image-based treatment planning: target volume segmentation and dose calculation in the presence of respiratory motion. Int. J. Radiat. Oncol. Biol. Phys. 61(5), 1535–1550 (2005).
- Chang JY, Komaki R, Lu C et al. Phase II study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer 117(20), 4707–4713 (2011).
- Engelsman M, Kooy HM. Target volume dose considerations in proton beam treatment planning for lung tumors. Med. Phys. 32(12), 3549–3557 (2005).
- Lee CH, Tait D, Nahum AE, Webb S. Comparison of proton therapy and conformal x-ray therapy in non-small cell lung cancer (NSCLC). Br. J. Radiol. 72(863), 1078–1084 (1999).
- Moyers MF, Miller DW, Bush DA, Slater JD. Methodologies and tools for proton beam design for lung tumors. Int. J. Radiat. Oncol. Biol. Phys. 49(5), 1429–1438 (2001).
- Park PC, Zhu XR, Lee AK et al. A beam-specific planning target volume (PTV) design for proton therapy to account for setup and range uncertainties. Int. J. Radiat. Oncol. Biol. Phys. 82(2), e329–e336 (2012).
- Paganetti H, Niemierko A, Ancukiewicz M et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 53(2), 407–421 (2002).
- Perez CA. Principles and Practice of Radiation Oncology (4th Edition). Perez CA, Brady LW, Halperin EC, Schmidt-Ullrich RK (Eds). Lippincott Williams & Wilkins, MD, USA, 105 (2004).
- Di Pietro C, Piro S, Tabbì G et al. Cellular and molecular effects of protons: apoptosis induction and potential implications for cancer therapy. Apoptosis 11(1), 57–66 (2006).
- Gerelchuluun A, Hong Z, Sun L et al. Induction of in situ DNA double-strand breaks and apoptosis by 200 MeV protons and 10 MV x-rays in human tumour cell lines. Int. J. Radiat. Biol. 87(1), 57–70 (2011).
- Timmerman R, Paulus R, Galvin J et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 303(11), 1070–1076 (2010).
- Nihei K, Ogino T, Ishikura S, Nishimura H. High-dose proton beam therapy for stage I non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 65(1), 107–111 (2006).
- Hata M, Tokuuye K, Kagei K et al. Hypofractionated high-dose proton beam therapy for stage I non-small-cell lung cancer: preliminary results of a Phase I/II clinical study. Int. J. Radiat. Oncol. Biol. Phys. 68(3), 786–793 (2007).
- Iwata H, Murakami M, Demizu Y et al. High-dose proton therapy and carbon-ion therapy for stage I nonsmall cell lung cancer. Cancer 116(10), 2476–2485 (2010).
- Bush DA, Slater JD, Shin BB, Cheek G, Miller DW, Slater JM. Hypofractionated proton beam radiotherapy for stage I lung cancer. Chest 126(4), 1198–1203 (2004).
- Nakayama H, Sugahara S, Tokita M et al. Proton beam therapy for patients with medically inoperable stage I non-small-cell lung cancer at the University of Tsukuba. Int. J. Radiat. Oncol. Biol. Phys. 78(2), 467–471 (2010).
- Grutters JP, Kessels AG, Pijls-Johannesma M, De Ruysscher D, Joore MA, Lambin P. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: a meta-analysis. Radiother. Oncol. 95(1), 32–40 (2010).
- Chang JY, Zhang X, Wang X et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 65(4), 1087–1096 (2006).
- Hoppe BS, Huh S, Flampouri S et al. Double-scattered proton-based stereotactic body radiotherapy for stage I lung cancer: a dosimetric comparison with photon-based stereotactic body radiotherapy. Radiother. Oncol. 97(3), 425–430 (2010).
- Register SP, Zhang X, Mohan R, Chang JY. Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage I non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 80(4), 1015–1022 (2011).
- Westover KD, Seco J, Adams JA et al. Proton SBRT for medically inoperable stage I NSCLC. J. Thorac. Oncol. 7(6), 1021–1025 (2012).
- Seco J, Panahandeh HR, Westover K, Adams J, Willers H. Treatment of non-small cell lung cancer patients with proton beam-based stereotactic body radiotherapy: dosimetric comparison with photon plans highlights importance of range uncertainty. Int. J. Radiat. Oncol. Biol. Phys. 83(1), 354–361 (2012).
- Stephans KL, Djemil T, Reddy CA et al. Comprehensive analysis of pulmonary function Test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J. Thorac. Oncol. 4(7), 838–844 (2009).
- Rancati T, Ceresoli GL, Gagliardi G, Schipani S, Cattaneo GM. Factors predicting radiation pneumonitis in lung cancer patients: a retrospective study. Radiother. Oncol. 67(3), 275–283 (2003).
- Hernando ML, Marks LB, Bentel GC et al. Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 51(3), 650–659 (2001).
- Movsas B, Raffin TA, Epstein AH, Link CJ Jr. Pulmonary radiation injury. Chest 111(4), 1061–1076 (1997).
- Cox JD, Azarnia N, Byhardt RW, Shin KH, Emami B, Pajak TF. A randomized Phase I/II trial of hyperfractionated radiation therapy with total doses of 60.0 Gy to 79.2 Gy: possible survival benefit with greater than or equal to 69.6 Gy in favorable patients with Radiation Therapy Oncology Group stage III non-small-cell lung carcinoma: report of Radiation Therapy Oncology Group 83-11. J. Clin. Oncol. 8(9), 1543–1555 (1990).
- Saunders M, Dische S, Barrett A, Harvey A, Gibson D, Parmar M. Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: a randomised multicentre trial. CHART Steering Committee. Lancet 350(9072), 161–165 (1997).
- Mehta MP, Tannehill SP, Adak S et al. Phase II trial of hyperfractionated accelerated radiation therapy for nonresectable non-small-cell lung cancer: results of Eastern Cooperative Oncology Group 4593. J. Clin. Oncol. 16(11), 3518–3523 (1998).
- Yuan S, Sun X, Li M et al. A randomized study of involved-field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage III nonsmall cell lung cancer. Am. J. Clin. Oncol. 30(3), 239–244 (2007).
- Socinski MA, Rosenman JG, Halle J et al. Dose-escalating conformal thoracic radiation therapy with induction and concurrent carboplatin/paclitaxel in unresectable stage IIIA/B nonsmall cell lung carcinoma: a modified Phase I/II trial. Cancer 92(5), 1213–1223 (2001).
- Socinski MA, Morris DE, Halle JS et al. Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small-cell lung cancer: a dose-escalation Phase I trial. J. Clin. Oncol. 22(21), 4341–4350 (2004).
- Socinski MA, Blackstock AW, Bogart JA et al. Randomized Phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J. Clin. Oncol. 26(15), 2457–2463 (2008).
- Rosenman JG, Halle JS, Socinski MA et al. High-dose conformal radiotherapy for treatment of stage IIIA/IIIB non-small-cell lung cancer: technical issues and results of a Phase I/II trial. Int. J. Radiat. Oncol. Biol. Phys. 54(2), 348–356 (2002).
- Blackstock AW, Ho C, Butler J et al. Phase Ia/Ib chemo-radiation trial of gemcitabine and dose-escalated thoracic radiation in patients with stage III A/B non-small cell lung cancer. J. Thorac. Oncol. 1(5), 434–440 (2006).
- Hayman JA, Martel MK, Ten Haken RK et al. Dose escalation in non-small-cell lung cancer using three-dimensional conformal radiation therapy: update of a Phase I trial. J. Clin. Oncol. 19(1), 127–136 (2001).
- Schild SE, McGinnis WL, Graham D et al. Results of a Phase I trial of concurrent chemotherapy and escalating doses of radiation for unresectable non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 65(4), 1106–1111 (2006).
- Machtay M, Bae K, Movsas B et al. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: an analysis of the Radiation Therapy Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 82(1), 425–434 (2012).
- Wang L, Correa CR, Zhao L et al. The effect of radiation dose and chemotherapy on overall survival in 237 patients with stage III non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 73(5), 1383–1390 (2009).
- Kong FM, Ten Haken RK, Schipper MJ et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int. J. Radiat. Oncol. Biol. Phys. 63(2), 324–333 (2005).
- Bradley RP, Komaki R, Masters G et al. A randomized Phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy +/− cetuximab for stage IIIa/IIIb non-small cell lung cancer: preliminary findings on radiation dose in RTOG 0617. Presented at: 53rd Annual Meeting of the American Society of Radiation Oncology. Miami, FL, USA, 2–6 October 2011.
- Cox JD. Are the results of RTOG 0617 mysterious? Int. J. Radiat. Oncol. Biol. Phys. 82(3), 1042–1044 (2012).
- Roelofs E, Engelsman M, Rasch C et al.; ROCOCO Consortium. Results of a multicentric in silico clinical trial (ROCOCO): comparing radiotherapy with photons and protons for non-small cell lung cancer. J. Thorac. Oncol. 7(1), 165–176 (2012).
- Nichols RC, Huh SN, Henderson RH et al. Proton radiation therapy offers reduced normal lung and bone marrow exposure for patients receiving dose-escalated radiation therapy for unresectable stage III non-small-cell lung cancer: a dosimetric study. Clin. Lung Cancer 12(4), 252–257 (2011).
- Salama JK, Stinchcombe TE, Gu L et al.; Cancer and Leukemia Group B. Pulmonary toxicity in stage III non-small cell lung cancer patients treated with high-dose (74 Gy) 3-dimensional conformal thoracic radiotherapy and concurrent chemotherapy following induction chemotherapy: a secondary analysis of Cancer and Leukemia Group B (CALGB) trial 30105. Int. J. Radiat. Oncol. Biol. Phys. 81(4), e269–e274 (2011).
- Koay EJ, Lege D, Mohan R, Komaki R, Cox JD, Chang JY. Adaptive/nonadaptive proton radiation planning and outcomes in a Phase II trial for locally advanced non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 84(5), 1093–1100 (2012).
- Oshiro Y, Mizumoto M, Okumura T et al. Results of proton beam therapy without concurrent chemotherapy for patients with unresectable stage III non-small cell lung cancer. J. Thorac. Oncol. 7(2), 370–375 (2012).
- Hoppe BS, Flampouri S, Henderson RH et al. Proton therapy with concurrent chemotherapy for non-small-cell lung cancer: technique and early results. Clin. Lung Cancer 13(5), 352–358 (2012).
- Suit H, Kooy H, Trofimov A et al. Should positive Phase III clinical trial data be required before proton beam therapy is more widely adopted? No. Radiother. Oncol. 86(2), 148–153 (2008).
- Bush DA. Proton radiation therapy for lung cancer: is there enough evidence? Oncology (Williston Park, NY) 24(11), 1052–1057 (2010).
- Onishi H, Shirato H, Nagata Y et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int. J. Radiat. Oncol. Biol. Phys. 81(5), 1352–1358 (2011).
- Louie AV, Rodrigues G, Hannouf M et al. Stereotactic body radiotherapy versus surgery for medically operable stage I non-small-cell lung cancer: a Markov model-based decision analysis. Int. J. Radiat. Oncol. Biol. Phys. 81(4), 964–973 (2011).
- Stuschke M, Kaiser A, Pöttgen C, Lübcke W, Farr J. Potentials of robust intensity modulated scanning proton plans for locally advanced lung cancer in comparison to intensity modulated photon plans. Radiother. Oncol. 104(1), 45–51 (2012).
- Meyer J, Bluett J, Amos R et al. Spot scanning proton beam therapy for prostate cancer: treatment planning technique and analysis of consequences of rotational and translational alignment errors. Int. J. Radiat. Oncol. Biol. Phys. 78(2), 428–434 (2010).
- Phillips MH, Pedroni E, Blattmann H, Boehringer T, Coray A, Scheib S. Effects of respiratory motion on dose uniformity with a charged particle scanning method. Phys. Med. Biol. 37(1), 223–234 (1992).
- Knopf AC, Hong TS, Lomax A. Scanned proton radiotherapy for mobile targets – the effectiveness of re-scanning in the context of different treatment planning approaches and for different motion characteristics. Phys. Med. Biol. 56(22), 7257–7271 (2011).
- Zenklusen SM, Pedroni E, Meer D, Bula C, Safai S. Preliminary investigations for the option to use fast uniform scanning with compensators on a gantry designed for IMPT. Med. Phys. 38(9), 5208–5216 (2011).
Website
- Particle therapy facilities in operation (incl.patient statistics) (2012). http://ptcog.web.psi.ch/ptcentres.html (Accessed 19 July 2012)