Abstract
Due to an unmet clinical need for treatment, the first in class proteasome inhibitor, bortezomib, moved from drug discovery to FDA approval in multiple myeloma in an unprecedented eight years. In the wake of this rapid approval arose a large number of questions about its mechanism of action and toxicity as well as its ultimate role in the treatment of this disease. In this article, we briefly review the preclinical and clinical development of the drug as the underpinning for a systematic review of the large number of clinical trials that are beginning to shed some light on the full therapeutic potential of bortezomib in myeloma. We conclude with our current understanding of the mechanism of action of this agent and a discussion of the novel proteasome inhibitors under development, as it will be progress in these areas that will ultimately determine the true potential of proteasome inhibition in myeloma.
Keywords:
The proteasome
The proteasome is a large, hollow cylindrical multi-enzymatic complex that is present in both the cytoplasm and the nucleus of all eukaryotic cells. It is necessary for the degradation of intracellular proteins in eukaryotic cells whereas extracellular/transmembrane proteins are typically degraded by the aggresome/lysosomal pathway.Citation1 The proteins degraded by the former pathway are involved in signal transduction pathways that regulate cell growth and proliferation including: cell-cycle-regulatory proteins (cyclins A, B, D, and E; p21 and p27), the tumor suppressor p53, NF-κB, and adhesion molecules.Citation4
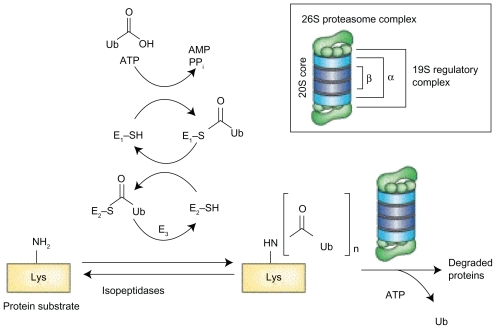
The formation of the 26S proteasome occurs in an ATP dependent fashion, when a 20S catalytic core is capped by a 19S regulatory subunit at both ends (see ).Citation1 The lysine residues of those proteins targeted for degradation are covalently modified with a polyubquitin protein chain, with each ubiquitin tag consisting of a 76 amino acid polypeptide. The ubiquitin chain is recognized by the lid-like structure of the 19S subunit and then removed. The target protein is then denatured in an energy dependent manner by the 6 ATPases at the base of the 19S subunit and threaded into the center of the 20S subunit.Citation2
Figure 1A Structure of 26S proteasome: the 26S proteasome is formed when the 20S catalytic core is capped by 19S regulatory subunits at both ends in an ATP dependent fashion.
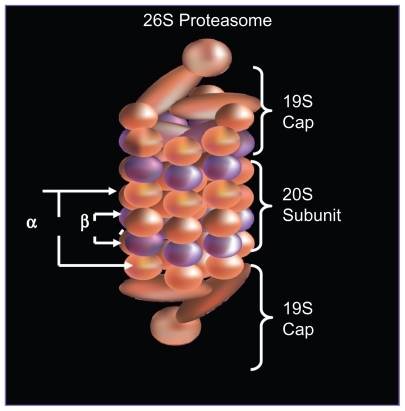
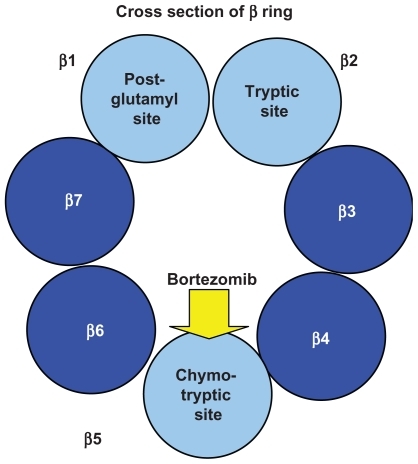
As shown in , the 20S subunit is itself comprised of four rings, 2 α and 2 β subunits. Within the channel at the center, threonine residues of the indicated β units wield catalytic activity comparable to three enzymes: chymotrypsin (β5), trypsin (β2), and post-glutamyl peptide hydrolase (β1).
Preclinical development of bortezomib
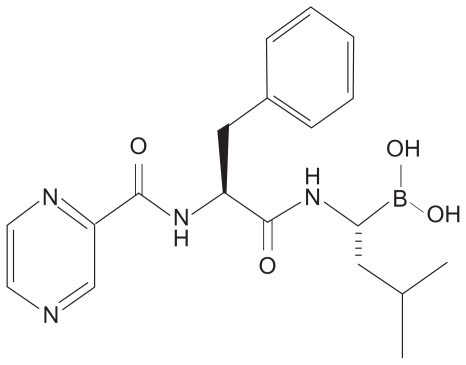
In 1993, the company Myogenics was founded by Alfred Goldberg to decrease muscle wasting/cachexia by inhibiting the ubiquitin–proteasome pathway. A team of enzymologists created the first inhibitors of the proteasome: peptide aldehyde analogs of the proteasome’s chymotrypsin-like substrates. Chemists then created a dipeptide boronic acid analog that would eventually come to be known as bortezomib ().Citation3
When applied to the National Cancer Institute’s 60-cell line screen, bortezomib demonstrated potent growth inhibition against a broad range of tumor types.Citation5 Importantly, confirmation was also obtained that the intended biologic target was being inhibited. Additional studies with human myeloma cell lines and freshly isolated from myeloma patients confirmed that bortezomib not only inhibited tumor proliferation but also induced apoptosis and overcame drug resistance.Citation6
The growth inhibition of bortezomib was extended to the in vivo setting using a human plasmacytoma xenograft mouse model. Relative to controls, bortezomib treatment resulted in improved overall survival.Citation7 A fluorogenic pharamacodynamic assay was developed to measure the relative chymotryptic and tryptic activities of the proteasome in peripheral blood mononuclear cells.Citation8 This assay showed that bortezomib-mediated inhibition of the chymotrypsin-like activity of the 26S mammalian proteasome () was dose-dependent and reversible,Citation7 thus helping guide dosing and optimize dose escalation in phase I studies.
Clinical development of bortezomib – relapsed/refractory multiple myeloma
In a phase I trial among patients with advanced hematological malignancies, bortezomib was noted to have activity in patients with refractory myeloma; among nine patients with multiple myeloma antitumor activity was noted in almost all patients including 1 patient achieving a complete response.Citation4 A subsequent, large, multicenter phase II trial involving 202 patients with relapsed, refractory myeloma yielded a 35% overall response rate which was comprised of a 4% complete remission (CR), 6% near CR, 18% partial remission (PR), and 7% minimal response (MR).Citation9 It was on the basis of this trial in large part, that bortezomib was approved by the United States Food and Drug Administration (FDA) in 2003, thus resulting in a remarkably short 8 years from drug discovery to FDA approval.
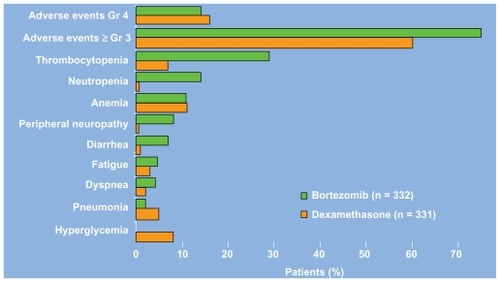
The phase III Assessment of Proteasome Inhibition for Extending Remissions (APEX) study compared bortezomib (1.3 mg/m2 on days 1,4, 8, and 11 by intravenous push for eight 3-week cycles) to high dose dexamethasone (40 mg days 1–4, 9–12, and 17–20 orally for four 5-week cycles and then days 1–4 for five 4-week cycles) in 669 patients with relapsed multiple myeloma. The study was halted on interim analysis because bortezomib treatment resulted in higher response rates (38 vs 18%), longer time to progression (6.22 months vs 3.49 months), and improved overall survival. The median time to response was 43 days in both groups.Citation10 In an updated analysis, based on a median follow up of 22 months, the median overall survival was 29.8 vs 23.7 months (P = 0.0272) despite a 62% crossover rate from dexamethasone to boretzomib.Citation11 As shown in , a comparison of the Grade 3/4 adverse events in each arm reveals that bortezomib treatment is associated with an increased incidence of thrombocytopenia, neutropenia, peripheral neuropathy, and diarrhea.Citation10 The thrombocytopenia and neuropathy are discussed in further detail below. Despite these toxicities, a prospective comparsion of health-related quality of life found improved outcomes with bortezomib.Citation12 Of note, subgroup analysis has also found no difference in safety or efficacy in patients with varying degrees of renal insufficiency.Citation13
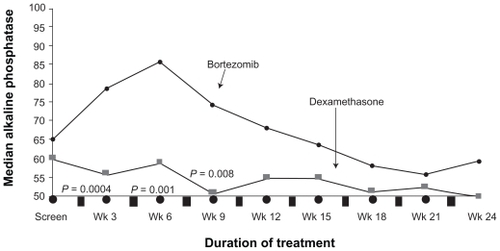
Bortezomib therapy also appears to have beneficial effects on the bone. When alkaline phosphatase levels were compared with responders and nonresponders in the APEX study, the most powerful predictor of a response was a 25% increase in alkaline phosphatase at week 6 (P < 0.0001) ().Citation14 Laboratory work has confirmed the ability of bortezomib to not only inhibit osteclast mediated bone destruction, but also directly induce bone formation.Citation15,Citation16 Interestingly, as shown in , the increase in alkaline phosphatase was not observed on the dexamethasone arm, even in the responders.Citation14 This increase has also been recently found to be associated with improved time to progression. Citation17
Figure 4 Median levels of alkaline phosphatase levels of patients with multiple myeloma who responded to treatment with bortezomib and dexamethasone in the APEX trial. Reproduced with permission from Zangari M, Esseltine D, Lee CK, et al. Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. Br J Haematol. 2005;131(1):71–73.Citation14 Copyright © 2005 John Wiley and Sons.
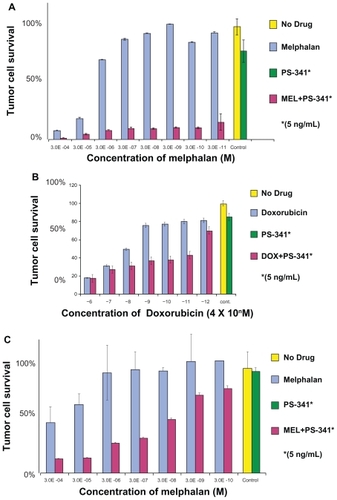
As the safety and efficacy results for bortezomib monotherapy were accumulating, combination therapy was being explored in the preclinical setting. Hideshima et al found that the growth inhibitory effects of bortezomib and dexamethasone on a myeloma cell line were additive ().Citation6 Ma et al found that the addition of a noncytotoxic dose of bortezomib to chemotherapeutic agents could increase the sensitivity of chemoresistant myeloma cells by 100,000 to 1,000,000-fold without affecting normal hematopoietic cells ().Citation18
Figure 5A Dexamethasone sensitive myeloma cells (MM.1S) cultured with 0.001 to 0.625 × 10−6 M. Dexamethasone in control media (□) and with bortezomib 0.0025 (▧) or 0.005 (■) × 10−6 M. Reproduced with permission from Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61(7): 3071–3076.Citation6 Copyright © 2001 American Association for Cancer Research.
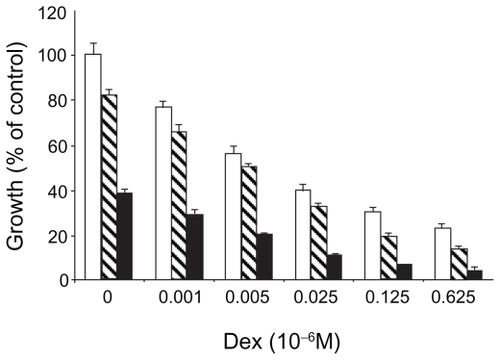
Figure 5B The addition of bortezomib to chemotherapeutic agents results in synergistic cytotoxicity in multiple myeloma cells: A) Melphalan-resistant cell line (RPMI8228/LR) treated for 24 hours with varying concentrations of melphalan alone or in combination with a noncytotoxic dose of bortezomib; B) Doxorubicin-resistant cell line (U266/dox4) treated for 24 hours with varying concentrations of doxorubicin alone or in combination with a noncytotoxic dose of bortezomib. C) Fresh myeloma cells treated with varying concentrations of melphalan alone or in combination with a noncytotoxic dose of bortezomib. Reproduced with permission from Ma MH, Yang HH, Parker K, et al. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res. 2003;9(3):1136–1144.Citation18 Copyright © 2003 American Association for Cancer Research.
The largest published phase III clinical trial combining bortezomib with another chemotherapeutic agent randomized 646 myeloma patients with 2 or more lines of prior therapy to receive either the standard dose/schedule of bortezomib alone or with liposomal doxorubicin (PLD) on Day 4. The combination therapy was associated with a higher incidence of grade 3/4 events 80 vs 64% (due to myelosuppression, constitutional and gastrointestinal symptoms, and hand foot syndrome). There was also no significant difference in response rates. However, the time to progression (9.3 vs 6.5 months, P = 0.000004) and overall survival at 15 months (76% vs 65%, P = 0.03) both favored bortezomib with PLD.Citation19 This steroid sparing regimen is an excellent treatment option especially for those patients intolerant of steroids due to psychosis or brittle diabetes.
The proteasome inhibitor bortezomib has now been studied in combination with each of the three other classes of drugs with activity in myeloma: steroids, immunomodulatory agents (IMids), and conventional chemotherapeutics (anthracyclines and alkylating agents). For those phase I/II studies with 30 or more evaluable patients, summaries of the recent response data of doublet (), triplet (), and multiagent () permutations of the four classes of drugs in relapsed/refractory myeloma are shown in the indicated tables.
Table 1 Clinical trials of bortezomib in doublet-drug combination regimens
Table 2 Clinical trials of bortezomib in triplet-drug combination regimens
Table 3 Clinical trials of bortezomib in multiple drug combination regimens
Bortezomib in previously untreated multiple myeloma
The only published phase III study of bortezomib in untreated myeloma is the Velcade as Initial Standard Therapy in Multiple Myeloma: Assessment with Melphalan Prednisone (VISTA) study. In this study, 682 nontransplant eligible patients with untreated myeloma were randomized to receive either melphalan and prednisone alone (MP) or with bortezomib (VMP) at the doses and schedule shown in .
Figure 6 Chemotherapy schedule of bortezomib, melphalan, and prednisone (VMP) and melphalan and prednisone (MP) in the VISTA trial.
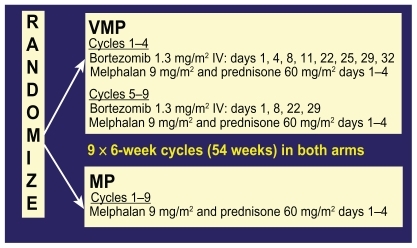
Overall response rate for VMP was 71% vs 35% for MP with a very impressive CR rate of 30% vs 4% (P < 0.001 for both comparisons). Of note, a 30% CR rate compares very favorably to the CR rates obtained for patients who receive high dose melphalan chemotherapy with autologous stem cell rescue (for which none of the patients in the VISTA study were eligible). With a median follow up of 16.3 months, the hazard ratio for overall survival for the VMP group was 0.61 (P = 0.008).Citation34
All of the following efficacy outcomes were also significantly better for the VMP group relative to MP: median time to first response (1.4 vs 4.2 months), duration of response (20 vs 13 months), and treatment-free interval (17 months vs 9 months). The improved outcomes were seen in all subgroups, including age >75, creatinine clearance <60, and high risk cytogenetics (translocation (t) (4;14), t(14,16), or chromosome 17 deletion).Citation34 Of note, the lack of effect of high risk cytogenetics on efficacy with borezomib-based regimens has been a consistent finding across all front line studies.Citation34–Citation37
Toxicities ≥ Grade 3 that were higher in the VMP arm included peripheral neuropathy (14 vs 0%), nausea/vomiting/diarrhea, fatigue/asthenia, and zoster. Herpes zoster was observed in 14% of VMP patients vs 4% in MP, but among patients receiving antiviral prophylaxis, the rate was 3%.Citation34
Of note, a recent analysis of varicella zoster virus (VZV) reactivation in the APEX study also found a significantly increased incidence in patients receiving bortezomib relative to dexamethasone 13% vs 5%, P = 0.0002.Citation38 Antiviral prophylaxis is therefore recommended for all myeloma patients receiving bortezomib based regimens. A variety of agents and regimens have been used including acyclovir 400 mg daily, valacyclovir 250 or 500 mg daily, famciclovir 500 mg daily.Citation39,Citation40 Doses should be decreased in the setting of renal insufficiency as the metabolites of these drugs can accumulate and cause profound neuropsychiatric changes.Citation41,Citation42 Finally, the varicella vaccine, which contains live attenuated virus, is not licensed for use in patients with neoplasms affecting the bone marrow or lymphatic systems.Citation43
Additional follow up data presented recently indicated that despite the fact that 43% of MP patients subsequently received bortezomib upon progression, intention to treat analysis still demonstrated increased overall survival for the VMP group. Moreover, there was no difference in response to IMiD-based second line treatments between the two groups.Citation44 The results of the VISTA study therefore demonstrate clearly improved efficacy with VMP without any adverse long term consequences of upfront bortezomib based regimens.
There are also several large phase III studies ongoing evaluating the use of bortezomib as induction therapy prior to stem cell transplantation (see ). The Franchophone Myeloma Intergroup (IFM) 2005-01 study randomized 482 patients to receive either bortezomib-dexamethasone (Vel-Dex) or the traditional VAD. Of the 442 evaluable patients, the CR rates were 10% vs 3%, CR + near CR 19% vs 8%, and ≥ PR 83 vs 66% without any impairment in stem cell harvest. Moreover, the higher quality of responses persisted after the first melphalan 200 mg/m2 followed by autologous stem cell rescue, with CR/near CR rates of 40 vs 22%, P = 0.0001.Citation36 Preliminary data from two other phase III studies comparing bortezomib in combination with doxorubicin and dexamethasone (PAD) to traditional VADCitation37 and bortezomib, thalidomide, and dexamethasone (VTD) to TDCitation35 also found improved CR/nCR rates (23% vs 9%, P < 0.015 and 55% vs 32%, P < 0.001 respectively) after autologous stem cell transplantation.
Table 4 Summary of phase II trials in previously untreated MM
These improvements in CR rates after transplant with bortezomib based induction therapies have clinical significance. Two large published phase III studies comparing single vs tandem autologous stem cell transplants in myeloma found that patients who did not achieve a CR/near CR after the first autologus stem cell transplant were the ones that could benefit from a second SCT.Citation48,Citation49 Therefore, the higher CR rates being obtained with novel induction regimens may obviate the need for a second autologous transplant – with its attendant mortality, morbidity, and cost.
While the details of the various bortezomib based front line regimens are beyond the scope of this review, a summary of the responses noted to date are shown in . With the understanding that response rates in single/few institution phase II studies are typically higher than those obtained in phase III multi-institutional settings, a regimen that stands out is bortezomib, lenalidomide, and dexamethasone (VRD). With 65 evaluable patients, the combination of bortezomib, lenalidomide, and dexamethasone resulted in a 100% response rate and a 38% CR/nCR rate.Citation50 A caveat of course, is that lenalidomide based induction regimens often result in inadequate stem cell harvests with granulocyte colony stimulating factor (GCSF) mobilization and therefore require cyclophosphamide or the recently FDA-approved CXCR inhibitor, plerixafor, to ensure adequate stem cell harvests.
Table 5 Phase I/II and II combination trials in untreated myeloma
Mechanism of action of bortezomib
While rational drug design and pharmacodynamic assays identified and confirmed the proteasome as the biologic target, without an understanding of the exact mechanism of action, the full therapeutic potential of proteasome inhibition cannot be realized. Research has focused on three possible themes that will be discussed below: the transcription factor NF-κB, the interaction of the pro-apoptotic factor NOXA and the c-myc oncogene, and finally, the transcription factor x-box binding protein 1 (XBP-1) and the unfolded protein response.
Initial focus was on the impact of bortezomib on NF-κB, which promotes tumor cell survival and proliferation. The inhibitor protein I-κB binds NF-κB in the cytoplasm, thereby rendering NF-κB inactive. A variety of cytokines and other cellular stimuli result in the phosphorylation and ubiquitination of I-κB by E3 ligase, thus targeting it for proteasome mediated degradation ().Citation2
Figure 7 NF-κB activation pathway. The inhibitor protein I-κB, when bound to NF-κB in the cytoplasm, renders NF-κB inactive. A variety of cellular stimuli result in the phosphorylation and ubiquitination of I-κB, thereby targeting it for proteasome mediated degradation. Bortezomib, by inhibiting the proteasome, results in increased I-κB inhibition of NF-κB, thus resulting in inhibition of tumor growth. Reproduced with permission from Adams J. Potential for proteasome inhibition in the treatment of cancer. Drug Discov Today. 2003;8(7):307–315.Citation3 Copyright © 2003 Elsevier.
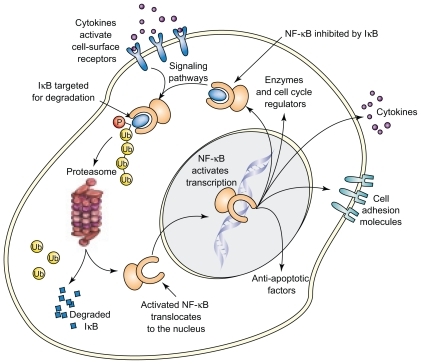
Bortezomib, by blocking the latter process, results in increased availability of I-κB to inhibit NF-κB, resulting in the inhibition of tumor cell growth. Gene expression profiling studies in patients with myeloma who responded to bortezomib treatment also highlighted pathways such as NF-κB activity and cell adhesion, thereby confirming preclinical studies.Citation69
Additional work by Hideshima et al revealed that bortezomib activation seemed to be dependent on the activation of c-Jun NH2-terminal kinase (JNK) and subsequently caspases-8 and caspase-3 that elicit DNA damage and apoptosis. In parallel, bortezomib was noted to be associated with the up-regulation of p53.Citation70 While these inital studies shed some light on the mechanism of action, it is unclear if the changes observed in NF-κB and JNK are a cause or the result of the death process. Indeed, more recent studies suggest the antimyeloma activity of proteasome inhibition is actually p53 independent.Citation71
When myeloma cell lines are exposed to bortezomib, the proapoptotic factor NOXA is induced in a concentration dependent manner accompanied by the activation of caspases. NOXA is also induced by p53 and other transcriptional factors such as hypoxia-inducible factor 1 (HIF-1) and E2F-1, consistent with its involvement in the response to many types of cellular stress. Human NOXA contains one BH3 (Bcl-2 homology 3) domain, which has a high affinity for the antiapoptotic factor Mcl-1. Because Mcl-1 is a target for ubiquitination, proteasome inhibition increases levels of Mcl-1. The induction of NOXA is therefore essential to override high Mcl-1 levels and allow for the activation of the apoptotic machinery in response to bortezomib.Citation72 Also, NOXA’s interaction with anti-apoptotic members of the Bcl-2 family causes release of cytochrome c into the cytosol, leading to the activation of caspases and induction of apoptosis ().Citation73
Figure 8 Alteration in levels of Mcl-1 and NOXA results in apoptosis. Proteasome inhibiton increases levels of the proapoptoic factor NOXA, which then can override the concurrent increase in the anti-apoptic factor Mcl-1, thereby inducing the activation of caspases, and resulting in apoptosis.
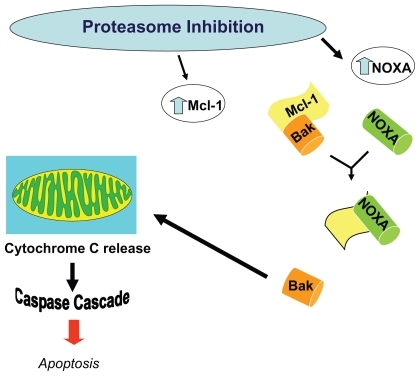
Bortezomib induction of NOXA is also seen in melanoma and mantle cell lymphoma cell lines, with antisense NOXA oligonucleotide (but not control) resulting in a decrease in bortezomib induced apoptosis.Citation71,Citation74 Of note, apoptosis/NOXA induction is not induced by conventional chemotherapeutic agents but is induced by other proteasome inhibitors (eg, MG132), suggesting a possible class specific effect.Citation73,Citation75 To understand why NOXA is preferentially induced in tumor cells, the myriad transcription factors with consensus binding sites at the NOXA promoter were restricted to those that are conserved (as NOXA itself is) across mammalian species and also dysregulated by proteasome inhibition and tumorogenesis. The oncogene c-myc emerged as a candidate mediator of tumor specificity. Indeed, when c-myc levels were decreased by RNA interference, the tumor cell-specific induction of NOXA was abrogated. Exogenous c-myc also increased the sensitivity of nonmalignant cells to proteasome inhibition by bortezomib.Citation72
The interaction of NOXA and c-myc also provides a possible rationale for the encouraging clinical data noted thus far when histone deacetylase (HDAC) inhibitors are combined with bortezomib. The transcriptional activity of c-myc at the NOXA promoter can be favored by chromatin remodeling or modification proteins (including histone acetyl transferases, with acetylated histone H3 being a classical cofactor for myc).Citation72 HDAC inhibition is also thought to interfere with the targeting of ubiquinated proteins via the aggresome for eventual autophagy/degradation by the lysozome, an alternate pathway to proteasome-mediated degradation.Citation76
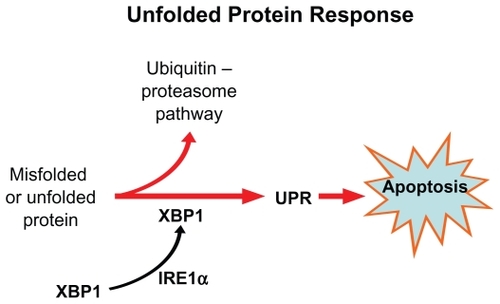
A third possible explanation for the specif icity of bortezomib for myeloma cells is based on the unfolded protein response (UPR). Plasma cells have highly developed rough endoplasmic reticulum (ER) and chaperone proteins that enable them to produce vast quantities of antibodies per second. If misfolded proteins accumulate in ER, the UPR signaling pathway is activated through its sensing mechanism IRE1α.Citation77 The IRE1 kinase, in turn, results in the removal of an intron from the transcription factor XBP1, resulting in a activated ie, spliced form XBP-1.Citation78 Interestingly XBP-1 is is highly expressed in plasma cells and is a prerequisite for transformation from antigen selected B cell to plasma cell.
Once the UPR is activated, the unfolded proteins are refolded by upregulation of the chaperone molecules or destroyed through cytosolic 26S proteasomes; otherwise, accumulation of unfolded protein results in apoptosis of the cell (). Proteasome inhibition triggers apoptosis by interfering with the UPR pathway, both at the sensing level as well as by preventing destruction of misfolded protein.Citation79
Pathophysiology and management of bortezomib toxicities
Thrombocytopenia
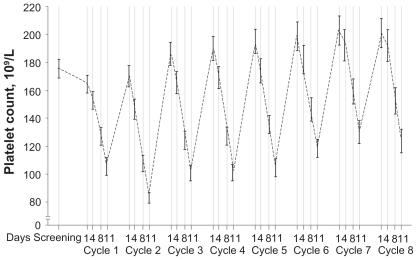
The thrombocytopenia associated with bortezomib therapy has been well characterized. The platelet count drops during Days 1 to 14 and then rapidly recovers to baseline level during Days 15 to 21 (). The mean reduction in relapsed/refractory patients is 60% and appears to be independent of the baseline platelet count, the concentration of the monoclonal protein, and bone marrow plasmacytosis. Murine studies demonstrated no cytotoxic effects on megakaryocytes, thus suggesting a mechanism distinct from traditional myelosuppressive chemotherapeutic agents.Citation80
Figure 10 Kinetics of thrombocytopenia associated with bortezomib therapy. Reproduced with permission from Lonial S, Waller EK, Richardson PG, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005;106(12):3777–3784.Citation80 Copyright © 2005 American Society of Hematololgy.
Neuropathy
When the proteasome is inhibited, proteins accumulate in aggresomes at the periphery of cells and then track centrally via microtubules towards the microtubule-organizing center (MTOC).Citation81 When the distribution of microtubules between polymerized and soluble fractions was compared following the treatment of neuroblastoma and myeloma cells with five proteasome inhibitors, the polymerized fraction increased from 41% to 68% to approximately 55% to 99%, for up to 144 hours after the proteasome inhibitor was removed. Immunofluorescence studies did not reveal microtubule bundles seen with taxanes, suggesting microtubule stabilization occurred by a mechanism different than direct drug binding.Citation82 Animal models have also found significant mitochondrial and endoplasmic reticulum damage in dorsal root ganglia.Citation83 Other postulated mechanisms of bortezomib associated neuropathy include mitochondrial dysregulation of calcium homeostasis or dysregulation of growth factors important for neuron survival.Citation84
Clinically, it is important to note the baseline rate of neuropathy in patients with relapsed/refractory myeloma. In the phase II SUMMIT and CREST studies with bortezomib, 81% of patients had symptoms by FACT/GOG-Ntx questionnaire and 83% by neurologists’ examination.Citation85 This likely reflects not only the side effects of prior treatments, but also a manifestation of the disease itself. While the likelihood of developing severe peripheral neuropathy (PN) was more frequent in those patients with baseline neuropathy, the overall occurrence was independent of baseline neuropathy.
In the phase III APEX trial, of the 37% of patients who experienced peripheral neuropathy (PN), 9% had grade ≥ 3. The neuropathy was typically sensory, although 2% of patients did experience motor neuropathy. The neuropathy does appear to be dose related with PN typically occurring by cycle 5 and then reaching a plateau by cycle 8, associated with cumulative bortezomib doses of 26 and 42 mg/m2 respectively.Citation86 Based on similar findings in previous studies, the APEX trial also incorporated dose-modification guidelines for PN (see ).
Sixty-eight percent of patients in the APEX study who had dose modification for grade ≥ 2 PN experienced improvement or resolution to baseline in their symptoms at a median of 110 days without any compromise in efficacy. The development of neuropathy was independent of age, prior therapies (including thalidomide and vincristine), and glucose intolerance/diabetes.Citation86
A recent publication described a case series of five patients with myeloma who received bortezomib and then developed severe motor involvement. Electrophysiological evaluations showed demyelinating or mixed axonal-demyelinating neuropathy with prominent motor involvement. Cerebrospinal fluid showed albumin-cytological dissociation. Importantly, all four patients treated with either steroids or intravenous immunoglobulin had improved outcomes, suggesting a possible immunologic cause of this neuropathy.Citation88 Therefore, the development of motor neuropathy merits prompt neurological consultation.
Particularly in the setting of combination therapy, attenuation in the dosing schedule eg, weekly treatment, appears to be associated with significantly less neurotoxicity. For example, the incidence of grade 3 or higher neuropathy with VMP decreased from 14% to 2% with twice weekly vs weekly bortezomib with preliminary outcome data showing no loss in efficacy.Citation46 Interestingly, patients treated with the combination of the heat shock protein (HSP)-90 inhibitor tanesipmycin and bortezomib have not developed Grade 3 PN, suggesting a possible neuroprotective effect of this novel agent.Citation89 Of note, development/exacerbation of PN has also not been observed to date with the novel proteasome inhibitor carfilzomib, suggesting that this may not be a class specific effect.Citation90,Citation91
Currently there is no proven effective prophylaxis for PN. A variety of agents are used for symptomatic relief of boretzomib associated PN including opioids, tricyclic antidepressants such as nortryptline, anticonvulsants such as gabapentin, serotonin-norepeinephrine reuptake inhibitors such as duloxetine, nonsteroidal anti-inflammatory agents, vitamins, and nutritional supplements such as α-lipoic acid, glutamine, and L-carnitine.Citation84 However, with recent data suggesting a possible decrease in the efficacy of bortezomib with concomitant vitamin CCitation92 and other supplements such as green teaCitation92, neither the effectiveness in symptom palliation nor the absence of an interaction with bortezomib has been clearly established in randomized clinical trials.
The future of proteasome inhibition
A protein is first identified to be degraded by the polyubqiuitination of lysine residues. The process consists of sequential ubquitin activation, conjugation, and protein ligation – each catalyzed by E1, E2, and E3 enzymes () – which creates the polyubiquitination chain. It appears that there is a family of small ubiquitin like modifiers such as Nedd8, SUMO, FAT10 and ISG15 that are also able to target proteins for degradation.Citation93 Each step of this process is therefore a putative therapeutic target. Efforts are underway to evaluate novel agents, with a Nedd8 activating enzyme inhibitor (MLN 4924) already in phase I clinical trials.Citation94
Figure 11 Protein ubiquitination that earmarks proteins for proteasome degradation requires three classes of enzymes: E1 for activation of ubiquitin, E2 carrier protein for conjugation, and finally the E3 ligase that transfers ubiquitin to a lysine residue on the target protein. Reproduced with permission from Adams J. Potential for proteasome inhibition in the treatment of cancer. Drug Discov Today. 2003;8(7):307–315.Citation3 Copyright © 2003 Elsevier.
Based on the pharmacaphore that interacts with the proteasome’s active site, proteasome inhibitors can be divided into five classes: peptide aldehydes, peptide boronotes, peptide vinyl sulfones, peptide epoxyketones, and the only nonpeptide group – β lactone inhibitors ().Citation2 The peptide aldehydes such as MG-132 are the first class to be studied and while cell permeable, they are not only rapidly oxidized and unstable, but also lack specificity with activity against nonproteasome enzymes such as serine and cysteine proteases.
Table 6 Recommended dose modification for bortezomib-related neuropathic pain and/or peripheral sensory neuropathyCitation87
The peptide boronates were derived by substitution of the aldehyde with boron to increase potency, selectivity, and stability.Citation2 Bortezomib is currently the only FDA approved proteasome inhibitor. Recently published preclinical data demonstrated activity comparable with bortezomib with another peptide boronate compound, CEP-18770, that is also water-soluble and orally bioavailable.Citation95 Bortezomib is also being used as a platform for phase I/II studies with numerous novel agents including an anti-IL6 antibody, heat shock protein inhibitors, and epigenetic modulators such as vorinostat or panobinostat. These novel agents may therefore shed light on mechanisms of bortezomib resistance. For example, in two different studies, three patients who were refractory to bortezomib had a response to bortezomib with the addition of a novel agent – either tanespimycin or vorinostat.Citation89,Citation96
There have been some recent developments in the epoxyketone class of proteasome inhibitors. Epoxomicin is a natural compound initially isolated from an Actinomycete strain and found to have antimelanoma activity in preclinical models.Citation97 Carfilzomib (formerly PR-171; Proteolix®), is a tetrapeptide epoxyketone related to epoxomicin. There are two components of this agent, a peptide portion that binds to the substrate binding pocket(s) of the proteasome with high affinity and a epoxyketone pharmacophore that interacts with the catalytic amino-terminal threonine residue and irreversibly inhibits proteasome activity. Relative to bortezomib, carfilzomib more selectively inhibits the chymotrypsin-like activity of the proteasome with less cross-reactivity at the caspase-like and trypsin-like sites. At doses of 15 mg/m2 or greater, there is > 80% proteasome inhibition in both red blood cells and peripheral blood mononuclear cells in humans. The ability to give this drug safely on consecutive days allows for sustained proteasome inhibition.Citation98 Preliminary data presented at the annual meeting of American Society of Hematology in 2008 from ongoing phase II studies indicate an overall response rate of greater than 50% and 26% in bortezomib-naïveCitation91 and bortezomib-exposedCitation90 patients with multiple myeloma, respectively. Cyclic thrombocytopenia was also noted but otherwise, the toxicity profile was different from bortezomib – increased creatinine and possible tumor lysis but no significant neuropathy.Citation90,Citation91,Citation99
Table 7 Classes of proteasome inhibitors
The first member of the β lactone class of proteasome inhibition that received attention was derived from lactacystin, produced by Streptomyces. It was highly unstable intracellularly but was more specific than the peptide aldehydes.Citation2 Salinosporamide A (NPI-0052), a product of a marine actinomycete Salinispora tropica, has a bicyclic ring structure similar to lactacystin, but with various substitutions.Citation100 Preclinical studies have shown that unlike bortezomib, NPI-0052 inhibits all three protease activities of the proteasome. It is also orally bioactive, a more potent inducer of apoptosis in myeloma cells than bortezomib, and demonstrates activity in bortezomib resistant cell lines as well.Citation101 Preliminary reports from ongoing phase I studies in a variety of tumors indicate that the drug appears to be well tolerated.Citation102–Citation104
The development of the first-in-class proteasome inhibitor bortezomib in multiple myeloma is a paradigm for the optimal interaction between the pharmaceutical industry, academic institutions, and patient advocacy groups. With ever increasing knowledge of the mechanism of action of this agent, the full therapeutic potential of this growing class of drugs can be realized.
Disclosure
Ajai Chari and Amitabha Mazumder have both received honoraria related to speakers’ bureau activities from Millennium Pharmaceuticals Inc., The Takeda Oncology Company. Sundar Jagannath has received honoraria related to Advisory Board Consultancy from Millennium Pharmaceuticals Inc., The Takeda Oncology Company.
References
- AdamsJThe proteasome: a suitable antineoplastic targetNat Rev Cancer20044534936015122206
- OrlowskiRZStinchcombeTEMitchellBSPhase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignanciesJ Clin Oncol200220224420442712431963
- AdamsJPotential for proteasome inhibition in the treatment of cancerDrug Discov Today20038730731512654543
- Sanchez-SerranoISuccess in translational research: lessons from the development of bortezomibNat Rev Drug Discov20065210711416518378
- AdamsJPalombellaVJSausvilleEAProteasome inhibitors: a novel class of potent and effective antitumor agentsCancer Res199959112615262210363983
- HideshimaTRichardsonPChauhanDThe proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cellsCancer Res20016173071307611306489
- LeBlancRCatleyLPHideshimaTProteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine modelCancer Res200262174996500012208752
- LightcapESMcCormackTAPienCSChauVAdamsJElliottPJProteasome inhibition measurements: clinical applicationClin Chem200046567368310794750
- RichardsonPGBarlogieBBerensonJA phase 2 study of bortezomib in relapsed, refractory myelomaN Engl J Med2003348262609261712826635
- RichardsonPGSonneveldPSchusterMWBortezomib or high-dose dexamethasone for relapsed multiple myelomaN Engl J Med2005352242487249815958804
- RichardsonPGSonneveldPSchusterMExtended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trialBlood2007110103557356017690257
- LeeSJRichardsonPGSonneveldPBortezomib is associated with better health-related quality of life than high-dose dexamethasone in patients with relapsed multiple myeloma: results from the APEX studyBr J Haematol2008143451151918986387
- San-MiguelJFRichardsonPGSonneveldPEfficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 studyLeukemia200822484284918200040
- ZangariMEsseltineDLeeCKResponse to bortezomib is associated to osteoblastic activation in patients with multiple myelomaBr J Haematol20051311717316173965
- GiulianiNMorandiFTagliaferriSThe proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patientsBlood2007110133433817371942
- TerposESezerOCroucherPDimopoulosMAMyeloma bone disease and proteasome inhibition therapiesBlood200711041098110417494860
- ZangariMEsseltineDCavalloFPredictive value of alkaline phosphatase for response and time to progression in bortezomib-treated multiple myeloma patientsAm J Hematol200782983183317546639
- MaMHYangHHParkerKThe proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agentsClin Cancer Res2003931136114412631619
- OrlowskiRZNaglerASonneveldPRandomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progressionJ Clin Oncol200725253892390117679727
- BerensonJRYangHHVescioRASafety and efficacy of bortezomib and melphalan combination in patients with relapsed or refractory multiple myeloma: updated results of a phase 1/2 study after longer follow-upAnn Hematol200887862363118463870
- PopatROakerveeHWilliamsCBortezomib, low-dose intravenous melphalan, and dexamethasone for patients with relapsed multiple myelomaBr J Haematol2009144688789419183191
- Pineda-RomanMZangariMvan RheeFVTD combination therapy with bortezomib-thalidomide-dexamethasone is highly effective in advanced and refractory multiple myelomaLeukemia20082271419142718432260
- ReeceDEPizaGTrudelSA Phase I–II Trial of Bortezomib Plus Oral Cyclophosphamide and Prednisone for Relapsed/Refractory Multiple MyelomaASH Annual Meeting Abstracts2006108113536
- KropffMBispingGSchuckEBortezomib in combination with intermediate-dose dexamethasone and continuous low-dose oral cyclophosphamide for relapsed multiple myelomaBr J Haematol2007138333033717614819
- HajekRZahradovaLGregoraEThe reduced intensity Cvd regimen: a good option with well balanced efficacy/toxicity ratio for elderly patients with poor status performanceASH Annual Meeting Abstracts20081116112113699
- LeeSSSuhCKimBSBortezomib, doxorubicin and dexamethasone (PAD) combination therapy followed by thalidomide and dexamethasone (TD) as a salvage treatment for relapsed multiple myeloma (MM): preliminary analysis of efficacy and safetyASH Annual Meeting Abstracts2007110112731
- PalumboAGayFBringhenSBortezomib, doxorubicin and dexamethasone in advanced multiple myelomaAnn Oncol20081961160116518326520
- RichardsonPJagannathSJakubowiakALenalidomide, bortezomib, and dexamethasone in patients with relapsed or relapsed/refractory multiple myeloma (MM): encouraging response rates and tolerability with correlation of outcome and adverse cytogenetics in a phase II studyASH Annual Meeting Abstracts2008112111742
- PoenischWBourgeoisMWangSYBortezomib in combination with bendamustine and prednisone in the treatment of patients with refractory/relapsed multiple myelomaASH Annual Meeting Abstracts2007110112723
- PalumboAAmbrosiniMTBenevoloGBortezomib, melphalan, prednisone, and thalidomide for relapsed multiple myelomaBlood200710972767277217148584
- TerposEHeathDJZervasKDimopoulosMAThe effect of bortezomib monotherapy and bortezomib-based regimens on bone metabolism in patients with relapsed/refractory multiple myelomaPaper presented at: Haematologica2007
- CiolliSLeoniFCasiniCBreschiCSantiniVBosiAThe addition of liposomal doxorubicin to bortezomib, thalidomide and dexamethasone significantly improves clinical outcome of advanced multiple myelomaBr J Haematol2008141681481918410447
- KimYKLeeJJSohnSKClinical Efficacy of VEL-CTD (bortezomib, cyclophosphamide, thalidomide, and dexamethasone) regimen in patients with relapsed or refractory multiple myeloma: a phase II studyASH Annual Meeting Abstracts2008112113693
- San MiguelJFSchlagRKhuagevaNKBortezomib plus melphalan and prednisone for initial treatment of multiple myelomaN Engl J Med2008359990691718753647
- CavoMTacchettiPPatriarcaFSuperior complete response rate and progression-free survival after autologous transplantation with up-front velcade-thalidomide-dexamethasone compared with thalidomide-dexamethasone in newly diagnosed multiple myelomaASH Annual Meeting Abstracts200811211158
- HarousseauJUpdated results: Randomized phase III IFM trial comparing Vel/Dex with VAD induction in MM in pts ≤ 65 yearsJoint ASH/ASCO Symposium200812
- SonneveldPvan der HoltBSchmidt-WolfIGHFirst analysis of HOVON-65/GMMG-HD4 randomized phase III trial comparing bortezomib, adriamycine, dexamethasone (pad) vs vad as induction treatment prior to high dose melphalan (HDM) in patients with newly diagnosed multiple myeloma (MM)ASH Annual Meeting Abstracts200811211653
- Chanan-KhanASonneveldPSchusterMWAnalysis of herpes zoster events among bortezomib-treated patients in the phase III APEX studyJ Clin Oncol200826294784479018711175
- VickreyEAllenSMehtaJSinghalSAcyclovir to prevent reactivation of varicella zoster virus (herpes zoster) in multiple myeloma patients receiving bortezomib therapyCancer2009115122923219090004
- PourLAdamZBuresovaLVaricella-zoster virus prophylaxis with low-dose acyclovir in patients with multiple myeloma treated with bortezomibClin Lymphoma Myeloma20099215115319406726
- AsahiTTsutsuiMWakasugiMValacyclovir neurotoxicity: clinical experience and review of the literatureEur J Neurol200916445746019187258
- HelldenALyckeJVanderTSvenssonJOOdar-CederlofIStahleLThe aciclovir metabolite CMMG is detectable in the CSF of subjects with neuropsychiatric symptoms during aciclovir and valaciclovir treatmentJ Antimicrob Chemother200657594594916540518
- MarinMGurisDChavesSSSchmidSSewardJFPrevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP)MMWR Recomm Rep200762256RR-414017585291
- MiguelJFSSchlagRKhuagevaNKUpdated follow-up and results of subsequent therapy in the phase III VISTA trial: bortezomib plus melphalan-prednisone versus melphalan-prednisone in newly diagnosed multiple myelomaASH Annual Meeting Abstracts1200811211650
- MateosMVOriolAMartinezJBortezomib (Velcade)-melphalan-prednisone (VMP) versus velcade-thalidomide-prednisone (VTP) in elderly untreated multiple myeloma patients: which is the best partner for velcade: an alkylating or an immunomodulator agent?ASH Annual Meeting Abstracts200811211651
- PalumboABringhenSRossiDA prospective, randomized, phase III study of bortezomib, melphalan, prednisone and thalidomide (VMPT) versus bortezomib, melphalan and prednisone (VMP) in elderly newly diagnosed myeloma patientsASH Annual Meeting Abstracts1200811211652
- RosinolLCibeiraMTMartinezJThalidomide/dexamethasone (TD) vs bortezomib(Velcade(R))/thalidomide/dexamethasone (VTD) vs VBMCP/VBAD/Velcade(R) as induction regimens prior autologous stem cell transplantation (ASCT) in younger patients with multiple myeloma (MM): first results of a prospective phase III PETHEMA/Gem trialASH Annual Meeting Abstracts200811211654
- AttalMHarousseauJLFaconTSingle versus double autologous stem-cell transplantation for multiple myelomaN Engl J Med2003349262495250214695409
- CavoMTosiPZamagniEProspective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical studyJ Clin Oncol200725172434244117485707
- RichardsonPLonialSJakubowiakALenalidomide, bortezomib, and dexamethasone in patients with newly diagnosed multiple myeloma: encouraging efficacy in high risk Groups with updated results of a phase I/II studyASH Annual Meeting Abstracts20081121192
- HarousseauJLAttalMLeleuXBortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II studyHaematologica200691111498150517043025
- CorsoABarbaranoLMangiacavalliSBortezomib with HIG-dose dexamethasone as first Line therapy in patients with multiple myeloma candidates to high-dose therapyASH Annual Meeting Abstracts2007110113595
- RosinolLOriolAMateosMVPhase II PETHEMA trial of alternating bortezomib and dexamethasone as induction regimen before autologous stem-cell transplantation in younger patients with multiple myeloma: efficacy and clinical implications of tumor response kineticsJ Clin Oncol200725284452445817785704
- JagannathSDurieBGMWolfJLLong-term follow-up of patients treated with bortezomib alone and in combination with dexamethasone as frontline therapy for multiple myelomaASH Annual Meeting Abstracts200610811796
- OrlowskiRZPetersonBLSanfordBBortezomib and pegylated liposomal doxorubicin as induction therapy for adult patients with symptomatic multiple myeloma: Cancer and Leukemia Group B Study 10301ASH Annual Meeting Abstracts200610811797
- JakubowiakAKendallTAl-ZoubiAInitial treatment with bortezomib (Velcade(R)), Doxil(R), and dexamethasone (VDD) is superior to thalidomide and dexamethasone (TD) as initial therapy prior to autologous stem cell transplantation (ASCT) for multiple myeloma (MM)ASH Annual Meeting Abstracts2008112113713
- PalumboAFalcoPGayFBortezomib-doxorubicin-dexamethasone as induction prior to reduced intensity autologous transplantation followed by lenalidomide as consolidation/maintenance in elderly untreated myeloma patientsASH Annual Meeting Abstracts200811211159
- BelchAReeceDEBahlisNJBortezomib [VELCADETM], pegylated liposomal doxorubicin [DOXIL/CAELYX(R)] and dexamethasone in the treatment of previously untreated multiple myeloma patients: impact on quality-of-lifeASH Annual Meeting Abstracts2007110113618
- LandauHHassounHCohenASequential administration of bortezomib, liposomal doxorubicin and dexamethasone (BDD) followed by thalidomide and dexamethasone (td) results in rapid control of untreated high-risk multiple myeloma (MM) and improves depth of responseASH Annual Meeting Abstracts2008112113712
- WangMGiraltSDelasalleKHandyBAlexanianRBortezomib in combination with thalidomide-dexamethasone for previously untreated multiple myelomaHematology200712323523917558699
- KaufmanJLGleasonCHeffnerLLonialSBortezomib, thalidomide, and dexamethasone as induction therapy for patients with symptomatic multiple myelomaASH Annual Meeting Abstracts2007110113605
- YoonSSKimHJChungJSSequential VAD (Vincristine, adriamycin, dexamethasone) and VTD (Bortezomib, thalidomide, dexamethasone) induction followed by high-dose therapy with autologous stem cell transplantation and maintenance treatment with bortezomib for newly diagnosed multiple myeloma: final analysis of phase II trialASH Annual Meeting Abstracts2008112113330
- BerensonJRYellinOWoytowitzDBortezomib, ascorbic acid and melphalan (bam) therapy for patients (pts) with newly diagnosed multiple myeloma (MM): an effective and well-tolerated frontline regimenASH Annual Meeting Abstracts2007110113602
- BensingerWJagannathSVescioRA phase II study of bortezomib (Velcade (R)), cyclophosphamide (Cytoxan(R)), thalidomide (Thalomid(R)) and dexamethasone as first-line therapy for multiple myelomaASH Annual Meeting Abstracts20081121194
- ReederCBReeceDEKukretiVCyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trialLeukemia2009
- KnopSLiebischPWandtHBortezomib, intravenous cyclophosphamide and dexamethasone (VelCD) for previously untreated multiple myeloma: an interim analysis of the German DSMM XIa TrialASH Annual Meeting Abstracts20081116112112776
- BarlogieBAnaissieEJShaughnessyJDJrNinety percent sustained complete response (CR) rate projected 4 years after onset of CR in gene expression profiling (GEP)-defined low-risk multiple myeloma (MM) treated with total therapy 3 (TT3): basis for GEP-risk-adapted TT4 and TT5ASH Annual Meeting Abstracts200811211162
- BarlogieBAnaissieEvan RheeFIncorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3Br J Haematol2007138217618517593024
- MulliganGMitsiadesCBryantBGene expression profiling and correlation with outcome in clinical trials of the proteasome inhibitor bortezomibBlood200710983177318817185464
- HideshimaTMitsiadesCAkiyamaMMolecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341Blood200310141530153412393500
- QinJZZiffraJStennettLProteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cellsCancer Res200565146282629316024630
- NikiforovMARiblettMTangWHTumor cell-selective regulation of NOXA by c-MYC in response to proteasome inhibitionProc Natl Acad Sci U S A200710449194881949318042711
- JulligMZhangWVFerreiraAStottNSMG132 induced apoptosis is associated with p53-independent induction of pro-apoptotic NOXA and transcriptional activity of beta-cateninApoptosis200611462764116673057
- Perez-GalanPRoueGVillamorNMontserratECampoEColomerDThe proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 statusBlood2006107125726416166592
- FernandezYVerhaegenMMillerTPDifferential regulation of NOXA in normal melanocytes and melanoma cells by proteasome inhibition: therapeutic implicationsCancer Res200565146294630416024631
- HideshimaTBradnerJEWongJSmall-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myelomaProc Natl Acad Sci U S A2005102248567857215937109
- DavenportELMooreHEDunlopASHeat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cellsBlood200711072641264917525289
- LeeAHIwakoshiNNAndersonKCGlimcherLHProteasome inhibitors disrupt the unfolded protein response in myeloma cellsProc Natl Acad Sci U S A2003100179946995112902539
- ObengEACarlsonLMGutmanDMHarringtonWJJrLeeKPBoiseLHProteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cellsBlood2006107124907491616507771
- LonialSWallerEKRichardsonPGRisk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myelomaBlood2005106123777378416099887
- JohnstonJAWardCLKopitoRRAggresomes: a cellular response to misfolded proteinsJ Cell Biol19981437188318989864362
- PoruchynskyMSSackettDLRobeyRWWardYAnnunziataCFojoTProteasome inhibitors increase tubulin polymerization and stabilization in tissue culture cells: a possible mechanism contributing to peripheral neuropathy and cellular toxicity following proteasome inhibitionCell Cycle20087794094918414063
- CavalettiGGilardiniACantaABortezomib-induced peripheral neurotoxicity: a neurophysiological and pathological study in the ratExp Neurol2007204131732517214983
- ArgyriouAAIconomouGKalofonosHPBortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literatureBlood200811251593159918574024
- RichardsonPGBriembergHJagannathSFrequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomibJ Clin Oncol200624193113312016754936
- RichardsonPGSonneveldPSchusterMWReversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guidelineBr J Haematol2009144689590319170677
- Millennium Pharmaceuticals IVelcadeTM (bortezomib) for injectionPrescribing informationCambridge, MAMillennium Pharmaceuticals, Inc2005
- RavagliaSCorsoAPiccoloGImmune-mediated neuropathies in myeloma patients treated with bortezomibClin Neurophysiol2008119112507251218829381
- RichardsonPGChanan-KhanALonialSTanespimycin (T) + bortezomib (BZ) in multiple myeloma (MM): confirmation of the recommended dose using a novel formulationASH Annual Meeting Abstracts2007110111165
- JagannathSVijRStewartAKInitial results of PX-171-003, an open-label, single-arm, phase II study of carfilzomib (CFZ) in patients with relapsed and refractory multiple myeloma (MM)ASH Annual Meeting Abstracts200811211864
- VijRWangMOrlowskiRInitial results of PX-171-004, an open-label, single-arm, phase II study of carfilzomib (CFZ) in patients with relapsed myeloma (MM)ASH Annual Meeting Abstracts200811211865
- PerroneGHideshimaTIkedaHAscorbic acid inhibits anti-tumor activity of bortezomib in vivoLeukemia2009
- YangYKitagakiJWangHHouDXPerantoniAOTargeting the ubiquitin-proteasome system for cancer therapyCancer Sci20091001242819037995
- SoucyTASmithPGMilhollenMAAn inhibitor of NEDD8-activating enzyme as a new approach to treat cancerNature2009458723973273619360080
- PivaRRuggeriBWilliamsMCEP-18770: A novel, orally active proteasome inhibitor with a tumor-selective pharmacologic profile competitive with bortezomibBlood200811152765277518057228
- WeberDBadrosAZJagannathSVorinostat plus bortezomib for the treatment of relapsed/refractory multiple myeloma: early clinical experienceASH Annual Meeting Abstracts200811211871
- HanadaMSugawaraKKanetaKEpoxomicin, a new antitumor agent of microbial originJ Antibiot (Tokyo)19924511174617521468981
- DemoSDKirkCJAujayMAAntitumor activity of PR-171, a novel irreversible inhibitor of the proteasomeCancer Res200767136383639117616698
- AlsinaMTrudelSValloneMMolineauxCKunkelLGoyAPhase 1 single agent antitumor activity of twice weekly consecutive day dosing of the proteasome inhibitor carfilzomib (PR-171) in hematologic malignanciesASH Annual Meeting Abstracts200711011411
- FelingRHBuchananGOMincerTJKauffmanCAJensenPRFenicalWSalinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus salinosporaAngew Chem Int Ed Engl200342335535712548698
- ChauhanDCatleyLLiGA novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from bortezomibCancer Cell20058540741916286248
- HamlinPAAghajanianCHongDFirst-in-human phase 1 dose escalation study of NPI-0052, a novel proteasome inhibitor, in patients with lymphoma and solid tumorASH Annual Meeting Abstracts2008112114939
- PriceTPadrikPTownsendAClinical trial of NPI-0052 (2nd generation proteasome inhibitor) in patients having advanced malignancies with expanded RP2D cohorts in lymphoma and CLLASH Annual Meeting Abstracts2008112114934
- RichardsonPHofmeisterCCZimmermanTMPhase 1 clinical trial of NPI-0052, a novel proteasome inhibitor in patients with multiple myelomaASH Annual Meeting Abstracts2008112112770