Abstract
Aim
To identify a potential efficacy–effectiveness gap and possible explanations (drivers of effectiveness) for differences between results of randomized controlled trials (RCTs) and observational studies investigating glucose-lowering drugs.
Methods
A systematic literature review was conducted in English language articles published between 1 January, 2000 and 31 January, 2015 describing either RCTs or observational studies comparing glucagon-like peptide-1 analogs (GLP-1) with insulin or comparing dipeptidyl peptidase-4 inhibitors (DPP-4i) with sulfonylurea, all with change in glycated hemoglobin (HbA1c) as outcome. Medline, Embase, Current Content, and Biosis were searched. Information on effect estimates, baseline characteristics of the study population, publication year, study duration, and number of patients, and for observational studies, characteristics related to confounding adjustment and selection- and information bias were extracted.
Results
From 312 hits, 11 RCTs and 7 observational studies comparing GLP-1 with insulin, and from 474 hits, 16 RCTs and 4 observational studies comparing DPP-4i with sulfonylurea were finally included. No differences were observed in baseline characteristics of the study populations (age, sex, body mass index, time since diagnosis of type 2 diabetes mellitus, and HbA1c) or effect sizes across study designs. Mean effect sizes ranged from −0.43 to 0.91 and from −0.80 to 1.13 in RCTs and observational studies, respectively, comparing GLP-1 with insulin, and from −0.13 to 2.70 and −0.20 to 0.30 in RCTs and observational studies, respectively, comparing DPP-4i and sulfonylurea. Generally, the identified observational studies held potential flaws with regard to confounding adjustment and selection- and information bias.
Conclusions
Neither potential drivers of effectiveness nor an efficacy–effectiveness gap were identified. However, the limited number of studies and potential problems with confounding adjustment, selection- and information bias in the observational studies, may have hidden a true efficacy-effectiveness gap.
Introduction
The beneficial effects of drugs can be divided into efficacy and effectiveness. The efficacy of a drug describes the biological effect and can be seen as the effect evaluated under optimal conditions in randomized controlled trials (RCTs). The effectiveness of a drug describes the effect under circumstances of routine clinical practice. The efficacy–effectiveness gap refers to the difference between the (in theory) largest possible effect of a drug and its effect in clinical practice.Citation1–Citation4 A comparison of RCTs and observational studies can be used as a model to investigate and better understand the efficacy–effectiveness gap.
The population in routine clinical practice may differ from the often highly selected study population included in RCTs,Citation5–Citation10 which could be one possible reason for an efficacy–effectiveness gap. Observational studies usually reflect the population seen in clinical practice, and also other factors such as the delivery of care, adherence to treatment, and time between treatment and assessment of the outcome are often more similar to ordinary clinical practice than that which is seen in RCTs because observational studies are often based on real-world data.Citation11 Discrepancies in the results from RCTs and observational studies may be due to biases in the observational study design,Citation12–Citation15 but may also be explained by an efficacy–effectiveness gap. An understanding of the efficacy–effectiveness gap is important for patients, health care professionals, payers, regulators, and the pharmaceutical industry to provide effective treatments.Citation3,Citation16
The aim of this literature review is to identify a potential efficacy–effectiveness gap, by comparing RCTs and observational studies investigating glucose-lowering drugs in relation to change in glycated hemoglobin (HbA1c), and if such a gap exists, to investigate whether it can be explained by differences in the baseline characteristics of the study populations or other features that characterize the RCTs and observational studies.
Methods
A systematic literature search was performed to identify RCTs and observational studies fulfilling the following inclusion criteria: published between 1 January, 2000 and 31 January, 2015 in English language and compared either glucagon-like peptide-1 analogs (GLP-1) with insulin or dipeptidyl peptidase-4 inhibitors (DPP-4i) with sulfonylurea, all with change in HbA1c as an outcome. The chosen comparator groups were to compare second-line (DPP-4i and sulfonylurea) and third-line (GLP-1 and insulin) treatments, respectively.Citation17 Especially, observational studies are difficult to identify, and therefore, more search terms were used to identify such studies, and covered both prospective and retrospective studies, as well as cohort and case–control studies. The key terms and the combination of these can be found in the supplementary material. The following databases were used: Medline, Embase, Current Content, and Biosis. The search strategy was developed by one of the reviewers (MZA) and a librarian. References of the identified studies were searched to identify additional relevant studies.
The studies identified through the literature search were screened on title and abstract by two reviewers independently. Disagreements were settled through discussions and consensus. Full text was read by a single reviewer, who extracted information on the baseline characteristics of the study population, other features that described the included studies, and effect estimate from text and tables in the included studies. Some of the hits from the search were abstracts published in relation to scientific conferences. Information from conference abstracts was not included in this review. If a conference abstract seemed relevant, an attempt was made to identify the published studies related to the conference abstract by web search and by contacting the authors of the conference abstract.
Post hoc, it was decided to exclude studies comparing DPP-4i with sulfonylurea during Ramadan in Muslim populations (three RCTs and six observational studies) because we did not want to compare across fasting and nonfasting studies and to exclude studies with fast-acting insulin (five RCTs) because we did not want to compare across fast-acting and basal insulins. Studies investigating mixed insulin (combination of fast-acting and intermediate/long-acting) were included.
Post hoc exclusion criteria were applied as we gained knowledge when working on the review. Importantly, none of the post hoc exclusion criteria are in conflict with the initial inclusion criteria and they only narrow the inclusion criteria further.
If the identified RCTs and the observational studies included treatment arms of other drugs or placebo, only information about the relevant treatment arms was extracted. If several publications were based on the same study population, but with different follow-up time, the information on patient characteristics was extracted once, while each effect size at different time points was extracted. If studies included several analyses, for example, intention-to-treat and per protocol, the analysis that was reported as the primary analysis was extracted. Two RCTsCitation18,Citation19 included a once-daily and a twice-daily insulin group; GLP-1 vs. twice-daily insulin is reported later. Two RCTsCitation20,Citation21 included a high and a low dose of GLP-1 and DPP-4i, respectively; high dose vs. comparator is reported later. Generally, the data extraction protocol was based on the Cochrane Handbook:Citation22 Baseline characteristics were extracted as mean and standard deviation (SD) or proportion. A few studies reported median and interquartile range, and in those cases, SD was derived by dividing interquartile range by 1.35.Citation22 The reported outcome is the difference in change in HbA1c between treatment groups. When extracting effect estimates, the following prioritization was used: 1) effect estimate and 95% confidence interval (CI) as written in text; 2) effect estimate and 95% CI as written in a table; 3) if, for example, one-sided interval was given, then the two-sided 95% CI was calculated; 4) if no effect size with CI was given, these were calculated from the effect estimate and SD or standard error of the mean (SEM) in each treatment group; 5) if no SD or SEM, but a p value was given, then z values were calculated, and from this SEM and 95% CI; and 6) if only an effect estimate was reported and no CI or a p value, only the point estimate was used.
For the observational studies, additional information was extracted: confounding adjustment, analysis of initiator by having a “wash-out” period, selection bias related to clear and reasonable inclusion criteria or handling of missing data, and information bias related to the assessment of exposure and outcome. Comprehensive methods to assess quality of observational studies, such as, for example, ACROBATENRSI,Citation23 were not deemed necessary because the aim was not to have an estimate of the overall treatment effect across studies, but rather to look at signals of an efficacy–effectiveness gap and potential drivers of such a gap. In relation to this, pooled estimates of the study characteristics and the effect estimates were not performed. The literature search and inclusion of studies did not strive to get homogeneous studies suitable for pooled estimates. Instead, baseline characteristics and effect estimates were handled descriptively. The overlap of patient characteristics and effect estimate was used to assess if difference was present across studies. A difference >0.4% units is acknowledged as a clinically meaningful difference in HbA1cCitation24 and was used to evaluate an efficacy–effectiveness gap.
Results
The search for studies comparing GLP-1 with insulin showed 312 hits, of which 19 publications were included. However, the three publications by Diamant et alCitation25–Citation27 were based on the same RCT, but with different follow-up time, and the study by Thayer et alCitation28 included two cohorts, which were reported separately later. Hence, 13 publications described 11 individual RCTsCitation18–Citation20,Citation25–Citation27,Citation29–Citation35 and 6 publications described 7 individual observational studiesCitation28,Citation36–Citation40 (). The study duration ranged from 16 to 156 weeks and from 26 to 102 weeks in RCTs and observational studies, respectively, and the number of participants ranged from 69 to 1028 and from 47 to 51,977, respectively. Among the 312 hits, 9 were conference abstracts of observational studies, of which 1 was among the included observational studies as a research article. The authors of the other conference abstracts were contacted; one author replied, and no additional full-text study was identified.
Figure 1 Flow chart.
Abbreviation: RCTs, randomized controlled trials.
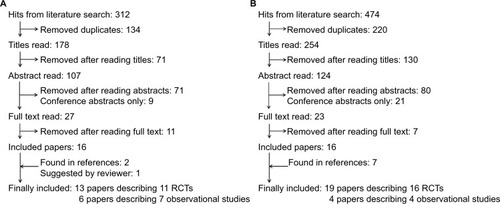
The search for studies comparing DPP-4i with sulfonylurea showed 474 hits, of which 23 publications were included. However, the publications by Nauck et al,Citation41 Seck et al,Citation42 Ferrannini et al,Citation43 and Matthews et al,Citation44 and the two publications by Göke et al,Citation45,Citation46 respectively, were based on the same RCTs with different follow-up time. Hence, 19 publications described 16 individual RCTsCitation21,Citation41–Citation58 and 4 publications described 4 individual observational studiesCitation59–Citation62 (). The study duration ranged from 4 to 104 weeks and from 24 to 52 weeks in RCTs and observational studies, respectively, and the number of participants ranged from 33 to 3118 and from 69 to 16,832, respectively. Among the 474 hits, 4 and 17 were conference abstracts of RCTs and observational studies, respectively, of which 2 were among the included RCTs as research articles. The authors of the other conference abstracts were contacted; none of them replied, and no additional full-text study was identified.
More detailed information on the included studies is found in Tables S1–S4.
holds information on study population characteristics of the 17 individual studies (10 RCTs and 7 observational studies) and the effect estimates from the 18 publications comparing GLP-1 with insulin. holds information on study population characteristics of the 20 individual studies (16 RCTs and 4 observational studies) and the effect estimates of the 23 publications comparing DPP-4i with sulfonylurea.
Table 1 Characteristics of RCTs and observational studies comparing glucagon-like peptide-1 with insulin
Table 2 Characteristics of RCTs and observational studies comparing dipeptidyl peptidase-4 inhibitors with sulfonylurea
Characteristics of observational studies
Of the 11 individual observational studies,Citation28,Citation36–Citation40,Citation59–Citation62 4 were prospectiveCitation39,Citation60–Citation62 and 7 were based on registries.Citation28,Citation36–Citation38,Citation40,Citation59 Information of exposure in the prospective studies was based on doctor’s records of prescription, whereas exposure in registry studies was based on databases with information on prescriptionCitation36–Citation38,Citation40,Citation59 or claims.Citation28 The outcome in all studies was based on the clinical measure of HbA1c. The inclusion criteria in the studies were primarily based on previous medication, but also age and comorbidity were used in most studies. The observational studies analyzed patients who initiated either GLP-1 or insulin, or DPP-4i or sulfonylurea, respectively. Five of the observational studies excluded patients if information was missing,Citation28,Citation36–Citation38 while the other six studies did not mention how missing data were handled.Citation39,Citation40,Citation59–Citation62 Five of the observational studies used multivariable regressionCitation38–Citation40,Citation60 or propensity score matchingCitation37 to adjust for potential confounding, although Karagianni et alCitation39 only included body mass index (BMI) and age in the model. Unadjusted effect estimates were reported in the remaining six observational studies.Citation28,Citation36,Citation59,Citation61,Citation62 Generally, the design of the included observational studies was deemed suboptimal regarding confounding adjustment and the potential for selection- and information bias. However, two observational studies – one studyCitation37 comparing GLP-1 with insulin and another studyCitation60 comparing DPP-4i and sulfonylurea – were explicit about the conducted analysis, including confounding adjustment, and gave information about possible selection bias and information bias. Neither the effect estimate nor the patient characteristics of these studiesCitation37,Citation60 were different from the other observational studies comparing GLP-1 with insulin or comparing DPP-4i and sulfonylurea, respectively.
Characteristics of the study populations across study designs
The study populations did not differ across RCTs and observational studies with regard to age, sex ratio, BMI, time since diagnosis of type 2 diabetes mellitus, and baseline HbA1c neither in the studies that compared GLP-1 with insulin nor in the studies that compared DPP-4i with sulfonylureas. Generally, this goes for both means and SDs. One exception is HbA1c among studies of GLP-1 and insulin, where the HbA1c distribution in the observational studies was more heterogeneous than in the RCTs. Also, a few outliers should be mentioned. Among studies comparing GLP-1 with insulin, the observational study by Bounthavong et alCitation40 included almost only men and BMI was low in the RCT by Inagaki et alCitation33 (explained by a Japanese population). The range of the distribution of HbA1c is generally wider in the observational studies than in the RCTs. This indicates that the study population is more heterogeneous with regard to HbA1c in the observational studies. However, the mean of HbA1c is of similar magnitude across study designs. An outlier among the studies comparing DPP-4i with sulfonylurea is the RCT by Shimoda et al,Citation58 which included a higher proportion of women compared to the other studies. Unfortunately, information on time since diagnosis of type 2 diabetes mellitus was only available in two of the observational studies comparing GLP-1 with insulin.
Effect estimates across study designs
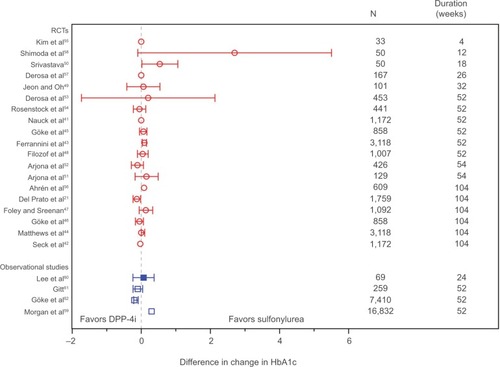
Effect estimates did not differ across RCTs and observational studies, both for studies comparing GLP-1 with insulin () and studies comparing DPP-4i with sulfonylurea (). Among studies comparing GLP-1 with insulin, a few studiesCitation18,Citation28,Citation36 reported findings outside the 95% CI of the other studies; in the observational study by Horton et alCitation36 and the two cohorts in the observational study by Thayer et al,Citation28 no adjustment for confounding was done. This could explain why the findings differ from those of the confounding adjusted observational studies and the RCTs. It should be noted that Thayer et alCitation28 did not aim for a comparison of effects across treatments. The RCT by Bergenstal et alCitation18 reported results outside the 95% CI of the other RCTs and must be seen as an outlier. Among studies comparing DPP-4i with sulfonylurea, the three observational studies reporting unadjusted effectsCitation59,Citation61,Citation62 show effect estimates of similar magnitude to the effect estimates in the confounding adjusted observational studyCitation60 and the RCTs.
Figure 2 Effect estimates of studies comparing glucagon-like peptide-1 with insulin.
Abbreviations: HbA1c, glycated hemoglobin; RCTs, randomized controlled trials.
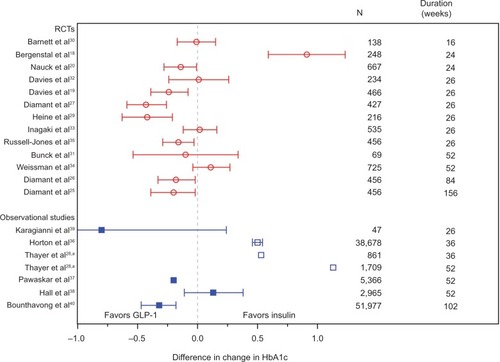
Figure 3 Effect estimates of studies comparing dipeptidyl peptidase-4 inhibitors with sulfonylurea.
Abbreviations: HbA1c, glycated hemoglobin; RCTs, randomized controlled trials.
The numbers from and are presented graphically in Figures S1 and S2. More information on the RCTs and observational studies is found in Tables S1–S4.
Discussion
No clear differences in the available baseline characteristics of the study populations and in the effect estimates of the identified RCTs and observational studies were observed in this review. Hence, no efficacy–effectiveness gap was observed and no drivers of effectiveness were identified.
Despite examples where results from RCTs and observational studies seem not to agree,Citation12–Citation15 reviews that have systematically compared the results from RCTs and observational studies have found that effect sizes from RCTs and observational studies are often similar or do not differ systematically across a range of medical subjectsCitation63,Citation64 and suggest that the theoretical efficacy–effectiveness gap may not be as widespread as often thought. This is in line with the findings in this review.
An efficacy–effectiveness gap with regard to DPP-4i (specifically vildagliptin) and sulfonylurea in relation to change in HbA1c has been investigated elsewhere;Citation65 the effect of the individual drug, that is, change from baseline of the two drugs separately, was compared across five RCTs and the one observational study. Ahrén et alCitation65 found that DPP-4i had a similar effect in the RCTs and the observational study, but that an efficacy–effectiveness gap may exist with regard to sulfonylurea because sulfonylurea proved more effective in RCTs than in the observational study. The study by Ahrén et alCitation65 is based on other data than this review because Ahrén et alCitation65 included RCTs that compared DPP-4i with placebo (only using data on the active arm), and because the observational data were based on the full EDGE study,Citation66 which was not included in this review because the EDGE study reported comparison of DPP-4i with other oral hypoglycemics and not specifically sulfonylurea. In this review article, the German part of the observational EDGE studyCitation62 was included. Also to be mentioned, it is unclear how Ahrén et alCitation65 identified the included studies, as it was not based on a systematic literature search as in this review. This review used the comparison of two drugs as outcome (change with DPP-4i subtracted from change with sulfonylurea) and did not assess the effect of the individual drugs (change for DPP-4i and sulfonylurea, respectively) as done by Ahrén et al.Citation65
Possible biases in this review could work in opposite directions, and thus hide an actual efficacy–effectiveness gap. No identification of an efficacy–effectiveness gap could be a net result of such biases. Possible biases in this review are described in the following points:
1) Unmeasured confounding is always a potential problem in observational studies, and several of the observational studies reported effects not adjusted for potential confounders. Selection bias may also have been a problem in the observational studies because inclusion criteria were only partly clear in the observational studies, and all observational studies either excluded participants with missing information or did not report how missing data were handled. From this it is clear that future observational studies in the investigated area of this review can be designed to a higher degree to avoid biases and include confounding adjustment in the analyses. A descriptive approach to identify key drivers of bias was used to assess the observational studies. As stated, the aim of this review was not to assess the quality of the studies in detail with a more comprehensive and validated tool. Rather, the descriptive approach was found sufficient to identify potential flaws in the observational studies. 2) The limited number of studies in this review may also have affected the findings. Especially, the number of observational studies was lower than the RCTs. One could speculate whether the use of a hard end point (e.g., death) would have led to a higher number of available observational studies. However, it would probably limit the amount of available RCTs. As to the number of available studies, publication bias may also have affected our results. Probably, publication bias will be most pronounced among observational studies. However, the effect estimates of the observational studies look fairly symmetric, when looking at and , which suggest no publication bias. However, a specific study on this topic is needed to draw final conclusions. It is important to note that effect estimates from the same RCTs at different follow-up time points are listed in and . However, as there was no overall effect estimated, we did not double count these studies in any pooled analysis. In the descriptive comparison of effect estimates, we wanted to make it complete, and, therefore, all effect estimates were listed. 3) Characteristics of the study populations and other features of the studies may differ in ways not quantified in the data extraction. The assessed characteristics were restricted to the information that was available in both the RCTs and the observational studies. The observational studies often included more information on patient characteristics than the RCTs, for example, distribution of comorbidities and comedication of the study population. Delivery of care and adherence to the treatments is an area where RCTs and observational studies may differ with a possible impact on treatment effect as, for example, seen in osteoporosis treatment.Citation67 However, such information was not available and, therefore, cannot be compared across study designs. Future studies based on patient-level data rather than systematic reviews may be better suited to investigate the potential drivers of effectiveness not observed in this review, for example comorbidity, comedication, delivery of care, and adherence to treatment. Studies on patient-level data are also useful to investigate effect modification of, for example, drug and patient characteristics, which will give insights in possible drivers of effectiveness. 4) It is possible that the observational studies were designed to be comparable with the RCTs with regard to, for example, the study population. If so, this would result in no efficacy–effectiveness gap because of differences in the study populations when compared in this review. However, this was neither explicitly stated in any of the observational studies nor could it be deduced from the listed inclusion criteria. 5) If the studies have had similar subgroup analyses across RCTs and observational studies, this could be used to investigate the potential efficacy–effectiveness gap even further. However, few subgroup analyses were conducted in the included studies, and not in a way that we could compare across study designs. 6) The results of this review should be interpreted in the light of GLP-1 and DPP-4i being analyzed on drug class level. It would require many more studies to do subgroup analyses on the individual drugs, and not all observational studies give information on drug names and doses. Tables S1–S4 hold the available information on drug names and doses investigated in the included studies.
In this review, HbA1c was used as outcome measure because it is the common effect measure of glucose-lowering drugs. It is important to note that this review did not aim to do a full evaluation of the included glucose-lowering drugs. Such evaluation should involve more parameters than solely change in HbA1c, for example cardiovascular events, hypoglycemic events, and weight change. We used this outcome measure as an example to study a potential efficacy–effectiveness gap. As described in the Methods section, pooled analyses were not the aim of this review. For pooled analyses to make sense, this would require more homogeneous studies, for example with regard to the duration of study, and it is likely that very few studies would be included in such analyses. Instead, the present review gives an insight into the published studies in this area, and with the inclusion of heterogenetic studies, for example with varying study duration, possible explanation of an efficacy–effectiveness gap was investigated.
To conclude, no efficacy–effectiveness gap between RCTs and observational studies comparing GLP-1 with insulin or DPP-4i with sulfonylurea was observed. However, the limited number of studies and potential problems with confounding adjustment, selection- and information bias in the observational studies, may have hidden a true efficacy-effectiveness gap. Hence, the existence of an efficacy-effectiveness gap cannot be fully excluded. No potential drivers of effectiveness were identified among age, sex, BMI, time since diagnosis of type 2 diabetes mellitus, baseline HbA1c, publication year, duration of study, and number of patients in the study.
Author contributions
MZA, RHHG and OHK conceived the study. MZA and EA designed data collection. MZA did data extraction. All authors analyzed data and interpreted the results. MZA drafted the manuscript. All authors revised the manuscript. All authors read and approved the final manuscript and agreed to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank Ida Dalgaard Pedersen from Novo Nordisk Global Information & Analysis (GLIA), Novo Nordisk A/S with help in structuring and executing the literature search. The research leading to these results was conducted as part of the GetReal consortium. For further information please refer to https://www.imi-getreal.eu/. This paper only reflects the personal views of the stated authors.
The work leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115546, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007–2013) and European Federation of Pharmaceutical Industries and Association (EFPIA) companies in kind contribution. In addition, as a special form of the IMI JU grant, University Medical Center Utrecht received a direct financial contribution from Novo Nordisk A/S to support work on this study. MZA and EA belong to EFPIA member companies in the IMI JU and costs related to their part in the research were carried by the respective company as in kind contribution under the IMI JU scheme.
Disclosure
MZA was employed by Novo Nordisk A/S as PostDoc in the IMI GetReal project. EA is employed by Novo Nordisk A/S and is a shareholder of Novo Nordisk A/S. RHHG, MSA and OHK report no conflicts of interest in this work.
References
- LuceBRDrummondMJonssonBEBM, HTA, and CER: clearing the confusionMilbank Q201088225627620579285
- EichlerHGBloechl-DaumBAbadieEBarnettDKonigFPearsonSRelative efficacy of drugs: an emerging issue between regulatory agencies and third-party payersNat Rev Drug Discov20109427729120186141
- SilvermanEEffectiveness/efficacy difference too often ignoredManag Care201322136
- NordonCKarcherHGroenwoldRHGetReal consortiumThe “Efficacy-Effectiveness Gap”: historical background and current conceptualizationValue Health2016191758126797239
- DavisCEGeneralizing from clinical trialsControl Clin Trials199415111148149768
- BaileyKRGeneralizing the results of randomized clinical trialsControl Clin Trials199415115238149769
- BrittonAMcKeeMBlackNMcPhersonKSandersonCBainCThreats to applicability of randomised trials: exclusions and selective participationJ Health Serv Res Policy19994211212110387403
- DowdRReckerRRHeaneyRPStudy subjects and ordinary patientsOsteoporos Int200011653353610982170
- KhanAYPreskornSHBakerBEffect of study criteria on recruitment and generalizability of the resultsJ Clin Psychopharmacol200525327127515876909
- Van SpallHGTorenAKissAFowlerRAEligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling reviewJAMA2007297111233124017374817
- SorensenHTLashTLRothmanKJBeyond randomized controlled trials: a critical comparison of trials with nonrandomized studiesHepatology20064451075108217058242
- LaupacisAMamdaniMObservational studies of treatment effectiveness: some cautionsAnn Intern Med20041401192392415172907
- LawlorDADavey SmithGEbrahimSCommentary: the hormone replacement-coronary heart disease conundrum: is this the death of observational epidemiology?Int J Epidemiol200433346446715166201
- FreidlinBKornELAssessing causal relationships between treatments and clinical outcomes: always read the fine printBone Marrow Transplant201247562663221625225
- BoykoEJObservational research–opportunities and limitationsJ Diabetes Complications201327664264824055326
- EichlerHGAbadieEBreckenridgeABridging the efficacy-effectiveness gap: a regulator’s perspective on addressing variability of drug responseNat Rev Drug Discov201110749550621720406
- The National Institute for Health and Care Excellence (NICE) [homepage on the Internet]Managing blood glucose in adults with type 2 diabetes Available from: https://pathways.nice.org.uk/pathways/type-2-diabetes-in-adults#path=view%3A/pathways/type-2-diabetes-in-adults/managing-blood-glucose-in-adults-with-type-2-diabetes.xml&content=view-node%3Anodes-insulin-based-treatmentsAccessed November 25, 2016
- BergenstalRLewinABaileyTEfficacy and safety of biphasic insulin aspart 70/30 versus exenatide in subjects with type 2 diabetes failing to achieve glycemic control with metformin and a sulfonylureaCurr Med Res Opin2009251657519210140
- DaviesMHellerSSreenanSOnce-weekly exenatide versus once- or twice-daily insulin detemir: randomized, open-label, clinical trial of efficacy and safety in patients with type 2 diabetes treated with metformin alone or in combination with sulfonylureasDiabetes Care20133651368137623275363
- NauckMHortonEAndjelkovicMT-emerge 5 Study GroupTaspoglutide, a once-weekly glucagon-like peptide 1 analogue, vs. insulin glargine titrated to target in patients with type 2 diabetes: an open-label randomized trialDiabet Med201330110911322937895
- Del PratoSCamisascaRWilsonCFleckPDurability of the efficacy and safety of alogliptin compared with glipizide in type 2 diabetes mellitus: a 2-year studyDiabetes Obes Metab201416121239124625132212
- HigginsJPTGreenSCochrane Collaboration Cochrane Handbook for Systematic Reviews of InterventionsChichester, EnglandWiley – Blackwell2008
- SterneJHigginsJReevesBA Cochrane Risk of Bias Assessment Tool: for Non-Randomized Studies of Interventions (ACROBAT-NRSI), Version 1.0.02492014 Available from: http://www.riskofbias.infoAccessed October 8, 2015
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER)Guidance for Industry Diabetes Mellitus: Developing Drugs and Therapeutic Biologics for Treatment and PreventionCDERSilver Spring, MD, USA2008
- DiamantMVan GaalLGuerciBExenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trialLancet Diabetes Endocrinol20142646447324731672
- DiamantMVan GaalLStranksSSafety and efficacy of once-weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeksDiabetes Care201235468368922357185
- DiamantMVan GaalLStranksSOnce weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trialLancet201037597332234224320609969
- ThayerSWeiWBuysmanEThe INITIATOR study: pilot data on real-world clinical and economic outcomes in US patients with type 2 diabetes initiating injectable therapyAdv Ther201330121128114024293131
- HeineRJVan GaalLFJohnsDExenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trialAnn Intern Med2005143855956916230722
- BarnettAHBurgerJJohnsDTolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trialClin Ther200729112333234818158075
- BunckMCDiamantMCornerAOne-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trialDiabetes Care200932576276819196887
- DaviesMJDonnellyRBarnettAHJonesSNicolayCKilcoyneAExenatide compared with long-acting insulin to achieve glycaemic control with minimal weight gain in patients with type 2 diabetes: results of the helping evaluate exenatide in patients with diabetes compared with long-acting insulin (HEELA) studyDiabetes Obes Metab200911121153116219930005
- InagakiNAtsumiYOuraTSaitoHImaokaTEfficacy and safety profile of exenatide once weekly compared with insulin once daily in Japanese patients with type 2 diabetes treated with oral antidiabetes drug(s): results from a 26-week, randomized, open-label, parallel-group, multicenter, noninferiority studyClin Ther20123491892190822884767
- WeissmanPNCarrMCYeJHARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylureaDiabetologia201457122475248425208756
- Russell-JonesDVaagASchmitzOLiraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study GroupLiraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trialDiabetologia200952102046205519688338
- HortonESSilbermanCDavisKLBerriaRWeight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort databaseDiabetes Care20103381759176520460445
- PawaskarMLiQHoogwerfBJMetabolic outcomes of matched patient populations initiating exenatide BID vs. insulin glargine in an ambulatory care settingDiabetes Obes Metab201214762663322321776
- HallGCMcMahonADDainMPWangEHomePDPrimary-care observational database study of the efficacy of GLP-1 receptor agonists and insulin in the UKDiabet Med201330668168623330649
- KaragianniPPolyzosSAKartaliNZografouISambanisCComparative efficacy of exenatide versus insulin glargine on glycemic control in type 2 diabetes mellitus patients inadequately treated with metformin monotherapyAdv Med Sci2013581384323640946
- BounthavongMTranJNGolshanSRetrospective cohort study evaluating exenatide twice daily and long-acting insulin analogs in a Veterans Health Administration population with type 2 diabetesDiabetes Metab201440428429125059703
- NauckMAMeiningerGShengDTerranellaLSteinPPSitagliptin Study GEfficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trialDiabetes Obes Metab20079219420517300595
- SeckTNauckMShengDSitagliptin Study 024 GroupSafety and efficacy of treatment with sitagliptin or glipizide in patients with type 2 diabetes inadequately controlled on metformin: a 2-year studyInt J Clin Pract201064556257620456211
- FerranniniEFonsecaVZinmanBFifty-two-week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapyDiabetes Obes Metab200911215716619125777
- MatthewsDRDejagerSAhrenBVildagliptin add-on to metformin produces similar efficacy and reduced hypoglycaemic risk compared with glimepiride, with no weight gain: results from a 2-year studyDiabetes Obes Metab201012978078920649630
- GökeBGallwitzBErikssonJHellqvistAGause-NilssonID1680C00001 InvestigatorsSaxagliptin is non-inferior to glipizide in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: a 52-week randomised controlled trialInt J Clin Pract201064121619163120846286
- GökeBGallwitzBErikssonJGHellqvistAGause-NilssonISaxagliptin vs. glipizide as add-on therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: long-term (52-week) extension of a 52-week randomised controlled trialInt J Clin Pract201367430731623638466
- FoleyJESreenanSEfficacy and safety comparison between the DPP-4 inhibitor vildagliptin and the sulfonylurea gliclazide after two years of monotherapy in drug-naive patients with type 2 diabetesHorm Metab Res2009411290590919705345
- FilozofCGautierJFA comparison of efficacy and safety of vildagliptin and gliclazide in combination with metformin in patients with type 2 diabetes inadequately controlled with metformin alone: a 52-week, randomized studyDiabet Med201027331832620536495
- JeonHJOhTKComparison of vildagliptin-metformin and glimepiride-metformin treatments in type 2 diabetic patientsDiabetes Metab J201135552953522111045
- SrivastavaSSaxenaGNKeshwaniPGuptaRComparing the efficacy and safety profile of sitagliptin versus glimepiride in patients of type 2 diabetes mellitus inadequately controlled with metformin aloneJ Assoc Physicians India2012602730
- Arjona FerreiraJCCorryDMogensenCEEfficacy and safety of sitagliptin in patients with type 2 diabetes and ESRD receiving dialysis: a 54-week randomized trialAm J Kidney Dis201361457958723352379
- Arjona FerreiraJCMarreMBarzilaiNEfficacy and safety of sitagliptin versus glipizide in patients with type 2 diabetes and moderate-to-severe chronic renal insufficiencyDiabetes Care20133651067107323248197
- DerosaGCiceroAFFranzettiIGA randomized, double-blind, comparative therapy evaluating sitagliptin versus glibenclamide in type 2 diabetes patients already treated with pioglitazone and metformin: a 3-year studyDiabetes Technol Ther201315321422223427864
- RosenstockJWilsonCFleckPAlogliptin versus glipizide monotherapy in elderly type 2 diabetes mellitus patients with mild hyperglycaemia: a prospective, double-blind, randomized, 1-year studyDiabetes Obes Metab2013151090691423531118
- KimHSShinJALeeSHA comparative study of the effects of a dipeptidyl peptidase-IV inhibitor and sulfonylurea on glucose variability in patients with type 2 diabetes with inadequate glycemic control on metforminDiabetes Technol Ther2013151081081624050737
- AhrénBJohnsonSLStewartMHARMONY 3 Study GroupHARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metforminDiabetes Care20143782141214824898304
- DerosaGBonaventuraABianchiLVildagliptin compared to glimepiride on post-prandial lipemia and on insulin resistance in type 2 diabetic patientsMetabolism201463795796724874591
- ShimodaSIwashitaSSekigamiTComparison of the efficacy of sitagliptin and glimepiride dose-up in Japanese patients with type 2 diabetes poorly controlled by sitagliptin and glimepiride in combinationJ Diabetes Investig201453320326
- MorganCLPooleCDEvansMBarnettAHJenkins-JonesSCurrieCJWhat next after metformin? a retrospective evaluation of the outcome of second-line, glucose-lowering therapies in people with type 2 diabetesJ Clin Endocrinol Metab201297124605461223076348
- LeeYKSongSOKimKJGlycemic effectiveness of metformin-based Ddual-combination therapies with sulphonylurea, pioglitazone, or DPP4-inhibitor in drug-naive Korean type 2 diabetic patientsDiabetes Metab J201337646547424404518
- GittAKBramlagePBinzCKreklerMDeegETschopeDPrognostic implications of DPP-4 inhibitor vs. sulfonylurea use on top of metformin in a real world setting – results of the 1 year follow-up of the prospective DiaRegis registryInt J Clin Pract201367101005101423981060
- GökeRGruenbergerJBBaderGDworakMReal-life efficacy and safety of vildagliptin compared with sulfonylureas as add-on to metformin in patients with type 2 diabetes mellitus in GermanyCurr Med Res Opin201430578578924328429
- PeinemannFTushabeDAKleijnenJUsing multiple types of studies in systematic reviews of health care interventions – a systematic reviewPLoS One2013812e8503524416098
- AnglemyerAHorvathHTBeroLHealthcare outcomes assessed with observational study designs compared with those assessed in randomized trialsCochrane Database Syst Rev20144MR000034
- AhrénBMathieuCBaderGSchweizerAFoleyJEEfficacy of vildagliptin versus sulfonylureas as add-on therapy to metformin: comparison of results from randomised controlled and observational studiesDiabetologia20145771304130724682379
- MathieuCBarnettAHBrathHEffectiveness and tolerability of second-line therapy with vildagliptin vs. other oral agents in type 2 diabetes: a real-life worldwide observational study (EDGE)Int J Clin Pract2013671094795623961850
- SirisESSelbyPLSaagKGBorgstromFHeringsRMSilvermanSLImpact of osteoporosis treatment adherence on fracture rates in North America and EuropeAm J Med2009122Suppl 2S3S13
