Abstract
Chemotherapeutic agents, endocrine therapy and radiotherapy used in the management of breast cancer are known to cause decreased bone mineral density, and thus, increased incidence of fractures. A majority (~60%) of the breast cancer patients in India are either estrogen (ER) or progesterone hormone receptor (PR) positive. Adjuvant treatment with aromatase inhibitors (AIs) is the treatment mainstay for hormone-sensitive disease in postmenopausal (PM) women, with reduced bone mineral density (BMD), which results in increased fracture rates. Zoledronic acid, alendronate, risedronate and denosumab have been the agents of choice for managing bone loss. Denosumab 60 mg is approved for gaining bone mass in women with breast cancer who are at high risk for fracture following adjuvant AI treatment. The phase III HALT-BC data indicate an improvement in BMD with denosumab and a 50% reduction in clinical fractures, with significant improvements seen at the lumbar spine, distal third of the radius, and total hip. Denosumab has several advantages over other bone modifying agents such as subcutaneous self-administration by the patient themselves, no requirement of hospitalization, no dose modifications in renal impairment, and low incidence of acute phase anaphylactic reactions. We review the available evidence of denosumab for managing bone loss in non-metastatic breast cancer patients.
Introduction
Globally, breast cancer is one of the most commonly diagnosed cancer, and the leading cause of mortality in women with cancer.Citation1,Citation2 Of all the cancer cases worldwide, 11.7% cases are breast cancer, of which 2,261,419 cases are newly diagnosed and 684,996 (6.9%) deaths reported, as per GLOBOCAN 2020.Citation3 In India, the incidence of breast cancer was 26.3% (178,361 cases) in 2020, which is highest among all other cancers in women.Citation4
Postmenopausal (PM) breast cancer accounts for ~80% of all newly diagnosed breast cancers.Citation5 Approximately 75–80% of the breast cancer cases are hormone-receptor (HR; either estrogen [ER] or progesterone [PR]) positive.Citation6,Citation7 In India, 50–60% of breast cancer patients are HR-positive.Citation8–Citation10 The American Society of Clinical Oncology and the European Society of Medical Oncology guidelines recommend oophorectomy or adjuvant therapy with endocrine agents in HR-positive disease to prevent recurrence or developing new breast cancer. These include selective ER modulators (SERMs), agonists for luteinizing hormone (LH)-releasing hormone (LHRH) and aromatase inhibitors (AIs).Citation6,Citation11–Citation13 The extensive use of adjuvant endocrine treatment has led to reduced mortality in HR-positive early breast cancer (EBC).Citation7
There is an increased risk of breast cancer occurrence in individuals with advanced age, particularly after 60 years,Citation14 who also have a high risk of developing osteoporosis. There is also a high risk of osteoporosis in breast cancer patients with low estrogen levels. Low estrogen levels cause an increased rate of bone resorption leading to decreased levels of bone mineral density (BMD), resulting in osteoporosis and increased fracture risk.Citation6,Citation15,Citation16
Bone Loss in Breast Cancer
In the healthy state, osteoblast-led bone formation and osteoclast-led resorption of bone are balanced,Citation17 but in breast cancer, there is an increased osteoclastic activity attributable to the raised transforming growth factor, cytokine, parathyroid hormone-related protein, tumor necrosis factor, insulin-like growth factor 1, and interleukin (IL)-1 and 6 levels.Citation18,Citation19 The increased risk of osteoporosis and fractures is observed in breast cancer patients with advanced age (>60 years), and low estrogen levels due to hormonal treatment or loss of ovarian function, especially in premenopausal women, due to several medications or surgical interventions.Citation6,Citation14–Citation16,Citation20
In PM women, a decreased bone density is observed due to natural physiological decline in estrogen levels.Citation21 The Women’s Health Initiative evaluated the incidence of fractures before and after cancer diagnosis in 146,959 PM women who did not have cancer history at baseline. A 9-year follow-up data reported a 55% higher fracture risk in women after diagnosis of breast cancer vs women who remained cancer-free.Citation5 Furthermore, chemotherapy, radiotherapy, endocrine therapy, and several other medications such as glucocorticoids have been linked with bone loss in PM breast cancer patients.Citation16,Citation22
Hormonal (Endocrine) and Anti-Estrogen Therapy-Induced Bone Loss
Gonadotropin-releasing hormone (GnRH) agonists decrease estrogen production in pre- or peri-menopausal women.Citation23 The ovarian suppression due to GnRH agonists (leuprolide, nafarelin, goserelin) reduces circulating estrogen leading to accelerated bone resorption and decreased BMD (~5%).Citation22,Citation24 Tamoxifen is a SERM anti-estrogen agent, which has been the cornerstone for managing the HR-positive disease, but a meta-analysis in PM women (n = 31,920) demonstrated that adjuvant AIs were superior over tamoxifen with 30% more reduction in the recurrence rate in ER-positive EBC.Citation25
AI Therapy: Bone Loss and Fracture Risk
The AIs are being used as a part of the treatment mainstay in PM women with hormone-sensitive EBC.Citation11 The AI use has demonstrated a 2- to 4-times greater decrease in the BMD as compared with physiologic postmenopausal BMD loss.Citation26 Several phase III randomized studies have reported a loss of up to 2.6% BMD in PM breast cancer patients within the first year of AI treatment ().Citation27–Citation31 In ATAC trial in PM women with EBC, the median percent change from baseline in BMD at 5-years with adjuvant anastrozole vs tamoxifen vs control (breast cancer women not receiving any treatment post-primary surgery) group were −6.08% vs +2.77% vs +1.35%, respectively, at the lumbar spine, and −7.24% vs +0.74% vs −2.81%, respectively, at the total hip.Citation32
Figure 1 Bone loss in women at 1 year. Data from Citation27–Citation31.
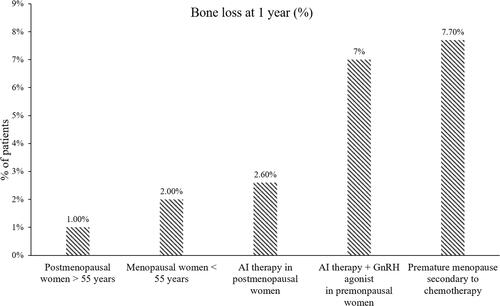
There is a high fracture risk in women receiving treatment with AI agents due to decreased estradiol in plasma as a result of blocking the activity of aromatase enzyme predominantly in the adipose tissues.Citation22,Citation33 Mincey et al identified 1354 patients receiving an AI and 11,014 controls via medical and pharmacy databases in a large managed care population and reported that the fracture rate was higher in patients receiving AIs vs controls (13.5% vs 10.3%, P = 0.001); the AI group had a 40% more risk of fractures. The incidence rate of fracture per 100 person-years was 35% higher for the AI group (incidence rate per 100 person-years: 8.6 for AI vs 6.36 for control groups).Citation34 Overall, the AI-associated bone loss was ≥2-fold higher vs healthy, age-matched, PM women, leading to significantly higher fracture rates regardless of the AI agent used.Citation35
Chemotherapy and Bone Loss
Adjuvant chemotherapy increases the loss of bone mass in patients with breast cancer. Burning et al reported that adjuvant chemotherapy with cyclophosphamide, methotrexate and 5-fluorouracil (CMF) led to premature ovarian failure in 71% of premenopausal women and had a significantly decreased BMD (1.17 g/cm2 vs 1.29 g/cm2) vs the controls (women pair-matched for age and year of breast cancer surgery who did not receive chemotherapy).Citation36 Bines et al reported the average chemotherapy-related amenorrhea rate at 68% in premenopausal breast cancer patients who were administered chemotherapy with adjuvant CMF.Citation37 The ovarian failure due to chemotherapy led to rapid bone loss in the lumbar spine (9.5%) and femoral neck (4.6%) within 2 years in premenopausal breast cancer patients.Citation38
Greep et al reported a significantly lower bone density at hips and spine in PM EBC patients receiving adjuvant chemotherapy vs patients who did not receive adjuvant chemotherapy.Citation39 In addition to an increase in the osteoclastic activity observed in breast cancer, increased bone resorption has been reported with several agents including taxanes, methotrexate, doxorubicin, cyclophosphamide, 5-fluorouracil and cisplatin.Citation16
Radiotherapy Induced Loss in Bone Mass
The use of radiotherapy in women with breast cancer can have adverse effects on bonesCitation40 with fractures reported.Citation41–Citation45 Though the precise mechanism of radiotherapy-induced loss in bone mass is uncertain, it is believed that radiotherapy through mechanisms of cell cycle arrest, altered differentiation, and increased apoptosis decrease active osteoblasts.Citation46 The use of radiotherapy for cervical, endometrial, and vaginal cancers has been associated with significant decrease in BMD.Citation44 In a study by Dybvik et al, 3% of women with breast cancer treated with breast radiotherapy required total hip replacement at 8 years.Citation45
Treatment for Loss of Bone Mass
To reduce the risk of fractures in cancer patients with non-metastatic disease and osteoporosis, treatment with bone-modifying agents (BMAs) may be offered as per the European Society of Medical Oncology (ESMO) guidelines.Citation26 The BMAs include intravenous (IV) or oral bisphosphonates and denosumab.Citation26 In addition, lifestyle interventions, exercise, optimized dietary vitamin D and calcium intake along with other pharmacological interventions are recommended.Citation26,Citation47 Several antiresorptive agents have emerged, which can reduce bone loss in patients with osteoporosis through inhibition of bone degeneration and/or acceleration of bone formation. The antiresorptive agents include bisphosphonates (alendronate, etidronate, ibandronate, risedronate, zoledronate), SERMs (tamoxifen, raloxifene) and hormonal (estrogens) agents.Citation25,Citation48,Citation49
In 2010, the US Food and Drug Administration (FDA) has approved denosumab, an inhibitor of the receptor activator of nuclear factor-kappa-Β (RANK)-ligand (RANKL).Citation50 As per the American Society of Clinical Oncology (ASCO) guidelines, oral or IV bisphosphonates and subcutaneous denosumab are efficacious options in this patient population.Citation26
Denosumab Prevents Bone Loss: Mechanism of Action
The RANKL is an essential mediator in bone destruction. Decreased estrogen levels cause an increased RANKL secretion with a simultaneous decrease in osteoprotegerin expression, thus, causing an increase in the osteoclast activity leading to reduction in the bone mass.Citation16 Denosumab inhibits the RANKL activity through binding with high specificity and affinity, resulting in diminished osteoclast recruitment, maturation and action, thus slowing down bone resorption.
What Do Guidelines Say?
As per the 2011 practical guidance by Hadji et al, intravenous zoledronic acid is a recommended agent, but denosumab and oral bisphosphonates could be considered in individual patients, for managing bone loss caused due to AI therapy in PM breast cancer patients. In 2017, an update of the practical guidance by Hadji et al included the use of denosumab along with bisphosphonates for the prevention of bone loss in breast cancer patients due to AI therapy.Citation51 The 2019 ASCO guidelines recommend use of BMAs, including denosumab, for nonmetastatic cancer patients with osteoporosis or who are at increased fracture risk.Citation26 As per the 2020 ESMO clinical practice guideline, denosumab is recommended as the choice of treatment for the prevention of fractures in postmenopausal women with EBC.Citation52 However, the 2019 St. Gallen consensus guidelines recommended bisphosphonate use in postmenopausal women with breast cancer, and substituting denosumab for bisphosphonates was not recommended.Citation53
Subcutaneous denosumab 60 mg 6 monthly is approved for the management of PM women with osteoporosis at high risk for fracture, women with glucocorticoid-induced osteoporosis at high risk for fracture and increased bone mass in women at high risk for fracture receiving adjuvant AI therapy for breast cancer.Citation50 We review the available evidence of denosumab administered at a dose of 60 mg as 6 monthly injections to manage the loss of bone mass in non-metastatic breast cancer (non-MBC) patients.
Denosumab for the Treatment of Bone Loss in PM Women with Low BMD
Denosumab reduces bone turnover markers and causes a rapid and sustainable increase in BMD in PM women with low BMD.Citation54 The long-term 10 years data of the FREEDOM trial and its 7-year open-label extension demonstrated that denosumab increased the BMD and decreased the risk of vertebral, nonvertebral and hip fractures in PM women with osteoporosis or low BMD.Citation55,Citation56
Denosumab in EBC Patients Receiving Adjuvant AI Therapy: Phase III Study
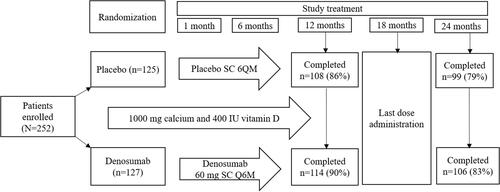
A phase III Hormone Ablation Bone Loss Trial in Breast Cancer (HALT-BC) trial in HR-positive non-MBC patients receiving placebo (n = 122) or subcutaneous 6-monthly denosumab 60 mg (n = 123) () for AI–induced bone loss demonstrated that BMD at lumbar spine significantly (P < 0.0001) increased with denosumab treatment after 12 (+5.5%) and 24 months (+7.6%) compared with placebo.Citation57 Lumbar spine BMD showed significant changes as early as 1 month with denosumab. These effects on BMD were independent of the AI duration before denosumab administration, the previous AI administered, or whether the patients had received tamoxifen previously, suggesting that denosumab was beneficial in these patient populations. Furthermore, there were no vertebral fractures reported whereas 6% of patients in each group had nonvertebral fractures.Citation57 A post hoc analysis to demonstrate the treatment effects on the preservation of BMD at 24 months showed >3% increase in the BMD at the lumbar spine in 80% patients receiving denosumab vs 13% patients receiving placebo; >6% increased BMD at the lumbar spine was seen in 50% patients receiving denosumab vs 3% receiving placebo. Denosumab showed increased BMD vs placebo at other sites after 2 years – total hip (4.7%), femoral neck (3.6%), and distal third of the radius (6.1%).Citation57
Figure 2 Study design: denosumab vs placebo in women receiving AI-therapy for breast cancer in phase III study by Ellis et al. Data from Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26(30):4875–4882. doi:10.1200/JCO.2008.16.3832.Citation57
Also, denosumab caused a rapid reduction in bone remodeling markers with 63% to 80% reductions in serum collagen type I cross-linked C-telopeptide (sCTx) levels and 71% to 73% reductions in procollagen I intact N-terminal (PINP) levels over 6 to 24 months during the study. The safety profile was comparable between denosumab and placebo groups.Citation57 The authors concluded that 6-monthly administration of denosumab led to a significant improvement in the BMD along with reduced bone turnover over 24 months, with similar rates of AEs vs placebo.Citation57
ABCSG-18 Trial
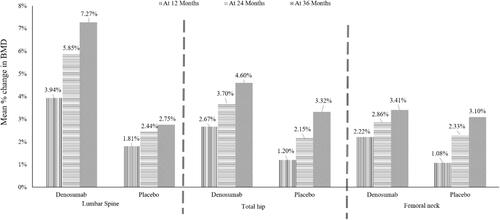
The ABCSG-18 trial studied the effects of 6-monthly denosumab 60 mg (n = 1711) or placebo (n = 1709) in HR-positive PM breast cancer patients receiving adjuvant AIs and showed that denosumab led to a 50% greater reduction in the risk of clinical fractures (hazards ratio: 0.50, 95% CI: 0.39–0.65; P < 0.0001) vs placebo. The losses in BMD at 36 months for denosumab vs placebo at the lumbar spine were 10% vs 74%, at the femoral neck were 22% vs 75% and at the total hip were 17% vs 78%. Furthermore, denosumab showed significantly greater (P < 0.0001) improvements in BMD vs placebo throughout the study (). The authors concluded that denosumab had significantly improved BMD and reduced fractures, which was independent of baseline BMD (T-score), age, HR receptor status and whether the patient had previously received AI or not.Citation58
Figure 3 Mean % changes in BMD at total lumbar spine, total hip, and femoral neck for denosumab versus placebo at 12, 24, and 36 months.
In a subsequent analysis of the ABSCG-18 trial, denosumab showed improved disease-free survival (DFS) vs placebo (14% vs 16.8%). The univariate analysis showed that denosumab significantly improved DFS in PM patients aged <60 years with ER and PR double-positive status (HR, 0.81 [0.67–0.98]) as compared with placebo. Overall, the researchers concluded that adjuvant denosumab was an effective treatment option irrespective of the baseline BMD status in PM women with HR +ve EBC.Citation59
Evidence from Asian Studies
In a phase II study, Nakatsukasa et al evaluated the effects of SC denosumab 60 mg given every 6 months on the BMD of lumbar spine and femoral necks in Japanese PM women (n = 100) with HR-positive postoperative breast cancer, who had low BMD (T-score −1.0 to −2.5) and who were scheduled or receiving AI treatment along with calcium and vitamin supplementation. Improvements in the BMD for the lumbar spine (4.7%), right femoral neck (2.4%), and left femoral neck (1.4%) were observed at 1 year. These improvements in BMD were independent of whether the patients received prior AI therapy or not. Furthermore, there were no incidences of non-traumatic fractures at 12 months in patients who received AI treatment and denosumab.Citation60
Denosumab treatment led to quick decreases in bone remodeling marker levels (tartrate-resistant acid phosphatase 5b [TRAP5b; 59.2%] and bone alkaline phosphatase [BAP; 52.2%]) at 1 year.Citation60 A subgroup analysis based on the risk factors for bone loss showed that BMI (<25, ≥25 kg/m2), time since menopause (≤5, >5 years), age (<65, ≥65 years), and baseline BMD T-score for right and left femoral necks (≤ −1.0, > −1.0) did not affect BMD when compared with previous AI therapy (before or with).Citation61
In a 12-month, nonrandomized, prospective study, Nakatsukasa et al evaluated SC 6-monthly denosumab 60 mg in Japanese PM HR-positive breast cancer patients (n = 102) with osteoporosis (T-score ≤ −2.5) who received adjuvant AI therapy. Approximately 58% of patients had initiated AI therapy before denosumab. The increases in BMD were 6.6% (95% CI: 5.7–7.6) for the lumbar spine, 3.3% (95% CI: 2.2–4.4) for the right femoral neck and 4.1% (95% CI: 2.7–5.5) for the left femoral neck. These improvements in the BMD were regardless of the previous AI therapy history. Also, no incidences of non-traumatic fractures were reported in patients who received AI treatment and denosumab. Reductions were observed with denosumab treatment in TRAP5b (59.5%) and BAP (49.8%) markers at 12 months.Citation62 In the 24-month follow-up of this study, denosumab treatment led to BMD improvements at the lumbar spine (7.0% [95% CI: 5.9–8.0]), right femoral neck (3.4% [95% CI: 2.4–4.5]) and the left femoral neck (3.6% [95% CI: 2.6–4.6]). There were no clinical fractures reported in patients receiving AI and denosumab.Citation63
Studies have evaluated the use of denosumab administration in patients currently receiving AI therapy or who had received AI therapy. There is little evidence suggesting the optimal time to initiate treatment with denosumab. From a retrospective case-control study in BC patients who received AI therapy categorized as ≤12 or >12 months of AI treatment, Scaturro et al concluded that early treatment with denosumab as compared to a delayed treatment might result in the prevention of incident fragility fractures of vertebra and hip, and a higher improvement in the lumbar spine and femoral neck T-scores.Citation64
Denosumab vs Zoledronic Acid
For IV zoledronic acid treatment, patients are required to visit hospitals every few months (3–12 months), which causes an additional burden related to transport, time for waiting, set up, 15-min infusion and monitoring.Citation65,Citation66 Furthermore, intravenous administration, monitoring of renal functions with required dose modifications, and acute-phase anaphylaxis reactions are a few other challenges with zoledronic acid treatment.Citation65–Citation67 Denosumab therapy overcomes these limitations as it is given subcutaneously, the patient can self-administer, thus improving the compliance, and it does not require hospitalization, has a lesser incidence of acute phase anaphylaxis reactions, while monitoring of renal functions and subsequent dose changes are not warranted.Citation52,Citation67–Citation69
A head-to-head comparison on the efficacy and safety of denosumab vs zoledronic acid in PM women with breast cancer is not available but denosumab has demonstrated a delayed time for developing skeletal-related events as compared with zoledronic acid in cancer patients with bone metastasis.Citation70
Mixed Treatment Meta-Analysis – Prevention of Fractures
Denosumab was compared with zoledronic acid for preventing fractures in PM EBC patients receiving AIs in a mixed treatment meta-analysis from five phase III studies (2 studies for denosumab and 3 studies for zoledronic acid; n = 5545). The risk of fractures was not different between immediate treatment (denosumab or zoledronic acid) vs delayed treatment (odds ratio [OR]: 0.78 and 0.88; respectively) at 12 months cut-off; however, it was reduced for immediate denosumab compared to delayed treatment which was not observed between immediate zoledronic acid vs delayed treatment (OR: 0.50 and 0.91; respectively) at the 36 months cut-off.Citation71
Denosumab vs Other Bone Modifying Agents (BMA) in Breast Cancer
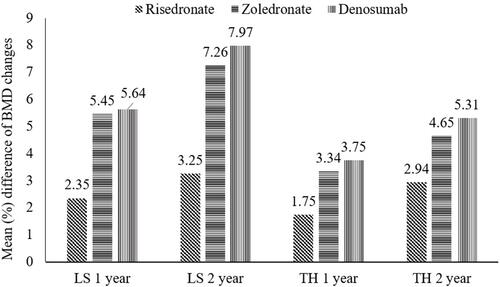
A recent meta‑analysis (n = 7699) compared different BMAs (denosumab: n = 1838, risedronate: n = 312, zoledronate: n = 1708, and no upfront treatment: n = 3841). The analysis reported that denosumab and zoledronate showed significantly increased BMD vs risedronate or no treatment groups at the lumbar spine and total hip at 12 and 24 months (). Denosumab led to a significant increase in BMDs at the total hip at 24 months vs zoledronate (0.66%).Citation72 The authors of the network meta-analysis concluded that denosumab treatment also led to an increase in the BMD in the cortical-bone-rich hip, along with significantly decreased risk of fracture.Citation72
Figure 4 Mean difference (95% CI) of BMD changes with each treatment compared with no upfront treatment.
Safety in Non-MBC Patients Receiving Adjuvant AI Therapy
The use of denosumab is contraindicated in patients with hypocalcemia and women of reproductive potential/pregnancy. The AEs of clinical interest with denosumab administration include osteonecrosis of the jaw (ONJ), hypocalcemia, multiple vertebral fractures after treatment stoppage, atypical femoral fractures, musculoskeletal pain, infections, skin reactions, and suppression of bone turnover. The AEs reported in the clinical studies of denosumab in PM women with breast cancer are back pain, musculoskeletal pain, hypercholesterolemia, pain in extremity, fatigue, cystitis, and arthralgia.Citation50,Citation57,Citation58,Citation60,Citation62,Citation63,Citation73 Furthermore, there were no reports of ONJ, hypocalcemia, or non-traumatic fractures in these clinical studies.Citation50,Citation57,Citation58,Citation60,Citation62,Citation63 enlists AEs with denosumab in non-metastatic breast cancer patients reported in clinical studies.
Table 1 AEs with Denosumab Treatment in Clinical Studies in Non-Metastatic Breast Cancer Patients
The ONJ is a rare but serious complication in PM women receiving bisphosphonates or denosumab. A systemic review of 3 randomized phase III studies demonstrated no significant (P = 0.11) difference in the incidence of ONJ between denosumab (52 ONJ cases in 2841 patients) and zoledronic acid (37 ONJ cases in 2836 patients).Citation74 Overall, ONJ incidence in clinical studies in patients with cancer ranges from 0.7% to 6.7% with bisphosphonatesCitation75 whereas it is 1.7% with denosumab.Citation76 Furthermore, a dental evaluation and suitable prophylactic dental measures are recommended before initiating denosumab therapy.Citation50 The increased multiple vertebral fracture risks after stoppage of denosumab remain a concern,Citation73,Citation77 and use of a bisphosphonate agent after stopping denosumab is recommended by the European Calcified Tissue Society to decrease this risk.Citation78
Denosumab: Dosage, Administration and Usage
Denosumab is supplied as a prefilled syringe (60 mg in 1 mL clear, colorless to pale yellow solution) and should be administered as a subcutaneous injection every 6 months. Denosumab should be removed from the refrigerator 15–30 minutes before administration. Once the drug is administered, a green safety guard which is present on the syringe needs to be manually slide over the needle and locked securely in place to minimize accidental needle sticks. However, caution is required that the green safety guard should be activated only after administration as if done before drug administration, it will lock and avert injection.Citation50
Denosumab Cost-Effectiveness
Financial constraints preclude many eligible patients from receiving appropriate treatment, particularly uninsured patients and in countries where health insurance is not common. The cost-effectiveness of denosumab versus bisphosphonates evaluated for the treatment of bone metastases from solid tumors by Ford et al concluded that denosumab was found to be cost-effective as compared with zoledronic acid but only with patient access scheme.Citation79 Several biosimilars of denosumab are being developed to provide a cost-effective alternative to Prolia® (innovator denosumab manufactured by Amgen, USA).Citation80 In India, biosimilar denosumab is approved and may provide a cost-effective alternative to Indian patients.
Summary
Bone loss and fractures are common complications of treatments used in breast cancer patients. Aromatase inhibitors are the standard of care for hormone receptor-positive early breast cancer, which are known to decrease the BMD and increase the risk of fractures. Bisphosphonates and denosumab are established agents in the management of bone health in cancer patients. Studies have confirmed that denosumab prevents loss of bone mass in HR-positive EBC patients. Denosumab prevents loss of bone mass, maintains bone mineral density, and reduces fracture risk. Denosumab 60 mg SC 6-monthly is recommended in women at high risk of fractures receiving adjuvant AI treatment for breast cancer. The availability of biosimilar denosumab in India may provide an alternative cost-effective option for these patients.
Key Clinical Takeaways
The use of hormonal therapy with adjuvant aromatase inhibitors (AI) increases the risk of bone loss and fractures in breast cancer patients.
The agents of choice for managing bone loss and preventing fracture include zoledronic acid, alendronate, risedronate and denosumab.
Denosumab (60 mg subcutaneous 6 monthly injections) is recommended for improving bone mass in women with breast cancer who receive adjuvant AI treatment and are at a high risk of fractures.
Denosumab has certain advantages over bisphosphonates that include no requirement of renal monitoring and dose modification in renally impaired patients, ease of self-administration (subcutaneous), and a tolerable safety profile.
Denosumab is not recommended in pregnancy and patients with hypocalcemia.
Due to the risk of hypocalcemia worsening, calcium and minerals (magnesium and phosphorus) should be monitored for 14 days after denosumab administration.
Serial dental examinations are suggested with suitable precautionary dentistry measures to avoid osteonecrosis of the jaw (ONJ).
When denosumab is planned to be stopped, a rapid transition to an alternative antiresorptive agent should be advocated to avoid the undue fracture risk.
Author Contributions
The author has made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The author reports no conflicts of interest in this work.
References
- Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016;17(S3):43–46. doi:10.7314/APJCP.2016.17.S3.43
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
- World Health Organization. International agency for research on cancer. The Global Cancer Observatory (Globocan 2020). World; 2021. Available from: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf. Accessed June 28, 2022.
- World Health Organization. International agency for research on cancer. The Global Cancer Observatory (Globocan 2020). India; 2021. Available from: https://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf. Accessed June 28, 2022.
- Chen Z, Maricic M, Aragaki AK, et al. Fracture risk increases after diagnosis of breast or other cancers in postmenopausal women: results from the Women’s Health Initiative. Osteoporos Int. 2009;20(4):527–536. doi:10.1007/s00198-008-0721-0
- Lipton A, Smith MR, Ellis GK, et al. Treatment-induced bone loss and fractures in cancer patients undergoing hormone ablation therapy: efficacy and safety of denosumab. Clin Med Insights Oncol. 2012;6:287–303. doi:10.4137/CMO.S8511
- Winer EP. Optimizing endocrine therapy for breast cancer. J Clin Oncol. 2005;23(8):1609–1610. doi:10.1200/JCO.2005.01.005
- Patnayak R, Jena A, Rukmangadha N, et al. Hormone receptor status (estrogen receptor, progesterone receptor), human epidermal growth factor-2 and p53 in South Indian breast cancer patients: a tertiary care center experience. Indian J Med Paediatr Oncol. 2015;36(2):117–122. doi:10.4103/0971-5851.158844
- Chatterjee K, Bhaumik G, Chattopadhyay B. Estrogen receptor and progesterone receptor status of breast cancer patients of eastern India: a multi-institutional study. South Asian J Cancer. 2018;7(1):5–6. doi:10.4103/sajc.sajc_60_17
- Desai SB, Moonim MT, Gill AK, et al. Hormone receptor status of breast cancer in India: a study of 798 tumours. Breast. 2000;9(5):267–270. doi:10.1054/brst.2000.0134
- Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: American society of clinical oncology clinical practice guideline update on ovarian suppression. J Clin Oncol. 2016;34(14):1689–1701. doi:10.1200/JCO.2015.65.9573
- Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v8–v30. doi:10.1093/annonc/mdv298
- Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23(3):619–629. doi:10.1200/JCO.2005.09.121
- Moser K, Patnick J, Beral V. Do women know that the risk of breast cancer increases with age? Br J Gen Pract. 2007;57(538):404–406.
- Planas Morin J, Morote Robles J. Skeletal complications of ADT: disease burden and treatment options. Asian J Androl. 2012;14(5):670–675. doi:10.1038/aja.2012.70
- Kalder M, Hadji P. Breast cancer and osteoporosis - management of cancer treatment-induced bone loss in postmenopausal women with breast cancer. Breast Care. 2014;9(5):312–317. doi:10.1159/000368843
- Boskey AL, Coleman R. Aging and bone. J Dent Res. 2010;89(12):1333–1348. doi:10.1177/0022034510377791
- Delmas PD, Fontana A. Bone loss induced by cancer treatment and its management. Eur J Cancer. 1998;34(2):260–262. doi:10.1016/S0959-8049(97)10135-6
- Hadji P, Gottschalk M, Ziller V, et al. Bone mass and the risk of breast cancer: the influence of cumulative exposure to oestrogen and reproductive correlates. Results of the Marburg breast cancer and osteoporosis trial (MABOT). Maturitas. 2007;56(3):312–321. doi:10.1016/j.maturitas.2006.09.005
- Ramaswamy B, Shapiro CL. Osteopenia and osteoporosis in women with breast cancer. Semin Oncol. 2003;30(6):763–775. doi:10.1053/j.seminoncol.2003.08.028
- Agostini D, Zeppa Donati S, Lucertini F, et al. Muscle and bone health in postmenopausal women: role of protein and Vitamin D supplementation combined with exercise training. Nutrients. 2018;10(8):1103. doi:10.3390/nu10081103
- Taxel P, Faircloth E, Idrees S, et al. Cancer treatment-induced bone loss in women with breast cancer and men with prostate cancer. J Endocr Soc. 2018;2(7):574–588. doi:10.1210/js.2018-00052
- Robertson JF, Blamey RW. The use of gonadotrophin-releasing hormone (GnRH) agonists in early and advanced breast cancer in pre- and perimenopausal women. Eur J Cancer. 2003;39(7):861–869. doi:10.1016/S0959-8049(02)00810-9
- Sverrisdóttir A, Fornander T, Jacobsson H, et al. Bone mineral density among premenopausal women with early breast cancer in a randomized trial of adjuvant endocrine therapy. J Clin Oncol. 2004;22(18):3694–3699. doi:10.1200/JCO.2004.08.148
- Dowsett M, Forbes JF, Bradley R, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–1352. doi:10.1016/S0140-6736(15)61074-1
- Shapiro CL, Van Poznak C, Lacchetti C, et al. Management of osteoporosis in survivors of adult cancers with nonmetastatic disease: ASCO clinical practice guideline. J Clin Oncol. 2019;37(31):2916–2946. doi:10.1200/JCO.19.01696
- Eastell R, Adams JE, Coleman RE, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol. 2008;26(7):1051–1057. doi:10.1200/JCO.2007.11.0726
- Gnant MF, Mlineritsch B, Luschin-Ebengreuth G, et al. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2007;25(7):820–828. doi:10.1200/JCO.2005.02.7102
- Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol. 2001;19(14):3306–3311. doi:10.1200/JCO.2001.19.14.3306
- Maillefert JF, Sibilia J, Michel F, et al. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161(4):1219–1222. doi:10.1016/S0022-5347(01)61639-2
- Gralow JR, Biermann JS, Farooki A, et al. NCCN task force report: bone health in cancer care. J Natl Compr Canc Netw. 2013;11(Suppl 3):S1–S50. doi:10.6004/jnccn.2013.0215
- Coleman RE. Effect of anastrozole on bone mineral density: 5-year results from the ’Arimidex’, Tamoxifen, Alone or in Combination (ATAC) trial. J Clin Oncol. 2006;24(18_suppl):511. doi:10.1200/jco.2006.24.18_suppl.511
- Hamilton A, Piccart M. The third-generation non-steroidal aromatase inhibitors: a review of their clinical benefits in the second-line hormonal treatment of advanced breast cancer. Ann Oncol. 1999;10(4):377–384. doi:10.1023/A:1008368300827
- Mincey BA, Duh MS, Thomas SK, et al. Risk of cancer treatment-associated bone loss and fractures among women with breast cancer receiving aromatase inhibitors. Clin Breast Cancer. 2006;7(2):127–132. doi:10.3816/CBC.2006.n.021
- Hadji P. Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit Rev Oncol Hematol. 2009;69(1):73–82. doi:10.1016/j.critrevonc.2008.07.013
- Bruning PF, Pit MJ, de Jong-Bakker M, et al. Bone mineral density after adjuvant chemotherapy for premenopausal breast cancer. Br J Cancer. 1990;61(2):308–310. doi:10.1038/bjc.1990.58
- Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14(5):1718–1729. doi:10.1200/JCO.1996.14.5.1718
- Saarto T, Blomqvist C, Välimäki M, et al. Chemical castration induced by adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy causes rapid bone loss that is reduced by clodronate: a randomized study in premenopausal breast cancer patients. J Clin Oncol. 1997;15(4):1341–1347. doi:10.1200/JCO.1997.15.4.1341
- Greep NC, Giuliano AE, Hansen NM, et al. The effects of adjuvant chemotherapy on bone density in postmenopausal women with early breast cancer. Am J Med. 2003;114(8):653–659. doi:10.1016/S0002-9343(03)00127-X
- Fontanges E, Fontana A, Delmas P. Osteoporosis and breast cancer. Joint Bone Spine. 2004;71(2):102–110. doi:10.1016/j.jbspin.2003.02.001
- Overgaard M. Spontaneous radiation-induced rib fractures in breast cancer patients treated with postmastectomy irradiation. A clinical radiobiological analysis of the influence of fraction size and dose-response relationships on late bone damage. Acta Oncol. 1988;27(2):117–122. doi:10.3109/02841868809090331
- Mavrogenis AF, Pala E, Romantini M, et al. Side effects of radiation in musculoskeletal oncology: clinical evaluation of radiation-induced fractures. Int J Immunopathol Pharmacol. 2011;24(1_suppl2):29–37. doi:10.1177/03946320110241S207
- Alouani E, Parent L, Massabeau C, et al. [Rib fracture following intra-operative radiotherapy for breast cancer. Case Report and local experience]. Cancer Radiother. 2020;24(1):64–66. French. doi:10.1016/j.canrad.2019.10.005
- Salcedo MP, Sood AK, Jhingran A, et al. Pelvic fractures and changes in bone mineral density after radiotherapy for cervical, endometrial, and vaginal cancer: a prospective study of 239 women. Cancer. 2020;126(11):2607–2613. doi:10.1002/cncr.32807
- Dybvik E, Furnes O, Fosså SD, et al. Pelvic irradiation does not increase the risk of Hip replacement in patients with gynecological cancer. A cohort study based on 8507 patients. Acta orthopaedica. 2014;85(6):652–656. doi:10.3109/17453674.2014.963784
- Wissing MD. Chemotherapy- and irradiation-induced bone loss in adults with solid tumors. Curr Osteoporos Rep. 2015;13(3):140–145. doi:10.1007/s11914-015-0266-z
- Reid DM, Doughty J, Eastell R, et al. Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat Rev. 2008;34(Suppl 1):S3–S18. doi:10.1016/j.ctrv.2008.03.007
- Novak S. [Antiresorptive agents in the treatment of osteoporosis]. Reumatizam. 2014;61(2):89–94. Croatian.
- Deal C. Osteoporosis therapies Bisphosphonates, SERMs, PTH, and new therapies. Clin Rev Bone Miner Metab. 2005;3(2):125–141. doi:10.1385/BMM:3:2:125
- Prolia (denosumab). Prescribing information. Accessed from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125320s205lbl.pdf. Accessed July 21, 2020.
- Hadji P, Aapro MS, Body -J-J, et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol. 2017;7:1–12. doi:10.1016/j.jbo.2017.03.001
- Coleman R, Hadji P, Body JJ, et al. Bone health in cancer: ESMO clinical practice guidelines†. Ann Oncol. 2020;31(12):1650–1663. doi:10.1016/j.annonc.2020.07.019
- Balic M, Thomssen C, Würstlein R, et al. Gallen/Vienna 2019: a brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care. 2019;14(2):103–110. doi:10.1159/000499931
- Lewiecki EM. Denosumab in postmenopausal osteoporosis: what the clinician needs to know. Ther Adv Musculoskelet Dis. 2009;1(1):13–26. doi:10.1177/1759720X09343221
- Cummings SR, Martin JS, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. doi:10.1056/NEJMoa0809493
- Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the Phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513–523. doi:10.1016/S2213-8587(17)30138-9
- Ellis GK, Bone HG, Chlebowski R, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26(30):4875–4882. doi:10.1200/JCO.2008.16.3832
- Gnant M, Pfeiler G, Dubsky PC, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9992):433–443. doi:10.1016/S0140-6736(15)60995-3
- Gnant M, Pfeiler G, Steger GG, et al. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):339–351. doi:10.1016/S1470-2045(18)30862-3
- Nakatsukasa K, Koyama H, Ouchi Y, et al. Effect of denosumab administration on low bone mineral density (T-score −1.0 to −2.5) in postmenopausal Japanese women receiving adjuvant aromatase inhibitors for non-metastatic breast cancer. J Bone Miner Metab. 2018;36(6):716–722. doi:10.1007/s00774-017-0884-x
- Nakatsukasa K, Koyama H, Ouchi Y, et al. Effect of denosumab on bone mineral density in Japanese women with osteopenia treated with aromatase inhibitors for breast cancer: subgroup analyses of a Phase II study. Ther Clin Risk Manag. 2018;14:1213–1218. doi:10.2147/TCRM.S167579
- Nakatsukasa K, Koyama H, Ouchi Y, et al. Effects of denosumab on bone mineral density in Japanese women with osteoporosis treated with aromatase inhibitors for breast cancer. J Bone Miner Metab. 2019;37(2):301–306. doi:10.1007/s00774-018-0917-0
- Nakatsukasa K, Koyama H, Ouchi Y, et al. Effect of denosumab on low bone mineral density in postmenopausal Japanese women receiving adjuvant aromatase inhibitors for non-metastatic breast cancer: 24-month results. Breast Cancer. 2019;26(1):106–112. doi:10.1007/s12282-018-0896-y
- Scaturro D, de Sire A, Terrana P, et al. Early Denosumab for the prevention of osteoporotic fractures in breast cancer women undergoing aromatase inhibitors: a case-control retrospective study. J Back Musculoskelet Rehabil. 2022;35(1):207–212. doi:10.3233/BMR-210012
- Poon Y, Pechlivanoglou P, Alibhai SMH, et al. Systematic review and network meta-analysis on the relative efficacy of osteoporotic medications: men with prostate cancer on continuous androgen-deprivation therapy to reduce risk of fragility fractures. BJU Int. 2018;121(1):17–28. doi:10.1111/bju.14015
- Khan AA, Morrison A, Hanley DA, et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res. 2015;30(1):3–23. doi:10.1002/jbmr.2405
- Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–5139. doi:10.1200/JCO.2010.29.7101
- Doria C, Leali PT, Solla F, et al. Denosumab is really effective in the treatment of osteoporosis secondary to hypogonadism in prostate carcinoma patients? A prospective randomized multicenter international study. Clin Cases Miner Bone Metab. 2016;13(3):195–199. doi:10.11138/ccmbm/2016.13.3.195
- Hadji P, Kyvernitakis I, Kann PH, et al. GRAND-4: the German retrospective analysis of long-term persistence in women with osteoporosis treated with bisphosphonates or denosumab. Osteoporos Int. 2016;27(10):2967–2978. doi:10.1007/s00198-016-3623-6
- Imai H, Saijo K, Yamada H, et al. Efficacy and safety of denosumab versus zoledronic acid in delaying skeletal-related events in patients with gastrointestinal cancer, pancreas-biliary system cancer, and other rare cancers. J Bone Oncol. 2017;6:37–40. doi:10.1016/j.jbo.2016.10.002
- Abdel-Rahman O. Denosumab versus zoledronic acid to prevent aromatase inhibitors-associated fractures in postmenopausal early breast cancer; a mixed treatment meta-analysis. Expert Rev Anticancer Ther. 2016;16(8):885–891. doi:10.1080/14737140.2016.1192466
- Miyashita H, Satoi S, Kuno T, et al. Bone modifying agents for bone loss in patients with aromatase inhibitor as adjuvant treatment for breast cancer; insights from a network meta-analysis. Breast Cancer Res Treat. 2020;181(2):279–289. doi:10.1007/s10549-020-05640-3
- Deeks ED, Deeks ED. Denosumab: a review in postmenopausal osteoporosis. Drugs Aging. 2018;35(2):163–173. doi:10.1007/s40266-018-0525-7
- Van den Wyngaert T, Wouters K, Huizing MT, et al. RANK ligand inhibition in bone metastatic cancer and risk of osteonecrosis of the jaw (ONJ): non bis in idem? Support Care Cancer. 2011;19(12):2035–2040. doi:10.1007/s00520-010-1061-0
- Qaisi M, Hargett J, Loeb M, et al. Denosumab related osteonecrosis of the jaw with spontaneous necrosis of the soft palate: report of a life threatening case. Case Rep Dent. 2016;2016:5070187. doi:10.1155/2016/5070187
- Qi WX, Tang LN, He AN, et al. Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: a meta-analysis of seven randomized controlled trials. Int J Clin Oncol. 2014;19(2):403–410. doi:10.1007/s10147-013-0561-6
- Gonzalez-Rodriguez E, Aubry-Rozier B, Stoll D, et al. Increased risk of multiple spontaneous vertebral fractures at denosumab discontinuation must be taken into account. J Clin Oncol. 2020;38(14):1641–1642. doi:10.1200/JCO.19.02678
- Tsourdi E, Langdahl B, Cohen-Solal M, et al. Discontinuation of Denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11–17. doi:10.1016/j.bone.2017.08.003
- Ford J, Cummins E, Sharma P, et al. Systematic review of the clinical effectiveness and cost-effectiveness, and economic evaluation, of denosumab for the treatment of bone metastases from solid tumours. Health Technol Assess. 2013;17(29):1–386. doi:10.3310/hta17290
- Chen H, Chen W, Yuan F, et al. Pharmacokinetics, pharmacodynamics, safety and immunogenicity of CMAB807, a new denosumab biosimilar, in healthy Chinese subjects. Front Pharmacol. 2022;13:821944. doi:10.3389/fphar.2022.821944
