ABSTRACT
In many of the alpine-treeline ecotones (ATE) of the Rocky Mountains, Pinus albicaulis (whitebark pine) is the most common conifer initiating tree islands through facilitation. We examined whether microsites leeward of P. albicaulis experience more moderate microclimate, less sky exposure, and more total soil carbon and nitrogen than other common types of leeward microsites. From July to September 2010, 2011, and 2012, in two study areas on the eastern Rocky Mountain Front, we compared microclimate, sky exposure, and total soil carbon and nitrogen leeward of four common microsites, P. albicaulis, Picea engelmannii (Engelmann spruce), rock, and unprotected (exposed). Microsites leeward of P. albicaulis did not consistently experience the most moderate microclimate, but both P. albicaulis and P. engelmannii leeward microsites had lower daily photosynthetically active radiation (PAR), lower average wind speeds, lower soil temperature maxima, and higher soil temperature minima. In general, conifer microsites had significantly lower values for sky exposure; but, the performance of each microsite type varied from micro to study-area scales. Our results highlight the importance of conifers as nurse objects for facilitating treeline community development in the ATE, and especially P. albicaulis because of its high abundance. High losses of P. albicaulis from infection by Cronartium ribicola may alter community dynamics and treeline response to climate warming.
Introduction
Recent studies have substantiated the importance of ecological facilitation in community processes (e.g., CitationCallaway et al., 2002; CitationBrooker et al., 2008). Facilitative interactions shape ecosystem structure at the population and community level in environments under high abiotic stress (CitationStachowicz, 2001; CitationBruno et al., 2003). “Nurse objects,” such as rocks or tree stumps, may also facilitate seed germination and plant establishment (e.g., CitationAngel Munguía-Ross and Sosa, 2008; CitationCastro et al., 2011).
Facilitation often creates a microsite with conditions such as microclimate better suited to seed germination or seedling survival (CitationSpittlehouse and Stathers, 1990; CitationMaher et al., 2005; CitationResler, 2006). Microclimates are defined as “small-scale climates” influenced by light intensity, precipitation, humidity, wind, air temperature, soil temperature, and soil moisture, usually reflecting regional macroclimate (CitationSpittlehouse and Stathers, 1990). Nurse objects and plants function as facilitators most often by moderating microclimate.
Interactions among species that are competitive in favorable environments often shift to facilitative under adverse conditions, according to the stress gradient hypothesis (CitationCallaway, 1998; CitationCallaway et al., 2002; CitationSthultz et al., 2007). Plants in facilitative roles mitigate harsh environments by moderating soil temperatures (CitationBreshears et al., 1998; CitationChambers, 2001), reducing solar radiation (CitationGermino and Smith, 1999; CitationGermino et al., 2002), increasing soil nutrients (CitationCallaway et al., 1991), and reducing wind speeds (CitationBaumeister and Callaway, 2006). For example, in the southwestern United States, a common shrub Fallugia paradoxa facilitates the establishment and survival of Pinus edulis at sites experiencing higher drought stress but not in sites of lower drought stress (CitationSthultz et al., 2007). At upper subalpine elevations in the Rocky Mountains of the United States, Abies lasiocarpia (subalpine fir) aggregate around mature Pinus albicaulis (whitebark pine), which provide protection in areas of high wind exposure (CitationCallaway, 1998). In general, the plant species that are important facilitators are prevalent, tolerate prevailing conditions, and are able to provide shelter (e.g., CitationCallaway et al., 2002; CitationBaumeister and Callaway, 2006; CitationSthultz et al., 2007). Facilitative interactions, however, vary in outcome and effectiveness among and within nurse plant species, depending on recipient plant life history stage, extent of disturbance, and harshness of the abiotic environment (CitationLortie and Turkington, 2008; CitationSoliveres et al., 2011; CitationMichalet et al., 2015).
Facilitative interactions are particularly important in moderating harsh microclimate in treeline communities, where seedling establishment and growth may be limited by low soil temperatures, short growing seasons, winter injury, intense solar radiation, nighttime sky exposure, and leaf loss from extreme winds (CitationStevens and Fox, 1991; CitationSveinbjörnsson et al., 1996; CitationCairns and Malanson, 1997; CitationKörner, 1998; CitationGermino and Smith, 1999; CitationHoch et al., 2002; CitationMaher et al., 2005). Low soil temperatures lead to photo-inhibition (CitationGermino and Smith, 1999) and slow root growth (CitationLandhäusser et al., 1996). Although photosynthesis rates generally increase as photosynthetically active radiation (PAR) increases, high ambient temperatures and full sunlight decrease survival (CitationGermino et al., 2002; CitationMoyes et al., 2013). Excess sky exposure also lowers soil moisture and results in colder nighttime temperatures from long-wave irradiance (CitationMaher et al., 2005; CitationGermino and Smith, 1999). High wind speeds and radiation reduce soil moisture as well (CitationHoltmeier and Broll, 1992; CitationHoltmeier, 2009). In the Rocky Mountains, soil moisture and the distribution of soil nutrients at treeline may differ among conifer species and with the proximity of trees or tree islands, resulting in differential soil fertility (CitationVan Miegroet et al., 2000; CitationSeastedt and Adams, 2001; CitationShiels and Sanford, 2001).
The alpine-treeline ecotone (ATE) in the Rocky Mountains represents the transition zone for conifers from upper subalpine forest communities to their upper elevational limits, where krummholz forms predominate (CitationHoltmeier, 2009). Community structure and composition of the ATE are shaped by local processes, including seed dispersal, seed germination, and seedling establishment, which in turn are influenced by microclimate, snow accumulation, and timing of snowmelt (CitationMoir et al., 1999; CitationAlftine and Malanson, 2004; CitationMaher and Germino, 2006; CitationMalanson et al., 2007; CitationBatllori et al., 2009). These processes encompass tree island initiation. This occurs when a solitary conifer facilitates the leeward establishment of another conifer (CitationBekker, 2005). Further conifer establishment builds a patch of closely associated trees, which we define as a tree island. Thus, seedling establishment may be aided by facilitation from conifers, other vegetation, nurse objects, or by topographic niches, which all moderate harsh conditions (CitationHoltmeier and Broll, 1992; CitationGermino et al., 2002; CitationMaher et al., 2005; CitationResler et al., 2005; CitationResler, 2006; CitationBatllori et al., 2009). Little is known, however, about the differences in leeward microclimate provided by different nurse objects or conifer species or how the quality of protection may vary relative to local climate or geographically.
In the ATE in the northern Rocky Mountains, P. albicaulis—a widely distributed subalpine conifer of the western United States and Canada—facilitates tree island development more often than associated krummholz conifers, with Picea engelmannii as the next most common initiator (CitationResler and Tomback, 2008; CitationTomback et al., 2014). P. albicaulis seeds are dispersed by Clark's nutcrackers (Nucifraga columbiana) throughout mountain terrain, including the ATE (CitationTomback, 1978, Citation1986, Citation2001; CitationHutchins and Lanner, 1982). Seeds cached by nutcrackers in the ATE near nurse objects and plants and in microtopography may be more protected than wind-dispersed seeds, providing germination and survival advantage (CitationMalanson et al., 2007; CitationTomback and Resler, 2007). However, P. albicaulis mortality from Cronartium ribicola, the non-native pathogen that causes white pine blister rust, is increasing in this region (CitationSmith et al., 2008, Citation2013; CitationSmith et al., 2011). Mortality of P. albicaulis reduces facilitative interactions at treeline (CitationTomback and Resler, 2007; CitationResler and Tomback, 2008; CitationSmith-McKenna et al., 2013), which potentially alters community development and the response of treeline to climate warming (CitationTomback and Resler, 2007; CitationSmith-McKenna et al., 2014).
There are two nonexclusive explanations for the prevalence of P. albicaulis as a tree island initiator: (1) P. albicaulis may occur as a solitary krummholz tree more frequently than other conifer species in the ATE (CitationResler and Tomback, 2008; CitationBlakeslee, 2012; CitationTomback et al., 2014) and thus has a higher probability of facilitating tree island development. However, higher abundance of solitary P. albicaulis does not always correspond to its dominance as a tree island initiator (CitationTomback et al., 2014; CitationResler et al., 2014). (2) P. albicaulis provides a more protective leeward microsite and moderate microclimate than other common conifers or nurse objects at treeline.
Here, we investigated the hypotheses that microsites leeward of P. albicaulis in the ATE experience less extreme microclimate, less sky exposure, and more total soil carbon and nitrogen than microsites leeward of similarly sized krummholz P. engelmannii and rocks, or in open, unprotected microsites. To test these hypotheses, we compared values for nine biophysical variables among four common microsite types in two treeline study areas on the climatically harsh Rocky Mountain eastern front. In particular, we propose that P. albicaulis leeward microsites experience lower air and soil temperature maxima, higher air and soil temperature minima, moister soils, lower cumulative daily PAR, lower wind and gust speeds, but also lower sky exposure and greater percentages of soil carbon and nitrogen.
Methods
Study Areas
Research was conducted at two ATE study areas separated by about 500 km and 3° latitude on the eastern Rocky Mountain Front, Montana, U.S.A., from 2010–2014 (). The Divide Mountain study area straddles the eastern boundary between Glacier National Park and Blackfeet Tribal Land (ca. 48°39′25″N, 113°23′45″W; elevation ca. 2200 m) and includes White Calf Mountain (White Calf), which lies directly south on the eastern edge of Glacier National Park, Montana (48°38′20.95″N, 113°24′08.72″W; elevation ca. 2270 m). The Line Creek Research Natural Area (Line Creek), Custer National Forest, lies on the east side of the Beartooth Plateau (ca. 45°01′47″N, 109°24′09″W; elevation ca. 2950 m). These study areas comprise ATE with conifers growing as solitary krummholz trees and in tree islands primarily composed of krummholz P. albicaulis, P. engelmannii, and A. lasiocarpia. Previous research in both study areas established that P. albicaulis is the majority tree island initiator, and P. engelmannii is the second most abundant conifer overall for both study areas (CitationResler and Tomback, 2008; CitationSmith-McKenna et al., 2013; CitationTomback et al., 2014: Wyoming Creek = Line Creek Research Natural Area). Data from randomly placed sampling transects indicated that the proportions of solitary trees of P. albicaulis were significantly greater than P. engelmannii or A. lasiocarpa on 16 of 19 transects at Divide Mountain and on 12 of 15 transects at Line Creek (CitationBlakeslee, 2012).
FIGURE 1. Study areas: Divide Mountain and White Calf Mountain on the Blackfeet Reservation and Glacier National Park, and Line Creek Research Natural Area in the Custer National Forest on the Beartooth Plateau in Montana. Map modified from Smith-McKenna et al. (Citation2013). White Calf is adjacent to and 2.5 km south of Divide Mountain.
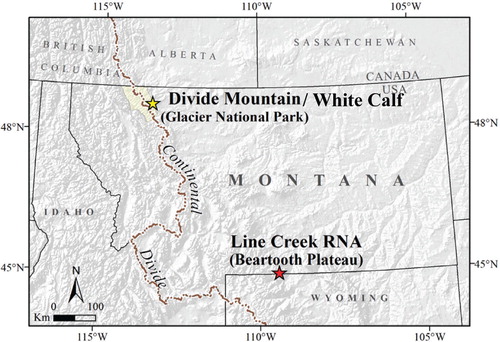
Study sites on Divide Mountain and White Calf are predominantly northeast-facing and on steep slopes (∼20°). The bedrock in this region comprises white limestone of the Altyn Formation (CitationLesica, 2002). The Line Creek study area is also predominantly northeast-facing, but less steep (∼11°), with a ridgeline providing west-facing topography. Soils are shallow, coarse, and relatively undeveloped (CitationNimlos et al., 1965). The local geology is characterized as an uplifted Precambrian granitic mass (CitationBevan, 1923).
Study Design
We defined a microsite as a circular space 20 cm in diameter and no taller than 15 cm directly leeward of a conifer or rock, or on open ground with no windward protection. Microclimate was recorded in microsites directly leeward of solitary, krummholz P. albicaulis and P. engelmannii, rocks—and in unprotected (exposed) microsites. Microsite types were grouped in blocks to reduce confounding variation in aspect and topography. Blocks consisted of a set of each microsite type occurring in close proximity (within 10 m). We sought nurse conifers of similar dimensions within a block, but the mean height of rocks (16–42 cm) and nurse trees (30–70 cm) depended on local availability (Appendix 1 [Table A1-1; note that appendices for this paper are available free with the online version of this paper]). In general, the mean tree heights for krummholz nurse conifers on Divide Mountain were nearly half the height of krummholz nurse conifers at Line Creek, reflecting environmental differences between the two study areas. In all cases, the heights were more than double the height of our defined microsite space.
Microsite position was determined by the direction of wind-flagging on branches of surrounding conifer tree islands. We defined the leeward microsite as directly opposite the source of wind causing the most prevalent branch flagging and tree sculpting at each study site. Selected microsites were not protected by nearby objects, trees, or tree islands. In 2010, we established two blocks at Divide Mountain and Line Creek, placing one on a northeast-facing slope and one on a west-facing slope (16 microsites total, 4 of each type). In 2011 and 2012, we focused on northeast-facing slopes, measuring the same three blocks at Divide Mountain and two blocks at Line Creek (20 microsites total, 5 of each type). Microsites at Divide Mountain typically supported a higher percent ground cover (75%–100%) and the understory plants Arctostaphylos uva-ursi, Dryas octopetala, and less commonly Poa alpina. Microsites at Line Creek supported less ground cover (50%–75%) and the plants Carex spp., Aster alpigenus, and Geum rossii. Conifer microsites differed from rock and unprotected microsites in that they often had a layer of conifer needle litter.
Microclimate Weather Station Setup
We measured the following microclimate characteristics at each microsite (Onset Computer HOBO sensors) from mid-July to mid-September, 2010 to 2012: PAR (µmol m-2 s-1) 7 cm above ground (S-LIA-M003) (2010 only), air temperature (°C) 10 cm above ground (S-THB-M002), soil temperature (°C) 3 cm below the ground (S-TMB-M002), and soil moisture (m3 m-3) 6 cm below ground (S-SMC-M005). We measured wind and gust speeds (m s-1) in 2011 and 2012 in each microsite with an anemometer (S-WSA-M003) 10 cm above ground level.
We constructed protective housing for data loggers and cables with vinyl gutter and PVC piping, to prevent damage from animals, weather, or wind-blown debris (Appendix 4 [Fig. A4-1]). We mounted the air temperature sensor within a temperature shield and PAR sensor by a specially designed bracket, both on a single wood block, 7.6 cm in height, secured in place by a metal stake. The anemometer was secured in the microsite by burying the stem. The data logger, placed 1 m away from the microsite, was mounted on plastic gutter pipe 30 cm above the ground for access and protection from run-off. The soil temperature and soil moisture sensors were positioned at least 4 cm from other sensors or anchoring points to avoid interference.
Using HOBOware Pro software (version 3.3.0), we set the Micro Station Data Loggers to record all microclimate variables every 15 minutes; the data loggers were launched from about mid-July to mid-September each year (dates differed by study area). Data loggers averaged wind speeds over each 15 minute interval from 1 minute observations. Gust speeds represented the highest wind speed over any 3 second period within each 15 minute logging interval. Because the data loggers had only four inputs, we eliminated PAR sensors in order to add wind anemometer sensors in 2011 and 2012.
Sky Exposure Measurements
We determined sky exposure for each leeward weather station microsite in 2010 and 2011 by positioning a Nikon D50 digital camera with 180° fisheye lens in each microsite and photographing the sky view. In 2014, we sampled sky exposure on the northeastern slope of White Calf, which has a greater expanse of ATE. We randomly selected 20 points in the ATE using Arc GIS (version 10.1). At each point, we found the nearest unsheltered solitary P. albicaulis, A. lasiocarpa (P. engelmannii was nearly absent on White Calf), rock, and exposed site. We determined the leeward microsite with respect to nearby tree flagging patterns and photographed sky exposure with the same digital camera and fisheye lens. Percent sky exposure was estimated digitally using Adobe Photoshop Elements 10 (2011) by selecting sky pixel counts and recording the percentage of sky pixels of total overhead pixels.
Soil Sampling
In the Divide Mountain and Line Creek study areas in 2011, we collected soil samples from 10 microsites leeward of P. albicaulis, P. engelmannii, rock, and from unprotected microsites, using a 2.56-cm-diameter soil corer, following the procedures of Tan (Citation2005). Each core was taken to below the O horizon (6 cm depth at Divide Mountain; 15 cm depth at Line Creek), the layer directly influencing seed and seedling growth (CitationBliss and Smith, 2006). In 2014, we sampled on White Calf, using the same 20 random points and 40 microsites selected for sky exposure photos to collect soil samples. Because of shallow soil layers and bedrock, we extracted soil using a steel spade to dig a V-shaped hole past the O-horizon (CitationTan, 2005). For all soil sampling, we transported soil samples in a cooler and refrigerated them until analysis.
In both years of soil sample collection, we dried, ground, and analyzed soil samples for percent total carbon and nitrogen using a LECO CN elemental analyzer at the EcoCore Analytical Services Lab at Colorado State University in Fort Collins, Colorado. Samples from both locations included high amounts of leaf litter, elevating the percentage of carbon beyond the limits of the elemental analyzer for soil determination; therefore, we designated these samples as vegetation for calibration.
Data Analysis
We used R statistical software, version 2.14.1 (CitationR Development Core Team, 2011) for all data analysis. General ground-level climate patterns between the two study areas were compared using microclimate data from unprotected (exposed) microsites.
For 2010, 2011, and 2012 microclimate data, we performed a two-factor analysis for each climate variable to determine if data differed with block. Seasonal trends in temperature and PAR confounded analyses; thus, we fit our daily values (temperature maximums or minimums, and PAR sums over time) to both linear and quadratic models, and selected the model with the lowest Akaike Information Criterion (AIC) score (Appendix 2). Residuals, representing the difference between the observed value and the model value, were retained for comparative analysis among microsite types (Appendix 4 [Fig. A4-2]). This model was used to detrend data and removed time as a dependent variable. Thus, all reported comparisons among microsites for air and soil temperature variables and PAR are based on residual data sets.
To determine whether our replicates represented “true” replicates, we determined the interaction effect of blocks on microsite type by ranking the maximum, average, or minimum daily values for soil moisture, wind, and gust speed (or the data set residuals for PAR and temperature) and then compared the ranked values among microsites and blocks with two-way analysis of variance (ANOVA). Ranked rather than raw data were used because assumptions of normality and homoscedasticity were not met. Then, we compared daily values of microclimate variables with Kruskal-Wallis one-way ANOVA among microsites either separately among blocks or combined if no significant interaction effect was detected. We performed pairwise Wilcoxon rank sum tests for post hoc analysis among pairs of microsite types.
For comparisons of numbers of soil freeze-thaw events among microsites for each study area, we used a chi-square goodness-of-fit test. We used the Shapiro-Wilk normality test to examine sky exposure and soil carbon and nitrogen data. The sky exposure data were not normally distributed, so we used a Kruskal-Wallis one-way ANOVA and pairwise Wilcoxon rank sum tests with Bonferroni corrections for post hoc analysis to compare the differences in median percentages of sky exposure among all microsite types. For soil samples from 2011, carbon and nitrogen data did not depart significantly from normality, and percentages of total carbon and nitrogen in soil were compared among microsites and between study areas with parametric one-way ANOVA, followed by Tukey HSD tests for post hoc analysis. Soil data from 2014 departed from normality, and we used Kruskal-Wallis one-way ANOVA to compare the soil sample results.
Summary statistics for all microclimate variables are reported in Appendix 1; AIC scores fitting models to microclimate data, in Appendix 2; post hoc tests, in Appendix 3; and figures comparing residual values, in Appendix 4.
Results
Microsite Air and Soil Temperature
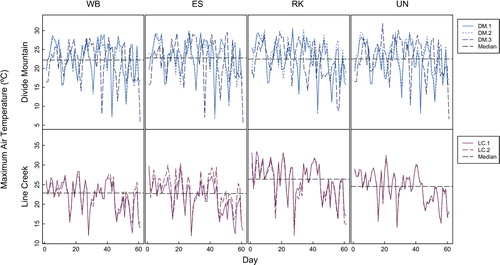
Air temperature varied with date at Divide Mountain and to greater extremes at Line Creek, trending seasonally from high values in July to lower values in September in both study areas in all three years (). At Divide Mountain, air temperature was not different in general at P. albicaulis microsites compared to other microsites. For northeast slope comparisons, from 2010 to 2012, median maximum air temperature did not differ among microsite types within more than half the block comparisons, and median minimum air temperature did not differ within any block comparisons (; Appendix 1 [Tables A1-2, A1-3, A1-4]; Appendix 3 [Tables A3-1, A3-5, A3-6]; Appendix 4 [Fig. A4-3]).
At Line Creek, maximum air temperatures differed across microsites within all blocks, but minimum air temperature differed within only one block (; Appendix 1 [Tables A1-2, A1-3, A1-4]; Appendix 3 [Tables A3-1, A3-5, A3-6]; Appendix 4 [Fig. A4-3]). P. albicaulis microsites at Line Creek, and especially P. engelmannii microsites, had lower maximum air temperature values than unprotected or rock microsites, and rock microsites had the highest maximum air temperatures (, ). P. engelmannii microsites had the highest minimum air temperatures.
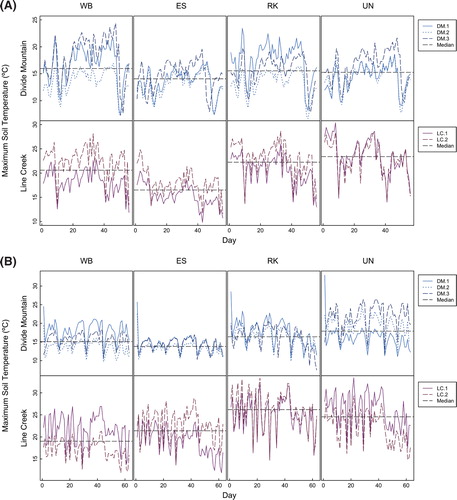
Soil temperatures similarly varied throughout the growing season for both study areas, with higher amplitude of variation at Line Creek; and, temperatures declined from higher to lower values by September (, parts A and B). At both study areas, microsites differed within nearly all blocks (). At Divide Mountain, P. albicaulis microsites did not have the lowest soil temperature maxima nor did they consistently have the highest soil temperature minima, but the conifer microsites together provided the lowest maximum and highest minimum soil temperatures (; Appendix 1 [Tables A1-5, A1-6, A1-7, A1-8]; Appendix 2 [Table A2-1]; Appendix 3 [Tables A3-1, A3-2, A3-3, A3-4]; Appendix 4 [Figs. A4-4, A4-5]).
At Line Creek, P. albicaulis did not consistently have the lowest soil temperature maxima or highest soil temperature minima, although the differences between the conifer microsites often were not significant (; Appendix 1 [Tables A1-5, A1-6, A1-7, A1-8]; Appendix 2 [Table A2-1]; Appendix 3 [Tables A3-1, A3-2, A3-3, A3-4]; Appendix 4 [Figs. A4-4, A4-5]). Both conifer microsites also had lower daily soil temperature variance in the two study areas than rock or exposed microsites, but P. engelmannii had the lowest variance in the majority of comparisons (; Appendix 3 [Tables A3-1, A3-4]).
FIGURE 2. Daily maximum air temperatures from the northeast slopes of Divide Mountain (DM) and Line Creek (LC) in 2012, which is representative of study results. Day 1 represents 4 July at Divide Mountain and 17 July at Line Creek. WB = Pinus albicaulis, ES = Picea engelmannii, RK = rock, and UN = unprotected (exposed) microsites.
PAR and Soil Moisture
PAR, measured only in 2010 on both west and northeast aspects, declined from July to September, with variation introduced by intermittent periods of rain and overcast days (). PAR differed significantly among microsites within all blocks in both study areas (; Appendix 1 [Table A1-9]; Appendix 2 [Table A2-3]; Appendix 3 [Tables A3-1, A3-7]; Appendix 4 [Fig. A4-7]). On Divide Mountain, P. engelmannii microsites had the lowest PAR values, with P. albicaulis microsites the next lowest. At Line Creek, the trend was reversed, with P. albicaulis microsites on both the west and northeast aspects experiencing the lowest PAR values, followed by P. engelmannii microsites.
Median daily average soil moisture differed significantly and inconsistently among microsites for most but not all blocks at Divide Mountain and Line Creek, although P. albicaulis had the highest median daily average overall for both study areas (; Appendix 1 [Table A1-10]; Appendix 3 [Tables A3-1, A3-10]; Appendix 4 [Fig. A4-6]). For some comparisons, neither conifer species had the highest average soil moisture, and they did not differ from each other in several post hoc tests (Appendix 3 [Table A3-10]).
Wind and Gust Speed
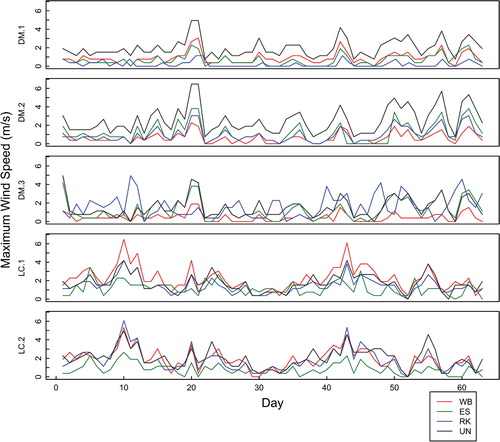
Median average daily wind speed in general was greater at Divide Mountain in 2011 but similar to that at Line Creek in 2012. Wind speed differed significantly across microsites within all blocks in both study areas (). Average wind speed was not consistently lowest in leeward P. albicaulis microsites within blocks (); however, on Divide Mountain the overall median average wind speed was lowest for P. albicaulis microsites (). At both study areas in 2011 and 2012, all protected microsites (conifer and rock microsites) experienced lower wind speeds than unprotected microsites, and the conifer microsites did not differ from one another in several post hoc tests (; ; Appendix 1 [Table A1-11]; Appendix 3 [Table A3-1, A3-8]; Appendix 4 [Fig. A4-8]).
TABLE 1 Median and range (minimum, maximum) of values for microclimate variables measured during 2010–2012 for each microsite type. (Values measured in 2010 from the west-facing aspect not included.) % diff = percent of Kruskal-Wallis One-way ANOVA comparisons among microsite blocks that were statistically different (Divide Mountain, n = 7 comparisons; Line Creek, n = 5 comparisons). WB = Pinus albicaulis, ES = Picea engelmannii, RK = rock, UN = unprotected (exposed).
Maximum wind gust speeds were not consistently lowest in P. albicaulis microsites. Although nearly all microsite comparisons within blocks differed significantly, the majority of the post hoc comparisons between P. albicaulis and P. engelmannii did not differ (Appendix 3 [Tables A3-1, A3-9]). On Divide Mountain, P. engelmannii microsites had the lowest maximum wind gust speeds and P. albicaulis the highest (). At Line Creek, P. engelmannii microsites consistently had the lowest maximum gust speeds (; ; Appendix 1 [Table A1-12]; Appendix 3 [Tables A3-1, A3-9]; Appendix 4 [Fig. A4-8]).
Sky Exposure and Soil Carbon and Nitrogen
Median sky exposure across the leeward weather station microsites differed significantly (χ 2 = 21.77, df = 3, P = 7.30e-05), varying from a low of 50.59% in P. albicaulis leeward microsites to 94.22% in unprotected microsites (). Post hoc tests indicated that conifer microsites did not differ from each other, although they differed significantly from rock and exposed microsites (Appendix 3 [Table A3-11]). Sky exposure data collected from random points in 2014 ranged from a low of 60.08% at P. albicaulis microsites to 92.50% at unprotected microsites, differing significantly across microsites (χ 2 = 48.61, df = 3, P = < 2e-16) (). Post hoc tests again indicated significant differences between conifer and nonconifer microsites (Z = 2.03, P = 0.0425), but no differences between P. albicaulis and A. lasiocarpa (Appendix 3 [Table A3-12]).
Percent total nitrogen and carbon were not consistently high in P. albicaulis leeward microsites or in conifer microsites for samples collected in 2012. At Divide Mountain, nitrogen ranged from a mean of 0.42% in P. engelmannii microsites to a mean of 0.48% in P. albicaulis microsites; at Line Creek, percent nitrogen ranged from a mean of 0.17% in P. albicaulis and rock microsites to 0.19% in P. engelmannii and unprotected microsites (). Carbon at Divide Mountain ranged from a mean of 8.58% in P. engelmannii microsites to 9.45% in unprotected microsites, and at Line Creek from 2.03% in rock microsites to 2.70% in P. albicaulis microsites (). Two-way ANOVA indicated that soil nitrogen (F = 0.84, df = 3, 74, P = 0.48) and carbon content (F = 0.27, df = 3, 74, P = 0.85) did not differ among the microsite types (Appendix 3 [Table A3-13]). For soils sampled on White Calf in 2014, however, P. albicaulis microsites had both the highest carbon and the highest nitrogen content, but the differences were not statistically significant (χ 2 =1.19, n = 20, P = 0.755, and χ 2 = 1.72, n = 20, P = 0.633, respectively) ().
Comparisons Between Study Areas
We characterized differences in general growing season microclimate between the two study areas based on data from the unprotected microsites on northeast-facing slopes (). Divide Mountain was on average warmer, wetter, and windier than Line Creek. Tree flagging and growth form sculpting indicated that prevailing winds were both strong and variable over short distances on Divide Mountain as a result of the complex topography. Tree growth forms were more strongly krummholz at Divide Mountain than at Line Creek.
Median daily average air temperatures were higher at Divide Mountain in 2011 and 2012, but median daily variance in temperature was greater at Line Creek in all three years. Line Creek experienced 15 soil freeze-thaw events in 2011 and 34 in 2012. Divide Mountain experienced significantly fewer soil freeze-thaw events—9 in 2011 (χ2 = 9.55, df = 1, P = 0.002) and 13 in 2012 (χ2 = 39.34, df = 1, P = 3.56e-10). Divide Mountain daily average wind speeds reached higher maxima than Line Creek and had greater variance in both years (, ).
Based on sampling in 2012, Divide Mountain soils had significantly higher mean percentages of total nitrogen and total carbon than Line Creek soils (, two-way ANOVA: Total nitrogen-microsite-study-area interaction effect, F = 0.7356, df = 7, 74, P = 0.5342; study area, F = 241.6890, df = 1, 74, P < 2e-16; and total carbon-microsite-study-area interaction effect, F = 0.2666, df = 7, 74, P = 0.8492; study area, F = 253.7934, df = 1, 74, P < 2e-16).
Discussion
Evaluating P. albicaulis Leeward Microsites
Given the prevalence of P. albicaulis as a tree island initiator in the northern Rocky Mountains, we hypothesized that microsites leeward of P. albicaulis experience less extreme microclimate, less sky exposure, and more total soil carbon and nitrogen than microsites leeward of P. engelmannii, rocks, or in unprotected microsites. We found that P. albicaulis leeward microsites did not consistently provide the most moderate microclimate and protective microsites, as defined by lower air and soil temperature maxima, higher air and soil temperature minima, moister soils, lower cumulative daily PAR, and lower wind and gust speeds. In fact, P. engelmannii microsites may produce a somewhat more favorable leeward microclimate.
FIGURE 3. (A) Top, daily maximum soil temperatures from the northeast slopes of Divide Mountain (DM) and Line Creek (LC) in 2011. Day 1 represents 9 July at Divide Mountain and 22 July at Line Creek. (B) Bottom, daily maximum soil temperatures from the northeast slopes of Divide Mountain and Line Creek in 2012. Day 1 represents 4 July at Divide Mountain and 17 July at Line Creek. Abbreviations as in .
P. albicaulis microsites, however, were marginally advantageous for other biophysical measurements. For the 2014 soil samples on White Calf, P. albicaulis microsites had greater percentages of soil carbon and nitrogen, which may indicate higher fertility in P. albicaulis microsites under some conditions across study areas. P. albicaulis leeward microsites also had slightly higher median average daily soil moisture than other microsite types. P. albicaulis microsites in the two sampling efforts experienced the lowest median percent sky exposure of all microsites—∼15% lower than P. engelmannii microsites on Divide Mountain and ∼10% lower than A. lasiocarpa microsites on White Calf. Although these differences were not significant in post hoc tests, they may be attributed to the longer needles and branches and more irregular, overhanging canopies of krummholz P albicaulis. P. albicaulis shoots and needles, measured at our ATE study areas, are significantly longer than those of P. engelmannii and A. lasiocarpa (CitationBlakeslee, 2012). A small reduction in sky exposure, a slight increase in soil moisture, or additional soil carbon or nitrogen provided by P. albicaulis microsites could improve leeward seedling survival.
FIGURE 4. Daily photosynthetically active radiation (PAR), recorded only in 2010 on northeast- and west-facing slopes of Divide Mountain (DM) and Line Creek (LC). Day 1 represents 8 July at Divide Mountain and 22 July at Line Creek. Abbreviations as in .
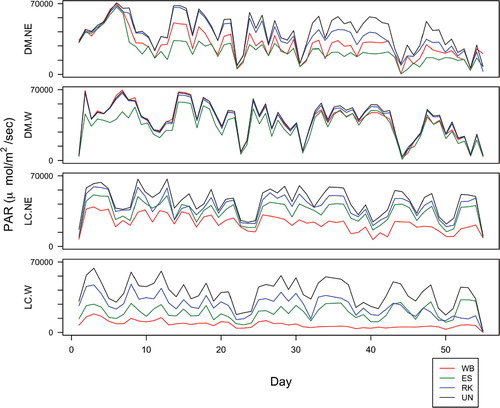
TABLE 2 Descriptive statistics for percent sky exposure for each microsite type associated with microclimate weather stations at Divide Mountain and Line Creek, and at randomly selected locations at White Calf. WB = Pinus albicaulis, ES = Picea engelmannii, SF = Abies lasiocarpa.
TABLE 3 Descriptive statistics for percent total nitrogen and carbon in soils sampled at Divide Mountain and Line Creek in 2011 and at randomly selected locations at White Calf in 2014. WB = Pinus albicaulis, ES = Picea engelmannii, SF = Abies lasiocarpa, Exposed = open.
If the microclimate protection offered by P. albicaulis leeward microsites is no greater than that offered by associated conifers, why is it the most frequent tree island initiator? First of all, the preceding information suggests that there may be some advantageous qualities to P. albicaulis leeward microsites beyond microclimate. However, the prevalence of P. albicaulis as a tree island initiator is generally predicted by its high relative abundance as a solitary tree, which may result from seed dispersal by Clark's nutcrackers to protective microsites at treeline and greater seedling hardiness. In fact, Tomback et al. (Citation2016) found that the proportional abundance of P. albicaulis among solitary trees in the ATE across 10 study areas predicted its proportional abundance as a tree island initiator.
With respect to hardiness, Bansal et al. (Citation2011) compared P. albicaulis and P. engelmannii seedlings grown at treeline and demonstrated that P. albicaulis had greater carbon gain, greater carbon use efficiency, and greater water-use efficiency in exposed microsites as well as greater resistance to low-temperature photo inhibition. Blakeslee (Citation2012) demonstrated that P. albicaulis were associated with less protective microsites for establishment and had higher vigor than other solitary krummholz trees. These factors may contribute both to higher relative abundance of P. albicaulis and more dependable protection.
TABLE 4 Comparisons of northeastern slope microclimate and overall average percent soil carbon and nitrogen between the Divide Mountain study area and Line Creek study area. These comparisons are based on data from unprotected (exposed) microsites. Microclimate data were collected from about mid-July though about mid-September in both study areas.
Importance of Protected Microsites
Previous studies at Rocky Mountain alpine treelines have suggested that conifers offer microclimatic protection, aiding in subsequent conifer establishment and ultimately the formation of tree islands (e.g., CitationMarr, 1977; CitationResler et al., 2005). Our results support this contention. Although species-specific differences in protection are not strongly evident, conifer microsites experienced measurably more moderated microclimate than rock and unprotected microsites. Unprotected microsites had higher daily average wind speeds, which can desiccate foliage and increase transpiration and soil evaporation rates, resulting in drought stress (CitationHoltmeier, 2009). Too much solar radiation, which heats soils to high temperatures and harms foliage, has been shown to kill young P. engelmannii and A. lasiocarpa seedlings (CitationCui and Smith, 1991; CitationGermino et al., 2002). Furthermore, we found that conifer microsites reduced sky exposure. For seedlings, more sky exposure may lead to excess solar radiation including PAR, but also lower soil moisture and colder nighttime temperatures from long-wave irradiance (e.g., CitationMaher et al., 2005; CitationGermino and Smith, 1999). Conifer microsites did not always have the highest soil moisture. This effect may result from reduced sky exposure: overhanging canopies could intercept precipitation and reduce infiltration in leeward microsites. A better determinant of local soil moisture might be microtopographic “concavities” and “convexities,” which influence moisture distribution regardless of type of nurse object (e.g., CitationSmith-McKenna et al., 2013).
FIGURE 5. Wind speeds 10 cm above the ground from microsites on the northeast slope of Divide Mountain (DM) and Line Creek (LC) for the 2012 growing season. Abbreviations as in .
Conceivably, the quality of facilitation provided by different microsite types could vary with conifer life history stage. In a companion study, Blakeslee (Citation2012) compared the protective quality of the same four treeline leeward microsites in the ATE on Divide Mountain (krummholz P. albicaulis and P. engelmannii, rock, and exposed site) by examining the germination and survival rates of sown seeds. Five P. engelmannii seeds from the appropriate seed transfer zone were sown in 20 replicates of each type of leeward microsite. Higher than expected germination occurred in rock microsites and fewer than expected in P. albicaulis microsites. However, the odds of cotyledon seedling survival during summer months in P. albicaulis microsites were about 7 times higher than for P. engelmannii microsites, 10 times higher than for rock, and 14 times higher than for exposed microsites.
Seedling survival in the ATE is generally low, especially for the first-year (CitationCui and Smith, 1991). Our microclimate results support previous findings that facilitative “nurse” objects, and especially conifers, potentially provide protection from extreme climate at treeline, enabling seedling establishment (CitationCallaway, 1998; CitationHättenschwiler and Smith, 1999; CitationGermino et al., 2002). P. albicaulis is the most prevalent solitary tree at both study areas, and thus potentially important for seedling establishment and community development (CitationBlakeslee, 2012).
Differences in Facilitation Requirements by Study Area
The two treeline study areas were characterized by different climate extremes that could impact conifer growth and survival. The ATE at Divide Mountain was moister, warmer, but windier than the ATE at Line Creek. Higher wind speeds and maximum gust speeds would explain more krummholz growth and severe windward needle kill at Divide Mountain than at Line Creek. However, Line Creek experienced greater air and soil temperature variance, more freeze-thaw events, and less moisture.
Amelioration of extreme temperatures and PAR may be more important for survival at Line Creek than at Divide Mountain. Cold soil temperatures limit seedling growth at treeline (CitationLandhäusser et al., 1996; CitationGermino and Smith, 1999). Although conifers tolerate low temperatures throughout the winter (CitationSakai and Okada, 1971), cold hardiness is reduced during the growing season (CitationKalberer et al., 2006). Frequent summer freezes may lead to cold injury (CitationChristersson and Fircks, 1988). Divide Mountain experienced higher median average wind speeds in 2011, and all nurse objects, but especially conifers, offered protection.
Variation in Facilitation by Microsite
We found that microclimate varied among similar microsite types between study areas, between slopes in the same study area, on the same slope aspect, and from year to year. This variation may result from numerous factors interacting, such as differences in regional climate and synoptic weather patterns between the two sites, topographically unique wind patterns (especially on Divide Mountain), slight variations in slope aspect and slope steepness, and differences in morphology and dimensions among nurse objects. All microclimate variables are affected, but particularly maximum soil temperature, daily average soil moisture, and wind speed and maximum gust speed. These results illustrate that the benefits of facilitation attributed to specific plants or nurse objects vary with specific environmental conditions, and we must be cautious about generalizations.
Conclusions
Our studies did not support the hypothesis that P. albicaulis leeward microsites experience the most moderate microclimate and suggest that other factors, such as additional protective microsite qualities and availability as a solitary tree, are responsible for the prevalence of P. albicaulis as the dominant tree island initiator in the ATE. Our results, however, underscore the ecological importance of conifers as nurse objects for facilitating treeline community development in the ATE, and especially P. albicaulis, given its high abundance in many harsh treeline communities. Previous studies in the ATE on the eastern slope of Glacier National Park documented infection rates of P. albicaulis by C. ribicola at ∼35% (CitationResler and Tomback, 2008), and within the park at an overall 47% (CitationSmith et al., 2011). P. albicaulis is a candidate species under the U.S. Endangered Species Act (CitationU.S. Fish and Wildlife Service, 2011) and listed as endangered in Canada (CitationGovernment of Canada, 2012). As P. albicaulis declines from C. ribicola, the establishment rate of tree islands may also decline, resulting in changes in ATE community structure and composition. In turn, these changes are likely to cause lags in expected upslope treeline migration in response to climate warming (CitationTomback and Resler, 2007; CitationSmith-McKenna et al., 2014).
uaar_a_11957908_sm0001.pdf
Download PDF (5.3 MB)Acknowledgments
We are grateful to Tara Carolin of Glacier National Park, Mark Magee of the Blackfeet Nation Land Office, Kent Houston of Shoshone National Forest, and Sam Foster of the USDA Forest Service Rocky Mountain Research Station for permits and for coordinating our research. We thank Libby Pansing, Solédad Diaz, and Aaron Wagner for assistance in the field, Emily Smith-McKenna for advice and information, and Charles Kwit for constructive suggestions. The Department of Integrative Biology, University of Colorado Denver, provided greenhouse space for testing weather station equipment. DFT prepared the manuscript while supported by a Charles Bullard Harvard Forest Fellowship. We are grateful to David Orwig and George Malanson for helpful comments on a previous draft of the manuscript. This study was supported by National Science Foundation grant 0850548 to Resler, Tomback, and G. P. Malanson. None of the authors has a conflict of interest to declare.
References Cited
- Alftine, K. J. , and Malanson, G. P. , 2004: Directional positive feedback and pattern at an alpine tree line. Journal of Vegetation Science , 15: 3–12.
- Angel Munguía-Ross, M. , and Sosa, V. J. , 2008: Nurseplants vs. nurse objects: effects of woody plants and rocky cavities on the recruitment of the Pilosocereus leucocephalus columnar cactus. Annals of Botany , 101: 175–185.
- Bansal, S. , Reinhardt, K. , and Germino, M. J. , 2011: Linking carbon balance to establishment patterns: comparison of whitebark pine and Engelmann spruce seedlings along an herb cover exposure gradient at treeline. Plant Ecology , 212: 219–228.
- Batllori, E. , Camarero, J. J. , Ninot, J. M. , and Gutiérrez, E. , 2009: Seedling recruitment, survival and facilitation in alpine Pinus uncinata tree line ecotones. Implications and potential responses to climate warming. Global Ecology and Biogeography , 18: 460–472.
- Baumeister, D. , and Callaway, R. M. , 2006: Facilitation by Pinus flexilis during succession: a hierarchy of mechanisms benefits other plant species. Ecology , 87: 1816–1830.
- Bekker, M. F. , 2005: Positive feedback between tree establishment and patterns of subalpine forest advancement, Glacier National Park, M.T., U.S.A. Arctic, Antarctic, and Alpine Research , 37: 97–107.
- Bevan, A. , 1923: Summary of the geology of the Beartooth Mountains, Montana. Journal of Geology , 31: 441–465.
- Blakeslee, S. C. , 2012: Assessing Whitebark Pine Vigor and Facilitation Roles in the Alpine Treeline Ecotone. M.S. thesis, Department of Integrative Biology, University of Colorado Denver.
- Bliss, D. , and Smith, H. , 2006: Penetration of light into soil and its role in the control of seed germination. Plant, Cell & Environment , 8: 475–483.
- Breshears, D. D. , Nyhan, J. W. , Heil, C. E. , and Wilcox, B. P. , 1998: Effects of woody plants on microclimate in a semiarid woodland: soil temperature and evaporation in canopy and intercanopy patches. International Journal of Plant Sciences , 159: 1010–1017.
- Brooker, R. W. , Maestre, F. T. , Callaway, R. M. , Lortie, C. L. , Cavieres, L. A. , Kunstler, G. , Liancourt, P. , Tielbörger, K. , Travis, J. M. J. , Anthelme, F. , Armas, C. , Coll, L. , Corcket, E. , Delzon, S. , Forey, E. , Kikvidze, Z. , Olofsson, J. , Pugnaire, F. , Quiroz, C. L. , Saccone, P. , Schiffers, K. , Seifan, M. , Touzard, B. , and Michalet, R. , 2008: Facilitation in plant communities: the past, the present, and the future. Journal of Ecology , 96: 18–34.
- Bruno, J. F. , Stachowicz, J. J. , and Bertness, M. D. , 2003: Inclusion of facilitation into ecological theory. Trends in Ecology and Evolution , 18: 119–125.
- Cairns, D. M. , and Malanson, G. P. , 1997: Environmental variables influencing the carbon balance at the alpine treeline: a modeling approach. Journal of Vegetation Science , 9: 679–692.
- Callaway, R. M. , 1998: Competition and facilitation on elevation gradients in subalpine forest of the northern Rocky Mountains, U.S.A. Oikos , 82: 561–573.
- Callaway, R. M. , Nadkarni, N. M. , and Mahall, B. E. , 1991: Facilitation and interference of Quercus douglasii on understory productivity in central California. Ecology , 72: 1484–1499.
- Callaway, R. M. , Brooker, R. W. , Choler, P. , Kikvidze, Z. , Lortie, C. J. , Michalet, R. , Paolini, L. , Pugnaire, F. I. , Newingham, B. , Aschehoug, E. T. , Armas, C. , Kikodze, D. , and Cook, B. J. , 2002: Positive interactions among alpine plants increase with stress. Nature , 417: 844–848.
- Castro, J. , Allen, C. D. , Molina-Morales, M. , Marañón-Jiménez, S. , Sánchez-Miranda, Á. , and Zamora, R. , 2011: Salvage logging versus the use of burnt wood as a nurse object to promote post-fire seedling establishment. Restoration Ecology , 19: 537–544.
- Chambers, J. C. , 2001: Pinus monophylla establishment in an expanding Pinus‐Juniperus woodland: Environmental conditions, facilitation and interacting factors. Journal of Vegetation Science , 12: 27–40.
- Christersson, L. , and Fircks, H. A.V. , 1988: Injuries to conifer seedlings caused by simulated summer frost and winter desiccation. Silva Fennica , 22: 195–201.
- Cui, M. , and Smith, W. K. , 1991: Photosynthesis, water relations and mortality in Abies lasiocarpa seedlings during natural establishment. Tree Physiology , 8: 37–46.
- Germino, M. J. , and Smith, W. K. , 1999: Sky exposure, crown architecture, and low-temperature photoinhibition in conifer seedlings at alpine treeline. Plant, Cell, & Environment , 22: 407–415.
- Germino, M. J. , Smith, W. K. , and Resor, A. C. , 2002: Conifer seedling distribution and survival in an alpine-treeline ecotone. Plant Ecology , 162: 157–168.
- Government of Canada, 2012: Order amending Schedule 1 to the Species at Risk Act. Canada Gazette Part II, Vol 146, No. 14, SOR/2012-113, <http://www.sararegistry.gc.ca/virtual_sara/files/orders/g2-14614i_e.pdf>, (accessed 20 June 2012).
- Hättenschwiler, S. , and Smith, W. K. , 1999: Seedling occurrence in alpine treeline conifers: a case study from the Central Rocky Mountains, USA. Acta Oecologica , 20: 219–224.
- Hoch, G. , Popp, M. , and Körner, C. , 2002: Altitudinal increase of mobile carbon pools in Pinus cembra suggests sink limitation of growth at the Swiss treeline. Oikos , 98: 361–374.
- Holtmeier, F. K. , 2009: Mountain Timberlines: Ecology, Patchiness, and Dynamics. Second edition. Dordrecht, Netherlands: Springer, Advances in Global Change Research 36.
- Holtmeier, F. K. , and Broll, G. , 1992: The influence of tree islands and microtopography on pedoecological conditions in the forest-alpine tundra ecotone on Niwot Ridge, Colorado Front Range, USA. Arctic and Alpine Research , 24: 216–228.
- Hutchins, H. E. , and Lanner, R. M. , 1982: The central role of Clark's nutcracker in the dispersal and establishment of whitebark pine. Oecologia , 55: 192–201.
- Kalberer, S. R. , Wisniewski, M. , and Arora, R. , 2006: Deacclimation and reacclimation of cold-hardy plants: current understanding and emerging concepts. Plant Science , 171: 3–16.
- Körner, C. , 1998: A re-assessment of high elevation treeline positions and their explanation. Oecologia , 115: 445–459.
- Landhäusser, S. M. , Wein, R. W. , and Lange, P. , 1996: Gas exchange and growth of three Arctic tree-line tree species under different soil temperature and drought preconditioning regimes. Canadian Journal of Botany , 74: 686–693.
- Lesica, P. , 2002: A Flora of Glacier National Park, Montana. Corvallis: Oregon State University Press.
- Lortie, C. J. , and Turkington, R. , 2008: Species-specific positive effects in an annual plant community. Oikos , 117: 1511–1521.
- Maher, E. L. , Germino, M. J. , and Hasselquist, N. J. , 2005: Interactive effects of tree and herb cover on survivorship, physiology, and microclimate of conifer seedlings at the alpine tree-line ecotone. Canadian Journal of Forest Research , 35: 567–574.
- Maher, E. L. , and Germino, M. J. , 2006: Microsite differentiation among conifer species during seedling establishment at alpine treeline. Écoscience , 13: 334–341.
- Malanson, G. P. , Butler, D. R. , Fagre, D. B. , Walsh, S. J. , Tomback, D. F. , Daniels, L. D. , Resler, L. M. , Smith, W. K. , Weiss, D. J. , Peterson, D. L. , Bunn, A. G. , Hiemstra, C. A. , Liptzin, D. , Bourgeron, P. S. , Shen, Z. , and Millar, C. I. , 2007: Alpine treeline of western North America: linking organism-to-landscape dynamics. Physical Geography , 28: 378–396.
- Marr, J. W. , 1977: The development and movement of tree islands near the upper limit of tree growth in the southern Rocky Mountains. Ecology , 58: 1159–1164.
- Michalet, R. , Maalouf, J.-P. , Choler, P. , Clément, B. , Rosebery, D. , Royer, J.-M. , Schöb, C. , and Lortie, C. J. , 2015: Competition, facilitation and environmental severity shape the relationship between local and regional species richness in plant communities. Ecography , 38: 335–345.
- Moir, W. H. , Rochelle, S. G. , and Schoettle, A. W. , 1999: Microscale patterns of tree establishment near upper treeline, Snowy Range, Wyoming, USA. Arctic, Antarctic, and Alpine Research , 31: 379–388.
- Moyes, A. B. , Castanha, C. , Germino, M. J. , and Kueppers, L. M. , 2013: Warming and the dependence of limber pine (Pinus flexilis) establishment on summer soil moisture within and above its current elevation range. Oecologia , 171: 271–282.
- Nimlos, T. J. , McConnel, R. C. , and Pattie, D. L. , 1965: Soil temperature and moisture regimes in Montana alpine soils. Northwest Science , 39: 129–137.
- R Development Core Team, 2011: R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- Resler, L. M. , 2006: Geomorphic controls of spatial pattern and process at alpine treeline. The Professional Geographer , 58: 124–138.
- Resler, L. M. , and Tomback, D. F. , 2008: Blister rust prevalence in krummholz whitebark pine: implications for treeline dynamics, northern Rocky Mountains, Montana, U.S.A. Arctic, Antarctic, and Alpine Research , 40: 161–170.
- Resler, L. M. , Butler, D. R. , and Malanson, G. P. , 2005: Topographic shelter and conifer establishment and mortality in an alpine environment, Glacier National Park, Montana. Physical Geography , 26: 112–125.
- Resler, L. M. , Shao, Y. , Tomback, D. F. , and Malanson, G. P. , 2014: Predicting the functional role and occurrence of whitebark pine (Pinus albicaulis) at alpine treeline: model accuracy and variable importance. Annals of the Association of American Geographers. doi http://dx.doi.org/10.1080/00045608.2014.910072.
- Sakai, A. , and Okada, S. , 1971: Freezing resistance of conifers. Silvae Genetics , 20: 91–97.
- Seastedt, T. R. , and Adams, G. A. , 2001: Effects of mobile tree islands on alpine tundra soils. Ecology , 82: 8–17.
- Shiels, A. B. , and Sanford, R. L., Jr. , 2001: Soil nutrient differences between two krummholz-form tree species and adjacent alpine tundra. Geoderma , 102: 205–217.
- Smith, C. M. , Wilson, B. , Rasheed, S. , Walker, R. C. , Carolin, T. , and Shepherd, R. , 2008: Whitebark pine and white pine blister rust in the Rocky Mountains of Canada and northern Montana. Canadian Journal of Forest Research , 38: 982–985.
- Smith, C. M. , Shepherd, B. , Gillies, C. , and Stuart-Smith, J. , 2013: Changes in blister rust infection and mortality in whitebark pine over time. Canadian Journal of Forest Research , 43: 90–96.
- Smith, E. K. , Resler, L. M. , Vance, E. A. , Carstensen, L. W. , and Kolivras, K. N. , 2011: Blister rust incidence in treeline whitebark pine, Glacier National Park, USA: environmental and topographic influences. Arctic, Antarctic, and Alpine Research , 43: 107–117.
- Smith-McKenna, E. K. , Resler, L. M. , Tomback, D. F. , Zhang, H. , and Malanson, G. P. , 2013: Topographic influences on the distribution of white pine blister rust in Pinus albicaulis treeline communities. Écoscience, 20: 215–229.
- Smith-McKenna, E. K. , Malanson, G. P. , Resler, L. M. , Carstensen, L. W. , Prisley, S. P. , and Tomback, D. F. , 2014: Cascading effects of feedbacks, disease, and climate change on alpine treeline dynamics. Environmental Modelling and Software , 62: 85–96.
- Soliveres, S. , Eldridge, D. J. , Maestre, F. T. , Bowker, M. A. , Tighe, M. , and Escudero, A. , 2011: Microhabitat amelioration and reduced competition among understory plants as drivers of facilitation across environmental gradients: towards a unifying framework. Perspectives in Plant Ecology, Evolution and Systematics , 13: 247–258.
- Spittlehouse, D. L. , and Stathers, R. J. , 1990: Seedling Microclimate. Victoria, British Columbia: BC Ministry of Forests, Land Management Report, no. 65.
- Stachowicz, J. J. , 2001: Mutualism, facilitation, and the structure of ecological communities. Bioscience , 51: 235–246.
- Stevens, G. C. , and Fox, J. F. , 1991: The causes of treeline. Annual Review of Ecology and Systematics , 22: 177–191.
- Sthultz, C. M. , Gehring, C. A. , and Whitham, T. G. , 2007: Shifts from competition to facilitation between a foundation tree and a pioneer shrub across spatial and temporal scales in a semiarid woodland. New Phytologist , 173: 135–145.
- Sveinbjörnsson, B. , Kauhanen, H. , and Nordell, O. , 1996: Treeline ecology of mountain birch in the Torneträsk area. Ecological Bulletins , 45: 65–70.
- Tan, K. , 2005: Soil Sampling, Preparation, and Analysis , Second edition. Boca Raton, Florida: Taylor and Francis.
- Tomback, D. F. , 1978: Foraging strategies of Clark's nutcracker. Living Bird , 16: 123–161.
- Tomback, D. F. , 1982: Dispersal of whitebark pine seeds by Clark's nutcracker: a mutualism hypothesis. Journal of Animal Ecology , 51: 451–467.
- Tomback, D. F. , 1986: Post-fire regeneration of krummholz whitebark pine: a consequence of nutcracker seed caching. Madrono , 33: 100–110.
- Tomback, D. F. , 2001: Clark's nutcracker: agent of regeneration. In Tomback, D. F. , Arno, S. F. , and Keane, R. E. (eds.), Whitebark Pine Communities: Ecology and Restoration. Washington, D.C.: Island Press, 89–104.
- Tomback, D. F. , and Resler, L. M. , 2007: Invasive pathogens and alpine treeline: consequences for treeline dynamics. Physical Geography , 28: 397–418.
- Tomback, D. F. , Chipman, K. G. , Resler, L. M. , Smith-McKenna, E. K. , and Smith, C. M. , 2014: Relative abundance and functional role of whitebark pine at treeline in the Northern Rocky Mountains. Arctic, Antarctic, and Alpine Research , 46: 116–126.
- Tomback, D. F. , Resler, L. M. , Keane, R. E. , Pansing, E. R. , Andrade, A. J. , and Wagner, A. C. , 2016: Community structure, biodiversity, and ecosystem services in treeline whitebark pine communities: potential impacts from a non-native pathogen. Forests , 7(1): doi http://dx.doi.org/10.3390/f7010021.
- U.S. Fish and Wildlife Service , 2011: Endangered and threatened wildlife and plants;12-month finding on a petition to list Pinus albicaulis as Endangered or Threatened with critical habitat. Federal Register , v. 76(136): 42631–42654.
- Van Miegroet, H. , Hysell, M. T. , and Johnson, A. D. , 2000: Soil microclimate and chemistry of spruce-fir tree islands in northern Utah. Soil Science Society of America Journal , 64: 1515–1525.
