Abstract
One of the most widely conserved hallmarks of aging is a decline in functional capabilities. Mobility loss is particularly burdensome due to its association with negative health outcomes, loss of independence and disability, and the heavy impact on quality of life. Recently, a new condition, physical frailty and sarcopenia, has been proposed to define a critical stage in the disabling cascade. Physical frailty and sarcopenia are characterized by weakness, slowness, and reduced muscle mass, yet with preserved ability to move independently. One of the strategies that have shown some benefits in combatting mobility loss and its consequences for older adults is physical activity. Here, we describe the opportunities and challenges for the development of physical activity interventions in people with physical frailty and sarcopenia. The aim of this article is to review age-related physio(patho)logical changes that impact mobility in old age and to provide recommendations and procedures in accordance with the available literature.
Introduction
One of the major challenges rising from the aging of the population is to avoid mobility impairment. Mobility is defined in a broad context by Webber et alCitation1 as the ability to move oneself (either independently or by using assistive devices or transportation) within environments that expand from one’s home to the neighborhood and to regions beyond. Approximately one third to one half of individuals aged 65 years or older report difficulties related to walking or climbing stairs.Citation1 Furthermore, mobility limitation during aging is associated with loss of strength and/or function that characterizes sarcopenia.Citation2–Citation4 Sarcopenia is described by the EWGSOP2 as a progressive and generalized skeletal muscle disorder that is associated with increased likelihood of adverse outcomes including falls, fractures, physical disability, and mortality.Citation5 Sarcopenia is now formally acknowledged as a muscle disease with an ICD-10-MC diagnosis code.Citation6 This medical syndrome of sarcopenia may reflect a gradual decline that impairs functional reserve in a dynamic process. On a parallel track, adverse events can drastically modify the health status of a person, which refers to the concept of frailty. An international group of experts has defined frailty as a clinical state in which there is an increase in an individual’s vulnerability for developing increased dependency and/or mortality when exposed to a stressor. Frailty can occur as the result of a range of diseases and medical conditions.Citation7,Citation8 In this context, a frail individual is characterized by weak functional abilities that occur large deterioration after a minor illness.Citation9 In order to counteract the effect of the aging process and avoid moving toward a state of frailty, functional reserve has to be strengthened, notably by physical activity. Physical activity is used as an umbrella term including exercise, leisure time physical activity, or even sports.Citation10 Physical activity is defined as any bodily movement produced by skeletal muscles that require energy expenditure, whereas exercise is a planned, structured, repetitive movement including progression with regard to intensity.Citation10
Frailty, especially associated with sarcopenia syndrome, is the main pivotal point to establish a preventive intervention program. Actually, there is evidence that without intervention, sarcopenia and frailty often lead to disability, falls, and a decline in quality of life.Citation11 There is also an increased risk of hospitalization and death.Citation7,Citation8 That is why prevention of loss of mobility becomes a priority in this population.
Regular physical activity, exercise, and leisure time physical activity including sports, combined with an adequate diet can prevent sarcopenia and consequently frailty. A multicomponent exercise training program, which includes aerobic, strength, and balance exercises, is considered to be the most effective tool for improving mobility and gait, increasing muscle mass and strength, decreasing falls, enhancing functional performance of activities of daily living, and improving quality of life.Citation12
This article first summarizes the functional alterations occurring in the sarcopenic and frail population. In the second part, the article highlights opportunities and challenges to prevent functional impairment, and mobility impairment through a physical activity program based on randomized clinical trials: LIFE and SPRINTT. Finally, the article provides recommendations for maintaining and improving mobility and functional performance in this specific population.
Physiological Changes in the Older Population: Sarcopenia and/or Frailty
Muscle Strength and Power
In old age, the most important impact on mobility arises from changes in muscle strength and power. “Strength” can be defined as the maximum force generation capacity of an individual, whereas “power” refers to the product of force and velocity of contraction.Citation13–Citation15 Several studies have demonstrated that strength capacities start to decline around the age of 30 yearsCitation16,Citation17 and that the decline increases at the rate of about 12% to 15% per decade after the fifth decade, with an even faster decline after 60 years.Citation18 Longitudinal studies have reported that strength decreases about 2.5% and 1.5% per year after sixty years old at the knee and elbow joints, respectively.Citation19 It has consequently been suggested that alteration of muscular strength could be muscle-specific.Citation20,Citation21 Skelton et al demonstrated that power declines with aging at an even more rapid rate than strength.Citation15 The origins of strength and power decline with aging are multifactorial. In two reviews, Clark and ManiniCitation22 and VandervoortCitation23 characterized the loss of neuromuscular strength with aging. In these reviews, the authors reported that neuromuscular strength could be directly influenced by changes in both the nervous system and the muscular system. Regarding the nervous system, the authors indicated that alteration of command drive,Citation24–Citation31 spinal reflex excitability,Citation32,Citation33 and motor unit discharge rateCitation34 can alter strength production in older people. In the muscular system, alterations were observed in muscle mass size,Citation3,Citation35 muscular architecture,Citation36 and excitation–contraction coupling,Citation37 which can be influenced by reduction of androgen secretion and growth factor.Citation38,Citation39 Studies over the last four decades have demonstrated that physiological deficiencies are clearly associated with functional performance.Citation15,Citation40-43
Related to physical function, there is evidence that knee extensor strength is an excellent predictor of dependency and survival, and that leg power is a stronger predictor of mobility loss than strength.Citation40,Citation44-46 Bean et alCitation40 found that leg power was strongly predictive of physical performance in 45 participants aged about 73 years (75% women), highlighting a significant relationship with stair climb time, chair stand, tandem gait time, habitual gait velocity, maximal gait velocity and Short Physical Performance Battery (SPPB) tests (). Other studies have reported that handgrip strength is strongly associated with lower limb muscle power and physical function of daily living.Citation47,Citation48 In conclusion, it can be stated that muscle strength and power decline at different rates, with power decreasing more rapidly than strength. While many factors contribute to strength and power decline, muscle mass is of key importance.
Table 1 Overview of Tests Available for the Assessment of Balance, Gait, and Lower Extremity Function
Muscle Mass
As previously mentioned, the combination of low muscle strength, low physical performance, and reduction of muscle mass characterize sarcopenia.Citation3–Citation5,Citation49 Sarcopenia is considered a critical point to determine the frail population and can be influenced by several factors. In a recent overview, Marzetti et alCitation3 reported that sarcopenia can be impacted by (i) personal factors such as age, early life events, low birth weight, and genetic characteristics, (ii) hormonal factors (eg, testosterone, estrogens, growth hormone, insulin-like growth factor-1), chronic low-grade inflammation, and mitochondrial dysfunction, (iii) lifestyle habits such as decrease in food and protein intake, sedentary behavior or reduced physical activity, alcohol abuse, tobacco use, and bed rest, (iv) chronic health conditions such as cognitive impairment, diabetes, and advanced stage organ diseases.
The literature has shown that after the seventh decade, muscle mass decreases by 4.7% and 3.7% per decade in men and women, respectively.Citation14 Using dual energy X-ray absorptiometry (DXA) to examine muscle mass in 433 individuals (180 women) aged 18–94 years, Kyle et alCitation50 reported that muscle mass is almost steady from 18 to 60 years, and declines after 60 years. In a magnetic resonance imagery (MRI) study in 468 individuals (200 women), Janssen et alCitation51 also reported that the rate of loss of muscle mass of upper limbs decreases less than twice the rate of loss of lower limbs. Further, men showed larger age-related muscle mass decrease compared to women.Citation52
Muscle mass can be estimated with several techniques such as anthropometric measurements, bioelectrical impedance analysis (BIA), computed tomography (CT), MRI, and DXA. Each of these techniques has advantages and disadvantages in terms of cost, availability, ease of use and time consumption.Citation3 Compared to the gold standard for quantifying muscle mass, ie CT and MRI, DXA may, due to the minimal radiation received by the patient, be the best way of differentiating fat and lean tissues. However, DXA scan is not portable, which limits its use in a large-scale population. To overcome this issue, BIA is inexpensive, easy to perform, readily reproducible, appropriate for ambulatory as well as bedridden patients, and more accurate than anthropometry. All in all, DXA could be considered as the best gold standard alternative to quantify muscle mass in research, and BIA as a valid portable alternative.
While the relationship between muscle mass decline and negative outcomes is still debated in the literature,Citation14 recent studies provide robust evidence that sarcopenia, including loss of muscle mass, is associated with falls, physical frailty, and disability.Citation3 Older adults with sarcopenia are reportedly less active than people without sarcopenia.Citation53,Citation54 Furthermore, different studies have shown that the older population with sarcopenia has a greater risk of death (whatever the causes) compared with nonsarcopenic people.Citation55–Citation59 From another perspective, the decrease in the circulating levels of specific hormones has been associated with both sarcopenia and osteoporosis, showing that the two processes follow a similar path.Citation60 An observational study recently carried out among 68 prefrail older persons aged between 65 and 95 years reported that those with osteosarcopenia were at higher risk of fractures or functional decline than those with either sarcopenia or osteoporosis.Citation61 Further, the authors showed that the performance on the handgrip strength, chair rise and sit-to-stand tests were significantly lower in patients with osteosarcopenia than in those with sarcopenia or osteoporosis alone. Finally, it has been reported that the sarcopenic older population was more than threefold likely to fall than those without sarcopenia.Citation62–Citation64
Balance and Postural Control
It has been well documented that postural control decreases and risk of falls increases with aging. As a result, falls are a common event in older adults, with about one third of persons 65 years and older experiencing a fall event yearly. This number increases to nearly 50% in the age group 80 years and above.Citation65 The decline in balance and postural control are multicausal. To maintain upright standing, the neuromuscular system has to generate appropriate muscular contraction involving complementary input from the visual, proprioceptive, exteroceptive and vestibular systems.Citation66–Citation71 Age-related alteration in these different systems results in decline of postural control.Citation72–Citation74 Experimental studies have shown that older persons are less able to regulate postural control when sensory information is manipulated or removed.Citation72,Citation74–Citation76 In a challenging condition, such as one-leg stance, maintaining upright standing is more energy-consuming in older than in their young counterparts.Citation77–Citation79 Strength capacity is also useful to discriminate fallers from nonfallers, showing that the former group is weaker than the latter.Citation80 It has also been shown that aging is associated with a progressive shift from spinal to supraspinal pathways to regulate leg muscle activity in an upright standing position.Citation81 This finding is supported by experimental studies reporting that the frail population could require more cognitive resources to maintain upright standing, and consequently increase the delay of postural control adjustment in comparison with healthy older adults.Citation82–Citation84 While there is no clear evidence of specific system alteration between frail and nonfrail, and sarcopenia vs nonsarcopenia populations, clinical studies have shown more conclusive results.
From a meta-analysis, Yeung et alCitation85 recently reported significant higher risk of falls in people with sarcopenia than without. Further, in a systematic review and meta-analysis of 10 studies with 10,073 participants, Zhang et alCitation86 concluded that sarcopenia is a risk factor for falls among community-dwelling older people, but not in nursing home residents. The authors provided several explanations for this counterintuitive finding.Citation87,Citation88 First, nursing-home residents may have limited mobility due to their poorer health status, which may moderate the effect of sarcopenia on falls.Citation89 In addition, in community-dwelling older adults falls were reported with questionnaires, whereas falls in nursing homes reported by nurses may be underestimated. With regard to gender, Zhang et alCitation86 indicated that old men with sarcopenia had a higher risk of falls than older persons in mixed gender groups. These results are supported by the fact that muscle mass decreases in men are generally twice as great than in women.Citation90,Citation91 In addition to sarcopenia, a systematic review and meta-analysis by KojimaCitation92 demonstrated that both frailty and prefrailty are significant predictors of future falls among community-dwelling older people. This could result from decreased functional reserve capacity in multiple physiologic systems and increased vulnerability to stressors such as accidents, disease symptoms, or adverse drug reactions.Citation93 All in all, the literature has demonstrated an age-related increased risk of falls, especially in persons with sarcopenia and the frail population. The increased risk of falls with aging is intrinsically linked to the deterioration of the dynamic balance generated during walking and, more generally, when in relation to mobility.
Walking and Mobility
Locomotion, defined as a motor action that changes the location of the entire body within the environment, is a common daily activity in human beings. Walking speed, representing the capacity of walking, declines slightly until the sixth decade, and decreases at a faster pace thereafter.Citation9,Citation94,Citation95 In a cross-sectional study, Samson et alCitation95 evaluated the preferred walking speed over a 12-meter walkway in 118 women and 121 men aged from 19 to 90 years. The authors found an age-related decrease of gait parameters with an alternation not only in walking speed, but also in stride length. However, modification of walking patterns with age has not appeared consistently throughout the literature. Compared to young adults, some studies have reported greater stride width in older adults,Citation96–Citation98 while other studies have indicated lesser stride width with older age.Citation99 Ko et alCitation100 revealed that stride width at preferred walking speed was narrower in middle-age (32–57 years) than in old adults (58–78 years) at maximum speed, while it was wider in oldest old group (79–93 years). The authors concluded that the walking pattern alteration could be different during the aging process, and could also depend on testing conditions. The impact of the methods used to obtain walking/gait speed was also addressed by Ng et alCitation101 and Wang et alCitation102 To explain alterations of walking capacity and patterns, different hypotheses have been put forward. In the Baltimore Longitudinal Study of Aging, a significant correlation between walking speed and maximal voluntary contraction of knee extensor was shown.Citation100 The authors’ interpretation was that a decrease of knee extensor muscle strength could explain the decline of walking speed with aging. Callisaya et alCitation103 found an association among white matter atrophy and walking speed, step length and cadence. The authors suggested that these results strengthened the evidence of a causal relationship between brain aging and walking decline. In a recent systematic review and meta-analysis, Peel et alCitation104 supported these results by reporting an association between gait speed performance and global cognitive function in community-dwelling older people throughout 16 longitudinal (15,662 participants) and 20 cross-sectional (13,848 participants) studies. This meta-analysis showed reduction of gait speed of 0.11 m/s in persons with cognitive impairment, 0.20 m/s in those with mild dementia, and of 0.41 m/s in those with moderate dementia, compared to cognitively intact older adults. In addition to cognitive functions, walking speed may be related to older people’s mobility.
While 85% of people at the age of 60 years have a normal gait, this proportion drops to 18% in people aged 85 years. In a predictive model study, Guralnik et alCitation179 reported that walking speed is a good predictor of daily living activity disability and mobility impairment. Furthermore, age-related walking speed decline has been associated with increased risk of falls,Citation105 quality of life,Citation106 health status,Citation107 physical function, and mobility,Citation108,Citation109 cognitive decline and dementia,Citation110–Citation112 and early mortality.Citation113 More specifically, Studenski et alCitation113 using individual data from 34,485 community-dwelling older adults (from nine cohort studies) with a follow-up longer than five years, evaluated the relationship between gait speed and survival. The authors reported that survival increases across the full range of gait speeds, with a gait speed of around 0.8 m/s at the median life expectancy at most ages for both sexes. They reported that gait speed, adjusted by age and sex, provided as accurate as predictions based on age, sex, use of mobility aids, and self-reported function or as predictions based on age, sex, chronic conditions, smoking history, blood pressure, body mass index, and hospitalization. All in all, the authors suggested that gait speed could be used in a simple way as an indicator of the health of older persons, and might help to identify populations that could benefit from preventive intervention. For all these reasons, walking speed has been identified as the sixth vital sign in geriatric assessment,Citation114 and could be the main indicator of mobility loss in the older population. In addition to motor function impairment, aging has an impact at the molecular and cellular level.
Molecular and Cellular Factors
Aging is accompanied by changes in molecular, cellular and organ level and modulated by genetic, behavioral and environmental factors. One of the most important factors in this area is the immune dysregulation producing high blood levels of pro-inflammatory immunogenetic stimulation.Citation115 High levels of circulating pro-inflammatory markers, eg Il-1, IL-6 as well as C-reactive protein, transforming growth factor ß and others are responsible for immune dysregulation and increased inflammation in older age. This pro-inflammatory status is often known as “inflammaging”.Citation115 Epidemiological studies have demonstrated the negative impact of inflammaging on cardiovascular disease, neurological disorder such as depression or dementia, and furthermore on global health indicators such as frailty, sarcopenia, and mobility limitation.Citation115
There is increasing evidence that low physical activity levels increases the accumulation of visceral fat, adipose infiltration by pro-inflammatory immune cells and persistent low-grade inflammation.Citation116 In his review article PhilippsCitation117 provided evidence that long-term physical activity modulates age-related cellular and molecular changes. Physical activity has a positive impact on the inflammatory processes and increases the resilience stress response.Citation117
Given that aging induces alterations in strength and power, muscle mass, balance and postural control, walking and mobility, physical activity or an exercise program provide opportunities to reduce and/or to stave off some of the negative effects of aging.
Opportunities
Evidence suggests that regular physical activity provides substantial health benefits and reduces risk of chronic diseases. In a systematic review, Paterson and WarburtonCitation118 demonstrated that inactive older persons ran an increased risk of functional limitations. Interestingly, the risk of physical limitations decreased on average about 20% if the person was only “somewhat active” (level 1 of physical activity). Moderate physical activity reduced even further the risk of functional limitations. It is commonly understood that any type of physical activity, even at low intensity, is one of the most effective strategies designed to counteract the onset of chronic diseases, eg Type II diabetes and cardiovascular disease,Citation119 and to support healthy aging. In addition, physical activity decreases mobility limitations, and supports independent status as well as quality of life.Citation120 TaylorCitation119 stated that physical activity should be regarded as an actual “medicine” due to its large array of health benefits. Hence, several recommendations for older persons to be physically active have been published.Citation121–Citation123 These recommendations propose similar advice: an older person should be physically active for about 30 min per day adding up to 150 min per week of moderate physical activity. One has to keep in mind that the term “physical activity” incorporates all movements that increase energy expenditure. Another important point in all of the recommendations addresses the intensity level by proposing moderate or even vigorous levels of physical activity, and the inclusion of strength and balance exercises.Citation121,Citation122 With regard to intensity, brisk walking may be considered as moderate and running or jogging as vigorous physical activity.Citation122 Nevertheless, most older adults rarely reach moderate-to-vigorous intensity on a daily basis and walking at light intensity constitutes the major part of physical activity in which they engage.Citation124 Knowing that additional health effects arise from engaging in a higher level of physical activity, especially in structured exercise programs,Citation119,Citation121 greater efforts should be made to increase the participation of older adults.
With regard to function, a recent review by Steffl et alCitation54 demonstrated that physical activity also has positive effects on physical function, eg modifying the sarcopenic process. This is in line with the findings by Marzetti et alCitation125 With regard to cognitive function, evidence is evolving that physical activity and exercise also have positive effects on cognitive capacities such as memory, attention, or executive function. Although the effects of physical activity on cognitive function vary with regard to physical activity modality (aerobic, strength, or other type), intensity (light, moderate, or vigorous), and various cognitive domains, evidence exists that older persons benefit from physical activity or exercise training with regard to cognitive functions and in some cases maintain this benefit over the long-term.Citation126–Citation130
With regard to the important issue of fall events in older persons, recent reviews have underlined the importance of targeted, planned, and structured exercise intervention including progressive strength training and challenging balance exercise.Citation131,Citation132 It is important to realize that in fall prevention the subcategory of exercise, not the broad-based term of physical activity, has demonstrated positive effects.
Over recent years another construct has been recognized as a key factor: sedentary behavior, which has been found to be an important risk factor for adverse events in older adults. In an EU project, the determinants of sedentary behavior were defined over the life span, and especially in older persons.Citation133 Sedentary behavior, or the formal construct of inactivity, has not only demonstrated negative health impact on an individual level, but has also generated deleterious economic and social consequences. Inactivity was responsible for about 5.3 to 5.7 million deaths globally from noncommunicable diseases, which could be prevented if people who were inactive were instead sufficiently active.Citation134 These findings are independent of physical activity levels.Citation135 Not surprisingly, in most recommendations reduction of sedentary behavior has been included, with the goal of reducing sitting time.Citation122 Several studies have demonstrated the negative health impact of sedentary behavior.Citation133,Citation136 Nevertheless, recent research has demonstrated that although replacing sedentary behavior with moderate-to-vigorous physical activity is associated with reduction of sarcopenia rates and with better performance across its determinants in a dose-response fashion, light physical activity also seemed protective.Citation137
In conclusion, these results suggest that even light physical activity, in which most older adults can partake, could contribute to healthy aging, by counteracting one of the most important contributors to functional loss, sarcopenia.Citation138 It is evident that physical activity has demonstrated beneficial health effects in older persons, whereas the other side of the coin—sedentary behavior—has only lately been recognized as equally important. Further research has demonstrated the positive effects of even a small increase in physical activity levels on health outcomes.Citation139 While regular physical activity offers clear opportunities to improve health, many challenges are encountered by the older population.
Challenges
Older persons constitute the most inactive cohort in population-based studies.Citation140,Citation141 In Australia, among persons aged 75 years and older, about 75% are reported not to meet recommended physical activity levels.Citation140 Older persons’ motivation to engage in physical activity is influenced by behavioral (eg perceptions, self-efficacy) and environmental factors (eg access, availability). Furthermore, different domains add to the complexity of engaging older persons in physical activity: type and intensity of physical activity, group or individual physical activity, organizational issues (morning or afternoon).Citation120,Citation140 The need to communicate and translate research findings on the positive effects of physical activity has been recognized lately by investigating the perception of older persons. Communication models such as the elaboration likelihood model (ELM) by Petty and CacioppoCitation142 or behavior-related psychological models such as the health belief model (HBM) or integrated behavior change model (ICBM) have been used. Most models of increased physical activity share as a central point self-efficacy, norms and values, as well as attitudes.Citation143 Being “too old” or “at my age, physical activity does not help” are attitudes that pose a barrier to adhering to physical activity recommendations.Citation144,Citation145 Low self-efficacy likewise poses a barrier for uptake or maintenance of physical activity or exercise programs.Citation146 Taking into account the specificities of the two domains, it is of utmost importance to phrase invitations in a positive manner and to address older persons’ perceptions of physical activity. In other words, older persons or future participants in research projects on exercise intervention should be persuaded that they are able to take part or carry out the proposed physical activity.Citation147
Several factors that prevent older persons from being physically active have been reported. In a systematic review, Baert et alCitation148 provided a theoretical framework for investigating barriers to physical activity in older adults. The social-ecological model used in this systematic review provides different levels to be addressed when investigating these barriers.Citation148 The model differentiates intrapersonal, interpersonal, community, and policy-based barriers. A similar differentiation method was used by Bauman et alCitation149 who also identified interpersonal, intrapersonal and extrapersonal factors such as environment and policy. On an intrapersonal level, for example, several factors have been identified as barriers. Baert et alCitation148 identified health status as a barrier for physical activity, which is in line with the findings of Newson and KempsCitation150 and Lubs et alCitation151 However, health status can act as an enabler and motivation to become physically active and thereby improve health outcomes.Citation152,Citation153
Further expertise to an increased level of physical activity or exercise programs may be found in the fall prevention research field.Citation154–Citation157 Elskamp et alCitation154 detected, on an individual level, the following reported reasons for nonactivity: lack of time, impaired mobility or “being too healthy”. Furthermore, one study showed that fall risk perception was related to fear of vulnerability, maintenance of autonomy, and interpretation of risk.Citation158
In conclusion, increasing physical activity or uptake of exercise intervention in older persons is a challenging and complex task. Several aspects of theoretical psychological models need to be taken into account, and positive wording as well as the highlighting of positive outcomes are of utmost importance in strengthening older persons’ belief that they can perform or take part in the proposed activity to follow the recommendations.
Recommendations and Procedures
Due to current demographic trends, health promotion and physical independence are crucial. The most recent recommendations advise aging people, frail or not, to perform a minimum of 30 min of moderate intensity physical activity such as fast pace walking, at least five days per week ().Citation159 Further, in a meta-analysis of more than one million men and women, Ekelund et alCitation160 reported that a high level of physical activity, equivalent to 60–75 min of moderate intensity per day, seemed to offset the increased mortality risk associated with prolonged sitting time. More specifically, the study found that the active older persons (about 60–75 min/day) who sit for more than eight hours daily have a significantly lower risk of mortality than people who sit for less than four hours per day with less physical activity (about five min/day). Based on these findings, physical activity can cancel out the deleterious effect of inactivity and be justified in older persons. To date, the largest and longest study on physical activity in the older population is the Lifestyle Interventions and Independence for Elders (LIFE) recently conducted in the US.Citation12 This multicenter randomized controlled trial (RCT) assessed the efficacy of a structured exercise program (designated by the authors as a physical activity program) compared to an educational group in preventing disability in 1635 sedentary and functionally limited persons older than 70 years, over a follow-up period of 2.6 years. In the LIFE study, physical intervention was based on a combination of walking (with a goal of 150 min/week), strength, flexibility and balance exercises at the center twice per week and at home once per week (weeks 1–4), twice per week (4–8 weeks) and up to four times per week (week 8–52). The exercise sessions were individualized, setting an overall target of 30 min at moderate intensity, 10 min of strength training of lower limbs, 10 min of balance training, and flexibility exercises. This study demonstrated that compared with a health education program, a structured moderate-intensity exercise program reduced major mobility disability, persistent mobility disability and a combined outcome of major mobility disability or death over 2.6 years of follow-up.
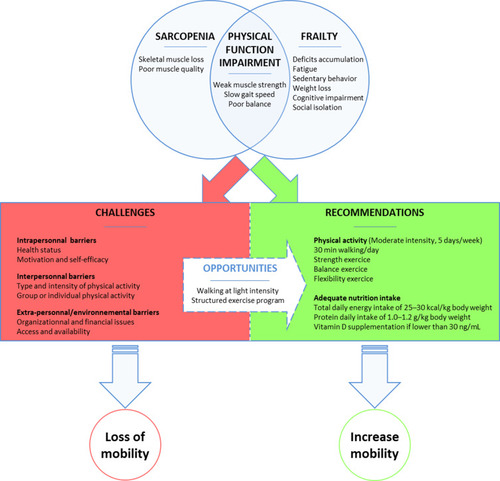
Figure 1 A Schematic representation summarizes the opportunities, the challenges, the recommendation of physical activity intervention to preserve mobility in older adults with physical frailty and sarcopenia.
Although no final results are available of the SPRINTT study yet, initial experiences in the challenges of recruitment in such a population have been reportedCitation161 after the study protocol had been published.Citation162 The SPRINTT study, a phase III, single-blind, multicenter RCT was designed to compare the efficacy of a multicomponent intervention (MCI) program (exercise intervention, nutritional counseling/dietary intervention, and information and communication technology intervention) versus a Healthy Aging Lifestyle Education (HALE) program in prevention of mobility impairment in initially nondisabled older persons with physical frailty and sarcopenia.Citation162 Emphasizing a combination of physical activity/exercise and nutritional intervention, the SPRINTT RCT differs from the LIFE study.Citation161 The MCI program was based on the exercise protocol of the LIFE studyCitation12 and consists of aerobic, strength, flexibility, and balance training.Citation12,Citation163 The physical activity/exercise program was designed to be performed both at the center (twice a week with an instructor) and at home (3–4 times/week).Citation125 As the primary goal is to enhance mobility, strength training intervention is mainly focused on lower extremity exercises, while upper body exercises are carried out at the end of the training session.
Nutrition is a major determinant of muscle health, physical function and overall well-being, especially in older persons.Citation164–Citation166 It follows that nutrition may play a role as a part of multicomponent interventions aimed at preserving muscle mass, counteracting physical performance decline and promoting robustness.Citation164,Citation167,Citation168 In this context, it is widely acknowledged that a combination of nutrition and exercise programs is one valuable approach to management of sarcopenia and the physical components of frailty.Citation164 Nutritional patterns conveying adequate daily energy, protein, as well as micronutrients (eg vitamins and plant-derived antioxidants), dietary fiber and healthy oils (in particular extra-virgin olive oil) have shown positive effects on muscle mass, physical function preservation and overall metabolic health.Citation169–Citation172 Several strategies may be implemented to design a nutritional plan for older adults involved in physical activity interventions aimed at maximizing the benefits of training and overcoming the traditional barriers that hamper its deployment in real life (ie lack of motivation, low adherence).Citation173,Citation174
In the SPRINTT RCT, a multifactorial approach was developed combining a physiologic/metabolic rationale with the educational/behavioral aspects of nutrition. The nutritional intervention of SPRINTT was designed to provide adequate quality and quantity of macro- and micronutrients tailored to the individual’s age, gender, health status, physical performance levels, comorbidities and therapies.Citation162 Individual preferences as well as a constant and timely dialogue between participants and nutritional “trainers” was implemented throughout the trial to increase adherence, motivation and nutritional awareness in older persons.Citation162 Two predefined nutritional targets were set according to expert recommendations: (1) a daily total energy intake of 25–30 kcal/kg body weight (corrected by ideal weight when appropriate); and (2) an average protein daily intake of at least 1.0–1.2 g/kg body weight.Citation175–Citation177 Vitamin D levels were also regularly monitored and supplementation was prescribed if serum concentrations of 25-hydroxyvitamin D (25-OH-D) were below 30 ng/mL (75 nmol/L).Citation178
Conclusion
Aging induces biological and functional decline at several levels: loss of muscle strength, loss of muscle mass, decline in balance, and subsequent loss of mobility. Sarcopenia and more widely frailty are critical points to address in preventive physical activity/exercise programs to avoid loss of mobility and physical performance. Physical activity and nutritional support to improve physical function and to prevent sarcopenia, frailty and disability are widely recommended in the literature. While the efficacy of long-term physical activity/exercise programs is to date available only in the LIFE study conducted in the US,Citation12 the SPRINTT study will provide new evidence of physical activity program feasibility and efficacy to prevent mobility impairment among sarcopenic and physically frail older adults.
Acknowledgments
The present work was funded by a grant from the Innovative Medicines Initiative—Joint Undertaking (IMI-JU 115621). The work was also partially supported by the nonprofit research foundation “Centro Studi Achille e Linda Lorenzon” and by intramural research grants from the Università Cattolica del Sacro Cuore (D3.2 2013 and D3.2 2015).
The authors thank all the participants and the staff involved in the trial for their enthusiastic participation and dedication. The authors would like to thank Jeffrey Arsham for his revision of the English-language manuscript.
Disclosure
All of the authors of the present work are partners of the SPRINTT consortium, which is partially funded by the European Federation of Pharmaceutical Industries and Associations (EFPIA). The authors report no potential conflicts of interest in this work.
References
- Webber SC, Porter MM, Menec VH. Mobility in older adults: a comprehensive framework. Gerontologist. 2010;50(4):443–450. doi:10.1093/geront/gnq01320145017
- Bülow J, Ulijaszek SJ, Holm L. Rejuvenation of the term sarcopenia. J Appl Physiol. 2019;126(1):255–256. doi:10.1152/japplphysiol.00400.201830001155
- Marzetti E, Calvani R, Tosato M, et al. Sarcopenia: an overview. Aging Clin Exp Res. 2017;29(1):11–17. doi:10.1007/s40520-016-0704-528155183
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5):990S–991S. doi:10.1093/jn/127.5.990S9164280
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601. doi:10.1093/ageing/afz046
- Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7(5):512–514. doi:10.1002/jcsm.1214727891296
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol a Biol Sci Med Sci. 2001;56(3):M146–156. doi:10.1093/gerona/56.3.m14611253156
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi:10.1016/j.jamda.2013.03.02223764209
- Hollman JH, McDade EM, Petersen RC. Normative spatiotemporal gait parameters in older adults. Gait Posture. 2011;34(1):111–118. doi:10.1016/j.gaitpost.2011.03.02421531139
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131.3920711
- Mijnarends DM, Luiking YC, Halfens RJG, et al. Muscle, health and costs: a glance at their relationship. J Nutr Health Aging. 2018;22(7):766–773. doi:10.1007/s12603-018-1058-930080217
- Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi:10.1001/jama.2014.561624866862
- Macaluso A, De Vito G. Muscle strength, power and adaptations to resistance training in older people. Eur J Appl Physiol. 2004;91(4):450–472. doi:10.1007/s00421-003-0991-314639481
- Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260. doi:10.3389/fphys.2012.0026022934016
- Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing. 1994;23(5):371–377. doi:10.1093/ageing/23.5.3717825481
- Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol. 1990;45(3):M82–88. doi:10.1093/geronj/45.3.m822335723
- Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol a Biol Sci Med Sci. 1997;52(5):B267–276. doi:10.1093/gerona/52a.5.b2679310077
- Vandervoort AA, McComas AJ. Contractile changes in opposing muscles of the human ankle joint with aging. J Appl Physiol. 1986;61(1):361–367. doi:10.1152/jappl.1986.61.1.3613525504
- Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88(4):1321–1326. doi:10.1152/jappl.2000.88.4.132110749826
- Winegard KJ, Hicks AL, Sale DG, Vandervoort AA. A 12-year follow-up study of ankle muscle function in older adults. J Gerontol a Biol Sci Med Sci. 1996;51(3):B202–207. doi:10.1093/gerona/51a.3.b2028630696
- Simoneau EM, Billot M, Martin A, Van Hoecke J. Antagonist mechanical contribution to resultant maximal torque at the ankle joint in young and older men. J Electromyogr Kinesiol. 2009;19(2):e123–131. doi:10.1016/j.jelekin.2007.11.00618164627
- Clark BC, Manini TM. Sarcopenia != Dynapenia. J Gerontol a Biol Sci Med Sci. 2008;63(8):829–834. doi:10.1093/gerona/63.8.82918772470
- Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25(1):17–25. doi:10.1002/mus.121511754180
- Billot M, Duclay J, Simoneau-Buessinger EM, Ballay Y, Martin A. Is co-contraction responsible for the decline in maximal knee joint torque in older males? Age (Dordr). 2014;36(2):899–910. doi:10.1007/s11357-014-9616-524445962
- Bilodeau M, Erb MD, Nichols JM, Joiner KL, Weeks JB. Fatigue of elbow flexor muscles in younger and older adults. Muscle Nerve. 2001;24(1):98–106. doi:10.1002/1097-4598(200101)24:1<98::AID-MUS11>3.0.CO;2-D11150971
- Jakobi JM, Rice CL. Voluntary muscle activation varies with age and muscle group. J Appl Physiol. 2002;93(2):457–462. doi:10.1152/japplphysiol.00012.200212133850
- Morse CI, Thom JM, Davis MG, Fox KR, Birch KM, Narici MV. Reduced plantarflexor specific torque in the elderly is associated with a lower activation capacity. Eur J Appl Physiol. 2004;92(1–2):219–226. doi:10.1007/s00421-004-1056-y15054662
- Stackhouse SK, Stevens JE, Lee SC, Pearce KM, Snyder-Mackler L, Binder-Macleod SA. Maximum voluntary activation in nonfatigued and fatigued muscle of young and elderly individuals. Phys Ther. 2001;81(5):1102–1109. doi:10.1093/ptj/81.5.110211319935
- Stevens JE, Binder-Macleod S, Snyder-Mackler L. Characterization of the human quadriceps muscle in active elders. Arch Phys Med Rehabil. 2001;82(7):973–978. doi:10.1053/apmr.2001.2399511441388
- Stevens JE, Stackhouse SK, Binder-Macleod SA, Snyder-Mackler L. Are voluntary muscle activation deficits in older adults meaningful? Muscle Nerve. 2003;27(1):99–101. doi:10.1002/mus.1027912508301
- Yue GH, Ranganathan VK, Siemionow V, Liu JZ, Sahgal V. Older adults exhibit a reduced ability to fully activate their biceps brachii muscle. J Gerontol a Biol Sci Med Sci. 1999;54(5):M249–253. doi:10.1093/gerona/54.5.m24910362008
- Hortobágyi T, Del Olmo MF, Rothwell JC. Age reduces cortical reciprocal inhibition in humans. Exp Brain Res. 2006;171(3):322–329. doi:10.1007/s00221-005-0274-916307241
- Kido A, Tanaka N, Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol. 2004;82(4):238–248. doi:10.1139/y04-01715181462
- Kamen G. Aging, resistance training, and motor unit discharge behavior. Can J Appl Physiol. 2005;30(3):341–351. doi:10.1139/h05-12616129898
- Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84(2–3):275–294. doi:10.1016/0022-510X(88)90132-33379447
- Narici MV, Maganaris CN. Adaptability of elderly human muscles and tendons to increased loading. J Anat. 2006;208(4):433–443. doi:10.1111/j.1469-7580.2006.00548.x16637869
- Delbono O. Regulation of excitation contraction coupling by insulin-like growth factor-1 in aging skeletal muscle. J Nutr Health Aging. 2000;4(3):162–164.10936903
- Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol a Biol Sci Med Sci. 2000;55(12):M716–724. doi:10.1093/gerona/55.12.m71611129393
- Welle S. Cellular and molecular basis of age-related sarcopenia. Can J Appl Physiol. 2002;27(1):19–41. doi:10.1139/h02-00211880689
- Bean JF, Kiely DK, Herman S, et al. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50(3):461–467. doi:10.1046/j.1532-5415.2002.50111.x11943041
- Hyatt RH, Whitelaw MN, Bhat A, Scott S, Maxwell JD. Association of muscle strength with functional status of elderly people. Age Ageing. 1990;19(5):330–336. doi:10.1093/ageing/19.5.3302251967
- Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40(1):4–12. doi:10.1097/JES.0b013e31823b5f1322016147
- Suzuki T, Bean JF, Fielding RA. Muscle power of the ankle flexors predicts functional performance in community-dwelling older women. J Am Geriatr Soc. 2001;49(9):1161–1167. doi:10.1046/j.1532-5415.2001.49232.x11559374
- Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol a Biol Sci Med Sci. 2003;58(8):728–733. doi:10.1093/gerona/58.8.m72812902531
- Byrne C, Faure C, Keene DJ, Lamb SE. Ageing, muscle power and physical function: a systematic review and implications for pragmatic training interventions. Sports Med. 2016;46(9):1311–1332. doi:10.1007/s40279-016-0489-x26893098
- Rantanen T, Avela J. Leg extension power and walking speed in very old people living independently. J Gerontol a Biol Sci Med Sci. 1997;52(4):M225–231. doi:10.1093/gerona/52a.4.m2259224434
- Cruz-Jentoft AJ, Landi F, Topinková E, Michel J-P. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010;13(1):1–7. doi:10.1097/MCO.0b013e328333c1c119915458
- Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95(5):1851–1860. doi:10.1152/japplphysiol.00246.200314555665
- Morley JE, Anker SD, von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology-update 2014. J Cachexia Sarcopenia Muscle. 2014;5(4):253–259. doi:10.1007/s13539-014-0161-y25425503
- Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr. 2001;55(8):663–672. doi:10.1038/sj.ejcn.160119811477465
- Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89(1):81–88. doi:10.1152/jappl.2000.89.1.8110904038
- Gallagher D, Visser M, De Meersman RE, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83(1):229–239. doi:10.1152/jappl.1997.83.1.2299216968
- Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91(4):1123S–1127S. doi:10.3945/ajcn.2010.28608A20164314
- Steffl M, Bohannon RW, Sontakova L, Tufano JJ, Shiells K, Holmerova I. Relationship between sarcopenia and physical activity in older people: a systematic review and meta-analysis. Clin Interv Aging. 2017;12:835–845. doi:10.2147/CIA.S13294028553092
- Cerri AP, Bellelli G, Mazzone A, et al. Sarcopenia and malnutrition in acutely ill hospitalized elderly: prevalence and outcomes. Clin Nutr. 2015;34(4):745–751. doi:10.1016/j.clnu.2014.08.01525263170
- Du Y, Karvellas CJ, Baracos V, Williams DC, Khadaroo RG; Acute Care and Emergency Surgery (ACES) Group. Sarcopenia is a predictor of outcomes in very elderly patients undergoing emergency surgery. Surgery. 2014;156(3):521–527. doi:10.1016/j.surg.2014.04.02724929435
- Landi F, Liperoti R, Fusco D, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc. 2012;13(2):121–126. doi:10.1016/j.jamda.2011.07.00421856243
- Landi F, Cruz-Jentoft AJ, Liperoti R, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing. 2013;42(2):203–209. doi:10.1093/ageing/afs19423321202
- Vetrano DL, Landi F, Volpato S, et al. Association of sarcopenia with short- and long-term mortality in older adults admitted to acute care wards: results from the CRIME study. J Gerontol a Biol Sci Med Sci. 2014;69(9):1154–1161. doi:10.1093/gerona/glu03424744390
- Tarantino U, Baldi J, Celi M, et al. Osteoporosis and sarcopenia: the connections. Aging Clin Exp Res. 2013;25(Suppl 1):S93–95. doi:10.1007/s40520-013-0097-724046056
- Drey M, Sieber CC, Bertsch T, Bauer JM, Schmidmaier R. FiAT intervention group. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res. 2016;28(5):895–899. doi:10.1007/s40520-015-0494-126563287
- Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31(5):652–658. doi:10.1016/j.clnu.2012.02.00722414775
- Tanimoto Y, Watanabe M, Sun W, et al. Sarcopenia and falls in community-dwelling elderly subjects in Japan: defining sarcopenia according to criteria of the European Working Group on sarcopenia in older people. Arch Gerontol Geriatr. 2014;59(2):295–299. doi:10.1016/j.archger.2014.04.01624852668
- Wu I-C, Lin -C-C, Hsiung CA, et al. Epidemiology of sarcopenia among community-dwelling older adults in Taiwan: a pooled analysis for a broader adoption of sarcopenia assessments. Geriatr Gerontol Int. 2014;14(Suppl 1):52–60. doi:10.1111/ggi.1219324450561
- Grossman DC, Curry SJ, Owens DK, et al.; US Preventive Services Task Force. Interventions to prevent falls in community-dwelling older adults: US preventive services task force recommendation statement. JAMA. 2018;319(16):1696–1704. doi:10.1001/jama.2018.3097.29710141
- Cathers I, Day BL, Fitzpatrick RC. Otolith and canal reflexes in human standing. J Physiol (Lond). 2005;563(1):229–234. doi:10.1113/jphysiol.2004.07952515618274
- Kavounoudias A, Roll R, Roll JP. The plantar sole is a “dynamometric map” for human balance control. Neuroreport. 1998;9(14):3247–3252. doi:10.1097/00001756-199810050-000219831459
- Paulus WM, Straube A, Brandt T. Visual stabilization of posture. Physiological stimulus characteristics and clinical aspects. Brain. 1984;107(4):1143–1163. doi:10.1093/brain/107.4.11436509312
- van Deursen RW, Simoneau GG. Foot and ankle sensory neuropathy, proprioception, and postural stability. J Orthop Sports Phys Ther. 1999;29(12):718–726. doi:10.2519/jospt.1999.29.12.71810612069
- Billot M, Handrigan GA, Simoneau M, Corbeil P, Teasdale N. Short term alteration of balance control after a reduction of plantar mechanoreceptor sensation through cooling. Neurosci Lett. 2013;535:40–44. doi:10.1016/j.neulet.2012.11.02223305721
- Billot M, Handrigan GA, Simoneau M, Teasdale N. Reduced plantar sole sensitivity induces balance control modifications to compensate ankle tendon vibration and vision deprivation. J Electromyogr Kinesiol. 2015;25(1):155–160. doi:10.1016/j.jelekin.2014.06.00324993669
- Hay L, Bard C, Fleury M, Teasdale N. Availability of visual and proprioceptive afferent messages and postural control in elderly adults. Exp Brain Res. 1996;108(1):129–139. doi:10.1007/BF02429108721161
- Manchester D, Woollacott M, Zederbauer-Hylton N, Marin O. Visual, vestibular and somatosensory contributions to balance control in the older adult. J Gerontol. 1989;44(4):M118–127. doi:10.1093/geronj/44.4.M1182786896
- Woollacott MH. Systems contributing to balance disorders in older adults. J Gerontol a Biol Sci Med Sci. 2000;55(8):M424–428. doi:10.1093/gerona/55.8.m42410952363
- Aartolahti E, Häkkinen A, Lönnroos E, Kautiainen H, Sulkava R, Hartikainen S. Relationship between functional vision and balance and mobility performance in community-dwelling older adults. Aging Clin Exp Res. 2013;25(5):545–552. doi:10.1007/s40520-013-0120-z24002802
- Saftari LN, Kwon O-S. Ageing vision and falls: a review. J Physiol Anthropol. 2018;37(1):11. doi:10.1186/s40101-018-0170-129685171
- Allum JHJ, Carpenter MG, Honegger F, Adkin AL, Bloem BR. Age-dependent variations in the directional sensitivity of balance corrections and compensatory arm movements in man. J Physiol (Lond). 2002;542(2):643–663. doi:10.1113/jphysiol.2001.01564412122159
- Billot M, Simoneau EM, Van Hoecke J, Martin A. Age-related relative increases in electromyography activity and torque according to the maximal capacity during upright standing. Eur J Appl Physiol. 2010;109(4):669–680. doi:10.1007/s00421-010-1397-720213469
- Nagai K, Yamada M, Uemura K, Yamada Y, Ichihashi N, Tsuboyama T. Differences in muscle coactivation during postural control between healthy older and young adults. Arch Gerontol Geriatr. 2011;53(3):338–343. doi:10.1016/j.archger.2011.01.00321310498
- Cattagni T, Scaglioni G, Laroche D, Van Hoecke J, Gremeaux V, Martin A. Ankle muscle strength discriminates fallers from non-fallers. Front Aging Neurosci. 2014;6:336. doi:10.3389/fnagi.2014.0033625566068
- Baudry S, Penzer F, Duchateau J. Vision and proprioception do not influence the excitability of the corticomotoneuronal pathway during upright standing in young and elderly adults. Neuroscience. 2014;268:247–254. doi:10.1016/j.neuroscience.2014.03.02624662846
- Martínez-Ramírez A, Lecumberri P, Gómez M, Rodriguez-Mañas L, García FJ, Izquierdo M. Frailty assessment based on wavelet analysis during quiet standing balance test. J Biomech. 2011;44(12):2213–2220. doi:10.1016/j.jbiomech.2011.06.00721719016
- Teasdale N, Bard C, LaRue J, Fleury M. On the cognitive penetrability of posture control. Exp Aging Res. 1993;19(1):1–13. doi:10.1080/036107393082539198444263
- Kubicki A, Bonnetblanc F, Petrement G, Ballay Y, Mourey F. Delayed postural control during self-generated perturbations in the frail older adults. Clin Interv Aging. 2012;7:65–75. doi:10.2147/CIA.S2835222423179
- Yeung SSY, Reijnierse EM, Pham VK, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10(3):485–500. doi:10.1002/jcsm.1241130993881
- Zhang X, Huang P, Dou Q, et al. Falls among older adults with sarcopenia dwelling in nursing home or community: a meta-analysis. Clin Nutr. 2019. doi:10.1016/j.clnu.2019.01.002
- Kim JH, Lim S, Choi SH, et al. Sarcopenia: an independent predictor of mortality in community-dwelling older Korean men. J Gerontol a Biol Sci Med Sci. 2014;69(10):1244–1252. doi:10.1093/gerona/glu05024721723
- Senior HE, Henwood TR, Beller EM, Mitchell GK, Keogh JWL. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas. 2015;82(4):418–423. doi:10.1016/j.maturitas.2015.08.00626341045
- Lord SR, March LM, Cameron ID, et al. Differing risk factors for falls in nursing home and intermediate-care residents who can and cannot stand unaided. J Am Geriatr Soc. 2003;51(11):1645–1650. doi:10.1046/j.1532-5415.2003.51518.x14687397
- Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol a Biol Sci Med Sci. 2006;61(10):1059–1064. doi:10.1093/gerona/61.10.105917077199
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589–598. doi:10.1210/jcem.87.2.820111836290
- Kojima G. Frailty as a predictor of future falls among community-dwelling older people: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16(12):1027–1033. doi:10.1016/j.jamda.2015.06.01826255098
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi:10.1016/S0140-6736(12)62167-923395245
- Laufer Y. Effect of age on characteristics of forward and backward gait at preferred and accelerated walking speed. J Gerontol a Biol Sci Med Sci. 2005;60(5):627–632. doi:10.1093/gerona/60.5.62715972616
- Samson MM, Crowe A, de Vreede PL, Dessens JA, Duursma SA, Verhaar HJ. Differences in gait parameters at a preferred walking speed in healthy subjects due to age, height and body weight. Aging (Milano). 2001;13(1):16–21. doi:10.1007/BF335148911292147
- Beauchet O, Allali G, Annweiler C, et al. Gait variability among healthy adults: low and high stride-to-stride variability are both a reflection of gait stability. Gerontology. 2009;55(6):702–706. doi:10.1159/00023590519713694
- Dean JC, Alexander NB, Kuo AD. The effect of lateral stabilization on walking in young and old adults. IEEE Trans Biomed Eng. 2007;54(11):1919–1926. doi:10.1109/TBME.2007.90103118018687
- Schrager MA, Kelly VE, Price R, Ferrucci L, Shumway-Cook A. The effects of age on medio-lateral stability during normal and narrow base walking. Gait Posture. 2008;28(3):466–471. doi:10.1016/j.gaitpost.2008.02.00918400500
- Blanke DJ, Hageman PA. Comparison of gait of young men and elderly men. Phys Ther. 1989;69(2):144–148. doi:10.1093/ptj/69.2.1442913584
- Ko S, Stenholm S, Metter EJ, Ferrucci L. Age-associated gait patterns and the role of lower extremity strength - results from the Baltimore longitudinal study of aging. Arch Gerontol Geriatr. 2012;55(2):474–479. doi:10.1016/j.archger.2012.04.00422564361
- Ng SSM, Au KKC, Chan ELW, et al. Effect of acceleration and deceleration distance on the walking speed of people with chronic stroke. J Rehabil Med. 2016;48(8):666–670. doi:10.2340/16501977-212427534654
- Wang C-Y, Chen T-R, Lin Y-H, Liu M-H, Chen Y-C. Gait speed measure: the effect of different measuring distances and the inclusion and exclusion of acceleration and deceleration. Percept Mot Skills. 2012;114(2):469–478. doi:10.2466/10.25.26.PMS.114.2.469-47822755452
- Callisaya ML, Beare R, Phan TG, et al. Brain structural change and gait decline: a longitudinal population-based study. J Am Geriatr Soc. 2013;61(7):1074–1079. doi:10.1111/jgs.1233123796055
- Peel NM, Alapatt LJ, Jones LV, Hubbard RE. The association between gait speed and cognitive status in community-dwelling older people: a systematic review and meta-analysis. J Gerontol a Biol Sci Med Sci. 2019;74(6):943–948. doi:10.1093/gerona/gly14029917045
- Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45(3):313–320. doi:10.1111/j.1532-5415.1997.tb00946.x9063277
- Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48(12):1618–1625. doi:10.1111/j.1532-5415.2000.tb03873.x11129752
- Cesari M, Kritchevsky SB, Penninx BWHJ, et al. Prognostic value of usual gait speed in well-functioning older people–results from the health, aging and body composition study. J Am Geriatr Soc. 2005;53(10):1675–1680. doi:10.1111/j.1532-5415.2005.53501.x16181165
- Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi:10.1001/jama.295.17.201816670410
- Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51(3):314–322. doi:10.1046/j.1532-5415.2003.51104.x12588574
- Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci.2013;68(8):929–937. doi: 10.1093/gerona/gls256
- Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78(9):929–935. doi:10.1136/jnnp.2006.10691417237140
- Watson NL, Rosano C, Boudreau RM, et al. Executive function, memory, and gait speed decline in well-functioning older adults. J Gerontol a Biol Sci Med Sci. 2010;65(10):1093–1100. doi:10.1093/gerona/glq11120581339
- Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi:10.1001/jama.2010.192321205966
- Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign.”. J Geriatr Phys Ther. 2009;32(2):46–49. doi:10.1519/00139143-200932020-0000220039582
- Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. doi:10.1038/s41569-018-0064-230065258
- Phillips C, Fahimi A. Immune and neuroprotective effects of physical activity on the brain in depression. Front Neurosci. 2018;12:498. doi:10.3389/fnins.2018.0049830093853
- Phillips C. Physical activity modulates common neuroplasticity substrates in major depressive and bipolar disorder. Neural Plast. 2017;2017:7014146. doi:10.1155/2017/701414628529805
- Paterson DH, Warburton DE. Physical activity and functional limitations in older adults: a systematic review related to Canada’s physical activity guidelines. Int J Behav Nutr Phys Act. 2010;7:38. doi:10.1186/1479-5868-7-3820459782
- Taylor D. Physical activity is medicine for older adults. Postgrad Med J. 2014;90(1059):26–32. doi:10.1136/postgradmedj-2012-13136624255119
- Amireault S, Baier JM, Spencer JR. Physical activity preferences among older adults: a systematic review. J Aging Phys Act. 2018;1–12. doi:10.1123/japa.2017-0234
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al.; American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi:10.1249/MSS.0b013e3181a0c95c.19516148
- Macera CA, Cavanaugh A, Bellettiere J. State of the Art Review: physical Activity and Older Adults. Am J Lifestyle Med. 2017;11(1):42–57. doi:10.1177/155982761557189730202313
- WHO. Global Recommendations on Physical Activity for Health. Geneva, Switzerland: World Health Organization; 2010.
- Bijnen FC, Feskens EJ, Caspersen CJ, Mosterd WL, Kromhout D. Age, period, and cohort effects on physical activity among elderly men during 10 years of follow-up: the Zutphen elderly study. J Gerontol a Biol Sci Med Sci. 1998;53(3):M235–241. doi:10.1093/gerona/53a.3.m2359597057
- Marzetti E, Calvani R, Tosato M, et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin Exp Res. 2017;29(1):35–42. doi:10.1007/s40520-016-0705-4
- Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res. 2013;2013:657508. doi:10.1155/2013/65750824102028
- Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol a Biol Sci Med Sci. 2006;61(11):1166–1170. doi:10.1093/gerona/61.11.116617167157
- Engeroff T, Ingmann T, Banzer W. Physical activity throughout the adult life span and domain-specific cognitive function in old age: a systematic review of cross-sectional and longitudinal data. Sports Med. 2018;48(6):1405–1436. doi:10.1007/s40279-018-0920-629667159
- Koščak Tivadar B. Physical activity improves cognition: possible explanations. Biogerontology. 2017;18(4):477–483. doi:10.1007/s10522-017-9708-628492999
- Sáez de Asteasu ML, Martínez-Velilla N, Zambom-Ferraresi F, Casas-Herrero Á, Izquierdo M. Role of physical exercise on cognitive function in healthy older adults: a systematic review of randomized clinical trials. Ageing Res Rev. 2017;37:117–134. doi:10.1016/j.arr.2017.05.00728587957
- Hopewell S, Adedire O, Copsey BJ, et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2018;7:CD012221. doi:10.1002/14651858.CD012221.pub230035305
- Sherrington C, Fairhall NJ, Wallbank GK, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2019;1:CD012424. doi:10.1002/14651858.CD012424.pub230703272
- Chastin SFM, Buck C, Freiberger E, et al. Systematic literature review of determinants of sedentary behaviour in older adults: a DEDIPAC study. Int J Behav Nutr Phys Act. 2015;12:127. doi:10.1186/s12966-015-0292-326437960
- Kohl HW, Craig CL, Lambert EV, et al. The pandemic of physical inactivity: global action for public health. Lancet. 2012;380(9838):294–305. doi:10.1016/S0140-6736(12)60898-822818941
- Koster A, Caserotti P, Patel KV, et al. Association of sedentary time with mortality independent of moderate to vigorous physical activity. PLoS One. 2012;7(6):e37696. doi:10.1371/journal.pone.003769622719846
- Lee I-M, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi:10.1016/S0140-6736(12)61031-922818936
- Sánchez-Sánchez JL, Mañas A, García-García FJ, et al. Sedentary behaviour, physical activity, and sarcopenia among older adults in the TSHA: isotemporal substitution model. J Cachexia Sarcopenia Muscle. 2019;10(1):188–198. doi:10.1002/jcsm.1236930920779
- Suetta C, Haddock B, Alcazar J, et al. The Copenhagen Sarcopenia Study: lean mass, strength, power, and physical function in a Danish cohort aged 20–93 years. J Cachexia Sarcopenia Muscle. 2019;10(6):1316–1329. doi:10.1002/jcsm.1247731419087
- Wen CP, Wai JPM, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–1253. doi:10.1016/S0140-6736(11)60749-621846575
- Franco MR, Tong A, Howard K, et al. Older people’s perspectives on participation in physical activity: a systematic review and thematic synthesis of qualitative literature. Br J Sports Med. 2015;49(19):1268–1276. doi:10.1136/bjsports-2014-09401525586911
- Sun F, Norman IJ, While AE. Physical activity in older people: a systematic review. BMC Public Health. 2013;13:449. doi:10.1186/1471-2458-13-44923648225
- Petty RE, Cacioppo JT. The elaboration likelihood model of persuasion. Adv Exp Soc Psychol. 1986;19:123–205.
- Hagger MS, Chatzisarantis NLD. An integrated behavior change model for physical activity. Exerc Sport Sci Rev. 2014;42(2):62–69. doi:10.1249/JES.000000000000000824508739
- Zunft HJ, Friebe D, Seppelt B, et al. Perceived benefits and barriers to physical activity in a nationally representative sample in the European Union. Public Health Nutr. 1999;2(1A):153–160. doi:10.1017/S136898009900020810933635
- Chang M, Leveille S, Cohen-Mansfield J, Guralnik JM. The association of physical-performance level with attitude toward exercise in older adults. J Aging Phys Act. 2003;11(2):254–264. doi:10.1123/japa.11.2.254
- Lee -L-L, Arthur A, Avis M. Using self-efficacy theory to develop interventions that help older people overcome psychological barriers to physical activity: a discussion paper. Int J Nurs Stud. 2008;45(11):1690–1699. doi:10.1016/j.ijnurstu.2008.02.01218501359
- Sales M, Levinger P, Polman R. Relationships between self perceptions and physical activity behaviour, fear of falling, and physical function among older adults. Eur Rev Aging Phys Act. 2017;14:17. doi:10.1186/s11556-017-0185-328943974
- Baert V, Gorus E, Mets T, Geerts C, Bautmans I. Motivators and barriers for physical activity in the oldest old: a systematic review. Ageing Res Rev. 2011;10(4):464–474. doi:10.1016/j.arr.2011.04.00121570493
- Bauman AE, Reis RS, Sallis JF, et al. Correlates of physical activity: why are some people physically active and others not? Lancet. 2012;380(9838):258–271. doi:10.1016/S0140-6736(12)60735-122818938
- Newson RS, Kemps EB. Factors that promote and prevent exercise engagement in older adults. J Aging Health. 2007;19(3):470–481. doi:10.1177/089826430730016917496245
- Lübs L, Peplies J, Drell C, Bammann K. Cross-sectional and longitudinal factors influencing physical activity of 65 to 75-year-olds: a pan European cohort study based on the survey of health, ageing and retirement in Europe (SHARE). BMC Geriatr. 2018;18(1):94. doi:10.1186/s12877-018-0781-829661154
- Kaleta D, Makowiec-Dabrowska T, Dziankowska-Zaborszczyk E, Jegier A. Physical activity and self-perceived health status. Int J Occup Med Environ Health. 2006;19(1):61–69. doi:10.2478/v10001-006-0005-x16881600
- Loprinzi PD, Frith E. Association between perceived physical activity and cognitive function in older adults. Psychol Rep. 2019;122(1):108–116. doi:10.1177/003329411775063229307247
- Elskamp ABM, Hartholt KA, Patka P, van Beeck EF, van der Cammen TJM. Why older people refuse to participate in falls prevention trials: a qualitative study. Exp Gerontol. 2012;47(4):342–345. doi:10.1016/j.exger.2012.01.00622310657
- Gardiner S, Glogowska M, Stoddart C, Pendlebury S, Lasserson D, Jackson D. Older people’s experiences of falling and perceived risk of falls in the community: a narrative synthesis of qualitative research. Int J Older People Nurs. 2017;12(4):e12151. doi:10.1111/opn.12151
- Hill KD, Day L, Haines TP. What factors influence community-dwelling older people’s intent to undertake multifactorial fall prevention programs? Clin Interv Aging. 2014;9:2045–2053. doi:10.2147/CIA.S7267925473276
- Yardley L, Donovan-Hall M, Francis K, Todd C. Attitudes and beliefs that predict older people’s intention to undertake strength and balance training. J Gerontol B Psychol Sci Soc Sci. 2007;62(2):P119–125. doi:10.1093/geronb/62.2.p11917379672
- McMahon S, Talley KM, Wyman JF. Older people’s perspectives on fall risk and fall prevention programs: a literature review. Int J Older People Nurs. 2011;6(4):289–298. doi:10.1111/j.1748-3743.2011.00299.x22078019
- Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–759. doi:10.1093/ageing/afu11525241753
- Ekelund U, Steene-Johannessen J, Brown WJ, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. 2016;388(10051):1302–1310. doi:10.1016/S0140-6736(16)30370-127475271
- Marzetti E, Cesari M, Calvani R, et al. The “Sarcopenia and physical frailty in older people: multi-component treatment strategies” (SPRINTT) randomized controlled trial: case finding, screening and characteristics of eligible participants. Exp Gerontol. 2018;113:48–57. doi:10.1016/j.exger.2018.09.01730261246
- Landi F, Cesari M, Calvani R, et al. The “Sarcopenia and physical frailty in older people: multi-component treatment strategies” (SPRINTT) randomized controlled trial: design and methods. Aging Clin Exp Res. 2017;29(1):89–100. doi:10.1007/s40520-016-0715-228144914
- Fielding RA, Rejeski WJ, Blair S, et al. The lifestyle interventions and independence for elders study: design and methods. J Gerontol a Biol Sci Med Sci. 2011;66(11):1226–1237. doi:10.1093/gerona/glr12321825283
- Calvani R, Miccheli A, Landi F, et al. Current nutritional recommendations and novel dietary strategies to manage sarcopenia. J Frailty Aging. 2013;2(1):38–53.26082911
- Azzolino D, Arosio B, Marzetti E, Calvani R, Cesari M. Nutritional status as a mediator of fatigue and its underlying mechanisms in older people. Nutrients. 2020;12(2):444. doi:10.3390/nu12020444
- Lorenzo-López L, Maseda A, de Labra C, Regueiro-Folgueira L, Rodríguez-Villamil JL, Millán-Calenti JC. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr. 2017;17(1):108. doi:10.1186/s12877-017-0496-228506216
- Robinson SM, Reginster JY, Rizzoli R, et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin Nutr. 2018;37(4):1121–1132. doi:10.1016/j.clnu.2017.08.01628927897
- Cruz-Jentoft AJ, Kiesswetter E, Drey M, Sieber CC. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res. 2017;29(1):43–48. doi:10.1007/s40520-016-0709-028155181
- Ntanasi E, Yannakoulia M, Kosmidis M-H, et al. Adherence to Mediterranean Diet and Frailty. J Am Med Dir Assoc. 2018;19(4):315–322.e2. doi:10.1016/j.jamda.2017.11.00529289542
- Montiel-Rojas D, Nilsson A, Santoro A, et al. Dietary fibre may mitigate sarcopenia risk: findings from the NU-AGE cohort of older European adults. Nutrients. 2020;12(4):1075. doi:10.3390/nu12041075
- Behrouzi P, Grootswagers P, Keizer PLC, et al. Dietary intakes of vegetable protein, folate, and vitamins B-6 and B-12 are partially correlated with physical functioning of Dutch older adults using copula graphical models. J Nutr. 2020;150(3):634–643. doi:10.1093/jn/nxz26931858107
- Ghosh TS, Rampelli S, Jeffery IB, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. 2020;69(7):1218–1228. doi:10.1136/gutjnl-2019-31965432066625
- Dedeyne L, Dewinter L, Lovik A, Verschueren S, Tournoy J, Gielen E. Nutritional and physical exercise programs for older people: program format preferences and (dis)incentives to participate. Clin Interv Aging. 2018;13:1259–1266. doi:10.2147/CIA.S15981930050293
- Hsieh T-J, Su S-C, Chen C-W, et al. Individualized home-based exercise and nutrition interventions improve frailty in older adults: a randomized controlled trial. Int J Behav Nutr Phys Act. 2019;16(1):119. doi:10.1186/s12966-019-0855-931791364
- Correia MITD, Hegazi RA, Higashiguchi T, et al. Evidence-based recommendations for addressing malnutrition in health care: an updated strategy from the feedM.E. Global study group. J Am Med Dir Assoc. 2014;15(8):544–550. doi:10.1016/j.jamda.2014.05.01124997720
- Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE study group. J Am Med Dir Assoc. 2013;14(8):542–559. doi:10.1016/j.jamda.2013.05.02123867520
- Deutz NEP, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN expert group. Clin Nutr. 2014;33(6):929–936. doi:10.1016/j.clnu.2014.04.00724814383
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adults. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62(1):147–152. doi:10.1111/jgs.12631.24350602
- Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol a Biol Sci Med Sci. 2000;55(4):M221–231. doi:10.1093/gerona/55.4.m22110811152
- Tinetti ME, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80(3):429–434. doi:10.1016/0002-9343(86)90717-53953620
- Vellas BJ, Wayne SJ, Romero L, Baumgartner RN, Rubenstein LZ, Garry PJ. One-leg balance is an important predictor of injurious falls in older persons. J Am Geriatr Soc. 1997;45(6):735–738. doi:10.1111/j.1532-5415.1997.tb01479.x9180669
- Berg K, Wood-Dauphine S, Williams JI, Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiother Can. 2009. doi:10.3138/ptc.41.6.304
- Rolland YM, Cesari M, Miller ME, Penninx BW, Atkinson HH, Pahor M. Reliability of the 400-m usual-pace walk test as an assessment of mobility limitation in older adults. J Am Geriatr Soc. 2004;52(6):972–976. doi:10.1111/j.1532-5415.2004.52267.x15161464
- Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80(7):837–841. doi:10.1016/s0003-9993(99)90236-810414771
- Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi:10.1111/j.1532-5415.1991.tb01616.x1991946
