Abstract
In green biological synthesis, metal nanoparticles are produced by plants or microorganisms. Since it is ecologically friendly, economically viable and sustainable, this method is preferable to other traditional ones. For their continuous groundbreaking advancements and myriad physiochemical and biological benefits, nanotechnologies have influenced various aspects of scientific fields. Metal nanoparticles (MNPs) are the field anchor for their outstanding optical, electrical and chemical capabilities that outperform their regular-sized counterparts. This review discusses the most current biosynthesized metal nanoparticles synthesized by various organisms and their biological applications along with the key elements involved in MNP green synthesis. The review is displayed in a manner that will impart assertiveness, help the researchers to open questions, and highlight many points for conducting future research.
Plain language summary
Metal nanoparticles are small sized particles with diameters ranging from 1 to 100 nm. These particles have favorable characteristics that made them substitute regular sized particles in various industrial fields. They can be prepared chemically or physically or biologically. Biological preparation of metal nanoparticles which is also known as green synthesis involves the use of different microbes and plant species. It's more beneficial than chemical and physical preparative methods, as it's considered cheap and environmentally sustainable. This review will summarize different forms of green synthesis of metal nanoparticles and their application in biomedical field.
Green & conventional synthesis of metal nanoparticles
Nanomedicine has solved many drawbacks in therapeutics and diagnositics.
Chemical and physical metal nanoparticles (MNPs) synthesizes are energy consuming and sometimes associated with accumulation of toxic byproducts.
Green biological MNPs synthesis offers an energy saving and cost effective alternative synthesizing procedures.
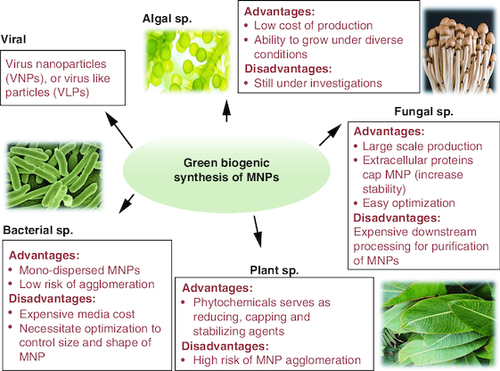
Green biological MNPs synthesis utilizes plants, bacteria, fungi, viruses and algae as MNPs nanofactories.
Green biological metal nanoparticles in biological applications
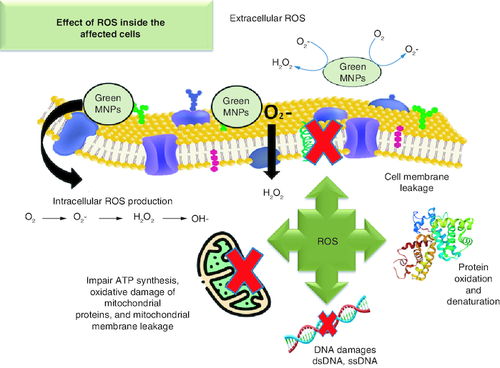
Green biological MNPs acquire anticancer and antimicrobial activities due to reactive oxygen species (ROS) generation.
Green biological MNPs attain desirable morphologies that enhance their biological activities.
AuNPs, AgNPs, ZnO-NPs, CuO-NPs, TiO2-NPs and IONPs derived from different biological sources acquire significant antimicrobial, anticancer and antioxidant properties.
Biogenic SeNPs are promising therapeutic agents when used alone or incombination with irradiation sources.
Biogenic PtNPs retrieved from fungal biomasses acquire promising physiochemical properties for various therapeutic applications.
Factors affecting green biological metal nanoparticles synthesis
Physical factors such as time, pH, temperature, concentration of precursor solution and biological biomasses affect the yield and physiochemical properties of biogenic MNPs.
Optimization of synthesizing technique is essential prior to scaling up production of biogenic MNPs.
Future prespectives in green metal nanoparticles synthesis & applications
Actinomycetes are potiential MNPs nanofactories that need to be investigated.
Statistical optimization techniques and genetic studies may improve optimization techniques and the scaling up of green MNP synthesis.
ADPT, hyperthermia and radiotherapies can be combined with green biogenic MNPs.
Toxological studies for Green MNPs should be properly investigated.
Nanomedicine has revolutionized traditional treatments and diagnostic approaches. It elevated the efficacy of drug delivery, biomarker detection sensitivity, diagnostic imaging and lowered drug-associated side effects [Citation1]. Several nano-based therapies have been already introduced into the market, particularly those targeting cancer and microbial pathogens [Citation2]. It's interesting to note that metal nanoparticles (MNPs) showed advantageous optical, morphological and physiochemical characteristics that opened up new opportunities for nanomedicine, notably in the field of targeted drug delivery systems. They serve as transporters for many medicinal substances, antibodies, nucleic acid fragments etc. Through various forms of bonding, their external surfaces operate as interactive areas that promote conjugation with different forms of bioactive compounds [Citation3,Citation4].
MNPs were created using numerous chemical, physical and biological synthesizing techniques. The chloroauric acid, chloroplatinic acid and silver nitrate are chemical reducing agents that are used in the fabrication of gold, platinum and silver nanoparticles, respectively. The physical synthesis of MNPs incorporates different forms of irradiation (such as gamma rays, UV), combustions (i.e. thermal) and pyrolysis techniques [Citation5]. Although MNP can be produced efficiently and inexpensively through chemical and physical means, current research is focused on creating green, environmentally friendly MNPs by incorporating biological processes [Citation6]. This could be explained by the dangerous byproducts and high energy requirements involved in the chemical synthesis of MNPs. Candidate MNPs for medicinal usage, is constrained by the possibility of contamination from the chemical solvents employed during the fabrication process [Citation7,Citation8]. The chemical solvents residues themselves could be carcinogenic, mutagenic and cytotoxic [Citation9]. Additionally, variations in the physiochemical characteristics and morphologies of chemically and physically manufactured MNPs are linked to various toxicities. Gold nanoparticles (15 nm) were lethal to fibroblasts, epithelial, macrophage and melanoma cell lines [Citation10]. Compared with neutrally charged nanoparticles, the positively charged gold nanoparticles reduced the cell survival of mouse breast cancer 4T1 cells by 50% [Citation11]. This is assigned to the increased electrostatic attraction with negatively charged cell surfaces and enhanced cellular uptake of these particles, which was associated with high levels of oxidative stress and intracellular reactive oxygen species (ROS) [Citation11]. Further, needle and plate shaped nano-sized hydroxyapatite are more hazardous than sphere- and rod-shaped nanoparticles due to their high cell membrane penetrative abilities that compromise cells integrity incurring high levels of cytotoxicity [Citation12]. In similar veins, nano spike titanium dioxide nanoparticles induced potassium efflux and inflammasome activation in macrophages and dendritic cells [Citation13].
Microbial or plant cellular mechanisms that reduce metal ions into their elemental form are used during green biological synthesis of MNPs. In contrast to the intracellular green MNPs synthesis, which involves delivering ions inside the microbial cell where they interact with enzymes to produce nanoparticles, the extracellular MNP synthesis traps the metal ions at external cell surfaces of microorganisms for conversion to its elemental form [Citation14,Citation15]. MNPs created through biological mechanisms are preferable to those created through chemical techniques in several ways [Citation16]. The biological processes use less energy and do not require expensive chemical precursors, making them a better option for the environment. Additionally, biogenic MNPs acquire favorable physiochemical characteristics, greater surface area to volume ratios, regular forms (spherical) and optimal sizes [Citation16,Citation17]. Green MNPs have demonstrated lesser toxicities and adverse effects than chemically induced MNPs. Green silver nanoparticles (AgNPs) elucidated a lower rise in a number of hematological indicators of treated fish when compared with chemically prepared AgNPs [Citation18]. At relatively modest doses (6.25–25 μl), the Ferula asafetida AgNPs were fatal to lung and cervical carcinoma cell lines and down-regulated the expression of PIK3CA and KRAS oncogenes, indicating their efficacy, safety and tolerability [Citation19]. When evaluated at low doses (10 mg/Kg), gold nanoparticles (AuNPs) isolated from Egyptian propolis extract exhibited notable antibacterial, anticancer, and anti-mycobacterial effects while evidencing minor toxicities and organ damage within albino rats [Citation20]. The Cymbopogon citratus iron oxide nanoparticles' reduced inhibitory effects on the motility, growth and reproduction rates of Caenorhabditis elegans worms served as evidence that they were biocompatible and safe for environmental usage [Citation21]. Applications of biological MNPs have included cancer therapy, antimicrobial agents and diagnostics [Citation22,Citation23]. Today, the paradigm change from conventional manufacturing methods to green MNP production is entwined with the concepts of sustainability and energy-saving principles [Citation24].
In this review, different methods of green MNP production through biological green processes will be briefly reviewed before moving on to the optimization techniques. It will also discuss the role of green MNPs as potential anticancer and antimicrobial agents. Future prospects for green MNPs synthesis and applications will be covered in the final section.
Nanoparticles in biological therapies
Numerous industries, including medicine, have experienced major transformations thanks to the introduction of nanotechnology. It enabled the creation of molecules within the submicroscopic ranges by manipulating atoms and taking advantage of naturally existing quantum phenomena within the nanoscale level. The fabricated nanoparticles have advantageous chemical, physical and biological features that made them outperform their bigger, or ‘bulk,’ counterparts' molecules [Citation25]. It is heavily influenced by developments in cellular biology and molecular genetics, and focuses on enhancing physiological processes at the nanoscale. These nanoscale molecules can easily internalize into the cells, and readily integrate with different cellular elements (e.g. receptors, nucleic acids, antibodies, cellular proteins) [Citation26]. Their nanoscale size also enables their simple diffusion across diverse tissues and organs and facile movement within blood circulation, which is vital for targeted administration of therapeutics and reduces their negative effects [Citation27]. Nanotherapeutics have proven superiority over traditional therapies in various aspects (absorption, toxicity and solubility) and been recently implemented in various medical settings, to enhance effectiveness, safety, sensitivity and personalization [Citation28].
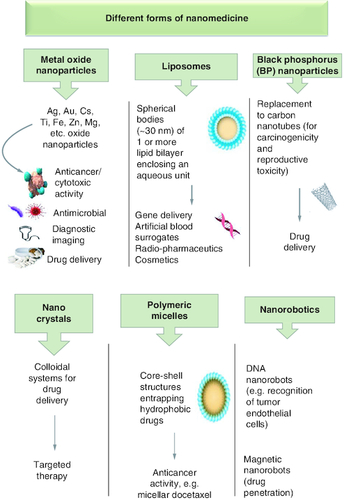
Nanomedicine has been implemented in three major medicinal applications, therapeutics, diagnostics and theragnostic. Therapeutic nanomedicine uses nanoparticles to treat ailments, while in diagnostic nanomedicine, nanoparticles aids in the early detection of diseases via noninvasive techniques or the evaluation of disease biomarkers. Theranostic nanomedicine is a cutting-edge form of nanotechnology that simultaneously performs therapy and diagnosis [Citation28]. There are various forms of nanoparticles that have been used in nanomedicine (). They can be made of inorganic materials such as metal oxide and black phosphorus nanoparticles, and carbon nanotubes, dendrimers, micelles, liposomes, nanocrystals, etc. They had been incorporated in various medicinal applications and proven more efficacious than conventional therapies. For instance, AgNPs of different sizes (1–100 nm) displayed antibacterial properties against pathogenic Gram-positive and -negative bacteria [Citation29]. They also acquire anticancer activity against various cancer lines (e.g. colon cancer cell HCT-116) [Citation30]. Gold nanoparticles (AuNPs) are known for their anticancer activities against various cancer cell lines (e.g. 5637 human bladder carcinoma cells) [Citation31]. Further, liposomal encapsulation of celecoxib reveals a threefold increase in tissue accumulation within BALB/c mice harbouring C26 colon cancer cells, suggesting the high biocompatibility of liposomal nanodrugs [Citation32]. The hazardous carbon nanotubes have been substituted by black phosphorus nanosheets (BPNS) for their high loading capacity and biocompatibility [Citation33].
Benefits & drawbacks of nanoparticles in nanomedicine
Nanomedicine offers several advantages over conventional treatments. Drugs and therapeutic agents can be delivered to specific disease areas, minimizing the chance of adverse effects and maximizing the therapeutic impact. To address ulcerative colitis, a dual-targeted nanoparticle-in-microparticle delivery system known as Asiatic acid-loaded delivery method (AA/CDM-BT-ALG) was developed to prevent early drug release and degradation in the gastrointestinal environment [Citation34]. Additionally, nanotechnology-based imaging techniques allow earlier and more accurate diagnosis of carcinoma metastasis [Citation35]. Nanochains made of iron oxide NPs coupled with cRGD enhanced the magnetic resonance imaging (MRI) signal and successfully detected micro-metastases of relatively low diameter (<0.5 mm) [Citation36]. Technetium-99m (99mTc) was utilized as a radionuclide label for MNPs (AuNPs) during gamma scintigraphy procedure. Within only 30 min of injecting 99mTc-AuNP radiotracer, gamma scintigraphy identified 4T1 breast carcinoma metastasis in in vivo animal models [Citation37].
NPs can improve drug delivery systems, reduces toxicities and cause undesirable side effects. For instance, piperlongumine (PL), an alkaloid that revealed pronounced cytotoxicity against cancer cells, had its water solubility and bioavailability increased by albumin nanoparticles (BSA-NPs) [Citation38]. Nanoparticles can also be used to deliver growth factors and regenerative agents to damaged tissues to help with tissue repair and regeneration. Tantalum nanoparticles (TaNPs) and nanoscale magnesium oxide (MgO) were added to poly caprolactone (PCL)-based periosteum replacement nanofiber to improve its osteogenic and angiogenic capabilities that may heal destroyed periosteum. The PCL/Ta/MgO nanofiber elucidated high cytocompatibility and enhanced osteogenesis and angiogenesis capabilities [Citation39].
Nevertheless, as the use of nanoparticles in medicine is still a subject of research, less is known about their long-term toxicity. For instance, carbon nanotubes are well-known for their extraordinary mechanical and electrical capabilities; however, they resemble asbestos which make them potentially harmful. For their graphitic nature, they are anticipated to be biologically persistent in the body [Citation40]. Coated magnetic nanoparticles including super-paramagnetic iron oxide (SPIONs) may aggregate within the tissues [Citation41]. Studies have also shown that exposure to metal nanoparticles over extended periods damages DNA, which predisposes to cancer and developmental toxicities. They can migrate across the dendrites and axons, inducing oxidative stress and inflammatory responses [Citation42]. Histological examination of mice brain tissues elucidated nanoparticle aggregates, but no obvious toxicity or functional disruptions were reported [Citation43]. Therefore, strict regulatory permissions are necessary prior to usage of MNPs in clinical trails which may delay their industrial usages [Citation44].
Conventional metal nanoparticles synthesis techniques & the introduction of green synthesis technology
Numerous chemical and physical processes are used for MNPs synthesis, but their biological relevance is restricted due to the usage of toxic compounds as reducing agents for precursor materials. According to Li et al., chemical and physical methodologies are complex and may accumulate toxic byproducts that are harmful to both environment and human health. In order to increase the therapeutic applications of MNPs, environmentally friendly and nontoxic manufacturing procedures are perquisite [Citation22]. The green syntheses of MNPs utilize safe, non-toxic and environmentally friendly reagents. Further, the green biogenic MNPs have advantageous physiochemical natures, higher stability and optimal dimensions [Citation45].
Green nano-biotechnology is generally defined as the use of microbes and plants to synthesize nanoparticles via intracellular or extracellular biological processes [Citation46]. It offers alternative pathways for creating stable, eco-friendly metal nanoparticles (green biogenic MNPs) () [Citation47]. The advantages and disadvantages for the commonly used chemical, physical and green approaches MNP synthesizing approaches are summarized in .
Table 1. Common methods for synthesizing green, physical and chemical metal nanoparticles, along with their benefits and drawbacks.
Green metal nanoparticles produced by plants species & their biological applications
Plants are regarded low-maintenance and cost-effective MNPs nanofactories [Citation76]. Various plant components have been used for MNPs production since their extracts are rich with phytochemicals that may act as reducing and stabilizing agents during MNPs production [Citation8,Citation76]. To synthesize nanoparticles through plants, the selected plant portion is thoroughly washed and boiled in distilled water. The precursor solution for the required MNP synthesis is mixed with the plant extract after consecutive steps of squeezing and filtration. Following the addition of MNP precursor solution, the color of the plant extract begins to change which indicates the formation of MNPs [Citation77].
Phytochemicals in plants biomasses act as both reducing and capping agents for metal nanoparticles [Citation78]. Functional moieties found in phytochemicals such as OH, carboxyl and ketones, act as bio-reducers for phyto-MNPs whereas plant proteins and amino acids act as capping agents. Terpenoids, alkaloids, tannins, phenolic acids and flavonoids are powerful reducing and stabilizing agents. Protein and amino acids capped MNPs elucidate favourable physiochemical properties and stability profiles and less prone to aggregation [Citation79].
The isoprene based terpenoids and flavonoids are capable of reducing both silver and gold salt solutions to nanoforms. The eugenol-rich clove extract harnessing hydroxyl groups bound to benzene rings together with potent electron-withdrawing groups at the ortho and para positions, methoxy and allyl groups, successfully reduced AuCl4 and AgNO3 to Ag and Au-NPs. The strong electron withdrawing groups, methoxy and allyl, surrounding the hydroxyl group enhanced the ability of eugenol to release protons making it an extremely potent reducing agent. Further, the release of proton by eugenol is stabilized by a resonating anionic form. The electrons are up-taken by both AuCl4 and AgNO3 generating the reduced forms Au0 and Ag0 [Citation80]. On the other hands, flavonoids rich Ocimum basilicum and Mangifera indica leaf extract emitted protons in their tautomeric state (transformation from enol to keto state) that reduced silver salts and generated AgNPs [Citation81]. Gold nanoparticles were retrieved from extracts of Magnolia kobus and Diopyros kaki following the reduction of aqueous HAuCl4 solution by the ketones, alcohols, amines, aldehydes and carboxylic acids [Citation82]. The hydroxyl group of quercetin in broccoli extract reduced the calcium ion to Ca(OH)2, which is then dried and calcined to CaO nanoparticles [Citation83]. Dihydromyricetin is also thought to be involved in the production of AuNPs through the oxidation of hydroxyl to carbonyl groups [Citation84]. Aqueous ginger/cardamom extracts are now recognized promising phyto-MNPs generators as they are opulent with various capping, reducing/stabilizing and chelating agents (e.g. ginerol, luteolin, quercetin, rutin, etc.). Using iron nitrate, cobalt nitrate and aqueous extracts of ginger and cardamom, crystalline cobalt ferrite of an average size 100 nm can be successfully synthesized [Citation85].
Alteration in plant extract content during phyto-MNPs production impact the morphological and physiochemical characteristics of MNPs. Higher plant extract concentrations yield spherical MNPs of uniform distribution, while lower concentrations yield bigger, poly-dispersed phyto-MNPs. In contrast to hexagonally formed AuNPs of >100 nm at low extract concentrations, Lin et al. reported the appearance of spherical AuNPs with a diameter <15 nm among high Morinda lucida leaf extract concentrations. This is assigned to relatively high concentration of phytochemicals in higher plant extract concentrations necessary for MNPs capping and prevention of aggregation [Citation86]. The chemical composition of the plant varies according to the plant part, wherefore; the section chosen for phytosynthesis will also affect MNPs properties. Appapalam and Panchamoorthy denoted the presence of regular sized AgNPs (<15 nm) in the Aerva lanata leave extract while larger AgNPs were prevalent among A. lanata flower extract [Citation87]. The A. lanata extracts are opulent with flavonoids (rutin, quercetin and kaempferol), polyphenols (gallic acid and ellagic acid) and saponins that were involved in the reduction of AgNO3 [Citation87]. Additionally, plant extract storage conditions also influences size and dispersion of phyto-MNPs. Storage conditions affect the phytochemicals stabilities regulating the bio-reduction and capping of biogenic MNPs which in turn affects the bio-reduction process. Elevating the storage temperatures raises the plant extract aging time and destabilizes the phytochemicals. This is associated with large poly-dispersed phyto-MNPs [Citation79]. summarizes the different types of MNPs synthesized by various plant extracts.
Table 2. Metal nanoparticles synthesized by different plant extracts.
Plant derived metal nanoparticles as anticancer agents
Numerous MNPs fabricated by plants species elucidated anticancer potential through the activation of apoptotic pathways and regression of angiogenesis. The AgNPs incur double or single DNA strand breaks and induce apoptosis. They also halt mitochondrial respiration blocking ATP synthesis and disrupt the activity of the RNA topoisomerase enzyme hindering gene transcription [Citation98]. Further, Houttuynia cordata CuONPs stimulated HeLa cells apoptosis by acting upon the PI3K/Akt signaling pathways [Citation99]. It is also thought that AuNPs are capable of binding to VEGF hindering its integration with VEGFR receptor which reduces angiogenesis. The VEGF/VEGFR-stimulated angiogenesis activates three downstream pathways, the MAPK, JNK/c-Jun and AKT signaling pathways. Downregulation of either VEGFR or VEGF disturbs these downstream pathways, which results in anti-angiogenesis effect [Citation100,Citation101].
MNPs results in the production of reactive oxygen species (ROS), which induces oxidative stress, dsDNA, protein denaturation and p53-mediated intrinsic apoptosis [Citation102]. Biogenic AgNPs upregulate the expression caspases 8, 9 and 3 apoptosis factors in lung cancer cell lines [Citation103]. Subcellular morphological alterations, such as cell shrinkage, clumping, dissociation, rounding and the generation of apoptotic bodies, are also linked to the use of MNPs against cancer cells [Citation104]. MNPs also induce membrane leakage as noted by extensive leakage of lactate dehydrogenase (LDH) from biogenic AgNPs treated cells [Citation105]. Moreover, biogenic AgNPs can change the tumor's metastatic properties and diminish the microvilli growth [Citation106].
Crude peel extract of Garcinia mangostana (GM) was used to fabricate gold–silver core shell nanoparticles (Au–AgNPs) for targeted delivery of protocatechuic acid (PCA). The dried extracts of GM were mixed with precursor salts, tetrachloroauric (III) acid (HAuCl4) and silver nitrate (AgNO3) to produce Au–AgNPs. The GM extract Au-AgNPs were spherical with only few irregularities. The IC50 values for GM Au-NPs and Au-AgNPs against colon carcinoma HCT116 cells were 82.99 and 24.36 g/ml, respectively. Loading the core shell Au-AgNPs with PCA was associated with eminent reduction in IC50 value. Against the HCT116 colon cancer cell line, 15.6 g/ml Au–Ag-PCA-NPs demonstrated effective suppression (70%) while remaining non-lethal to normal colon CCD112 cells denoting their selectivity and superiority [Citation107]. In similar manners, Fe3O4 nanoparticles (Fe3O4-NPs) were generated using the fruit peel extract of Garcinia mangostana. The produced Fe3O4-NPs were lethal to colon cancer HCT116 cells and benign to normal colon CCD112 cells indicating that G. mangostana fruit peel extracts can be utilised as an inexpensive stabilising and capping agent for the manufacture of Fe3O4-NPs [Citation108].
AuNPs and AgNPs can also be synthesized by GM pericarp waste extract. In this green synthesis process, the hydroxyl groups prevalent among GM extract acted as reducing agents for the reduction of gold or silver salts. The GM extracts produced spherical AuNPs (15.37 nm) and asymmetric AgNPs (13–31 nm). Interestingly, only GM-AgNPs were cytotoxic to lung carcinoma A549 and mouse fibroblast NIH3T3 cells lines. GM-AuNPs, on the other hand, may be used as drug-delivery carriers since they are benign to cultivated cell lines [Citation109].
Biofabricated CuO-NPs with anticancer activity were retrieved after utilizing the fruit extract of Prunus nepalensis. The CuO-NPs were crystalline in nature (∼35–50 nm) and were able to upregulate p53, Bax, caspase-3 and caspase-9 genes and down regulate Ras and Myc oncogenes in in vitro cultured MCF-7 cells [Citation110]. In similar manners, bio-fabricated CuO-NPs by Prunus mahaleb Leaf extracts were spherical (20–30 nm) with antioxidant, anticancer, anti-mutagenicity and antimicrobial potential against Gram-positive bacteria [Citation111]. Pimenta dioica biofabricated CuO-NPs were spherical (20–50 nm) with prominent antibacterial activity when tested on S. aureus, S. mutans, P. aeroginosa and E. coli. They also exhibited lethality to in vitro cultured human colorectal cancer cells and anti-diabetic potentiality through in vitro alpha amylase inhibitory assay [Citation112].
The Raphanus sativus var. Longipinnatus biofabricated ZnO-NP exerted anticancer activity against A549 cell lines [Citation113]. The reducing agents found in C. crista seed extract, such as alcohol, phenol and alkyl halides, enabled the biofabrication of crystalline ZnO-NPs conffering significant antibacterial activities against 14 microorganisms, cytotoxicity against HeLa, MCF-7 and regular fibroblast cell lines [Citation114]. Uniform spherical green AgNPs with antioxidant and anticancer capabilities against the alveolar carcinoma cells (A549 cells) can be obtained from Leucus aspera extract [Citation115].
Yuan et al. have retrieved nickel nanoparticles (Ni-NPs) from the extracts of Alhagi maurorum (camelthorn) with anticancer activity against various malignant ovarian carcinoma cells while being benign to normal HUVEC cells [Citation116]. In similar manners, Huang et al. biofabricated spherical Ni-NPs with potent anti-ovarian caner activity from the aqueous extract of Fumaria officinalis [Citation117].
Plant derived antimicrobial metal nanoparticles
One of the most urgent challenges of our decade is the surge of antibiotic resistance. Cassia fistula and Melia azadarach ZnO-NPs (spherical, 3–68 nm) elucidate antibacterial potential against E. coli and S. aureus. Standard antibiotics and biogenic ZnO-NPs were evaluated, and the results revealed that the later had a stronger antibacterial vigor [Citation118]. In similar manners, Cassia alata spherical ZnO-NPs (∼60 nm) elucidated antibacterial activity against E. coli with an IC50 value of 20 μg/ml [Citation119]. The dried leaves extracts of C. auriculata generate spherical biogenic ZnO-NPs (20–30 nm) that repressed B. subtilis, K. pneumoniae, P. aeruginosa and Proteus mirabilis [Citation120]. Sustainable ZnO-NPs purified from the ethanol extract of Maesa indica Roxb. Sweet (ME) aerial parts exhibit potent antiviral activity against coronavirus 229E [Citation121].
Silver has long been recognized for its ability to combat numerous bacterial types. A range of pathogenic bacteria were successfully suppressed by Carissa carandas AgNPs [Citation122]. Stevia leaf and Saudi Arabian desert plant Sisymbrium irio AgNPs suppressed the growth of MDR Gram-negative pathogens [Citation123]. The Chenopodium formosanum-mediated AuNPs fabrication yielded AuNPs (6–8 nm) with bactericidal activity against E. coli and Staphylococcus aureus [Citation124]. Listeria monocytogenes and Serratia marcescens were likewise sensitive to AuNPs purified from Trachyspermum ammi seed extract [Citation125]. Ginkgo biloba AgNPs exerted promising anti-HCov-229E and mild anti-MERS-CoV [Citation126].
The protozoan vector-borne disease, leishmaniasis, affects about 350 million people. Leishmaniasis chemotherapeutics regimens are associated with unfavorable side effects. Biogenic AuNPs retrieved from the aqueous extract of Rhazya stricta decne acquires anti-leishmanial activity against Leishmania tropica (HTD7) amastigotes [Citation127].
Further, all types of in vitro cultured ginseng cell lines reduced silver nitrate into spherical AgNPs. Fusarium graminearum, F. avenaceum, F. poae and F. sporotrichioides are wheat affecting fungal pathogens that were successfully eradicated with AgNPs purified from Panax ginseng's hairy root extracts [Citation128]. In similar veins, the pathogenic bacteria Agrobacterium rhizogenes, Bacillus subtilis, A. tumefaciens and E. coli are repressed by Aristolochia manshuriensis AgNPs [Citation129]. S. aureus and K. pneumoniae are susceptible to Rhazya stricta AgNPs extract [Citation130].
Plant derived antioxidant metal nanoparticles
The extreme oxidative stress can incur oxidative damage to a variety of cell macromolecules, including DNA, proteins and membrane lipids, resulting in degenerative illnesses and ageing [Citation131]. The Harrisonia abyssinica AgNPs [Citation132], Canthium inerme AgNPs and AuNPs [Citation133], Stevia leaf extract NiO-NPs [Citation134], acquire antioxidant potential.
Green metal nanoparticles produced by bacterial species their biological applications
Microbial cells can be MNPs bio-factories. They are more frequently utilized for MNPs production for their environmental abundance and exceptional adaptability. Physical and chemical bacterial growth conditions can be easily altered to achieve utmost MNPs production performances [Citation74,Citation135]. Different bacterial species can be utilized for extracellular and intracellular manufacture of MNPs [Citation74,Citation135].
The mechanism of MNPs synthesis often varies among microorganisms, but in general, the process starts with the microbes entrapping metal ions on its external surface or within, followed by bioreduction to MNPs. Typically, entrapment is mediated by electrostatic attraction forces. The bioreduction process is initiated by different intracellular enzymes (e.g. NADH-dependent reductase enzyme) [Citation136]. The extracellular (EC) production of MNPs is the commonly utilized pathway. In EC biofabrication, microbial cell filtrates containing reducing and stabilizing agents are co-incubated with aqueous metallic salt precursor solutions. When metal ion reduction occurs, a visible color change takes place [Citation137]. In case, intracellular technique (IC) is utilized for biogenic MNPs production, the bacterial biomass itself is co-cultivated with the metal precursor salt solution where ion transport ports mediate the metal reduction to MNPs [Citation138]. Bio-fabricated MNPs can be microscopically identified within the periplasmic space, cell wall and cytoplasm of bacterial cells. Intracellular production of AuNPs was observed among Thermomonospora sp. and Rhodococcus sp. [Citation139].
Furthermore, bacterial supernatants or cultures are capable of capping nanoparticles which halts MNPs aggregation and agglomeration. Capping reduces the MNPs surface energies and maintains their size <100 nm [Citation140]. It's also noted that Gram-positive bacteria have higher silver ion reduction tendencies than the gram-negative bacteria [Citation140]. The Gram-positive Lactobacillus sp. are fast and effective AgNPs generators [Citation141]. Co-cultivation of the alkalified Lactobacillus sp., Pediococcus pentosaceus, Enterococcus faecium and Lactococcus garvieae biomass with diamine silver complex generates AgNPs [Citation140]. enumerates the features and prerequisite manufacturing methods for the most common bacterially produced MNPs.
Table 3. Characteristics and fabrication techniques for the most commonly synthesized bacterial metal nanoparticles.
Bacterial derived antimicrobial metal nanoparticles
MNP interacts with a variety of microbial targets. Generation of ROS can induce DNA breaks, disrupt proteins translation and harm other essential components (). In comparison to traditional antibiotics, nano-antimicrobial agents are both affordable and safe in infectious diseases treatments [Citation74]. MNPs antibacterial activity is heavily influenced by their size and structure. Particles ranging from 1–10 nm exert stronger bactericidal activity than their larger counterparts [Citation162]. Different MNPs with effective antibacterial capabilities were synthesized these included the gold (Au), silver (Ag), titanium (Ti), zinc and magnesium (Mg). Additionally, antibiotic-loaded MNPs enhance the pharmacokinetics of the medicine [Citation163].
Bacterial derived antimicrobial silver nanoparticles
Bacterial cell wall may be damaged by direct contact with AgNPs, resulting in cellular leakage and cell death. AgNPs are more hazardous to bacteria when they are <10 nm [Citation164]. Pits arise when AgNPs (12 nm) build up on the cell wall of E. coli, according to earlier research by Sondi and Salopek-Sondi. These pits lower the E. coli outer membrane stability contributing to cellular leakage and LPS and membrane proteins escape [Citation165]. Since Gram-positive and Gram-negative bacteria have different cell wall architectures, Gram-negative bacteria are typically more vulnerable to Ag+ invasion. This might be attributed to the fact that gram-positive bacteria have thick cell walls with several peptidoglycan layers that operate as a barrier to the entry of Ag+ ions [Citation166,Citation167]. The AgNPs shape and geometry also influences their bactericidal effect. When compared with spherical and rod-shaped AgNP, triangular silver nanoplates exhibit the best biocidal activity [Citation167].
The culture filtrates of endophytic bacterium Bacillus siamensis synthesize AgNPs from aqueous silver nitrate (AgNO3) when incubated at 30 °C for 24 h. The resultant AgNPs were spherical (25–50 nm) with strong bactericidal effect at 20 μg/ml against the plant pathogens Xanthomonas oryzae and Acidovorax oryzae [Citation168]. Silver ion tolerant Bacillus ROM6 synthesizes AgNPs when their bacterial supernatant was co-incubated with AgNO3 at 30 °C for 48 h. The resultant AgNPs are mono-dispersed and spherical (<25 nm). These nanoparticles are bactericidal to S. aureus, E. coli, P. aeruginosa and A. baumannii with minimum inhibitory concentration (MIC) ranging from 1.4 to 5.6 μg/ml. They have also exhibited good stability (∼180 days) [Citation169]. Co-incubation of K. pneumoniae supernatants with AgNO3 at 37 °C for 24 h generates spherical AgNPs with bactericidal activity [Citation170]. In similar manners, spherical mono-dispersed AgNPs (13.8 nm) were retrieved from the culture extracts of Lactobacillus sp. strain LCM5. The retrieved AgNPs are bactericidal to Gram-negative bacteria Chromobacterium violaceum with an inhibition zone 18 ± 0.69 mm diameter [Citation171].
Bacterial derived antimicrobial gold nanoparticles
According to Schrofel et al., AuNPs prevent the binding of tRNA to the ribosomal subunit [Citation172]. They also block unwinding of DNA and mRNA transcription [Citation173]. The bimetallic Ag-Au-NPs purified from Shewanella oneidensis co-cultivated with HAuCl4 and AgNO3 at 37°C are spherical (20 nm) and bactericidal to pathogenic strains, E. coli, P. aeruginosa, E. faecalis and S. aureus. The lowest recorded MIC is 30 μM and at 10 μM, Ag-Au-NPs elucidate anti-biofilm activity [Citation174].
AuNPs can be retrieved from cultural extracts of hyperthermophilic Caldicellulosiruptor changbaiensis co-incubated with HAuCl4.3H2O for 12 h. The AuNPs have a special quality that set them apart from chemically synthesized AuNPs where the smallest AuNPs attained the highest peroxidase activity over a wide pH range. C. changbaiensis AuNPs have impressive antibacterial capabilities and excellent anti-bioflim activities in vitro and in vivo assays [Citation175]. In similar manners, extracellular spherical AuNPs (20.93 nm) with prominent antioxidant activity are retrieved from cell free extracts of the marine bacterium Paracoccus haeundaensis BC74171 [Citation176].
Bacterial derived antibacterial ZnO, & Fe2O3 nanoparticles
The zinc-tolerant Lactobacillus plantarum TA4 cell-biomass (CB) and supernatant (CFS) are potential nanofactories for ZnO-NPs generation. With particles sizes of 291.1 and 191.8 nm, the biogenic ZnO-NPs have flower-like pattern for ZnO-NPs-CFS and irregular shape for ZnO NPs-CB. They are regarded biocompatible on Vero cell line at low doses and antibacterial to pathogenic bacteria [Citation177]. Using an extracellular approach, spherical iron oxide nanoparticles (IONPs) are created by Proteus vulgaris ATCC-29905. Interestingly, P. vulgaris IONPs are bactericidal to methicillin-resistant S. aureus. The IOPNs have also halted the migration of HT-29 cancer cell in scratch assays and were cytotoxic to glioblastoma cell lines [Citation178].
Secondary metabolites of Bacillus cereus HMH1 are also used to extracellularly synthesize spherical IONPs (29.3 nm) [Citation161]. In similar manners, Staphylococcus warneri mediate IONP capping by extracellular polysaccharide. The polysaccharide capped IOPNs elucidates high biocompatibility [Citation179]. Spherical IONPs (15 nm) are also produced by Lactobacillus casei [Citation180]. Streptomyces sp. (SRT12) was employed by Rajeswaran et al. to create quasi-spherical IONPs (65.0–86.7 nm) that exhibit strong antibacterial and antioxidant activities [Citation181].
Bacterial-derived anticancer metal nanoparticles
Gold nanoparticles are biosynthesized by marine bacteria Vibrio alginolyticus. These AuNPs acquire strong inhibitory effects on colon cancer cell proliferation with an IC50 score of 15 μg/ml. The AuNPs induce apoptotic mediated cellular death and exerts free radical scavenging activity that denotes antioxidant potential [Citation182].
Green metal nanoparticles produced by fungal species & their biological applications
For their high metal resistance and ability to bio-accumulate metals intracellularly, Fungi are utilized as reducing and stabilizing agents for the production of MNPs. Fungus may readily grow in large industrial scale and generate MNPs with precise sizes and morphologies. Fungal-mediated MNPs production can either be intracellular or extracellular. The metal precursor is added to the mycelial culture biomass in the case of intracellular synthesis. Post synthesis, the nanoparticles must be extracted using chemical processing, centrifugation and filtration to disrupt the biomass and liberate MNPs. On contrast, extracellular fungal biosynthesis of MNPs necessitates the addition of metal precursor to the aqueous mycelial cultures filtrate that solely contains the fungal biomolecules and secondary metabolites. Extracellular fungal biosynthesis of MNPs is the most advantageous techniques since no special processes are needed to purify MNPs from the cells [Citation75].
The enzymes present within the fungal cell extract can reduce metal ions precursors. Nicotinamide adenine dinucleotide (NADH) and NADH-dependent nitrate reductase are involved in the reduction of Ag+ ions into AgNPs [Citation183]. By employing Fusarium oxysporum to create AgNPs, Durán et al. hypothesized that the nitrate reductase enzyme and anthraquinones are responsible for the reduction of silver ions [Citation184]. Further, quinones are also involved in the creation of fungal nanoparticles [Citation185].
According to Hietzschold et al., NADPH alone mediates fungal-mediated nanoparticle formation, which negates the need for optimal nitrate reductase enzyme conditions. This will allow the synthesis of nanoparticles through a variety of species that don't produce reductase enzyme [Citation186]. It's also crucial to note that extracellular metabolites served as stabilizing and capping agents during MNPs production [Citation187]. summarizes the characteristics and fabrications techniques for the most commonly fungal synthesized MNPs.
Table 4. Characteristics and fabrications techniques for the most commonly fungal synthesized metal nanoparticles.
Fungal-derived antimicrobial metal nanoparticles
The biggest problem with external fracture fixation is pin-tract infections (PTIs) where the inserted pin allows bacterial and biofilm adherence [Citation205]. Most treatments have proven ineffective and pin removal is necessary. In an effort to overcome PTIs, recent developments suggest the usage of anti-infective coatings with antibacterial activity [Citation206]. Liang et al. facilitated an ecofriendly large-scale production of SeNPs using Aureobasidium pullulans. Following cultivation, Aureobasidium pullulans supernatant was mixed with sodium selenite under shaking condition at 25 °C for 72 h. prior to SeNPs harvest. Granular SeNPs (50–70 nm) were obtained and doped on silver coating to create silver-selenium (Ag-Se) coatings. The bactericidal activity of Ag-Se-NPs coatings on S. aureus and E. coli were comparable to commercial AgNPs and Ag-PTFE-coated surfaces. They were also corrosion resistant [Citation207].
The mycelia extract of Aspergillus melleus can fabricate biogenic AgNPs with eminent anticancer and antibacterial vigor. On MG-63 cells, A. melleus AgNPs have significant cytotoxic capability. S. aureus and E. coli were also sensitive to A. melleus AgNPs' [Citation208]. The mycelia extract of a soil derived Aspergillus niger can synthesize ZnO-NP with promising anticancer and antibacterial activity. The purified ZnO-NPs are rod shaped (11–17 nm) with bactericidal activity against S. aureus, Salmonella enterica, Bacillus cereus, and E. coli, Aspergillus niger. Additionally, Vancomycin and A. niger ZnO-NPs demonstrated strong synergistic antibacterial vigor against tested organisms. They also acquire potent anticancer activity against A549 cell line with an IC50 score of 50 μg/ml [Citation209]. A. niger ZnO-NPs also exerts strong antioxidant and cytotoxicitc effects on human hepatocellular carcinoma cells (HepG2) [Citation210].
Due to their distinctive physico-chemical (catalytic, magnetic, and optical) and biological (antimicrobial, antioxidant and anticancer) capabilities, platinum nanoparticles (PtNPs) are of tremendous interest in a variety of technical and biomedical sectors. Bio-fabrication of PtNPs by F. oxysporum is studied by Riddin et al. to identify optimal condition for biosynthesis. Intracellular, extracellularly as well as hyphael surface synthesizes generated different shapes of PtNPs (hexagons, pentagons, circles, squares and rectangles with average sizes 10–100 nm). However, only the extracellular production was found to be statistically significant [Citation211]. Using F. oxysporum filtrate, Gupta and Chundawat produced face-centered cubic PtNPs (25 nm) with antibacterial and photocatalytic activities [Citation212]. Highly diffusible non-aggregating PtNPs (5–40 nm) are also achievable from Penicillium chrysogenum culture filtrate [Citation213]. Neurospora crassa is another ascomycota that can synthesis platinum nanoparticles with different sizes and shapes. Extracellular PtNPs (4–35 nm) and spherical nanoaggregates (20–110 nm) are formed when mycelial biomass was incubated with H2PtCl6. Further, round single crystal nanoagglomerates (17 to 76 nm) and individual single crystals (2–3 nm) can also be produced using mycelial extract of Neurospora crassa [Citation214]. Further, the phytopathogenic fungus Alternaria alternata's culture filtrate has been used to produce nanoplatinum of quasi-spherical, rectangular, tetrahedral, hexagonal and polygonal morphologies (∼135 nm) [Citation215].
When combined with ciprofloxacin, Candida albicans silver nanoparticles produced by Bhat et al. exerted effective antibacterial vigor against various bacterial pathogens. C. albicans AgNPs are bactericidal and synergistic effects were observed when combined with ciprofloxacin [Citation216]. Butyl acrylate caped AgNPs purified from culture extracts of Alternaria alternate were doped in cotton to generate nanoparticle treated cotton that can combat E. coli and S. aureus. The cottons impregnated with biogenic AgNPs inhibit bacterial growth at different concentrations [Citation217]. The oropharyngeal Candida glabrata isolates synthesize AgNPs with exquisite bactericidal and fungicidal activity [Citation218].
Biogenic AgNPs have also demonstrated antiviral properties. Using Alternaria sp., Fusarium oxysporum, Curvularia sp., Chaetomium indicum, and Phoma sp., Gaikwad et al. created silver nanoparticles that inhibited the reproduction of HSV-1, HSV-2 and HPIV-3 in cell cultures. The most efficient and least harmful nanoparticles were generated by F. oxysporum, Curvularia sp. and C. indicum [Citation219]. When tested on the larvae and pupae of the dengue vector mosquito Aedes aegypti, Sundaravadivelan and Padmanabhan biogenic AgNPs purified from the filtrate of Trichoderma harzianum exerted dose dependent larvicidal effects [Citation220]. Another study by Banu and Balasubramanian examined the effectiveness of AgNPs retrieved from the entomopathogenic fungus Isaria fumosorosea in controlling the mosquito species Culex quinquefasciatus and A. aegypti. Isaria fumosorosea AgNPs demonstrated larvicidal effects [Citation221].
Fungal-derived anticancer metal nanoparticles
Intracellular fabrication of AgNPs facilitated by co-cultivation of A. niger biomass with AgNO3 yields capped spherical nanoparticles with anticancer activities. These AgNPs suppressed 70.2% of the HeLa cell growth [Citation222]. Nassar et al. bio-fabricated SeNPs from the culture filtrates of endophytic Penicillium verhagenii co-incubated with metal salt precursor (Na2SO3) at 25 °C overnight. The purified SeNPs were spherical and crystalline in nature. Interestingly, the biogenic P. verhagenii SeNPs elucidated antimicrobial vigor against various Gram negative and Gram-positive strains with MIC scores ranging from 12.5–100 μg/ml. It was also noted that P. verhagenii SeNPs acquired a dose dependent antioxidant DPPH-scavenging capability. Moreover, the P. verhagenii SeNPs were lethal to in vitro cultured PC3 and MCF7 cancer cell lines with IC50 values of 225 and 283 μg/ml, respectively, and biocompatible with normal WI38 and Vero cell lines. They were also larvicidal to Aedes albopictus instar larvae [Citation223].
Fouda et al. utilized the endophytic fungal species Penicillium crustosum to achieve spherically crystalline SeNPs. These SeNPs elucidated antimicrobial activities while experimenting with different bacterial strains that were enhanced with light irradiation. It also acquired anticancer activity when incubated with in vitro cultured T47D and HepG2 cell lines. Their anticancer activity was also enhanced following exposure to light where the cell viability % scored in dark conditions was reduced from 95 and 93% to 84 and 46%, respectively. Impact of light irradiation was also noted when the IC50 scores were lowered from 109 to 70 μg/ml for the T47D and 19 to 4.8 μg/ml for the HepG2 post light irradiation. Irradiation also elevated SeNPs photoluminescence activity adding a dye degrading ability to its activity profile. The Penicillium crustosum SeNPs elucidated exceptional durability and reusability [Citation224]. The filtered biomass of marine Fungi, Hamigera Pallida, is also utilized to synthesize biogenic AgNPs from AgNO3. The resultant AgNPs were spherical (3–16 nm) with significant antibacterial vigor against different bacterial strains and potent antioxidant capabilities. With an IC50 value of 66 μg/ml, the AgNPs are cytotoxic to human breast cancer (MCF-7) cell line [Citation225].
The antibacterial and anticancer potential of AgNPs produced by Fusarium oxysporum was also assessed by Husseiny et al. The nanoparticles suppressed tumor cell line (MCF-7), E. coli, and S. aureus. According to Husseiny et al., the effect was attributed to the silver nanoparticles' involvement in the disruption of the mitochondrial respiratory chain, which produced reactive oxygen species and prevented the synthesis of ATP [Citation226].
Green metal nanoparticles produced by viruses
Virus can be used in bio-reduction of metal ions to MNPs and can create 3D nanomaterials for targeted medication delivery [Citation227]. Viral modification allows the deposition of materials inside the capsid core of viruses. Viruses can also produce nano-conjugates and nano-composites. For instance, AuNPs and AgNPs (32 nm) are fabricated by the phytopathogenic virus Squash leaf curl China virus (SLCCNV). These eco-friendly SLCCNV-metallic-hybrid nanoparticles are produced within 5 min period only. The nanoparticles elucidated remarkable electrical conductivity which made them eligible for several biomedical applications [Citation228].
Small amounts of tobacco mosaic virus (TMV) and bovine papillomavirus (BPV) were added to different kinds of plant extracts as additives to enhance the production of nanoparticles. A notable rise in the metal ion reduction capacity of aqueous plant extract was observed post addition of these viruses [Citation24]. Genetically modified TMV, MBP-TMV, can produce distinct gold nanoparticles (10–40 nm) when mixed with 3 mM potassium tetrachloroaurate. The MBA-TMV-AuNPs elucidated high stability and crystalline structure [Citation229]. To construct gold nanoparticles with interparticle spacing, several variants of the Cowpea Mosaic Virus (CPMV) have been employed as scaffolds for the attachment of 2 and 5 nm AuNPs through the creation of gold–sulfur bonds at certain sites on the virus. The generated AuNPs suggest that CPMV mutants can serve as nanoscale scaffolds [Citation230]. In chemotherapy and photothermal therapy, viruses were utilized to transport medications and control release. Red Clover Necrotic Mosaic Virus (RCNMV) was used by Cao et al. to create nanoparticles that release doxorubicin during chemotherapy [Citation231].
Green metal nanoparticles produced by algal species & their biological applications
Phycosynthesis of MNPs is mediated by algal species. Algae are polyphyletic, autotrophic and photosynthetic eukaryotic creatures found in marine, and freshwater environments and on damp rocks. They are classed according to their morphological traits into microalgae and macroalgae. MNPs phycosynthesis is facilitated by the high abundance of polyunsaturated fatty acids, chlorophyll, carotenoids, tocopherols, polyphenols and phycobilins that act as stabilizing/capping and reducing agents for nanoparticles [Citation232]. Extracellular MNPs phycosynthesis is initiated by mixing metal ion precursor solution with the boiled algal extract until the color changes [Citation233]. It's the best method for the generation of MNPs by algal biomasses. Intracellular MNP production by algae involves the usage of the live algal biomass itself (after collection and washing) followed by sonication to harvest the intracellular MNPs [Citation234]. Here, the NADPH dependent reductase serves as reducing agent. Intracellular synthesis of AuNPs is initiated by the co-cultivation of Ulva intestinalis and Rhizoclonium fontinale with chloroauric acid at 20 °C for 72 h until the green color turned purple [Citation232,Citation235]. Color changes observed in Klebsormidium flaccidum in a silica gel suspension denotes the reduction of gold precursor which is confirmed by presence of dark-colored patches in thylakoid membrane TEM examination [Citation232,Citation233,Citation235].
Recently, ultrasonic irradiation-assisted synthesis (UIAS) of MNPs by Cyanobacterium sp. elucidated accelerated chemical reactions rates and eased MNPs extraction processes. This is attributed to the cavitation collapses induced by ultrasonic waves causing severe local heating (5000 °C) and high pressures (2000 °A) within the liquid reaction mixture that markedly shortened MNP synthesis reaction time [Citation236]. Euglenoids and diatoms, as well as other types of algae like Phaeophyceae, Cyanophyceae and Rhodophyceae, have been used in the fabrication of metallic NPs including palladium, gold, iron and silver [Citation233]. The brown algae species Turbinaria conoides and Padina tetrastromaticaare seaweed are AgNPs producers. The produced AgNPs are spherical in shape (96 nm) and bactericidal [Citation237]. The spherical poly-dispersed AuNPs are also fabricated by Sargassum myriocystum, Sargassum wightii, Cystoseira baccata, Stereospermum marginatum, Ecklonia cava and Padina gymnospora [Citation238].
The Lemanea fluviatilis, marine red algae, are potential producers of AuNPs using chloroauric acid [Citation233]. Red algae Gracilaria edulis are capable of producing bimetallic Ag–Au nanoparticles with powerful anti-cancer activity against human breast cancer cells utilizing various molar ratios of HAuCl4 and AgNO3 [Citation239]. The blue algae, Spirulina platensis, secrete both AgNPs and Au-NPs of spherical, octahedral and cubic shapes utilizing different proteins and peptides as reducing agents [Citation240]. Chlamydomonas reinhardtii promotes the synthesis of cadmium sulphide bimetallic nanoparticles (CdSNPs), which have numerous uses in fields including biosensors, LEDs, and photo-catalysis [Citation241]. The green macroalgae are capable of producing metallic nanoparticles. One of the most advantageous types of green macroalgae, Ulva fasciata, was used to produce ZnO-NPs with antibacterial qualities [Citation242]. In a different investigation, octahedral ZnO-NPs and spherical AgNPs were produced using Gracilaria edulis [Citation243].
Algal-derived antimicrobial metal nanoparticles
Padina tetrastromatica AgNPs are bactericidal to B. subtilis, K. planticola and P. aeruginosa [Citation244]. Caulerpa serrulata AgNPs acquire stable and colloidal morphologies with remarkable antimicrobial activity [Citation245]. In similar vein, AgNPs retrieved from aqueous extract of Pithophora oedogonia are bactericidal to a number of bacterial pathogens, including B. subtilis, Micrococcus luteus, V. cholerae, S. aureus and E. coli [Citation246]. The Stoechospermum marginatum derived AuNPs elucidate superior antibacterial activity against E. faecalis when compared with the standard tetracycline antibiotic [Citation247]. The Dictyosphaerium sp. and Pectinodesmus sp. AgNPs are also anti-bacterial to 14 bacterial species, antifungal to C. albicans, and cytotoxic toward hepatocellular carcinoma (HepG2), breast cancer (MCF7) and Newcastle disease virus (NDV) in Huh7-infected cells [Citation248]. Additionally, Venkatesan et al. showed that the brown seaweed Ecklonia cava extracts utilized for biogenic fabrication of AgNPs have significant anti-bacterial activity against Escherichia coli and Staphylococcus aureus and antioxidant apoptotic activity toward in vitro cultured human cervical carcinoma (HeLa) cells [Citation249].
Factor affecting green synthesis of metal nanoparticles
Adjustment of several physical conditions including metal precursor concentration, pH, temperature, culture biomass and reaction time is crucial to optimize the production of MNPs through biological processes. Variation in the pH during phyto-synthesis of MNPs is associated with variation in MNPs particle size. Acidic pH is associated with higher yield of large sized MNPs while smaller MNPs particle sizes are observed in alkaline medium pH [Citation250]. Biogenic AgNPs tend to be spherically shaped; however, at pH 5 and 11, biogenic AgNPs tend to acquire triangular and hexagonal forms. Further, AgNPs retrieved from alkaline and neutral medium tends to be more stable as raising the pH increases the number of negative charges on their external surfaces. Negatively charged AgNPs have reduced tendencies to aggregation due to electrostatic repulsion [Citation251]. Altering pH (2 to 10) during the AgNPs synthesis by brown algae, Sargassum angustifolium, was associated with stable uniformly sized AgNPs at alkaline pHs (pH 10) [Citation252].
Different reaction times are associated with differences in MNPs yield and particle size. The extracts of Ananas comosus generate MNPs within 2 min but optimal spherical MNPs (12 nm) are observed within 5 min of bioreduction [Citation253]. Furthermore, Aboelfetoh et al. (2017) demonstrated that the increasing the contact time between Caulerpa serrulata and silver ion (Ag+) at room temperature results in rapid synthesis of non-agglomerated AgNPs [Citation235]. The growth kinetics of AgNPs generated by aqueous extract of Azadirachta indica (Neem tree) leaves were studied by Prathna et al. [Citation254]. The reaction was carried out for 4 h while being examined using UV-Vis (ultraviolet-visible) spectrometry. Within 2 h, the first peak of the UV-Vis spectrum became visible, indicating synthesis of MNPs within the size range 10 to 35 nm. Neem AgNPs were spherically shaped (∼20 nm) after 2 h. The size of Neem AgNPs rose to 36.6 nm when the reaction time reached 4 h [Citation254]. Further, the reaction time affects the aggregation rate and magnetic properties of MNPs. It was reported by Karade et al. that increasing the reaction time was associated with larger MNPs and enhancement in saturation magnetization [Citation255].
Incubation temperature also affects synthesis rate and morphologies of biogenic MNPs. Higher MNP production rates were observed upon increasing temperatures [Citation256]. Fast reduction of biogenic AgNPs by Vitex agnus-castus leaves extract took place at 40 °C while higher AgNPs yield occurred at higher temperatures (60–80°C) [Citation257]. Varying the concentration of metal ion precursor and biological biomasses also affect the overall performance and yield of MNPs. The concentration of metal ion salt precursor plays a significant role in determining the size of biogenic MNPs, since higher concentrations are associated with MNPs agglomerations and aggregation [Citation258]. Bio-fabrication of MNPs is also highly influenced by concentration of reducing agents in the biological biomasses. For instance, production of AuNPs by the Aloe vera leaf extracts was highly dependent on the concentration of multiple twinned particles (MTPs) [Citation259]. Furthermore, leveling up extract concentrations of marine green macroalga Caulerpa serrulata to a solution of constant concentration of silver nitrate at room temperature lowered the average size of AgNPs [Citation235].
The choice of the organism and its storage condition are also crucial to consider during green biogenic synthesis of MNPs. It's crucial to determine the right inoculum size and whether adding a biocatalyst to the culture filtrates can help with the bio-reduction of metal ions. Additionally, using complete microbial or plant cells is preferred since they have their own enzymes or co-factors (such NADH, NADPH or FAD) that can operate as biocatalysts [Citation234].
Challenges & optimizations of green metal nanoparticles
There are some obstacles that stand in the way of industrial and commercial application of green biological synthesis of MNPs. First, maintenance of the size, shape regularity and dispersion of green synthesized MNPs is essential and requires optimization. It is well established that the behavior and properties of MNPs are significantly influenced by the particle size and dispersity (size distribution). A monodispersed population of MNPs is required for commercial applications as opposed to the poly-dispersed MNPs [Citation260]. It's also known that dispersion level and size of MNPs affect the overall biomedical activity. For instance, anticancer efficacy of 50 nm silica NPs-drug conjugate was higher than that of 20 and 200 nm [Citation261]. Smaller-sized AgNPs were more successful at inducing apoptosis than larger-sized AgNPs [Citation262]. Mono-dispersed MNPs can be produced using a variety of optimization strategies, including adjusting the pH, temperature, precursor salt content and culture growth conditions [Citation24]. summarizes some of the optimization strategies applied to maintain regularity of green synthesized nanoparticles.
Table 5. Examples of optimization strategies implemented to enhance characteristics of green metal nanoparticles.
Future perspective in the green synthesis of metal nanoparticles
Terpenoids, alkaloids, polyketides and flavones are abundant in marine and soil actinomycetes. Marine actinomycetes secondary metabolites share chemical similarities with a number of phytochemicals that various algal and plant species used during the green MNPs synthesis [Citation270]. Actinomycetes are the least studied green nano-factories. Therefore, marine actinomycetes may be introduced as new generation in green MNPs nanofactories [Citation271]. Khalil et al. utilized the silver tolerant Nocardiopsis dasonvillei KY772427, for the fabrication of biogenic AgNPs. Interestingly, Nocardia sp. AgNPs are mono-dispersed, spherical and acquired negative surface charge. They exhibited synergistic effects with antimicrobial agents (peracillin-tazobactam and fluconazole) against pathogenic Gram-positive (S. aureus), Gram-negative bacteria (P. aeruginosa), and fungal strains (A. niger, C. albicans). The Nocardia AgNPs also acquired potent insecticidal activities against Macrosiphum rosae, antioxidant and anticancer activities against CaCO-2 cells [Citation272]. The culture filtrates of actinomycete strains retrieved from sea sponge Crella cyathophora successfully generated AgNPs with significant antibacterial activity against P. aeruginosa and E. cloacae [Citation273].
In a similar vein, Abd El-Ghany et al. isolated biogenic AgNPs from rare silver resistant actinomycete strains, Glutamicibacter nicotianae SNPRA1 and Leucobacter aridicollis SNPRA2. The SNPRA1 and SNPRA2 AgNPs were fungicidal to A. flavus and Aspergillus ochraceus with acceptable biocompatibility on human skin fibroblast cells suggesting their safety and tolerability [Citation274]. The Streptomyces sp. SSUT88A AgNPs elucidate potent antibacterial vigor that outperformed commercially available AgNPs against multidrug resistant Acinetobacter baumannii. It was also effective against other Gram-negative species, including the E. coli, K. pneumoniae and P. aeruginosa [Citation275]. Zhao et al. utilized the culture supernatants of marine endophytic actinomycetes CKV1 as reducing and stabilizing agents for copper sulfate (CuSO4.5H20) to generate green CuO-NPs. The CKV1 CuO-NPs were spherical (∼20 nm) and elucidated eminent antibacterial vigor against biofilm producing Gram-negative E. coli and P. mirabilis. The CKV1 CuO-NPs are also lethal to in vitro cultured A549 lung carcinoma cell lines [Citation276].
Statistical optimizations utilizing response surface methodology (RSM) and multifactorial design are supportive methods that have been newly undertaken for the production optimization of various secondary antibacterial metabolites [Citation277,Citation278], antifungal metabolites [Citation279] and biosurfactants [Citation280,Citation281]. Therefore, this approach is promising during the optimization procedures of green synthesized nanoparticles.
Introduction of whole genome analyses during green MNP synthesis is essential. The metabolic characteristics of synthesizing strains will be better understood with the aid of thorough genomic investigations, which will also assist the identification of enzymes/metabolites involved in metal ion reduction and ease the scaling up of green MNP synthesis [Citation282]. Using a bacterial cell-free extract (CFE) facilitate easy purification of MNPs, much easier to standardize, optimize and replicate during large scale productions. The CFEs are also opulent with proteins, amino acids, peptides and enzymes that can act as the phytochemicals, serving as capping, reducing and stabilizing agents [Citation283].
Future perspective for green metal nanoparticles in biological therapies
Biogenic SeNP nanoparticles that showed enhanced activities post exposure to light irradiations can be successfully coupled with antimicrobial photodynamic therapy (ADPT). The ADPT is a promising non-antibiotic based method that's utilized to combat MDR infectious diseases. Briefly, ADPT depends on a photosensitizer (PS), a nontoxic dye, along with a safe visible light. When the PS is irradiated, ROS are released and several cellular components are irreversibly damaged. Due to the multi-target nature of ROS species, the chances of developing microbial resistance against ADPT are almost absent [Citation284]. Previous reports denoted the effectiveness of combining several MNPs with ADPT. TiO2 nanoparticles are considered photocatalytic antimicrobial agents and have been used in various coatings. Upon irradiating TiO2 coated surfaces with UV rays at 385 nm, free radicals are released initiating bacterial lysis. UV irradiated TiO2-NPs solutions might be an effective strategy to get rid of viruses including hepatitis B, influenza subtypes, poliovirus and coronavirus. Fungi such as A. niger, C. albicans and Cryptosporidium parvum can be effectively eliminated by UV irradiated TiO2 [Citation285]. Since Fouda et al. had previously reported that UV irradiation of biogenic SeNP enhanced both its cytotoxic and antibacterial activities, conjugating these nanoparticles with ADPT would be beneficial to combat MDR bacteria [Citation224].
For their intriguing physiochemical and optical characteristics, green MNPs (Fe, Co, Ni, Ag, Au, Mn) can replace chemically synthesized MNPs in cancer combination therapies in particular hyperthermia and radiotherapy. The most important variables that affect hyperthermic intensity of MNPs are particle size, NP dispersity and magnetic moment [Citation286]. It has been previously reported that gold nanoshells improved the efficacy of thermal therapy in combination with radiotherapy for their unique optical, electrical, and drug loading capacities [Citation287]. The cobalt ferrites (CoFe2O4), manganese ferrite (MnFe2O4), nickel ferrite (NiFe2O4), and lithium ferrite (Li0.5Fe2.5O4), provide the foundation for another set of MNPs used in hyperthermia treatment for their quite high heating capability (1300–1600 W/g) [Citation288].
Additionally, the toxicities of green MNPs should be taken into consideration. In order to fully comprehend their mode of action and associated side effects, more in vivo studies must be carried out. Kobylinska et al. green FeCl3/FeSO4/CoCl2 mixture purified from the haiy root extract of Artemisia tilesii exhibited supermagnetic properties with no phyto-toxicities against Cichorium intybus and Lactuca sativa plants which are known for their distinguishably high and active seed germination rates. The seeds are thought to be more sensitive to metal toxicities and the study denotes that green oxide MNPs have no effect of germination rates of seeds suggesting their environmental safety [Citation289].
Conclusion
Incorporating MNPs into various biological processes has become standard practice that aim to improve therapeutic outcomes and overcome some of the drawbacks associated with many conventional therapies. To overcome the drawbacks of conventional chemical and physical MNPs synthesis, a new area of nanotechnology utilizes green synthesis to create MNPs have emerged. Since it avoids various hazardous elements associated with both chemically and physically synthesizing procedures, green synthesis is extremely advantageous for nanomedicine. It is efficient, eco-friendly and simple to execute. By utilizing a variety of species and carrying out the synthesis under varying temperature, pH, biomass, and metal precursor concentration conditions, it is feasible to produce MNPs with favorable physicochemical and biological features. Numerous biogenic MNPs have shown a variety of biological activities in diverse in vitro investigations, nevertheless these MNPs face a number of challenges when it comes to maximizing their synthesis and figuring out their mode of action and associated side effects. Overall, green manufacturing of metal/metal oxide nanoparticles supports a variety of biological therapies, including the control of antioxidant, antifungal and antibacterial activities.
Author contributions
Conceptualization, RN Morgan & KM Aboshanab, formal analysis, RN Morgan and KM Aboshanab, writing – original draft preparation RN Morgan; writing – review and editing, KM Aboshanab, supervision, KM Aboshanab, All authors read and approved the manuscript.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Acknowledgments
The authors express their appreciation to the National Centre for Radiation Research and Technology (NCRRT), Drug Radiation Research Department, Egyptian Atomic Energy Authority (EAEA), and Department of Microbiology and Immunology, Faculty of Pharmacy, Ain Shams University Cairo, Egypt for providing any type of facility and support whenever needed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Haleem A, Javaid M, Singh RP, Rab S, Suman R. Applications of nanotechnology in medical field: a brief review. Global Health J. 7(2), 70–77 (2023).
- Shan X, Gong X, Li J, Wen J, Li Y, Zhang Z. Current approaches of nanomedicines in the market and various stage of clinical translation. Acta Pharm Sin B. 12(7), 3028–3048 (2022).
- Chandrakala V, Aruna V, Angajala G. Review on metal nanoparticles as nanocarriers: current challenges and perspectives in drug delivery systems. Emergent Mater. 5(6), 1593–1615 (2022).
- Ovais M, Zia N, Khalil AT, Ayaz M, Khalil A, Ahmad I. Nanoantibiotics: recent developments and future prospects. Front. Clin. Drug Res. Anti Infect. 5, 158 (2019).
- Karunakaran H, Krithikadatta J, Doble M. Local and systemic adverse effects of nanoparticles incorporated in dental materials- a critical review. Saudi Dent. J. doi: 10.1016/j.sdentj.2023.08.013 (2023).
- Patel SK, Choi SH, Kang YC, Lee J-K. Large-scale aerosol-assisted synthesis of biofriendly Fe2O3 yolk–shell particles: a promising support for enzyme immobilization. Nanoscale 8(12), 6728–6738 (2016).
- Mohamed HEA, Afridi S, Khalil AT, Ali M, Zohra T, Salman M et al. Bio-redox potential of Hyphaene thebaica in bio-fabrication of ultrafine maghemite phase iron oxide nanoparticles (Fe2O3 NPs) for therapeutic applications. Mater Sci. Eng. C Mater. Biol. Appl. 112, 110890 (2020).
- Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 13(10), 2638–2650 (2011).
- Nath D, Banerjee P. Green nanotechnology - a new hope for medical biology. Environ. Toxicol. Pharmacol. 36(3), 997–1014 (2013).
- Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U et al. Size-dependent cytotoxicity of gold nanoparticles. Small. 3(11), 1941–1949 (2007).
- Sukhanova A, Bozrova S, Sokolov P, Berestovoy M, Karaulov A, Nabiev I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 713(1), 44 (2018).
- Zhao X, Ng S, Heng BC, Guo J, Ma L, Tan TT et al. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch. Toxicol. 87(6), 1037–1052 (2013).
- Wang J, Chen HJ, Hang T, Yu Y, Liu G, He G et al. Physical activation of innate immunity by spiky particles. Nat. Nanotechnol. 13(11), 1078–1086 (2018).
- Zhang X, Yan S, Tyagi RD, Surampalli RY. Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere. 82(4), 489–494 (2011).
- Ovais M, Khalil AT, Ayaz M, Ahmad I. Biosynthesized metallic nanoparticles as emerging cancer theranostics agents. Nanotheranostics: Applications and Limitations. Springer International Publishing, Cham 229–244 (2019).
- Chouke PB, Shrirame T, Potbhare AK, Mondal A, Chaudhary AR, Mondal S et al. Bioinspired metal/metal oxide nanoparticles: a road map to potential applications. Mater Today Adv. 16, 100314 (2022).
- Bhattacharya R, Mukherjee P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 60(11), 1289–1306 (2008).
- Younas W, Khan FU, Zaman M, Lin D, Zuberi A, Wang Y. Toxicity of synthesized silver nanoparticles in a widespread fish: a comparison between green and chemical. Sci. Total Environ. 845, 157366 (2022).
- Bin Saeed HA, Daghestani MH, Ambreen K, Daghestani MH, Al-Zahrani SA, Alobaid H et al. Low dose of green synthesized silver nanoparticles is sufficient to cause strong cytotoxicity via its cytotoxic efficiency and modulatory effects on the expression of pik3ca and kras oncogenes, in lung and cervical cancer cells. J. Clust. Sci. 34(5), 2471–2485 (2023).
- Aljohani FS, Hamed MT, Bakr BA, Shahin YH, Abu-Serie MM, Awaad AK et al. In vivo bio-distribution and acute toxicity evaluation of greenly synthesized ultra-small gold nanoparticles with different biological activities. Sci. Rep. 12(1), 6269 (2022).
- Patiño-Ruiz D, Sánchez-Botero L, Tejeda-Benitez L, Hinestroza J, Herrera A. Green synthesis of iron oxide nanoparticles using Cymbopogon citratus extract and sodium carbonate salt: nanotoxicological considerations for potential environmental applications. Environ. Nanotechnol. Monit. Manag. 14, 100377 (2020).
- Li X, Xu H, Chen Z-S, Chen G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011, 270974 (2011).
- Borehalli Mayegowda S, Roy A, N G M, Pandit S, Alghamdi S, Almehmadi M et al. Eco-friendly synthesized nanoparticles as antimicrobial agents: an updated review. Front Cell Infect Microbiol. 13, 1224778 (2023).
- Khalil AT, Ovais M, Iqbal J, Ali A, Ayaz M, Abbas M et al. Microbes-mediated synthesis strategies of metal nanoparticles and their potential role in cancer therapeutics. Semin. Cancer Biol. 86, 693–705 (2022).
- Ventola CL. The nanomedicine revolution: part 1: emerging concepts. P T. 37(9), 512–525 (2012).
- Patra CR, Bhattacharya R, Mukhopadhyay D, Mukherjee P. Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Adv Drug Deliv Rev. 62(3), 346–361 (2010).
- Nikolova MP, Joshi PB, Chavali MS. Updates on biogenic metallic and metal oxide nanoparticles: therapy, drug delivery and cytotoxicity. Pharmaceutics 15(6), 1650 (2023).
- Kotcherlakota R, Das S, Patra CR. Chapter 16 - Therapeutic applications of green-synthesized silver nanoparticles. Green Synthesis, Characterization and Applications of Nanoparticles. Elsevier, NY, USA, 389–428 (2019).
- Ahmad T, Wani IA, Manzoor N, Ahmed J, Asiri AM. Biosynthesis, structural characterization and antimicrobial activity of gold and silver nanoparticles. Colloids Surf. B. Biointerfaces 107, 227–234 (2013).
- Acharya D, Satapathy S, Somu P, Parida UK, Mishra G. Apoptotic effect and anticancer activity of biosynthesized silver nanoparticles from Marine Algae Chaetomorpha linum extract against human colon cancer cell HCT-116. Biol. Trace Elem. Res. 199(5), 1812–1822 (2021).
- Daei S, Ziamajidi N, Abbasalipourkabir R, Khanaki K, Bahreini F. Anticancer effects of gold nanoparticles by inducing apoptosis in bladder cancer 5637 cells. Biol. Trace Elem. Res. 200(6), 2673–2683 (2022).
- Matbou Riahi M, Sahebkar A, Sadri K, Nikoofal-Sahlabadi S, Jaafari MR. Stable and sustained release liposomal formulations of celecoxib: in vitro and in vivo anti-tumor evaluation. Int. J. Pharm. 540(1–2), 89–97 (2018).
- Yang X, Wang D, Zhu J, Xue L, Ou C, Wang W et al. Functional black phosphorus nanosheets for mitochondria-targeting photothermal/photodynamic synergistic cancer therapy. Chem. Sci. 10(13), 3779–3785 (2019).
- Zhang Y, Wu Y, Yan Y, Ma Y, Tu L, Shao J et al. Dual-targeted nanoparticle-in-microparticle system for ulcerative colitis therapy. Adv. Healthc. Mater. e2301518 (2023).
- Ağçeli GK, Hammachi H, Kodal SP, Cihangir N, Aksu Z. A novel approach to synthesize TiO2 nanoparticles: biosynthesis by using Streptomyces sp. HC1. J. Inorg. Organomet. Polym. Mater. 30(8), 3221–3229 (2020).
- Mu Q, Wang H, Zhang M. Nanoparticles for imaging and treatment of metastatic breast cancer. Expert Opin. Drug Deliv. 14(1), 123–136 (2017).
- Peiris PM, Deb P, Doolittle E, Doron G, Goldberg A, Govender P et al. Vascular targeting of a gold nanoparticle to breast cancer metastasis. J. Pharm. Sci. 104(8), 2600–2610 (2015).
- Niu S, Li X, Guo Z, Wan D, Liu Y, Li L et al. A strategy to improve the solubility and bioavailability of the insoluble drug piperlongumine through albumin nanoparticles. Pak. J. Pharm. Sci. 36(2), 483–490 (2023).
- Nan J, Liu W, Zhang K, Sun Y, Hu Y, Lei P. Tantalum and magnesium nanoparticles enhance the biomimetic properties and osteo-angiogenic effects of PCL membranes. Front. Bioeng. Biotechnol. 10, 1038250 (2022).
- Girbes ARJ, Robert R, Marik PE. The dose makes the poison. Intensive Care Med. 42(4), 632 (2016).
- Markides H, Rotherham M, El Haj AJ. Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J. Nanomater. 2012, 614094 (2012).
- Durnev AD. Toxicology of nanoparticles. Bull. Exp. Biol. Med. 145(1), 72–74 (2008).
- Kim JS, Yoon TJ, Yu KN, Kim BG, Park SJ, Kim HW et al. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol. Sci. 89(1), 338–347 (2006).
- Elespuru R, Pfuhler S, Aardema MJ, Chen T, Doak SH, Doherty A et al. Genotoxicity assessment of nanomaterials: recommendations on best practices, assays, and methods. Toxicol. Sci. 164(2), 391–416 (2018).
- Mousavi SM, Hashemi SA, Ghasemi Y, Atapour A, Amani AM, Savar Dashtaki A et al. Green synthesis of silver nanoparticles toward bio and medical applications: review study. Artif. Cells Nanomed. Biotechnol. 46(Suppl. 3), S855–S872 (2018).
- Aswani R, Radhakrishnan EK. Chapter 5 - Green approaches for nanotechnology. Green Functionalized Nanomaterials for Environmental Applications. Elsevier, NY, USA, 129–154 (2022).
- Ijaz I, Gilani E, Nazir A, Bukhari A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 13(3), 223–245 (2020).
- Li H, Li Y, Zhang Y, Liang C, Wang H, Li B et al. Fabrication and properties of carbon-encapsulated cobalt nanoparticles over nacl by cvd. Nanoscale Res. Lett. 11(1), 432 (2016).
- Wang K, Li Y, Wang H, Qian Z, Zhu X, Hussain S et al. CdSSe Nano-Flowers for ultrasensitive raman detection of antibiotics. Molecules. 28(7), 2980 (2023).
- Carlsson J-O, Martin PM. Chapter 7 - Chemical Vapor Deposition. Handbook of Deposition Technologies for Films and Coatings. (3rd Edition). 314–363 William Andrew Publishing, MA, USA (2010).
- Xu L, Tetreault AR, Khaligh HH, Goldthorpe IA, Wettig SD, Pope MA. Continuous Langmuir-Blodgett deposition and transfer by controlled edge-to-edge assembly of floating 2d materials. Langmuir. 35(1), 51–59 (2019).
- Swierczewski M, Bürgi T. Langmuir and Langmuir-Blodgett films of gold and silver nanoparticles. Langmuir. 39(6), 2135–2151 (2023).
- Tahir MB, Rafique M, Rafique MS, Nawaz T, Rizwan M, Tanveer M. Chapter 8 - Photocatalytic nanomaterials for degradation of organic pollutants and heavy metals. Nanotechnology and Photocatalysis for Environmental Applications. Elsevier, NY, USA, 119–138 (2020).
- Majumder S. Synthesis methods of nanomaterials for visible light photocatalysis. Nanostructured Materials for Visible Light Photocatalysis. Elsevier, NY, USA, 47–113 (2022).
- Phakatkar AH, Saray MT, Rasul MG, Sorokina LV, Ritter TG, Shokuhfar T et al. Ultrafast synthesis of high entropy oxide nanoparticles by flame spray pyrolysis. Langmuir. 37(30), 9059–9068 (2021).
- Bokov D, Turki Jalil A, Chupradit S, Suksatan W, Javed Ansari M, Shewael IH et al. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv Mat Sci Eng. 2021, 5102014 (2021).
- Gonçalves MC. Sol-gel silica nanoparticles in medicine: a natural choice. design, synthesis and products. Molecules. 23(8), 2021 (2018).
- González CMO, Morales EMC, Tellez ADMN, Quezada TES, Kharissova OV, Méndez-Rojas MA. Chapter 18 - CO2 capture by MOFs. Handbook of Greener Synthesis of Nanomaterials and Compounds. Elsevier, NY, USA, 407–448 (2021).
- Ghoshal T, Biswas S, Paul M, De SK. Synthesis of ZnO nanoparticles by solvothermal method and their ammonia sensing properties. J. Nanosci. Nanotechnol. 9(10), 5973–5980 (2009).
- Bajaj NS, Joshi RA. Chapter 3 - Energy materials: synthesis and characterization techniques. Energy Materials. Elsevier, NY, USA, 61–82 (2021).
- de la Fuente-Jiménez JL, Rodríguez-Rivas CI, Mitre-Aguilar IB, Torres-Copado A, García-López EA, Herrera-Celis J et al. A Comparative and critical analysis for in vitro cytotoxic evaluation of magneto-crystalline zinc ferrite nanoparticles using mtt, crystal violet, ldh, and apoptosis assay. Int. J. Mol. Sci. 24(16), 12860 (2023).
- Malik MA, Wani MY, Hashim MA. Microemulsion method: a novel route to synthesize organic and inorganic nanomaterials: 1st Nano Update. Arab. J. Chem. 5(4), 397–417 (2012).
- Chen Y, Zhang F, Wang Q, Lin H, Tong R, An N et al. The synthesis of LA-Fe3O4@PDA-PEG-DOX for photothermal therapy-chemotherapy. Dalton Trans. 47(7), 2435–2443 (2018).
- Han H, Huang Z, Lee W. Metal-assisted chemical etching of silicon and nanotechnology applications. Nano Today. 9(3), 271–304 (2014).
- Liang LY, Kung YH, Hsiao VKS, Chu CC. Reduction of nitroaromatics by gold nanoparticles on porous silicon fabricated using metal-assisted chemical etching. Nanomaterials. 13(11), 1805 (2023).
- Balachandran A, Sreenilayam SP, Madanan K, Thomas S, Brabazon D. Nanoparticle production via laser ablation synthesis in solution method and printed electronic application - A brief review. Results Eng. 16, 100646 (2022).
- Sportelli MC, Izzi M, Volpe A, Clemente M, Picca RA, Ancona A et al. The Pros and Cons of the Use of Laser Ablation Synthesis for the Production of Silver Nano-Antimicrobials. Antibiotics. 7(3), 67 (2018).
- Dawber M. Sputtering techniques for epitaxial growth of complex oxides. Epitaxial Growth of Complex Metal Oxides. Woodhead Publishing, NY, USA, 31–45 (2015).
- Zhu M, Nguyen MT, Chau YR, Deng L, Yonezawa T. Pt/Ag Solid Solution Alloy Nanoparticles in Miscibility Gaps Synthesized by Cosputtering onto Liquid Polymers. Langmuir. 37(19), 6096–6105 (2021).
- Virji MA, Stefaniak AB. A Review of Engineered Nanomaterial Manufacturing Processes and Associated Exposures. Comprehensive Materials Processing. Elsevier, NY, USA, 103–125 (2014).
- Toozandehjani M, Matori KA, Ostovan F, Abdul Aziz S, Mamat MS. Effect of Milling Time on the Microstructure, Physical and Mechanical Properties of Al-Al2O3 Nanocomposite Synthesized by Ball Milling and Powder Metallurgy. Materials. 10(11), 1232 (2017).
- Hano C, Abbasi BH. Plant-Based Green Synthesis of Nanoparticles: Production, Characterization and Applications. Biomolecules. 12(1), 31 (2021).
- Ovais M, Khalil AT, Ayaz M, Ahmad I, Nethi SK, Mukherjee S. Biosynthesis of metal nanoparticles via microbial enzymes: a mechanistic approach. Int. J. Mol. Sci. 19(12), 4100 (2018).
- Busi S, Rajkumari J. Chapter 15 - Microbially synthesized nanoparticles as next generation antimicrobials: scope and applications. Nanoparticles in Pharmacotherapy. William Andrew Publishing, NY, USA 485–524 (2019).
- Guilger-Casagrande M, de Lima R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 7, 287 (2019).
- Shahid M, Dumat C, Khalid S, Schreck E, Xiong T, Niazi NK. Foliar heavy metal uptake, toxicity and detoxification in plants: a comparison of foliar and root metal uptake. J. Hazard Mater. 325, 36–58 (2017).
- Nande A, Raut S, Michalska-Domanska M, Dhoble SJ. Green synthesis of nanomaterials using plant extract: A Review. Curr. Pharm. Biotechnol. 22(13), 1794–1811 (2021).
- Adeyemi JO, Oriola AO, Onwudiwe DC, Oyedeji AO. Plant extracts mediated metal-based nanoparticles: synthesis and biological applications. Biomolecules. 12(5), 627 (2022).
- Paiva-Santos AC, Herdade AM, Guerra C, Peixoto D, Pereira-Silva M, Zeinali M et al. Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. Int. J. Pharm. 597, 120311 (2021).
- Singh A, Talat M, Singh D, Srivastava ON. Biosynthesis of gold and silver nanoparticles by natural precursor clove and their functionalization with amine group. J. Nanopart. Res. 12, 1667–1675 (2010).
- Pirtarighat S, Ghannadnia M, Baghshahi S. Biosynthesis of silver nanoparticles using Ocimum basilicum cultured under controlled conditions for bactericidal application. Mater Sci. Eng. C. Mater. Biol. Appl. 98, 250–255 (2019).
- Song JY, Jang H-K, Kim BS. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 44(10), 1133–1138 (2009).
- Osuntokun J, Onwudiwe DC, Ebenso EE. Aqueous extract of broccoli mediated synthesis of CaO nanoparticles and its application in the photocatalytic degradation of bromocrescol green. IET Nanobiotechnol. 12(7), 888–894 (2018).
- Guo Q, Guo Q, Yuan J, Zeng J. Biosynthesis of gold nanoparticles using a kind of flavonol: dihydromyricetin. Colloids Surf A Physicochem. Eng. Asp. 441, 127–132 (2014).
- Tamboli QY, Patange SM, Mohanta YK, Sharma R, Zakde KR. Green synthesis of cobalt ferrite nanoparticles: an emerging material for environmental and biomedical applications. J. Nanomater. 2023, 9770212 (2023).
- Lin Q, Hong X, Zhang D, Jin H. Biosynthesis of size-controlled gold nanoparticles using M. lucida leaf extract and their penetration studies on human skin for plastic surgery applications. J. Photochem. Photobiol. Biol. 199, 111591 (2019).
- Thanganadar Appapalam S, Panchamoorthy R. Aerva lanata mediated phytofabrication of silver nanoparticles and evaluation of their antibacterial activity against wound associated bacteria. J. Taiwan Inst. Chem. Eng. 78, 539–551 (2017).
- Simon S, Sibuyi NRS, Fadaka AO, Meyer S, Josephs J, Onani MO et al. Biomedical applications of plant extract-synthesized silver nanoparticles. Biomedicines. 10(11), 2792 (2022).
- Akter M, Sikder MT, Rahman MM, Ullah A, Hossain KFB, Banik S et al. A systematic review on silver nanoparticles-induced cytotoxicity: physicochemical properties and perspectives. J. Adv. Res. 9, 1–16 (2018).
- Bharadwaj KK, Rabha B, Pati S, Sarkar T, Choudhury BK, Barman A et al. Green synthesis of gold nanoparticles using plant extracts as beneficial prospect for cancer theranostics. Molecules 26(21), 6389 (2021).
- Keijok WJ, Pereira RHA, Alvarez LAC, Prado AR, da Silva AR, Ribeiro J et al. Controlled biosynthesis of gold nanoparticles with Coffea arabica using factorial design. Sci Rep. 9(1), 16019 (2019).
- Boomi P, Poorani GP, Selvam S, Palanisamy S, Jegatheeswaran S, Anand K et al. Green biosynthesis of gold nanoparticles using Croton sparsiflorus leaves extract and evaluation of UV protection, antibacterial and anticancer applications. Appl. Organomet. Chem. 34(5), e5574 (2020).
- Ambika S, Sundrarajan M. Antibacterial behaviour of Vitex negundo extract assisted ZnO nanoparticles against pathogenic bacteria. J. Photochem. Photobiol. B. 146, 52–57 (2015).
- Bala N, Saha S, Chakraborty M, Maiti M, Das S, Basu R et al. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 5(7), 4993–5003 (2015).
- Osibe DA, Aoyagi H. Use of Catharanthus roseus cell cultures for the synthesis of metal nanoparticles. Methods Mol. Biol. 2469, 55–64 (2022).
- Kalaiselvi A, Roopan S, Madhumitha G, Chidambaram R, Vijay G. Synthesis and characterization of palladium nanoparticles using Catharanthus roseus leaf extract and its application in the photo-catalytic degradation. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 135, 116–119 (2015).
- Fahmy SA, Preis E, Bakowsky U, Azzazy HME. Platinum nanoparticles: green synthesis and biomedical applications. Molecules 25(21), 4981 (2020).
- Azizi M, Ghourchian H, Yazdian F, Bagherifam S, Bekhradnia S, Nyström B. Anti-cancerous effect of albumin coated silver nanoparticles on MDA-MB 231 human breast cancer cell line. Sci Rep. 7(1), 5178 (2017).
- Chen H, Feng X, Gao L, Mickymaray S, Paramasivam A, Abdulaziz Alfaiz F et al. Inhibiting the PI3K/AKT/mTOR signalling pathway with copper oxide nanoparticles from Houttuynia cordata plant: attenuating the proliferation of cervical cancer cells. Artif Cells Nanomed. Biotechnol. 49(1), 240–249 (2021).
- Pan Y, Wu Q, Qin L, Cai J, Du B. Gold nanoparticles inhibit VEGF165-induced migration and tube formation of endothelial cells via the Akt pathway. Biomed. Res. Int. 2014, 418624 (2014).
- Arvizo RR, Saha S, Wang E, Robertson JD, Bhattacharya R, Mukherjee P. Inhibition of tumor growth and metastasis by a self-therapeutic nanoparticle. Proc. Natl Acad Sci. U S A. 110(17), 6700–6705 (2013).
- Fageria L, Pareek V, Dilip RV, Bhargava A, Pasha SS, Laskar IR et al. Biosynthesized protein-capped silver nanoparticles induce ros-dependent proapoptotic signals and prosurvival autophagy in cancer cells. ACS Omega. 2(4), 1489–1504 (2017).
- Krishnamoorthy V, Chidambaram A, Saranya T, Ramalingam V, Sakthivel R, Vilwanathan R. Avicennia marina engineered nanoparticles regulate p53 dependent intrinsic apoptosis signaling pathway in adenocarcinoma lung cancer. Process Biochem. 94, 349–358 (2020).
- Jeyaraj M, Renganathan A, Sathishkumar G, Ganapathi A, Premkumar K. Biogenic metal nanoformulations induce Bax/Bcl2 and caspase mediated mitochondrial dysfunction in human breast cancer cells (MCF 7). RSC Adva. 5(3), 2159–2166 (2015).
- Gurunathan S, Qasim M, Park C, Yoo H, Choi DY, Song H et al. Cytotoxicity and transcriptomic analysis of silver nanoparticles in mouse embryonic fibroblast cells. Int. J. Mol. Sci. 19(11), 3618 (2018).
- Hamida RS, Albasher G, Bin-Meferij MM. Oxidative stress and apoptotic responses elicited by nostoc-synthesized silver nanoparticles against different cancer cell lines. Cancers 12(8), 2099 (2020).
- Lee KX, Shameli K, Nagao Y, Yew YP, Teow SY, Moeini H. Potential use of gold-silver core-shell nanoparticles derived from Garcinia mangostana peel for anticancer compound, protocatechuic acid delivery. Front. Mol. Biosci. 9, 997471 (2022).
- Yusefi M, Shameli K, Su Yee O, Teow SY, Hedayatnasab Z, Jahangirian H et al. Green synthesis of Fe3O4 nanoparticles stabilized by a Garcinia mangostana fruit peel extract for hyperthermia and anticancer activities. Int. J. Nanomedicine. 16, 2515–2532 (2021).
- Park JS, Ahn EY, Park Y. Asymmetric dumbbell-shaped silver nanoparticles and spherical gold nanoparticles green-synthesized by mangosteen (Garcinia mangostana) pericarp waste extracts. Int J Nanomedicine. 12, 6895–6908 (2017).
- Biresaw SS, Taneja P. Copper nanoparticles green synthesis and characterization as anticancer potential in breast cancer cells (MCF7) derived from Prunus nepalensis phytochemicals. Mater Today: Proc. 49, 3501–3509 (2022).
- Dashtizadeh Z, Jookar Kashi F, Ashrafi M. Phytosynthesis of copper nanoparticles using Prunus mahaleb L. and its biological activity. Mater Today Commun. 27, 102456 (2021).
- Pillai RR, Sreelekshmi PB, Meera AP. Enhanced biological performance of green sythesized copper oxide nanoparticles using Pimenta dioica leaf extract. Mater Today: Proc. 50, 163–172 (2022).
- Umamaheswari A, Prabu SL, John SA, Puratchikody A. Green synthesis of zinc oxide nanoparticles using leaf extracts of Raphanus sativus var. Longipinnatus and evaluation of their anticancer property in A549 cell lines. Biotechnol. Rep. 29, e00595 (2021).
- Donga S, Chanda S. Caesalpinia crista seeds mediated green synthesis of zinc oxide nanoparticles for antibacterial, antioxidant, and anticancer activities. BioNanoScience. 12(2), 451–462 (2022).
- Zhang H, Li T, Luo W, Peng GX, Xiong J. Green synthesis of Ag nanoparticles from Leucus aspera and its application in anticancer activity against alveolar cancer. J. Exp. Nanosci. 17(1), 47–60 (2022).
- Yuan C, Jiang B, Xu X, Wan Y, Wang L, Chen J. Anti-human ovarian cancer and cytotoxicity effects of nickel nanoparticles green-synthesized by Alhagi maurorum leaf aqueous extract. J. Exp. Nanosci. 17(1), 113–125 (2022).
- Huang Y, Zhu C, Xie R, Ni M. Green synthesis of nickel nanoparticles using Fumaria officinalis as a novel chemotherapeutic drug for the treatment of ovarian cancer. J. Exp. Nanosci. 16(1), 368–381 (2021).
- Naseer M, Aslam U, Khalid B, Chen B. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 10(1), 9055 (2020).
- Happy A, Soumya M, Venkat Kumar S, Rajeshkumar S, Sheba RD, Lakshmi T et al. Phyto-assisted synthesis of zinc oxide nanoparticles using Cassia alata and its antibacterial activity against Escherichia coli. Biochem. Biophys. Rep. 17, 208–211 (2019).
- Ramesh P, Saravanan K, Manogar P, Johnson J, Vinoth E, Mayakannan M. Green synthesis and characterization of biocompatible zinc oxide nanoparticles and evaluation of its antibacterial potential. Sens Bio-Sens Res. 31, 100399 (2021).
- Abdelgawad FAM, El-Hawary SS, Abd El-Kader EM, Alshehri SA, Rabeh MA, El-Mosallamy A et al. Phytochemical profiling and antiviral activity of green sustainable nanoparticles derived from Maesa indica (Roxb.) sweet against human Coronavirus 229E. Plants. 12(15), 2813 (2023).
- Singh R, Hano C, Nath G, Sharma B. Green biosynthesis of silver nanoparticles using leaf extract of Carissa carandas L. and their antioxidant and antimicrobial activity against human pathogenic bacteria. Biomolecules. 11(2), 299 (2021).
- Mallikarjuna K, Nasif O, Ali Alharbi S, Chinni SV, Reddy LV, Reddy MRV et al. Phytogenic synthesis of Pd-Ag/rGO nanostructures using stevia leaf extract for photocatalytic H2 production and antibacterial studies. Biomolecules. 11(2), 190 (2021).
- Chen M-N, Chan C-F, Huang S-L, Lin Y-S. Green biosynthesis of gold nanoparticles using Chenopodium formosanum shell extract and analysis of the particles' antibacterial properties. J. Sci. Food Agric. 99(7), 3693–3702 (2019).
- Perveen K, Husain FM, Qais FA, Khan A, Razak S, Afsar T et al. Microwave-assisted rapid green synthesis of gold nanoparticles using seed extract of Trachyspermum ammi: ROS mediated biofilm inhibition and anticancer activity. Biomolecules. 11(2), 197 (2021).
- Elshazly EH, Nasr A, Elnosary ME, Gouda GA, Mohamed H, Song Y. Identifying the Anti-MERS-CoV and Anti-HcoV-229E potential drugs from the ginkgo biloba leaves extract and its eco-friendly synthesis of silver nanoparticles. Molecules. 28(3), 1375 (2023).
- Saleem K, Khursheed Z, Hano C, Anjum I, Anjum S. Applications of nanomaterials in leishmaniasis: a focus on recent advances and challenges. Nanomaterials. 9(12), 1749 (2019).
- Yugay Y, Rusapetova T, Mashtalyar D, Grigorchuk V, Vasyutkina E, Kudinova O et al. Biomimetic synthesis of functional silver nanoparticles using hairy roots of Panax ginseng for wheat pathogenic fungi treatment. Colloids Surf B Biointerfaces. 207, 112031 (2021).
- Yugay YA, Sorokina MR, Grigorchuk VP, Rusapetova TV, Silant'ev VE, Egorova AE et al. Biosynthesis of functional silver nanoparticles using callus and hairy root cultures of Aristolochia manshuriensis. J. Funct. Biomater. 14(9), 451 (2023).
- Bawazeer S, Rauf A, Emran TB, Aljohani ASM, Alhumaydhi FA, Khan Z et al. Biogenic synthesis of silver nanoparticles using Rhazya stricta extracts and evaluation of its biological activities. J. Nanomater. 2022, 7365931 (2022).
- Hano C, Tungmunnithum D. Plant polyphenols, more than just simple natural antioxidants: oxidative stress, aging and age-related diseases. Medicines. 7(5), 26 (2020).
- Melkamu WW, Bitew LT. Green synthesis of silver nanoparticles using Hagenia abyssinica (Bruce) JF Gmel plant leaf extract and their antibacterial and anti-oxidant activities. Heliyon. 7(11), e08459 (2021).
- Khan SA, Shahid S, Lee C-S. Green synthesis of gold and silver nanoparticles using leaf extract of Clerodendrum inerme; characterization, antimicrobial, and antioxidant activities. Biomolecules. 10(6), 835 (2020).
- Srihasam S, Thyagarajan K, Korivi M, Lebaka VR, Mallem SPR. Phytogenic generation of NiO nanoparticles using Stevia leaf extract and evaluation of their in-vitro antioxidant and antimicrobial properties. Biomolecules. 10(1), 89 (2020).
- Alavi M, Moradi M. Different antibacterial and photocatalyst functions for herbal and bacterial synthesized silver and copper/copper oxide nanoparticles/nanocomposites: a review. Inorg Chem Commun. 142, 109590 (2022).
- Ali J, Ali N, Wang L, Waseem H, Pan G. Revisiting the mechanistic pathways for bacterial mediated synthesis of noble metal nanoparticles. J. Microbiol. Methods. 159, 18–25 (2019).
- Hossain A, Hong X, Ibrahim E, Li B, Sun G, Meng Y et al. Green synthesis of silver nanoparticles with culture supernatant of a bacterium Pseudomonas rhodesiae and their antibacterial activity against soft rot pathogen Dickeya dadantii. Molecules. 24(12), 2303 (2019).
- Molnár Z, Bódai V, Szakacs G, Erdélyi B, Fogarassy Z, Sáfrán G et al. Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci Rep. 8(1), 3943 (2018).
- Ahmad A, Senapati S, Khan MI, Kumar R, Ramani R, Srinivas V et al. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology. 14(7), 824 (2003).
- Perni S, Hakala V, Prokopovich P. Biogenic synthesis of antimicrobial silver nanoparticles capped with l-cysteine. Colloids Surf A Physicochem Eng Asp. 460, 219–224 (2014).
- Sintubin L, De Windt W, Dick J, Mast J, van der Ha D, Verstraete W et al. Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl. Microbiol. Biotechnol. 84(4), 741–749 (2009).
- Das VL, Thomas R, Varghese RT, Soniya EV, Mathew J, Radhakrishnan EK. Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. Biotech. 4(2), 121–126 (2014).
- Deljou A, Goudarzi S. Green Extracellular synthesis of the silver nanoparticles using Thermophilic Bacillus Sp. AZ1 and its antimicrobial activity against several human pathogenetic bacteria. Iran J Biotechnol. 14(2), 25–32 (2016).
- Abd Ali MA, Shareef AA. Antibacterial activity of silver nanoparticles derived from extracellular Extract of Enterococcus aerogenes against dental disease bacteria isolated. Regen Eng Transl Med. (2023) doi: 10.1007/s40883-023-00304-2
- Altinsoy BD, Şeker Karatoprak G, Ocsoy I. Extracellular directed ag NPs formation and investigation of their antimicrobial and cytotoxic properties. Saudi Pharm J. 27(1), 9–16 (2019).
- Ameen F, AlYahya S, Govarthanan M, Aljahdali N, Al-Enazi N, Alsamhary K et al. Soil bacteria Cupriavidus sp. mediates the extracellular synthesis of antibacterial silver nanoparticles. J. Mol. Struct. 1202, 127233 (2020).
- Iqtedar M, Aslam M, Akhyar M, Shehzaad A, Abdullah R, Kaleem A. Extracellular biosynthesis, characterization, optimization of silver nanoparticles (AgNPs) using Bacillus mojavensis BTCB15 and its antimicrobial activity against multidrug resistant pathogens. Prep. Biochem. Biotechnol. 49(2), 136–142 (2019).
- Korbekandi H, Iravani S, Abbasi S. Optimization of biological synthesis of silver nanoparticles using Lactobacillus casei subsp. casei. J Chem Technol Biotechnol. 87(7), 932–937 (2012).
- Zada S, Ahmad A, Khan S, Iqbal A, Ahmad S, Ali H et al. Biofabrication of gold nanoparticles by Lyptolyngbya JSC-1 extract as super reducing and stabilizing agents: synthesis, characterization and antibacterial activity. Microb. Pathog. 114, 116–123 (2018).
- Cherian T, Maity D, Rajendra Kumar RT, Balasubramani G, Ragavendran C, Yalla S et al. Green chemistry based gold nanoparticles synthesis using the marine bacterium Lysinibacillus odysseyi PBCW2 and their multitudinous activities. Nanomaterials. 12(17), 2940 (2022).
- Manivasagan P, Alam MS, Kang K-H, Kwak M, Kim S-K. Extracellular synthesis of gold bionanoparticles by Nocardiopsis sp. and evaluation of its antimicrobial, antioxidant and cytotoxic activities. Bioprocess Biosyst Eng. 38(6), 1167–1177 (2015).
- Vairavel M, Devaraj E, Shanmugam R. An eco-friendly synthesis of Enterococcus sp. mediated gold nanoparticle induces cytotoxicity in human colorectal cancer cells. Environ Sci Pollut Res. 27, 8166–8175 (2020).
- Waghmode MS, Gunjal AB, Mulla JA, Patil NN, Nawani NN. Studies on the titanium dioxide nanoparticles: biosynthesis, applications and remediation. SN Appl Sci. 1(4), 310 (2019).
- Prasad K, Jha AK, Kulkarni A. Lactobacillus assisted synthesis of titanium nanoparticles. Nanoscale Res Lett. 2, 248–250 (2007).
- Al-Kordy HM, Sabry SA, Mabrouk ME. Statistical optimization of experimental parameters for extracellular synthesis of zinc oxide nanoparticles by a novel haloalaliphilic Alkalibacillus sp. W7. Sci rep. 11(1), 10924 (2021).
- Busi S, Rajkumari J, Pattnaik S, Parasuraman P, Hnamte S. Extracellular synthesis of zinc oxide nanoparticles using Acinetobacter schindleri SIZ7 and its antimicrobial property against foodborne pathogens. J Microbiol Biotechnol Food Sci. 5(5), 407 (2016).
- Kundu D, Hazra C, Chatterjee A, Chaudhari A, Mishra S. Extracellular biosynthesis of zinc oxide nanoparticles using Rhodococcus pyridinivorans NT2: multifunctional textile finishing, biosafety evaluation and in vitro drug delivery in colon carcinoma. J Photochem Photobiol B Biol. 140, 194–204 (2014).
- Eltarahony M, Zaki S, Abd-El-Haleem D. Concurrent synthesis of zero-and one-dimensional, spherical, rod-, needle-, and wire-shaped CuO nanoparticles by Proteus mirabilis 10B. J. Nanomater. 2018, 1–14 (2018).
- Talebian S, Shahnavaz B, Nejabat M, Abolhassani Y, Rassouli FB. Bacterial-mediated synthesis and characterization of copper oxide nanoparticles with antibacterial, antioxidant, and anticancer potentials. Front Bioeng Biotechnol. 11, 1140010 (2023).
- Abdel-Aziz MM, Emam TM, Elsherbiny EA. Bioactivity of magnesium oxide nanoparticles synthesized from cell filtrate of endobacterium Burkholderia rinojensis against Fusarium oxysporum. Mater Sci Eng. C. 109, 110617 (2020).
- Fatemi M, Mollania N, Momeni-Moghaddam M, Sadeghifar F. Extracellular biosynthesis of magnetic iron oxide nanoparticles by Bacillus cereus strain HMH1: characterization and in vitro cytotoxicity analysis on MCF-7 and 3T3 cell lines. J Biotechnol. 270, 1–11 (2018).
- Allaker RP, Memarzadeh K. Nanoparticles and the control of oral infections. Int. J Antimicrob Agents. 43(2), 95–104 (2014).
- Baymiller M, Huang F, Rogelj S. Rapid one-step synthesis of gold nanoparticles using the ubiquitous coenzyme NADH. Matters (2017) doi: 10.19185/matters.201705000007
- Liao C, Li Y, Tjong SC. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int J Mol Sci. 20(2), 449 (2019).
- Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 275(1), 177–182 (2004).
- Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 73(6), 1712–1720 (2007).
- Lee HJ, Lee SG, Oh EJ, Chung HY, Han SI, Kim EJ et al. Antimicrobial polyethyleneimine-silver nanoparticles in a stable colloidal dispersion. Colloids Surf B. 88(1), 505–511 (2011).
- Ibrahim E, Fouad H, Zhang M, Zhang Y, Qiu W, Yan C et al. Biosynthesis of silver nanoparticles using endophytic bacteria and their role in inhibition of rice pathogenic bacteria and plant growth promotion. RSC Adv. 9(50), 29293–29299 (2019).
- Esmail R, Afshar A, Morteza M, Abolfazl A, Akhondi E. Synthesis of silver nanoparticles with high efficiency and stability by culture supernatant of Bacillus ROM6 isolated from Zarshouran gold mine and evaluating its antibacterial effects. BMC Microbiol. 22(1), 97 (2022).
- Saleh MN, Alwan SK. Bio-synthesis of silver nanoparticles from bacteria Klebsiella pneumonia: their characterization and antibacterial studies. J Phys Conf Ser. 1664(1), 012115 (2020).
- Matei A, Matei S, Matei G-M, Cogălniceanu G, Cornea CP. Biosynthesis of silver nanoparticles mediated by culture filtrate of lactic acid bacteria, characterization and antifungal activity. Eurobiotech J. 4(2), 97–103 (2020).
- Schröfel A, Kratošová G, Šafařík I, Šafaříková M, Raška I, Shor LM. Applications of biosynthesized metallic nanoparticles - a review. Acta Biomater. 10(10), 4023–4042 (2014).
- Rudramurthy GR, Swamy MK, Sinniah UR, Ghasemzadeh A. Nanoparticles: alternatives against drug-resistant pathogenic microbes. Molecules. 21(7), 836 (2016).
- Ramasamy M, Lee JH, Lee J. Potent antimicrobial and antibiofilm activities of bacteriogenically synthesized gold-silver nanoparticles against pathogenic bacteria and their physiochemical characterizations. J. Biomater. Appl. 31(3), 366–378 (2016).
- Bing W, Sun H, Wang F, Song Y, Ren J. Hydrogen-producing hyperthermophilic bacteria synthesized size-controllable fine gold nanoparticles with excellence for eradicating biofilm and antibacterial applications. J Mater Chem B. 6(28), 4602–4609 (2018).
- Patil MP, Kang M-J, Niyonizigiye I, Singh A, Kim J-O, Seo YB et al. Extracellular synthesis of gold nanoparticles using the marine bacterium Paracoccus haeundaensis BC74171T and evaluation of their antioxidant activity and antiproliferative effect on normal and cancer cell lines. Colloids Surf B. 183, 110455 (2019).
- Mohd Yusof H, Mohamad R, Zaidan UH, Rahman NA. Sustainable microbial cell nanofactory for zinc oxide nanoparticles production by zinc-tolerant probiotic Lactobacillus plantarum strain TA4. Microb Cell Fact. 19(1), 10 (2020).
- Majeed S, Danish M, Mohamad Ibrahim MN, Sekeri SH, Ansari MT, Nanda A et al. Bacteria mediated synthesis of iron oxide nanoparticles and their antibacterial, antioxidant, cytocompatibility properties. J Clust Sci. 32(4), 1083–1094 (2021).
- Kianpour S, Ebrahiminezhad A, Deyhimi M, Negahdaripour M, Raee MJ, Mohkam M et al. Structural characterization of polysaccharide-coated iron oxide nanoparticles produced by Staphylococcus warneri, isolated from a thermal spring. J Basic Microbiol. 59(6), 569–578 (2019).
- Fani M, Ghandehari F, Rezaee M. Biosynthesis of iron oxide nanoparticles by cytoplasmic extract of bacteria lactobacillus fermentum. J Med Chem Sci. 1(2), 28–30 (2018).
- Rajeswaran S, Somasundaram Thirugnanasambandan S, Dewangan NK, Moorthy RK, Kandasamy S, Vilwanathan R. Multifarious pharmacological applications of green routed eco-friendly iron nanoparticles synthesized by Streptomyces Sp.(SRT12). Biol Trace Elem Res. 194, 273–283 (2020).
- Shunmugam R, Renukadevi Balusamy S, Kumar V, Menon S, Lakshmi T, Perumalsamy H. Biosynthesis of gold nanoparticles using marine microbe (Vibrio alginolyticus) and its anticancer and antioxidant analysis. J King Saud Univ Sci. 33(1), 101260 (2021).
- Zomorodian K, Pourshahid S, Sadatsharifi A, Mehryar P, Pakshir K, Rahimi MJ et al. Biosynthesis and characterization of silver nanoparticles by Aspergillus Species. Biomed Res Int. 2016, 5435397 (2016).
- Durán N, Marcato PD, Alves OL, Souza GI, Esposito E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nanobiotechnology. 3, 8 (2005).
- Anil Kumar S, Abyaneh MK, Gosavi SW, Kulkarni SK, Pasricha R, Ahmad A et al. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol Lett. 29(3), 439–445 (2007).
- Hietzschold S, Walter A, Davis C, Taylor A, Sepunaru L. Does nitrate reductase play a role in silver nanoparticle synthesis? evidence for nadph as the sole reducing agent. ACS Sustain Chem Eng. 7(9), 8070–8076 (2019).
- Rai M, Bonde S, Golinska P, Trzcińska-Wencel J, Gade A, Abd-Elsalam KA et al. Fusarium as a Novel Fungus for the Synthesis of Nanoparticles: Mechanism and Applications. J Fungi. 7(2), 139 (2021).
- Halkai KR, Mudda JA, Shivanna V, Patil V, Rathod V, Halkai R. Cytotoxicity evaluation of fungal-derived silver nanoparticles on human gingival fibroblast cell line: an in vitro study. J Conserv Dent. 22(2), 160–163 (2019).
- Halkai KR, Halkai R, Mudda JA, Shivanna V, Rathod V. Antibiofilm efficacy of biosynthesized silver nanoparticles against endodontic-periodontal pathogens: an in vitro study. J Conserv Dent. 21(6), 662–666 (2018).
- Alves MF, Paschoal ACC, Klimeck TDMF, Kuligovski C, Marcon BH, de Aguiar AM et al. Biological synthesis of low cytotoxicity silver nanoparticles (AgNPs) by the fungus chaetomium thermophilum—sustainable nanotechnology. J Fungi. 8(6), 605 (2022).
- Kobashigawa JM, Robles CA, Martínez Ricci ML, Carmarán CC. Influence of strong bases on the synthesis of silver nanoparticles (AgNPs) using the ligninolytic fungi Trametes trogii. Saudi J Biol Sci. 26(7), 1331–1337 (2019).
- Elegbede JA, Lateef A, Azeez MA, Asafa TB, Yekeen TA, Oladipo IC et al. Fungal xylanases-mediated synthesis of silver nanoparticles for catalytic and biomedical applications. IET Nanobiotechnol. 12(6), 857–863 (2018).
- Ameen F, Al-Homaidan AA, Al-Sabri A, Almansob A, AlNAdhari S. Anti-oxidant, anti-fungal and cytotoxic effects of silver nanoparticles synthesized using marine fungus Cladosporium halotolerans. Appl Nanosci. 13(1), 623–631 (2023).
- Raman J, Reddy GR, Lakshmanan H, Selvaraj V, Gajendran B, Nanjian R et al. Mycosynthesis and characterization of silver nanoparticles from Pleurotus djamor var. roseus and their in vitro cytotoxicity effect on PC3 cells. Proc Biochem. 50(1), 140–147 (2015).
- Nagajyothi PC, Sreekanth TVM, Lee J-I, Lee KD. Mycosynthesis: antibacterial, antioxidant and antiproliferative activities of silver nanoparticles synthesized from Inonotus obliquus (Chaga mushroom) extract. J Photochem Photobiol B Biol. 130, 299–304 (2014).
- Soni N, Prakash S. Efficacy of fungus mediated silver and gold nanoparticles against Aedes aegypti larvae. Parasitol. Res. 110, 175–184 (2012).
- Zhang X, Qu Y, Shen W, Wang J, Li H, Zhang Z et al. Biogenic synthesis of gold nanoparticles by yeast Magnusiomyces ingens LH-F1 for catalytic reduction of nitrophenols. Colloids Surf A Physicochem Eng Asp. 497, 280–285 (2016).
- Vala AK. Exploration on green synthesis of gold nanoparticles by a marine-derived fungus Aspergillus sydowii. Environ Prog Sustain. 34(1), 194–197 (2015).
- Kitching M, Choudhary P, Inguva S, Guo Y, Ramani M, Das SK et al. Fungal surface protein mediated one-pot synthesis of stable and hemocompatible gold nanoparticles. Enzyme Microb. Technol. 95, 76–84 (2016).
- Rajakumar G, Rahuman AA, Roopan SM, Khanna VG, Elango G, Kamaraj C et al. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim Acta A Mol Biomol Spectrosc. 91, 23–29 (2012).
- Peiris M, Gunasekara T, Jayaweera P, Fernando S. TiO2 nanoparticles from Baker's yeast: a potent antimicrobial. J Microbial Biotechnol. 28(10), 1664–1670 (2018).
- Tarafdar A, Raliya R, Wang W-N, Biswas P, Tarafdar J. Green synthesis of TiO2 nanoparticle using Aspergillus tubingensis. Adv Sci Eng Med. 5(9), 943–949 (2013).
- Ranjani VA, Rani GT, Sowjanya M, Preethi M, Srinivas M, Nikhil M. Yeast mediated synthesis of iron oxide nano particles: its characterization and evaluation of antibacterial activity. Int Res J Pharm Med sci. 5(5), 12–16 (2022).
- Ali A, Elewa N, Elbostany N, Shetaia Y, Swilm M. Antimicrobial potentiality of green synthesized iron oxide nanoparticles by Penicillium roqueforti. Egypt. J Basic Appl Sci. 59(1), 29–43 (2021).
- Ceroni D, Grumetz C, Desvachez O, Pusateri S, Dunand P, Samara E. From prevention of pin-tract infection to treatment of osteomyelitis during paediatric external fixation. J Child Orthop. 10(6), 605–612 (2016).
- Qu H, Knabe C, Radin S, Garino J, Ducheyne P. Percutaneous external fixator pins with bactericidal micron-thin sol-gel films for the prevention of pin tract infection. Biomaterials 62, 95–105 (2015).
- Liang X, Zhang S, Gadd GM, McGrath J, Rooney DW, Zhao Q. Fungal-derived selenium nanoparticles and their potential applications in electroless silver coatings for preventing pin-tract infections. Regen Biomater. 9(1), rbac013 (2022).
- Skanda S, Bharadwaj PSJ, Datta Darshan VM, Sivaramakrishnan V, Vijayakumar BS. Proficient mycogenic synthesis of silver nanoparticles by soil derived fungus Aspergillus melleus SSS-10 with cytotoxic and antibacterial potency. J. Microbiol. Meth. 199, 106517 (2022).
- Majeed S, Danish M, Norazmi FSB. Fungal derived zinc oxide nanoparticles and their antibacterial and anticancer activities against human Alveoli lung cancer A-549 cell line. Adv Sci Eng Med. 10(6), 551–556 (2018).
- Gao Y, Arokia Vijaya Anand M, Ramachandran V, Karthikkumar V, Shalini V, Vijayalakshmi S et al. Biofabrication of Zinc Oxide Nanoparticles from Aspergillus niger, Their Antioxidant, Antimicrobial and Anticancer Activity. J Clust Sci. 30(4), 937–946 (2019).
- Riddin TL, Gericke M, Whiteley CG. Analysis of the inter- and extracellular formation of platinum nanoparticles by Fusarium oxysporum f. sp. lycopersici using response surface methodology. Nanotechnology. 17(14), 3482–3489 (2006).
- Gupta K, Chundawat TS. Bio-inspired synthesis of platinum nanoparticles from fungus Fusarium oxysporum: its characteristics, potential antimicrobial, antioxidant and photocatalytic activities. Mater Res Express. 6(10), 1050d6 (2019).
- Subramaniyan SA, Sheet S, Vinothkannan M, Yoo DJ, Lee YS, Belal SA et al. One-Pot Facile Synthesis of Pt Nanoparticles Using Cultural Filtrate of Microgravity Simulated Grown P. chrysogenum and Their Activity on Bacteria and Cancer Cells. J Nanosci Nanotechnol. 18(5), 3110–3125 (2018).
- Castro-Longoria E, Moreno-Velázquez SD, Vilchis-Nestor AR, Arenas-Berumen E, Avalos-Borja M. Production of platinum nanoparticles and nanoaggregates using Neurospora crassa. J Microbiol Biotechnol. 22(7), 1000–1004 (2012).
- Sarkar J, Acharya K. Alternaria alternata culture filtrate mediated bioreduction of chloroplatinate to platinum nanoparticles. Synth React Inorg met. 47, 00–00 (2016).
- Nanda A, Bhat M, Nayak BK. Evaluation of Bactericidal Activity of Biologically synthesised silver nanoparticles from Candida albicans in combination with ciprofloxacin. Mater Today. 2, 4395–4401 (2015).
- Ibrahim HMM, Hassan MS. Characterization and antimicrobial properties of cotton fabric loaded with green synthesized silver nanoparticles. Carbohydr Polym. 151, 841–850 (2016).
- Jalal M, Ansari MA, Alzohairy MA, Ali SG, Khan HM, Almatroudi A et al. Biosynthesis of Silver Nanoparticles from Oropharyngeal Candida glabrata Isolates and Their Antimicrobial Activity against Clinical Strains of Bacteria and Fungi. Nanomaterials 8(8), 586 (2018).
- Gaikwad S, Ingle A, Gade A, Rai M, Falanga A, Incoronato N et al. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomedicine 4303–4314 (2013).
- Sundaravadivelan C, Padmanabhan MN. Effect of mycosynthesized silver nanoparticles from filtrate of Trichoderma harzianum against larvae and pupa of dengue vector Aedes aegypti L. Environ Sci Pollut Res. 21(6), 4624–4633 (2014).
- Banu AN, Balasubramanian C. Optimization and synthesis of silver nanoparticles using Isaria fumosorosea against human vector mosquitoes. Parasitol. Res. 113, 3843–3851 (2014).
- Lan Chi NT, Veeraragavan GR, Brindhadevi K, Chinnathambi A, Salmen SH, Alharbi SA et al. Fungi fabrication, characterization, and anticancer activity of silver nanoparticles using metals resistant Aspergillus niger. Environ. Res. 208, 112721 (2022).
- Nassar A-RA, Eid AM, Atta HM, El Naghy WS, Fouda A. Exploring the antimicrobial, antioxidant, anticancer, biocompatibility, and larvicidal activities of selenium nanoparticles fabricated by endophytic fungal strain Penicillium verhagenii. Sci Rep. 13(1), 9054 (2023).
- Fouda A, Hassan SE-D, Eid AM, Abdel-Rahman MA, Hamza MF. Light enhanced the antimicrobial, anticancer, and catalytic activities of selenium nanoparticles fabricated by endophytic fungal strain, Penicillium crustosum EP-1. Sci Rep. 12(1), 11834 (2022).
- Mistry H, Thakor R, Bariya H. Biogenesis and characterization of proficient silver nanoparticles employing marine procured fungi Hamigera pallida and assessment of their antioxidative, antimicrobial and anticancer potency. Biotechnol Lett. 44(9), 1097–1107 (2022).
- Husseiny SM, Salah TA, Anter HA. Biosynthesis of size controlled silver nanoparticles by Fusarium oxysporum, their antibacterial and antitumor activities. Beni-Suef univ j basic appl sci. 4(3), 225–231 (2015).
- Zeng Q, Wen H, Wen Q, Chen X, Wang Y, Xuan W et al. Cucumber mosaic virus as drug delivery vehicle for doxorubicin. Biomaterials 34(19), 4632–4642 (2013).
- Thangavelu RM, Ganapathy R, Ramasamy P, Krishnan K. Fabrication of virus metal hybrid nanomaterials: an ideal reference for bio semiconductor. Arab J Chem. 13(1), 2750–2765 (2020).
- Love AJ, Makarov VV, Sinitsyna OV, Shaw J, Yaminsky IV, Kalinina NO et al. A Genetically modified tobacco mosaic virus that can produce gold nanoparticles from a metal salt precursor. Front Plant Sci. 6, 984 (2015).
- Blum AS, Soto CM, Wilson CD, Cole JD, Kim M, Gnade B et al. Cowpea Mosaic Virus as a Scaffold for 3-D Patterning of Gold Nanoparticles. Nano Lett. 4(5), 867–870 (2004).
- Cao J, Guenther RH, Sit TL, Opperman CH, Lommel SA, Willoughby JA. Loading and release mechanism of red clover necrotic mosaic virus derived plant viral nanoparticles for drug delivery of doxorubicin. Small. 10(24), 5126–5136 (2014).
- Khanna P, Kaur A, Goyal D. Algae-based metallic nanoparticles: synthesis, characterization and applications. J Microbiol Methods. 163, 105656 (2019).
- Behera M, Behera PR, Bhuyan PP, Singh L, Pradhan B. Algal nanoparticles and their antibacterial activity: current research status and future prospectives. Drugs and Drug Candidates. 2(3), 554–570 (2023).
- Uzair B, Liaqat A, Iqbal H, Menaa B, Razzaq A, Thiripuranathar G et al. Green and cost-effective synthesis of metallic nanoparticles by algae: safe methods for translational medicine. Bioengineering 16, 7(4), 129 (2020).
- Aboelfetoh EF, El-Shenody RA, Ghobara MM. Eco-friendly synthesis of silver nanoparticles using green algae (Caulerpa serrulata): reaction optimization, catalytic and antibacterial activities. Environ Monit Assess. 189(7), 349 (2017).
- Kumar B, Smita K, Sánchez E, Guerra S, Cumbal L. Ecofriendly ultrasound-assisted rapid synthesis of gold nanoparticles using Calothrix algae. Adv Nat Sci: Nanosci Nanotechnol. 7(2), 025013 (2016).
- Shanmugam R, Kannan C, Gurusamy A. Synthesis and Characterization of Antimicrobial Silver Nanoparticles Using Marine Brown Seaweed Padina tetrastromatica. Drug Invent Today. 4(10), 511–513 (2012).
- Khodashenas B, Ghorbani HR. Synthesis of silver nanoparticles with different shapes. Arab J Chem. 12(8), 1823–1838 (2019).
- Ramakritinan C, Kaarunya E, Shankar S, Kumaraguru A. Antibacterial effects of Ag, Au and bimetallic (Ag-Au) nanoparticles synthesized from red algae. Solid State Phenom. 201, 211–230 (2013).
- Chaudhary R, Nawaz K, Khan AK et al. An Overview of the Algae-Mediated Biosynthesis of Nanoparticles and Their Biomedical Applications. Biomolecules. 10(11), 1498 (2020).
- Rao MD, Pennathur G. Green synthesis and characterization of cadmium sulphide nanoparticles from Chlamydomonas reinhardtii and their application as photocatalysts. Mater Res Bull. 85, 64–73 (2017).
- Fouda A, Eid AM, Abdelkareem A, Said HA, El-Belely EF, Alkhalifah DHM et al. Phyco-synthesized zinc oxide nanoparticles using marine macroalgae, Ulva fasciata Delile, characterization, antibacterial activity, photocatalysis, and tanning wastewater treatment. Catalysts. 12(7), 756 (2022).
- Madhiyazhagan P, Murugan K, Kumar AN, Nataraj T, Subramaniam J, Chandramohan B et al. One pot synthesis of silver nanocrystals using the seaweed Gracilaria edulis: biophysical characterization and potential against the filariasis vector Culex quinquefasciatus and the midge Chironomus circumdatus. J Appl Phycol. 29, 649–659 (2017).
- Sampath S, Madhavan Y, Muralidharan M, Sunderam V, Lawrance AV, Muthupandian S. A review on algal mediated synthesis of metal and metal oxide nanoparticles and their emerging biomedical potential. J Biotechnol. 360, 92–109 (2022).
- AlNadhari S, Al-Enazi NM, Alshehrei F, Ameen F. A review on biogenic synthesis of metal nanoparticles using marine algae and its applications. Environ. Res. 194, 110672 (2021).
- Sinha SN, Paul D, Halder N, Sengupta D, Patra SK. Green synthesis of silver nanoparticles using fresh water green alga Pithophora oedogonia (Mont.) Wittrock and evaluation of their antibacterial activity. Appl Nanosci. 5, 703–709 (2015).
- Shen P, Yin Z, Qu G, Wang C. 11 - Fucoidan and Its Health Benefits. In: Bioactive Seaweeds for Food Applications. Qin Y ( Ed.). 223–238 Academic Press, London, UK (2018).
- Khalid M, Khalid N, Ahmed I, Hanif R, Ismail M, Janjua H. Comparative studies of three novel fresh water microalgae strains for synthesis of silver nanoparticles: insights of characterization, antibacterial, cytotoxicity and antiviral activities. J Appl Phycol. 29(4), 1851–1863 (2017).
- Venkatesan J, Kim SK, Shim MS. Antimicrobial, Antioxidant, and Anticancer Activities of Biosynthesized Silver Nanoparticles Using Marine Algae Ecklonia cava. Nanomaterials 6, 6(12), (2016).
- Dubey SP, Lahtinen M, Sillanpää M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 45(7), 1065–1071 (2010).
- Handayani W, Ningrum AS, Imawan C. The Role of pH in Synthesis Silver Nanoparticles Using Pometia pinnata (Matoa) Leaves Extract as Bioreductor. J Phys Conf Ser. 1428(1), 012021 (2020).
- Ghaemi M, Gholamipour S. Controllable Synthesis and Characterization of Silver Nanoparticles Using Sargassum Angostifolium. Iran J Chem Chem Eng. 36(1), 1–10 (2017).
- Ahmad N, Sharma S. Green synthesis of silver nanoparticles using extracts of Ananas comosus. Green and Sustainable Chemistry. 2(4), 141–147 (2012).
- Prathna T, Chandrasekaran N, Raichur AM, Mukherjee A. Kinetic evolution studies of silver nanoparticles in a bio-based green synthesis process. Colloids Surf A Physicochem Eng Asp. 377(1–3), 212–216 (2011).
- Karade VC, Dongale TD, Sahoo SC, Kollu P, Chougale AD, Patil PS et al. Effect of reaction time on structural and magnetic properties of green-synthesized magnetic nanoparticles. J Phys Chem Solids. 120, 161–166 (2018).
- Rai A, Singh A, Ahmad A, Sastry M. Role of Halide Ions and Temperature on the Morphology of Biologically Synthesized Gold Nanotriangles. Langmuir. 22(2), 736–741 (2006).
- Stavinskaya O, Laguta I, Fesenko T, Krumova M. Effect of Temperature on Green Synthesis of Silver Nanoparticles Using Vitex Agnus-castus Extract. Chem J Mold. 14, 117–121 (2019).
- Gupta M, Tomar RS, Mishra RK. Factors affecting biosynthesis of green nanoparticles. Our Heritage. 68(30), 10530–10555 (2020).
- Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloevera plant extract. Biotechnol. Prog. 22(2), 577–583 (2006).
- Guilger-Casagrande M, de Lima R. Synthesis of silver nanoparticles mediated by fungi: a review. Front Bioeng Biotechnol. 7, 287 (2019).
- Tang L, Yang X, Yin Q, Cai K, Wang H, Chaudhury I et al. Investigating the optimal size of anticancer nanomedicine. Proc Natl Acad Sci U S A. 111(43), 15344–15349 (2014).
- Mishra AR, Zheng J, Tang X, Goering PL. Silver nanoparticle-induced autophagic-lysosomal disruption and nlrp3-inflammasome activation in HepG2 cells is size-dependent. Toxicol Sci. 150(2), 473–487 (2016).
- Abdelmoneim HM, Taha TH, Elnouby MS, AbuShady HM. Extracellular biosynthesis, OVAT/statistical optimization, and characterization of silver nanoparticles (AgNPs) using Leclercia adecarboxylata THHM and its antimicrobial activity. Microb Cell Fact. 21(1), 277 (2022).
- El-Bendary MA, Afifi SS, Moharam ME, Elsoud MMA, Gawdat NA. Optimization of Bacillus subtilis NRC1 growth conditions using response surface methodology for sustainable biosynthesis of gold nanoparticles. Sci Rep. 12(1), 20882 (2022).
- Safaei M, Mozaffari HR, Moradpoor H, Imani MM, Sharifi R, Golshah A. Optimization of green synthesis of selenium nanoparticles and evaluation of their antifungal activity against oral Candida albicans infection. Adv Mater Sci Eng. 2022, 1376998 (2022).
- Ma L, Su W, Liu JX, Zeng XX, Huang Z, Li W et al. Optimization for extracellular biosynthesis of silver nanoparticles by Penicillium aculeatum Su1 and their antimicrobial activity and cytotoxic effect compared with silver ions. Mater Sci Eng C Mater Biol Appl. 77, 963–971 (2017).
- Krishnamoorthi R, Bharathakumar S, Malaikozhundan B, Mahalingam PU. Mycofabrication of gold nanoparticles: optimization, characterization, stabilization and evaluation of its antimicrobial potential on selected human pathogens. Biocatal Agric Biotechnol. 35, 102107 (2021).
- Liaqat N, Jahan N, Khalil Ur R, Anwar T, Qureshi H. Green synthesized silver nanoparticles: optimization, characterization, antimicrobial activity, and cytotoxicity study by hemolysis assay. Front Chem. 10, 952006 (2021).
- Dube P, Meyer S, Madiehe A, Meyer M. Antibacterial activity of biogenic silver and gold nanoparticles synthesized from Salvia africana-lutea and Sutherlandia frutescens. Nanotechnology. 31(50), 505607 (2020).
- Morgan RN, Ali AA, Alshahrani MY, Aboshanab KM. New Insights on Biological Activities, Chemical Compositions, and Classifications of Marine Actinomycetes Antifouling Agents. Microorganisms. 11(10), 2444 (2023).
- Kumari S, Tehri N, Gahlaut A, Hooda V. Actinomycetes mediated synthesis, characterization, and applications of metallic nanoparticles. Inorg Nano Met. 51(10), 1386–1395 (2020).
- Khalil MA, El-Shanshoury AER, Alghamdi MA, Alsalmi FA, Mohamed SF, Sun J et al. Biosynthesis of Silver Nanoparticles by Marine Actinobacterium Nocardiopsis dassonvillei and Exploring Their Therapeutic Potentials. Front Microbiol. 12, 705673 (2021).
- Hamed AA, Kabary H, Khedr M, Emam AN. Antibiofilm, antimicrobial and cytotoxic activity of extracellular green-synthesized silver nanoparticles by two marine-derived actinomycete. RSC adv. 10(17), 10361–10367 (2020).
- Abd El-Ghany MN, Hamdi SA, Korany SM, Elbaz RM, Emam AN, Farahat MG. Biogenic Silver Nanoparticles Produced by Soil Rare Actinomycetes and Their Significant Effect on Aspergillus-derived mycotoxins. Microorganisms. 11(4), 1006 (2023).
- Rosyidah A, Weeranantanapan O, Chudapongse N, Limphirat W, Nantapong N. Streptomyces chiangmaiensis SSUT88A mediated green synthesis of silver nanoparticles: characterization and evaluation of antibacterial action against clinical drug-resistant strains. RSC Adv. 12(7), 4336–4345 (2022).
- Zhao H, Maruthupandy M, Al-mekhlafi FA, Chackaravarthi G, Ramachandran G, Chelliah CK. Biological synthesis of copper oxide nanoparticles using marine endophytic actinomycetes and evaluation of biofilm producing bacteria and A549 lung cancer cells. J King Saud Univ Sci. 34(3), 101866 (2022).
- El-Housseiny GS, Ibrahim AA, Yassien MA, Aboshanab KM. Production and statistical optimization of Paromomycin by Streptomyces rimosus NRRL 2455 in solid state fermentation. BMC Microbiol. 21(1), 34 (2021).
- Ibrahim AA, El-Housseiny GS, Aboshanab KM, Yassien MA, Hassouna NA. Paromomycin production from Streptomyces rimosus NRRL 2455: statistical optimization and new synergistic antibiotic combinations against multidrug resistant pathogens. BMC Microbiol. 19(1), 18 (2019).
- El-Sayed SE, Abdelaziz NA, El-Housseiny GS, Aboshanab KM. Octadecyl 3-(3, 5-di-tert-butyl-4-hydroxyphenyl) propanoate, an antifungal metabolite of Alcaligenes faecalis strain MT332429 optimized through response surface methodology. Appl. Microbiol. Biotechnol. 104(24), 10755–10768 (2020).
- El-Housseiny GS, Aboulwafa MM, Aboshanab KA, Hassouna NAH. Optimization of Rhamnolipid Production by P. aeruginosa Isolate P6. J Surfactants Deterg. 19(5), 943–955 (2016).
- El-Housseiny GS, Aboshanab KM, Aboulwafa MM, Hassouna NA. Structural and Physicochemical Characterization of Rhamnolipids produced by Pseudomonas aeruginosa P6. AMB Express. 10(1), 201 (2020).
- Riyadi FA, Tahir AA, Yusof N, Sabri NSA, Noor M, Akhir F et al. Enzymatic and genetic characterization of lignin depolymerization by Streptomyces sp. S6 isolated from a tropical environment. Sci Rep 10(1), 7813 (2020).
- Della-Flora IK, de Andrade CJ. Biosynthesis of metallic nanoparticles by bacterial cell-free extract. Nanoscale. 15(34), 13886–13908 (2023).
- Sperandio FF, Huang YY, Hamblin MR. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat Antiinfect Drug Discov. 8(2), 108–120 (2013).
- Monmaturapoj N, Sri-On A, Klinsukhon W, Boonnak K, Prahsarn C. Antiviral activity of multifunctional composite based on TiO2-modified hydroxyapatite. Mater Sci Eng C Mater Biol Appl. 92, 96–102 (2018).
- Ondeck C, Habib A, Ohodnicki P, Miller K, Sawyer C, Chaudhary P et al. Theory of magnetic fluid heating with an alternating magnetic field with temperature dependent materials properties for self-regulated heating. J Appl Phys 105(7), 07B324 (2009).
- Farzin A, Etesami SA, Quint J, Memic A, Tamayol A. Magnetic Nanoparticles in Cancer Therapy and Diagnosis. Adv Healthc Mater. 9(9), e1901058 (2020).
- Cruz MM, Ferreira LP, Ramos J, Mendo SG, Alves AF, Godinho M et al. Enhanced magnetic hyperthermia of CoFe2O4 and MnFe2O4 nanoparticles. J Alloys Compd. 703, 370–380 (2017).
- Kobylinska N, Klymchuk D, Khaynakova O, Duplij V, Matvieieva N. Morphology-Controlled Green Synthesis of Magnetic Nanoparticles Using Extracts of ‘Hairy’ Roots: Environmental Application and Toxicity Evaluation. Nanomaterials. 12(23), 4231 (2022).