Introduction
Alzheimer’s disease (AD) is the most common type of dementia in the aging population characterized by a progressive decline of cognition that brings a significant burden for patients and society. The exact pathogenesis of AD is still elusive.Citation1 AD progresses along a continuum from the preclinical stage, mild cognitive, and then dementia stages. Converging lines of evidence from research studies reveal that underlying pathological changes due to AD exist in the decades before symptom onset.Citation2 However, a large portion of patients remains undiagnosed at the early stages of AD in clinical practice. The early and accurate identification of AD underlying pathology is fundamental for the diagnosis, disease monitoring, and management of AD patients. Notably, early diagnosis of AD is a critical step forward for the clinical trial, which facilitates the development of disease-modifying therapies. In recent years, immense affords have been made to illustrate the early pathological changes of AD.Citation3 Progress in fluid biomarkers and image analyses facilitates the early and accurate diagnostic process for AD. Based on the evidence of several core pathological changes of AD, including amyloid-β (Aβ) deposition, phosphorylated tau (p-tau), and neurodegeneration, a research framework for AD defining was proposed in 2018.Citation4 However, positron emission tomography (PET) and cerebrospinal fluid (CSF) biomarkers evaluations have several limitations, including high cost, insufficient accessibility, and invasiveness, stint them as a first-line AD diagnostic evaluation. Emerging blood-based biomarkers are an exciting development in this field, since they may provide a convenient, cost-effective, and less invasive screening tool.Citation5
Current Situation of AD Early Diagnosis
AD has been considered as a continuous biological continuum that is identified by several kinds of biomarkers. In 2011, the National Institute on Aging and Alzheimer’s Association (NIA-AA) proposed diagnostic guidelines for AD, including the pre-symptomatic and symptomatic stages of AD.Citation6 In 2018, the NIA-AA further proposed a research framework to biologically define AD by AT(N) biomarker profiles.Citation4 The research framework listed validated biomarkers of AD pathology include: Aβ and p-tau PET; the CSF concentration of Aβ42; the CSF Aβ42/Aβ40 ratio; the CSF concentrations of total tau (t-tau) and p-tau181. Due to the limitations of PET and CSF, a blood test for AD screening would be a significant step toward earlier intervention and less society burden. Although blood-based biomarkers (ie, Aβ, p-tau) are still not recommended in clinical practice which need to be further standardized and validated.Citation7 The recommendations for use of blood biomarkers in AD proposed by the Alzheimer’s Association suggest that blood-based biomarkers can be used in specialized memory clinics for cognitively impaired individuals but the results still need to be confirmed by CSF or PET.Citation8
Blood-Based Biomarkers of AD
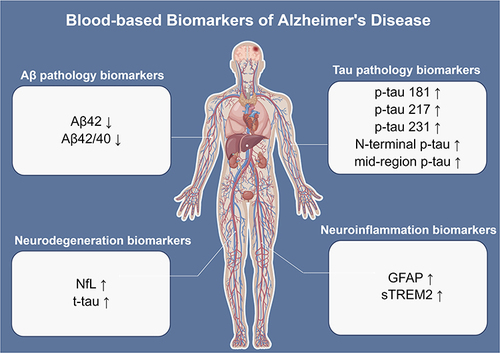
Several blood biomarkers include the plasma Aβ42, Aβ42/40 ratio, p-tau, t-tau, neurofilament light polypeptide (NfL), glial fibrillary acidic protein (GFAP), and soluble triggering receptor expressed on myeloid cells 2 (sTREM2) have been considered as the promising biomarkers of AD (). Their roles in diagnostic, progress monitoring, and prognosis have been intensively discussed in recent years.
Figure 1 Overview of the most promising blood-based biomarkers of AD. The biomarkers can be divided into four aspects: Aβ pathology (Aβ42 and Aβ42/40 ratio), tau pathology (p-tau181, p-tau217, p-tau231, N-terminal p-tau, and mid-region p-tau), neurodegeneration (NfL and t-tau), and neuroinflammation (GFAP and sTREM2). The figure was made by Figdraw (ID: RAWSR4a31d).
Blood-Based Aβ Pathology Biomarkers
Initial studies used enzyme linked immunosorbent assays (ELISA) immunoassays to assess plasma levels of Aβ42 which showed negative association with AD.Citation9 Subsequently, one study using single-molecule array (Simoa) reported that reduced concentration of plasma Aβ42 was observed in AD compared to controls, but plasma Aβ42 showed negative correlation with brain amyloid deposition which measured by PET.Citation10 Later, several studies using sensitive assays such as Simoa, electrochemiluminescence immunoassays (Elecsys) and Immunoprecipitation-Mass Spectrometry (IP/MS) assays further confirmed that the decreased plasma Aβ42 and Aβ42/Aβ40 ratios in AD and subjective cognitive decline (SCD).Citation11,Citation12 More recently, a head-to-head comparison study evaluated the performance of eight plasma Aβ42/40 IP/MS based assays to identify abnormal CSF Aβ42/40 and Aβ-PET status. The results showed that certain MS-based methods had best performance for brain Aβ pathology detection.Citation13 Plasma Aβ42/Aβ40 has been considered as a promising blood-based biomarker for AD screening. However, there is lack of evidence that the plasma Aβ42/40 can differentiate AD from non-AD dementias currently. Considering the availability and relative high cost, both Simoa and IP/MS based assays still need to be optimized in several aspects before they can be used in screening for AD in large populations.
Blood Based Tau Pathology Biomarkers
P-tau is a key component of neurofibrillary tangles in AD brain. CSF p-tau181 is the most commonly studied soluble p-tau.Citation14 Similar to Aβ, various new developed sensitive assays (ie, Simoa) were used to assay p-tau181 in recent years. Many researchers found plasma p-tau181 was significantly elevated in AD, compared with cognitively normal controls, and other non-AD dementia patients. Furthermore, plasma p-tau181 has a positive association with tau-PET and longitudinal cognitive decline in AD patients.Citation15 It also showed that p-tau181 could predict AD pathology 8 years prior to post-mortem,Citation16 indicating that p-tau 181 is a practicable blood-based biomarker of AD. However, a recent study reported plasma p-tau181 was also elevated in patients with amyotrophic lateral sclerosis (ALS).Citation17 More recently, several studies reported that other forms of p-tau such as tau phosphorylated at threonine 217 (p-tau217) and tau phosphorylated at threonine 231 (p-tau231) had better diagnostic performances than p-tau181 as a diagnostic biomarker of AD.Citation18,Citation19 Then, a following study compared the diagnostic performance of three types of CSF p-tau biomarkers, N-terminal-directed p-tau217 (N-p-tau217), N-terminal-directed p-tau181 (N-p-tau181) and standard mid-region p-tau181 (Mid-p-tau181), found that N-p-tau217 and N-p-tau181 had a better diagnostic accuracy than Mid-p-tau181.Citation13 Furthermore, plasma p-tau217 could accurately determine AD, and discriminated non-AD dementia.Citation20,Citation21 Plasma p-tau217 is also correlated with Aβ and p-tau pathology in the brain and a good predicter of AD and cognitive decline in MCI, making it become one of the most promising blood-based AD biomarkers.Citation22
Blood Based Neurodegeneration Biomarkers (NfL, t-tau)
The concentration of plasma t-tau was elevated in patients with AD compared with cognitively normal controls, but t-tau measured in plasma do not correspond to CSF measures.Citation23 Furthermore, plasma t-tau elevation is not specific in AD, and is also found in several other neurodegenerative diseases.Citation24 CSF NfL is one of the most promising neurodegenerative biomarkers. Notably, plasma NfL corresponds well to CSF measures, making it a good blood-based biomarker.Citation25 Considering NfL being increased in multiple neurological disorders such as ALS, frontotemporal lobar degeneration (FTLD) and multiple system atrophy (MSA), it is considered to be a non-specific marker of neuronal neurodegeneration or injury and it has poor diagnostic performance for the separation of AD dementia and non-AD dementia. Recently, several studies found that NfL could serve as a biomarker for disease severity evaluation, and treatment effects monitoring.Citation5 Furthermore, patients with neurodegenerative diseases who have higher levels of NfL are associated with faster disease progression.Citation26
Blood Based Neuroinflammation Biomarkers (GFAP, and sTREM2)
Multiple lines of evidence indicate that neuroinflammation plays an important role in AD. Several necroinflammation related biomarkers such as GFAP, sTREM2, YKL-40, and S100 calcium-binding protein B (S100B) have been assessed in AD.
Previous studies have revealed that YKL-40 is also a biomarker linked to cardiovascular disease and diabetes, and its levels in serum can be altered by many other conditions.Citation27,Citation28 S100B is expressed in astrocytes and oligodendrocytes. Studies conducted at single-centers have not demonstrated any consistent evidence that S100B in plasma or serum can be used to specifically diagnose AD.Citation29 In recent years, the importance of blood GFAP and sTREM2 in the early identification of Alzheimer’s Disease has been increasingly recognized. GFAP is a well-known marker of astroglia activation. It has been reported that both CSF and serum GFAP concentrations were significantly increased in AD compared to cognitively unimpaired (CU) participants and correlates with cognitive impairment.Citation30,Citation31 Furthermore, plasma GFAP levels were significantly elevated in Aβ+ CU participants compared to Aβ- CU participants,Citation32 and it could predict clinical AD risk.Citation33 In addition, one study with small sample scale reported that serum GFAP could discriminate AD from FTLD patients (with 89% sensitivity and a specificity of 79%).Citation30 However, in another study, higher serum GFAP level is also found in FTLD and can serve as a disease severity biomarker for FTLD.Citation34 A recent study reported that plasma GFAP was correlated with both longitudinal Aβ-PET and cognitive decline, suggesting that the elevation of GFAP was a response to Aβ aggregation.Citation35 From this perspective, plasma GFAP is also considered an Aβ related biomarker. Another biomarker related to neuroinflammation is sTREM2, which regulates microglial function. It was found that plasma sTREM2 was significantly correlated with CSF sTREM2.Citation36 It indicates periphery could reflect the central system. A study reported the level of sTREM2 in plasma was highest in MCI.Citation37 However, the plasma sTREM2 concentrations between AD patients and controls are still ambiguous.Citation38 Several researches focused on the relationship between the plasma sTREM2 and other AD biomarkers. It was reported that plasma sTREM2 was significantly associated with CSF Aβ42, but not t-tau and p-tau in AD participants.Citation36 And another study investigated that plasma sTREM2 was independently related to tau-positive scan and white matter hyperintensity volume in AD and cerebral amyloid angiopathy.Citation39 Furthermore, plasma sTREM2 is also considered a potential biomarker in FTLD,Citation40 Parkinson’s disease,Citation41 and cerebrovascular injury.Citation42
Blood-Based Biomarkers’ Panel
Many studies reported that some other neurodegenerative diseases also showed positive Aβ or p-tau pathological changes pattern which emerges as comorbidities. More and more researchers realized that Aβ and p-tau represent only a fraction of the complicated pathophysiology underlying AD. In this context, systemic and comprehensive workflows were explored by several large MS-based proteomic analysis studies and revealed a number of combined blood-based potential early diagnostic biomarkers which were more specific as compared with single protein.Citation43 However, the inaccessibility of blood-based biomarkers panel detection impedes its use as the screening test for AD.
Conclusions and Future Prospects
It has been over a hundred years since the first AD patient was reported. However, immense challenges still exist in the early diagnosis, pathogenesis, and treatment of the disease. Owing to the development of sensitive and precise assays, the field of blood-based biomarkers advanced rapidly in recent years. As the discovery of effective disease-modifying therapies remains a critical need for patients with this devastating disease, blood-based biomarkers will be crucial for optimizing clinical trial strategies by facilitating early diagnosis and precise management. The usage of specific and sensitive blood-based biomarkers will help us move to a new era that AD could be better intervened in an early stage. However, tremendous efforts were needed to further validate blood-based biomarkers in unselected large-scale ethnically diverse populations. In addition, future efforts are also needed to develop and validate blood-based biomarkers for non-AD dementias in order to better discriminate the different types of dementia. If the blood-based biomarkers can achieve comparable high performance like CSF or PET testing, suspected AD patients will be easily determined at primary care settings at an early stage, which will greatly improve the situation of early diagnosis of AD.
Disclosure
The authors report no conflicts of interest in this work.
Additional information
Funding
References
- Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577–1590. doi:10.1016/S0140-6736(20)32205-4
- Palmqvist S, Insel PS, Stomrud E, et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer’s disease. EMBO Mol Med. 2019;11(12):e11170. doi:10.15252/emmm.201911170
- Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179(2):312–339. doi:10.1016/j.cell.2019.09.001
- Jack CR, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dementia. 2018;14(4):535–562. doi:10.1016/j.jalz.2018.02.018
- Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet. 2022;21(1):66–77. doi:10.1016/S1474-4422(21)00361-6
- McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dementia. 2011;7(3):263–269. doi:10.1016/j.jalz.2011.03.005
- Dubois B, Villain N, Frisoni GB, et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group. Lancet. 2021;20(6):484–496. doi:10.1016/S1474-4422(21)00066-1
- Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimers Dementia. 2022;2022:154.
- Hansson O, Zetterberg H, Vanmechelen E, et al. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment. Neurobiol Aging. 2010;31(3):357–367. doi:10.1016/j.neurobiolaging.2008.03.027
- Janelidze S, Stomrud E, Palmqvist S, et al. Plasma beta-amyloid in Alzheimer’s disease and vascular disease. Sci Rep. 2016;6:26801. doi:10.1038/srep26801
- Verberk IMW, Slot RE, Verfaillie SCJ, et al. Plasma amyloid as prescreener for the earliest Alzheimer pathological changes. Ann Neurol. 2018;84(5):648–658. doi:10.1002/ana.25334
- Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature. 2018;554(7691):249–254. doi:10.1038/nature25456
- Janelidze S, Teunissen CE, Zetterberg H, et al. Head-to-head comparison of 8 plasma amyloid-beta 42/40 assays in Alzheimer disease. JAMA Neurol. 2021;78(11):1375–1382. doi:10.1001/jamaneurol.2021.3180
- Karikari TK, Benedet AL, Ashton NJ, et al. Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer’s disease neuroimaging initiative. Mol Psychiatry. 2021;26(2):429–442. doi:10.1038/s41380-020-00923-z
- Lin RR, Xue YY, Li XY, Chen YH, Tao QQ, Wu ZY. Optimal combinations of AT(N) biomarkers to determine longitudinal cognition in the Alzheimer’s disease. Front Aging Neurosci. 2021;13:718959. doi:10.3389/fnagi.2021.718959
- Lantero Rodriguez J, Karikari TK, Suarez-Calvet M, et al. Plasma p-tau181 accurately predicts Alzheimer’s disease pathology at least 8 years prior to post-mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol. 2020;140(3):267–278. doi:10.1007/s00401-020-02195-x
- Cousins KAQ, Shaw LM, Shellikeri S, et al. Elevated plasma phosphorylated tau 181 in amyotrophic lateral sclerosis. Ann Neurol. 2022;92(5):807–818. doi:10.1002/ana.26462
- Janelidze S, Stomrud E, Smith R, et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat Commun. 2020;11(1):1683. doi:10.1038/s41467-020-15436-0
- Bayoumy S, Verberk IMW, den Dulk B, et al. Clinical and analytical comparison of six Simoa assays for plasma P-tau isoforms P-tau181, P-tau217, and P-tau231. Alzheimers Res Ther. 2021;13(1):198. doi:10.1186/s13195-021-00939-9
- Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324(8):772–781. doi:10.1001/jama.2020.12134
- Thijssen EH, La Joie R, Strom A, et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet. 2021;20(9):739–752. doi:10.1016/S1474-4422(21)00214-3
- Abbasi J. Alzheimer blood test using tau biomarker is in development. JAMA. 2020;323(14):1336.
- Shahim P, Zetterberg H, Simren J, et al. Association of plasma biomarker levels with their CSF concentration and the number and severity of concussions in professional athletes. Neurology. 2022;99(4):e347–e354. doi:10.1212/WNL.0000000000200615
- Garcia-Moreno H, Prudencio M, Thomas-Black G, et al. Tau and neurofilament light-chain as fluid biomarkers in spinocerebellar ataxia type 3. Eur J Neurol. 2022;29(8):2439–2452. doi:10.1111/ene.15373
- Li QF, Dong Y, Yang L, et al. Neurofilament light chain is a promising serum biomarker in spinocerebellar ataxia type 3. Mol Neurodegener. 2019;14(1):39. doi:10.1186/s13024-019-0338-0
- Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat Med. 2019;25(2):277–283. doi:10.1038/s41591-018-0304-3
- Rathcke CN, Vestergaard H. YKL-40--an emerging biomarker in cardiovascular disease and diabetes. Cardiovasc Diabetol. 2009;8:61. doi:10.1186/1475-2840-8-61
- Perez MF, Atuegwu NC, Mortensen EM, Oncken C. The inflammatory biomarker YKL-40 is elevated in the serum, but not the sputum, of E-cigarette users. Exp Lung Res. 2021;47(2):55–66. doi:10.1080/01902148.2020.1847216
- Steiner J, Bogerts B, Schroeter ML, Bernstein HG. S100B protein in neurodegenerative disorders. Clin Chem Lab Med. 2011;49(3):409–424. doi:10.1515/CCLM.2011.083
- Oeckl P, Halbgebauer S, Anderl-Straub S, et al. Glial fibrillary acidic protein in serum is increased in Alzheimer’s disease and correlates with cognitive impairment. J Alzheimers Dis. 2019;67(2):481–488. doi:10.3233/JAD-180325
- Abu-Rumeileh S, Steinacker P, Polischi B, et al. CSF biomarkers of neuroinflammation in distinct forms and subtypes of neurodegenerative dementia. Alzheimers Res Ther. 2019;12(1):2. doi:10.1186/s13195-019-0562-4
- Chatterjee P, Pedrini S, Stoops E, et al. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl Psychiatry. 2021;11(1):27. doi:10.1038/s41398-020-01137-1
- Beyer L, Stocker H, Rujescu D, et al. Amyloid-beta misfolding and GFAP predict risk of clinical Alzheimer’s disease diagnosis within 17 years. Alzheimers Dementia. 2022. doi:10.1002/alz.12745
- Benussi A, Ashton NJ, Karikari TK, et al. Serum Glial Fibrillary Acidic Protein (GFAP) is a marker of disease severity in frontotemporal lobar degeneration. J Alzheimers Dis. 2020;77(3):1129–1141.
- Pereira JB, Janelidze S, Smith R, et al. Plasma GFAP is an early marker of amyloid-beta but not tau pathology in Alzheimer’s disease. Brain. 2021;144(11):3505–3516. doi:10.1093/brain/awab223
- Ferri E, Rossi PD, Geraci A, Ciccone S, Cesari M, Arosio B. The sTREM2 concentrations in the blood: a marker of neurodegeneration? Front Mol Biosci. 2020;7:627931. doi:10.3389/fmolb.2020.627931
- Weber GE, Khrestian M, Tuason ED, et al. Peripheral sTREM2-related inflammatory activity alterations in early-stage Alzheimer’s disease. J Immunol. 2022;208(10):2283–2299. doi:10.4049/jimmunol.2100771
- Liu D, Cao B, Zhao Y, et al. Soluble TREM2 changes during the clinical course of Alzheimer’s disease: a meta-analysis. Neurosci Lett. 2018;686:10–16. doi:10.1016/j.neulet.2018.08.038
- Tsai HH, Chen YF, Yen RF, et al. Plasma soluble TREM2 is associated with white matter lesions independent of amyloid and tau. Brain. 2021;144(11):3371–3380. doi:10.1093/brain/awab332
- Kleinberger G, Yamanishi Y, Suarez-Calvet M, et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med. 2014;6(243):243ra286. doi:10.1126/scitranslmed.3009093
- Wilson EN, Swarovski MS, Linortner P, et al. Soluble TREM2 is elevated in Parkinson’s disease subgroups with increased CSF tau. Brain. 2020;143(3):932–943. doi:10.1093/brain/awaa021
- Lu Y, Zhao Y, Zhang Q, et al. Soluble TREM2 is associated with death and cardiovascular events after acute ischemic stroke: an observational study from CATIS. J Neuroinflammation. 2022;19(1):88. doi:10.1186/s12974-022-02440-y
- Bader JM, Geyer PE, Muller JB, et al. Proteome profiling in cerebrospinal fluid reveals novel biomarkers of Alzheimer’s disease. Mol Syst Biol. 2020;16(6):e9356. doi:10.15252/msb.20199356
