Abstract
The skin is a dynamic and complex organ that relies on the interrelation among different cell types, macromolecules, and signaling pathways. Further, the skin has interactions with its own appendages and other organs such as the sebaceous glands and hair follicles, the kidney, and adrenal glands; systems such as the central nervous system; and axes such as the hypothalamic–pituitary–adrenal axis. These continuous connections give the skin its versatility, and when an injury is caused, some triggers start a cascade of events designed to restore its integrity. Nowadays, it is known that this psychoneuroimmune–endocrine intercommunication modulates both the homeostatic condition and the healing process. In this sense, the skin conditions before a trauma, whether of endogenous (acne) or exogenous origin (injury or surgical incision), could regulate the process of tissue repair. Most skin diseases such as psoriasis and atopic dermatitis, among others, have in their pathophysiology a psychogenic component that triggers integrated actions in the nervous, immune, and endocrine systems. However, fibroproliferative disorders of wound healing, such as hypertrophic scar and keloid, are not yet included in this listing, despite showing correlation with stress, especially with the psychosocial character. This review, by understanding the “brain–skin connection”, presents evidence that allows us to understand the keloid as a psychomediated disease.
Introduction
Keloid is considered a fibroproliferative disorder of skin wound healing, and its pathophysiological mechanisms are not fully known. As keloid only occurs in humans in which the psychological chronic stress component is already demonstrated, it is now also reported from the “psychomediation” perspective.
Skin scar diseases cannot be understood merely as esthetic complaints. They are associated with a variety of physiological reactions that can be modulated by many events, such as psychological ones. It begins with understanding about the interconnection between the skin and the central nervous system at an embryonic level. Their common origin is the ectoderm. Thus, depending on the reference, it can be said that the skin would be the external surface of the brain, or the brain would be the skin’s deepest layer.Citation1
Some skin diseases such as psoriasis,Citation2 atopic dermatitis,Citation3 seborrheic dermatitis,Citation4 herpes simplex,Citation5 vitiligo,Citation6,Citation7 acne,Citation8 alopecia,Citation9 and urticariaCitation10 are already included in the “psychophysiological disorders” category.Citation11 Keloid and hypertrophic scars should also be included in this category because of the impact of psychoemotional stress in their clinical features. The trigger, the maintenance, and/or the worsening of the signs and symptoms of these skin scar diseases are closely related to a stressful event (internal/psychological and/or external/environmental)Citation12 that activates neuroimmune–endocrine circuits.Citation13
In this context, this review aims to open up new fronts for the comprehension of this pathological scar, looking for a better understanding of the patient as a whole in a psychoneuroimmune–endocrine way.Citation14,Citation15
Psychoneuroimmune–endocrine systems and the skin
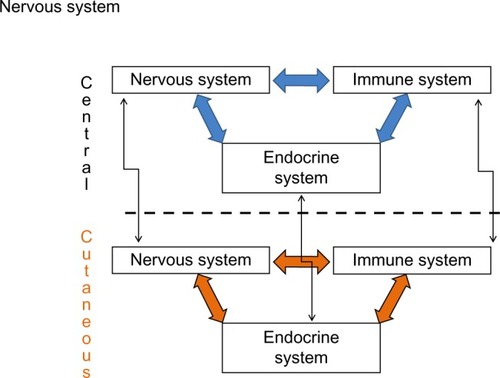
The skin has several elements of the nervous,Citation16 immune,Citation17 and endocrine systems.Citation18 Some examples are corticotropin-releasing hormone (CRH) production by keratinocytes, melanocytes, and pilosebaceous units;Citation19 adrenocorticotropic hormone (ACTH) and α-melanocyte-stimulating hormone (α-MSH) by keratinocytes, melanocytes, pilosebaceous units, fibroblasts, and endothelial cells;Citation20 and cortisol by keratinocytes and pilosebaceous units, among other hormones.Citation21 Cytokines and growth factors such as interleukin 1 (IL-1) are also synthesized by keratinocytes, melanocytes, fibroblasts, and endothelial cells; IL-6 by the keratinocytes, fibroblasts, pilosebaceous units, and endothelial cells; tumor necrosis factor-α (TNF-α) by keratinocytes and melanocytes;Citation22,Citation23 and interferon-γ by keratinocytes and fibroblasts, among other elements of character, mostly proinflammatory. Therefore, the skin has a power plant of its own, with the capacity to produce locally all these factors related to other systems, speaking a common biochemical language and communicating via a complete bidirectional circuit involving shared ligands.Citation24 In addition to this local and bidirectional communication among systems, there is also a central–peripheral communication ().Citation25
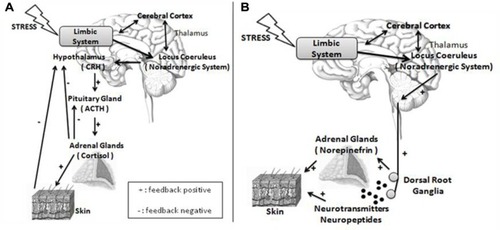
In this review, regulatory events of the skin homeostasis will be studied under the prism of psychogenic stimuli.Citation26 Besides the 5-hydroxytryptamine, acetylcholine, and inflammatory cytokines, signaling from areas of the limbic system (hippocampus, amygdale, and medial prefrontal cortex)Citation27 is able to release CRH from the hypothalamus paraventricular nuclei (). Adding to its classical performance in the pituitary gland, releasing ACTH, which acts on the adrenal glands cortex, providing cortisol, the CHR also elicits sympathetic responses in the locus coeruleus of the brain stem, releasing norepinephrine by peripheral nerve endings, and norepinephrine and epinephrine by the adrenal glands medulla (). The activation of the sympathetic nervous system also enables the production of other substances, including the catecholamines.
Figure 2 Nervous and endocrine pathways under stress.
Abbreviations: CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone.
The skin is able to express CRH as its receptors (CRH-R). The CRH-R1α isoform is the predominant CRH receptor in the skin, and it is expressed in all major cell populations of epidermis, dermis, and subcutaneous tissue. By contrast, CRH-R2 is expressed predominantly in hair follicles, sebaceous and eccrine glands, and muscle and blood vessels.Citation28 In addition to CRH, the skin also expresses urocortin I and urocortin II messenger ribonucleic acid.Citation28 CRH-R1 binds to urocortin I but not to urocortin II, while CRH-R2 binds to urocortin II but not to urocortin I,Citation29,Citation30 leading to the belief that the skin has a depth of responsiveness and interaction to the environment that is little understood. Finally, skin produces the precursor protein proopiomelanocortin protein (POMC) and POMC-derived peptides that give rise to ACTH and other polypeptide products.Citation19,Citation31 Keloid fibroblasts express POMC.Citation32
Ito et alCitation33 have shown that human hair follicles can synthesize cortisol, and its synthesis is regulated by endogenous feedback controls. Thus, the skin apparently has a peripheral equivalent to the hypothalamic–pituitary–adrenal (HPA) axis that is fully functional. The peripheral skin HPA axis may coordinate or fine-tune peripheral stress responses with the central HPA axis. In addition to expressing components of the HPA axis, the skin also produces a number of other neuroendocrine signals, including prolactin,Citation34–Citation36 melatonin,Citation37 and catecholamines.Citation38
Besides the HPA axis, the skin is highly innervated with sensory nerves that produce neurotrophins and neuropeptides. Sensory nerves derive from the dorsal root ganglion in the skin, and C-fibers form the cutaneous sensory nervous system. Psychological stress leads to increased concentrations of cutaneous nerve growth factor (NGF).Citation39 NGF has a number of biological activities, including 1) axon sprouting of peptidergic and sympathetic neurons; 2) promoting cross-talk between neural cells, glia, and immune cells; and 3) facilitating monocyte/macrophage migration through vascular endothelium.Citation40 NGF upregulates substance P (SP) nerve fibers in the dermis of stressed mice. Calcitonin gene-related peptide (CGRP), a potent vasodilator, is also upregulated in response to NGF.Citation39 SP and CGRP have different distributions within the skin, with SP nerve fibers detected in the dermis and subcutis, and CGRP nerve fibers are in the epidermis around the distal hair follicle and the arrector pili muscle.Citation41
From this evidence, there is no way to understand skin diseases and their symptoms decoupled from psychological aspects. Clinical and experimental data support the assertion that the minds can start, stop, and influence biological events in the skin.Citation42
Psychoneuroimmune–endocrine system and keloid
At the moment of the loss of skin integrity
Considering the three super-systems, the nervous system is the one that responds more rapidly to noxious stimuli in the skin, due to its electrical nature. After a skin wound (endogenous origin such as acne, or exogenous such as a surgical incision or a mosquito bite), the loss of cutaneous integrity generates a “short circuit” in the skin battery, changing the outer skin pole to positive; this is responsible for generating an electrical current at the site of injury and initiating eletrotaxia. This current remains present until the skin regains its integrity by wound healing, restoring its natural negative polarization. This current of injury helps eletrotaxia (primarily neutrophils and monocytes) initiate tissue repair (for better understanding of these definitions, see Hochman et alCitation43).
At the same time as the nerve endings directly influence the bioelectrical condition, they also lead afferences to the dorsal root ganglion, reporting the cutaneous status. Some of these stimuli reach the cerebral cortex. The efferent response is the neuropeptides and neurotransmitters release. The neuroelectric and neuropeptidergic components of the healing are inseparable parts of the neurogenic inflammation. The neurogenic inflammation level dictates the intensity of the subsequent phases of the healing.
Akaishi et alCitation44 proposed a neurogenic inflammation hypothesis for keloid formation. Other authors have also followed this perspective.Citation45–Citation47 However, all of them have analyzed just the peripheral and local nature of the lesion as a result of stressful stimuli (skin stretch and mechanotransduction). Considering the existence of central–peripheral ways of communication between the central and the cutaneous nervous system, it is possible to extrapolate the opposite route, based on the same grounds, in which central nervous stimuli would trigger a similar response in the skin. The neurogenic inflammation level of the healing process is directly related to the amount and the functionality of cutaneous nerve fibers. KadanoffCitation48 was the pioneer in demonstrating the relationship of innervation with keloid. Hochman et alCitation49 showed higher density and depth of nerve fibers in the keloid than the skin. A higher density of nerve fibers was also demonstrated in hypertrophic scars when compared to normal scars.Citation50–Citation52 In turn, the lower density of nerve fibers in the keloid epidermis could be related to chronic stimulation of conductive nerve endings of itching, similar to the fibers of the nociceptive stimuli, and it would be an autoregulatory mechanism for modulation of intensity and persistence of sensory inputs.Citation53 In this context, botulinum toxin has recently come to prominence as a form of treatment.Citation54–Citation57 For the future, capsaicin, the functional denervator, may also appear as an alternative.Citation58
Before the loss of skin integrity
The basal skin condition is a very important factor in healing disorders. The cutaneous inflammatory status is influenced, among other factors, by ultraviolet radiation,Citation12,Citation59 feeding,Citation60–Citation62 obesity and metabolic syndrome, and stress of psychological origin.Citation63 This could explain the bizarre temporal character of keloid, in that an individual can develop it in different periods of their life.Citation64 Black skin absorbs more ultraviolet radiation than the other skin color types. This is a disadvantage because the more radiation absorbed, the more skin inflammation, increasing this important risk factor which corresponds to a higher prevalence of keloids in this population.
The immune response and the immune system can be modulated by the interactions between essential fatty acids, eicosanoids, and free radicals coming from the diet. For the essential fatty acids, if there are enhanced arachidonic acid levels plus deficiency of precursors (linoleic acid, g-linolenic acid, and dihomo-g-linolenic acid) and some inflammatory competitors (dihomo-g-linolenic acid and eicosapentaenoic acid), it will result in an inevitable overproduction of proinflammatory metabolites, which enhances a pathologic inflammation, both systemic and local, in the skin. This scenario is seen in patients with keloid.Citation65
Psychological stress can amplify both the bioelectric and neuropeptidergic components of the neurogenic phase of the healing inflammation. Sweat and sebaceous secretion as a result of emotional factorsCitation66,Citation67 increase the electrical conductivity of the deeper layers and the impedance of the stratum corneum, respectively, increasing the current of injury and the eletrotaxia at the moment of injury. On the other hand, the stress chronically stimulates the neuroimmune–endocrine circuit and the sensory system.Citation68–Citation70 The binding of neurotransmitters, neuropeptides, and neurohormones (acetylcholine, CGRP, substance P, CRH) in the mast cells receptors makes them degranulate. From the degranulation, several proinflam-matory mediators such as IL-1, IL-6, growth factors (NGF, vascular endothelial growth factor [VEGF], transforming growth factor-β [TGF-β]), TNF-α, and histamine are released and refeed the cycle, creating a chronic inflammatory condition.Citation12 The intimate relationship and interaction between mast cells and myofibroblasts support the important role of mast cells and their mediators in the pathogenesis of the keloid.Citation71,Citation72 TGF-β,Citation73–Citation76 VEGF,Citation74 histamine,Citation77 TNF-α,Citation78 and IL-6Citation79–Citation81 have already been implicated in the pathogenesis and symptomatology of the keloid.Citation82
In summary, stressed patients exacerbate the neuroimmune–endocrine system, and that causes basal inflammatory metabolic activity on the individual skin, which enhances the neurogenic inflammation phase during the healing process. In other words, people bring on the inflammation themselves when their “nerves are on edge”.Citation43
Furtado et alCitation83 were the first to show a direct correlation between stress and keloid. Patients with fibroproliferative scars who were candidates for surgical resection and postoperative radiotherapy underwent evaluation of psychological stress on the day before the surgery. The parameters evaluated were pain and itching, quality of life, perceived stress, depression and anxiety – through questionnaires and scales – salivary cortisol, and minimum and maximum galvanic skin response (GSR) at rest and under stress (when filling in questionnaires). The patients were evaluated postoperatively at 3, 6, 9, and 12 months. Each time the patients returned to the medical service, two specialists classified the lesions in nonrelapsed and relapsed patients. The relapsed group presented higher values in GSR during the stress situation. The chance of relapse increased 34% for each increase of 1,000 arbitrary units, at maximum GSR in stress. Thus, psychological stress increases the chance of relapse in postoperative keloid.
Final considerations
Keloid management is still considered a challenge for physicians and researchers. This is a healing benign neoplasm exclusive to humans whose therapeutic modalities are unsatisfactory and present a high rate of recurrence.Citation84,Citation85 Several hypotheses regarding its physiopathogenesis have been postulated.Citation44,Citation86–Citation89 Currently, a genetic cause has received the greater prominence, with a large number of publications about it.Citation90–Citation92
However, it must be understood that genetic and environmental influences are interconnected in a complex way. Currently, a widely accepted model is the stress–diathesis model, according to which stressful situations (the precipitating factor) will affect people in different ways due to inherited vulnerabilities (a predisposing factor). Moreover, according to this model, a genetic predisposition to disease development (“diathesis”) may remain latent until stress events, which can be represented by psychosocial factors interacting with biological characteristics of a person, make the disease unfold.Citation42
Environmental factors are certainly critical in defining phenotypes during early development, and they continue to influence phenotypes throughout the life cycle of an organism. Nearly every aspect of development and behavior is affected by both personal experience, that is gained through the environment, and the genetic makeup. Environmental factors can affect and alter gene expression, while genes can define how people respond to different environments. Indeed, it is actually superficial to debate whether nature or nurture is more important. In truth, the relationship between genetic determinants and the environment is so completely entwined that you cannot look at an individual and judge which contribution is more valuable. Together, the continual interplay of both genes and ever-changing environmental factors determines who people are.
Keloid and hypertrophic scars are different phenotypic expressions of the same phenomenon and can exemplify the complexity of the disorder.Citation92 It is certain that the treatment of these healing disorders cannot focus on one single aspect. It is necessary to understand the wide spectrum of communication between the three super-systems. The release of hormones, neural transmitters, substances, and immune cells creates an inflammatory environment that stimulates the fibrogenesis (the expression of proinflammatory genes are inserted in this context).Citation89,Citation93–Citation95 On the other hand, personality and coping styles reflect individual differences in appraisal and response to stressors that may influence neuroimmune–endocrine functions.
Thus, skin disorders should be interpreted in a holistic way; in other words, a good practice evaluates the individual as a whole, under a psychoneuroimmune–endocrine functional perspective. Since most, or perhaps all, cases of cutaneous somatization have been long neglected, they now become the target of research in which the psychogenic part has gained prominence, because there is no dermatosis in which the psychological factor is not involved.Citation43 The trigger to wound healing depends on neurogenic factors (electrical and neurosecretory) which, in turn, are premodulated by psychological aspects and adjusted by immune and endocrine factors. Any change in these circuits may interfere in the normal formation of a scar, resulting in wound healing deficit (atrophic scars and ulcerations) until hyperproliferative healing (hypertrophic scars and keloid).
Based on these principles, the treatment of patients with keloid should be conducted by a multidisciplinary team. The joint and integrated approach of physicians, psychologists, nurses, nutritionists, and physical therapists knowledgeable of its psychoneuroimmune–endocrine etiology may be able to provide relief to those who suffer from a deformative, stigmatizing disease that is still without a cure.
Acknowledgments
Tribute is paid to the great master Professor Bernardo Hochman. This is a posthumous tribute to him, he devoted much of his life to studying, understanding, teaching, and disseminating what the keloid really is. We will remember forever his words “If the eyes are the mirror of the soul, the skin is the mirror of the mind.”
Disclosure
The authors report no conflicts of interest in this work.
References
- JuhanDJob’s Body. Skin as Surface of the BrainBarrytown, NYStation Hill Press, Inc20133541
- HallJMCruserDPodawiltzAMummertDIJonesHMummertMEPsychological stress and the cutaneous immune response: roles of the HPA axis and the sympathetic nervous system in atopic dermatitis and psoriasisDermatol Res Pract2012201240390822969795
- SuárezALFeramiscoJDKooJSteinhoffMPsychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updatesActa Derm Venereol201292171522101513
- MiseryLTouboulSVinçotCStress and seborrheic dermatitisAnn Dermatol Venereol20071341183383718033062
- ChidaYMaoXDoes psychosocial stress predict symptomatic herpes simplex virus recurrence? A meta-analytic investigation on prospective studiesBrain Behav Immun200923791792519409481
- ManolacheLBeneaVStress in patients with alopecia areata and vitiligoJ Eur Acad Dermatol Venereol200721792192817659001
- YuRHuangYZhangXZhouYPotential role of neurogenic inflammatory factors in the pathogenesis of vitiligoJ Cutan Med Surg201216423024422784515
- YosipovitchGTangMDawnAGStudy of psychological stress, sebum production and acnevulgaris in adolescentsActa Derm Venereol200787213513917340019
- HadshiewIMFoitzikKArckPCPausRBurden of hair loss: stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopeciaJ Invest Dermatol200412345545715304082
- ArckPPausRFrom the brain-skin connection: the neuroendocrine-immune misalliance of stress and itchNeuroimmunomodulation2006135–634735617709957
- Lugović-MihićLLjubešićLMihićJVuković-CvetkovićVTroskotNŠitumMPsychoneuroimmunologic aspects of skin diseasesActa Clin Croat20135233734524558766
- ArckPCSlominskiATheoharidesTCPetersEMPausRNeuroimmunology of stress: skin takes center stageJ Invest Dermatol200612681697170416845409
- PausRTheoharidesTCArckPCNeuroimmunoendocrine circuitry of the ‘brain-skin connection’Trends Immunol2006271323916269267
- AderRCohenNFeltenDPsychoneuroimmunology: interactions between the nervous system and the immune systemLancet19953458942991037815892
- FurtadoFHochmanBFerraraSFWhat factors affect the quality of life of patients with keloids?Rev Assoc Med Bras200955670070420191224
- RoostermanDGoergeTSchneiderSWBunnettNWSteinhoffMNeuronal control of skin function: the skin as a neuroimmunoendocrine organPhysiol Rev20068641309137917015491
- BosJDKapsenbergMLThe skin immune system: progress in cutaneous biologyImmunol Today199314275788447935
- ZouboulisCCHuman skin: an independent peripheral endocrine organHorm Res2000545–623024211595811
- KonoMNagataHUmemuraSKawanaSOsamuraRYIn situ expression of corticotrophin-releasing hormone (CRH) and proopiomelanocortin (POMC) genes in human skinFASEB J200115122297229911511529
- WakamatsuKGrahamACookDThodyAJCharacterisation of ACTH peptides in human skin and their activation of the melanocortin-1 receptorPigment Cell Res19971052882979359624
- SlominskiAZbytekBNikolakisGSteroidogenesis in the skin: implications for local immune functionsJ Steroid Biochem Mol Biol201313710712323435015
- LugerTAEpidermal cytokinesActa Derm Venereol Suppl (Stockh)198915161762696309
- AnselJPerryPBrownJCytokine modulation of keratinocyte cytokinesJ Invest Dermatol199094Suppl 6101S107S2161884
- BlalockJEThe immune system as the sixth senseJ Inter Med20052572126138
- FerreiraLMGragnaniAFurtadoFHochmanBControl of the skin scarring responseAn Acad Bras Cienc200981362362919722029
- SlominskiATZmijewskiMAZbytekBTobinDJTheoharidesTCRivierJKey role of CRF in the skin stress response systemEndocr Rev201334682788423939821
- JankordRHermanJPLimbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stressAnn N Y Acad Sci20081148647319120092
- SlominskiAPisarchikATobinDJMazurkiewiczJEWortsmanJDifferential expression of a cutaneous corticotropin-releasing hormone systemEndocrinology2004145294195014605004
- HsuSYHsuehAJHuman stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptorNat Med20017560561111329063
- GrammatopoulosDKChrousosGPFunctional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonistsTrends Endocrinol Metab2002131043644412431840
- SlominskiASzczesniewskiAWortsmanJLiquid chromatography-mass spectrometry detection of corticotropin-releasing hormone and proopiomelanocortin-derived peptides in human skinJ Clin Endocrinol Metab200085103582358811061505
- TeofoliPMotokiKLottiTMUittoJMauvielAPropiomelanocortin (POMC) gene expression by normal skin and keloid fibroblasts in culture: modulation by cytokinesExp Dermatol1997631111159226132
- ItoNItoTKrommingaAHuman hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisolFASEB J200519101332133415946990
- FoitzikKKrauseKNixonAJProlactin and its receptor are expressed in murine hair follicle epithelium, show hair cycle-dependent expression, and induce catagenAm J Pathol200316251611162112707045
- LanganEAFoitzik-LauKGoffinVRamotYPausRProlactin: an emerging force along the cutaneous-endocrine axisTrends Endocrinol Metab201021956957720598901
- RamotYBíróTTiedeSProlactin – a novel neuroendocrine regulator of human keratin expression in situFASEB J20102461768177920103718
- SlominskiATobinDJZmijewskiMAWortsmanJPausRMelatonin in the skin: synthesis, metabolism and functionsTrends Endocrinol Metab2008191172418155917
- WeiheESchützBHartschuhWAnlaufMSchäferMKEidenLECoexpression of cholinergic and noradrenergic phenotypes in human and nonhuman autonomic nervous systemJ Comp Neurol2005492337037916217790
- JoachimRAKuhlmeiADinhQTNeuronal plasticity of the “brain-skin connection”: stress-triggered up-regulation of neuropeptides in dorsal root ganglia and skin via nerve growth factor-dependent pathwaysJ Mol Med (Berl)200785121369137817639286
- Levi-MontalciniRSkaperSDDal TosoRPetrelliLLeonANerve growth factor: from neurotrophin to neurokineTrends Neurosci199619115145208931279
- PetersEMBotchkarevVABotchkarevaNVTobinDJPausRHair-cycle-associated remodeling of the peptidergic innervations of murine skin, and hair growth modulation byneuropeptidesJ Invest Dermatol2001116223624511179999
- UrpeMBuggianiGLottiTStress and psychoneuroimmunologic factors in dermatologyDermatol Clin200523460961716112436
- HochmanBFurtadoFIsoldiFCNishiokaMAFerreiraLMPsychological stress and skin wound healing: new highlightsCavalcantiLAzevedoSPsychology of StressNew York, NYNova Science Publishers Inc2013148 Available from: https://www.novapublishers.com/catalog/product_info.php?products_id=42015&osCsid=a79df5592f9331df1e8f8cf43e6796c1Accessed September 24, 2014
- AkaishiSOgawaRHyakusokuHKeloid and hypertrophic scar: neurogenic inflammation hypothesesMed Hypotheses2008711323818406540
- YagmurCAkaishiSOgawaRGunerenEMechanical receptor-related mechanisms in scar management: a review and hypothesisPlast Reconstr Surg2010126242643420375759
- OgawaRMechanobiology of scarringWound Repair Regen201119Suppl 1s2s921793962
- OgawaRHsuCKMechanobiological dysregulation of the epidermis and dermis in skin disorders and in degenerationJ Cell Mol Med201317781782223672502
- KadanoffDNeurotization and innervation of the human scar-keloidsZ Haut Geschlechtskr196944219259305396789
- HochmanBNahasFXSobralCSNerve fibres: a possible role in keloid pathogenesisBr J Dermatol2008158365165218205867
- ParkhouseNCroweRMcGroutherDABurnstockGPainful hypertrophic scarring and neuropeptidesLancet1992340883214101360111
- CroweRParkhouseNMcGroutherDBurnstockGNeuropeptide-containing nerves in painful hypertrophic human scar tissueBr J Dermatol199413044444527514432
- ZhangLQLaatoMInnervation of normal and hypertrophic human scars and experimental wounds in the ratAnn Chir Gynaecol200121590293212041924
- TeyHLMaddisonBWangHCutaneous innervation and itch in keloidsActa Derm Venereol201292552953122378106
- ZhiboXMiaoboZIntralesional botulinum toxin type A injection as a new treatment measure for keloidsPlast Reconstr Surg20091245275e277e
- UyesugiBLippincottBDaveSTreatment of a painful keloid with botulinum toxin type AAm J Phys Med Rehabil201089215315519884811
- RobinsonAJKhadimMFKhanKKeloid scars and treatment with botulinum toxin type A: the Belfast experienceJ Plast Reconstr Aesthet Surg201366343944023092904
- WilsonAMEradication of keloids: surgical excision followed by a single injection of intralesional 5-fluorouracil andbotulinum toxinCan J Plast Surg2013212879124431948
- KimLRWhelpdaleKZurowskiMPomeranzBSympathetic denervation impairs epidermal healing in cutaneous woundsWound Repair Regen1998631942019776863
- ScholzenTEBrzoskaTKaldenDHEffect of ultraviolet light on the release of neuropeptides and neuroendocrine hormones in the skin: mediators of photodermatitis and cutaneous inflammationJ Investig Dermatol Symp Proc1999415560
- LouwLThe keloid phenomenon: progress toward a solutionClin Anat200720131416944532
- MeeranSMSinghTNagyTRKatiyarSKHigh-fat diet exacerbates inflammation and cell survival signals in the skin of ultraviolet B-irradiated C57BL/6 miceToxicol Appl Pharmacol2009241330331019747500
- FerreiraACHochmanBFurtadoFBonattiSFerreiraLMKeloids: a new challenge for nutritionNutr Rev201068740941720591108
- DhabharFSPsychological stress and immunoprotection versus immunopathology in the skinClin Dermatol2013311183023245970
- HochmanBVilas BôasFCMarianoMFerreirasLMKeloid heterograft in the hamster (Mesocricetus auratus) cheek pouch, BrazilActa Cir Bras200520320021216033178
- NirodiCSDevalarajaRNanneyLBChemokine and chemokine receptor expression in keloid and normal fibroblastsWound Repair Regen20008537138211115149
- WilkeKMartinATerstegenLBielSSA short history of sweat gland biologyInt J Cosmetic Sci2007293169179
- ZouboulisCCThe sebaceous glandHautarzt201061646746820512305
- LugerTALottiTNeuropeptides: role in inflammatory skin diseasesJ Eur Acad Dermatol Venereol19981032072119643321
- HarvimaITNilssonGNaukkarinenARole of mast cells and sensory nerves in skin inflammationG Ital Dermatol Venereol2010145219520420467393
- HarvimaITNilssonGStress, the neuroendocrine system and mast cells: current understanding of their role in psoriasisExpert Rev Clin Immunol20128323524122390488
- LeeYSVijayasingamSMast cells and myofibroblasts in keloid: a light microscopic, immunohistochemical and ultrastructural studyAnn Acad Med Singapore19952469029058839007
- ShakerSAAyuobNNHajrahNHCell talk: a phenomenon observed in the keloid scar by immunohistochemical studyAppl Immunohistochem Mol Morphol201119215315920881838
- MessadiDVLeABergSHuangGZhuangWBertolamiCNEffect of TGF-beta 1 on PDGF receptors expression in human scar fibroblastsFront Biosci19983a16a229450987
- FujiwaraMMuragakiYOoshimaAUpregulation of transforming growth factor-beta1 and vascular endothelial growth factor in cultured keloid fibroblasts: relevance to angiogenic activityArch Dermatol Res2005297416116916184401
- CampanerABFerreiraLMGragnaniABruderJMCusickJLMorganJRUpregulation of TGF-beta1 expression may be necessary but is not sufficient for excessive scarringJ Invest Dermatol200612651168117616498396
- AbdouAGMaraeeAHAl-BaraAMDiabWMImmunohistochemical expression of TGF-β1 in keloids and hypertrophic scarsAm J Dermatopathol2011331849120559120
- KikuchiKKadonoTTakeharaKEffects of various growth factors and histamine on cultured keloid fibroblastsDermatology19951901487894095
- McCauleyRLChopraVLiYYHerndonDNRobsonMCAltered cytokine production in black patients with keloidsJ Clin Immunol19921243003081512303
- GhazizadehMTosaMShimizuHHyakusokuHKawanamiOFunctional implications of the IL-6 signaling pathway in keloid pathogenesisJ Invest Dermatol200712719810517024100
- GhazizadehMEssential role of IL-6 signaling pathway in keloid pathogenesisJ Nippon Med Sch2007741112217384473
- UittoJIL-6 signaling pathway in keloids: a target for pharmacologic intervention?J Invest Dermatol200712716817170717
- EishiKBaeSJOgawaFHamasakiYShimizuKKatayamaISilicone gel sheets relieve pain and pruritus with clinical improvement of keloid: possible target of mast cellsJ Dermatolog Treat200314424825214660274
- FurtadoFHochmanBFarberPLMullerMCHayashiLFFerreiraLMPsychological stress as a risk factor for postoperative keloid recurrenceJ Psychosom Res201272428228722405222
- DuraniPBayatALevels of evidence for the treatment of keloid diseaseJ Plast Reconstr Aesthet Surg200861141717644502
- GauglitzGGManagement of keloids and hypertrophic scars: current and emerging optionsClin Cosmet Investig Dermatol20136103114
- AlonsoPERiojaLFPeraCKeloids: a viral hypothesisMed Hypotheses20087015616617509771
- de MesquitaCJAbout strawberry, crab claws, and the Sir James Black’s invention. Hypothesis: can we battle keloids with propranolol?Med Hypotheses20107435335919758768
- HuangCAkaishiSHyakusokuHOgawaRAre keloid and hypertrophic scar different forms of the same disorder? A fibroproliferative skin disorder hypothesis based on keloid findingsInt Wound J Epub11222012
- DongXMaoSWenHUpregulation of proinflammatory genes in skin lesions may be the cause of keloid formation [review]Biomed Rep20131683383624649037
- ShihBBayatAGenetics of keloid scarringArch Dermatol Res2010302531933920130896
- HalimASEmamiASalahshourifarIKannanTPKeloid scarring: understanding the genetic basis, advances, and prospectsArch Plast Surg201239318418922783524
- BurdAHuangLHypertrophic response and keloid diathesis: two very different forms of scarPlast Reconstr Surg20051167150e157e
- NirodiCSDevalarajaRNanneyLBChemokine and chemokine receptor expression in keloid and normal fibroblastsWound Repair Regen20008537138211115149
- ZhangQYamazaTKellyAPTumor-like stem cells derived from human keloid are governed by the inflammatory niche driven by IL-17/IL-6 axisPLoS One2009411e779819907660
- QuMSongNChaiGWuXLiuWPathological niche environment transforms dermal stem cells to keloid stem cells: a hypothesis of keloid formation and developmentMed Hypotheses201381580781224074897