Abstract
Stroke is one of the leading causes of death in industrialized countries for people older than 65 years of age. The reasons are still unclear. A reduction of endogenous mechanisms against ischemic insults has been proposed to explain this phenomenon. The “cerebral” ischemic preconditioning mechanism is characterized by a brief episode of ischemia that renders the brain more resistant against subsequent longer ischemic events. This ischemic tolerance has been shown in numerous experimental models of cerebral ischemia. This protective mechanism seems to be reduced with aging both in experimental and clinical studies. Alterations of mediators released and/or intracellular pathways may be responsible for age-related ischemic preconditioning reduction. Agents able to mimic the “cerebral” preconditioning effect may represent a new powerful tool for the treatment of acute ischemic stroke in the elderly. In this article, animal and human cerebral ischemic preconditioning, its age-related difference, and its potential therapeutical applications are discussed.
Introduction
Stroke and myocardial infarction account for more than 85% of death in patients older than 65 years.Citation1,Citation2 Accordingly, the incidence of acute stroke and myocardial infarction increases exponentially with age.Citation3 The reasons for the high mortality for stroke and myocardial infarction in elderly patients are not clear although several factors seem to contribute to this phenomenon – risk factors such as hypertension, hyperlipidemia, and diabetes, age-related prothrombotic changes in the hemostatic system, a less aggressive therapy including thrombolytic therapy, and the presence of comorbidities.Citation4 So, why is mortality for stroke and coronary artery disease higher in the elderly? This might be due, at least in part, to the age-related reduction of an endogenous powerful protective mechanism against ischemia. Aging can be defined as a progressive functional decline of an intrinsic, inevitable, and irreversible age-related process of loss of viability and increase in vulnerability.Citation5 Thus, one possibility is that the efficacy of endogenous protective mechanisms against ischemic disease might decrease with aging.Citation6
Ischemic preconditioning
Ischemic preconditioning (IPC) is defined as brief episodes of ischemia followed by a long period of ischemia, representing the most powerful endogenous mechanism against the injurious effects of ischemia.Citation7 Several studies in animals and humans have clearly demonstrated the capacity of IPC to protect organs against ischemic injury, including the brain.Citation8,Citation9 Moreover, IPC is classified as “early” when the protective effect occurs immediately during the first 1–3 hours after the ischemic episode (“first window”), and as “delayed” when the protective effect arises 24–72 hours after the ischemic episode (“second window”).Citation10,Citation11 Other forms of preconditioning have been recently discovered: “remote preconditioning” (in which ischemia in one region of an organ causes protection in a remote region of the same or of a different organ)Citation12 and “postconditioning” in which brief episodes of artery occlusion and reperfusion at the onset of reperfusion after a sustained ischemic insult confer organ protection against ischemic–reperfusion injury.Citation13 IPC has been widely investigated in clinical cardiology. Preinfarction angina, warm-up phenomenon, and transluminal coronary angioplasty are considered the clinical equivalents of IPC.Citation10,Citation11 It is important to note that the incidence of mortality and cardiogenic shock is reduced in patients with preinfarction angina.Citation14
IPC mechanism
IPC mechanism has been widely investigated in the heart.Citation15–Citation18 From activation of G-protein coupled receptors by adenosine, norepinephrine, bradykinin, and opioids, phosphoinositide-3-kinase/protein kinase B is activated with subsequent downstream activation of nitric oxide synthase and nitric oxide formation, and guanylate cyclase, protein kinase G, and protein kinase C (PKC) activation in acute ischemia. All these events lead to the opening of the mitochondrial adenosine triphosphate-dependent potassium channels through ɛPKC. The opening of mitochondrial adenosine triphosphate-dependent potassium channels results in an influx of potassium that causes swelling of the mitochondria, which is thought to lead to the production of reactive oxygen species. Reactive oxygen species formation results in p38 mitogen-activated kinase and PKC activation and subsequent “priming” of mitochondrial permeability transition pores. During early reperfusion after the preconditioning stimulus, activation of G-protein coupled receptors or of growth factor receptors results in activation of the reperfusion injury salvage kinase program. The reperfusion injury salvage kinase program involves the parallel activation of the phosphoinositide-3-kinase/protein kinase B and the extracellular regulated kinase system with downstream p70 ribosomal protein S6 kinase and glycogen synthase kinase 3β activation leading to the inhibition of mitochondrial permeability transition pore opening with an increased survival of myocardial cells.Citation15–Citation18
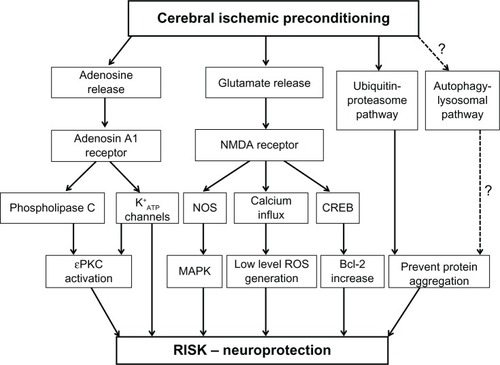
IPC has also been found in the brain as demonstrated by mammalian in vivo and in vitro studies.Citation19 A first demonstration of cerebral IPC has been found by inducing a sublethal ischemic stress, which protects against subsequent lethal ischemic injury in the hippocampal CA1 of transient global models of gerbils.Citation20 Cerebral IPC was also confirmed in different experimental models, ie, transient focal or global ischemia rat model,Citation21–Citation23 focal ischemia model,Citation24 blood–brain barrier models,Citation25 in cultured neurons,Citation26,Citation27 in brain endothelial cells,Citation28 and in oxygen–glucose-deprived rat hippocampal slices.Citation29 Cerebral IPC was also demonstrated in a study of 12 adult patients with aneurismal subarachnoid hemorrhage by 2-minute proximal temporary artery occlusion followed by 30 minutes of reperfusion.Citation30 In this study, a multi-parameter catheter inserted in the artery demonstrated an improvement of oxygen tension, carbon dioxide, tension, and pH in tissue at risk of ischemia during temporary artery occlusion. Many stimuli, stressors, and chemicals may be considered “cerebral” preconditioning triggers. Hyperthermia, hypothermia, hypoxia, oxygen species, inflammatory cytokines, spreading depression, mitochondrial inhibition, resveratrol, volatile anesthetics, adenosine triphosphate-sensitive potassium channel openers, antibiotics, myelin basic proteins, and E-selectin induce resistance to ischemic injury in the brain.Citation7,Citation8 Moreover, IPC leads to a release of adenosine and glutamate into the extracellular space. In particular, glutamate, which is a physiological excitatory neurotransmitter, binds to ionotropic glutamate receptors such as N-methyl-D-aspartate receptor and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid.Citation31 Excessive activation of glutamate receptors, particularly the N-methyl-D-aspartate receptor, causes a massive calcium influx that in turn activates several cell injury processes involving protease and lipase. These pathways, which are a part of the so-called glutamate– calcium theory, are believed to be central to the mechanism of cerebral ischemic tolerance and reperfusion injury salvage kinase activation ().
Figure 1 Signaling pathways involved in cerebral ischemic preconditioning. Triggering pathways include activation of the NMDA and adenosine A1 receptors which in turn are involved in activating some intracellular signaling pathways such as MAPKs, PKC, bcl-2, heat shock proteins, ubiquitin–proteasome pathway, and autophagic-lysosomal pathway. These pathways probably involve activation of the RISK program.
Abbreviations: ATP, adenosine triphosphate; bcl-2, b-cell lymphoma 2; CREB, cyclic adenosine monophosphate responsive element binding protein; MAPK, mitogen-activated protein kinase; NMDA receptor, N-methyl-D-aspartate receptor; NOS, nitric oxide synthase; PKC, protein kinase C; RISK, reperfusion injury salvage kinase; ROS, reactive oxygen species.
Transient ischemic attacks and cerebral IPC
Several clinical reports support the existence of cerebral IPC in the human brain. The more typical clinical equivalent of cerebral IPC is Transient ischemic attack (TIA), which determines better outcomes in patients with ischemic stroke than those without TIA.Citation32–Citation36 On the other hand, vascular comorbidities highly prevalent in the elderly such as atrial fibrillation, valvular heart disease, coronary artery disease, and chronic heart failure are significant risk factors for TIA development.Citation37 Thus, whether TIA preserves its protective effect represents a critical point in the prognosis of ischemic stroke in the elderly.
In a retrospective case–control study in 148 adult stroke patients with and without antecedent TIA, favorable clinical outcomes were significantly associated with the presence of prior TIA.Citation32 Among 2,490 stroke patients younger than 65 years, 293 patients who had had ipsilateral TIA a few days before cerebral ischemic injury had favorable neurologic outcomes compared to patients without prior TIA.Citation33 Prior TIA was associated with favorable outcomes in nonlacunar stroke in another study of 1,753 patients younger than 65 years of age.Citation36 In addition, improved National Institutes of Health Stroke Scale score was demonstrated in adult patients who had a TIA 1–7 days before ischemic stroke.Citation38 Finally, magnetic resonance images of patients with ischemic cerebral damage with and without TIA before stroke demonstrated that the extension of the lesion was clearly smaller in the patients with TIA prior to stroke.Citation35 In contrast, in a cohort of 180 patients with TIA and subsequent ischemic stroke within 90 days of TIA, there was no association between duration of TIA – used as a surrogate for degree of ischemia – and likelihood of disability from a subsequent stroke. Furthermore, there was no difference in rates of disability among patients with strokes occurring within 1 day, 1 to <7 days, and 7–90 days after TIA.Citation39
Age-related reduction of IPC
Experimental and clinical studies have demonstrated an age-related reduction of IPC-mediated protection in the aged heart.Citation4 Age-related reduction of IPC was first demonstrated in the isolated and perfused rat heart from 24-month-old rats subjected to an IPC with a short period of ischemia (2 minutes) followed by 10 minutes of reperfusion ().Citation4 Preinfarction angina exerted a beneficial effect on in-hospital outcomes in adult patients younger than 65 years of age but not in those patients 65 years of age or older (). Thus, IPC seems to be lost in senescent patients.Citation4 Interestingly, caloric restriction and physical activity are able to restore IPC in aged hearts in both animals and humans.Citation40 Several mechanisms have been proposed to explain the age-related reduction of IPC-mediated protection in the heart. An age-related reduction of norepinephrine release in response to “cardiac” IPC stimulus is one of the most accredited hypotheses.Citation4 Moreover, several studies demonstrated an age-related impairment of IPC trigger and transduction mechanisms, eg, adenosine, PKC, and mitochondria metabolism.Citation4
Age-related reduction of cerebral IPC
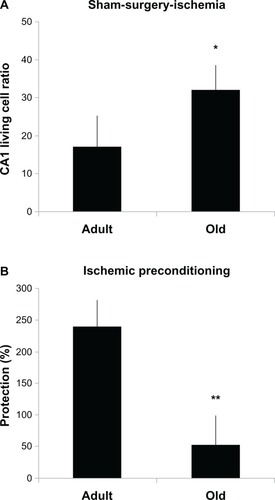
The protective effect of IPC in the brain has been well established in several animal studies.Citation41 However, whether IPC is present in the aged brain is still unknown.Citation42 In young animals, IPC protects CA1 hippocampal neurons against global ischemia.Citation20 In a population of aged (18- to 20-month-old) gerbils, 10 days after ischemia there was over 80% protection of CA1 neurons in ischemic preconditioned animals compared with 6% in ischemic gerbils. CA1 cell survival declined to approximately 75% of sham values at 60 days after ischemia.Citation43 Thus, IPC seems to provide a substantial neuroprotection in aged gerbils. However, the study did not compare young and aged gerbils. In a study where aged rats (4- and 24-months) were subjected to IPC (3-minute ischemia) followed by 10-minute ischemia, the assessments of histology and the immunoreactivity of N-methyl-D-aspartic acid receptor 1 and caspase-3 active peptide in the hippocampal CA1 region were performed 8 days after full ischemia. The study showed that the degree of cerebral protection against full ischemia was reduced in the aged and preconditioned rats compared with the young rats ().Citation44
Figure 2 CA1 “living cell ratio” was greater in the aged sham-surgery–ischemia group than in the young group (32% ± 6% versus 17% ± 5%, *P < 0.05) (A), whereas the degree of protection against full ischemia afforded by cerebral ischemic preconditioning was reduced in the aged compared with the young (53% ± 17% versus 241% ± 25%, **P < 0.0001) (B).
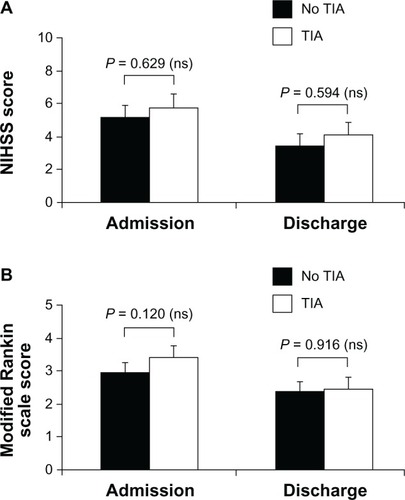
Similar to “cardiac” IPC, the clinical equivalent of “cerebral” IPC is TIA, a brief ischemic event occurring before a prolonged ischemic period in the same vascular territory leading to ischemic stroke.Citation41,Citation45,Citation46 Studies conducted in patients with stroke younger than 65 years reported age-favorable clinical outcomes associated with prior TIA.Citation32–Citation36,Citation39 At present, data on “cerebral” IPC in elderly patients with stroke are limited. In a study of 203 patients aged 65 years or older with diagnosis of acute ischemic stroke, no significant differences in the admission or discharge National Institutes of Health Stroke Status and modified Rankin scores were observed between patients who had TIA (n = 42, 21%) within 72 hours of stroke onset and those without TIA (n = 161, 79%) ().Citation47
Figure 3 Neurologic status evaluated according to NIHSS (A) and disability assessed with modified Rankin scale (B) in elderly patients with or without TIA before stroke.
Abbreviations: NIHSS, National Institutes of Health Stroke Scale; ns, not significant; TIA, transient ischemic attack.
Reduction of “cerebral” IPC protection in the aged brain may be due to the composite mechanisms that characterize the aged brain, ie, loss in number of neurons, impairment in the mitochondria function with an increase in reactive oxygen species production, alteration in gene expression and metabolic regulation, and alteration in intracellular calcium homeostasis. All these modifications make the organ more susceptible to stress such as ischemia.Citation48 In particular, a gene expression profile following middle cerebral anterior occlusion shows reduced transcriptional activity, proapoptotic genes, and downregulation of axonogenesis and neurogenesis in the periinfarct area, which is more pronounced in aged versus young animals.Citation49 These findings suggest that an aging brain is capable of upregulating gene expression, but the response is often blunted and temporally uncoordinated in response to cerebral ischemia.Citation50
Therapeutical attempts
It is now established that IPC may be useful in cardiac surgery and cardiac transplantation as an adjunctive protective mechanism.Citation11 The development and/or discovery of drugs or techniques able to mimic the IPC protective effect in the brain are still not ready for implementation in clinical practice.Citation51 PKC isozyme-selective activators and inhibitors have been proposed as potential therapeutic compounds in both the heart and brain because of their capacity to mediate beneficial or injurious effects in response to ischemic insult.Citation49 In particular, ψɛ-receptors for activated C kinase, an ɛPKC-selective peptide activator, injected intravenously 30 minutes before induction of global cerebral ischemia conferred neuroprotection in the CA1 region of the rat hippocampus.Citation52 In contrast, it has been demonstrated that rats treated with δPKC specific inhibitor (δV1-1) prior to cerebral ischemia exhibited improved perfusion after 24 hours and less hippocampal CA1 neuronal death 7 days after arterial occlusion.Citation53 These data suggest an opposite role of δPKC compared to ɛPKC in the mechanism of IPC-mediated neuroprotection. Finally, resveratrol, a natural polyphenol found in grapes and wine, was able to mimic IPC inducing neuroprotection against ischemia in both in vitro and in vivo studies.Citation54 In fact, resveratrol and IPC were able to increase sirtuin 1 activity and decrease uncoupling protein 2 at the mitochondrial level with the result of an increase in hippocampal mitochondrial oxygen consumption rates. The positive effects on restoring “cardiac” IPC in an aging heart by exercise training and caloric restriction has not been evaluated yet in the aging brain and awaits confirmation in a clinical trial.Citation4
Current studies and perspectives
The reduction of cerebral IPC with age represents a strong rationale to induce IPC in elderly patients to therefore decrease stroke-related mortality. However, IPC application in clinical practice is still a future prospect. Based on the protective effect of intravenous administration of erythropoietin and its participation in ischemic tolerance, a multi-center randomized trial with acute ischemic stroke patients was performed to test the protective effects of recombinant erythropoietin administered within the 6-hour window after stroke onset.Citation55 Unfortunately, the primary outcome measure failed to show a beneficial effect. Prior to carotid endarterectomyCitation56 and after subarachnoid hemorrhage,Citation57 the safety and feasibility of remote ischemic limb preconditioning has already been demonstrated in patients. Recently, the National Heart, Lung, and Blood Institute organized a preclinical consortium named the Consortium for Preclinical Assessment of Cardioprotective Therapies (CAESAR). This consortium will conduct studies by screening promising therapies and identifying those that are truly effective in relevant experimental models and thus most likely to be effective in patients.Citation58,Citation59 As underlined previously, IPC was first identified in the heart and successively in the brain. It is likely that some of the processes essential for the development of IPC are the same in both tissues.
Conclusion
Accumulating evidence clearly shows that preconditioning of the brain with a short period of ischemia activates specific pathways able to protect the brain from a prolonged ischemic insult and improves outcomes if the ischemic events occur. However, this powerful protective mechanism seems to be reduced in aging brains in both animal and human studies. The possible absence of preconditioning capacity in the brain may account, in part, for the higher mortality from stroke observed in elderly patients. Agents able to mimic the “cerebral” preconditioning effect may represent a new and powerful therapeutic option for the treatment of acute ischemic stroke in the elderly. Further studies are needed to establish the age-related reduction of “cerebral” IPC in the aging brain and translate these discoveries to clinical practice.
Acknowledgments
Tatjana Rundek and David Della-Morte were supported by the NIH/NINDS K24 NS 062737 grant.
Disclosure
The authors report no conflicts of interest in this work.
References
- DeFrancesCJHallMJPodgornikMNAdvance Data From Vital and Health Statistics No 359: 2003 National Hospital Discharge SurveyHyattsville, MDNational Center for Health Statistics2005 Available from: http://www.cdc.gov/nchs/data/ad/ad359.pdfAccessed July 7, 2013
- TruelsenTPiechowski-JozwiakBBonitaRMathersCBogousslavskyJBoysenGStroke incidence and prevalence in Europe: a review of available dataEur J Neurol200613658159816796582
- GoASMozaffarianDRogerVLAmerican Heart Association Statistics Committee and Stroke Statistics SubcommitteeExecutive summary: heart disease and stroke statistics – 2013 update: a report from the American Heart AssociationCirculation2013127114315223283859
- AbetePCacciatoreFTestaGIschemic preconditioning in the aging heart: from bench to bedsideAgeing Res Rev20109215316219615470
- ComfortABiological theories of agingHum Dev19701321271395465656
- JahangirASagarSTerzicAAging and cardioprotectionJ Appl Physiol200710362120212817717116
- MurryCEJenningsRBReimerKAPreconditioning with ischemia: a delay of lethal cell injury in ischemic myocardiumCirculation1986745112411363769170
- DirnaglUSimonRPHallenbeckJMIschemic tolerance and endogenous neuroprotectionTrends Neurosci200326524825412744841
- LiuXQShengRQinZHThe neuroprotective mechanism of brain ischemic preconditioningActa Pharmacol Sin20093081071108019617892
- KlonerRABolliRMarbanEReinlibLBraunwaldEMedical and cellular implications of stunning, hibernation, and preconditioning: an NHLBI workshopCirculation19989718184818679603540
- YellonDMDowneyJMPreconditioning the myocardium: from cellular physiology to clinical cardiologyPhysiol Rev20038341113115114506302
- PrzyklenkKWhittakerPRemote ischemic preconditioning: current knowledge, unresolved questions, and future prioritiesJ Cardiovasc Pharmacol Ther2011163–425525921821525
- Vinten-JohansenJShiWPerconditioning and postconditioning: current knowledge, knowledge gaps, barriers to adoption, and future directionsJ Cardiovasc Pharmacol Ther2011163–426026621821526
- KlonerRAShookTPrzyklenkKPrevious angina alters in-hospital outcome in TIMI 4: a clinical correlate to preconditioning?Circulation199591137457805217
- HausenloyDJYellonDMSurvival kinases in ischemic preconditioning and postconditioningCardiovasc Res200670224025316545352
- HalestrapAPClarkeSJKhaliulinIThe role of mitochondria in protection of the heart by preconditioningBiochim Biophys Acta2007176781007103117631856
- HeuschGBoenglerKSchulzRCardioprotection: nitric oxide, protein kinases, and mitochondriaCirculation2008118191915191918981312
- HausenloyDJOngSBYellonDMThe mitochondrial permeability transition pore as a target for preconditioning and postconditioningBasic Res Cardiol2009104218920219242644
- Perez-PinzonMANeuroprotective effects of ischemic preconditioning in brain mitochondria following cerebral ischemiaJ Bioenerg Biomembr200436432332715377866
- KitagawaKMatsumotoMTagayaM“Ischemic tolerance” phenomenon found in the brainBrain Res1990528121242245337
- StaglianoNEPerez-PinzonMAMoskowitzMAHuangPLFocal ischemic preconditioning induces rapid tolerance to middle cerebral artery occlusion in miceJ Cereb Blood Flow Metab199919775776110413030
- NishiSTakiWUemuraYIschemic tolerance due to the induction of HSP70 in a rat ischemic recirculation modelBrain Res199361522812888364736
- DaveKRSaulIBustoRGinsbergMDSickTJPerez-PinzonMAIschemic preconditioning preserves mitochondrial function after global cerebral ischemia in rat hippocampusJ Cereb Blood Flow Metab200121121401141011740201
- ChenYRuetzlerCPandipatiSMucosal tolerance to E-selectin provides cell-mediated protection against ischemic brain injuryProc Natl Acad Sci U S A200310025151071511214645708
- GesueteROrsiniFZanierERGlial cells drive preconditioning-induced blood–brain barrier protectionStroke20114251445145321474800
- GrabbMCChoiDWIschemic tolerance in murine cortical cell culture: critical role for NMDA receptorsJ Neurosci19991951657166210024352
- BlondeauNWidmannCLazdunskiMHeurteauxCActivation of the nuclear factor-κB is a key event in brain toleranceJ Neurosci200121134668467711425894
- AndjelkovicAVStamatovicSMKeepRFThe protective effects of preconditioning on cerebral endothelial cells in vitroJ Cereb Blood Flow Metab200323111348135514600442
- XuGPDaveKRViveroRSchmidt-KastnerRSickTJPerez-PinzonMAImprovement in neuronal survival after ischemic preconditioning in hippocampal slice culturesBrain Res2002952215315812376175
- ChanMTBoetRNgSCPoonWSGinTEffect of ischemic preconditioning on brain tissue gases and pH during temporary cerebral artery occlusionActa Neurochir Suppl200595939616463828
- BondALodgeDHicksCAWardMAO’NeillMJNMDA receptor antagonism, but not AMPA receptor antagonism attenuates induced ischemic tolerance in the gerbil hippocampusEur J Pharmacol19993802–3919910513567
- WeihMKallenbergKBergkAAttenuated stroke severity after prodromal TIA: a role for ischemic tolerance in the brain?Stroke19993091851185410471435
- MoncayoJde FreitasGRBogousslavskyJAltieriMvan MelleGDo transient ischemic attacks have a neuroprotective effect?Neurology200054112089209410851368
- CastilloJMoroMABlancoMThe release of tumor necrosis factor-α is associated with ischemic tolerance in human strokeAnn Neurol200354681181914681891
- WegenerSGottschalkBJovanovicVMRI in Acute Stroke Study Group of the German Competence Network StrokeTransient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging studyStroke200435361662114963288
- ArboixACabezaNGarcia-ErolesLRelevance of transient ischemic attack to early neurological recovery after nonlacunar ischemic strokeCerebrovasc Dis200418430431115331877
- GaliziaGAbetePMussiCRole of early symptoms in assessment of syncope in elderly people: results from the Italian group for the study of syncope in the elderlyJ Am Geriatr Soc2009571182319054186
- SchallerBIschemic preconditioning as induction of ischemic tolerance after transient ischemic attacks in human brain: its clinical relevanceNeurosci Lett2005377320621115755527
- JohnstonSCIschemic preconditioning from transient ischemic attacks? Data from the Northern California TIA StudyStroke20043511 Suppl 12680268215388902
- AbetePFerraraNCacciatoreFHigh level of physical activity preserves the cardioprotective effect of preinfarction angina in elderly patientsJ Am Coll Cardiol20013851357136511691508
- Perez-PinzonMAMechanisms of neuroprotection during ischemic preconditioning: lessons from anoxic toleranceComp Biochem Physiol A Mol Integr Physiol2007147229129917045830
- Della-MorteDCasoVGuadagniFA continuous debate about cerebral ischemic preconditioning in the elderlyJ Neurol Neurophysiol20112101e
- DowdenJCorbettDIschemic preconditioning in 18- to 20-month-old gerbils: long-term survival with functional outcome measuresStroke19993061240124610356107
- HeZCrookJEMeschiaJFBrottTJDicksonDWMcKinneyMAging blunts ischemic preconditioning-induced neuroprotection following transient global ischemia in ratsCurr Neurovasc Res20052536537416375718
- SchallerBGrafRCerebral ischemic preconditioning. An experimental phenomenon or a clinical important entity of stroke prevention?J Neurol2002249111503151112420088
- KitagawaKIschemic tolerance in the brain: endogenous adaptive machinery against ischemic stressJ Neurosci Res20129051043105422302606
- Della-MorteDAbetePGallucciFTransient ischemic attack before nonlacunar ischemic stroke in the elderlyJ Stroke Cerebrovasc Dis200817525726218755403
- ShankarSKBiology of aging brainIndian J Pathol Microbiol201053459560421045377
- BudasGRChurchillENMochly-RosenDCardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injuryPharmacol Res200755652353617576073
- Della-MorteDGuadagniFPalmirottaRGenetics of ischemic stroke, stroke-related risk factors, stroke precursors and treatmentsPharmacogenomics201213559561322462751
- KochSSaccoRLPerez-PinzonMAPreconditioning the brain: moving on to the next frontier of neurotherapeuticsStroke20124361455145722461331
- Della-MorteDRavalAPDaveKRLinHWPerez-PinzonMAPost-ischemic activation of protein kinase C ɛ protects the hippocampus from cerebral ischemic injury via alterations in cerebral blood flowNeurosci Lett2012487215816220951185
- LinHWDella-MorteDThompsonJWDifferential effects of δ and ɛ protein kinase C in modulation of postischemic cerebral blood flowAdv Exp Med Biol2012737636922259083
- Della-MorteDDaveKRDeFazioRABaoYCRavalAPPerez-PinzonMAResveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathwayNeuroscience20091593993100219356683
- EhrenreichHWeissenbornKPrangeHEPO Stroke Trial GroupRecombinant human erythropoietin in the treatment of acute ischemic strokeStroke20094012e647e65619834012
- WalshSRNouraeiSATangTYSadatUCarpenterRHGauntMERemote ischemic preconditioning for cerebral and cardiac protection during carotid endarterectomy: results from a pilot randomized clinical trialVasc Endovascular Surg201044643443920484064
- KochSKatsnelsonMDongCPerez-PinzonMRemote ischemic limb preconditioning after subarachnoid hemorrhage: a Phase Ib study of safety and feasibilityStroke20114251387139121415404
- LeferDJBolliRDevelopment of an NIH Consortium for Preclinical Assessment of Cardioprotective Therapies (CAESAR): a paradigm shift in studies of infarct size limitationJ Cardiovasc Pharmacol Ther2011163–433233921821536
- AbetePTestaGDella-MorteDTreatment for chronic heart failure in the elderly: current practice and problemsHeart Fail Rev20131845295123124913