ABSTRACT
We analyzed diversity patterns of alpine tundra ecosystems along environmental gradients. We hypothesized that alpine diversity is affected by climate at local and regional scales, nutrient availability, soil moisture, and disturbance related to herbivory. In all, 232 samples in 11 study areas in Troms and Finnmark counties were analyzed with regard to α- and β-diversity of vascular plants and lichens. Relationships between α-diversity and environmental variables were analyzed by regression trees. β-diversity defined as species turnover was investigated using indirect ordination methods. Sites with non-acidic soil parent material showed highest species densities. Lowest species numbers were typical for extreme topographic positions. Heavily grazed samples showed less species numbers and coverage percentage of vegetation. The number of graminoid species was found to be highest in areas of high grazing pressure. We concluded that α-diversity was controlled by growing season, snow cover, pH, soil moisture, disturbance, temperature, and precipitation, stressing the importance of multi-factorial approaches in diversity studies. Determinants of β-diversity were predominantly local environmental conditions, whereas regional conditions were less important.
Introduction
Mountains are widely recognized as containing highly diverse and species rich ecosystems (e.g., CitationDiaz et al., 2003; CitationKörner, 2004). Topographic gradients condensed over short distances are capable of producing unique hotspots of biodiversity. Commonly, environmental heterogeneity is expected to be positively correlated with species numbers (CitationRosenzweig, 1995; CitationKörner, 2004). But, at the same time, high mountain ecosystems are in general quite sensitive and highly vulnerable to environmental changes, for instance slow land degradation due to human activities with all the attendant socioeconomic consequences (CitationMesserli and Ives, 1997). Furthermore, many studies suggest that high elevation environments are among the most sensitive to climatic changes occurring on a global scale. Relatively small perturbations in global processes can cascade down to produce large changes in most of the interdependent patterns and processes, from the hydrological cycle to the complex fauna and flora, and the people that depend on those resources (e.g., CitationThompson, 2000; CitationBeniston, 2003). The human welfare and especially the water supply depend directly or indirectly on the functional integrity of mountain ecosystems with vegetation as their key component (CitationKörner, 2004). As such, plant diversity provides an insurance against system failure by functional redundancy in traits (CitationKörner, 2004). The understanding and explanation of mechanisms that control diversity is critical for predicting changes in diversity patterns resulting from changes in land use or a changing climate (CitationWalker, 1995).
Patterns of spatial and temporal variations in species richness and diversity as well as their relationship to underlying environmental variables within arctic and alpine regions have recently attracted considerable interest by ecologists and biogeographers (CitationHeikkinen and Neuvonen, 1997; CitationGough et al., 2000; CitationMoser et al., 2005). The compressed climatic gradient, sharp ecotones, and altitudinal gradients are useful for investigating patterns in species richness, as a changing climate may lead to the migration of species (CitationLomolino, 2001; CitationGrytnes, 2003; CitationFosaa, 2004; CitationSánchez-Gonzáles and López-Mata, 2005).
Focusing on determinants of species diversity, an appropriate consideration of scaling issues is inevitable (CitationRosenzweig, 1995). CitationKörner (1995) defined in his assessment of causes of alpine plant diversity a set of “sieves” acting at different spatial scales. In a global context, harsh environmental conditions present in arctic-alpine landscapes require plants to possess evolutionary adaptations to low temperatures and short growing seasons, resulting in a small overall species pool (CitationKörner, 2003). At finer scales, environmental heterogeneity gains importance. At regional scale, soil parent material (CitationMolau, 2003) and grazing pressure are likely to affect diversity patterns. For instance, moderate herbivory in productive areas is assumed to increase biodiversity, whereas diversity is reduced due to herbivory in less productive habitats (CitationAustrheim and Eriksson, 2001). At local scale, differentiations in topography and associated changes in snow cover and soil moisture are important determinants of diversity (e.g., CitationGould and Walker, 1999; CitationKörner, 2004). Thus, at local scale, both sets of determinants, regional and local as well as their interaction, may control diversity and need to be taken into account to understand diversity patterns (CitationGough et al., 2000; CitationHolten, 2003).
CitationPausas and Austin (2001) point out that species richness patterns in relation to the environment need to be understood before drawing conclusions on the effect of biodiversity in ecosystem processes. They criticize that despite species richness being governed by two or more environmental gradients, diversity studies in relation to environmental gradients have mainly been single-factor studies. CitationPausas and Austin (2001) concluded with a suggestion of potential approaches when studying species richness and its relation to the environment: (a) Appropriate variables related to resources availability and to direct environmental variables have to be chosen. Numerous different causal variables may be correlated with the same indirect gradient, precluding any interpretation. (b) Multidimensional gradients using non-linear regression techniques have to be studied, as multidimensional, non-linear patterns are common. (c) Species richness has to be separated into the richness of different vegetation types.
Within this case study, we responded to these suggestions. Diversity patterns in alpine tundra ecosystems of northern Norway were described and related to the environment. We hypothesized diversity to be affected by:
climatic variables such as near-surface temperature conditions and snow cover at local scale and the oceanic-continental gradient at regional scale (CitationLeathwick et al., 1998; CitationVirtanen et al., 2003; CitationHolten, 2003; CitationKörner, 2004),
nutrient availability (CitationGould and Walker, 1999; CitationMolau, 2003; CitationVirtanen et al., 2003; CitationKörner, 2004),
soil moisture (CitationLeathwick et al., 1998; CitationKörner, 2004), and
disturbance related to herbivory (CitationAustrheim and Eriksson, 2001; Citationden Herder et al., 2003; CitationNagy et al., 2003; CitationOlofsson et al., 2001, Citation2004; Citationvan der Wal et al., 2004).
Both α-diversity and β-diversity were studied based on vascular plants and lichens.
Materials and Methods
Study Areas
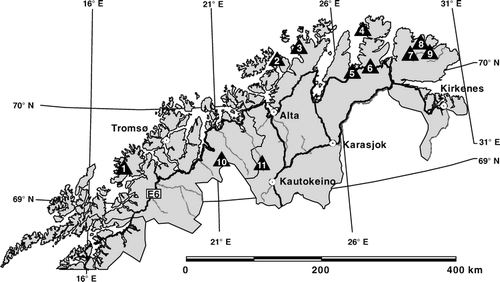
The study was carried out in northern Norway, within Troms and Finnmark counties. The choice of the study areas was based on two underlying broad-scaled gradients. Firstly, the pronounced climatic differentiation between the oceanic coastal mountains and the more continental interior areas, and secondly, different densities of reindeer. The resulting 11 study areas () are described with regard to geographic position, altitude, climate (CitationMoen, 1999), geology (CitationSigmond et al., 1984), and reindeer densities using mean and maximum values per square kilometer of the last 20 years according to CitationReindriftsforvaltningen (2005) in .
TABLE 1 Characteristics of investigated areas in northern Norway.
Substrates were composed of glacial till and consisted of sands and silty sands, varying by high contents of the skeleton fractions. Organic substrates such as alpine mires and bogs covered huge areas in the depressions and on wet slopes. Depressions at higher elevations had very thin organic layers. Permafrost was not found down to 100 cm depths.
Field Sampling
Surveys of vegetation were conducted along transects within each of the study areas. Transects were chosen with regard to fine-scale topographical gradients, i.e. representing the main topographic positions ridge, upper slope, mid-slope, lower slope, foot-slope, and depression. Corresponding vegetation types discriminated by snow cover and soil moisture were identified based on species composition (CitationDahl, 1987; ) and represented by several samples using 1 m2 plots. All were sampled with the same method and identical plot size, ensuring that the patterns both within and between transects were not a result of different sampling strategies. The small size of sample plots was used because it was possible to have a large number of samples within both, each vegetation type and each transect, and to sample several transects. Thus, the small size of sample plots was a compromise. The specific location of samples within each vegetation type was drawn at random, with the constraints that the next sample should be at least 2 m apart. Relevés were conducted according to CitationBraun-Blanquet (1964) recording coverage percentage for (a) any single plant species, (b) aggregated species groups (lichens, mosses, herbs, graminoids, woody plants), and (c) total vegetation. The nomenclature followed CitationLid and Lid (1994) for species of vascular plants, CitationKrog et al. (1994) for the lichen flora, and CitationFrisvoll et al. (1995) for bryophytes. Unfortunately, several bryophytes could be determined down to genus-level only, since for some genera (e.g. Sphagnum) possibilities of visual identification in the field did not allow any differentiation on the species level. Hence we decided to exclude all bryophytes from analyses of species richness. One hundred sixty-three species of vascular plants and lichens were recorded and used for further analyses.
TABLE 2 Vegetation types with number of samples (subscript a refers to acidic samples, and subscript b to non-acidic samples).
In all, 232 samples were studied. Out of these samples, 16 were situated along a reindeer-fence at Ifjordfjellet (study area 6) separating apparently heavily grazed areas from less grazed areas, but official reindeer densities were not available. On each side of the fence, but at least 25 m apart from it to prevent direct fence-effects, 8 samples were chosen from similar topographic positions and corresponding vegetation types, resulting in couples of each, two ridges, two upper slopes, three mid-slopes and one lower slope. Out of the whole data set, two subsets containing acidic samples (n = 202) and non-acidic samples (n = 30) were derived according to soil parent material () that served as the basis for further analyses.
Furthermore, 32 environmental variables for each sample were measured or indirectly estimated (). Relief was described regarding topographic position, curvature, slope, and aspect. Characterization of soil moisture conditions was based on uncalibrated hand-held TDR-measurements. Soil profiles were analyzed as to their horizon combination. Furthermore, root density and pH-value (H2O) at 5, 15, and 30 cm depth were recorded. Techniques and tools of environmental variable analysis were applied and structured after CitationAG Boden (1996), modified for arctic-alpine landscapes according to CitationSCWG (1998). Climatic variables such as annual minimum, annual mean, and annual maximum air temperature (2 m above the ground surface), annual temperature amplitude, annual heat sum (>5 °C), growing season (number of days with mean temperature >5 °C), and annual precipitation sums were transposed from neighboring (50 km radius) official meteorological stations (CitationDNMI, 2006) for each study area, but not further differentiated for single sites. Correction for altitudinal differences between meteorological stations and study areas were applied to both temperature using a lapse rate of 0.6 K (100 m−1) and precipitation using an increase of 10% (100 m−1) (CitationFørland et al., 1996). A rough estimate of duration of snow cover for each sample was derived from values given by CitationMoen (1999), corrected for topographic position based on field experiences of the authors and UEB model runs (CitationTarboton and Luce, 1996) for central Norway, i.e. snow cover on foot-slopes was assumed to last 30 days longer than at mid-slopes and 60 days longer than at ridges, respectively. Furthermore, potential radiation during the summer period was calculated for each sampling site (CitationStumböck, 1995).
TABLE 3 Environmental variables recorded and used for statistical analyses.
Data Analysis
Data analysis was based upon α-diversity and β-diversity and its environmental determination. α-diversity was expressed by (a) the number of species (bryophytes excluded) within each 1 m2 sample (species density in sensu CitationWhittaker, 1975), and (b) the Shannon-Wiener-Index “H” for each sample (CitationMagurran, 1988). Since the number of individual plants generally used for calculation was not counted in this study, values of coverage percentage were used instead. H was calculated using MVSP 3.1 (CitationKovach Computing Services, 2001). Relationships between α-diversity and environmental variables were analyzed by regression tree analysis (RTA) as implemented within SYSTAT 11 (CitationSystat Software Inc., 2004). Regression tree analysis does not involve a priori assumptions about any particular type of relationship between the variables, and hence is useful for capturing nonlinear relationships and facilitating interpretation of the results (CitationDe’ath and Fabricius, 2000). Analyses were based on the entire data set of 232 samples which were in case of species density and the Shannon-Wiener-Index aggregated to 6 vegetation types.
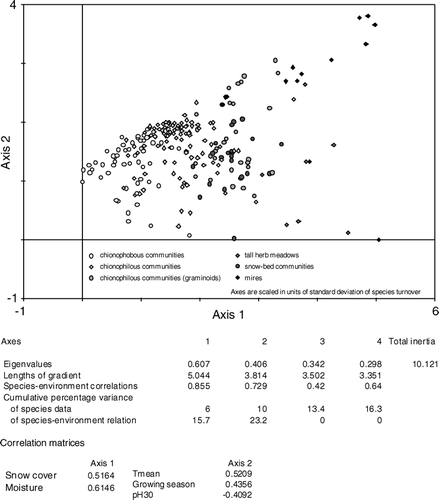
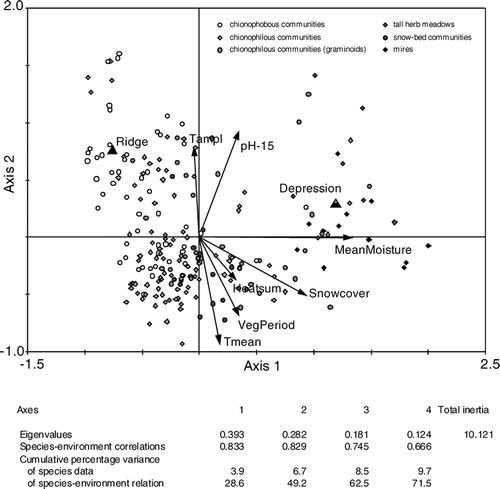
β-diversity as the degree of differentiation among communities within a landscape or the species turnover between samples (CitationWalker, 1995; CitationWhittaker et al., 2001) was investigated using indirect ordination methods. Detrended correspondence analysis (DCA; CitationHill and Gauch, 1980) was applied to the species data set using CANOCO 4.5 (Citationter Braak and Smilauer, 2004) to visualize differences in species composition and species turnover between samples. The ordination arranges the samples as points in a scatter diagram that are ecologically structured. Clustered points correspond to plots that are compositionally similar, whereas those that are further apart are more dissimilar. DCA was used with detrending by segments and non-linear rescaling (CitationKnox, 1989; CitationØkland, 1990). Due to this rescaling, the axes are scaled in units of standard deviation of species turnover. A secondary result is the total β-diversity, expressed by the length of gradients. The axes were also ecologically interpreted by correlation analyses to environmental variables. Furthermore, a direct ordination method, canonical correspondence analysis (CCA; Citationter Braak, 1986), was applied to the species and environmental data set to extract environmental variables that determine species composition and hence also differentiations among samples. Significant environmental variables were chosen by automatic forward selection routines.
Results
Patterns of α-Diversity
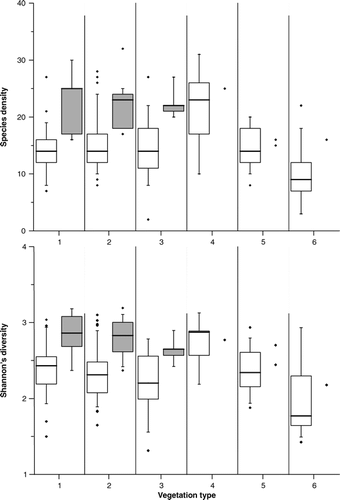
Study of patterns of the α-diversity expressed as species density and the Shannon-Wiener-Index was based on all 232 sites. They were differentiated for acidic and non-acidic soil parent material and for visualization purposes aggregated into 6 vegetation types (). Results are presented as boxplots with median, first and third quartile, 5–95% range, as well as outliers for all vegetation types (). Note that due to the small sample size for some vegetation types on non-acidic substrates, only the results of types 1, 2, and 3 could be handled as a sound statistical evaluation. Samples with non-acidic soil parent material showed generally higher species densities and higher values of the Shannon-Wiener-Index (significant at 0.001 levels) than their acidic counterparts. Mires showed lowest species densities with a median of 9 species, whereas tall herb meadows were found to be the communities with highest species densities (median of 23 species). The vegetation types 1, 2, 3, and 5, which represent a gradient in snow-shelter, did not show significant differences in species density and the Shannon-Wiener-Index.
Environmental Determination of α-Diversity
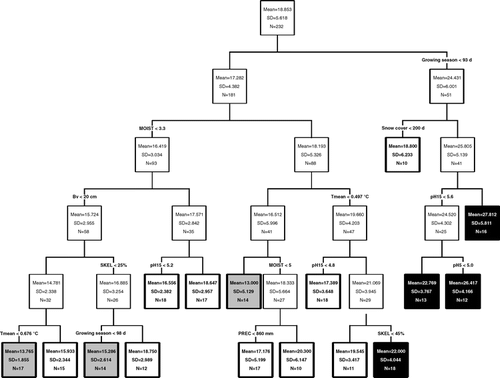
Regression tree analysis including the entire data set of samples and environmental variables revealed a regression tree containing 16 terminal nodes with an overall proportional reduction in error of 0.619 (). These nodes are discriminated by species density and are hence not comparable to vegetation types. Listed in order of importance, species density was controlled by a combination of growing season, snow cover, pH-value, soil moisture, disturbance as indicated by thickness of Bv-horizon and bare soil cover, temperature, and precipitation. Samples characterized by a short growing season (<93 d) with long lasting snow cover and high pH values showed highest species densities (right branch of the tree in ), whereas very wet samples with low mean temperatures showed lowest densities. Moist samples with a long growing season (>93 d) were differentiated by disturbance. Unstable conditions corresponded to low species densities (left branch of the tree in ).
Effect of Herbivory on α-Diversity
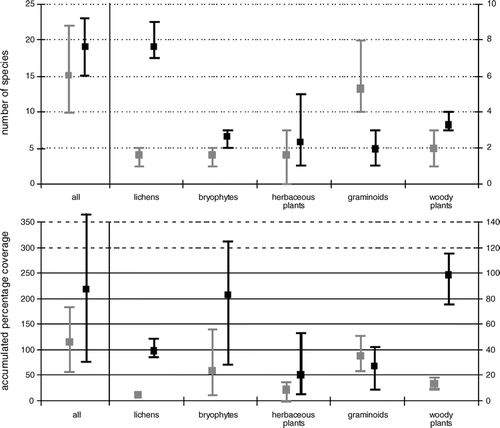
Regression tree analysis revealed no clear effects of herbivory on α-diversity. Nevertheless, sample differentiation by disturbance regimes gave first clues on a possible influence. The effect became more evident when studied on a small subset of samples on both sides of a reindeer-fence (heavily grazed/less grazed), as shown in . Note that even if the results are presented here and evaluated in the Discussion section, the number of samples was too small to consider this as a rigorous statistical evaluation of this topic. Heavily grazed samples showed an overall decrease in species numbers (mean values) as well as coverage percentage of vegetation (mean values) compared to less grazed samples (significance level p < 0.05). A more differentiated view revealed a strong decline in lichen species (p < 0.001) and lichen coverage (p < 0.05), and less pronounced in bryophyte genera and cover (p < 0.05). Moreover, coverage of woody plants decreased significantly (p < 0.05). In contrast, graminoids were supported by heavy grazing as the species number increased significantly (p < 0.05) combined with a slight increase in coverage.
β-Diversity
Species turnover between samples was investigated using DCA. The resulting scatter plot of samples is presented in . Long gradients of the axes (axis 1 = 5.044, axis 2 = 3.814) indicated a large species turnover among samples and hence a large β-diversity. The first and most important axis (eigenvalue 0.607) was found to be correlated with snow cover and soil moisture as already indicated by the arrangement of vegetation type clusters along this axis. The second axis (eigenvalue 0.406) correlated with mean temperature, length of growing season, and pH-value at 30 cm depth. Hence, local conditions represented by the first axis were found to be of superior importance controlling β-diversity, whereas regional conditions as represented by the second axis were less important. A corresponding CCA biplot of samples and environmental variables supported these findings (). Samples were arranged according to local moisture and snow cover gradients (longest arrows) as well as regional mean temperatures, temperature amplitude, and growing season gradients. The gradient of pH at 15 cm depth combined both local and regional influences. In contrast to the specific case along the reindeer-fence, for the entire set of samples, herbivory did not contribute significantly to the differentiation among samples.
Discussion
The findings of our study stress the importance of multi-factorial determinants of species diversity (CitationHeikkinen and Neuvonen, 1997; CitationGough et al., 2000; CitationPausas and Austin, 2001). We found that α-diversity patterns in alpine tundra landscapes at different spatial scales are determined by specific environmental variable constellations (CitationWalker, 2000; CitationKörner, 2003; CitationLöffler, 2007). In accordance with earlier studies, non-acidic soils corresponded with generally higher diversity at a regional scale (CitationSætersdal and Birks, 1997; CitationMolau, 2003; CitationMoser et al., 2005). In contrast to these and other findings from the Alaskan tundra (CitationGough et al., 2000), we could not explain diversity using corresponding pH-values only. Instead, the regional effect of soil acidity was found to be strongly modified by local effects of soil moisture and snow cover. Also CitationHolten (2003) found in the southern Scandes regional conditions often overruled by microhabitat conditions. Samples at gentle slope positions with intermediate soil moisture, sheltered by snow cover against low winter temperatures, showed higher diversity than samples at extreme topographic positions, i.e. ridges and depressions. These findings correspond to studies of CitationVirtanen et al (2003) who reported snow-protected communities to be highly diverse, followed by exposed and snow bed communities. CitationHeikkinen and Neuvonen (1997) found also notable high numbers of species in snow beds in subarctic Finland. Thus, we support suggestions that both regional and local effects on diversity have to be considered (CitationGough et al., 2000).
In general, the effect of herbivory on the maintenance of plant diversity in alpine environments is inadequately understood (CitationErschbamer et al., 2003). CitationProulx and Mazumder (1998) hypothesized that grazing decreases species richness in nutrient-poor environments while in nutrient rich environments plant species richness is increased. According to CitationErschbamer et al. (2003) alpine environments offer an excellent model system to further test general productivity-diversity hypotheses. Our study revealed clues on an overall reduction of diversity and percentage cover of vegetation due to heavy grazing at nutrient-poor sites. We found only the number of graminoid species that could be supported by grazing pressure, whereas lichens suffered most. Former studies proved that combined trampling and grazing by reindeer led to an almost complete removal of the lichen carpet (CitationLöffler, 2000, Citation2004; CitationBoudreau and Payette, 2004). Furthermore, negative effects of grazing on lichens, bryophytes, herbaceous plants and woody plants were found, whereas graminoids benefited from herbivory (CitationCrête et al., 2001). According to Citationvan der Wal et al. (2004) grazing and trampling were instrumental in reducing the depth of the moss layer, which raised soil temperature from which graminoids benefited in particular. Thus, an increased herbivory impact allowed the development of grass-rich tundra at the expense of lichens, bryophytes and woody plants (e.g. CitationOlofsson et al., 2001). Interestingly, similar results were achieved in our approach using a relatively small number of samples.
In contrast to our hypotheses, that the oceanic-continental gradient determines the β-diversity of plant species, we found a large species turnover primarily due to local topographic heterogeneity, whereas regional conditions were of minor importance. This high level of β-diversity in alpine tundra landscapes is thus due to topographic heterogeneity and associated differentiations in microclimatic conditions, soil moisture, soil types, and snow cover (CitationRichter, 2001).
Concluding, we related diversity patterns of vascular plants, bryophytes, and lichens to their environmental conditions as an example of an alpine tundra ecosystem. Facing the revealed importance of local conditions, the explained variance is expected to suffer from the lack of sample-specific microclimatic data. Moreover, biotic interspecific competition was assumed to gain importance when regarding one-square-meter plots (CitationGough et al., 2000), but remained unaccounted for. Methodically, this study emphasized the necessity to account for several determinants of diversity and their analyses in a non-linear way, as multidimensional, non-linear patterns are too common in nature.
Acknowledgments
The German Research Association (DFG) and Color Line AS, Oslo, kindly supported the research in northern Norway. A research grant to J. Löffler was provided by the Stifterverband für die Deutsche Wissenschaft. The authors thank Dr. A. Blume for field assistance, Dr. O.-D. Finch, Dr. D. Wundram, and O. Rößler for critical discussions on the manuscript. Two anonymous reviewers kindly improved the paper.
References Cited
- AG Boden, 1996. Bodenkundliche Kartieranleitung Stuttgart Schweizerbart.
- Austrheim, G. and O. Eriksson . 2001. Plant species diversity and grazing in the Scandinavian mountains—Patterns and processes at different spatial scales. Ecography 24:683–695.
- Beniston, M. 2003. Climatic change in mountain regions. A review of possible impacts. Climatic Change 59:5–31.
- Boudreau, S. and S. Payette . 2004. Caribou-induced changes in species dominance of lichen woodlands: an analysis of plant remains. American Journal of Botany 91:3 422–429.
- Braun-Blanquet, J. 1964. Pflanzensoziologie: Grundzüge der Vegetationskunde Wien Springer.
- Crête, M. , J-P. Ouellet , and L. Lesage . 2001. Comparative effects on plants of caribou/reindeer, moose and white-tailed deer herbivory. Arctic 54:4 407–417.
- Dahl, E. 1956. Rondane. Mountain vegetation in south Norway and its relation to the environment. Skrifter utgitt av Det Norske videnskaps-akademi i Oslo. I. Matematisk-naturvidenskapelig klasse 3, Oslo.
- Dahl, E. 1987. Alpine-subalpine plant communities of south Scandinavia. Phytocoenologia 15:4 455–484.
- De’ath, G. and K. E. Fabricius . 2000. Classification and regression trees: a powerful yet simple technique for the analysis of complex ecological data. Ecology 81:3178–3192.
- den Herder, M. , M-M. Kytöviita , and P. Niemelä . 2003. Growth of reindeer lichens and effects of reindeer grazing on ground cover vegetation in a Scots pine forest and a subarctic heathland in Finnish Lapland. Ecography 26:3–12.
- Diaz, H. F. , M. Grosjean , and L. Graumlich . 2003. Climate variability and change in high elevation regions: past, present and future. Climatic Change 59:1–4.
- DNMI 2006. Climatic data for Norway (http://www.eklima.no). Accessed 15 September 2006.
- Erschbamer, B. , R. Virtanen , and L. Nagy . 2003. The impact of vertebrate grazers on vegetation in European high mountains. In Nagy, L. , G. Grabherr , C. Körner , and D. B. A. Thompson , editors. Alpine biodiversity in Europe. Ecological Studies 167 Berlin Springer. 379–396.
- Førland, E. J. , P. Allerup , B. Dahlström , E. Elomaa , T. Jónsson , H. Madsen , J. Perälä , P. Rissanen , H. Vedin , and F. Vejen . 1996. Manual for operational correction of Nordic precipitation data. DNMI-Report 24/96.
- Fosaa, A. M. 2004. Biodiversity patterns of vascular plant species in mountain vegetation in the Faeroe Islands. Diversity and Distributions 10:217–223.
- Frisvoll, A. A. , A. Elvebakk , K. I. Flatberg , and R. H. Økland . 1995. Sjekkliste over norke mosar. vitskapleg og norsk namneverk. Norsk institute for naturforskining (NINA) Temahefte 4:1–104.
- Gough, L. , G. R. Shaver , J. Carroll , D. L. Royer , and J. A. Laundre . 2000. Vascular plant species richness in Alaskan arctic tundra: the importance of soil pH. Journal of Ecology 88:54–66.
- Gould, W. A. and M. D. Walker . 1999. Plant communities and landscape diversity along a Canadian Arctic river. Journal of Vegetation Science 10:537–548.
- Grytnes, J. A. 2003. Species-richness patterns of vascular plants along seven altitudinal transects in Norway. Ecography 26:291–300.
- Heikkinen, R. K. and S. Neuvonen . 1997. Species richness of vascular plants in the subarctic landscape of northern Finland: modelling relationships to the environment. Biodiversity and Conservation 6:1181–1201.
- Hill, M. O. and H. G. Gauch . 1980. Detrended correspondence analysis: an improved ordination technique. Vegetatio 42:47–58.
- Holten, J. I. 2003. Altitude ranges and spatial patterns of alpine plants in northern Europe. In Nagy, L. , G. Grabherr , C. Körner , and D. B. A. Thompson , editors. Alpine biodiversity in Europe. Ecological Studies 167 Berlin Springer. 173–184.
- Knox, R. 1989. Effects of detrending and rescaling on correspondence analysis: solution stability and accuracy. Vegetatio 83:129–136.
- Körner, C. 1995. Alpine plant diversity: a global survey and functional interpretations. In Chapin, F. S. and C. Körner , editors. Arctic and alpine biodiversity. Ecological Studies 113 Berlin Springer. 45–62.
- Körner, C. 2003. Alpine plant life: functional plant ecology of high mountain ecosystems Berlin Springer.
- Körner, C. 2004. Mountain biodiversity, its causes and function. Ambio Special Report 13. 11–17.
- Kovach Computing Services 2001. Multivariate statistical package MVSP 3.1 (http://www.kovcomp.com/). Accessed 28 April 2006.
- Krog, H. , H. Østhagen , and T. Tønsberg . 1994. Lavflora. Norske busk- og bladlav Oslo Universitetsforlaget.
- Leathwick, J. R. , B. R. Burns , and B. D. Clarkson . 1998. Environmental correlates of tree alpha-diversity in New Zealand primary forests. Ecography 21:235–246.
- Lid, J. and D. T. Lid . 1994. Norsk flora Oslo Det Norske Samlaget.
- Löffler, J. 2000. High mountain ecosystems and landscape degradation in northern Norway. Mountain Research and Development 20:356–363.
- Löffler, J. 2004. Degradation of high mountain ecosystems in northern Europe. Journal of Mountain Science 2:97–115.
- Löffler, J. 2007. Reindeer grazing changes diversity patterns in arctic-alpine landscapes of northern Norway. Die Erde 138:215–233.
- Lomolino, M. V. 2001. Elevation gradients of species-diversity: historical and prospective views. Global Ecology and Biogeography 10:9–13.
- Magurran, A. E. 1988. Ecological diversity and its measurement London Croom Helm.
- Messerli, B. and J. D. Ives . 1997. Mountains of the world: A global priority New York Parthenon.
- Moen, A. 1999. National atlas of Norway: vegetation Hønefoss Norwegian Mapping Authority.
- Molau, U. 2003. Overview: patterns in diversity. In Nagy, L. , G. Grabherr , C. Körner , and D. B. A. Thompson , editors. Alpine biodiversity in Europe. Ecological Studies 167 Berlin Springer. 125–132.
- Moser, D. , S. Dullinger , T. Englisch , H. Niklfeld , C. Plutzar , N. Sauberer , H. G. Zechmeister , and G. Grabherr . 2005. Environmental determinants of vascular plant species richness in the Austrian Alps. Journal of Biogeography 32:1117–1127.
- Nagy, L. , G. Grabherr , C. Körner , and D. B. A. Thompson . 2003. Alpine biodiversity in space and time: a synthesis. In Nagy, L. , G. Grabherr , C. Körner , and D. B. A. Thompson , editors. Alpine biodiversity in Europe. Ecological Studies 167 Berlin Springer. 453–464.
- Nordhagen, R. 1936. Versuch einer Einteilung der subalpinen-alpinen Vegetation Norwegens. Bergen Bergens Museums Ărbok. Naturvidensk, rekke 1936(7):. 1–88.
- Nordhagen, R. 1943. Sikilsdalen og Norges Fjellbeiter, en plantessosiologisk monografi. Bergen Bergens Museums Skrifter. 22 pp.
- Olofsson, J. , H. Kitti , P. Rautiainen , S. Stark , and L. Oksanen . 2001. Effects of summer grazing by reindeer on composition of vegetation, productivity and nitrogen cycling. Ecography 24:13–24.
- Olofsson, J. , S. Stark , and L. Oksanen . 2004. Reindeer influence on ecosystem processes in the tundra. Oikos 105:386–396.
- Økland, R. H. 1990. Vegetation ecology: theory, methods and applications with reference to Fennoscandia. Sommerfeltia Supplement 1:1–233.
- Pausas, J. G. and M. P. Austin . 2001. Patterns of plant species richness in relation to different environments: an appraisal. Journal of Vegetation Science 12:153–166.
- Proulx, M. and A. Mazumder . 1998. Reversal of grazing impact on plant species richness in nutrient-poor vs. nutrient-rich ecosystems. Ecology 79:2581–2592.
- Reindriftsforvaltningen 2005. Ressursregnskap for reindriftsnæringen. For reindriftsåret 1. april 2003–31. mars 2004 Alta Reindriftsforvaltningen.
- Richter, M. 2001. Zonal features of phytodiversity under natural conditions and under human impact—A comparative survey. In Barthlott, W. and M. Winiger , editors. Biodiversity. A challenge for development research and policy Berlin Springer. 83–109.
- Rosenzweig, M. L. 1995. Species diversity in space and time Cambridge Cambridge University Press.
- Sætersdal, M. and H. J. B. Birks . 1997. A comparative ecological study of Norwegian mountain plants in relation to possible future climatic change. Journal of Biogeography 24:127–152.
- Sánchez-Gonzáles, A. and L. López-Mata . 2005. Plant species richness and diversity along an altitudinal gradient in the Sierra Nevada, Mexico. Diversity and Distributions 11:567–575.
- SCWG [Soil Classification Working Group] 1998. The Canadian system of soil classification Ottawa Research Branch Agriculture and Agri-Food, Publication 1646.
- Sigmond, E. , M. Gustavson , and D. Roberts . 1984. Berggrunnskart over Norge—M. 1∶1.000.000 Trondheim Norges geologiske undersøkelse.
- Stumböck, M. 1995. Ein computergestütztes Modell zur Berechnung der potentiellen Glo-balstrahlung im Mikrorelief und seine Anwendung in Südgrönland. Bremen Norden. 10:37–46.
- Systat Software Inc 2004. SYSTAT 11 (http://www.systat.com). Accessed 13 October 2006.
- Tarboton, D. G. and C. H. Luce . 1996. Utah energy balance snow accumulation and melt model (UEB). Computer model technical description and users guide. Utah Water Research Laboratory and USDA Forest Service Intermountain Research Station (http://www.engineering.usu.edu/cee/faculty/dtarb/snow/snowrep.pdf). Accessed 15 September 2006.
- ter Braak, C. J. F. 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167–1179.
- ter Braak, C. J. F. and P. Smilauer . 2004. Canoco for Windows 4.5 (http://www.canoco.com). Accessed 13 October 2006.
- Thompson, L. G. 2000. Ice core evidence for climate changes in the tropics: implications for our future. Quaternary Science Reviews 19:19–35.
- van der Wal, R. , R. D. Bardgett , K. A. Harrison , and A. Stien . 2004. Vertebrate herbivores and ecosystem control: cascading effects of faeces on tundra ecosystems. Ecography 27:242–252.
- Virtanen, R. , T. Dirnböck , S. Dullinger , G. Grabherr , H. Pauli , M. Staudinger , and L. Villar . 2003. Patterns in plant species richness of European high mountain vegetation. In Nagy, L. , G. Grabherr , C. Körner , and D. B. A. Thompson , editors. Alpine biodiversity in Europe. Ecological Studies 167. Berlin Springer. 149–172.
- Walker, D. A. 2000. Hierarchical subdivision of Arctic tundra based on vegetation response to climate, parent material and topography. Global Change Biology 6:Suppl. 1 19–34.
- Walker, M. D. 1995. Patterns and causes of Arctic plant community diversity. In Chapin, F. S. and C. Körner , editors. Arctic and alpine biodiversity. Ecological Studies 113 Berlin Springer. 3–20.
- Whittaker, R. H. 1975. Communities and ecosystems Houndmills Macmillan.
- Whittaker, R. J. , K. J. Willis , and R. Field . 2001. Scale and species richness: towards a general, hierarchical theory of species diversity. Journal of Biogeography 28:453–470.