Abstract
The complexity of aging is hard to be captured. However, apart from its tissue-specific features, a structural and functional progressive decline of the whole organism that leads to death, often preceded by a phase of chronic morbidity, characterizes the common process of aging. Therefore, the research goal of scientists in the field moved from the search for strategies able to extend longevity to those ensuring healthy aging associated with a longer lifespan referred to as “healthspan”. The aging process is plastic and can be tuned by multiple mechanisms including dietary and genetic interventions. To date, the most robust approach, efficient in warding off the cellular markers of aging, is calorie restriction (CR). Here, after a preliminary presentation of the major debate originated by CR, we concisely overviewed the recent results of CR treatment on humans. We also provided an update on the molecular mechanisms involved by CR and the effects on some of the age-associated cellular markers. We finally reviewed a number of tested CR mimetics and concluded with an evaluation of future applications of such dietary approach.
Introduction
The natural and multifactorial process of aging affects all organisms. Although presenting tissue-specific features, the trait of a progressive structural and functional decline with advancing age, ultimately leading to death, is unanimously shared. Often the lifelong experiences of aged people make them an invaluable reservoir of wisdom for younger people. However, the negative correlates of aging (eg, physical and cognitive decline) make such older persons progressively less independent in self-sustaining and more needy of support from society.
The constantly “graying” of Western countries has already led to a heavy economic social burden because the lifespan extension reached in the past decades has often been accompanied by the chronicity of age-related diseases. This indicated the need for reframing the goal of extended longevity to that of healthy aging associated with a longer lifespan, referred to as “healthspan”.Citation1 A great deal of research demonstrated that aging has not a unique cause, whereas multiple mechanisms tune up the whole aging process.Citation2 Thanks to the pioneering work carried out on the Caenorhabditis elegans model by Kenyon et al, it became clear that the aging process is plastic and capable of being accelerated or attenuated by a range of dietary and genetic interventions.Citation3 This paved the way to the search of lifestyle interventions aimed at promoting healthy aging through prevention or delay of age-associated dysfunctions.
According to several authors,Citation2,Citation4–Citation6 a successful approach to healthy aging should be able to counteract the following nine cellular markers of aging: 1) telomere erosion, 2) epigenetic alterations, 3) stem cells depletion, 4) cellular senescence, 5) mitochondrial dysfunction, 6) genomic instability, 7) proteostasis imbalance, 8) impaired nutrient sensing, and 9) abnormal intercellular communication. To date, the most robust intervention efficient in warding off the aforementioned cellular markers of aging is calorie restriction (CR) that involves the administration of a well-balanced, nutrient-dense diet that reduces calorie intake by 20%–40% without malnutrition.Citation7 CR has a dramatic effect (two- to threefold) in extending both median and maximal lifespan in rodents, and it prevents or delays the onset of various age-related diseases such as obesity, type-2 diabetes, neurodegeneration, cardiomyopathy, and cancer.Citation8
Here, after a preliminary presentation of the major debate originated by CR, we concisely overviewed the recent results of CR treatment on humans. We also provided an update on the molecular mechanisms involved by CR and the effects on some of the age-associated cellular markers. We finally reviewed a number of tested CR mimetics and concluded with an evaluation of future applications of such dietary approach.
CR: the issue of the real cause
The unified hypothesis about CR and longevity by Sinclair proposed that CR might increase the survival capability of the organism by evoking a highly conserved stress response.Citation9 The “Hormesis hypothesis of CR” provided further support to such a model suggesting that the adaptive responses of cell and organs, induced by a moderate stress, prevent worse damage caused by a stronger similar stress.Citation10–Citation13 Since the initial experimental reports on CR, a major debate was arisen about the nature of the cause of the extended longevity namely whether it was due to the reduction of protein intakeCitation14 or to that of calories.Citation15 For a long period, the identification of reduced calorie intake as a responsible factor for increased longevity prevailed.Citation16 In the 2000 decade, works on insects (Drosophila melanogaster) by Partridge et alCitation17,Citation18 have reopened the question about the relevance of amino acids and proteins for the CR impact on longevity. It has been argued that the apparent effect of the decreased energy intake was really due to an altered ratio between protein and non-protein components of the diets.Citation19,Citation20 A novel approach, the “geometric framework” or “nutritional geometry”,Citation20–Citation23 provided a helpful tool for dissecting the consequences of composite nutritional manipulations of the diet on different phenotypic parameters including lifespan. Recent massive work has been performed on ad libitum-fed mice changing the protein to carbohydrate ratio (25 different diets) and then reporting the animals’ responses, including lifespan, in a two-dimensional plot.Citation24 Evidence suggested that a decline in protein to carbohydrate ratio is the factor mostly affecting longevity. The clear indication drawn by Solon-Biet et alCitation24 derived, however, from an intake restriction originated by additional indigestible cellulose that diluted the diet. Such diet “dilution” might have deeply affected the neuropeptide pathways linking restriction to lifespan benefits by inducing responses markedly different in comparison with those elicited by the simple reduction of food mass.Citation25 Reanalysis of the insect results with the “geometric framework” tool led to suggest that something substantially different might be happening in these animals through protein restriction (PR) with respect to what a reduced calorie intake alone might induce in rodents.Citation26 A recent application by Speakman et alCitation27 of conventional statistical analyses as well as of the nutritional geometric framework approach to the literature data, through a very accurate and thoughtful survey, has prompted again to the conclusion that CR impact on rodents longevity is exerted independently of PR occurring or not and that it is due to calorie deficit. The evident contrast with previous experimental work by Solon-Biet et alCitation24 has been reconciled, confirming the completely different effect of CR plus PR on rodents lifespan versus that of PR alone and demonstrating that reduced calories and not reduced protein intake cause the food restriction effect on longevity.Citation27 A more recent contribution to the longstanding debate on the nature of the real cause of the benefits of CR has been made by Simpson et alCitation28 by applying a nutritional geometry approach to the meta-analysis of previous reports on rodents and insects. In particular, this last study has questioned the conclusion drawn by Speakman et al,Citation27 about the unique relevance of the reduced intake of calories versus that of protein to increase lifespan, indicating a series of factors (analysis of various rodents strains with different ages of diet onset, absence of the exact composition of all included diets, and impossible evaluation of the “protein leverage” effect occurring in ad libitum-fed animals exposed to a low-protein diet) that might have been underestimated, thus leading to a not-straightforward final evaluation. It has been also marked that Speakman et al,Citation27 however, acknowledged a positive impact on longevity of the protein amount reduced below that of the reference diets and that the opposite conclusions might have derived from the difficult interpretation of the nutritional interventions by conventional instruments.Citation28 Therefore, the application of the nutritional geometry approach might be very useful and might allow to modify the original question about the optimum diet for lifespan in order to include multiple variations in the intake of macronutrients and calories. Overall, experimental results of geometric framework studiesCitation24,Citation29 are consistent with that of extensive meta-analyses.Citation27,Citation30 In fact, they all agree about the occurrence of the lowest risk of mortality with a protein content between 10 and 30% of total calories, while mortality increases together with protein content. This has shed a new light on CR, suggesting that it does not deal simply with a quantitative reduction in energy, but also with a qualitative change in macronutrients. In agreement with this, it might be helpful to introduce here the distinction between CR, implying a decreased total calorie intake, namely glucose dilution for yeast, dilution of bacterial density for worms, simple food dilution for fruit fly or food restriction for mammals, and dietary restriction (DR), involving the reduced intake of specific nutrients, each characterized by its respective caloric value. Such latter intervention causes a change in the total calorie intake as well as in the ratio between macronutrients, often in the proteins to carbohydrates ratio, which appears to be crucial for the impact on longevity.Citation31,Citation32 In particular, the application of DR regimens has allowed a more detailed definition of the assayed diet making more straightforward the interpretation of the experimental results and clarifying the relevance of specific nutrients.Citation31
Results of CR treatment in humans
Results from three ongoing long-term studies on primates indicate an increased healthspan in animals treated with CR.Citation33–Citation36 The CR-mediated influences on disease prevention and longevity have yet to be clarified, but the beneficial consequences of CR on human metabolic and molecular adaptations have been already extensively analyzed. The most thorough and extended review of the results obtained from various kinds of CR regimens applied to humans is the recent analysis by Most et al.Citation8
Short-term CR interventions in humans
The first reported CR study on humans involved a 10-week 20% CR that led to a reduced resting metabolic rate per kilogram of fat-free mass (FFM),Citation37 thus showing that the energy expended per FFM could be changed through a metabolic adaptation. A reduction in both systolic and diastolic blood pressure valuesCitation38 and in glucose concentrations was demonstrated in the CR group.Citation39 Then the first controlled clinical trials of CR with adequate nutrient provision in healthy, non-obese humans were started by the US National Institute of Aging through the CALERIE-1 (Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy) study. The project included three pilot studies carried out, respectively, at Pennington Biomedical Center in Louisiana, at Washington University in St Louis, and at Tufts University in Boston, and designed to evaluate feasibility and effects of CR on metabolic health after 6 monthsCitation40,Citation41 or 12 months.Citation42 In these pilot studies, CR obtained a negative energy balance through different situations: 1) reduced CR, 2) increased exercise energy expenditure, or 3) combination of CR and exercise. Enrolled subjects’ fasting insulin concentrations decreased after CR and insulin sensitivity improved by 40% (p=0.08).Citation43 In the CALERIE-1 trial, different markers related to cardiovascular diseases (CVDs) (eg, blood pressure, low-density lipoprotein [LDL], high-density lipoprotein [HDL], fibrinogen, and others) were not affected by CR, but it was calculated that the 25% CR diet for 6 months reduced by 29% the 10-year risk for CVD.Citation44 In addition to this, a reduction in markers of oxidative stress (DNA damage and superoxide dismutase activity) was describedCitation45 for the first time. The following Phase II multi-center trial (CALERIE-2 study) was performed to analyze whether 2-year 25% CR in leaner and younger men and women was efficient and safe. Therefore, 220 healthy, young, and middle-aged (21–51 years old), non-obese men and women were examined for the efficacy of CR on energy metabolism, metabolic adaptations, immune function, chronic disease risk factors, and quality of life.Citation46 Since the major contributor of metabolic rate is FFM, biopsies studies from CR-treated skeletal muscle could help to shed light on the molecular mechanisms of CR-induced metabolic adaptation. Mitochondrial biogenesis was promoted by CR in skeletal muscle. After 1-year of exposition to 20% CR, or to 20% increased energy expenditure because of endurance exercise, or to a healthy lifestyle,Citation42 the CR-induced weight loss improved insulin sensitivity, increased adiponectin, and reduced the serum concentrations of leptin, insulin, LDL cholesterol, and C-reactive protein.Citation47,Citation48 Moreover, 30% CR sustained for 2 years, reducing energy intake to 75% of baseline (25% CR), resulted in adaptive changes identical to those reported in rodents subjected to CR.Citation49 The 2-year of CR intervention was adopted also because the duration 6 months and 1 year in CALERIE-1 pilot trials was evaluated as not sufficient to induce in humans several of the metabolic and hormonal changes thought to favor rodents longevity. It has been demonstrated by the results of this large trial that mild CR can improve cardiometabolic risk factors, well below conventional clinical thresholds, even when healthy lean or slightly overweight young and middle-aged men and women are exposed. Total cholesterol, LDL cholesterol, triglycerides, C-reactive protein, tumor necrosis factor (TNF)-α, and blood pressure decreased significantly and HDL cholesterol increased in the CR group, even in people with normal risk factors.Citation49
Long-term CR interventions in humans
At present, only the collection of data recorded from the members of the Calorie Restriction Society, who have imposed on themselves a regimen of severe CR with optimal nutrition (CRON), believing to extend in this way their healthy lifespan, provides direct evidence that CR may affect the aging process in humans. These very lean men and women (body mass index 19.7±1.8 kg/m2) voluntarily restricted their caloric intake (1,800 kcal/d) for an average of 15 years and consumed ~30% less energy than a counterpart (matched for age, sex, and socioeconomic status) fed with a regular Western diet. It is important that the CRON diet meets all recommendations for essential nutrients and is very rich in vegetable fiber and low glycemic foods including a wide variety of phytochemicals, which may modulate metabolic health.Citation50 CRON data on longevity and mortality are not yet available, but the collected findings indicate that moderate/severe CR in humans induces the metabolic and molecular changes described in long-lived CR animals.Citation8 Furthermore, such CR with optimal intake of nutrients has decreased metabolic and hormonal risk factors for type-2 diabetes, CVD, stroke, cancer, and vascular dementia in the participants.Citation50 The total cholesterol–HDL cholesterol ratio was 2.6 and the range of triglycerides was 50 mg/dL. Systolic and diastolic blood pressure was 110/70 mmHg, even in people in their late 70s, and C-reactive protein was almost undetectable.Citation50,Citation51 Serum TNF-α, interleukin (IL) 6, fasting glucose, and insulin showed low values, and insulin sensitivity (HOMA-IR) improved.Citation52 The variability of heart rate in the CRON practitioners was comparable with normal values of healthy men and women 20 years younger.Citation53 Differently from the CALERIE trials, most hormonal adaptations described in long-lived CR rodents were found in subjects exposed to severe CR. It is relevant that studies about CR with adequate nutrition and behavioral support to participants have not reported an influence of CR on psychological and mental capacity both as a 20% CR for 10 weeksCitation38 and 25% CR for 6 months in CALERIE-1.Citation54 In the longer CALERIE-2 trial, bone mass significantly was reduced at sites of osteoporotic fractures as the hip and femoral neck and the lumbar spine.Citation55 Such data might limit the application of CR for older persons, eventually affected by accelerated bone loss. However, results from CRON practitioners are consistent with those from animal studies indicating that CR reduces bone mineral density but improves quality and strength of bones through their reduced turnover and prevention of secondary hyperparathyroidism.Citation56 Furthermore, absolute maximal aerobic capacity per kilogram of body mass was maintained or increased during CR, suggesting a positive effect of the body weight loss on physical functioning. Effectively, also quality of life improved according to scores from survey on physical component, depression, and physical functioning.Citation49
CR: effects and mechanisms
CR has pleiotropic effects, and by improving multiple metabolic pathways, it generates benefits for the whole organism. In particular, some indications deriving from a thorough investigation of the CR counteracting action, with respects to the aforementioned cellular markers of aging, will be concisely outlined in the present paragraph, whereas a more detailed discussion of the specific mechanisms involved will be developed in the following paragraphs.
Genomic instability and epigenetic alterations
Telomere shortening and high levels of DNA damage (both at nuclear and mitochondrial level), including mutations, DNA breaks, and chromosomal rearrangements, are typical age-related alterations. With aging, the DNA repair capacity decreases causing genome instability.Citation57 CR has a positive effect on the DNA repair and telomere machinery, thereby promoting genomic stability and healthy longevity.Citation58 However, the genome is not unique in shaping cellular homeostasis, health, and aging, as “epigenetics” has unveiled mechanisms that adjust gene expression and directly affect disease/phenotypes, without changing DNA sequences. Epigenetic marks can be added/removed on histones or the DNA itself, modulating chromatin remodeling and gene expression according to various environmental signals.Citation59 There exist some cellular pathways that are able to sense nutrients and/or energy levels and also to influence processes like epigenome remodeling, gene expression, protein activity, and organelle integrity.Citation2 Such pathways are deeply involved in modulating aging and age-related diseases and in mediating the CR beneficial effects promoting proteostasis balance, genome stability, and stem cell survival.Citation60 However, all CR mechanisms have not been yet clarified.
Proteostasis imbalance
Another mark of cellular aging is related to the crucial functional role performed by proteins inside cells where these molecules integrate all physiological pathways. Therefore, the stability of proteins (proteostasis) indicates the protection of protein structure and function, against environmental and internal stressors, operated by the cell. Vulnerability in proteostasis correlates with age-related changes and longevity rates among species.Citation61 An intricate proteostasis network including synthesis of proteins, activity of chaperones, autophagy, unfolded-protein response, and ubiquitin-proteasome pathway, counteracts, inside cells and mitochondria, protein misfolding and unfolding, thus allowing a regular protein turnover.Citation62 Effectively, CR appears associated with an increase in relevant chaperones and autophagic mediators active in protein quality control and removal of dysfunctional proteins and organelles.
Mitochondrial dysfunction, oxidative damage, and impaired nutrient sensing
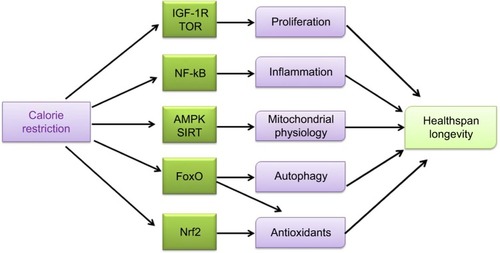
Other two hallmarks among CR effects are reduction of oxidative damage and modulation of mitochondrial activity. With dietary treatment, endogenous antioxidants are induced and cell membranes peroxidability index is modulated, thereby decreasing oxidative damage.Citation6 Pathways able to sense nutrients and/or energy levels – namely AMP-dependent kinase (AMPK) and sirtuins, the nicotinamide adenine dinucleotide (NAD)+-dependent deacetylases that regulate the activity of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), and forkhead box (FoxO) proteins, involved in mitochondrial biogenesis, turnover, and oxidative metabolism – are activated to induce mitochondrial activity with CR. Interestingly, two different pathways, target of rapamycin (TOR) inhibition and AMPK activation, which are both sensitive to energy/nutrient status, can induce FoxO activation. In general, CR decreases nutrient uptake that unbalances metabolism and requires the interaction of several regulatory pathways to reach a new equilibrium. A new balance is obtained via the coordination of growth-associated pathways, such as the insulin/insulin-like growth factor-1 (IGF-1) signaling (IIS) pathway and TOR, which are downregulated, and those activating a more efficient respiratory metabolism such as AMPK and sirtuins that are induced. This means that the diet-reduced calorie intake increases metabolic efficiency and protects against cellular damage and remodeling mechanisms, thereby reducing less efficient metabolism and synthetic pathways. It seems that an evolutionarily conserved response aiming to avoid useless expenditure of energy and to recycle structures for the organisms’ survival is activated by CR. Therefore, CR-induced block of IGF-1 receptor-dependent pathways and TOR-dependent activities inhibits processes involved in cell proliferation and glycolysis. Recently, it has been shown that CR elicits also an anti-inflammatory effect, by inhibiting nuclear factor-kB (NF-kB) activity and by reducing pro-inflammatory profile of aging. Therefore, CR-related extension in healthspan and longevity derives from the interrelated contributions of all the mechanisms described here ().
Figure 1 Calorie restriction (CR) impacts various cellular pathways and induces responses of the whole organism, leading to a more efficient metabolism, a higher protection against cellular damage, and the activation of remodeling mechanisms, whereas less efficient metabolism and synthetic pathways are blocked. CR inhibits processes involved in cell proliferation and glycolysis by blocking IGF-1 receptor-dependent pathways and TOR-dependent activities. CR exerts an anti-inflammatory effect by inhibiting nuclear factor-kB (NF-kB) activity. CR also decreases the production of ROS and increases mitochondrial biogenesis through different pathways (AMPK, sirtuins, and eNOS) leading to an improved mitochondrial physiology. The CR-induced activation of FoxOs implies the resumption of autophagy and mitophagy and the risen expression of antioxidants. CR also evokes activation of the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) that increases the expression of mitochondrial and cell antioxidant enzymes. Any of these processes participates in the CR-related increase in improving healthspan and longevity.
Management of reactive oxygen species (ROS), lipid peroxidation, and mitochondrial fitness
A relevant increase in ROS level, reduction in antioxidant defenses, and appearance of mitochondrial dysfunction, implying accumulation of oxidative damage to mitochondrial DNA, proteins, and lipids, are usually associated with aging.Citation63–Citation65 However, age-related mitochondrial dysfunction exceeds just the increased oxidative damage because it affects other key mitochondrial functions such as biogenesis, turnover, dynamics, and protein quality control.Citation66–Citation70 On the other side, the “Hormesis hypothesis” has highlighted the relevance of ROS as signaling molecules in several cellular processes among which lifespan extension is included, which is attained via the regulation of nuclear genes expression by retrograde signaling pathways.Citation71 Aging correlates with increased mitochondrial and cell ROS production and with increased degree of fatty acid unsaturation in membranes, due to a major fraction of more oxidizable polyunsaturated fatty acids.Citation72 Such two factors, together with a reduction in membrane-linked antioxidant enzymes, trigger age-increased lipid peroxidationCitation73 that impacts on various cell processes. CR in mitochondria reduces the membrane potential and the production of ROS, as well as it counteracts the age-related membranes deterioration by changing the saturation/unsaturation index of fatty acids with positive effects on oxidative damage and membrane fluidity. In particular, it has been recently demonstrated that the fatty acid types introduced with the diet may influence the CR effect.Citation74 As for the age-related increase of ROS, these reactive species, prevalently produced inside the cells by the mitochondrial electron transport chain, are released in higher amounts by the damaged mitochondria, which become also inefficient for energy production. In particular, age-related mitochondrial dysfunction affects the assembling of respiratory complexes in supercomplexes, involved in ROS regulation, thus increasing ROS production.Citation75 Furthermore, it has been demonstrated that the relevance of a cascade that, through the activation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), increases the expression of NAD(P)H dehydrogenase and quinone 1 (Nqo1) and ensures the maintenance of mitochondrial membrane potential compromised during aging in mice.Citation76 CR at the plasma membrane also induces the expression of coenzyme Q-dependent enzymes in old rodents but not in young animals,Citation77,Citation78 suggesting that the age of onset of CR might be important for organisms. Age-related mitochondrial dysfunction impairs mitochondrial biogenesis especially in those tissues largely dependent on oxidative energy for their metabolism such as muscle, brain, or heart.Citation79 CR induces mitochondrial biogenesis in human heart and skeletal muscle and in rodents liver and skeletal muscleCitation66,Citation78,Citation80–Citation82 by means of at least three different pathways that have been analyzed in muscle: induction of endothelial nitric oxide synthase (eNOS), activation of PGC-1α, and adiponectin-dependent activation of the SIRT1/AMPK axis. The CR-related induction of NOS in mice and human muscleCitation45,Citation83 is very relevant for the sequential activation of mitochondrial biogenesis. SIRT1 is also essential since it activates eNOS by deacetylation,Citation84 thus favoring mitochondrial biogenesis. However, mitochondrial biogenesis, dynamics, and genome copy number as well as oxidative phosphorylation are all controlled by the master regulator PGC-1αCitation85 that adapts mitochondrial population to energy needs. In fact, more recently, it has been suggested that the beneficial impact of CR on aging might be reached through the attenuation of age-related mitochondrial decline obtained by a complex crosstalk among energy-sensing pathways.Citation86 In fact, PGC-1α activity is directly modulated at transcriptional and posttranslational levelCitation87 by two major cell metabolic sensors namely AMPK and SIRT1, through phosphorylation and deacetylation.Citation88,Citation89 Also a factor important in age-related mitochondrial dysfunction is the accumulation of damaged organelles due to reduced mitochondrial turnover by inhibition of mitophagy, specifically dedicated to the removal of damaged mitochondria.Citation65 Effectively, the renovation of mitochondrial network is very relevant in the CR-dependent healthspan increaseCitation90 and in counteracting the aging process.Citation91
CR: the contribution of genetics and epigenetics of longevity
The studies about genes influencing lifespan led to the conclusion that human longevity can be influenced by about 25% by genetic factors, whereas environmental factors strongly affect lifespan by targeting relevant pathways in which alterations of the expression pattern are induced,Citation1 also by changes in chromatin structure.Citation92,Citation93 The object of epigenetics is “chromatin” and the mechanisms through which it modulates gene expression. Such mechanisms operate by means of changes in the chromatin structure that may involve DNA methylation profile, addition/removal of methyl groups to/from cytosines, involvement of histones via posttranslational modifications (methylation, acetylation, or phosphorylation), and nucleosomes positioning via ATP-dependent chromatin remodelers. The presence of a marked DNA methylation and specific histone modifications can render chromatin so tight and compact (closed) to become poorly accessible to the transcription machinery. Vice versa, unmethylated DNA and a different array of histone modifications can favor a looser or “open” form of DNA that might be actively transcribed. With aging, the epigenome undergoes remodeling at both the histone and DNA levels and the hope is that, by ensuring epigenetic stability, a disease-free lifespan might be obtained.Citation94 It appears likely the existence of a crosstalk between the chromatin and metabolome making of chromatin the nexus where the balance between fecundity and longevity is decided.Citation95 Effectively, chromatin and metabolome are very closely linked through metabolic cofactors. Products of the tricarboxylic acid (TCA) cycle, glycolysis, and β-oxidation feed into chromatin-modifying enzymes and adjust their activity, adapting epigenetic responses to the metabolic phenotype of the cell.Citation95 CR implies large changes in metabolic cofactors likely altering the cellular availability of enzyme co-factors and substrates, such as s-adenosyl-methionine, acetyl-CoA, and NAD+, so much to affect all enzymes using these factors, among which many control methylation and acetylation reactions. It appears that age-related decline may be causally related to age-dependent loss of cell-specific transcriptional regulation and genomic integrity due to changes in the genome methylation profile.Citation96 Such changes might be attenuated by CR, as short-term CR has reduced age-dependent alterations of methylation in the promoters of genes involved in age-associated degenerative phenotypes in 25-month-old rats.Citation97 CR also works by inhibiting anabolic pathways, allowing cells to invest resources for maintenance and stress resistance, which improve health and longevity, via alteration of programs of gene expression. CR, in fact, increases the activity of histone deacetylases, particularly SIRT1, which is localized to age-associated genes such as p16 in CR, thus contributing to their repression through histone H3 hypoacetylation. This effect increases the replicative lifespan of human fibroblasts in vitro.Citation98 Histone acetylation should favor gene activation through cumulative neutralization of histones’ positive charges, leading to disruption of nucleosomes and finally to generation of a more “open” chromatin structure.Citation99 Modified histone acetylation profiles, particularly hyperacetylation of histones, have been linked to aging phenotypes. However, we now begin to understand that histone acetylation plays a more nuanced role in gene regulation, with site-specific changes in histone acetylation being as important as more global alterations.Citation100 Few recent reports have demonstrated that longevity interventions can attenuate age-related chromatin decline, but the specific crucial alterations that impact on healthspan await to be highlighted by further studies.Citation94
Sirtuins
The role of sirtuins pathway in regulating lifespan was discovered in yeast,Citation101 but the family of these NAD+-dependent protein deacetylases is present in all organisms where intervenes favoring longevityCitation102 and, by removing acetyl residues, it regulates the activity of many enzymes. In humans, this family of ubiquitously expressed proteins comprises seven members, each featuring a specific localization. The most thoroughly studied SIRT1 regulates gene expression and the function of various proteins active in different molecular pathways. Furthermore, SIRT1 and the nuclear SIRT6 and 7 act as DNA repairing enzymes,Citation103 whereas SIRT3, SIRT4, and SIRT5 regulate mitochondrial functions. Sirtuins are strongly involved by CR as reported in mammals, where the diet induces in many organs the expression and the activity of such enzymes, and several CR-related metabolic changes are implied by sirtuins’ activities.Citation104 In particular, the deacetylating activity of sirtuins is coupled to NAD+ hydrolysis, thus originating the deacetylated substrate, O-acetyl-ADP-ribose and nicotinamide, a sirtuin inhibitor. Sirtuins depend on NAD+ for their activity, which link them to the availability of this co-substrate and to the cell energy balance; therefore, shifts in the NAD+/NADH ratio are directly detected by sirtuins acting as nutrient and metabolic sensors that influence enzymes to rapidly recuperate cell homeostasis.Citation105,Citation106 When NAD+ accumulates, such as during scarcity of nutrients, especially glucose, sirtuins are activated. These deacetylases affect both epigenome and acetylome since they remove acetyl groups from the lysine residues of histones and non-histone proteins. In particular, acetylome modulation has recently gained a great consideration because of the constantly growing number of proteins regulated by acetylation/deacetylation, involved in various crucial cellular processes like cell cycle, metabolism regulation, antioxidant protection, autophagy, and more. Therefore, the relevance of sirtuins for CR impact on longevity and healthspan has been thoroughly analyzed.Citation107 In particular, the CR-activated SIRT1 senses NAD+ abundance and deacetylates specific histone-acetylated residues, acting as an epigenetic regulator, but at the same time, by deacetylating transcription factors such as NF-kB, PGC-1α and FOXO3a regulate cell expression.Citation108
For example, CR induces in mice and human muscle the SIRT1-mediated activation of eNOSCitation45,Citation83 that is essential for promoting mitochondrial biogenesis. This is also favored through the activation of PGC-1α obtained by means of the SIRT1-mediated deacetylation.Citation88,Citation89 Thus, regulators of metabolism such as sirtuins are involved in the multiple effects of CR also through the complex network between their activities modulating mitochondrial biogenesis, dynamics, and turnover. However, after the initial general consensus, the need of sirtuins’ activation for CR efficacy has been objectedCitation109 and the overall anti-aging activity of sirtuins has been questioned because pro-aging actions of specific sirtuins (Sir2 in S. cerevisiae and SirT1 in murine neurons) have been demonstrated.Citation110,Citation111 It has been reported that the ablation of the SirT1 gene reduced age-related oxidative stress markers in the neurons of old mice, thus supporting its neuronal pro-aging role. Such ablation, although, induced very serious consequences for the whole organism namely severe developmental defects and a shorter lifespan in both ad libitum-fed and restricted animals that confirmed the SirT1 general protective role.Citation111,Citation112 More recently, the debate about the relevance of sirtuins activity for lifespan has continued with negativeCitation113,Citation114 and positiveCitation102,Citation115 reports that led to the final assessment of sirtuins as evolutionarily conserved regulators of aging/longevity.Citation116 In particular, the control over longevity exerted by sirtuins appears very tightly linked to cell NAD+ levels and this has led to include NAD+ among the CR mimetics (see the following specific section). Another pathway responsible for the involvement of sirtuins in the modulation of aging and related conditions acts through the age-dependent nuclear genome instability driving the activity of SIRT1, SIRT6, and SIRT7 that participate in nuclear DNA repair and regulate mitochondrial homeostasis and mitochondrial unfolded protein response,Citation117 a signaling pathway contributing to longevity.Citation115 DNA damage and repair are also relevant because their balance addresses the cell destiny toward apoptosis and death or autophagy/mitophagy and survival. Sir2 in yeast and SIRT1 in mammals have been reported to activate, respectively, mitophagyCitation118 and autophagy.Citation119 It has also been demonstrated that SIRT1 interacts cooperatively with the energy sensor pathway of AMPK (see the following section) to promote mitophagy, likely through a reciprocal feedback loop.Citation120 This suggests that SIRT1 may protect from aging by inhibiting apoptosis and maintaining mitochondrial integrity at the expenses of NAD+ cellular content. The regulation of NAD+ content depends, however, also on the competition between two NAD+-consuming enzymes namely SIRT1 and poly(ADP-ribose) polymerase 1 (PARP1), with the last activated to repair DNA damages. Therefore, when the damage level is so high to induce PARP1, this uses NAD+ up, which is not anymore available for SIRT1Citation121 and causes inhibition of the sirtuin and the subsequent reduction of autophagy and mitophagy. The finding that in some premature aging diseases, where defective mitophagy should trigger pathology progression, the restoration of the NAD+–SIRT1 (SIR2.1) pathway has improved healthspanCitation122,Citation123 strongly supports the link between sirtuins and autophagy/mitophagy.
IGF/TOR/FoxO and APMK pathways
The target of IGF-1 signaling (IIS) pathway is sensitive to nutrients and integrates growth/survival signals with glucose abundance. Aging appears modulated by changes in the insulin/IGF-1 receptor signaling system,Citation124–Citation126 as longevity is enhanced by a decrease in IIS signaling in worms, flies, and mice. The IIS pathway includes, among the main constituents, the gene expression regulatory factors FoxOs, whose localization either in the cytoplasm or in the nucleus depends on nutrient conditions. The IGF-1 signaling pathway is activated by nutrients abundance and induces activation of TOR and ribosomal protein 6 kinase (S6K) with sequential protein synthesis, while inhibiting FoxO-mediated transcription. These factors are relevant for CR impact on lifespan, as longevity in both C. elegans and D. melanogaster is reduced by the inhibition of the FoxO orthologue daf-16 by IGF-1-dependent signaling.Citation3 The relevance of FoxO for the induction of various CR protective mechanisms is supported by its involvement in the activation of several pathways induced by stress response genes.Citation127 CR decreases IGF-1, insulin, and glucose plasma levels in rodentsCitation128 and also in humans.Citation48 The bacteria Streptomyces hygroscopicus produces the antibiotic rapamycin, largely used for immunosuppression and cancer therapy. Later on, in mammals, the material target of rapamycin was pointed out as the mammalian target of rapamycin (mTOR) gene. The mTOR is a major metabolic switch that controls cell resources assignment so that growth factors and amino acid availability activate it to promote cell anabolism, while conditions of nutrient deprivation or rapamycin treatment repress it to favor cellular catabolism, through derepression of autophagic programs and drastic reduction of protein synthesis to free up cytosolic resources. Insulin, amino acids, and hormones trigger the product of mTOR that is a serine/threonine protein kinase involved in the regulation of relevant cell processes such as growth, proliferation, motility, survival, protein and lipid synthesis, autophagy, inflammation, mitochondrial function, and glucose metabolism.Citation129,Citation130 TOR kinase functions through the formation of two distinct complexes: TORC1 and TORC2, with only TORC1 being rapamycin sensitive in yeast and mammals, although prolonged rapamycin treatment can also inhibit TORC2.Citation131 Thus TORC1 activation after glucose input or insulin/IGF signaling or abundant amino acids availability has an opposite effect compared to that of sirtuins that are supposed to reduce TORC1 signalingCitation130 as stress signals or energy deficiencies do.Citation132 The activation of TORC1 induces protein translation and cell growth, whereas its inhibition stops growth and elicits stress responses as autophagy.Citation129 Reduced mTOR signaling has been demonstrated to extend lifespan in different organisms.Citation133 The mTOR inhibition has been largely identified as a longevity assurance mechanism,Citation133 and the availability of rapamycin and other mTOR inhibitors makes this pathway a valuable target for interventions to extend healthspan.Citation134 An increase in the AMP/ATP ratio, such as in conditions when cells are scarce of glucose, activates the very sensitive energy sensor AMPK, leading to reduced ATP utilization and raised energy production, whereas its activity is reduced when the cell energy load is high, such as in the conditions of low AMP/ATP ratio.Citation135 CR activates the AMPK pathway in heart, liver, and skeletal muscle of rodents,Citation136 and the AMPK impact on lifespan, described in several organisms from yeast to mammals, can be obtained through the activation of FoxO factors, the main constituents of the IIS pathwayCitation137 (). A longer lifespan is linked to an increased AMPK activity, while a reduced longevity is linked with its inhibition.Citation138,Citation139 However, in mammals, AMPK activity is not induced by CR, thereby raising doubts about its importance in these organisms.Citation140
Figure 2 Molecular markers of aging involved in the pleiotropic effects of calorie restriction. Several markers characterizing aged cells are indicated by the affected molecules or functions. In the nucleus, aging implies the following: telomeres erosion, genomic instability, and epigenetic alterations (indicated by methylation [m] of histones H [Hm] and DNA [DNAm] or acetylation [ac] of histones [Hac]) with involvement of sirtuins and other modifying enzymes. In the mitochondria, age-related mitochondrial dysfunction leads to reduced ATP production and increased ROS presence. In the cytoplasm, age-dependent proteostasis imbalance causes an abnormal protein turnover with functional consequences. In the cytoplasm, aging also affects other pathways (eg, mTOR, IIS, AMPK, sirtuins, FoxOs) with dual effects namely on metabolism as well as on chromatin remodeling and regulation of gene expression, causing impaired nutrient/energy sensing that leads to different alterations, also due to reciprocal interrelationships.
![Figure 2 Molecular markers of aging involved in the pleiotropic effects of calorie restriction. Several markers characterizing aged cells are indicated by the affected molecules or functions. In the nucleus, aging implies the following: telomeres erosion, genomic instability, and epigenetic alterations (indicated by methylation [m] of histones H [Hm] and DNA [DNAm] or acetylation [ac] of histones [Hac]) with involvement of sirtuins and other modifying enzymes. In the mitochondria, age-related mitochondrial dysfunction leads to reduced ATP production and increased ROS presence. In the cytoplasm, age-dependent proteostasis imbalance causes an abnormal protein turnover with functional consequences. In the cytoplasm, aging also affects other pathways (eg, mTOR, IIS, AMPK, sirtuins, FoxOs) with dual effects namely on metabolism as well as on chromatin remodeling and regulation of gene expression, causing impaired nutrient/energy sensing that leads to different alterations, also due to reciprocal interrelationships.](/cms/asset/4ad989b3-24cf-4c53-ae3e-fe2674cc459f/dcia_a_126458_f0002_c.jpg)
Inflammation and CR
Aging is also characterized by a condition of relevant inflammation, demonstrated by the age-associated rise of several pro-inflammatory factors including TNF-α, interferon-γ, IL-1β, and IL-18.Citation141–Citation143 Furthermore, induction of such chronic inflammation during aging can be favored by age-related elevation of oxidative stress that also appears to raise the incidence of age-related diseases.Citation144,Citation145 Another feature of aging with pathological consequences is the increased production of danger-associated molecular patterns that, originated by damage accumulated with age, are recognized by immune receptors and induce the activation of the inflammasome,Citation146,Citation147 leading to chronic inflammation often accompanying different age-associated diseases.Citation148 Such chronic inflammation associated with aging has been identified by the new idea of “inflammaging”.Citation149 The CR-positive effects on inflammation have been demonstrated by the reduction of inflammation and insulin resistance obtained in a rat model of age-associated inflammationCitation150 as well as by the regulation of GSH redox status and expression of NF-κB, SIRT1, peroxisome proliferator-activated receptors, and FoxOs.Citation151
CR mimetics
A workshop entitled “Interventions to Slow Aging in Humans: Are We Ready?” was held in Erice, Italy, on October 2013, in order to define the more comprehensive set of pathways involved in aging and of safe counteracting strategies to slow the whole process and extend healthspan. Multiple interventions and final indications were very thoroughly exposed in the review,Citation130 to which it is referred for deeper analyses and that was a major source for this section. In fact, a relevant concern about the feasibility of CR for most people, especially in the developed countries, deals with the severity of such regimen that has prompted the search for less drastic diets and safe drugs that may obtain the CR benefits with a more practical treatment. In recent years, growing attention has been dedicated to compounds, of different chemical natures, able to induce some of the effects evoked by CR in various organisms and thus grouped as CR mimetics. Such molecules share the ability to activate one or more pathways normally induced by CR.Citation6 Studies in animals demonstrated that a decrease in IGF-1 levels or IGF-1 action can extend lifespan. The obese, long-living mice without the growth hormone receptor (GHR−/−) have reduced levels of IGF-1 and decreased risk for cancer and diabetes while are sensitive to insulin.Citation152,Citation153 In patients suffering from the GHR-deficient Laron syndrome, similar results were obtained. In order to pharmacologically decrease IGF-1 action, designed drugs can target cells/tissues that produce or respond to GH and/or IGF-1. Among such drugs, somatostatin analogues are not only able to decrease serum GH levels and IGF-1 levels, but they also abolish secretion of other endocrine hormones, including insulin, which makes their use inappropriate to prolong healthspan.Citation130 Another approved drug for treating acromegaly is the GHR antagonist pegvisomantCitation154,Citation155 that inhibits GH action by binding to and blocking the GHR.Citation156 Thus, pegvisomant might be assayed for longevity and healthy aging effects. Sirtuins are involved in pathways implying health benefits and are a relevant drug target. Efficient sirtuin-activating compounds (STACs) are plant-derived metabolites, such as flavones, stilbenes, chalcones, and anthocyanidins, that activate SIRT1 in vitro.Citation157 Resveratrol (3,5,40-trihydroxystilbene) is still the strongest natural STACCitation158 and the best CR mimeticCitation13 since it decreases insulin secretion and insulin level while enhancing sensitivity to insulin, reduces fat mass, promotes mitochondrial biogenesis and oxidative phosphorylation, raises the NAD+/NADH ratio thus inducing sirtuins, elevates AMPK activity, and supports mitophagy and autophagy.Citation13 It has been recently reported that obese humans treated with resveratrol present effects similar to those induced by CR.Citation159 In mice kept on a high fat diet, resveratrol increases lifespanCitation160 as the synthetic activators SRT1720 and SRT2104 also prolong the lifespan of mice fed either a high-calorie or a low-calorie diet and both protect from tissue age-related changes.Citation161 In preclinical trials, STACs have also shown to be very promising to cope with age-associated diseases and complications.Citation158 To activate sirtuins in an alternate way, their need for NAD+ can be satisfied by providing NAD+ precursors or by inducing NAD+ biosynthetic enzymesCitation162 or by inhibiting NAD+ hydrolase CD38.Citation163 However, due to its multiple connections with energy/nutrient sensing pathways, mTOR signaling is a major candidate for targeted intervention to prolong healthspan. Many lifespan-extending interventions in model organisms, such as protein and CR/DR, decreased insulin/IGF signaling, activation of AMP kinase and possibly of sirtuins, reduce mTORC1 signaling,Citation133,Citation164 thereby raising the question of whether their positive effects depend on mTORC1 inactivation. Also rapamycin (sirolimus), probably the best characterized inhibitor of mTOR, has gained consideration because it increases lifespan and improves healthspan in several organisms.Citation165 It has been demonstrated that stress, nutrient, and growth factors elicit responses in the conserved family of kinases of TOR proteins. The mimetic action of rapamycin is justified by the extended longevity attained by CR through downregulation of TOR in yeast and other organisms, as well as in mice.Citation166–Citation168 Furthermore, inhibition of TOR increases FoxO-dependent autophagy that is crucial to extend lifespan in yeastCitation169 and in C. elegans.Citation170 However, rapamycin has serious side effects (eg, hyperglycemia, hyperinsulinemia, insulin resistance, and proliferative defects in hematopoietic lineages),Citation171 which limit its use as an anti-aging drug.
In 1998, 2-deoxy-d-glucose (2DG), an inhibitor of glycolysis, was indicated by Lane et alCitation172 as another CR mimetic since it increased C. elegans lifespanCitation173 and produced in rats various CR-like consequences such as reduced insulin levelCitation174 and increased glucocorticoids concentrations.Citation175 But long-term administration of 2DG can induce cardiotoxicity and increased tumorigenesis in adrenal medulla.Citation176 Another target of CR mimetics is AMPK activation that induces insulin sensitivity resulting in increased glucose uptake in skeletal muscles, reduced hepatic glucose production, and generalized raised fatty acid oxidation.Citation177 Activators of AMPK as biguanides and thiazolidinediones have been developed. Metformin, a biguanine drug used for type-2 diabetes therapy, likely through AMPK activation, increases the lifespan of C. elegans and D. melanogaster.Citation178 CR-like effects such as enhanced insulin sensitivity, reduced plasma levels of LDL and cholesterol, increased antioxidant protection, and reduced chronic inflammation are induced by low doses of metformin in mice.Citation6 The consideration of metformin has grown also because of its association with the induction of SIRT1-mediated autophagy independently of AMPK.Citation179 However, consideration of metformin for anti-aging clinical trials requires a deeper understanding of its mechanisms of action.Citation180 This presentation of the pharmacological CR mimetics is not absolutely exhaustive, as approaches directed to mitigate telomere erosion, counteract epigenetic alterations, reduce stem cells depletion, and ward off cellular senescence have also been discussed in this paper reporting about the aforementioned meeting,Citation130 but they exceed the limits of this review. Furthermore, recently, accumulating data have suggested that severe CR is not necessary, but different dietary interventions such as intermittent fasting, prolonged fasting, restricted feeding, and protein or selective amino acid restriction may recapture some of the positive results of severe CR in modulating certain anti-aging pathways.Citation60 In fact, revitalization of mice endocrine, immune, and nervous systems and improvements in biomarkers of human diseases (diabetes, CVD, and cancer) have been obtained with intermittent CR, as a result of subjecting mice and humans to fasting for few days per week, without major negative consequences.Citation181 Although all the effects involved by intermittent or prolonged fasting are not yet known, preliminary clinical trials in humans support these approaches as safe and efficacious to be tested in anti-aging studies.Citation182 However, the fasting interventions have to be carefully applied because they can be dangerous for old people or patients receiving insulin or insulin-like drugs. Therefore, the alternate group of dietary interventions, namely the restriction of specific nutrients, has recently gained growing consideration. In fact, studies in humans, analyzing the composition of the diet, have reported very interesting findings. The evaluation performed by Pedersen et alCitation183 led to the indication that an increase in all-cause mortality, type-2 diabetes mellitus, and CVD is possibly associated with low carbohydrate, high protein diets. The worst consequences were linked with animal-based proteins and other animal product–based diets, while vegetable-based proteins and other vegetable product–based diets were able to reverse the tendency. A later NHANES study published by Levine et alCitation184 has showed, in agreement with previous data, that the problems implicated by a higher protein diet in people under 65 years of age are eliminated by the consumption of vegetable- rather than animal-based protein. In participants aged 50–65 years with the highest protein intake (more than 20% of calories from protein), all-cause mortality increased by 75% and cancer and diabetes mortality increased four times. However, in participants over 65 years, a reverse association occurred as subjects consuming the highest protein intake had a 28% reduction in mortality. Therefore, the source of protein may be as crucial as the amount of protein in the diet. However, it seems that the ratio of dietary protein to other macronutrient calorie sources (eg, carbohydrates and fats), likely affecting the regulation of mTORC1 signaling,Citation24 is mainly responsible for the longevity benefits of PR.Citation130 Other studies have focused on the DR of specific essential amino acids as that in which the reduction of branched chain amino acids to the levels found in a low protein diet caused an improvement of mouse metabolic health similar to that obtained with a low protein diet.Citation185 Restriction of another essential amino acid, methionine, extends the longevity of flies, rats, and miceCitation186–Citation188 and reverses the metabolic dysfunction in aged mice.Citation186 The mechanisms involved in the sensing of amino acid levels are not fully understood, but they certainly include the activation of GCN2 (general control non-derepressible 2) and mTOR. In mammals, while mTOR is induced by abundance of amino acids, particularly leucine, GCN2 responds to the absence of several distinct amino acids. In conclusion, although much research remains to be done, it appears that restriction of dietary protein contributes to the metabolic benefits of a CR diet and that alterations in protein quality may mimic some of the CR beneficial effects.Citation189 This type of intervention, which does not reduce calorie intake, may extend the benefits of CR to a much broader population. However, CR or PR in the elderly has to be applied cautiously, as the preservation of muscle and bone mass is a priority in the aged to delay or prevent sarcopenia that is a major driver of frailty and loss of independence.Citation190,Citation191 Indeed, an increasing amount of data suggests that in the elderly protein intake may need to be increased to preserve muscle mass.Citation192–Citation196 Effectively, high protein diets, particularly in association with exercise, can improve muscle mass, if not function, in old age, by promoting anabolic responses in muscles.Citation197,Citation198 Therefore, recent findings suggest that there are valid alternates to conventional CR and that diets including altered protein contents demonstrate proteins’ activity in the regulation of longevity through the positive impact on metabolic health.Citation199
Future applications
Overall, the results from multiple studies show that both reduced calories intake (CR) and the ratio between macronutrients, namely the protein to carbohydrates ratio, positively impact lifespan. In particular, it seems that dietary restriction of proteins or other individual nutrients (DR), with respects to carbohydrates, produces an effect on longevity independent on that of CR. DR appears to be efficacious at a much lower degree and is induced in a more severe range of restriction (50%–85%: relative to a reference intake of 18%–26% protein in the diet) than that affected by CR (10%–65% relative to ad libitum intake).Citation27 Available data from the CRON practitioners confirm the positive effects of a reduced calorie intake on various parameters usually affected by age-related decline. However, it needs to be stressed that the CRON regimen is very rich in essential nutrients, vegetable fiber, low glycemic foods, and phytochemicals, which may modulate metabolic health. Furthermore, a dietetic indication, shared by all recent studies,Citation27,Citation28,Citation30 suggests the opportunity of a reduced protein intake (between 10 and 30% of total calories) to lower the risk of mortality. However, Nakagawa et alCitation30 also showed that very low level of protein was associated with reduced viability. It has been demonstrated that CR and DR have a large impact on healthspan and longevity through the involvement of multiple mechanisms affecting several metabolic pathways in tissues and organs, which have only begun to be dissected in details. It needs also to be considered that although DR can be a more feasible dietary regimen than severe CR, the pharmacological search for CR mimetics has developed a number of drugs that are already or next to be tested in clinical studies on humans and that will open a new, more walkable avenue toward increased healthspan. But in a nutshell, a non-severe reduction of the calorie with particular attention to a reduced protein intake versus a high carbohydrate one in not-aged subjects and a high protein diet in old persons might help people to better cope with a large number of age-related dysfunctions and diseases.
Acknowledgments
This research was supported by grants to AMSL (University of Bari-Progetti di Ateneo, 2012) and grant to AMSL (Istituto Banco di Napoli-Fondazione, 2015). We thank Flavio Fracasso, MS, for assisting with the figures.
Disclosure
The authors report no conflicts of interest in this work.
References
- PassarinoGDe RangoFMontesantoAHuman longevity: genetics or lifestyle? It takes two to tangoImmun Ageing201613121727053941
- CarmonaJJMichanSBiology of healthy aging and longevityRev Invest Clin201668171627028172
- KenyonCJThe genetics of ageingNature201046450451220336132
- Lopez-OtinCBlascoMAPartridgeLSerranoMKroemerGThe hallmarks of agingCell20131531194121723746838
- KennedyBKBergerSLBrunetAGeroscience: linking aging to chronic diseaseCell201415970971325417146
- Lopez-LluchGNavasPCalorie restriction as an intervention in agingJ Physiol201659482043206026607973
- FontanaLModulating human aging and age-associated diseasesBiochim Biophys Acta20091790101133113819364477
- MostJTostiVRedmanLMFontanaLCalorie restriction in humans: an updateAgeing Res Rev201739364527544442
- SinclairDAToward a unified theory of caloric restriction and longevity regulationMech Ageing Dev2005126987100215893363
- MattsonMPDietary factors, hormesis and healthAgeing Res Rev20087434817913594
- RattanSIHormesis in agingAgeing Res Rev20087637817964227
- MartinsIGalluzziLKroemerGHormesis, cell death and agingAging (Albany NY)2011382182821931183
- TestaGBiasiFPoliGChiarpottoECalorie restriction and dietary restriction mimetics: a strategy for improving healthy aging and longevityCurr Pharm Des2014202950297724079773
- SlonakerJRThe effect of different per cents of protein in the diet VII. Life span and cause of deathAm J Physiol19312266275
- McCayCMCrowellMFMaynardLAThe effect of retarded growth upon the length of life and upon the ultimate body sizeJ Nutr1935106379
- MasoroESubfield history: caloric restriction, slowing aging, and extending lifeSci Aging Knowledge Environ200320038RE212844547
- MairWPiperMDPartridgeLCalories do not explain extension of life span by dietary restriction in DrosophilaPLoS Biol20053e22316000018
- PiperMDPartridgeLDietary restriction in Drosophila: delayed aging or experimental artefact?PLoS Genet2007273e57
- RaubenheimerDSimpsonSJMayntzDNutrition, ecology and nutritional ecology: toward an integrated frameworkFunct Ecol200923416
- PiperMDPartridgeLRaubenheimerDSimpsonSJDietary restriction and aging: a unifying perspectiveCell Metab20111415416021803286
- SimpsonSJRaubenheimerDA multilevel analysis of feeding-behavior – the geometry of nutritional decisionsPhilos Trans R Soc Lond Ser B Biol Sci1993342381402
- SimpsonSJRaubenheimerDCaloric restriction and aging revisited: the need for a geometric analysis of the nutritional bases of agingJ Gerontol A Biol Sci Med Sci20076270771317634316
- RaubenheimerDSimpsonSJTaitAHMatch and mismatch: conservation, physiology, nutritional ecology and the timescales of biological adaptationPhilos Trans R Soc Lond Ser B Biol Sci20123671628164622566672
- Solon-BietSMMcMahonACBallardJWThe ratio of macro-nutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed miceCell Metab20141941843024606899
- LusseauDMitchellSEBarrosCThe effects of graded levels of calorie restriction: IV. Non-linear change in behavioural phenotype of mice in response to short-term calorie restrictionSci Rep201551319826306002
- TaorminaGMirisolaMGCalorie restriction in mammals and simple model organismsBiomed Res Int2014201430869024883306
- SpeakmanJRMitchellSEMazidiMCalories or protein? The effect of dietary restriction on lifespan in rodents is explained by calories aloneExp Gerontol201686283827006163
- SimpsonSJLe CouteurDGRaubenheimerDDietary protein, aging and nutritional geometryAgeing Res Rev201739788628274839
- Le CouteurDGSolon-BietSCoggerVCThe impact of low protein, high carbohydrate diets on aging and lifespanCell Mol Life Sci2016731237125226718486
- NakagawaSLagiszMHectorKLSpencerHGComparative and meta-analytic insights into life-extension via dietary restrictionAging Cell20121140140922268691
- KapahiPKaeberleinMHansenMDietary restriction and lifespan: lessons from invertebrate modelsAgeing Res Rev20173931428007498
- LushchakOStrilbytskaOPiskovatskaVStoreyKBKoliadaAVaisermanAThe role of the TOR pathway in mediating the link between nutrition and longevityMech Ageing Dev201716412713828315697
- ZainalTAOberleyTDAllisonDBSzwedaLIWeindruchRCaloric restriction of rhesus monkeys lowers oxidative damage in skeletal muscleFASEB J200014121825183610973932
- BlackAAllisonDBShapsesSACalorie restriction and skeletal mass in rhesus monkeys (Macaca mulatta): evidence for an effect mediated through changes in body sizeJ Gerontol A Biol Sci Med Sci2001563B98B10711253152
- MattisonJARothGSBeasleyTMImpact of caloric restriction on health and survival in rhesus monkeys from the NIA studyNature201248931832122932268
- ColmanRJBeasleyTMKemnitzJWJohnsonSCWeindruchRAndersonRMCaloric restriction reduces age-related and all-cause mortality in rhesus monkeysNat Commun20145355724691430
- Velthuis-te WierikEJWesterterpKRvan den BergHImpact of a moderately energy-restricted diet on energy metabolism and body composition in non-obese menInt J Obes Relat Metab Disord1995193183247647823
- Velthuis-te WierikEJvan den BergHSchaafsmaGHendriksHFBrouwerAEnergy restriction, a useful intervention to retard human ageing? Results of a feasibility studyEur J Clin Nutr1994481381488194495
- Velthuis-te WierikEJvan LeeuwenREHendriksHFShort-term moderate energy restriction does not affect indicators of oxidative stress and genotoxicity in humansJ Nutr1995125263126397562100
- DasSKGilhoolyCHGoldenJKLong-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trialAm J Clin Nutr2007851023103017413101
- HeilbronnLKde JongeLFrisardMIEffect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trialJAMA20062951539154816595757
- RacetteSBWeissEPVillarealDTOne year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissueJ Gerontol Ser A Biol Sci Med Sci20066194395016960025
- Larson-MeyerDEHeilbronnLKRedmanLMEffect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjectsDiabetes Care2006291337134416732018
- LefevreMRedmanLMHeilbronnLKCaloric restriction alone and with exercise improves CVD risk in healthy non-obese individualsAtherosclerosis200920320621318602635
- CivitareseAECarlingSHeilbronnLKCalorie restriction increases muscle mitochondrial biogenesis in healthy humansPLoS Med20074e7617341128
- RochonJBalesCWRavussinEDesign and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energyJ Gerontol Ser A Biol Sci Med Sci2011669710820923909
- FontanaLVillarealDTWeissEPCalorie restriction or exercise: effects on coronary heart disease risk factors. A randomized: controlled trialAm J Physiol Endocrinol Metab2007293E197E20217389710
- WeissEPRacetteSBVillarealDTImprovements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trialAm J Clin Nutr2006841033104217093155
- RavussinERedmanLMRochonJA 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevityJ Gerontol Ser A Biol Sci Med Sci2015701097110426187233
- FontanaLMeyerTEKleinSHolloszyJOLong-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humansProc Natl Acad Sci U S A20041016659666315096581
- MeyerTEKovacsSJEhsaniAAKleinSHolloszyJOFontanaLLong-term caloric restriction ameliorates the decline in diastolic function in humansJ Am Coll Cardiol20064739840216412867
- FontanaLKleinSHolloszyJOEffects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine productionAge2010329710819904628
- SteinPKSoareAMeyerTECangemiRHolloszyJOFontanaLCaloric restriction may reverse age-related autonomic decline in humansAging Cell20121164465022510429
- MartinCKAntonSDHanHExamination of cognitive function during six months of calorie restriction: results of a randomized controlled trialRejuvenation Res20071017919017518698
- VillarealDTFontanaLDasSKEffect of two-year caloric restriction on bone metabolism and bone mineral density in non-obese younger adults: a randomized clinical trialJ Bone Miner Res201631405126332798
- KaluDNHardinRRCockerhamRYuBPNorlingBKEganJWLifelong food restriction prevents senile osteopenia and hyperparathyroidism in F344 ratsMech Ageing Dev1984261031126748753
- LuTPanYKaoS-YGene regulation and DNA damage in the ageing human brainNature200442988389115190254
- VeraEBernardes de JesusBForondaMFloresJMBlascoMATelomerase reverse transcriptase synergizes with calorie restriction to increase health span and extend mouse longevityPLoS One20138e5376023349740
- BenayounBAPollinaEABrunetAEpigenetic regulation of ageing: linking environmental inputs to genomic stabilityNat Rev Mol Cell Biol20151659361026373265
- FontanaLPartridgeLPromoting health and longevity through diet: from model organisms to humansCell201516110611825815989
- TaylorRCDillinAAging as an event of proteostasis collapseCold Spring Harb Perspect Biol20113a00444
- DokladnyKZuhlMNMandellMRegulatory coordination between two major intracellular homeostatic systems: heat shock response and autophagyJ Biol Chem2013288149591497223576438
- PesceVCormioAFracassoFAge-related mitochondrial genotypic and phenotypic alterations in human skeletal muscleFree Radic Biol Med200130111223123311368920
- LezzaAMFallacaraFPPesceVLeeuwenburghCCantatorePGadaletaMNLocalization of abasic sites and single-strand breaks in mitochondrial DNA from brain of aged rat, treated or not with caloric restriction dietNeurochem Res200833122609261418946734
- ChistiakovDASobeninIARevinVVOrekhovANBobryshevYVMitochondrial aging and age-related dysfunction of mitochondriaBiomed Res Int2014201423846324818134
- PiccaAPesceVFracassoFJosephAMLeeuwenburghCLezzaAMAging and calorie restriction oppositely affect mitochondrial biogenesis through TFAM binding at both origins of mitochondrial DNA replication in rat liverPLoS One201389e7464424058615
- PiccaAFracassoFPesceVAge- and calorie restriction-related changes in rat brain mitochondrial DNA and TFAM bindingAge (Dordr)20133551607162022945739
- PiccaALezzaAMRegulation of mitochondrial biogenesis through TFAM-mitochondrial DNA interactions: useful insights from aging and calorie restriction studiesMitochondrion20152511677526437364
- RugarliETrifunovicAIs mitochondrial free radical theory of aging getting old?Biochim Biophys Acta20151847111345134626282072
- PiccaAPesceVSiragoGFracassoFLeeuwenburghCLezzaAM“What makes some rats live so long?” The mitochondrial contribution to longevity through balance of mitochondrial dynamics and mtDNA contentExp Gerontol20168512334027620821
- RistowMSchmeisserSExtending life span by increasing oxidative stressFree Radic Biol Med20115132733621619928
- BarjaGThe mitochondrial free radical theory of agingProg Mol Biol Transl Sci201412712725149212
- YuBPMembrane alteration as a basis of aging and the protective effects of calorie restrictionMech Ageing Dev20051261003101015893361
- Lopez-DominguezJARamseyJJTranDThe influence of dietary fat source on life span in calorie restricted miceJ Gerontol A Biol Sci Med Sci2015701181118825313149
- GenovaMLLenazGThe interplay between respiratory supercomplexes and ROS in agingAntioxid Redox Signal20152320823825711676
- KwonJHanEBuiCBAssurance of mitochondrial integrity and mammalian longevity by the p62-Keap1-Nrf2-Nqo1 cascadeEMBO Rep20121315015622222206
- HyunDHEmersonSSJoDGMattsonMPde CaboRCalorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during agingProc Natl Acad Sci U S A2006103199081991217167053
- Rodriguez-BiesENavasPLopez-LluchGAge-dependent effect of every-other-day feeding and aerobic exercise in ubiquinone levels and related antioxidant activities in mice muscleJ Gerontol A Biol Sci Med Sci201570334324496576
- ShortKRBigelowMLKahlJDecline in skeletal muscle mitochondrial function with aging in humansProc Natl Acad Sci U S A20051025618562315800038
- FeigeJNLagougeMAuwerxJDietary manipulation of mouse metabolismCurr Protoc Mol Biol200866 Chapter 29:Unit 29B.5
- BaurJAChenDChiniENDietary restriction: standing up for sirtuinsScience20103291012101320798296
- PiccaAPesceVFracassoFJosephA-MLeeuwenburghCLezzaAMSA comparison among the tissue-specific effects of aging and calorie restriction on TFAM amount and TFAM-binding activity to mtDNA in ratBiochim Biophys Acta201418402184219124631828
- NisoliETonelloCCardileACalorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOSScience200531031431716224023
- MattagajasinghIKimCSNaqviASIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthaseProc Natl Acad Sci U S A2007104148551486017785417
- HandschinCSpiegelmanBMPeroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolismEndocr Rev20062772873517018837
- RuetenikABarrientosADietary restriction, mitochondrial function and aging: from yeast to humansBiochim Biophys Acta201518471434144725979234
- Martin-MontalvoAde CaboRMitochondrial metabolic reprogramming induced by calorie restrictionAntioxid Redox Signal20131931032022901095
- NemotoSFergussonMMFinkelTSIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1αJ Biol Chem2005280164561646015716268
- JagerSHandschinCSt-PierreJSpiegelmanBMAMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alphaProc Natl Acad Sci U S A2007104120171202217609368
- Lopez-LluchGIrustaPMNavasPde CaboRMitochondrial biogenesis and healthy agingExp Gerontol20084381381918662766
- WeberTAReichertASImpaired quality control of mitochondria: aging from a new perspectiveExp Gerontol20104550351120451598
- ProllaTAMattsonMPMolecular mechanisms of brain aging and neurodegenerative disorders: lessons from dietary restrictionTrends Neurosci20012411 SupplS21S3111881742
- GhoshSWandersDStoneKPVanNTCortezCCGettysTWA systems biology analysis of the unique and overlapping transcriptional responses to caloric restriction and dietary methionine restriction in ratsFASEB J20142862577259024571921
- FieldAEAdamsPDTargeting chromatin aging – the epigenetic impact of longevity-associated interventionsExp Gerontol2017948293327986499
- GutPVerdinEThe nexus of chromatin regulation and intermediary metabolismNature201350248949824153302
- RakyanVKDownTAMaslauSHuman aging-associated DNA hypermethylation occurs preferentially at bivalent chromatin domainsGenome Res20102043443920219945
- KimCHLeeEKChoiYJShort-term calorie restriction ameliorates genome wide, age-related alterations in DNA methylationAging Cell20161510741081
- LiYTollefsbolTOP16INK4A suppression by glucose restriction contributes to human cellular lifespan extension through SIRT1-mediated epigenetic and genetic mechanismsPLoS One201162e1742121390332
- ZentnerGEHenikoffSRegulation of nucleosome dynamics by histone modificationsNat Struct Mol Biol20132025926623463310
- PelegSFellerCLadurnerAGImhofAThe metabolic impact on histone acetylation and transcription in ageingTrends Biochem Sci20164170071127283514
- KaeberleinMMcVeyMGuarenteLThe SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanismsGenes Dev1999132570258010521401
- SatohABraceCSRensingNSirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LHCell Metab20131841643024011076
- VazquezBNThackrayJKSerranoLSirtuins and DNA damage repair: SIRT7 comes to playNucleus2017810711528406750
- ImaiSGuarenteLTen years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseasesTrends Pharmacol Sci20103121222020226541
- YangHYangTBaurJANutrient-sensitive mitochondrial NAD+ levels dictate cell survivalCell20071301095110717889652
- VerdinENAD+ in aging, metabolism, and neurodegenerationScience20153501208121326785480
- GuarenteLCalorie restriction and sirtuins revisitedGenes Dev2013272072208524115767
- Fernandez-MarcosPJAuwerxJRegulation of PGC-1α, a nodal regulator of mitochondrial biogenesisAm J Clin Nutr201193884S890S21289221
- KaeberleinMKirklandKTFieldsSKennedyBKSir2-independent life span extension by calorie restriction in yeastPLoS Biol200429E29615328540
- FabrizioPGattazzoCBattistellaLSir2 blocks extreme lifespan extensionCell200512365566716286010
- LiYXuWMcBurneyMWLongoVDSirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neuronsCell Metab20088384818590691
- LongoVDLinking sirtuins, IGF-I signaling, and starvationExp Gerontol200944707418638538
- HerranzDMunoz-MartinMCanameroMSirt1 improves healthy ageing and protects from metabolic syndrome-associated cancerNat Commun20101320975665
- BurnettCValentiniSCabreiroFAbsence of effects of Sir2 overexpression on lifespan in C. elegans and DrosophilaNature201147748248521938067
- MouchiroudLHoutkooperRHMoullanNThe NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signalingCell201315443044123870130
- ImaiSIGuarenteLIt takes two to tango: NAD+ and sirtuins in aging/longevity controlNPJ Aging Mech Dis201621601728721271
- FangEFScheibye-KnudsenMChuaKFMattsonMPCroteauDLBohrVANuclear DNA damage signalling to mitochondria in ageingNat Rev Mol Cell Biol20161730832126956196
- Sampaio-MarquesBFelgueirasCSilvaASNCA (α-synuclein)-induced toxicity in yeast cells is dependent on sirtuin 2 (Sir2)-mediated mitophagyAutophagy201281494150922914317
- HuangRXuYWanWDeacetylation of nuclear LC3 drives autophagy initiation under starvationMol Cell20155745646625601754
- PriceNLGomesAPLingAJSIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial functionCell Metab20121567569022560220
- GibsonBAKrausWLNew insights into the molecular and cellular functions of poly(ADP-ribose) and PARPsNat Rev Mol Cell Biol20121341142422713970
- FangEFScheibye-KnudsenMBraceLEDefective mitophagy in XPA via PARP-1 hyperactivation and NAD+/SIRT1 reductionCell2014157488289624813611
- Scheibye-KnudsenMMitchellSJFangEFA high fat diet and NAD+ rescue premature aging in Cockayne syndromeCell Metab201420584085525440059
- TatarMBartkeAAntebiAThe endocrine regulation of aging by insulin-like signalsScience20032991346135112610294
- SelmanCLingardSChoudhuryAIEvidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null miceFASEB J20082280781817928362
- KenyonCThe first long-lived mutants: discovery of the insulin/IGF-1 pathway for ageingPhilos Trans R Soc Lond B Biol Sci201136691621115525
- GotoTTakanoMTranscriptional role of FOXO1 in drug resistance through antioxidant defense systemsAdv Exp Med Biol200966517117920429424
- ArgentinoDPDominiciFPAl-RegaieyKBonkowskiMSBartkeATurynDEffects of long-term caloric restriction on early steps of the insulin-signaling system in mouse skeletal muscleJ Gerontol A Biol Sci Med Sci200560283415741279
- LaplanteMSabatiniDMmTOR signaling in growth control and diseaseCell201214927429322500797
- LongoVDAntebiABartkeAInterventions to slow aging in humans: are we ready?Aging Cell201514449751025902704
- SarbassovDDAliSMSenguptaSProlonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKBMol Cell20062215916816603397
- KimYMKimDHdRAGging amino acid-mTORC1 signaling by SH3BP4Mol Cell20133516
- JohnsonSCRabinovitchPSKaeberleinMmTOR is a key modulator of ageing and age-related diseaseNature201349333834523325216
- HeissCSpyridopoulosIHaendelerJInterventions to slow cardiovascular aging: dietary restriction, drugs and novel moleculesExp Gerontol Epub2017627
- HardieDGSensing of energy and nutrients by AMP-activated protein kinaseAm J Clin Nutr201193891S896S21325438
- CantòCAuwerxJCalorie Restriction: is AMPK a key sensor and effector?Physiology20112621422421841070
- GreerELDowlatshahiDBankoMRAn AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegansCurr Biol2007171646165617900900
- ApfeldJO’ConnorGMcDonaghTDiStefanoPSCurtisRThe AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegansGenes Dev2004183004300915574588
- TohyamaDYamaguchiAA critical role of SNF1A/dAMPKalpha (Drosophila AMP-activated protein kinase alpha) in muscle on longevity and stress resistance in Drosophila melanogasterBiochem Biophys Res Commun201039411211820184862
- GonzalezAAKumarRMulliganJDDavisAJWeindruchRSaupeKWMetabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activityAm J Physiol Endocrinol Metab2004287E1032E103715251868
- ChungHYKimHJKimKWChoiJSYuBPMolecular inflammation hypothesis of aging based on the anti-aging mechanism of calorie restrictionMicrosc Res Tech20025926427212424787
- SalvioliSCapriMValensinSTieriPMontiDOttavianiEFranceschiCInflamm-aging, cytokines and aging: state of the art, new hypotheses on the role of mitochondria and new perspectives from systems biologyCurr Pharm Des2006123161317116918441
- GotoMInflammaging (inflammation + aging): a driving force for human aging based on an evolutionarily antagonistic pleiotropy theory?Biosci Trends2008221823020103932
- SolanaRPawelecGTarazonaRAging and innate immunityImmunity20062449149416713963
- CandoreGCarusoCJirilloEMagroneTVastoSLow grade inflammation as a common pathogenetic denominator in age-related diseases: novel drug targets for anti-ageing strategies and successful ageing achievementCurr Pharm Des20101658459620388068
- FeldmanNRotter-MaskowitzAOkunEDAMPs as mediators of sterile inflammation in aging-related pathologiesAgeing Res Rev201524293925641058
- PiccaALezzaAMSLeeuwenburghCFueling inflamm-aging through mitochondrial dysfunction: mechanisms and molecular targetsInt J Mol Sci2017185 pii: E933
- PawelecGGoldeckDDerhovanessianEInflammation, ageing and chronic diseaseCurr Opin Immunol201429232824762450
- FranceschiCCampisiJChronic inflammation (inflammaging) and its potential contribution to age-associated diseasesJ Gerontol A Biol Sci Med Sci201469 Suppl1S4S924833586
- HorrilloDSierraJArribasCAge-associated development of inflammation in Wistar rats: effects of caloric restrictionArch Physiol Biochem201111714015021635187
- ChungHYLeeEKChoiYJMolecular inflammation as an underlying mechanism of the aging process and age-related diseasesJ Dent Res20119083084021447699
- ZhouYXuBCMaheshwariHGA mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse)Proc Natl Acad Sci U S A19979413215132209371826
- IkenoYHubbardGBLeeSReduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout miceJ Gerontol A Biol Sci Med Sci20096452252919228785
- TrainerPJDrakeWMKatznelsonLTreatment of acromegaly with the growth hormone-receptor antagonist pegvisomantN Engl J Med20003421171117710770982
- Van der LelyAJBillerBMBrueTLong-term safety of pegvisomant in patients with acromegaly: comprehensive review of 1288 subjects in ACROSTUDYJ Clin Endocrinol Metab2012971589159722362824
- KopchickJJParkinsonCStevensECTrainerPJGrowth hormone receptor antagonists: discovery, development, and use in patients with acromegalyEndocr Rev20022362364612372843
- HowitzKTBittermanKJCohenHYSmall molecule activators of sirtuins extend Saccharomyces cerevisiae lifespanNature200342519119612939617
- HubbardBPSinclairDASmall molecule SIRT1 activators for the treatment of aging and age-related diseasesTrends Pharmacol Sci20143514615424439680
- TimmersSKoningsEBiletLCalorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humansCell Metab20111461262222055504
- PearsonKJBaurJALewisKNResveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life spanCell Metab2008815716818599363
- MinorRKBaurJAGomesAPSRT1720 improves survival and healthspan of obese miceSci Rep201117022355589
- WangGHanTNijhawanDP7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvageCell20141581324133425215490
- CantoCHoutkooperRHPirinenEThe NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet induced obesityCell Metab20121583884722682224
- KennedyBKPennypackerJKDrugs that modulate aging: the promising yet difficult path aheadTransl Res201416345646524316383
- WilkinsonJEBurmeisterLBrooksSVRapamycin slows aging in miceAging Cell20121167568222587563
- SharpZDAging and TOR: interwoven in the fabric of lifeCell Mol Life Sci20116858759720960025
- HarrisonDEStrongRSharpZDRapamycin fed late in life extends lifespan in genetically heterogeneous miceNature200946039239519587680
- SelmanCTulletJMWieserDRibosomal protein S6 kinase 1 signaling regulates mammalian life spanScience200932614014419797661
- KamadaYSekitoTOhsumiYAutophagy in yeast: a TOR-mediated response to nutrient starvationCurr Top Microbiol Immunol2004279738414560952
- TothMLSigmondTBorsosELongevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegansAutophagy2008433033818219227
- SoefjeSAKarnadABrennerAJCommon toxicities of mammalian target of rapamycin inhibitorsTarget Oncol2011612512921499766
- LaneMAIngramDKRothGS2-Deoxy-D-glucose feeding in rats mimics physiologic effects of calorie restrictionJ Anti Aging Med19981327337
- SchulzTJZarseKVoigtAUrbanNBirringerMRistowMGlucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stressCell Metab2007628029317908557
- IngramDKRothGSGlycolytic inhibition as a strategy for developing calorie restriction mimeticsExp Gerontol20114614815421167272
- WanRCamandolaSMattsonMPDietary supplementation with 2-deoxy-D-glucose improves cardiovascular and neuroendocrine stress adaptation in ratsAm J Physiol Heart Circ Physiol2004287H1186H119315317676
- MinorRKSmithDLJrSossongAMChronic ingestion of 2-deoxy-D-glucose induces cardiac vacuolization and increases mortality in ratsToxicol Appl Pharmacol201024333233920026095
- RudermanNBCarlingDPrentkiMCacicedoJMAMPK, insulin resistance, and the metabolic syndromeJ Clin Investig20131232764277223863634
- SlackCFoleyAPartridgeLActivation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in DrosophilaPLoS One20127e4769923077661
- SongYMLeeYHKimJWMetformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathwayAutophagy201511465925484077
- MadirajuAKErionDMRahimiYMetformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenaseNature201451054254624847880
- BrandhorstSChoiIYWeiMA periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspanCell Metab2015221869926094889
- LongoVDMattsonMPFasting: molecular mechanisms and clinical applicationsCell Metab20141918119224440038
- PedersenANKondrupJBorsheimEHealth effects of protein intake in healthy adults: a systematic literature reviewFood Nutr Res201357121245
- LevineMESuarezJABrandhorstSLow protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older populationCell Metab20141940741724606898
- FontanaLCummingsNEArriola ApeloSIDecreased consumption of branched-chain amino acids improves metabolic healthCell Rep20161652053027346343
- LeesEKKrolEGrantLMethionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21Aging Cell20141381782724935677
- MillerRABuehnerGChangYHarperJMSiglerRSmith-WheelockMMethionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistanceAging Cell2005411912515924568
- OrentreichNMatiasJRDeFeliceAZimmermanJALow methionine ingestion by rats extends life spanJ Nutr19931232692748429371
- CummingsNELammingDWRegulation of metabolic health and aging by nutrient-sensitive signaling pathwaysMol Cell Endocrinol2017455132227884780
- JensenMDRyanDHApovianCM2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American college of cardiology/American heart association task force on practice guidelines and the obesity societyJ Am Coll Cardiol2014632985302324239920
- LandiFCalvaniRCesariMSarcopenia as the biological substrate of physical frailtyClin Geriatr Med20153136737426195096
- NowsonCO’ConnellSProtein requirements and recommendations for older people: a reviewNutrients201576874689926287239
- Paddon-JonesDRasmussenBBDietary protein recommendations and the prevention of sarcopeniaCurr Opin Clin Nutr Metab Care200912869019057193
- WolfeRRMillerSLMillerKBOptimal protein intake in the elderlyClin Nutr20082767568418819733
- Le CouteurDGSolon-BietSWahlDDietary protein, ageing and the Okinawan ratioAge Ageing20164544344727130207
- VolpiECampbellWWDwyerJTIs the optimal level of protein intake for older adults greater than the recommended dietary allowance?J Gerontol A Biol Sci Med Sci20136867768123183903
- BaumJIKimIYWolfeRRProtein consumption and the elderly: what is the optimal level of intake?Nutrients20168359378
- MooreDRKeeping older muscle young through dietary protein and physical activityAdv Nutr20145599S607S25469405
- Solon-BietSMMitchellSJde CaboRRaubenheimerDLe CouteurDGSimpsonSJMacronutrients and caloric intake in health and longevityJ Endocrinol20152261R17R2826021555
